Introduction
The gold standard of therapy for locally invasive
bladder cancer (T2-4, N0M0) is radical cystectomy. However,
approximately 80% of cases of bladder cancer are diagnosed in
individuals over the age of 60 (1). Many of these patients may be at risk
of various conditions such as heart and lung disease (2), and are therefore deemed poor
candidates for surgery because of their medical comorbidities.
Trimodality therapy involving radical transurethral
resection, chemotherapy and radiation therapy has long been
attempted as an alternative approach for patients who require
cystectomy. The Radiation Therapy Oncology Group (RTOG) has
completed six prospective protocols entering 415 patients with
T2-T4a muscle-invasive bladder cancer who were candidates for
cystectomy. However, none of the protocols achieved a 5-year
survival rate of more than 60% (3–9).
Moreover, the 5-year bladder intact survival rates were less than
50% in most of studies (8–11). Improvement of the survival rate
with bladder preservation may require a new method, which allows to
deliver a higher dosage of anticancer agent specifically into the
tumor, without causing systemic side-effects.
We have developed a novel bladder preservation
therapy [referred to hereafter as the ‘OMC (Osaka Medical College)
regimen’] involving balloon-occluded arterial infusion (BOAI) of an
anticancer agent and concurrent hemodialysis (HD). This allows the
anticancer agent to accumulate at a high concentration at the site
of the tumor while ensuring that the systemic concentration remains
low. We have previously reported that all elderly patients (aged 98
years) completed this regimen, and that more than 90% of patients
with locally advanced urothelial bladder cancer who were treated in
this way achieved CR (12). The
OMC regimen, which enables short hospital stay, can be regarded as
a curative therapy for elderly patients, not only those for whom
total cystectomy is indicated, but also those of whom total
cystectomy is not feasible because of age, performance status or
other reasons and who are considered physically incapable of
tolerating the chemotherapeutic regimens that are usually applied
clinically. Herein, we describe this novel approach and its
outcomes to date for elderly patients (n=89) in comparison with
total cystectomy (n=45).
Patients and methods
Eligibility criteria
Eligible patients were aged over 70 years and had
histologically confirmed stage CIS, T2 or T3 muscle-invasive
bladder cancer without distant metastasis. Imaging studies,
including chest computed tomography (CT) scan, abdominal/pelvic
magnetic resonance imaging (MRI) and CT scan, and bone scintigraphy
were performed before the start of therapy. All patients who
received the OMC regimen had an absolute neutrophil count (ANC) of
1,500/μl, platelet count 100,000/μl, creatinine 3.0 mg/dl, a
bilirubin level 3 times the institutional upper limit of the normal
range, an AST level 4 times the institutional upper limit of the
normal range, an Eastern Cooperative Oncology Group (ECOG)
performance status of 0–2, and no prior radiotherapy or systemic
therapy for bladder cancer. The study was reviewed and approved by
the institutional review board of Osaka Medical College. Patients
were informed of the investigational nature of the study and
provided written informed consent before study enrollment.
Study design and treatment
Before study entry, patients underwent transurethral
resection of the bladder tumor (TURBT) to establish the diagnosis.
We primarily recommended total cystectomy when surgery was
feasible. However, the OMC regimen was offered as another treatment
option whenever total cystectomy was not feasible because of
advanced age, performance status or other reasons. Patients were
assigned to receive the OMC regimen at 4 to 5 weeks after TURBT to
allow adequate healing.
Assessability, toxicity and response
criteria
Pretreatment evaluation included a complete history
and physical examination, performance status assessment, complete
differential blood cell count, electrolytes, blood urea nitrogen,
serum creatinine, liver function parameters, and appropriate
imaging studies to assess the extent of disease. During treatment,
patients were seen weekly at our department, when their weight was
recorded and toxicity was monitored using the National Cancer
Institute’s Common Terminology Criteria for adverse events v4.0
(CTCAE). At 6 weeks, patients underwent repeat transurethral
resection of the site of the original tumor, ultrasound-guided
whole-layer biopsy, and urine cytology, as well as MRI and CT scan
of the pelvis, and were evaluated for their response to this
therapy. CR was defined as complete disappearance of all measurable
and evaluable disease. Duration of response was defined as the
period from documentation of the response until evidence of disease
recurrence. Survival was the period from study entry until patient
death. Patients who achieved CR were observed using our follow-up
protocol. However, any evidence of residual tumor in the bladder
was deemed as treatment failure, and such patients were primarily
advised to undergo total cystectomy when possible, but otherwise to
undergo secondary BOAI with a higher dosage of cisplatin or
gemcitabine (1,600 mg), as a salvage therapy. Patients who were
found to have only a superficial amount of remaining tumor
underwent intravesical injection of bacillus Calmette-Guerin
(BCG).
Follow-up
All patients were followed up on the basis of
monthly urine cytology, together with cystoscopy, biopsy and
imaging studies, every three months for 2 years, including chest CT
scan, abdominal/pelvic MRI and CT scan, and bone scintigraphy, and
at 6-month intervals thereafter.
Statistics
Simple as well as multiple regression analyses were
conducted to evaluate the significance of the following variables
as risk factors of treatment failure: age, sex, patient performance
status, tumor stage and tumor size, and significance of complete
resection of tumor and hydronephrosis due to tumors are also
evaluated. The life table probabilities of overall survival and
progression-free survival were determined using Kaplan-Meier
analysis and log-rank test. The Cox proportional hazards regression
analysis was conducted to assess the associations of each factor as
described above. Differences at p<0.05 were considered to be
statistically significant.
