Introduction
Casein kinase II (CK2), which is pleiotropic
aserine/threonine protein kinase composed of 2 catalytic subunits
(αα, α′α′ or αα′) and 2 regulatory subunits (β), is ubiquitously
expressed and highly conserved in cells. Through phosphorylation to
more than 300 proteins in cells, CK2 is an important regulator of
intracellular signalling pathways (1), and exerts many roles in cellular
processes, including gene expression, protein synthesis, cell
proliferation and apoptosis (2).
CK2 has been regarded as a potential candidate for
targeted therapy for cancers because dysregulation of CK2 in
association with other proteins increases oncogenic potential of
cells (3). In transgenic mice,
overexpression of CK2α subunits is reportedly associated with the
development of lymphoma (4) and
adenocarcinomas of the mammary gland (5). Overexpression of CK2 has been
reported in a variety of human cancers, including acute myeloid
leukaemia (6), mammary gland
(5), prostate (7), lung (8), head and neck (9), and kidney cancer (10), and also correlates with metastatic
potential, undifferentiated histological type and poor clinical
outcome in human cancers. Various CK2 inhibitors have been
discovered. For example, TBB (4,5,6,7 tetrabrome benzotriazole)
(11) and its derivatives
(12,13) have been shown to induce apoptosis
in human cancer cells. A potent and selective orally bioavailable
small molecule inhibitor of CK2, CX-4945, is being tested in a
clinical trial (14).
We previously showed that a novel CK2 inhibitor,
hematein (3,4,10,6a-tetrahydroxy-7, 6 adihydroindeno [2,1-c]
chroman-9-one), inhibited cancer cell growth and was noted to have
a high selectivity towards CK2 among a kinase panel of 48 kinases
(15). Hematein is a natural
compound from Caesalpinia sappan with a molecular weight of
300.26 Da, and has been used in oriental medicine as an analgesic
and anti-inflammatory agent (16).
It is also used in histochemical staining (17). Hematein has the in vitro
IC50 value of 0.74 μM on CK2 kinase activity,
which is comparable to other CK2 inhibitors (12). However, the effect of hematein on
tumor growth in animal models and the binding mode of hematein to
CK2 remain unknown. We therefore examined the inhibitory effects of
hematein on lung cancer tumor growth in a murine xenograft model
and used molecular docking to elucidate how hematein binds to
CK2.
Materials and methods
Cell culture
A427 (HTB-53) cell line was purchased from American
Type Culture Collection (Manassas, VA). Cells were grown in
complete growth medium (Roswell Park Memorial Institute)
supplemented with 10% fetal bovine serum, 10 units/ ml penicillin
and 10 μg/ml streptomycin at 37°C and 5% CO2.
Cell viability assay
The toxicity of hematein was evaluated by
CellTiter-Glo luminescent cell viability assay (Promega, Madison,
WI) was used to evaluate the cytotoxicity of hematein according to
the manufacturer’s manual (15).
In brief, after incubation with indicated amount of compounds for
48 h, 100 μl of the CellTiter-Glo reagent was added directly
to culture wells. The luminescence produced by the
luciferase-catalyzed reaction of luciferin and ATP was measured
using a luminometer.
Colony formation assay
A427 lung cancer cells (5×102) were
plated in 10 cm culture dishes and incubated in complete medium
with indicated concentrations of hematein (Sciencelab. com, Inc.,
Houston, TX) for 14 days. The colonies were then stained with 0.1%
crystal violet, and colonies of greater than 50 cells were counted.
Results were expressed as relative colony formation: percentage of
the number of colonies relative to the control group. Three
independent experiments were performed.
Western blot analysis
After treatment with indicated concentrations of
hematein for 48 h, whole cell proteins were extracted from A427
cells with M-PER Mammalian Protein Extraction Reagent (Pierce,
Rockfold, IL) added to Phosphatase Inhibitor Cocktail Set II
(Calbiochem, San Diego, CA) and Complete Protease Inhibitor
Cocktails (Roche, Switzerland) according to manufacturer’s
protocols. Proteins were separated on 4–15% gradient sodium dodecyl
sulfate (SDS)-polyacrylamide gels and transferred to Immobilon-P
membranes (Millipore, Billerica, MA). The following primary
antibodies were used: Akt, PARP, survivin (Cell Signaling
Technology, Danvers, MA), phospho-Akt S129 (Abcam Inc., Cambridge,
MA) and β-actin (Sigma, St. Louis, MO). After primary antibody and
antigen complexes were bound to specific secondary antibodies, an
enhanced chemiluminescence (ECL) blotting analysis system (GE
Healthcare Life Sciences, Piscataway, NJ) was used for
antigen-antibody detection. Densitometry of western blot analysis
was calculated by using ImageJ (v1.44m for Windows, National
Institutes of Health).
