Introduction
Renal cell carcinoma (RCC), the most common
malignant tumor of the kidney, accounts for 2–3% of adult
malignancies. It causes about 102,000 deaths worldwide per year
(1–3). Several molecular-targeting agents for
RCC have been developed; the multi-tyrosine kinase inhibitors
sorafenib and sunitinib, and the mammalian target of rapamycin
(mTOR) inhibitors everolimus and temsirolimus. One of the major
activities of these agents against RCC has been believed to be
their angiogenesis-inhibitory effect. Despite the success of these
agents, drug resistance is an urgent problem, which underscores the
need for new treatment strategies to improve clinical outcomes
(4–6).
Histone deacetylase (HDAC) inhibitors are promising
anticancer agents that induce growth arrest, differentiation and
apoptosis in various types of tumor cell lines (7,8). We
identified OBP-801, also known as YM753, as a novel HDAC inhibitor
with attractive pharmacodynamic and pharmacokinetic properties by
screening for a p21WAF1/Cip1-inducing agent (9). OBP-801 exerted the most potent
HDAC-inhibitory activity tested; it was about 50 times more
effective than SAHA, the most clinically used HDAC inhibitor
(9).
Phosphatidylinositol 3-kinase (PI3K) is a major
signaling component downstream of growth factor receptor tyrosine
kinases (10).
Phosphatidylinositol 3,4,5-trisphosphate (PIP3) generated by PI3K
at the cell membrane is a lipid second messenger and contributes to
the activation of the serinethreonine protein kinase Akt (10,11).
The PI3K-Akt signaling pathway is a key regulator of cell growth
through many downstream targets. Therefore, the PI3K inhibitor
LY294002 can inhibit cell growth and cause apoptosis also in RCC
cells (11–15).
Previous reports showed that co-treatment with an
HDAC inhibitor and a PI3K inhibitor was effective against ovarian
cancer, cervical cancer, non-small cell lung cancer, colon cancer,
chronic myeloid leukemia and cutaneous T-cell lymphoma by
downregulating XIAP and Mcl-1 (16–21).
The PI3K-Akt pathway is well known to be upregulated in most RCC,
but the combination of a PI3K inhibitor and an HDAC inhibitor has
not been examined in RCC cells. Therefore, we examined if this
combination was effective on RCC and found that the co-treatment of
the PI3K inhibitor LY294002 with the novel HDAC inhibitor OBP-801
drastically induced apoptosis through the strong suppression of
survivin as well as XIAP via ROS production. This is the first
report that the downregulation of survivin at least partially
contributes to the synergistic effect of the HDAC inhibitor with
the PI3K inhibitor.
Materials and methods
Reagents
OBP-801 (Oncolys BioPharma, Tokyo, Japan), LY294002
(Cell Signaling Technology, Beverly, MA, USA), SAHA (Biomol
Research Laboratories, Plymouth Meeting, PA, USA) and zVAD-fmk
(R&D Systems, Minneapolis, MN, USA) were dissolved in DMSO.
N-acetyl-L-cysteine (NAC) was purchased from Nacalai Tesque
(Kyoto, Japan).
Cell culture
Human renal cancer 786-O and ACHN cell lines were
maintained in RPMI-1640 and DMEM, respectively. Culture media were
supplemented with 10% FBS, glutamine (2 mM for RPMI-1640 and 4 mM
for DMEM), 100 U/ml penicillin and 100 μg/ml streptomycin.
Cell cultures were incubated at 37°C in a humidified atmosphere of
5% CO2.
Cell viability assay
The number of viable cells was determined using a
Cell Counting kit-8 assay according to the manufacturer’s
instructions (Dojindo Laboratories, Kumamoto, Japan). After the
incubation of cells for 72 h with the indicated concentrations of
OBP-801 or LY294002, the kit reagent WST-8 was added to the medium
and cells were incubated for a further 4 h. The absorbance of
samples (450 nm) was determined using a scanning multiwell
spectrophotometer (DS Pharma Biomedical, Osaka, Japan).
Detection of apoptosis
DNA fragmentation was quantified by the percentage
of hypodiploid DNA (sub-G1). Cells were harvested from culture
dishes, washed with PBS and treated with PBS containing 0.1% Triton
X-100. Cells were then treated with RNase A (Sigma, St. Louis, MO,
USA) and the nuclei were stained with propidium iodide (Sigma). DNA
content was measured using a FACSCalibur flow cytometer and
CellQuest software (Becton-Dickinson, Franklin Lakes, NJ, USA). For
each experiment, 10,000 events were analyzed.
Western blot analysis
Western blot analysis was carried out as described
previously (22). The following
antibodies were purchased from the indicated sources: rabbit
polyclonal antibodies for anti-survivin (R&D Systems),
anti-caspase-3 (Cell Signaling Technology), anti-Bcl-2 (Abcam,
Cambridge, UK), anti-BAX and anti-Bcl-xL (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) and mouse monoclonal antibodies for anti-XIAP
(R&D Systems), anti-caspase-8, -9 (MBL, Nagoya, Japan) and
anti-β-actin (Sigma) were used as primary antibodies. The signal
was detected using a Chemilumi-one chemiluminescent kit (Nacalai
Tesque).
Plasmid DNA transfection
The pCMV6-XL5 control, pCMV6-XL5/survivin and
pCMV6-XL5/XIAP plasmid constructs were purchased from OriGene
Technologies (Rockville, MD, USA). 786-O cells were seeded at
1×105 cells per well in 6-well plates without
antibiotics. After 24 h, plasmid DNA (4 μg) was transfected
into cells using HilyMax transfection reagent (Dojindo
Laboratories) according to the manufacturer’s instructions. Four
hours after the transfection, the medium was replaced with fresh
medium and cells were treated with or without OBP-801/LY294002 for
48 h and then harvested.
Measurement of intercellular ROS
For the measurement of ROS production, cells were
treated with 10 μM
5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate
acetyl ester (CM-H2DCFDA) (Molecular Probes, Carlsbad,
CA, USA). After 30 min of incubation with CM-H2DCFDA,
fluorescence was monitored in the FL-1 channel by FACSCalibur using
the CellQuest software.