Results
Patient characteristics
Between 1997 and 2013, 89 (63 males and 26 females)
were treated with the OMC regimen, and 45 (39 males and 6 females)
underwent radical cystectomy. The characteristics of the patients
in these two treatment groups are shown in Table I. For preoperative clinical
staging, we used a simplified form of the 2002 TNM classification
to stage bladder tumors as Tis, T1, T2 and T3 (13). To make a valid comparison,
preoperative clinical staging and not the pathologic stage after
cystectomy was used to compare the two treatment groups, thus
avoiding stage migration that may occur after pathologic staging
(14).
 | Table I.Patient characteristics of both
groups. |
Table I.
Patient characteristics of both
groups.
| Characteristics | OMC regimen | Total cystectomy | P-value |
|---|
| Age, median (range
years) | 77 (70–91) | 74 (70–89) | 0.0003 |
| Sex | | | |
| Male (%) | 63 (70.8%) | 36 (86.7%) | 0.0469 |
| Female (%) | 26 (29.2%) | 9 (13.3%) | |
| ECOG performance
status | | | |
| 0 | 35 (39.4%) | 13 (28.9%) | NS, 0.4063 |
| 1 | 39 (43.8%) | 25 (55.6%) | |
| 2 | 15 (16.8%) | 7 (15.6%) | |
| Clinical stage | | | |
| T-stage | | | |
| Cis | 5 (5.6%) | 3 (6.6%) | NS, 0.7929 |
| T2 | 47 (52.8%) | 26 (57.8%) | |
| T3 | 37 (41.6%) | 16 (35.6%) | |
Treatment details
OMC regimen group
Patients assigned to the OMC regimen group underwent
transurethral resection of the bladder tumor (TURBT) to establish
the diagnosis. They were then scheduled to receive the OMC regimen
4 to 5 weeks after TURBT to allow adequate healing. We administered
100, 200 or 300 mg of cisplatin as a single bolus according to the
criteria described in Table
II.
 | Table II.Criteria for the administration of
cisplatin. |
Table II.
Criteria for the administration of
cisplatin.
| In the initially
enrolled 11 patients |
| 100 mg | Renal function (sCr
>1.3) or age (>75 years) |
| 200 mg | Renal function (sCr
<1.3) with [age (60–74 years) and T-stage (T2 or T3)] |
| 300 mg | Renal function (sCr
<1.3) with [age (<60 years) or T-stage: T4] |
| In the latest 151
patients |
| 100 mg | All patients |
For the intra-arterial infusion procedure, we used
an intra-arterial catheter equipped with two occlusion balloons
(size: 6 Fr., M6F-28-70-TBSB4-ST, Clinical Supply, Tokyo, Japan).
The catheter was introduced into the posterior trunk of the
internal iliac artery through the femoral arterial approach, and
after the distal balloon had passed through the furcation of the
anterior trunk of the internal iliac artery, both the distal and
proximal balloons were inflated and immobilized, so that the
anterior trunk of the internal iliac artery, which lies upstream of
the target vessels (the ‘vesical arteries’) was isolated between
the balloons. At this time, using digital subtraction angiography
(DSA), it was confirmed that the injected agent did not enter the
superior gluteal artery and that there was no back-flow into the
internal iliac artery, while the tumor was markedly stained due to
active flow of injected contrast medium into the urinary bladder.
The extracorporeal circuit used in the treatment was illustrated in
our previous paper (15). Various
amounts of cisplatin (100, 200 or 300 mg) were locally infused
through the catheter over a 1-h period (Table I). Simultaneously, HD was performed
via two double-lumen catheters (size: 12 Fr., Argyle®,
Tyco Healthcare, Tokyo, Japan) placed in the bilateral common iliac
veins for 2 h after the start of arterial infusion. The catheters
were connected to a hollow-fiber dialyzer (APS150, Asahi, Tokyo,
Japan) with a membrane area of 1.0–1.5 m2 according to
the weight of each patient. The blood flow rate was 180–250 ml/min
and the hemodialysis-fluid flow rate was 500 ml/min.
Radiation therapy was administered to the whole
pelvis using a CT-planned three-dimensional conformal technique to
a total of 60 Gy: 50 Gy (2 Gy/day × 25 days) followed by 10 Gy (2
Gy/day × 5 days) of local irradiation to the bladder. Patients were
treated with the bladder empty. The planned target volume for the
bladder included the gross target volume (bladder plus any
extravesical tumor) with a 1-cm expansion. At 6 weeks, patients
underwent repeat transurethral resection of the site of the
original tumor, ultrasound-guided whole-layer biopsy, and urine
cytology, as well as MRI and CT scan of the pelvis, and the
response to this therapy was then evaluated.
Radical cystectomy group
Among the 45 patients in the radical cystectomy
group, 27 patients underwent uretero-cutaneostomy, 12 underwent
ileal conduit formation, 4 underwent continent urinary diversion
with ileal-neobladder formation (Hartmann’s method), 1 underwent
Indiana pouch formation, and the remaining 1 underwent
uretero-sigmoidostomy performed at the time of radical cystectomy.
Standard pelvic lymphadenectomy was performed in 39 patients, five
patients underwent iliac sampling, and one patient was not studied
in sufficient detail to allow assessment of the level of lymph node
dissection. As not all of the histology reports mentioned the
number of lymph nodes examined, it was not possible to precisely
evaluate the extent of dissection. There were no significant
differences in cause-specific or overall survival between the
patients who underwent nodal dissection and the patients who did
not. Urethrectomy was performed in 9 patients at the time of
radical cystectomy because of the presence of extensive carcinoma
in situ or multifocal bladder tumors.
