Introduction
Cancer is the leading cause of death in economically
developed countries and the second leading cause of death in
developing countries (1). Breast
cancer is the most frequently diagnosed cancer in females.
Worldwide, more than a million women are diagnosed with breast
cancer in 2008, accounting for 14% (458,400) of the total cancer
deaths (2). Maintaining a healthy
body weight, increasing physical activity and minimizing alcohol
intake are the best available strategies to reduce the risk of
developing breast cancer (3).
KISS1 was originally identified as a
metastasis suppressor gene in human melanoma and breast carcinoma
cells (4). The KISS1 gene
is located on chromosome 1 near q32.1 with regulatory elements
localized in chromosome 6 at 6q16.3-q23 (5). The KISS1 gene encodes a
precursor protein that is processed into several related peptides,
generically named kisspeptins (6,7),
where the major product appears to be a 54-amino acid peptide,
named kisspeptin-54 or metastin. In addition, three natural
peptides of 14-, 11- and 10-amino acids have been also identified,
sharing a common 10-amino acid C-terminal region (8). Cogent data support that loss of KISS1
expression has been associated with the progression and metastasis
of various tumors, including esophageal, brain, breast, ovarian and
melanoma (9). KISS1 was
recently shown epigenetically silenced by hypermethylation in
bladder cancer (10) and
colorectal cancer (11).
Circulating tumor cells (CTCs) captured from
peripheral blood were recently shown to predict disease outcome and
therapy response in cancer patients (12). The enumeration of CTCs at different
time-points during treatment has proven to be a reliable surrogate
marker of treatment response and a potential alternative for
non-invasive therapy monitoring (13). EpCAM as well as cytokeratin
expressing cells can be found in peripheral blood of advanced
cancer patients but are rare in healthy donors (14). Breast cancer cells of all grades
typically express the epithelial cytokeratins CK7, CK8, CK18 and
CK19 (15).
In this report, we evaluated whether KISS-1
expression and methylation patterns differed in breast cancer cell
lines, MDA-MB-231 and MDA-MB-157. Then we evaluated the inhibition
potential of KISS1 protein on tumor growth in a breast cancer
model, as evidenced by tumor size, tumor weight and circulating
tumor cells. Furthermore, mechanism(s) of an integrated KISS1
protein, as well as one truncated KISS1 protein, were examined in a
breast cancer model. It is anticipated that the results obtained
will provide novel insight into potential strategies for the
treatment of breast cancer.
Materials and methods
Cell culture
MDA-MB-231 and MDA-MB-157 cell lines were purchased
from the American Type Culture Collection (Rockville, MD, USA) and
maintained in DMEM supplemented with 10% FCS and antibiotics (100
μM penicillin and 100 μM streptomycin). Cells were
maintained in a humidified cell incubator with 5% CO2 at
37°C.
DNA extraction, sodium bisulfite
conversion and methylation-specific PCR (MSP)
Cells were incubated with medium containing 10
μM 5-aza-2′-deoxycytidine (5-aza-dC) (Sigma-Aldrich,
Carlsbad, CA, USA) for 48 h. Then we isolated DNA and carried out
MSP as the method described below. Genomic DNA was extracted from
cells using a TissueGen DNA kit according to the manufacturer’s
instructions (CWbiotech, Beijing, China). The concentration and
purity of the DNA were determined by absorbance at 260 and 280 nm
by NanoDrop™ 1000 Spectrophotometer (Thermo Scientific, Wilmington,
NC, USA). Sodium bisulfite modification for the extracted DNA was
performed using an EZ DNA Methylation™ kit strictly according to
the manufacturer’s instructions (Zymo Research, Orange, CA, USA).
Sequencing results confirmed that >99.0% of cytosine residues
were converted. The bisulfite-converted DNA was resuspended in 10
μl elution buffer and stored at −80°C until the samples were
ready for analysis. The 5 μl PCR mixture contained 10 ng
bisulfite-treated DNA, 25 mM dNTP, 0.2 U of Hot Start Taq DNA
polymerase (Takara, Dalian, China) and a 1 μM mixture of
forward and reverse primers. The primer sequences for the
methylated KISS1 gene were 5′-CGGGTTGGAAGTTTTAGC-3′ (sense)
and 5′-GCTTCGACAAACGAAAAAC-3′ (antisense) and for the unmethylated
allele were 5′-TTTTGGGTTGGAAGTTTTAGT-3′ (sense) and
5′-ACTTCAACAAACAAAAAACAAC-3′ (antisense). The PCR products were
separated in 2% agarose gel with ethidium bromide and visualized
under a UV imaging system (UVP, Upland, CA, USA).
RNA isolation and reverse
transcriptase-polymerase chain reaction (RT-PCR)
RNA (1 μg) was reverse-transcribed using AMV
reverse transcriptase (Promega, Madison, WI, USA) and amplified
using specific primers and conditions for KISS1. The
KISS1 primers used were: 5′-ATGAACTCACTGGTTTCTTGGCAG-3′
(sense) and 5′-TCACTGCCCCGCACCTG-3′ (antisense). GAPDH was
used as an internal normalization control. The GAPDH primers
used were: 5′-GAAGGCTGGGGCTCATTT-3′ (sense) and
5′-GGGGCCATCCACAGTCTT-3′ (antisense). PCR amplification of cDNA was
performed in reaction volumes of 15 μl. Finally, products
were resolved by 1% agarose gel electrophoresis and visualized by
ethidium bromide staining and a UV imaging system (UVP).
siRNA and plasmid transfections
MDA-MB-231 cells were seeded in 10-cm dishes and
grown overnight to 70% confluency, trypsinized and transfected with
siRNA targeting KISS1, or a non-targeting construct, using
Amaxa nucleofector (Lonza, Portsmouth, NH, USA). For the
preparation of truncated KISS1 proteins, the relevant sequences
were amplified from full-length KISS1 by PCR using primers that
included designed restriction sites (Table I), then digested with the relevant
restriction enzymes and ligated into pcDNA3.1. MDA-MB-231 cells
were seeded overnight and transfected with either pcDNA3.1 or
pcDNA-3.1-KISS1 fragments, using Lipofectamine™ 2000 according to
the procedures of the manufacturer (Invitrogen, Carlsbad, CA,
USA).
 | Table I.Primers used to generate integrated
and truncated forms of KISS1. |
Table I.
