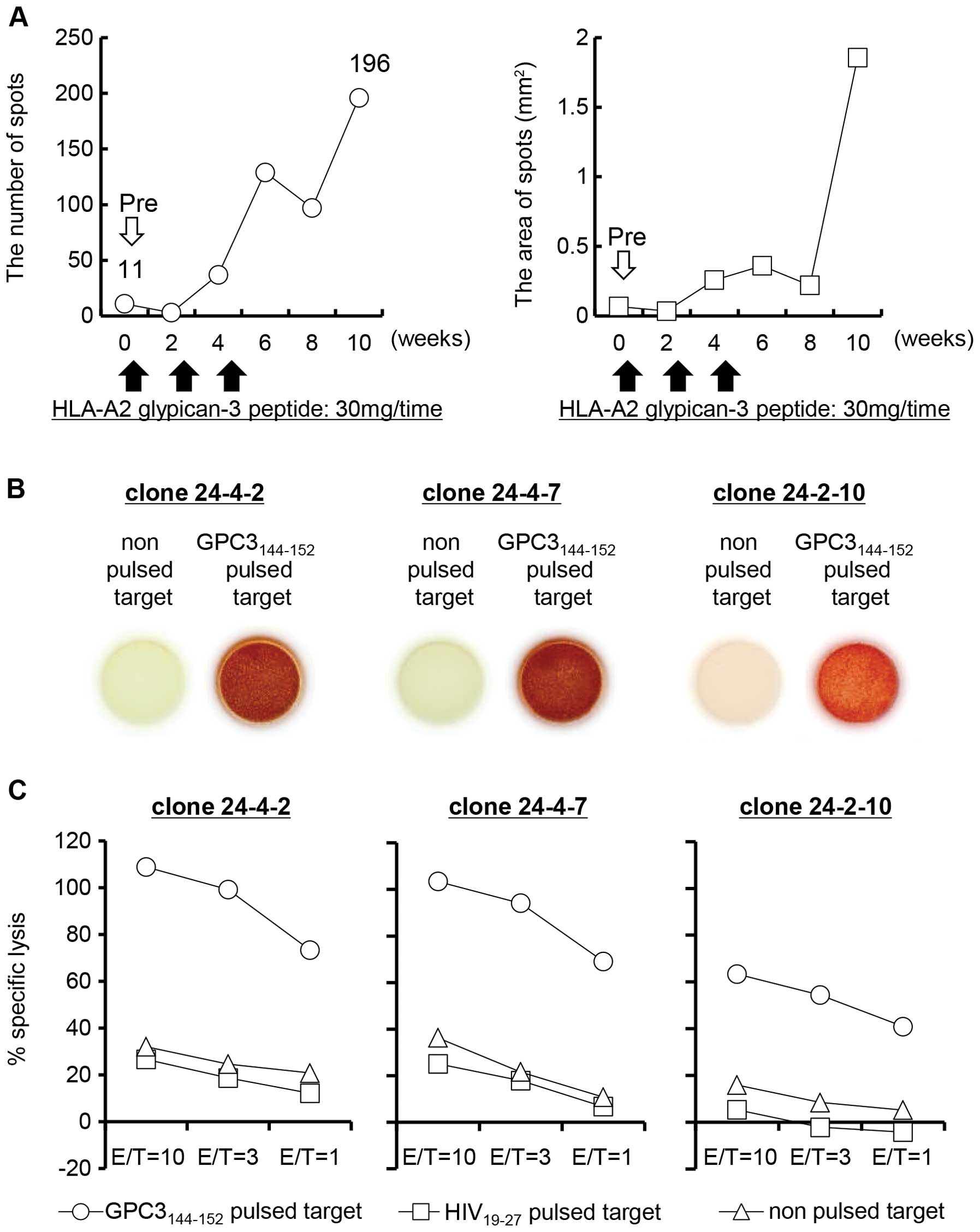
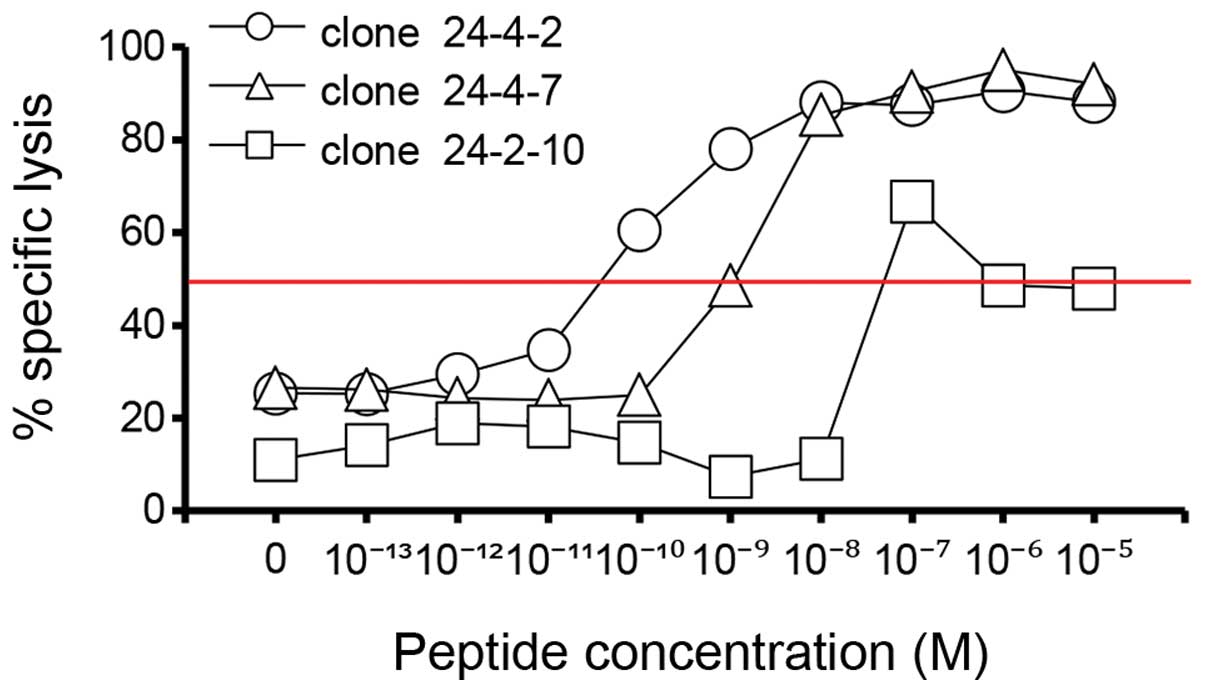
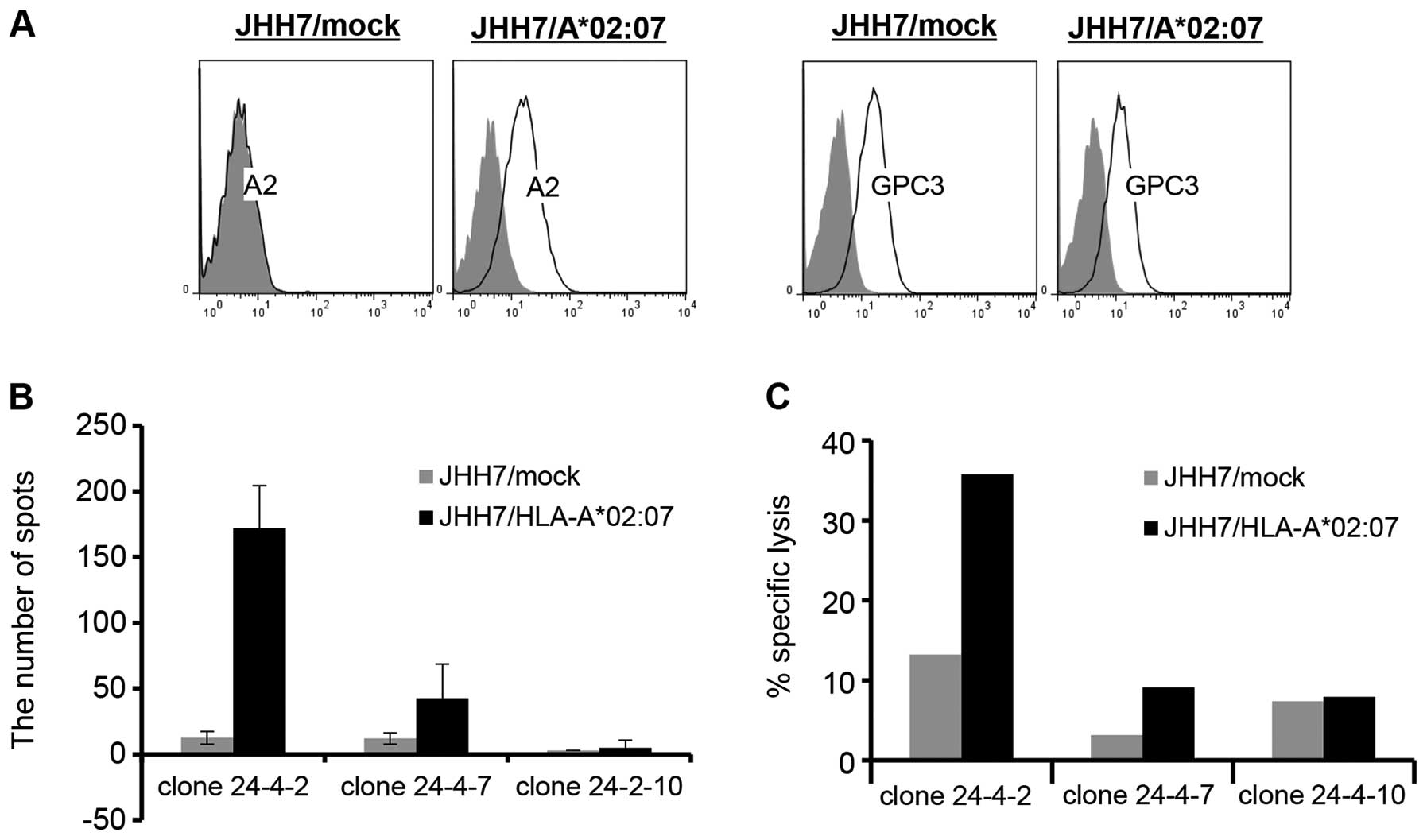
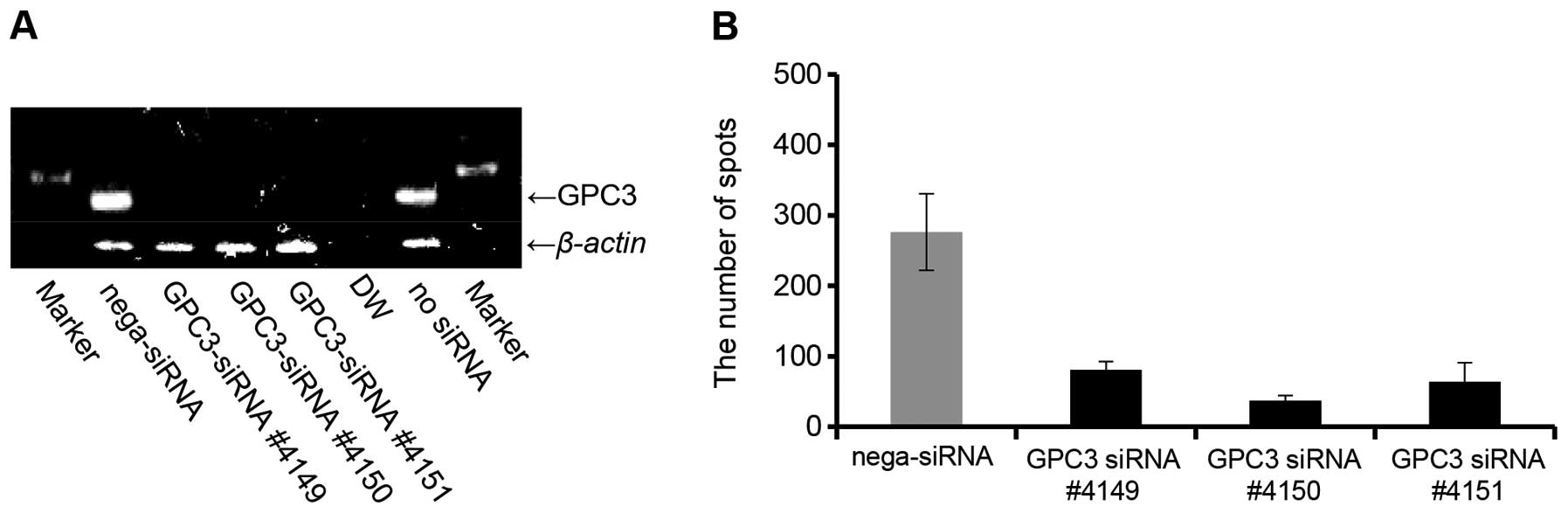
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zidan A, Scheuerlein H, Schüle S,
Settmacher U and Rauchfuss F: Epidemiological pattern of hepatitis
B and hepatitis C as etiological agents for hepatocellular
carcinoma in iran and worldwide. Hepat Mon. 12:e68942012.PubMed/NCBI
|
|
4.