Response to the OMC regimen
Table III
summarizes the treatment response, duration of response, and
patient characteristics, including sex, age, tumor stage, tumor
size, involvement of hydronephrosis, performance status and success
or failure of complete TURBT. Overall, 81 of the 89 patients
(91.0%, 95% CI, 83.1–91.0%) achieved a complete response as defined
by the absence of persistent disease revealed by cystoscopy,
biopsy, and urine cytology after therapy (Table III). More than 96% of patients with
CR were able to retain their urinary bladder with no evidence of
recurrent disease or distant metastasis within a mean follow-up
period of 166 weeks (range, 16–818 weeks; 1st to 3rd Qu = 69–245
weeks) from the completion of therapy.
 | Table III.Response at 3 months after treatment
and current outcome. |
Table III.
Response at 3 months after treatment
and current outcome.
| CR
| PR
| SD
| PD
|
|---|
| No | % | 95%-CI | No | % | 95%-CI | No | % | 95%-CI | No | % | 95%-CI |
|---|
| Total number of
patients | 81 | 91.0 | 83.1–96.0 | 4 | 4.49 | 1.24–11.1 | 2 | 2.25 | 0.27–7.88 | 2 | 2.25 | 0.27–7.88 |
| Duration of
response | | | | | | | | | | | | |
| Mean, range | 164, 16–818
weeks | 124, 36–231
weeks | 56, 18–94
weeks | 0 weeks |
| 1st, 3rd Qu | 67, 237 weeks | 83, 156 weeks | 37, 75 weeks | 0 weeks |
| Recurrence | 3 | 3.70 | 0.77–10.4 | 0 | 0 | 0–60.2 | 2 | 100 | 15.8–100 | | | |
| Death | 4 | 4.94 | 1.36–12.2 | 1 | 25.0 | 0.63–80.6 | 2 | 100 | 15.8–100 | 2 | 100 | 15.8–100 |
| Age (mean,
range) | 77, 70–91
years | 77, 73–78
years | 79, 76–81
years | 78, 77–79
years |
| Sex | | | | | | | | | | | | |
| Male | 58 | 71.6 | 60.5–81.1 | 3 | 75.0 | 19.4–99.4 | 1 | 50.0 | 12.6–98.7 | 1 | 50.0 | 12.6–98.7 |
| Female | 23 | 28.4 | 18.9–39.5 | 1 | 25.0 | 0.63–80.6 | 1 | 50.0 | 12.6–98.7 | 1 | 50.0 | 12.6–98.7 |
| Categories | | | | | | | | | | | | |
| T-stage | | | | | | | | | | | | |
| Tis | 5 | 6.17 | 2.03–13.8 | 0 | 0 | 0–60.2 | 0 | 0 | 0–84.2 | 0 | 0 | 0–84.2 |
| 2 | 46 | 56.8 | 45.3–67.8 | 1 | 25.0 | 0.63–80.6 | 0 | 0 | 0–84.2 | 0 | 0 | 0–84.2 |
| 3 | 30 | 37.0 | 26.6–48.5 | 3 | 75.0 | 19.4–99.4 | 2 | 100 | 15.8–100 | 2 | 100 | 15.8–100 |
| Tumor size | | | | | | | | | | | | |
| ≤3 cm | 41 | 50.6 | 39.3–61.9 | 2 | 50.0 | 6.76–93.2 | 0 | 0 | 0–84.2 | 1 | 50.0 | 12.6–98.7 |
| 3–5 cm | 38 | 46.9 | 35.7–58.3 | 1 | 25.0 | 0.63–80.6 | 2 | 100 | 15.8–100 | 1 | 50.0 | 12.6–98.7 |
| >5 cm | 2 | 2.47 | 0.30–8.64 | 1 | 25.0 | 0.63–80.6 | 0 | 0 | 0–84.2 | 0 | 0 | 0–84.2 |
| Hydro | | | | | | | | | | | | |
| (+) | 3 | 3.70 | 0.77–10.4 | 2 | 50.0 | 6.76–93.2 | 1 | 50.0 | 12.6–98.7 | 0 | 0 | 0–84.2 |
| (−) | 78 | 96.3 | 89.6–99.2 | 2 | 50.0 | 6.76–93.2 | 1 | 50.0 | 12.6–98.7 | 0 | 0 | 0–84.2 |
| Comp-TUR | | | | | | | | | | | | |
| (+) | 58 | 71.6 | 60.5–81.1 | 2 | 50.0 | 6.76–93.2 | 1 | 50.0 | 12.6–98.7 | 1 | 50.0 | 12.6–98.7 |
| (−) | 23 | 28.4 | 18.9–39.5 | 2 | 50.0 | 6.76–93.2 | 1 | 50.0 | 12.60–98.7 | 1 | 50.0 | 12.6–98.7 |
| PS | | | | | | | | | | | | |
| 0 | 33 | 40.7 | 29.9–52.2 | 1 | 25.0 | 0.63–80.6 | 0 | 0 | 0–84.2 | 1 | 50.0 | 12.6–98.7 |
| 1 | 33 | 40.7 | 29.9–52.2 | 3 | 75.0 | 19.4–99.4 | 2 | 100 | 15.8–100 | 1 | 50.0 | 12.6–98.7 |
| 2 | 15 | 18.5 | 10.8–28.7 | 0 | 0 | 0–60.2 | 0 | 0 | 0–84.2 | 0 | 0 | 0–84.2 |
The univariate as well as multivariate regression
analyses revealed that a hydronephrosis and tumor histology (UC)
are the only risk factor for treatment failure, while tumor stage,
tumor size, and failure of complete resection of tumor, those
usually have been reported as risk factors for treatment failure of
bladder preservation therapy, were not selected (Table IV).
 | Table IV.Risk factors for treatment failure in
the OMC regimen group. |
Table IV.