Primers used to generate integrated
and truncated forms of KISS1.
| Region of KISS1
amplified | Primers
(5′-3′) | Product (bp) |
|---|
| aa 1–121
(integrated) | F,
AAGCTTATGAACTCACTGGTTTCTTGGC
R, GGATTCCTTCGCGTCCGGCTTCCTCAAG | 381 |
| aa 20–121
(truncated) | F,
AAGCTTGAGCCATTA GAAAAGGTG
R, GGATTCCTTCGCGTCCGGCTTCCTCAAG | 324 |
Immunofluorescence assays
Cells were washed with PBS, fixed with 4%
paraformaldehyde, permeabilized in 2% Triton X-100 for 10 min, then
blocked with 5% bovine serum albumin in PBS containing 2% Triton
X-100 for 1 h. Anti-KISS1 antibodies (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) were incubated with cells for 2 h at room
temperature to identify the location of intact KISS1 proteins, or
truncated KISS1 fragments. Cells were then washed with PBS and
incubated with Alexa Fluor® 488 goat anti-rabbit IgG for
2 h at RT. To determine whether or not a lamellipodium was present,
cells were stained for actin and actin-associated proteins using
Fluorophore-conjugated phalloidin (Invitrogen). Cells were examined
and photographed using a laser confocal microscope (Olympus, Tokyo,
Japan).
Apoptosis assay
For the apoptosis assay, equal numbers of cells were
seeded in 6-cm plates. Following the manufacturer’s instructions
(Apoptosis Detection kit, KeyGen, Nanjing, China), cells were
trypsinized, washed twice with cold PBS, then resuspended in 200
μl binding buffer. Annexin V-FITC was added to a final
concentration of 0.5 μg/ml. Additionally samples were
incubated at room temperature in the dark. After 20 min, 300
μl binding buffer containing 0.5 μg/ml PI was added
and samples were immediately analyzed on a FACSCalibur flow
cytometer (Becton-Dickinson Medical Devices, Shanghai, China).
Cells in the stages of early apoptosis were defined as
FITC+/PI− cells.
Gelatin zymography
Fifty micrograms of protein was applied to 10%
polyacrylamide gels with 1% gelatin incorporated as a substrate for
gelatinolytic proteases. After running the gel the SDS was removed
by washing twice in 2.5% Triton X-100 for 30 min. The gels were
incubated overnight in zymography development buffer containing 50
mM Tris-HCl (pH 7.4), 2 mM NaN3 and 5 mM
CaCl2. After development the gels were stained for 3 h
in 45% methanol/10% glacial acetic acid containing 1% (w/v).
Coomassie Blue R-250 and subsequently partially destained with the
same solution without dye. The gelatinolytic activity of each MMP
was qualitatively evaluated as a clear band against the blue
stained gelatin background.
Xenograft assays
NOD SCID mice (NOD.CB17-Prkdcscid/NcrCrl) that were
3–5-weeks-old were purchased from Charles River (MA, USA). All
animals were housed four to a plastic cage with filter top. The
animal room was controlled at 20±2°C, 50±10% humidity and a 12-h
light/dark cycle. MDA-MB-231 cells (4×107 in 200
μl) or MDA-MB-157 cells (5×107 in 200 μl)
were subcutaneously injected into the axilla of each mouse. After
the tumor diameters reached 3–5 mm, the mice injected with
MDA-MB-157 cells were divided randomly into three groups and
received a 200 μl intratumoral injection of siRNA, or
scramble. The mice injected with MDA-MB-231 cells were divided
randomly into four groups and received a 200 μl intratumoral
injection of pCDNA-3.1, pCDNA-3.1-KISS1-T1 or pCDNA-3.1-KISS1-T2.
Two injections were administered at 9 a.m. and 4 p.m. every three
days. Tumor growth was then monitored for 30 days. Every five days
until the end of the experiment, one mouse from each group was
randomly selected to be sacrificed by CO2 asphyxiation
and tumors were harvested and weighed. The tissue was fixed in 4%
paraformaldehyde for histopathology analysis. All experiments with
animals were performed according to the guidelines of China Medical
University Ethics Committee.
Immunohistochemical staining (IHC)
Immunohistochemical staining was performed on
4-μm sections obtained from formalin-fixed,
paraffin-embedded blocks. Endogenous peroxidase activity was
blocked with 3% hydrogen peroxide for 30 min. Antigen retrieval was
carried out in citrate buffer (10 mM, pH 6.0) for 30 min at 95°C in
a microwave oven. Sections were incubated with primary antibody at
4°C overnight. Signaling pathway proteins were probed with:
anti-ERK, anti-phospho-ERK, anti-MEK and anti-phospho-MEK
antibodies (Santa Cruz). Afterward, sections were incubated with a
biotinylated secondary antibody and then exposed to a streptavidin
complex (HRP). Positive reactions were visualized with 3,
3′-diaminobenzidine tetrahydrochloride (DAB, Sigma), followed by
counterstaining with hematoxylin. Sections treated without primary
antibodies were used as negative controls.
Survival curves
Additional mice (n=140) were used to establish
xenografts to obtain survival curves. Mice with xenografted tumors
(as described above) that reached 3–5 mm in diameter were divided
into seven groups (n=20 for each). Survival was monitored until the
experiments were terminated due to heavy tumor burden.