Combination index
We calculated the combination index for OBP-801 and
LY294002 using CalcuSyn 2.0 software (Biosoft, Great Shelford,
UK).
Statistical analysis
Data were expressed as mean ± SD of three
determinations. Statistical analysis was performed using the
Student’s t-test. Samples were considered significantly different
at P<0.05.
Results
The combination of OBP-801 and LY294002
synergistically inhibits cell growth and induces apoptosis in RCC
786-O cells
To examine the growth-inhibitory effect of OBP-801
or LY294002 alone, we assessed the viable cell number of 786-O
cells after 72 h of treatment with the indicated concentrations of
agents. Each agent was effective against cell growth in a
dose-dependent manner in 786-O and ACHN cells (Fig. 1A). Interestingly, co-treatment with
low-dose OBP-801 and LY294002 synergistically inhibited cell growth
less than that of cells treated with either single agent in 786-O
and ACHN cells (Fig. 1B).
Moreover, the combination index (CI) values for OBP-801 and
LY294002 were <1.0, indicating a synergistic effect for the
inhibition of cell growth (Fig.
1C). To clarify the mechanisms of synergistic inhibitory
effects on cell growth by the combination of OBP-801 and LY294002,
we investigated the effects of the combination on apoptosis by
measuring the sub-G1 population. OBP-801 or LY294002 alone only
weakly induced apoptosis, but the co-treatment with OBP-801 and
LY294002 more remarkably induced apoptosis in 786-O cells (Fig. 1D). These results indicate that the
combination of OBP-801 and LY294002 synergistically inhibits cell
growth and induces apoptosis in 786-O cells.
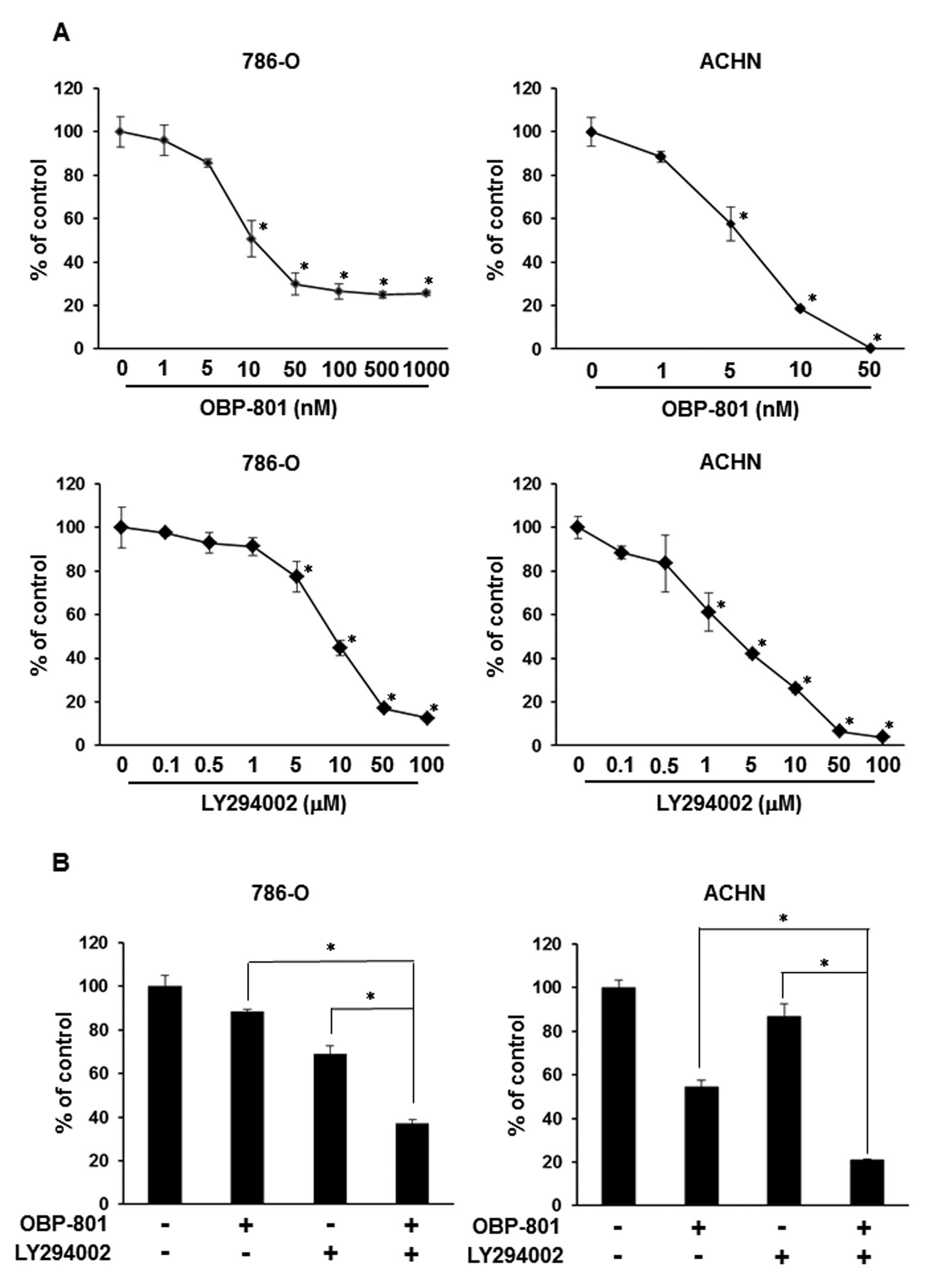 | Figure 1.OBP-801 and LY294002 synergistically