Transient transfection and luciferase
reporter assay
The TOP/ FOP Flash reporter assay was performed to
evaluate the TCF/LEF transcriptional activity induced by the Wnt
canonical pathway. Three independent transfection experiments were
performed in triplet using the Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The A427 cells were transfected with 8 μg Super 8×TOPflash
or 8 μg Super 8×FOPflash plasmid (a kindly gift from
Professor Randall Moon, Howard Hughes Medical Institute and
Department of Pharmacology, University of Washington, Seattle, WA,
USA), the pRL-TK plasmid (Promega) was co-transfected to normalize
for transfection efficiency. Twenty-four hours after transfection,
cells were treated with hematein (50 or 100 μM) for 24 h.
Luciferase activity was then assayed using the
Dual-Luciferase® Reporter Assay System (Promega) with a
luminometer.
Murine xenograft model
After approval was obtained from our institutional
animal care and use committee, groups of 6 female athymic BALB/c
nude mice (6-week-old), received subcutaneous injections of
4×106 A427 cells in the flank area with a volume of 100
μl PBS with 25% matrigel (BD Biosciences, Bedford, MA).
Seven days later, tumors had formed. The mice then received
intraperitoneal injections twice a week with 50 mg/kg of hematein
or 5% DMSO dissolved in PBS as the control. Tumor size was
determined twice a week for 6 weeks, and tumor volume was
calculated on the basis of width (x) and length (y):
x2y/2, where x < y. Seven
weeks after injection of A427 lung cancer cells, mice were
sacrificed. The heart, liver, lung and kidney were resected, fixed
and stained with hematoxylin and eosin according to standard
methods. All slides were reviewed by a pathologist and were were
photographed using a Zeiss AxioCam camera with Zeiss AxioVision
software.
Immunohistochemistry
The formalin-fixed and paraffin-embedded tumors were
sliced into 5 μm sections and were deparaffinized in xylene
and then rehydrated in graded alcohol. Antigen retrieval was
performed by steaming the tissue sections in citrate buffer (10 mM,
0.05% Tween-20, pH 6.0) for 20 min. Slides were then washed in TBS
plus 0.025% Triton X-100, blocked in 10% normal serum with 1% BSA
in TBS for 2 h at room temperature, and then incubated in the
primary antibody overnight at 4°C. The rabbit polyclonal cleaved
caspase-3 antibody (Cell Signaling, Boston, MA) was used as primary
antibody at a 1–300 dilution in TBS with 1% BSA. Following TBST
washes, endogenous peroxidase activity was then quenched with 0.3%
hydrogen peroxide in TBS. Mouse and Rabbit Specific HRP/DAB (ABC)
detection IHC kit (Abcam) kit was then used according to the
manufacturer’s protocol. Detection was achieved using a
biotinylated anti-rabbit secondary antibody and DAB chromogen. The
sections were counterstained with hematoxylin before being mounted
with organic media and glass slides.
Molecular docking of hematein to
CK2α
DOCK 3.5.54 was used to predict the binding pose of
hematein in both the canonical ATP binding site and the allosteric
DRB site of CK2α (18–20). DRB
(5,6-dichloro-1-b-D-ribofuranosylbenzimidazole) was used to
generate the docking environment and matching spheres. The most
favourable conformation was chosen from four predicted
conformations of hematein against each site. The docking results
were further verified by another docking program, Accelrys
Discovery Studio 2.5.
Statistical analysis
The data shown represent mean values ± standard
error of mean (SEM). Student’s t-test was used to compare tumor
size. Statistical analysis was carried out using SPSS (version
14.0, Chicago, IL). Two-sided p-values <0.05 were considered
statistically significant.