Isolation and enumeration of circulating
tumor cells
The CellSearch® system (Veridex LLC,
Warren, NJ, USA) is the only US Food and Drug
Administration-cleared test for CTC enumeration in clinical
practice (16). Blood samples (7.5
ml) from breast mouse models were drawn into CellSave®
tubes (Veridex LLC), which were maintained at RT and processed
within 72 h of collection. CTCs were defined as nucleated
EpCAM-positive cells, lacking CD45 but expressing cytoplasmic
cytokeratins 8, 18 and 19. All CTC evaluations were performed by
qualified and trained personnel.
Enzyme-linked immuno sorbent assay
(ELISA)
Blood samples for metastin determination were
collected in ethylenediamine tetraacetate tubes, placed on ice and
centrifuged at 3,000 rpm for 20 min. Recovered serum was stored at
−80°C in aliquots until assayed. All samples were measured in
duplicate. Circulating serum metastin was determined using a
sandwich enzyme immunoassay (Human Metastin ELISA Phoenix
Pharmaceuticals, Burlingame, CA, USA) with a sensitivity of 0.12
ng/ml.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Differences between groups were analyzed using Student’s
t-test for continuous variables. Statistical analysis was performed
using Statistical Package for the Social Sciences (SPSS, version
17.0; SPSS, Inc.) and significance was established at
P<0.05.
Results
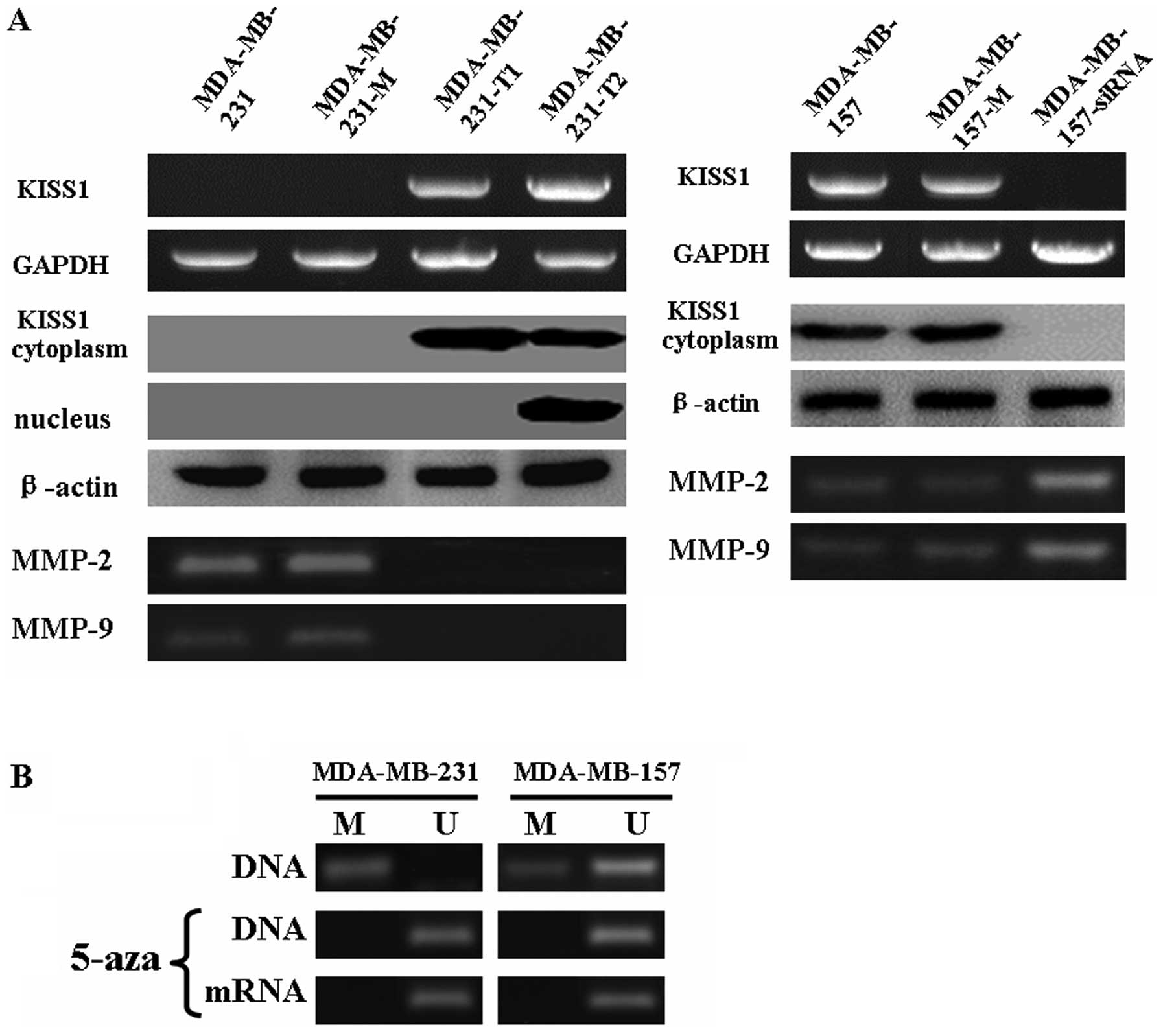
The mRNA and protein levels of KISS1 were
evaluated in MDA-MB-231 and MDA-MB-157 cells after treatment
We compared endogenous KISS1 protein level in two
breast cancer cell lines, MDA-MB-231 and MDA-MB-157. MDA-MB-231
cells showed relatively low KISS1 expression levels, whereas the
MDA-MB-157 lines expressed relatively stronger KISS1 protein levels
(Fig. 1A). A further link between
methylation and gene silencing was established by the treatment of
hypermethylated (MDA-MB-231) and hypomethylated (MDA-MB-157) cell
lines with a DNA demethylating drug. Exposure of the two cell lines
to 5-aza-2′-deoxycytidine (AZA) increased the expression of KISS1
at the transcript level (Fig. 1B).
