Introduction
Esophageal squamous cell carcinoma (ESCC) is a
devastating disease characterized by distinctly high incidence and
mortality rates. Epidemiological evidence shows that ESCC is the
5th most common cause of death from cancer in men (1). Although great improvements have been
made in the diagnostics, surgical treatment, chemotherapy, and
radiotherapy for esophageal cancer, the overall survival rate of
esophageal cancer remains poor, with a 5-year survival rate of
15–34% (2–5). Therefore, it is necessary to
investigate the molecular mechanisms related to ESCC development
and progression. We sought to identify novel tumor-related genes
and clarify their roles in order to elucidate the mechanisms of
initiation and progression of ESCC that may ultimately lead to
early diagnosis and increased patient survival.
Integrins are a family of transmembrane glycoprotein
adhesion receptors that mediate cell-matrix and cell-cell adhesion
(6). They are heterodimeric
glycoproteins with 18α subunits and 8β subunits, which can
associate to form 24 unique integrin heterodimers (7). Numerous studies have reported that
integrins are involved in various intracellular pathways, including
those involved in cell adhesion, migration, polarity, survival,
growth, and death, suggesting their important role in cancer
(8–10). Furthermore, integrins have been
shown to be differentially regulated during tumor growth and
progression, making them potential targets for cancer diagnostics
and therapy. Although many integrins contribute to tumor
progression, integrin alpha 6 (ITGA6), in particular, has been
implicated in breast cancer progression in several studies
(11–14). ITGA6 is synthesized as a 140-kDa
precursor that is converted into 2 disulfide-linked polypeptides of
120 and 25 kDa by endoproteolytic cleavage of the C-terminal domain
(15). The ITGA6 subunit can form
heterodimers with either the β1 or β4 integrin subunit to form α6β1
and α6β4 integrins, respectively. Both α6β1 and α6β4 integrins
function as receptors for the laminin receptor family of
extracellular matrix proteins. A recent study showed that
expression of certain integrins is highly upregulated in ESCC
tissue and cell lines (16).
However, neither ITGA6 regulation nor its function has been
previously examined in ESCC.
In this study, we have shown that ITGA6 is highly
expressed in ESCC and plays a role in the progression of cancer
cells by regulating the proliferation and invasiveness of these
cells. Furthermore, we have demonstrated the prognostic and
therapeutic potential of ITGA6 by in vitro and in
vivo analyses.
Materials and methods
Tissue samples
This study was approved by the institutional review
board of the Korea Cancer Center Hospital. Ten ESCC tissue samples
were obtained from patients undergoing surgery at the Korea Cancer
Center Hospital, Seoul, Korea. All the specimens were
histologically diagnosed as squamous cell carcinoma.
Total RNA preparation and RT-PCR
Total RNA was isolated from ESCC cell lines, frozen
normal esophagus epithelial and tumor tissues from patients with
ESCC by using the RNeasy Mini Kit (Qiagen, Cambridge, MA, USA)
according to the manufacturer’s instructions. A total of 2
μg RNA from each sample was subjected to reverse
transcription to produce cDNAs using Oligo-dT primers and
Superscript II (Invitrogen, Carlsbad, CA, USA). RT-PCR was carried
out with sets of synthesized primers specific to ITGA6; GAPDH was
used as an internal control. PCR reactions were optimized for the
number of cycles and annealing temperature to obtain an
appropriately sized amplicon.
Cell culture
Human ESCC cell lines, TE-1, TE-2, TE-4, TE-5, TE-6,
TE-8, TE-9, TE-10, TE-11, TE-14, and TE-15, and human primary
esophageal epithelial cells, Het-1A, were purchased from RIKEN,
Japan and ATCC, USA, respectively. All cells were cultured in
RPMI-1640 medium supplemented with 10% (v/v) FBS and antibiotics
(100 units/ml penicillin and 100 μg/ml streptomycin) and
were grown at 37°C under 5% CO2 and 95% air.
siRNA treatment
Two siRNAs specific for human ITGA6 were purchased
from Ambion. TE-8 cells were transfected with ITGA6 and control
siRNA by using Lipofectamine 2000 (Invitrogen) according to the
manufacturer’s instructions.
Cell proliferation assay
Cells (4×103/well) were seeded in 96-well
plates and incubated with 0.1 ml of RPMI-1640 supplemented with 10%
FBS. At the indicated time points, cells were incubated with
CellTiter 96 Aqueous (MTS) solution (Promega, Madison, WI, USA) in
serum-free RPMI-1640. After 2 h of incubation with CellTiter 96
Aqueous solution, the colored MTS products in the supernatant were
measured using a Microplate Reader at 490 nm absorbance.
Migration and invasion assay
Cells (5×105/well) were seeded in a
24-well plate and incubated with fresh medium. After overnight
incubation, a wound was introduced by scraping the monolayer with a
1-ml micropipette tip. The cells were washed twice with PBS to
remove debris, incubated for 24 h in RPMI-1640 supplemented with
10% FBS, and evaluated by light microscopy. Invasion assays were
performed using the 24-well Transwell system (8 μm pore
size, BD Biosciences). Cells were starved in serum-free medium
overnight, trypsinized, and washed 3 times with RPMI-1640
containing 1% FBS. Then, 2×103 cells in 1% FBS-RPMI-1640
were seeded into the upper chamber, and 600 μl of RPMI-1640
containing 10% FBS was placed in the lower chamber. After 24-h
incubation, the cells remaining in the upper chamber were removed.