Results
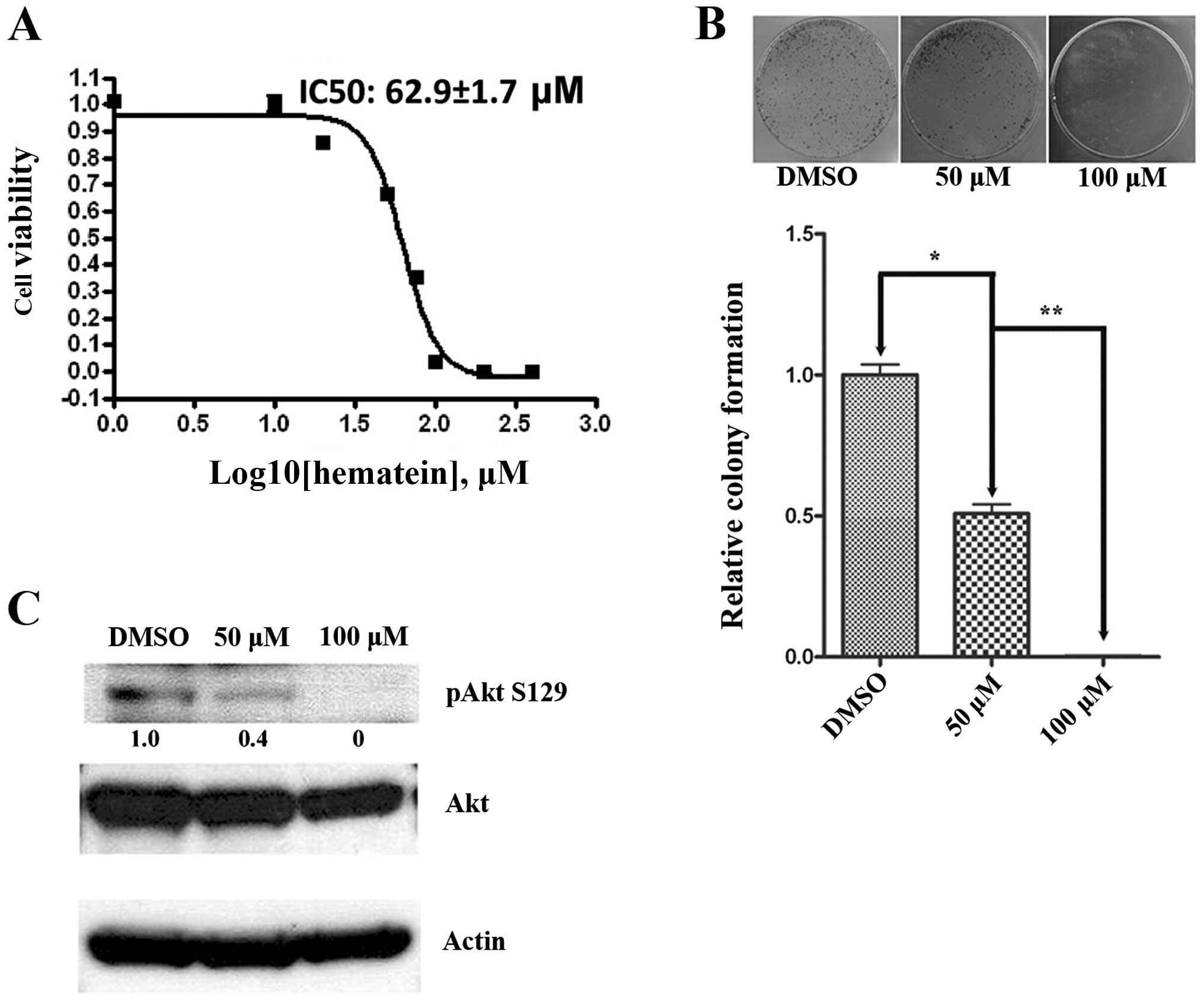
Hematein inhibits cells growth, and
CK2-specific Akt phosphorylation in A427 lung cancer cells
The A427 lung cancer cell line was chosen for in
vitro study because it showed the lowest IC50 for
hematein of several cell lines that we previously tested. The
IC50 of hematein is 62.9±1.7 μM for the A427 lung
cancer cell line (15) (Fig. 1A). To evaluate the inhibitory
effect of hematein on cell growth, we used the anchorage-dependent
colony formation assay. After culture in 50 and 100 μM of
hematein for 14 days, colony formation decreased significantly in
A427 lung cancer cells when compared to cells treated with DMSO
(Fig. 1B). Since CK2 was reported
to constitutively phosphorylate and upregulate Akt S129, which is a
specific phosphorylation site for CK2, in vitro and in
vivo (4). The phosphorylation
of Akt-S129 (Fig. 1C) was
evaluated, and a dose-dependent decrease of the phosphorylation of
Akt-S129 after hematein treatment was observed in A427 lung cancer
cells.