Based on the level of KISS1 protein in MDA-MB-231 and MDA-MB-157
cells, MDA-MB-231 cells were transfected with KISS1 fragments that
included: integrated (aa 1–121) and truncated (aa 20–121).
MDA-MB-157 cells were transfected with KISS1 siRNA. After
transfection, KISS1 mRNA and protein levels were significantly
changed compared to untransfected cells by using RT-PCR and western
blotting, respectively (Fig. 1A).
These results collectively suggested that the transfection was
successful. Interesting, the results of immunofluorescence analysis
showed integrated KISS1 protein was localized in the cytoplasm of
transfected MDA-MB-231 cells, however, truncated KISS1 protein was
mainly localized in the nucleus (Fig.
1C).
The effects of KISS1 protein on breast
cancer cells in vitro
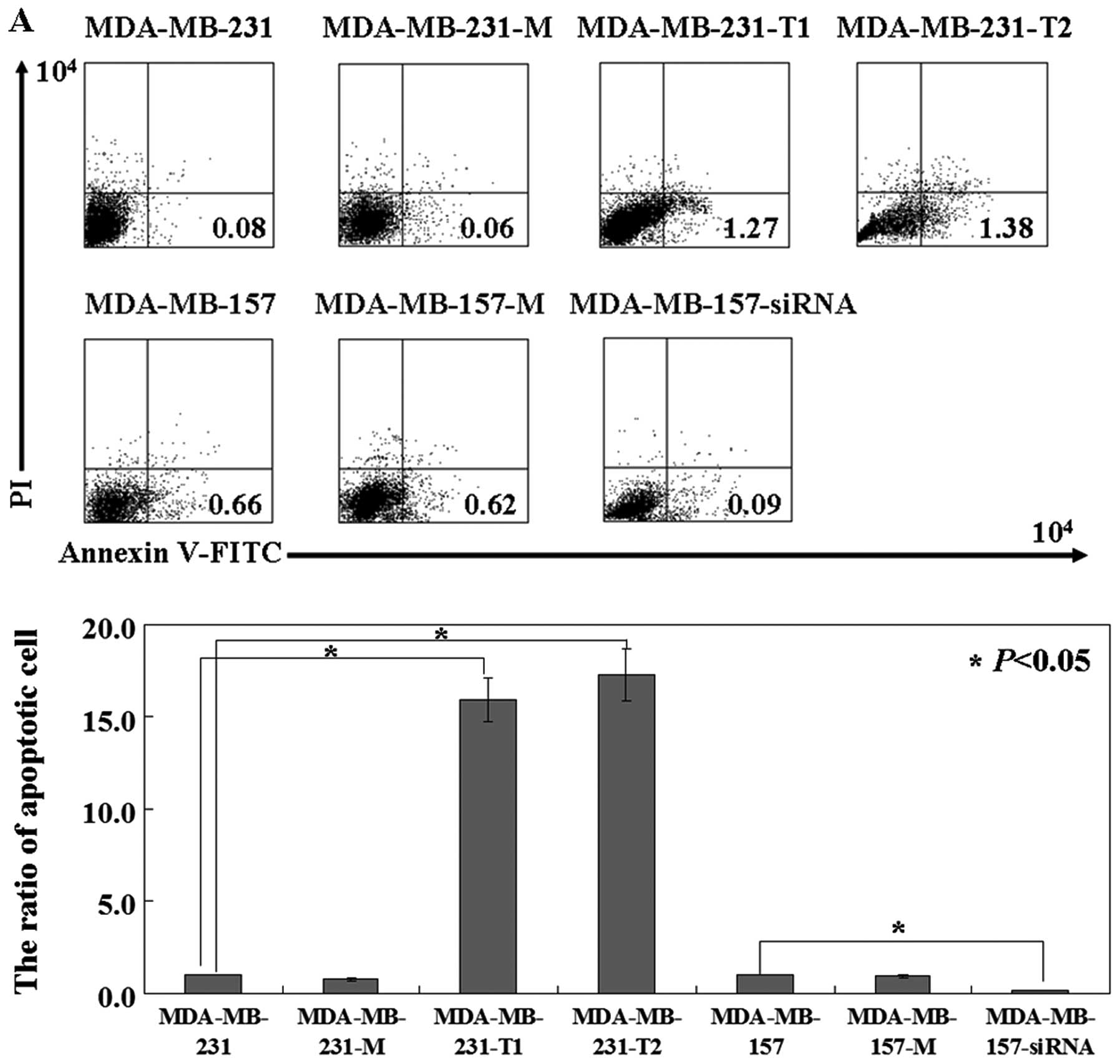
To detect apoptotic cells, Annexin V-FITC and PI
double staining was performed. In MDA-MB-231 cells expressing
intact or truncated KISS1, the apoptotic ratio was 4–5 times higher
than that of untransfected ones (P<0.05; Fig. 2A). The effect of KISS1-expression
on the motility of MDA-MB-231 cells was determined by wound-healing
assay and transwell assay. The percentage wound closure of the
MDA-MB-231 cells transfected with intact (89.4%) or truncated KISS1
(88.7%) was decreased when compared to the untreated (66.4%) and
the mock transfected (63.6%) cells (P<0.05; Fig. 2B). Transwell assay also showed that
less KISS1 positive cells migrated to the lower side of the
membrane than negative ones (P<0.05; Fig. 2C). Furthermore, we found the
activity of MMP-2 and MMP-9 was inhibited by both intact and
truncated KISS1 proteins (Fig. 1A,
blue stripe). Conversely, MDA-MB-157 cells treated with KISS1 siRNA
showed less apoptotic ratio than untreated ones (P<0.05;
Fig. 2A). Motility was
significantly increased in KISS1 siRNA treated cells compared to
KISS1 scramble treated or untreated cells (P<0.05; Fig. 2B and C). Additionally, forced KISS1
overexpression could cause weaker lamellipodia formation in
MDA-MB-231 cells, labeled with phalloidin immunostaining.