Risk factors for treatment failure in
the OMC regimen group.
| Category | Univariate
| Multivariate
|
|---|
| Odds ratio | P-value | Odds ratio | P-value |
|---|
| Hydronephrosis | (+) vs. (−) | 15.63 | 0.0034 | 200 | 0.0045 |
| T-stage (T3) | T3 vs. <T3 | 11.90 | 0.0235 | 14.71 | 0.0488 |
| Tumor
histology | UC vs. non-UC | 13.17 | 0.0176 | 56.38 | 0.0426 |
| Tumor size | ≥3 cm vs. <3
cm | 1.708 | 0.4830 | 10.42 | 0.2238 |
| Tumor size | ≥5 cm vs. <5
cm | 5.650 | 0.1787 | NV | NV |
| Tumor number | Cont. variable | 2.002 | 0.1307 | 4.533 | 0.0912 |
| Complete TUR | Yes vs. no | 1.513 | 0.5911 | 2.290 | 0.6316 |
| Performance
status | 2 vs. 0−1 | 485,376 | 0.9798 | 290,745 | 0.9985 |
| Sex | Male vs.
female | 2.500 | 0.1749 | 2.985 | 0.2628 |
| Age | Cont. variable | 1.513 | 0.5911 | 1.111 | 0.4653 |
Comparison of survival between the two
groups
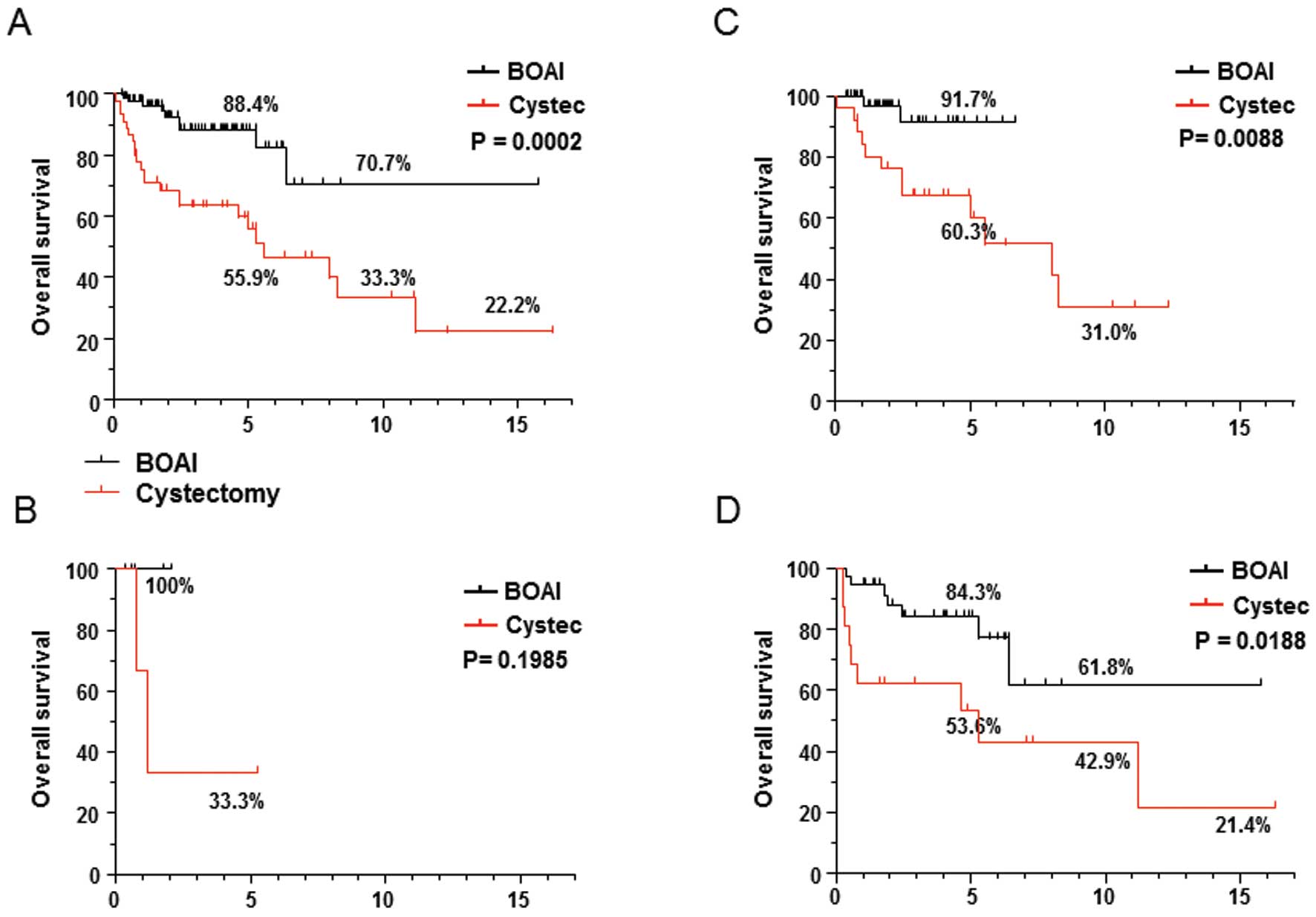
Overall survival
Overall survival was significantly improved in the
OMC regimen group, with 5- and 10-year survival rates of 88.4 and
70.7%, respectively as compared to 59.9 and 33.3% in the cystectomy
group, p=0.0002, Fig. 1A).