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar
|
|
6.
|
Kim HY and Park JW: Molecularly targeted
therapies for hepatocellular carcinoma: Sorafenib as a stepping
stone. Dig Dis. 29:303–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Morimoto M, Numata K, Kondo M, et al:
Higher discontinuation and lower survival rates are likely in
elderly Japanese patients with advanced hepatocellular carcinoma
receiving sorafenib. Hepatol Res. 41:296–302. 2011. View Article : Google Scholar
|
|
8.
|
Greten TF, Manns MP and Korangy F:
Immunotherapy of hepatocellular carcinoma. J Hepatol. 45:868–878.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mizukoshi E, Nakamoto Y, Arai K, et al:
Comparative analysis of various tumor-associated antigen-specific
t-cell responses in patients with hepatocellular carcinoma.
Hepatology. 53:1206–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Filmus J, Shi W, Wong ZM and Wong MJ:
Identification of a new membrane-bound heparan sulphate
proteoglycan. Biochem J. 311:561–565. 1995.
|
|
11.
|
Filmus J and Selleck SB: Glypicans:
proteoglycans with a surprise. J Clin Invest. 108:497–501. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nakatsura T, Yoshitake Y, Senju S, et al:
Glypican-3, over-expressed specifically in human hepatocellular
carcinoma, is a novel tumor marker. Biochem Biophys Res Commun.
306:16–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Capurro M, Wanless IR, Sherman M, Deboer
G, Shi W, Miyoshi E and Filmus J: Glypican-3: a novel serum and
histochemical marker for hepatocellular carcinoma.
Gastroenterology. 125:89–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nakatsura T and Nishimura Y: Usefulness of
the novel oncofetal antigen glypican-3 for diagnosis of
hepatocellular carcinoma and melanoma. BioDrugs. 19:71–77. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Shirakawa H, Kuronuma T, Nishimura Y, et
al: Glypican-3 is a useful diagnostic marker for a component of
hepatocellular carcinoma in human liver cancer. Int J Oncol.
34:649–656. 2009.PubMed/NCBI
|
|
16.
|
Shirakawa H, Suzuki H, Shimomura M, et al:
Glypican-3 expression is correlated with poor prognosis in
hepatocellular carcinoma. Cancer Sci. 100:1403–1407. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Motomura Y, Senju S, Nakatsura T, et al:
Embryonic stem cell-derived dendritic cells expressing glypican-3,
a recently identified oncofetal antigen, induce protective immunity
against highly metastatic mouse melanoma, B16-F10. Cancer Res.
66:2414–2422. 2006. View Article : Google Scholar
|
|
18.
|
Motomura Y, Ikuta Y, Kuronuma T, et al:
HLA-A2 and -A24-restricted glypican-3-derived peptide vaccine
induces specific CTLs: preclinical study using mice. Int J Oncol.
32:985–990. 2008.PubMed/NCBI
|
|
19.
|
Iwama T, Horie K, Yoshikawa T, et al:
Identification of an H2-Kb or H2-Db restricted and
glypican-3-derived cytotoxic T-lymphocyte epitope peptide. Int J
Oncol. 42:831–838. 2013.PubMed/NCBI
|
|
20.
|
Komori H, Nakatsura T, Senju S, et al:
Identification of HLA-A2- or HLA-A24-restricted CTL epitopes
possibly useful for glypican-3-specific immunotherapy of
hepatocellular carcinoma. Clin Cancer Res. 12:2689–2697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Nakatsura T, Komori H, Kubo T, et al:
Mouse homologue of a novel human oncofetal antigen, glypican-3,
evokes T-cell-mediated tumor rejection without autoimmune reactions
in mice. Clin Cancer Res. 10:8630–8640. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Imanish T, Akaza T, Kimura A, Tokunaga K
and Gojobori T: Allele and haplotype frequencies for HLA and
complement loci in various ethnic groups. HLA. 1991, 1. Tsuji K,
Aizawa M and Sasazuki T: Oxford University Press; Oxford: pp.