Downregulation of KISS1 protein in MDA-MB-157 cells showed stronger
lamellipodia formation (Fig.
1C).
The antitumor activity of KISS1 protein
in breast cancer mouse model
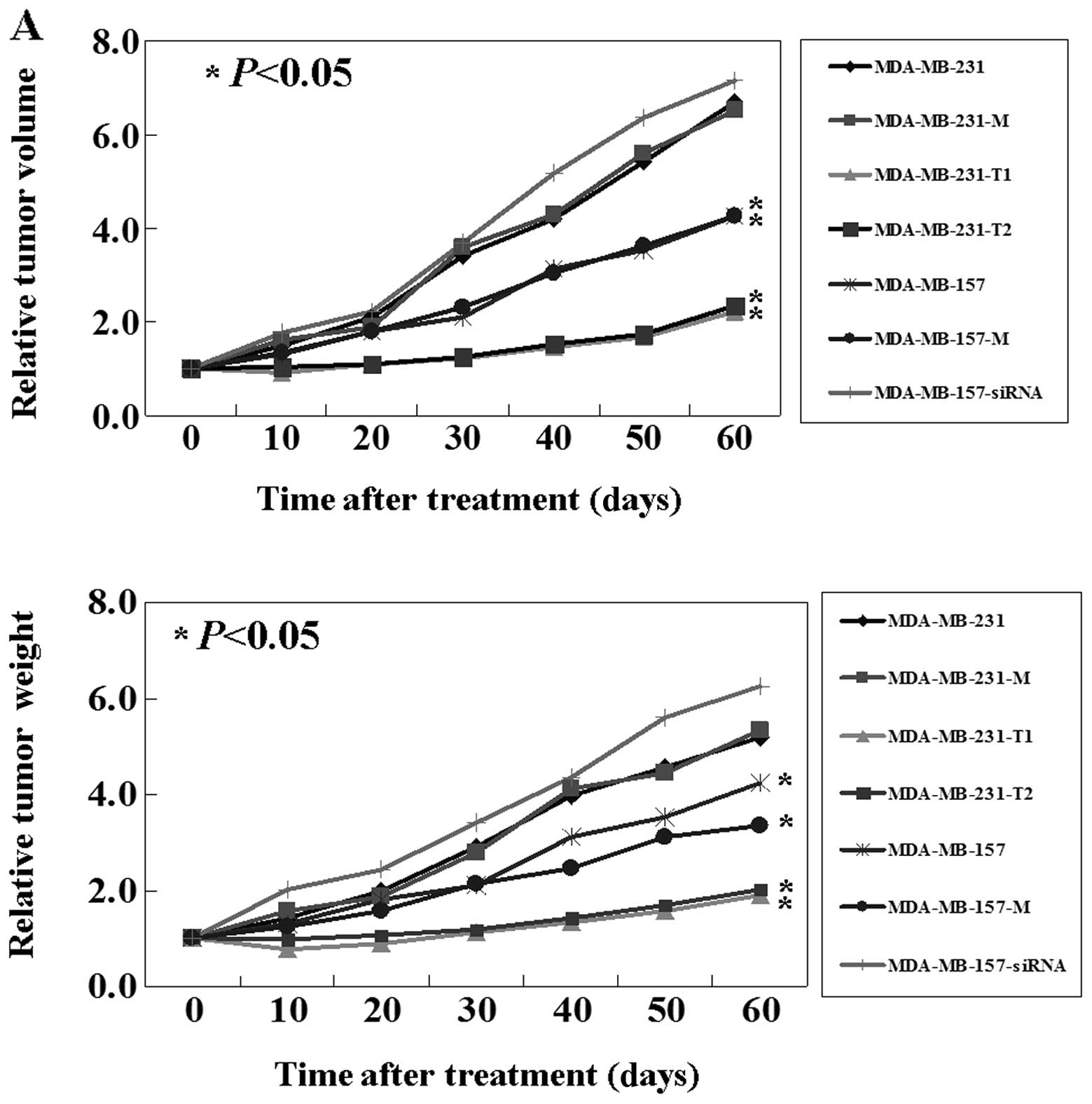
As shown above, KISS1 mediates an inhibitory effect
on breast cancer cells in vitro. The antitumor properties of
KISS1 were further evaluated using breast cancer mouse models. As
shown in the Fig. 3A, upper panel,
from days 10 to 60, compared to untreated MDA-MB-231 cells and mock
treated ones, intact or truncated KISS1 treated cells had a
significant lower tumor volume (P<0.05). Correspondingly, the
weights of intact or truncated KISS1 treated tumors also were lower
than that of untreated and mock treated tumors (P<0.05; Fig. 3A lower panel). In addition, the
survival rate of mice with tumors treated with intact or truncated
KISS1 was significantly improved. By the end of the experiment,
16/20 of mice in the intact or truncated KISS1 group were still
alive, while all of the mice in the untreated and mock groups had
died (Fig. 3B). Conversely,
results using KISS1-knockdown MDA-MB-157 mouse models were
comparable to results using the KISS1-upregulation MDA-MB-231 mouse
models (P<0.05; Fig. 3).
Correlation of metastin serum levels or
mouse model prognosis and CTCs
A statistically significant difference was observed
between circulating metastin levels in each group (P<0.05;
Fig. 3C). Intact KISS1 treated
group showed a higher serum metastin level than untreated group and
mock treated group. However, truncated KISS1 treated group showed
no changes compared with untreated group (P>0.05; Fig. 3C). CTC detection remains a big
technical challenge despite the continuing development of many new
exciting technologies (17). In
this study, we isolated and enumerated the circulating tumor cells
in mouse models by using the CellSearch system. KISS1-positive
models showed less CTCs in peripheral blood than KISS1-negative
ones (P<0.05; Fig. 3D). The
plasma level of metastin and the number of CTCs were significantly
positively correlated (r=−0.981, P<0.001; Fig. 3E). To further investigate the
relationship between CTC number and prognosis we defined 3 risk
groups, a low (CTC <10), a medium (CTC 10–30) and a high-risk
group (CTC >30). A significantly different survival rate between
the low and the medium risk as well as between the medium and the
high-risk groups in the Cox model was confirmed (P<0.05;
Fig. 3F).