Fig. 1B, C and D show OS ratio of
patients in each T-stage of CIS, T2 and T3, respectively. The OS
ratio in patients with T2 as well as T3 tumors was significantly
better in the OMC regimen group, although statistically
significance has not seen in patients in with CIS (despite of 100%
of OS), because of short follow-up period.
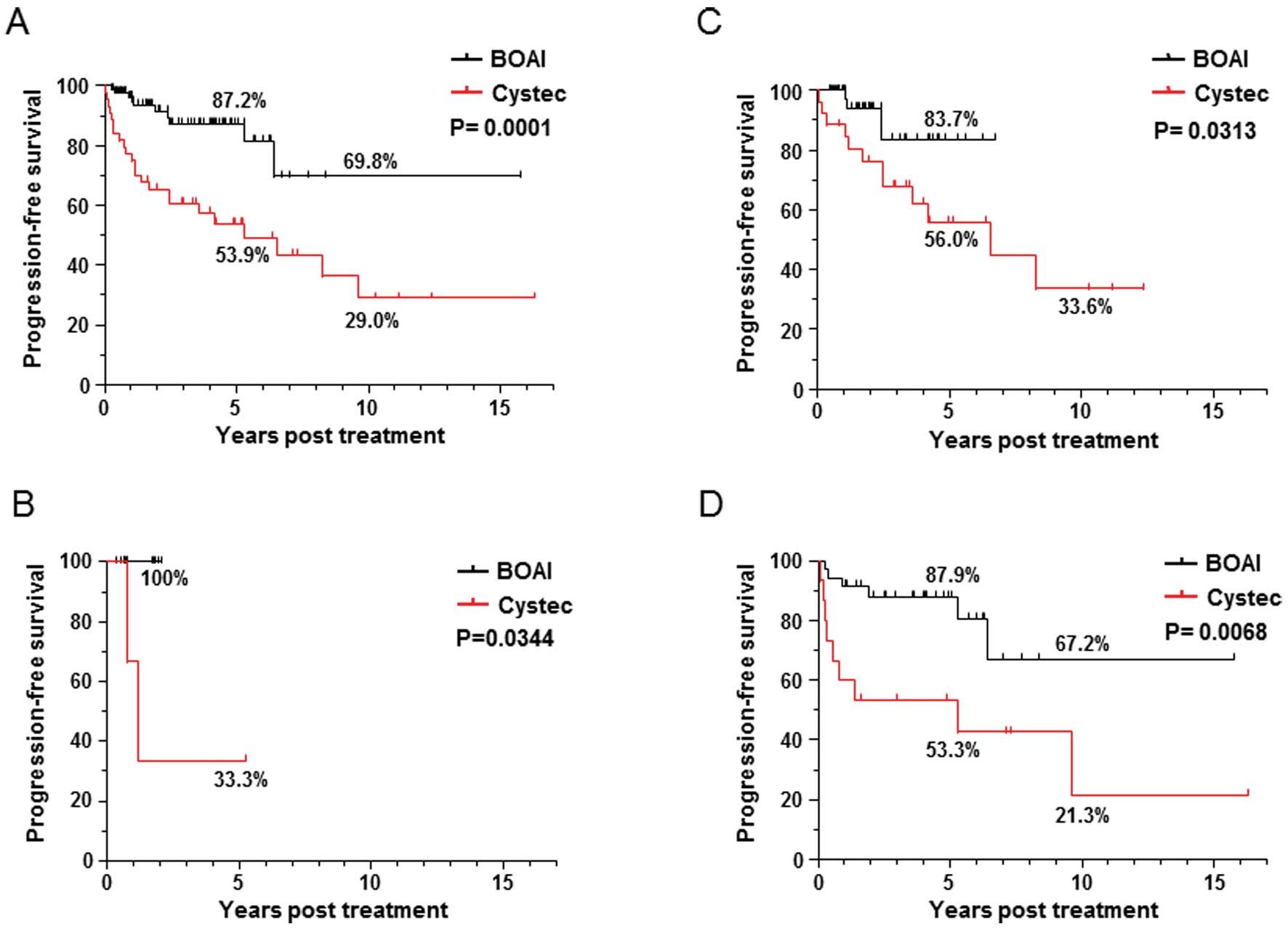
Progresion-free survival
The progression-free survival ratio was also
significantly better in the OMC regimen group in total patients, as
well as in each patients with CIS, T2 and T3 tumors (Fig. 2). In all patients, more than 87.2
and 69.8% of patients have been surviving with their functioning
bladder at 5- and 10-years, respectively; this is the most
important issue for the bladder preservation therapy.