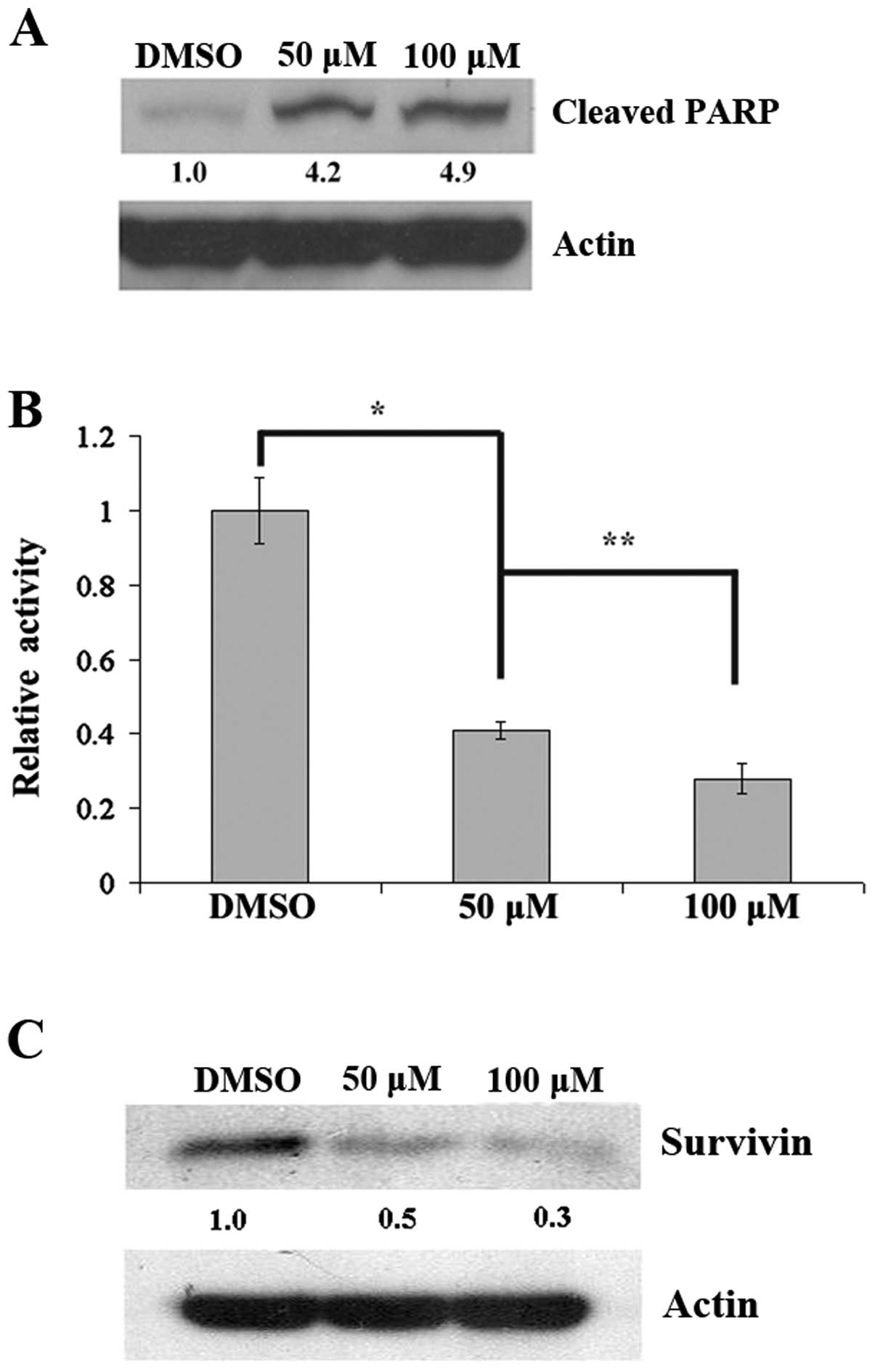
Hematein inhibits the Wnt canonical
pathway, and induces apoptosis in A427 lung cancer cells
To determine cleaved PARP as a late event in
apoptosis after inhibition of CK2 by hematein, cells were treated
with hematein for 48 h. We found that cleaved PARP increased in
A427 lung cancer cells after treatment with hematein (Fig. 2A), which indicated increased
apoptosis. In addition, down-regulation of the Wnt canonical
pathway was further confirmed by a dose-dependent decrease of
TOP/FOP luciferase activity (Fig.
2B) and survivin (Fig.
2C).