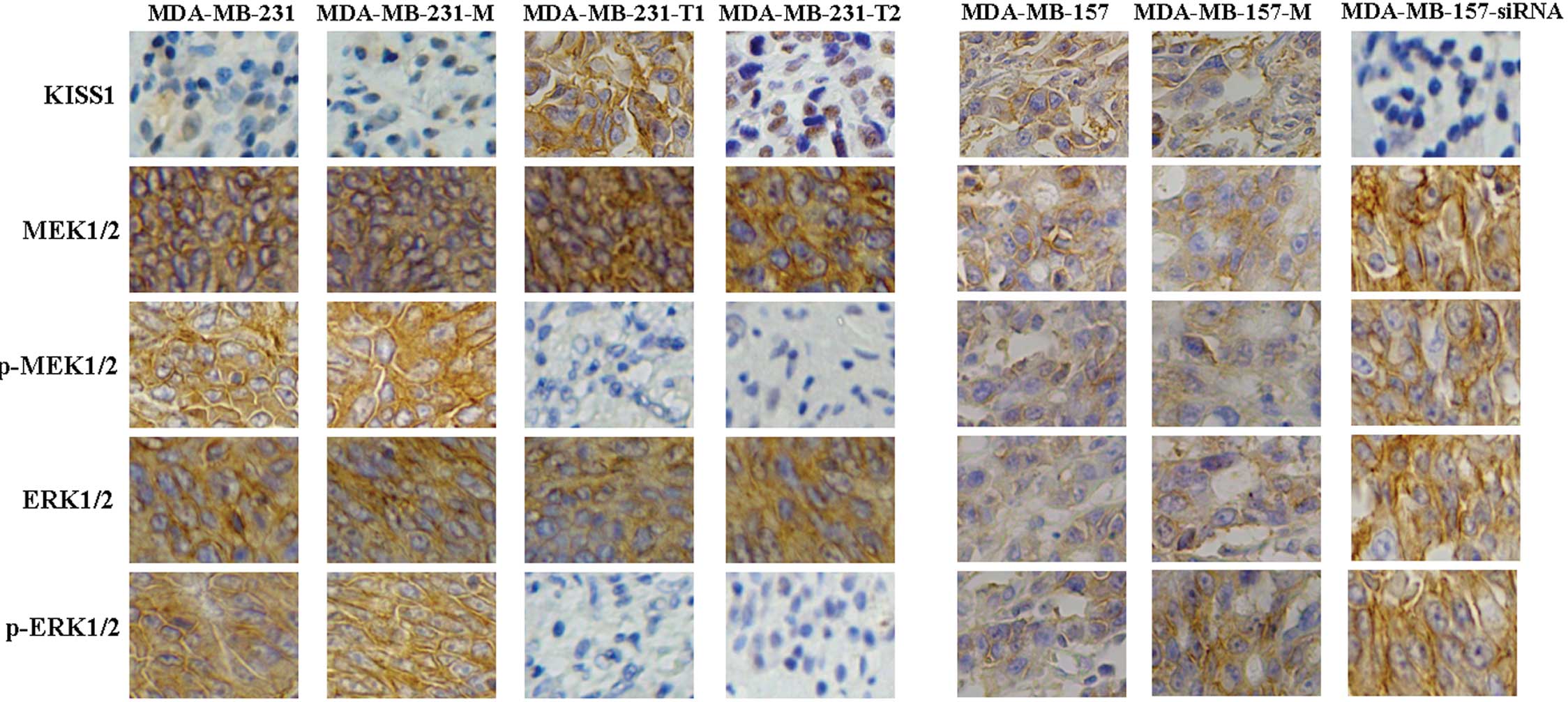
KISS1 fragments suppress the MEK/ERK
signaling pathway
To identify the mechanism of apoptosis induced by
KISS1, immunohistochemical staining assays were performed to detect
changes in possible signaling pathway proteins. While total levels
of MEK and ERK showed no changes, the levels of phospho-MEK and
phospho-ERK were observed to be significantly lower in
KISS-positive cells versus negative ones (Fig. 4). Interesting, both intact and
truncated KISS1 could inhibit the MEK/ERK signaling pathway in
breast cancer cells. Activation of phospho-MEK and phospho-ERK were
detected, while total levels of Ras, Raf, MEK and ERK were
unchanged after MDA-MB-157 models treated with KISS siRNA (Fig. 4). In combination, these results
suggest that KISS1 induced apoptosis in breast cancer cells by
suppressing the MEK/ERK signaling pathway.
Discussion
Downregulation of KISS1 expression is able to
increase tumor progression and poor prognosis in many cancers, such
as pancreatic cancer, breast cancer, bladder cancer, brain cancer,
epithelial ovarian cancer and gastric cancer (18–23).