Predictors of patient survival
selected using univariate and multivariate Cox regression analyses
in OMC regimen group
We investigated the significance of each factor,
including sex, age, tumor stage, tumor size, tumor pathology
(non-UC vs. UC), involvement of hydronephrosis, performance status
(2 vs. 0–1), and success or failure of complete TURBT as a
predictor of overall survival and progression-free survival using
the Cox regression model. As shown in Table V, univariate Cox regression
analysis selected hydronephrosis, tumor histology and greater than
5 cm of tumor size as the significant factors affecting both
overall and progression-free survival, while tumor stage and
failure of complete resection of tumor have not been selected.
Multivariate Cox regression analysis also selected hydronephrosis
and tumor histology as the significant factors affecting both
overall and progression-free survival, while tumor size, tumor
stage and failure of complete resection of tumor have not been
selected.
 | Table V.Predictors of overall survival and
progression-free survival in the OMC regimen group evaluated by
univariate and multivariate Cox regression analyses. |
Table V.
Predictors of overall survival and
progression-free survival in the OMC regimen group evaluated by
univariate and multivariate Cox regression analyses.
A, Overall
survival.
|
| Category | Univariate
| Multivariate
|
| Odds ratio | P-value | Odds ratio | P-value |
|
| Hydronephrosis | (+) vs. (−) | 9.524 | 0.0081 | 23.81 | 0.0068 |
| Histology | UC vs. non-UC | 7.649 | 0.0031 | 44.08 | 0.0093 |
| T-stage | T3 vs. <T3 | 1.887 | 0.3154 | 2.469 | 0.3339 |
| Tumor size | ≥3 cm vs. <3
cm | 1.500 | 0.5727 | 1.428 | 0.7964 |
| Tumor size | ≥5 cm vs. <5
cm | 11.63 | 0.0035 | 5.974 | 0.1253 |
| Tumor number | Cont. variable | 1.387 | 0.4103 | 1.880 | 0.3157 |
| Complete TUR | Yes vs. no | 1.005 | 0.9938 | 1.051 | 0.9741 |
| Performance
status | 2 vs. 0–1 | 1.376 | 0.6925 | 2.766 | 0.3883 |
| Sex | Male vs.
female | 2.372 | 0.1392 | 1.539 | 0.5813 |
| Age | Cont. variable | 1.061 | 0.4597 | 1.259 | 0.0644 |
|
B, Progression-free
survival.
|
| Category | Univariate
| Multivariate
|
| Odds ratio | P-value | Odds ratio | P-value |
|
| Hydronephrosis | (+) vs. (−) | 9.173 | 0.0016 | 10.99 | 0.0195 |
| Histology | UC vs. non-UC | 7.649 | 0.0031 | 12.492 | 0.0112 |
| T-stage | T3 vs. <T3 | 1.887 | 0.3154 | 1.323 | 0.6849 |
| Tumor size | ≥3 cm vs. <3
cm | 1.583 | 0.4590 | 2.659 | 0.2486 |
| Tumor size | ≥5 cm vs. <5
cm | 62.29 | 0.0158 | 6.098 | 0.1809 |
| Tumor number | Cont. variable | 1.115 | 0.7458 | 1.063 | 0.9011 |
| Complete TUR | Yes vs. no | 1.456 | 0.5601 | 5.291 | 0.0989 |
| Performance
status | 2 vs. 0–1 | 1.543 | 0.5167 | 1.443 | 0.6853 |
| Sex | Male vs.
female | 2.372 | 0.1392 | 1.143 | 0.8582 |
| Age | Cont. variable | 1.022 | 0.7476 | 1.128 | 0.1426 |
Toxicity
The most significant outcome of the OMC regimen was
that its related toxicity was markedly less severe than those
reported for other protocols, as shown in Table VI. None of the patients suffered
grade III or more severe toxicity. Eight patients (8.99%, 95%
confidence interval (CI), 3.96–16.9%) experienced grade I
blood/bone-marrow toxicity, 15 (16.9%, 95%CI, 9.75–26.3%) had
gastrointestinal toxicity, and 3 (2.25%, 95%CI, 0.27–7.88%) had
neuropathy. The duration of blood/bone-marrow toxicity, including
granulocytopenia and anemia, was relatively short: median duration
was 5 days for granulocytopenia (range 3–9 days) and 4 days for
anemia (range 2–10 days). No patient received granulocyte
colony-stimulating factor or transfusion of red blood cells.
Gastrointestinal toxicity included anorexia in 10 patients,
constipation in 6, diarrhea in 5, nausea in 11, and vomiting in 1,
but all symptoms disappeared within 6 days after intra-arterial
infusion. One patient experienced grade II neuropathy in the
peroneal nerve area, but this disappeared by the 12 months after
the treatment. There were no other adverse reactions such as renal
failure, genitourinary toxicity, radiation cystitis or
life-threatening complications.
 | Table VI.Toxicity. |
Table VI.
Toxicity.
| Toxicity | Grades
| Duration (days)
|
|---|
| 1 No. (%) | 2 No. (%) | 3–4 No. (%) | <3 No. (%) | 3–7 No. (%) | >7 No. (%) |
|---|
| Blood/bone
marrow | | | | | | |
| Total | 6 (6.7) | 2 (2.2) | 0 | | | |
|
Granurocytepenia | 5 (5.6) | 0 | 0 | 0 | 4 (4.5) | 1 (1.1) |
| Anemia | 6 (6.7) | 2 (2.2) | 0 | 3 (3.4) | 4 (4.5) | 1 (1.1) |
|
Gastrointestinal | | | | | | |
| Total | 15 (16.9) | 0 | 0 | | | |
| Anorexia | 10 (11.2) | 0 | 0 | 6 (6.7) | 4 (4.5) | 0 |
|
Constipation | 6 (6.7) | 0 | 0 | 5 (5.6) | 1 (1.1) | 0 |
| Diarrhea | 5 (5.6) | 0 | 0 | 2 (2.2) | 3 (3.4) | 0 |
| Nausea | 11 (12.4) | 0 | 0 | 6 (6.7) | 5 (5.6) | 0 |
| Vomiting | 1 (1.1) | 0 | 0 | 0 | 1 (1.1) | 0 |
| Neuropathy | 2 (2.2) | 1 (1.1) | 00 | 0 | 0 | 3 (3.4) |
Discussion
Optimal initial treatment for muscle-invasive
bladder cancer in elderly patients has been a subject of debate.