inhibit cell growth and induce apoptosis. (A) 786-O or ACHN cells
were treated with DMSO alone (control) or the indicated
concentrations of OBP-801 or LY294002. After incubation for 72 h,
viable cells were counted using Cell Counting kit-8. Columns, means
of triplicate data; bars, SD; *P<0.05, significantly
different from that by DMSO. (B) 786-O or ACHN cells were treated
with 4 nM OBP-801 and/or 5 μM LY294002. After incubation for
72 h, viable cells were counted using Cell Counting kit-8. Columns,
means of triplicate data; bars, SD; *P<0.05,
significantly different from that by either single agent. (C) The
combination index (CI) of OBP-801 and LY294002 was calculated in
786-O cells. (D) 786-O cells were treated with 4 nM OBP-801 and/or
5 μM LY294002 for 48 or 72 h. Apoptosis (sub-G1) was
determined using flow cytometry analysis. Columns, means of
triplicate data; bars, SD; *P<0.05. |
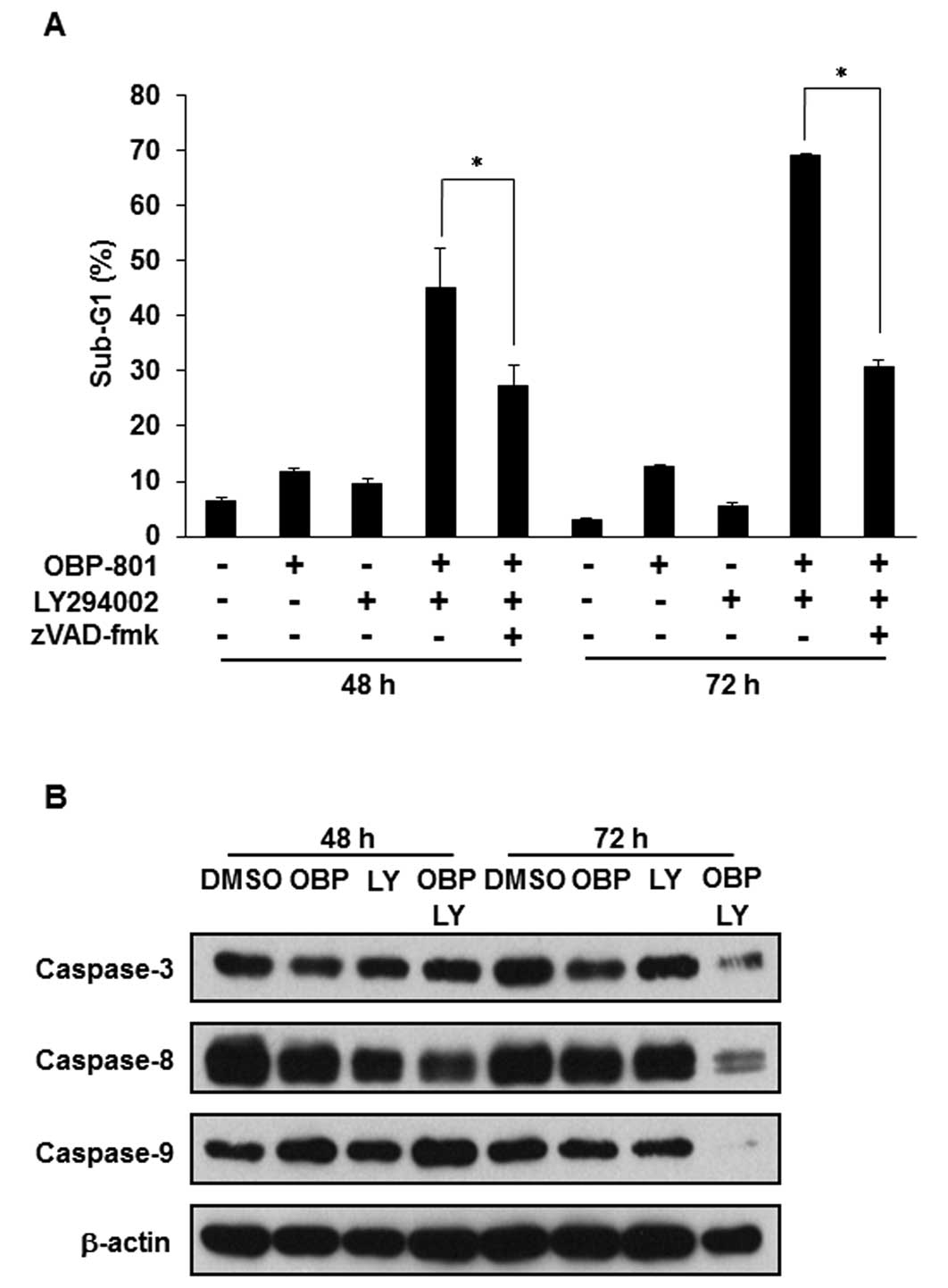
The combination of OBP-801 and LY294002
induces caspase-dependent apoptosis in 786-O cells
We investigated whether the apoptosis induced by the
combination of OBP-801 and LY294002 depends on caspases using the
pan-caspase inhibitor zVAD-fmk. Treatment with zVAD-fmk effectively
inhibited the apoptosis induced by the co-treatment with OBP-801
and LY294002 (Fig. 2A).
Additionally, we performed western blotting of caspase-3, -8 and
-9. Treatment with OBP-801 or LY294002 alone did not induce the
cleavage of caspases, but the co-treatment with OBP-801 and
LY294002 induced caspase cleavage (Fig. 2B). These results suggest that the
combination of OBP-801 and LY294002 induces apoptosis dependent on
caspase in 786-O cells.