A recent study showed that KISS1 expression is decreased in human
breast cancer, particularly in patients with aggressive tumors and
with mortality (24). As noted in
the introduction, kisspeptin is a product of the KISS1 gene which
encodes a 145-amino acid precursor. Posttranslational modifications
of this peptide result in a C-terminally amidated 54-amino acid
peptide and several shorter fragments (e.g. kisspeptin-10,
kiss-peptin-13, kisspeptin-14) (6). However, the biological activities of
these fragments were not very clear. In this study, we aimed to
explicate the distinct roles of KISS1 different fragments in breast
cancer cell. We studied two breast cancer cell lines, MDA-MB-231
and MDA-MB-157. Consistent with Teng et al (25), we confirmed MDA-MB-231 cells showed
relatively low KISS1 expression levels, whereas the MDA-MB-157
expressed relatively stronger KISS1 protein levels. We found that
KISS1 was aberrantly silenced by CpG island promoter
hypermethylation. In bladder tumors (10) and colorectal tumors (11), the loss of KISS1 expression was
attributed to epigenetic silencing by hypermethylation. Then we
upregulated KISS1 protein expression in MDA-MB-231 cells by
eukaryotic transfection and downregulated KISS1 protein expression
in MDA-MB-157 cells by RNA interference. MDA-MB-231 cells after
treatment showed a higher apoptotic ratio and a lower mobility than
untreated ones. Previous studies showed that an inverse
relationship between of KISS1 and MMP-9 expressions (26,27).
We also confirmed that upregulation of KISS1 expression leads to
loss of MMP-9 expression in breast cancer cell lines.
The main purpose of this study was to identify the
roles of KISS1 protein distinct domains. Especially, nuclear export
signal of KISS1 protein has not been formally identified.
Interesting, we found KISS1 protein without residues 1–19 was
unable to shift from nucleus to cytoplasm entirely. The results
indicated that the nuclear export signal of KISS1 may be included
in residues 1–19. However, KISS1 protein without residues 1–19 also
showed antitumor activity. Both intact KISS1 protein and
KISS1Δ20-121 induced apoptosis, inhibited mobility and caused
weaker lamellipodia formation. In vivo, tumor volumes and
tumor weights were found to be reduced in xenografts established
from treated cells versus untreated cells. Correspondingly,
survival times were also longer for treated cell xenograft models.
Previous studies have shown that patients with negative expression
of KISS1 protein were correlated with poor disease-specific
survival by using Kaplan-Meier curve survival analysis (10,11).
Metastasis is a multi-stage process that selects for circulating
tumor cells (CTCs) that can infiltrate, survive in and colonize
distant organs (28). Several
studies have suggested that the presence of CTCs that have survived
therapy might reflect a failure of systemic therapy (29,30).
Consistent with previous studies, we also found a negative
correlation between the plasma level of metastin and the number of
CTCs in xenograft models.
The mechanism of KISS1 sup pression remains unclear.
KISS1 protein could induce Ca2+ in receptor-transfected
CHO cells, as well as phosphorylation of ERK1/2 and weak
phosphoryla tion of p38/MAPK (31). Yan et al (32) found that metastin inhibits motility
by repressing the transcription of MMP-9 via the induction of
cytosolic IκBα. Another study showed that metastin induces
excessive formation of focal adhesions and stress fibers in
hOT7T175-transfected B16/BL6 and induces the phosphorylation of FAK
and paxillin (33). A further
important finding of this study was that KISS1 induced apoptosis in
breast cancer cells by suppressing the MEK/ERK signaling pathway.
The Ras/Raf/MEK/MAPK pathway has been associated with a more
aggressive disease or poor prognosis in variety of cancer systems
(34,35). Inactivation of the MEK/ERK pathway
has been observed to increase cell death in studies of commonly
used chemotherapy agents in vitro during the past few years
(36,37).
Taken together, both intact KISS1 protein and
KISS1-Δ20-121 were observed to mediate similar antitumor effects in
breast cancer cell line and these involved suppression of the
Ras/Raf/MEK/ERK signaling pathway. Moreover, following the
inhibition of this signaling pathway, breast cancer cell lines
showed higher apoptotic ratio, lower mobility and weaker
lamellipodia formation in vitro. In vivo, tumor volumes and
tumor weights were found to be reduced after KISS1 treatment.
Additionally a negative correlation was confirmed between the
plasma level of metastin and the number of CTCs in xenograft
models. These results provide valuable insight into potential novel
treatments for breast cancer.
Acknowledgements
We are indebted to Liu Ning for his
helpful criticism of the manuscript and excellent technical
assistance.
References
|
1.
|
Salomon JA, Vos T, Hogan DR, et al: Common
values in assessing health outcomes from disease and injury:
disability weights measurement study for the Global Burden of
Disease Study 2010. Lancet. 380:2129–2143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
3.
|
Kushi LH, Byers T, Doyle C, et al:
American Cancer Society Guidelines on Nutrition and Physical
Activity for cancer prevention: reducing the risk of cancer with
healthy food choices and physical activity. CA Cancer J Clin.
56:254–281; quiz 313–314. 2006. View Article : Google Scholar
|
|
4.
|
Lee JH, Miele ME, Hicks D, et al: KiSS-1,
a novel human malignant melanoma metastasis-suppressor gene. J Natl
Cancer Inst. 88:1731–1737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Makri A, Pissimissis N, Lembessis P, et
al: The kisspeptin (KISS-1)/GPR54 system in cancer biology. Cancer
Treat Rev. 34:682–692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ohtaki T, Shintani Y, Honda S, et al:
Metastasis suppressor gene KiSS-1 encodes peptide ligand of a
G-protein-coupled receptor. Nature. 411:613–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Muir AI, Chamberlain L, Elshourbagy NA, et
al: AXOR12, a novel human G protein coupled receptor, activated by
the peptide KiSS-1. J Biol Chem. 276:28969–28975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kotani M, Detheux M, Vandenbogaerde A, et
al: The metastasis suppressor gene KiSS-1 encodes kisspeptins, the
natural ligands of the orphan G protein-coupled receptor GPR54. J
Biol Chem. 276:34631–34636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nash KT and Welch DR: The KISS1 metastasis
suppressor: mechanistic insights and clinical utility. Front
Biosci. 11:647–659. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cebrian V, Fierro M, Orenes-Piñero E, et
al: KISS1 methylation and expression as tumor stratification
biomarkers and clinical outcome prognosticators for bladder cancer
patients. Am J Pathol. 179:540–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Moya P, Esteban S, Fernandez-Suarez A, et
al: KiSS-1 methylation and protein expression patterns contribute
to diagnostic and prognostic assessments in tissue specimens for
colorectal cancer. Tumour Biol. 34:471–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cristofanilli M, Hayes DF, Budd GT, et al:
Circulating tumor cells: a novel prognostic factor for newly
diagnosed metastatic breast cancer. J Clin Oncol. 23:1420–1430.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cristofanilli M, Budd GT, Ellis MJ, et al:
Circulating tumor cells, disease progression, and survival in
metastatic breast cancer. N Engl J Med. 351:781–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Osta WA, Chen Y, Mikhitarian K, et al:
EpCAM is overexpressed in breast cancer and is a potential target
for breast cancer gene therapy. Cancer Res. 64:5818–5824. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gusterson BA, Ross DT, Heath VJ, et al:
Basal cytokeratins and their relationship to the cellular origin
and functional classification of breast cancer. Breast Cancer Res.