Some urologists or radiologists have recommended bladder-sparing
trimodality approaches with aggressive transurethral resection of
the bladder tumor (TURBT) and radiochemotherapy, while others have
advocated immediate cystectomy (3–7).
Nielsen et al reviewed the records of 888 patients with
transitional cell carcinoma who underwent radical cystectomy and
pelvic lymphadenectomy for localized disease at three institutions
(16), and found that advanced age
was independently and significantly associated with more
pathologically advanced disease and poorer bladder-specific
mortality after surgery. The actuarial 5-year overall survival rate
of such patients over 70 years of age has reportedly ranged from 35
to 60% (3–9). A highly effective, but non- or
minimally invasive therapy that conserves the bladder is therefore
needed.
BOAI allows delivery of an extremely high
concentration of anticancer agent to the bladder and surrounding
pelvic region (17–19). In addition, severe hypoxia in the
target region resulting from BOAI may play a role in the marked
antitumor effect, as several basic studies have demonstrated that
hypoxia greatly enhances the effectiveness of cisplatin (20,21).
Enhanced radiosensitivity of the cancer cells due to the
BOAI-induced high concentration of cisplatin may also contribute
significantly to the good response achieved. Such theories may be
supported by the present outcomes; more than 90% of patients
achieved CR, and most of the CR patients (96%) survived with their
intact bladder; the 5- and 10-year bladder intact survival rates
were 87.2 and 69.8%, respectively. Additionally, the findings from
our in vivo experiments, demonstrating that BOAI provided an
extremely high concentration of anticancer agent to the bladder and
surrounding pelvic region, as well as to the para-aortic lymphatic
tissues (data not shown) may also contribute to the excellent
outcomes even better than cystectomy.
The other advantage of the OMC regimen, especially
pertinent for the elderly patients, is a significant reduction of
systemic side-effects. Cisplatin exerts its antitumor activity via
the non-protein-bound form, which reduces steeply after
administration: its half-life is normally less than 60 min, and
reaches to below the detection limit 4 h after administration
(22,23). The most important point of OMC
regimen is a removal of non-protein-bound Pt immediately after
administration of cisplatin by performing HD via the common iliac
veins, which accomplish efficient drainage of cisplatin immediately
after passage through the tumor. HD is specifically efficient for
cisplatin elimination, since the molecular weight of
protein-unbound cisplatin is approximately 300, similar to that of
creatinine. Moreover, the anatomic structure and blood supply of
the bladder may largely account for the efficient drainage of
cisplatin achieved with this approach. As the urinary bladder is
situated at the base of the pelvis, the relatively close circuit
formed by the internal iliac artery, bladder and common iliac veins
may contribute to efficient drainage of the anticancer agent, thus
increasing the elimination efficiency without influencing the
systemic circulation. Indeed, we have previously found that >95%
of free Pt was efficiently eliminated by HD, which may allow the
present outcomes that all elderly patients, even over 90 years old
patients, completed this regimen. Thus, it is noteworthy that this
therapy will improve the feasibility of radical cure without the
need for cystectomy in patients for whom such surgery would
otherwise be necessary, and also facilitate potential cure in
patients whose condition would normally rule out this likelihood
and for whom, otherwise, merely palliative treatment would seem the
only option.
In conclusions, the OMC regimen, which delivers an
extremely high concentration of anticancer agent to the site of a
tumor without causing systemic adverse effects, can be regarded as
a curative therapy for elderly patients, not only those for whom
total cystectomy is indicated, but also those of whom total
cystectomy is not feasible because of age, performance status or
other reasons and who are considered physically incapable of
tolerating the chemotherapeutic regimens that are usually applied
clinically.
Abbreviations:
|
ANC
|
absolute neutrophil count;
|
|
BOAI
|
balloon-occluded arterial
infusion;
|
|
CIS
|
carcinoma in situ;
|
|
CTCAE
|
common terminology criteria for
adverse events;
|
|
DSA
|
digital subtraction angiography;
|
|
ECOG
|
eastern cooperative oncology
group;
|
|
HD
|
hemodialysis;
|
|
Qu
|
quartile;
|
|
RTOG
|
radiation therapy oncology group;
|
|
TURBT
|
transurethral resection of bladder
tumor;
|
|
UC
|
urothelial carcinoma
|
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2.
|
Johansson SL and Cohen SM: Epidemiology
and etiology of bladder cancer. Semin Surg Oncol. 13:291–298. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Tran E, Souhami L, Tanguay S and Rajan R:
Bladder conservation treatment in the elderly population: results
and prognostic factors of muscle-invasive bladder cancer. Am J Clin
Oncol. 32:333–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Weizer AZ, Joshi D, Daignault S, et al:
Performance status is a predictor of overall survival of elderly
patients with muscle invasive bladder cancer. J Urol.