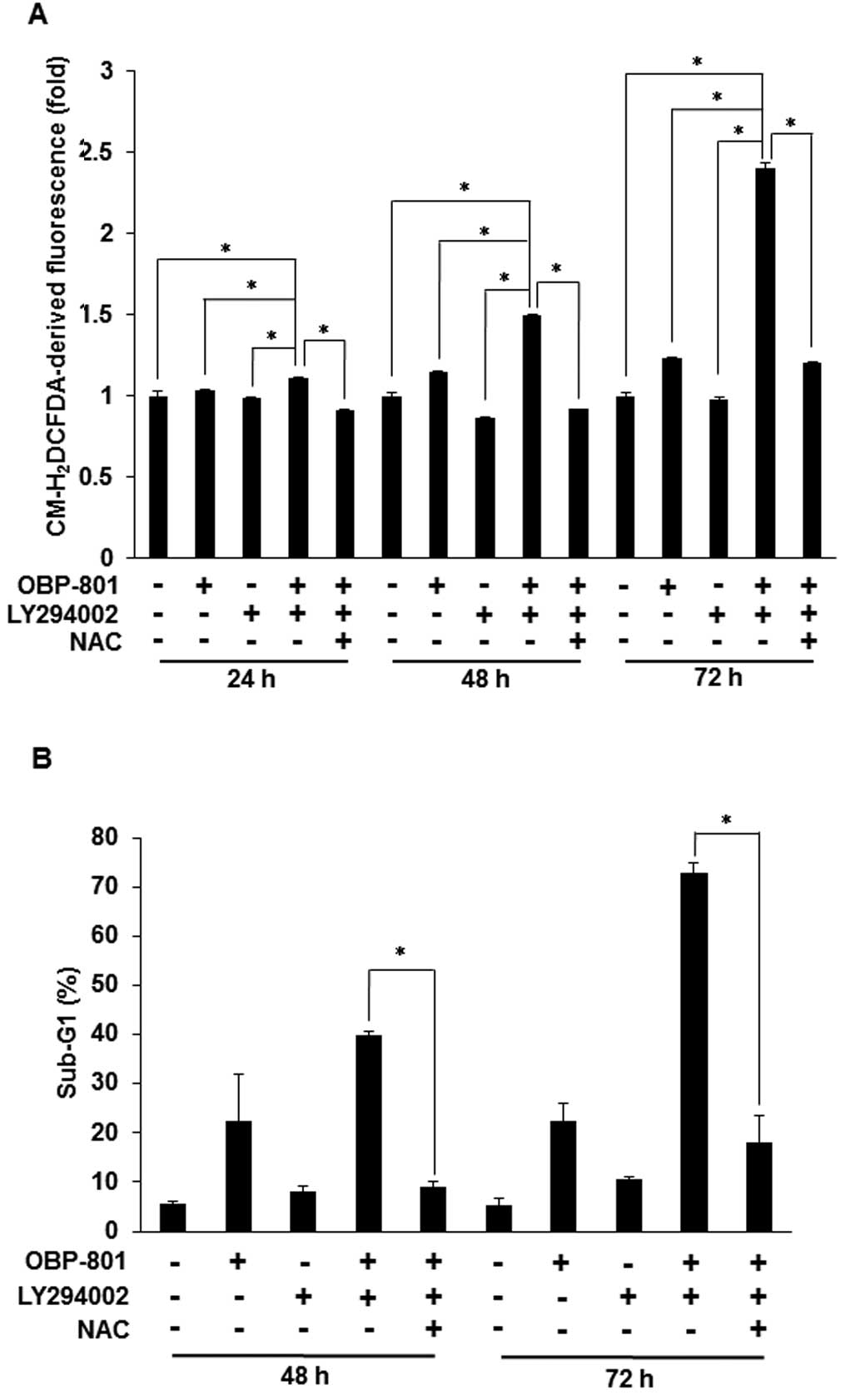
ROS are responsible for the apoptosis
induced by the combination of OBP-801 and LY294002 in 786-O
cells
It has been reported that the apoptosis induced by
the combination of a HDAC inhibitor and a PI3K inhibitor is
associated with the intracellular accumulation of ROS (19). We also found that the co-treatment
with OBP-801 and LY294002 induced intracellular ROS and the free
radical scavenger, NAC, blocked the intracellular ROS induced by
the co-treatment in 786-O cells (Fig.
3A). Moreover, NAC blocked OBP-801/LY294002-induced apoptosis
in 786-O cells (Fig. 3B). These
results suggest that the apoptosis induced by the combination of
OBP-801 and LY294002 is dependent on ROS production.
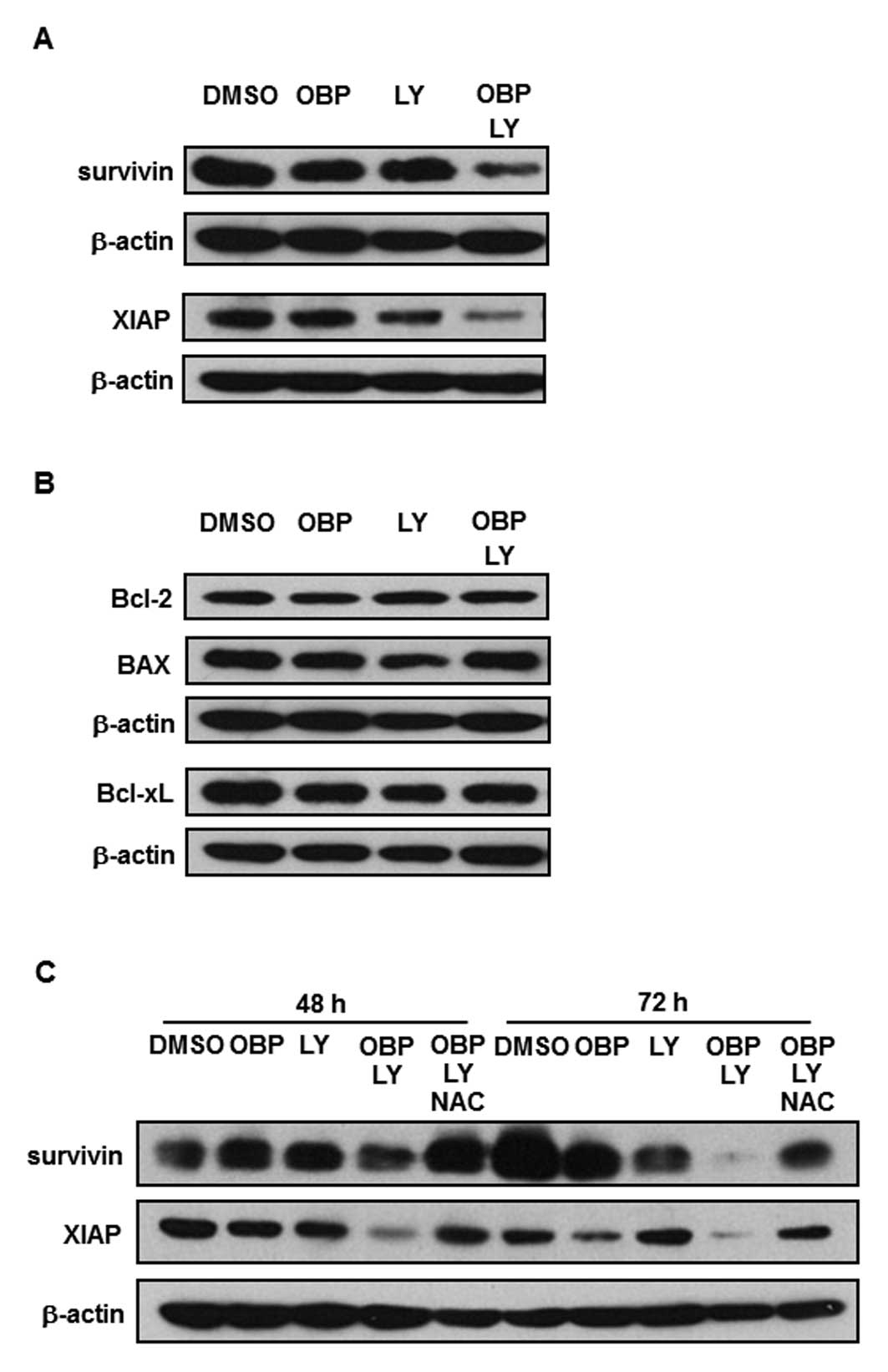
The combination of OBP-801 and LY294002
decreases protein levels of survivin and XIAP through ROS
generation in 786-O cells
To clarify the molecular mechanism of the apoptosis
induced by the combination of OBP-801 and LY294002, we performed
western blot analysis. As shown in Fig. 4A, the expression of anti-apoptotic
molecules such as survivin and XIAP with the co-treatment of
OBP-801 and LY294002 was significantly lower than with either