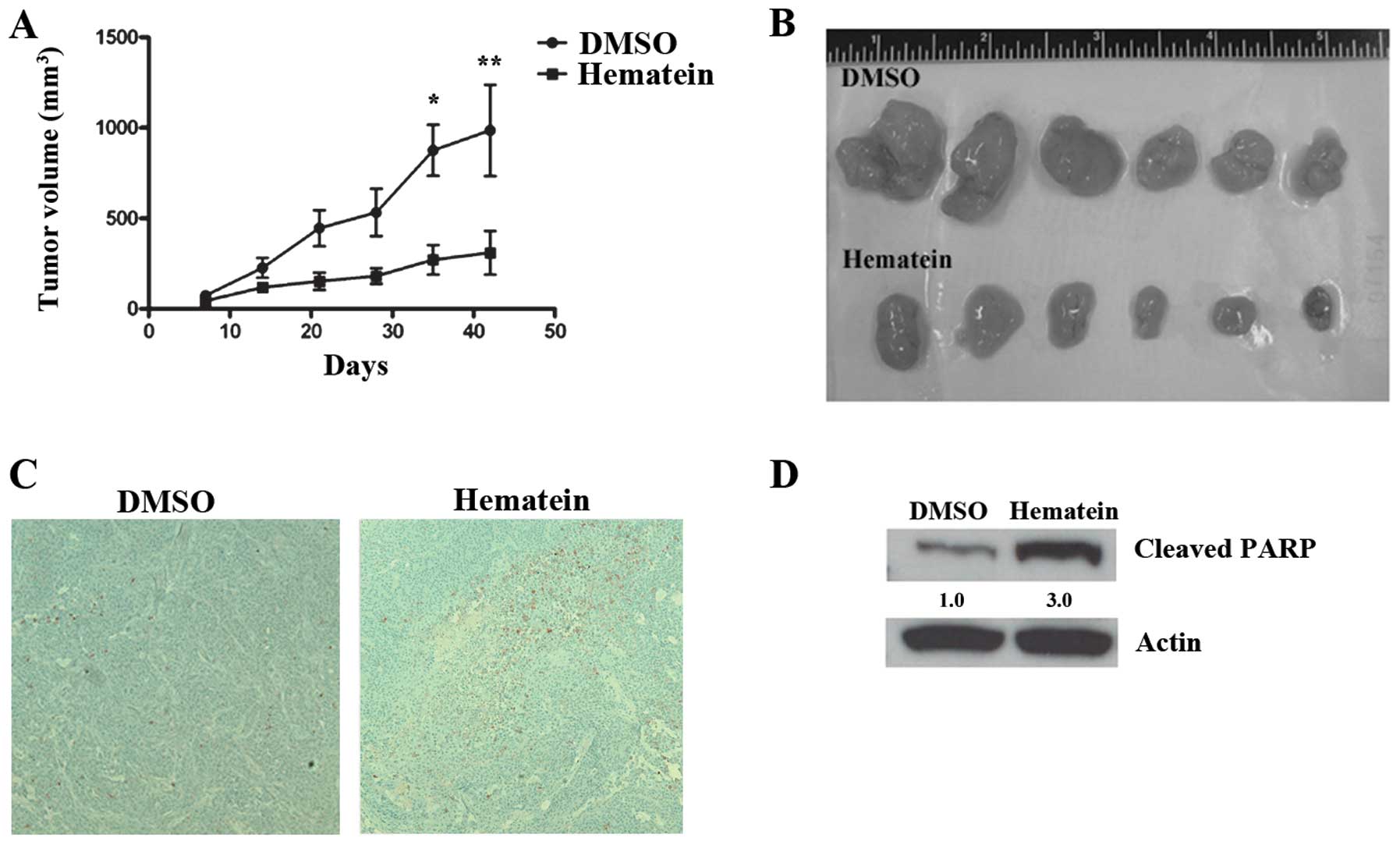
Hematein inhibits tumor growth in A427
lung cancer cell xenografts
Since hematein inhibited growth in A427 lung cancer
cells, we conducted an in vivo study using a murine
xenograft model to evaluate the inhibitory effect of hematein on
tumor growth. One week after 4×106 A427 lung cancer
cells were injected subcutaneously into flank areas of nude mice,
hematein was injected intraperitoneally at a dosage of 50 mg/kg
twice a week. Six and seven weeks after injection of A427 lung
cancer cells, tumor volumes decreased significantly in the group
treated with hematein when compared to the group treated with DMSO
(Fig. 3A and B). Cleaved caspase-3
and cleaved PARP proteins increased in tumors treated with hematein
(Fig. 3C and D).