1065–1220. 1992
|
|
23.
|
Sidney J, Grey HM, Kubo RT and Sette A:
Practical, biochemical and evolutionary implications of the
discovery of HLA class I supermotifs. Immunol Today. 17:261–266.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yasuda N, Tsuji K, Aizawa M, et al: HLA
antigens in Japanese populations. Am J Hum Genet. 28:390–399.
1976.
|
|
25.
|
Ellis JM, Henson V, Slack R, Ng J,
Hartzman RJ and Katovich Hurley C: Frequencies of HLA-A2 alleles in
five U.S. population groups. Predominance of A*02011 and
identification of HLA-A*0231. Hum Immunol. 61:334–340.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mehra NK, Jaini R, Rajalingam R,
Balamurugan A and Kaur G: Molecular diversity of
HLA-A*02 in Asian Indians: predominance of
A*0211. Tissue Antigens. 57:502–507. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sawada Y, Yoshikawa T, Nobuoka D, et al:
Phase I trial of a glypican-3-derived peptide vaccine for advanced
hepatocellular carcinoma: immunologic evidence and potential for
improving overall survival. Clin Cancer Res. 18:3686–3696. 2012.
View Article : Google Scholar
|
|
28.
|
Yoshikawa T, Nakatsugawa M, Suzuki S, et
al: HLA-A2-restricted glypican-3 peptide-specific CTL clones
induced by peptide vaccine show high avidity and antigen-specific
killing activity against tumor cells. Cancer Sci. 102:918–925.
2011. View Article : Google Scholar
|
|
29.
|
Nobuoka D, Yoshikawa T, Sawada Y, Fujiwara
T and Nakatsura T: Peptide vaccines for hepatocellular carcinoma.
Hum Vaccin Immunother. 9:210–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Sawada Y, Sakai M, Yoshikawa T, Ofuji K
and Nakatsura T: A glypican-3-derived peptide vaccine against
hepatocellular carcinoma. Oncoimmunology. 1:1448–1450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Purbhoo MA, Li Y, Sutton DH, et al: The
HLA A*0201-restricted hTERT(540–548) peptide is not
detected on tumor cells by a CTL clone or a high-affinity T-cell
receptor. Mol Cancer Ther. 6:2081–2091. 2007.PubMed/NCBI
|
|
32.
|
Nakatsugawa M, Horie K, Yoshikawa T, et
al: Identification of an HLA-A*0201-restricted cytotoxic
T lymphocyte epitope from the lung carcinoma antigen, Lengsin. Int
J Oncol. 39:1041–1049. 2011.
|
|
33.
|
Guo Y, Zhu Y and Sun S: Identification and
functional studies of HLA-A0201 restricted CTL epitopes in the X
protein of hepatitis B virus. Acta Virol. 55:107–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
McKee MD, Roszkowski JJ and Nishimura MI:
T cell avidity and tumor recognition: implications and therapeutic
strategies. J Transl Med. 3:352005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Harada Y and Kawase I: Single cell-based T
cell receptor gene analysis reveals existence of expanded WT1
(Wilms’ tumor gene) product-specific T cell clones in leukemia
patients but not healthy volunteers. Med J Osaka Univ. 50:1–12.
2007.
|
|
36.
|
Tanaka-Harada Y, Kawakami M, Oka Y, et al:
Biased usage of BV gene families of T-cell receptors of WT1 (Wilms’
tumor gene)-specific CD8+ T cells in patients with
myeloid malignancies. Cancer Sci. 101:594–600. 2010.
|
|
37.
|
Morimoto S, Oka Y, Tsuboi A, et al: Biased
usage of T cell receptor β-chain variable region genes of Wilms’
tumor gene (WT1)-specific CD8+ T cells in patients with
solid tumors and healthy donors. Cancer Sci. 103:408–414. 2012.
|
|
38.
|
Valmori D, Dutoil V, Lienard D, et al:
Tetramer-guided analysis of TCR beta-chain usage reveals a large
repertoire of melan-A-specific CD8+ T cells in melanoma
patients. J Immunol. 165:533–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Mandruzzato S, Rossi E, Bernardi F, et al:
Large and dissimilar repertoire of Melan-A/MART-1-specific CTL in
metastatic lesions and blood of a melanoma patient. J Immunol.