single agent. Bcl-2, BAX and Bcl-xL protein levels were not
affected by the co-treatment with OBP-801 and LY294002 (Fig. 4B). Next, we investigated further
whether ROS generation could cause the downregulation of survivin
and XIAP. As shown in Fig. 4C, the
downregulation of survivin and XIAP was restored by NAC treatment.
These results suggest that the downregulation of survivin and XIAP
induced by the combination of OBP-801 and LY294002 is
ROS-dependent.
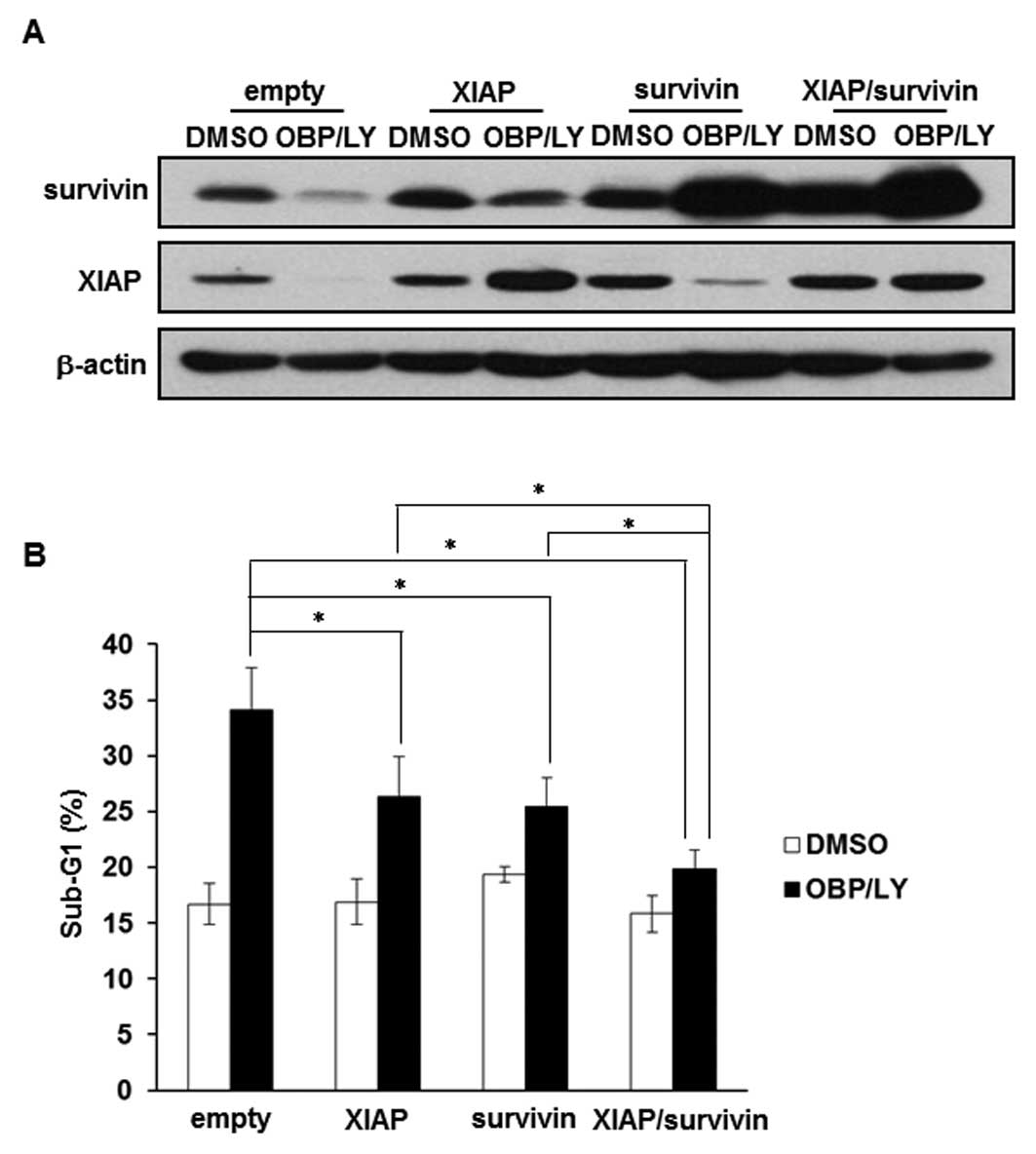
Downregulation of survivin and XIAP is
involved in the apoptosis induced by the combination of OBP-801 and
LY294002
We examined whether overexpression of survivin and
XIAP contributed to the resistance to the co-treatment with OBP-801
and LY294002. The effects of the overexpression of survivin and
XIAP were confirmed by western blotting (Fig. 5A). As shown in Fig. 5B, the overexpression of survivin or
XIAP partially suppressed OBP-801/LY294002-induced apoptosis,
whereas the co-expression of survivin and XIAP considerably
suppressed it. These results suggest that the combination of
OBP-801 and LY294002 causes apoptosis at least partially through
the downregulation of survivin and XIAP in 786-O cells.