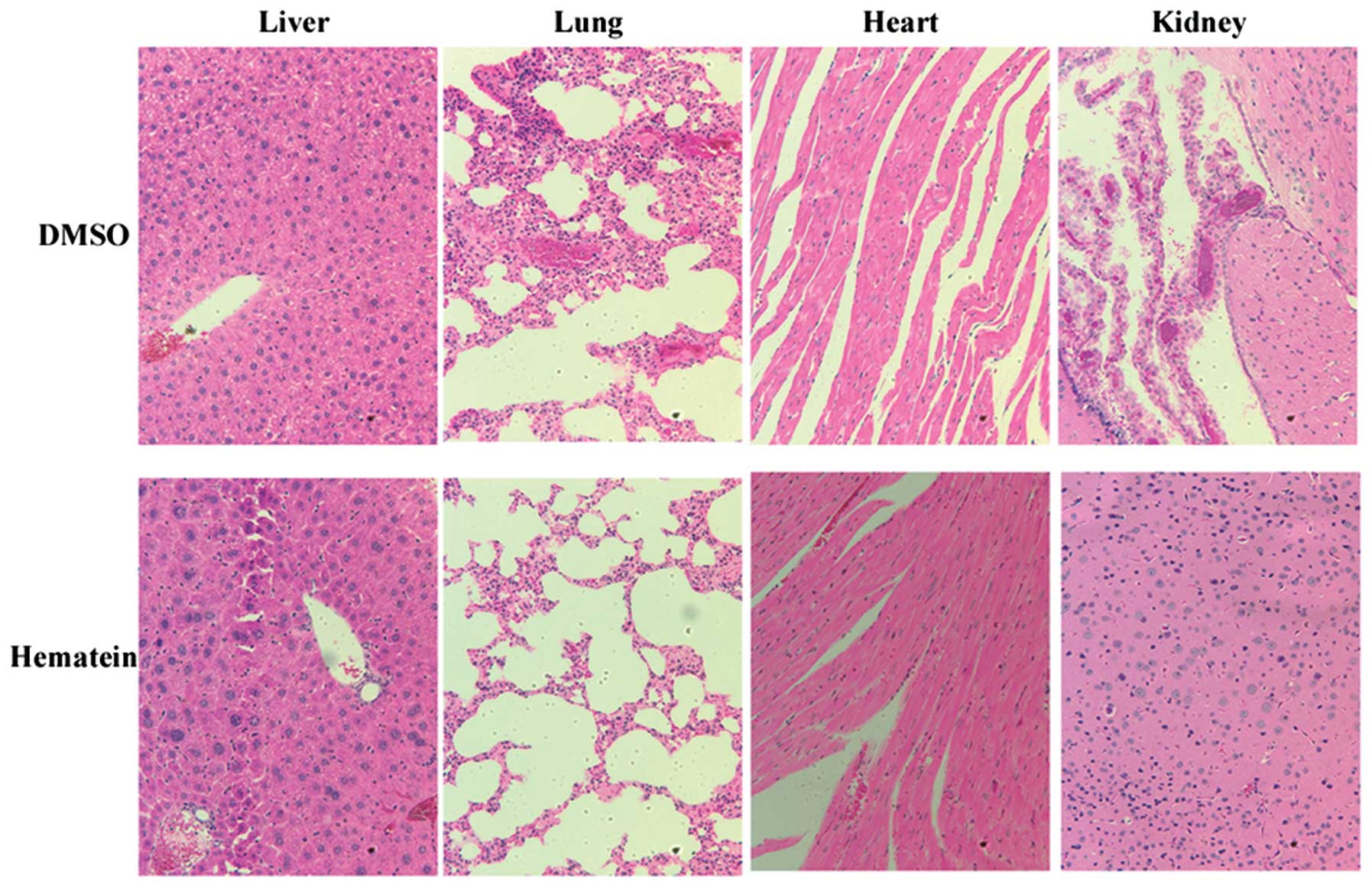
Hematein has minor toxicity to
organs
Histpathologic review of organs resected seven weeks
after mice received injections of A427 lung cancer cells showed no
obvious damage in heart, liver, lung and kidney (Fig. 4). No organ damage was observed in
hematein treated groups when compared with DMSO treatment groups.
These results showed the safety of hematein in animals studied.
Hematein has durable binding sites to
CK2
To elucidate the binding of hematein to CK2α enzyme,
virtual molecular docking was performed. Two docking programs (DOCK
3.5.54 and Accelrys Discovery Studio 2.5) were used to predict the
potential docking sites of hematein to CK2α enzyme. Similar docking
sites were noted by the two docking programs. Docking sites similar
to those of an often-used CK2 inhibitor,
5,6-dichloro-1-b-D-ribofuranosylbenzimidazole (DRB), were noted in
hematein (21). Hematein docked to
the canonical ATP binding site of CK2α (Fig. 5A and C). However, hematein also
docked well to an allosteric site (Fig. 5B and D), which reportedly serves as
a CK2α and CK2β interface. We previously found that hematein is an
ATP non-competitive inhibitor of CK2 (15), which may be explained by molecular
docking of hematein to the allosteric site of CK2α preferentially
in the hematein and CK2 complex.
Discussion
Our study shows that hematein inhibited growth and
Akt/ PKB Ser129 phosphorylation and increased apoptosis in lung
cancer cells. Hematein also inhibited tumor growth in a murine
xenograft model of lung cancer without obvious toxicity to the mice
tested. Molecular docking showed durable binding sites of hematein
to CK2α.