177:1287–1293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hoshi S, Shintaku I, Suzuki K, et al:
Bladder preservation by internal iliac arterial infusion
chemotherapy and irradiation in T3 bladder carcinoma patients over
the age of 70 years. Tohoku J Exp Med. 192:249–258. 2000.PubMed/NCBI
|
|
6.
|
Kaufman DS, Winter KA, Shipley WU, et al:
The initial results in muscle-invading bladder cancer of RTOG
95-06: phase I/II trial of transurethral surgery plus radiation
therapy with concurrent cisplatin and 5-fluorouracil followed by
selective bladder preservation or cystectomy depending on the
initial response. Oncologist. 5:471–476. 2000.
|
|
7.
|
Rodel C, Grabenbauer GG, Kuhn R, et al:
Combined-modality treatment and selective organ preservation in
invasive bladder cancer: long-term results. J Clin Oncol.
20:3061–3071. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shipley WU, Winter KA, Kaufman DS, et al:
Phase III trial of neoadjuvant chemotherapy in patients with
invasive bladder cancer treated with selective bladder preservation
by combined radiation therapy and chemotherapy: initial results of
Radiation Therapy Oncology Group 89-03. J Clin Oncol. 16:3576–3583.
1998.
|
|
9.
|
Tester W, Caplan R, Heaney J, et al:
Neoadjuvant combined modality program with selective organ
preservation for invasive bladder cancer: results of Radiation
Therapy Oncology Group phase II trial 8802. J Clin Oncol.
14:119–126. 1996.
|
|
10.
|
Hagan MP, Winter KA, Kaufman DS, et al:
RTOG 97-06: initial report of a phase I–II trial of selective
bladder conservation using TURBT, twice-daily accelerated
irradiation sensitized with cisplatin, and adjuvant MCV combination
chemotherapy. Int J Radiat Oncol Biol Phys. 57:665–672.
2003.PubMed/NCBI
|
|
11.
|
Kaufman DS, Winter KA, Shipley WU, et al:
Phase I–II RTOG study (99-06) of patients with muscle-invasive
bladder cancer undergoing transurethral surgery, paclitaxel,
cisplatin, and twice-daily radiotherapy followed by selective
bladder preservation or radical cystectomy and adjuvant
chemotherapy. Urology. 73:833–837. 2009.
|
|
12.
|
Azuma H, Inamoto T, Ibuki N, et al: Novel
bladder preservation therapy for locally invasive bladder cancer:
combined therapy using balloon-occluded arterial infusion of
anticancer agent and hemodialysis with concurrent radiation. Int J
Oncol. 37:773–785. 2010. View Article : Google Scholar
|
|
13.
|
Greene FL, Page DL, Fleming ID, Fritz A,
Balch CM, Haller DG and Morrow M: AJCC Cancer Staging Manual. 6th
edit. Springer Verlag; New York, NY: 2002, View Article : Google Scholar
|
|
14.
|
Ficcara V, Dalpiaz O, Alrabi N, Novara G,
Galfano A and Artibani W: Correlation between clinical and
pathological staging in a series of radical cystectomies for
bladder carcinoma. BJU Int. 95:786–790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Azuma H, Inamoto T, Ibuki N, et al:
Utility of the novel bladder preservation therapy,
BOAI-CDDP-radiation (OMC-regimen), for elderly patients with
invasive bladder cancer. Int J Oncol. 38:13–24. 2011.PubMed/NCBI
|
|
16.
|
Nielsen ME, Shariat SF, Karakiewicz PI, et
al: Advanced age is associated with poorer bladder cancer-specific
survival in patients treated with radical cystectomy. Eur Urol.
51:699–708. 2007. View Article : Google Scholar
|
|
17.
|
Collins JM: Pharmacokinetic rationale for
intraarterial therapy. Cancer Chemotherapy, Challenges for the
Future. 4. Kimura K: Excerpta Medica; Amsterdam: 1989
|
|
18.
|
Mitsuzane K, Kawabata M, Terada M, Nomura
S, Sato M and Yamada R: Balloon-occluded arterial infusion as
chemotherapy in bladder cancer-long-term results. Gan To Kagaku
Ryoho. 17:1701–1704. 1990.(In Japanese).
|
|
19.
|
Cvitkovic E, Spaulding J, Bethune V,
Martin J and Whitmore WF: Improvement of
cis-dichlorodiammineplatinum (NSC 119875): therapeutic index in an
animal model. Cancer. 39:1357–1361. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Douple EB and Richmond RC: A review of
platinum complex biochemistry suggests a rationale for combined
platinum-radiotherapy. Int J Radiat Oncol Biol Phys. 8:1335–1339.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Abbott DW, Freeman ML and Holt JT:
Double-strand break repair deficiency and radiation sensitivity in
BRCA2 mutant cancer cells. J Natl Cancer Inst. 90:978–985. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Belt RJ, Himmelstein KJ, Patton TF,
Bannister SJ, Sternson LA and Repta AJ: Pharmacokinetics of
non-protein-bound platinum species following administration of
cis-dichlorodiammineplatinum(II). Cancer Treat Rep. 63:1515–1521.
1979.PubMed/NCBI
|
|
23.
|
Himmelstein KJ, Patton TF, Belt RJ, Taylor
S, Repta AJ and Sternson LA: Clinical kinetics on intact cisplatin
and some related species. Clin Pharmacol Ther. 29:658–664. 1981.
View Article : Google Scholar : PubMed/NCBI
|