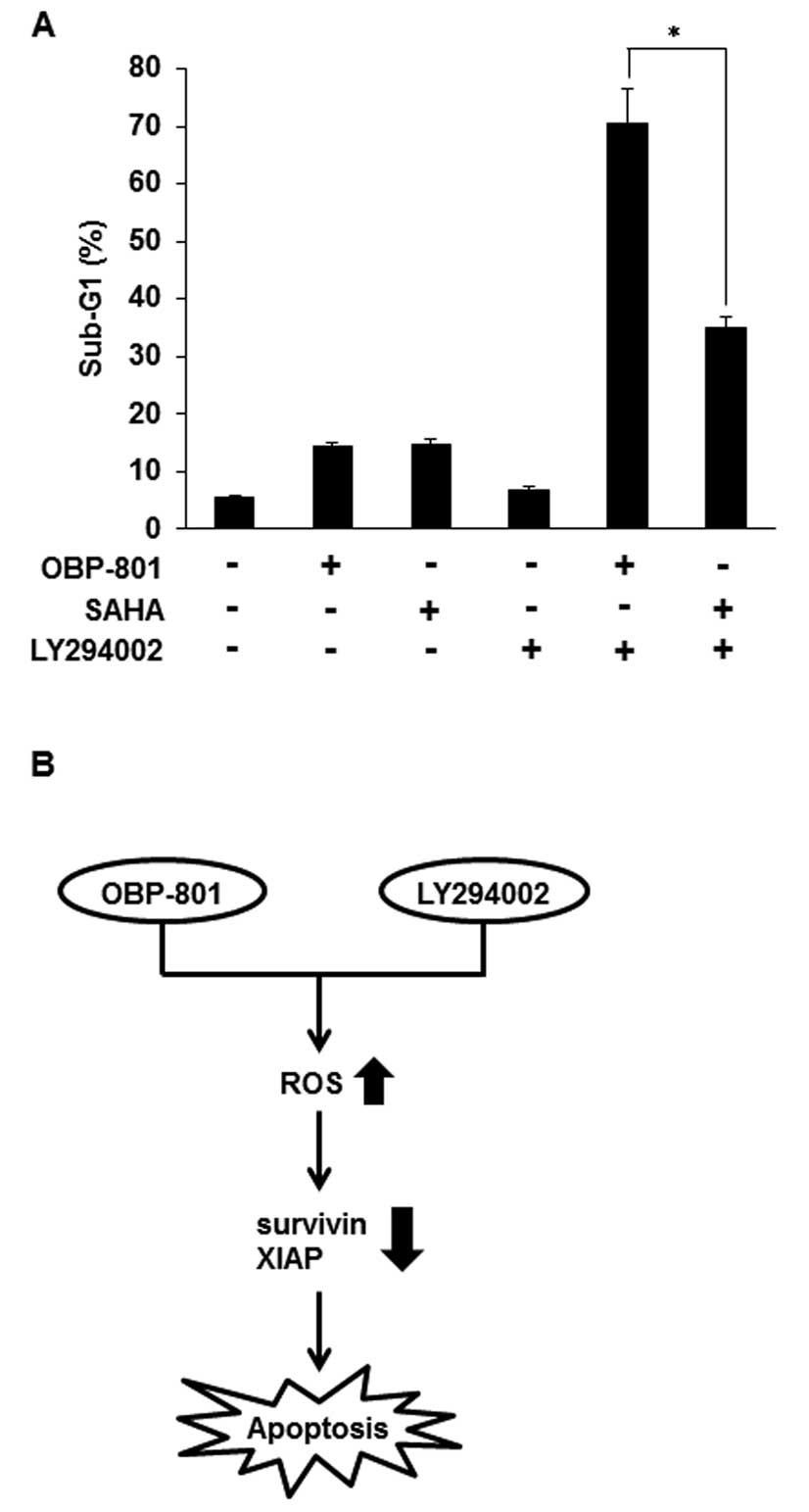
In the combination with LY294002, OBP-801
more strongly induces apoptosis than SAHA in 786-O cells
Suberoylanilide hydroxamic acid (SAHA) is the most
clinically used HDAC inhibitor (23). To compare OBP-801 and SAHA in
combination with LY294002, we analyzed sub-G1 by flow cytometry. As
shown in Fig. 6A, OBP-801 or SAHA
alone almost equally induced apoptosis, but co-treatment with
OBP-801 and LY294002 more remarkably induced apoptosis than that
with SAHA and LY294002 in 786-O cells. These results indicate that
OBP-801 is significantly more effective than SAHA in the
combination with LY294002 in 786-O cells.
Discussion
HDAC inhibitors have been reported to have potent
anticancer activity in various cancer types, but their role as
monotherapies appears to be limited. Considering the pleiotropic
effects of HDAC inhibitors against malignant tumors, their true
therapeutic potential most likely lies in combinations with other
anticancer drugs (24). Recent
clinical trials have indicated that HDAC inhibitors enhance the
antitumor activities of several conventional chemotherapeutic and
molecular-target drugs (25–27).
Additionally, a previous study showed that HDACs were highly
expressed in RCC, suggesting that HDAC inhibitors may be effective
on RCC (28).