Previously, Akt/PKB Ser129 was reported to play a
role in constitutive activation of Akt/PKB pathway by CK2 (22), which promotes cell survival through
activation of anti-apoptotic pathways such as the NF-κB pathway and
suppression of caspase activity (23). Treatment of a variety of cancer
cells with cell-permeable CK2 inhibitors such as TBB, IQA and DMAT
reportedly induce apotosis (11,13,24).
We previously found that hematein has high selectivity for
inhibition of CK2 kinase activity among a panel of protein kinases
(15). Like other reported CK2
inhibitors, hematein induces apoptosis in cancer cells at least
partially through inhibition of Akt/PKB pathway by down-regulation
of CK2 kinase and then decreased phosphorylation of Akt/PKB Ser129.
CK2 has been reported to promote cancer cell survival by increasing
β-catenin-Tcf/Lef-mediated transcription and then increased
expression of survivin (25). It
has been reported recently that CK2α-specific enhancement of
β-catenin transcriptional activity as well as cell survival may
depend on Akt/PKB Ser129 hyperactivation by CK2 (26). Our study showed that in addition to
inhibiting phosphorylation of Akt/PKB Ser129, hematein also
inhibited the Wnt canonical pathway, which is confirmed by
decreased TOP/FOP luciferase activity and survivin after treatment
with hematein.
We previously reported that hematein is an ATP
non-competitive and partially reversible CK2 inhibitor (15). The molecular docking analysis
performed in the present study further elucidates this
characteristic of hematein by showing that hematein binds to the
canonical ATP binding site of CK2α, and to an allosteric site of
CK2α, which is similar to the reported binding site of DRB. The
allosteric site for hematein is a hydrophobic pocket at the outer
surface of the N-terminal β sheet of CK2α and serves as a CK2α and
CK2β interface (19). A recently
reported class of novel allosteric small molecule inhibitors of
CK2, azonaphthalene derivatives, has similar structures and ATP
non-competitive features as hematein (27). The effect that these inhibitors
have on CK2 is due to large conformational change of CK2α upon
binding of these inhibitors. As a result, hematein may exert its
inhibitory effect on CK2 through similar mechanisms. However, X-ray
crystallographic analysis of the co-structure of CK2α-hematein
complex will be required to precisely reveal the binding site of
hematein.
In conclusion, we showed antitumor effects of
hematein in A427 lung cancer cells and a xenograft nude mouse model
of lung cancer. The therapeutic potential of hematein is emphasized
by its efficacy at inhibiting lung cancer cells growth and inducing
apoptosis. Furthermore, docking studies showed that hematein has
durable binding sites to CK2 and may act as an allosteric inhibitor
to CK2.
Acknowledgements
The present work was supported by NIH
grant 5 R01 CA140654-03 (to L.Y.). We are grateful for support from
the Kazan, McClain, Abrams, Fernandez, Lyons, Greenwood, Harley
& Oberman Foundation, Inc.; the Estate of Robert Griffiths; the
Jeffrey and Karen Peterson Family Foundation; Paul and Michelle
Zygielbaum; the Estate of Norman Mancini; and the Barbara Isackson
Lung Cancer Research Fund. We thank Pamela Derish from the
Department of Surgery at the University of California, San
Francisco, for editorial review of this manuscript.
References
|
1.
|
Meggio F and Pinna LA:
One-thousand-and-one substrates of protein kinase CK2? FASEB J.
17:349–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pinna LA: The raison d’etre of
constitutively active protein kinases: the lesson of CK2. Acc Chem
Res. 36:378–384. 2003.
|
|
3.
|
Tawfic S, Yu S, Wang H, Faust R, Davis A
and Ahmed K: Protein kinase CK2 signal in neoplasia. Histol
Histopathol. 16:573–582. 2001.PubMed/NCBI
|
|
4.
|
Seldin DC and Leder P: Casein kinase II
alpha transgene-induced murine lymphoma: relation to theileriosis
in cattle. Science. 267:894–897. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Landesman-Bollag E, Song DH, Romieu-Mourez
R, et al: Protein kinase CK2: signaling and tumorigenesis in the
mammary gland. Mol Cell Biochem. 227:153–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kim JS, Eom JI, Cheong JW, et al: Protein
kinase CK2alpha as an unfavorable prognostic marker and novel
therapeutic target in acute myeloid leukemia. Clin Cancer Res.