7:143–148. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Allard WJ, Matera J, Miller MC, et al:
Tumor cells circulate in the peripheral blood of all major
carcinomas but not in healthy subjects or patients with
nonmalignant diseases. Clin Cancer Res. 10:6897–6904. 2004.
View Article : Google Scholar
|
|
17.
|
Pantel K, Brakenhoff RH and Brandt B:
Detection, clinical relevance and specific biological properties of
disseminating tumor cells. Nat Rev Cancer. 8:329–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Masui T, Doi R, Mori T, et al: Metastin
and its variant forms suppress migration of pancreatic cancer
cells. Biochem Biophys Res Commun. 315:85–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Martin TA, Watkins G and Jiang WG: KiSS-1
expression in human breast cancer. Clin Exp Metastasis. 22:503–511.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Nicolle G, Comperat E, Nicolaïew N, et al:
Metastin (KISS-1) and metastin-coupled receptor (GPR54) expression
in transitional cell carcinoma of the bladder. Ann Oncol.
18:605–606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zohrabian VM, Nandu H, Gulati N, et al:
Gene expression profiling of metastatic brain cancer. Oncol Rep.
18:321–328. 2007.PubMed/NCBI
|
|
22.
|
Hata K, Dhar DK, Watanabe Y, et al:
Expression of metastin and a G-protein-coupled receptor (AXOR12) in
epithelial ovarian cancer. Eur J Cancer. 43:1452–1459. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yamashita S, Tsujino Y, Moriguchi K, et
al: Chemical genomic screening for methylation-silenced genes in
gastric cancer cell lines using 5-aza-2′-deoxycytidine treatment
and oligonucleotide microarray. Cancer Sci. 97:64–71.
2006.PubMed/NCBI
|
|
24.
|
Mooez S, Malik FA, Kayani MA, et al:
Expressional alterations and transcript isoforms of metastasis
suppressor genes (KAI1 and KiSS1) in breast cancer patients. Asian
Pac J Cancer Prev. 12:2785–2791. 2011.PubMed/NCBI
|
|
25.
|
Teng Y, Liu M and Cowell JK: Functional
interrelationship between the WASF3 and KISS1 metastasis-associated
genes in breast cancer cells. Int J Cancer. 129:2825–2835. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Dhar DK, Naora H, Kubota H, et al:
Downregulation of KiSS-1 expression is responsible for tumor
invasion and worse prognosis in gastric carcinoma. Int J Cancer.
111:868–872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zheng S, Chang Y, Hodges KB, et al:
Expression of KISS1 and MMP-9 in non-small cell lung cancer and
their relations to metastasis and survival. Anticancer Res.
30:713–718. 2010.PubMed/NCBI
|
|
28.
|
Pantel K and Brakenhoff RH: Dissecting the
metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Müller V, Stahmann N, Riethdorf S, et al:
Circulating tumor cells in breast cancer: correlation to bone
marrow micrometastases, heterogeneous response to systemic therapy
and low proliferative activity. Clin Cancer Res. 11:3678–3685.
2005.
|
|
30.
|
Slade MJ, Payne R, Riethdorf S, et al:
Comparison of bone marrow, disseminated tumour cells and
bloodcirculating tumour cells in breast cancer patients after
primary treatment. Br J Cancer. 100:160–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lee YR, Tsunekawa K, Moon MJ, et al:
Molecular evolution of multiple forms of kisspeptins and GPR54
receptors in vertebrates. Endocrinology. 150:2837–2846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yan C, Wang H and Boyd DD: KISS-1
represses 92-kDa type IV collagenase expression by down-regulating
NF-kappa B binding to the promoter as a consequence of Ikappa
Balpha-induced block of p65/p50 nuclear translocation. J Biol Chem.
276:1164–1172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Mandache E, Gherghiceanu M, Serafinceanu
C, et al: Myofibroblast involvement in tubular basement membrane
remodeling in type II diabetic nephropathy. Rom J Morphol Embryol.
52:75–79. 2011.PubMed/NCBI
|
|
34.
|
Reungwetwattana T, Weroha SJ and Molina
JR: Oncogenic pathways, molecularly targeted therapies, and
highlighted clinical trials in non-small-cell lung cancer (NSCLC).
Clin Lung Cancer. 13:252–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Salomon DS, Brandt R and Ciardiello F:
Epidermal growth factor-related peptides and their receptors in
human malignancies. Crit Rev Oncol Hematol. 19:183–232. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Woessmann W, Chen X and Borkhardt A:
Ras-mediated activation of ERK by cisplatin induces cell death
independently of p53 in osteosarcoma and neuroblastoma cell lines.
Cancer Chemother Pharmacol. 50:397–404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Bacus SS, Gudkov AV, Lowe M, et al:
Taxol-induced apoptosis depends on MAP kinase pathways (ERK and
p38) and is independent of p53. Oncogene. 20:147–155. 2001.
View Article : Google Scholar : PubMed/NCBI
|