Recently, the mTOR inhibitors everolimus and
temsirolimus have been clinically used as a treatment against RCC,
but are not curative. mTOR is known to inhibit the insulin receptor
substrate-1 (IRS-1), which plays a key role in transmitting signals
from insulin-like growth factor-I (IGF-I) receptors to the PI3K-Akt
pathway. Therefore, mTOR inhibitors reactivate the PI3K-Akt pathway
resulting in resistance (29). We
then selected a PI3K inhibitor as a combination-therapeutic partner
of the HDAC inhibitor.
We showed that the OBP-801/LY294002 co-treatment
specifically downregulated survivin and XIAP proteins in 786-O
cells (Fig. 4A). Furthermore, the
overexpression of survivin and/or XIAP reduced the apoptotic
response to OBP-801/LY294002 co-treatment, indicating that the
suppression of survivin and XIAP may be attributed to OBP-801/
LY294002-induced apoptosis (Fig.
5B). This is the first report that the downregulation of
survivin at least partially contributes to the synergistic effect
of an HDAC inhibitor and a PI3K inhibitor. Survivin is a member of
the inhibitor of apoptosis (IAP) family and has multiple functions
such as the regulation of mitosis and apoptosis (30,31).
High expression levels of survivin have been reported to contribute
to the resistance of several anticancer agents such as paclitaxel,
etoposide and cisplatin (31).
Moreover, it has been reported that the overexpression of survivin
is associated with disease progression in RCC and may be a
prognostic marker in RCC (32).
These reports provide the rationale for survivin-targeted therapy
in patients with RCC. Therefore, our results show that the
combination of OBP-801 with a PI3K inhibitor is promising for the
treatment for RCC.
We have additionally found that ROS production
contributes to the downregulation of survivin and XIAP by the
OBP-801/LY294002 co-treatment (Fig.
4C). It has been also reported that the expression of
IAP-family proteins including survivin and XIAP is upregulated by
nuclear factor-κB (NF-κB) (33–35)
and that NF-κB activity is suppressed by ROS (36). Therefore, the downregulation of
survivin and XIAP in this study might be caused by the
ROS-dependent suppression of NF-κB activity.
A recent report has shown that the combination of
OBP-801 and LY294002 synergistically induces apoptosis through the
upregulation of Bim with accumulation of ROS in human endometrial
carcinoma HEC-1A cells (37).
However, in our experiments, OBP-801/LY294002 co-treatment did not
induce Bim expression in RCC 786-O cells (data not shown). These
results suggest that there are different mechanisms of apoptosis
induced by the OBP-801/LY294002 co-treatment between the two cell
lines.
Our results showed that OBP-801 more markedly
induced apoptosis than SAHA, the most clinically used HDAC
inhibitor, when combined with LY294002 (Fig. 6A). Interestingly, the co-treatment
with SAHA and LY294002 did not decrease the expression of survivin
and XIAP proteins (data not shown). Therefore, the mechanism for
differences in the efficacy of both agents may be attributed to the
downregulation of survivin and XIAP.
In conclusion, we demonstrated that the novel HDAC
inhibitor OBP-801 and the PI3K inhibitor LY294002 synergistically
induced apoptosis by ROS-dependent downregulation of survivin and
XIAP in 786-O cells (Fig. 6B).
These observations raise the possibility that the combination of
OBP-801 and PI3K inhibitors may be promising for the treatment of
RCC.
Abbreviations:
|
RCC
|
renal cell carcinoma;
|
|
HDAC
|
histone deacetylase;
|
|
PI3K
|
phosphatidylinositol 3-kinase;
|
|
ROS
|
reactive oxygen species;
|
|
NAC
|
N-acetyl-L-cysteine
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein;
|
|
SAHA
|
suberoylanilide hydroxamic acid
|
|
mTOR
|
mammalian target of rapamycin;
|
|
PIP3
|
phosphatidylinositol
3,4,5-trisphosphate;
|
|
IAP
|
inhibitor of apoptosis;
|
|
IRS-1
|
insulin receptor substrate-1;
|
|
IGF-I
|
insulin-like growth factor-I;
|
|
NF-κB
|
nuclear factor-κB
|
Acknowledgements
We thank Drs Y. Sowa, S. Yogosawa, M.
Tomosugi and M. Koyama for their useful discussion. This study was
partly supported by the Japanese Ministry of Education, Culture,
Sports, Science and Technology.
References
|
1.
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kanno T, Kamba T, Yamasaki T, et al: JunB
promotes cell invasion and angiogenesis in VHL-defective renal cell
carcinoma. Oncogene. 31:3098–3110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mahalingam D, Medina EC, Esquivel JA II,
et al: Vorinostat enhances the activity of temsirolimus in renal
cell carcinoma through suppression of survivin levels. Clin Cancer
Res. 16:141–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Carew JS, Esquivel JA II, Espitia CM, et
al: ELR510444 inhibits tumor growth and angiogenesis by abrogating
HIF activity and disrupting microtubules in renal cell carcinoma.
PloS One. 7:e311202012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sosman JA, Puzanov I and Atkins MB:
Opportunities and obstacles to combination targeted therapy in
renal cell cancer. Clin Cancer Res. 13:S764–S769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Marks PA, Richon VM and Rifkind RA:
Histone deacetylase inhibitors: inducers of differentiation or
apoptosis of transformed cells. J Natl Cancer Inst. 92:1210–1216.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Koyama M, Izutani Y, Goda AE, et al:
Histone deacetylase inhibitors and
15-deoxy-Delta12,14-prostaglandin J2 synergistically induce
apoptosis. Clin Cancer Res. 16:2320–2332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shindoh N, Mori M, Terada Y, et al: YM753,
a novel histone deacetylase inhibitor, exhibits antitumor activity
with selective, sustained accumulation of acetylated histones in
tumors in the WiDr xenograft model. Int J Oncol. 32:545–555.
2008.
|
|
10.
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sourbier C, Lindner V, Lang H, et al: The
phosphoinositide 3-kinase/Akt pathway: a new target in human renal
cell carcinoma therapy. Cancer Res. 66:5130–5142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
13.
|
Kulik G, Carson JP, Vomastek T, et al:
Tumor necrosis factor alpha induces BID cleavage and bypasses
antiapoptotic signals in prostate cancer LNCaP cells. Cancer Res.