169:4017–4024. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Zhou J, Dudley ME, Rosenberg SA and
Robbins PF: Selective growth, in vitro and in vivo, of individual T
cell clones from tumor-infiltrating lymphocytes obtained from
patients with melanoma. J Immunol. 173:7622–7629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Akiyama Y, Maruyama K, Tai S, et al:
Characterization of a MAGE-1-derived HLA-A24 epitope-specific CTL
line from a Japanese metastatic melanoma patient. Anticancer Res.
29:647–655. 2009.PubMed/NCBI
|
|
42.
|
Saper MA, Bjorkman PJ and Wiley DC:
Refined structure of the human histocompatibility antigen HLA-A2 at
2.6 A resolution. J Mol Biol. 219:277–319. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Madden DR, Garboczi DN and Wiley DC: The
antigenic identity of peptide-MHC complexes: a comparison of the
conformations of five viral peptides presented by HLA-A2. Cell.
75:693–708. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Sidney J, del Guercio MF, Southwood S,
Hermanson G, Maewal A, Appella E and Sette A: The HLA-A0207 peptide
binding repertoire is limited to a subset of the A0201 repertoire.
Hum Immunol. 58:12–20. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Rivoltini L, Loftus DJ, Barracchini K, et
al: Binding and presentation of peptides derived from melanoma
antigens MART-1 and glycoprotein-100 by HLA-A2 subtypes:
implications for peptide-based immunotherapy. J Immunol.
156:3882–3891. 1996.PubMed/NCBI
|
|
46.
|
Sette A and Sidney J: HLA supertypes and
supermotifs: a functional perspective on HLA polymorphism. Curr
Opin Immunol. 10:478–482. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Ito M, Shichijo S, Tsuda N, Ochi M,
Harashima N, Saito N and Itoh K: Molecular basis of T cell-mediated
recognition of pancreatic cancer cells. Cancer Res. 61:2038–2046.
2001.PubMed/NCBI
|
|
48.
|
Nonaka Y, Tsuda N, Shichijo S, et al:
Recognition of ADP-ribosylation factor 4-like by HLA-A2-restricted
and tumor-reactive cytotoxic T lymphocytes from patients with brain
tumors. Tissue Antigens. 60:319–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Sawada Y, Yoshikawa T, Fujii S, et al:
Remarkable tumor lysis in a hepatocellular carcinoma patient
immediately following glypican-3-derived peptide vaccination: an
autopsy case. Hum Vaccin Immunother. 9:Mar 6–2013.(Epub ahead of
print).
|
|
50.
|
Krausa P, Brywka M III, Savage D, et al:
Genetic polymorphism within HLA-A*02: significant
allelic variation revealed in different populations. Tissue
Antigens. 45:223–231. 1995.PubMed/NCBI
|
|
51.
|
Chang CX, Tan AT, Or MY, et al:
Conditional ligands for Asian HLA variants facilitate the
definition of CD8(+) T-cell responses in acute and chronic viral
diseases. Eur J Immunol. 43:1109–1120. 2013.PubMed/NCBI
|
|
52.
|
Chen KY, Liu J and Ren EC: Structural and
functional distinctiveness of HLA-A2 allelic variants. Immunol Res.
53:182–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Shieh DC, Lin DT, Yang BS, Kuan HL and Kao
KJ: High frequency of HLA-A*0207 subtype in Chinese
population. Transfusion. 36:818–821. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Cheng LH, Jin SZ, Gao SQ, Li Z, Zou HY,
Wang DM and Wu GG: Difference in HLAA*02 allele
distribution between Han populations in south and north China. Di
Yi Jun Yi Da Xue Xue Bao. 25:321–324. 2005.(In Chinese).
|
|
55.
|
Mohana Devi S, Balachandar V, Arun M,
Suresh Kumar S, Balamurali Krishnan B and Sasikala K: Analysis of
genetic damage and gene polymorphism in hepatocellular carcinoma
(HCC) patients in a South Indian population. Dig Dis Sci.
58:759–767. 2013.PubMed/NCBI
|