13:1019–1028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Laramas M, Pasquier D, Filhol O, Ringeisen
F, Descotes JL and Cochet C: Nuclear localization of protein kinase
CK2 catalytic subunit (CK2alpha) is associated with poor prognostic
factors in human prostate cancer. Eur J Cancer. 43:928–934. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
O-charoenrat P, Rusch V, Talbot SG, et al:
Casein kinase II alpha subunit and C1-inhibitor are independent
predictors of outcome in patients with squamous cell carcinoma of
the lung. Clin Cancer Res. 10:5792–5803. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gapany M, Faust RA, Tawfic S, Davis A,
Adams GL and Ahmed K: Association of elevated protein kinase CK2
activity with aggressive behavior of squamous cell carcinoma of the
head and neck. Mol Med. 1:659–666. 1995.PubMed/NCBI
|
|
10.
|
Stalter G, Siemer S, Becht E, Ziegler M,
Remberger K and Issinger OG: Asymmetric expression of protein
kinase CK2 subunits in human kidney tumors. Biochem Biophys Res
Commun. 202:141–147. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ruzzene M, Penzo D and Pinna LA: Protein
kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces
apoptosis and caspase-dependent degradation of haematopoietic
lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J.
364:41–47. 2002.
|
|
12.
|
Pagano MA, Meggio F, Ruzzene M,
Andrzejewska M, Kazimierczuk Z and Pinna LA:
2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel
powerful and selective inhibitor of protein kinase CK2. Biochem
Biophys Res Commun. 321:1040–1044. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sarno S, De Moliner E, Ruzzene M, et al:
Biochemical and three-dimensional-structural study of the specific
inhibition of protein kinase CK2 by
[5-oxo-5,6-dihydroindolo-(1,2-a)quinazolin-7-yl] acetic acid (IQA).
Biochem J. 374:639–646. 2003.PubMed/NCBI
|
|
14.
|
Siddiqui-Jain A, Drygin D, Streiner N, et
al: CX-4945, an orally bioavailable selective inhibitor of protein
kinase CK2, inhibits prosurvival and angiogenic signaling and
exhibits antitumor efficacy. Cancer Res. 70:10288–10298. 2010.
View Article : Google Scholar
|
|
15.
|
Hung MS, Xu Z, Lin YC, et al:
Identification of hematein as a novel inhibitor of protein kinase
CK2 from a natural product library. BMC Cancer. 9:1352009.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Oh SR, Kim DS, Lee IS, Jung KY, Lee JJ and
Lee HK: Anti-complementary activity of constituents from the
heartwood of Caesalpinia sappan. Planta Med. 64:456–458. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bettinger C and Zimmermann HW: New
investigations on hematoxylin, hematein, and hematein-aluminium
complexes. II. Hematein-aluminium complexes and hemalum staining.
Histochemistry. 96:215–228. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lorber DM and Shoichet BK: Hierarchical
docking of databases of multiple ligand conformations. Curr Top Med
Chem. 5:739–749. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Raaf J, Brunstein E, Issinger OG and
Niefind K: The CK2 alpha/ CK2 beta interface of human protein
kinase CK2 harbors a binding pocket for small molecules. Chem Biol.
15:111–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Meng EC, Shoichet BK and Kuntz ID:
Automated docking with grid-based energy evaluation. J Computat
Chem. 13:505–524. 1992. View Article : Google Scholar
|
|
21.
|
Raaf J, Issinger OG and Niefind K:
Insights from soft X-rays: the chlorine and sulfur sub-structures
of a CK2alpha/DRB complex. Mol Cell Biochem. 316:15–23. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Di Maira G, Salvi M, Arrigoni G, et al:
Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell
Death Differ. 12:668–677. 2005.PubMed/NCBI
|
|
23.
|
Duncan JS and Litchfield DW: Too much of a
good thing: the role of protein kinase CK2 in tumorigenesis and
prospects for therapeutic inhibition of CK2. Biochim Biophys Acta.
1784:33–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yde CW, Frogne T, Lykkesfeldt AE, Fichtner
I, Issinger OG and Stenvang J: Induction of cell death in
antiestrogen resistant human breast cancer cells by the protein
kinase CK2 inhibitor DMAT. Cancer Lett. 256:229–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tapia JC, Torres VA, Rodriguez DA, Leyton
L and Quest AF: Casein kinase 2 (CK2) increases survivin expression
via enhanced beta-catenin-T cell factor/lymphoid enhancer binding
factor-dependent transcription. Proc Natl Acad Sci USA.
103:15079–15084. 2006. View Article : Google Scholar
|
|
26.
|
Ponce DP, Yefi R, Cabello P, et al: CK2
functionally interacts with AKT/PKB to promote the
beta-catenin-dependent expression of survivin and enhance cell
survival. Mol Cell Biochem. 356:127–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Moucadel V, Prudent R, Sautel CF, et al:
Antitumoral activity of allosteric inhibitors of protein kinase
CK2. Oncotarget. 2:997–1010. 2011.PubMed/NCBI
|