61:2713–2719. 2001.PubMed/NCBI
|
|
14.
|
Izuishi K, Kato K, Ogura T, Kinoshita T
and Esumi H: Remarkable tolerance of tumor cells to nutrient
deprivation: possible new biochemical target for cancer therapy.
Cancer Res. 60:6201–6207. 2000.PubMed/NCBI
|
|
15.
|
Lee CM, Fuhrman CB, Planelles V, et al:
Phosphatidylinositol 3-kinase inhibition by LY294002
radiosensitizes human cervical cancer cell lines. Clin Cancer Res.
12:250–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zhou C, Qiu L, Sun Y, et al: Inhibition of
EGFR/PI3K/AKT cell survival pathway promotes TSA’s effect on cell
death and migration in human ovarian cancer cells. Int J Oncol.
29:269–278. 2006.PubMed/NCBI
|
|
17.
|
Wang Q, Li N, Wang X, Kim MM and Evers BM:
Augmentation of sodium butyrate-induced apoptosis by
phosphatidylinositol 3′-kinase inhibition in the KM20 human colon
cancer cell line. Clin Cancer Res. 8:1940–1947. 2002.
|
|
18.
|
Park JK, Cho CH, Ramachandran S, et al:
Augmentation of sodium butyrate-induced apoptosis by
phosphatidylinositol 3-kinase inhibition in the human cervical
cancer cell-line. Cancer Res Treat. 38:112–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ozaki K, Kosugi M, Baba N, et al: Blockade
of the ERK or PI3K-Akt signaling pathway enhances the cytotoxicity
of histone deacetylase inhibitors in tumor cells resistant to
gefitinib or imatinib. Biochem Biophys Res Commun. 391:1610–1615.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wozniak MB, Villuendas R, Bischoff JR, et
al: Vorinostat interferes with the signaling transduction pathway
of T-cell receptor and synergizes with phosphoinositide-3 kinase
inhibitors in cutaneous T-cell lymphoma. Haematologica. 95:613–621.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Rahmani M, Yu C, Reese E, et al:
Inhibition of PI-3 kinase sensitizes human leukemic cells to
histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP
kinase inactivation and abrogation of p21(CIP1/WAF1) induction
rather than AKT inhibition. Oncogene. 22:6231–6242. 2003.
View Article : Google Scholar
|
|
22.
|
Nakata S, Yoshida T, Horinaka M, Shiraishi
T, Wakada M and Sakai T: Histone deacetylase inhibitors upregulate
death receptor 5/TRAIL-R2 and sensitize apoptosis induced by
TRAIL/APO2-L in human malignant tumor cells. Oncogene.
23:6261–6271. 2004. View Article : Google Scholar
|
|
23.
|
Carew JS, Giles FJ and Nawrocki ST:
Histone deacetylase inhibitors: mechanisms of cell death and
promise in combination cancer therapy. Cancer Lett. 269:7–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Bots M and Johnstone RW: Rational
combinations using HDAC inhibitors. Clin Cancer Res. 15:3970–3977.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ramaswamy B, Fiskus W, Cohen B, et al:
Phase I–II study of vorinostat plus paclitaxel and bevacizumab in
metastatic breast cancer: evidence for vorinostat-induced tubulin
acetylation and Hsp90 inhibition in vivo. Breast Cancer Res Treat.
132:1063–1072. 2012.
|
|
26.
|
Rathkopf D, Wong BY, Ross RW, et al: A
phase I study of oral panobinostat alone and in combination with
docetaxel in patients with castration-resistant prostate cancer.
Cancer Chemother Pharmacol. 66:181–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Chinnaiyan P, Chowdhary S, Potthast L, et
al: Phase I trial of vorinostat combined with bevacizumab and
CPT-11 in recurrent glioblastoma. Neurooncology. 14:93–100.
2012.PubMed/NCBI
|
|
28.
|
Fritzsche FR, Weichert W, Roske A, et al:
Class I histone deacetylases 1, 2 and 3 are highly expressed in
renal cell cancer. BMC Cancer. 8:3812008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Hudes GR: Targeting mTOR in renal cell
carcinoma. Cancer. 115:2313–2320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Revi Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Krambeck AE, Dong H, Thompson RH, et al:
Survivin and b7-h1 are collaborative predictors of survival and
represent potential therapeutic targets for patients with renal
cell carcinoma. Clin Cancer Res. 13:1749–1756. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Taniguchi H, Horinaka M, Yoshida T, et al:
Targeting the Glyoxalase pathway enhances TRAIL efficacy in cancer
cells by downregulating the expression of antiapoptotic molecules.
Mol Cancer Ther. 11:2294–2300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Zhu L, Fukuda S, Cordis G, Das DK and
Maulik N: Anti-apoptotic protein survivin plays a significant role
in tubular morphogenesis of human coronary arteriolar endothelial
cells by hypoxic preconditioning. FEBS Lett. 508:369–374. 2001.
View Article : Google Scholar
|
|
35.
|
Stehlik C, de Martin R, Kumabashiri I,
Schmid JA, Binder BR and Lipp J: Nuclear factor
(NF)-kappaB-regulated X-chromosome-linked iap gene expression
protects endothelial cells from tumor necrosis factor alpha-induced
apoptosis. J Exp Med. 188:211–216. 1998. View Article : Google Scholar
|
|
36.
|
Qu Y, Wang J, Ray PS, et al:
Thioredoxin-like 2 regulates human cancer cell growth and
metastasis via redox homeostasis and NF-kappaB signaling. J Clin
Invest. 121:212–225. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Yoshioka T, Yogosawa S, Yamada T, Kitawaki
J and Sakai T: Combination of a novel HDAC inhibitor OBP-801/YM753
and a PI3K inhibitor LY294002 synergistically induces apoptosis in
human endometrial carcinoma cells due to increase of Bim with
accumulation of ROS. Gynecol Oncol. 129:425–432. 2013. View Article : Google Scholar : PubMed/NCBI
|