The cells on the lower surface of the membrane were fixed in 4%
paraformaldehyde, stained with 0.5% crystal violet, and scored.
Colony formation assay
For colony formation assays, cells were plated into
three 6-well cell culture plates and incubated for 14 days. The
plates were washed with PBS and stained with 0.2% crystal violet
staining solution. For the colony formation assay in soft agar,
cells (0.5×103 cells/well) were suspended in RPMI
containing 0.3% agarose and 10% FBS and layered on RPMI containing
0.8% agarose and 10% FBS in a 6-well plate. The colonies were
analyzed morphologically using nitrotetrazolium blue chloride
(NBT), and the number of colonies formed per well after 14 days of
incubation was quantified.
Western blotting and antibodies
Protein extraction from frozen tissues and cell
lines was performed using RIPA buffer (Thermo Fisher Scientific,
Waltham, MA, USA) containing protease inhibitors (Roche, Basel,
Switzerland). Lysates of the same amounts of protein were separated
on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose
membranes. Antibodies were purchased from Santa Cruz Biotechnology
(ITGB4 and ITGB1) and Cell Signaling Technology (ITGA6). Membranes
were developed with the ECL detection system (Thermo Fisher
Scientific) after incubation with peroxidase-conjugated secondary
antibodies.
Immunoprecipitation and FACS
analysis
Cells were washed once with PBS and lysed for 30 min
on ice in RIPA buffer. Cell debris was removed by centrifugation.
Cell lysates were incubated at 4°C overnight with either anti-ITGB4
or rat IgG as a negative control. Immune complexes were
precipitated with Sepharose beads and washed with washing buffer.
Samples were resolved by electrophoresis on 10% SDS-polyacrylamide
gels, transferred onto nitrocellulose membranes, and probed with
anti-ITGA6 followed by a peroxidase-conjugated secondary antibody.
Immunoreactive bands were visualized using the ECL detection
system. To measure cell surface ITGA6 and ITGB4 expression, the
suspended cells were incubated for 1 h on ice with 1 μg of
primary antibodies per 3×103 cells, washed and stained
with FITC-conjugated secondary antibodies, and scanned using a
FACSCalibur cytometer (Becton-Dickinson, San Jose, CA, USA).
Blocking antibody experiments
For the ITGA6 functional study, TE-8 cells were
exposed to ITGA6 blocking antibody for 3 days; cell proliferation
was measured with CellTiter 96 Aqueous solution and cell invasion
by a Matrigel Transwell assay. For studies involving ITGA6 blocking
antibody (GoH3, MAB1378, Millipore), 2 doses of ITGA6 (1 or 10
μg/ml) were used, and the results were compared with those
obtained using a rat isotype control antibody (10 μg/ml,
Invitrogen).
Radioiodination of anti-human ITGA6
antibody
The Iodogen-coated tube method (Pierce) was used for
radiolabeling anti-human ITGA6 antibody (hITGA6 Ab, 3H1512, Santa
Cruz Biotechnology) with 125I (Perkin-Elmer).
Radiolabeled antibody was purified by gel filtration on a PD-10
column (GE Healthcare Life Sciences) and sterilized by filtration
(0.22 μm; Millipore Co). The radiolabeling yield and
radiochemical purity were determined with instant thin layer
chromatography-silica gel (Gelman Scientific) in the stationary
phase and acetone in the mobile phase.
Biodistribution study
Female athymic mice (BALB/c nu/nu; 5–6 weeks old;
17–23 g) were obtained from Japan SLC, Inc. Tumors were grown after
subcutaneous injection of 1×107 human esophageal
squamous cell carcinoma cells (TE-8) in the right flank. After 28
days, 125I-hITGA6 Ab (20 g/740 kBq) was injected into
the tail vein of mice bearing a TE-8 tumor. For each time point, 4
mice were sacrificed to collect and weigh blood, tumors, and
tissues. Radioactivity was measured with a scintillation counter.
The percentage of injected radioactivity dose per gram of tissue (%
ID/g) was calculated.
Statistical analysis
All data were obtained from at least 3 independent
experiments and are expressed as the mean ± standard deviation
values. Statistical significance was analyzed by Student’s
t-test.
Results
ITGA6 is overexpressed in ESCC tissues
and ESCC cell lines
In our previous study, we analyzed gene expression
profiles for samples obtained from ESCC patients (17) by using specific criteria to
identify genes associated with cell surface expression. Among the
genes identified on comparison between normal and tumor tissues, we
selected 7 upregulated and cell surface-expressed genes (ARL6IP,
LAPTM5, ITGA6, SCARB2, MSN, CDH13, and T1A-2; p<8e-07). After
validation using RT-PCR, we selected ITGA6 as a target molecule for
ESCC.
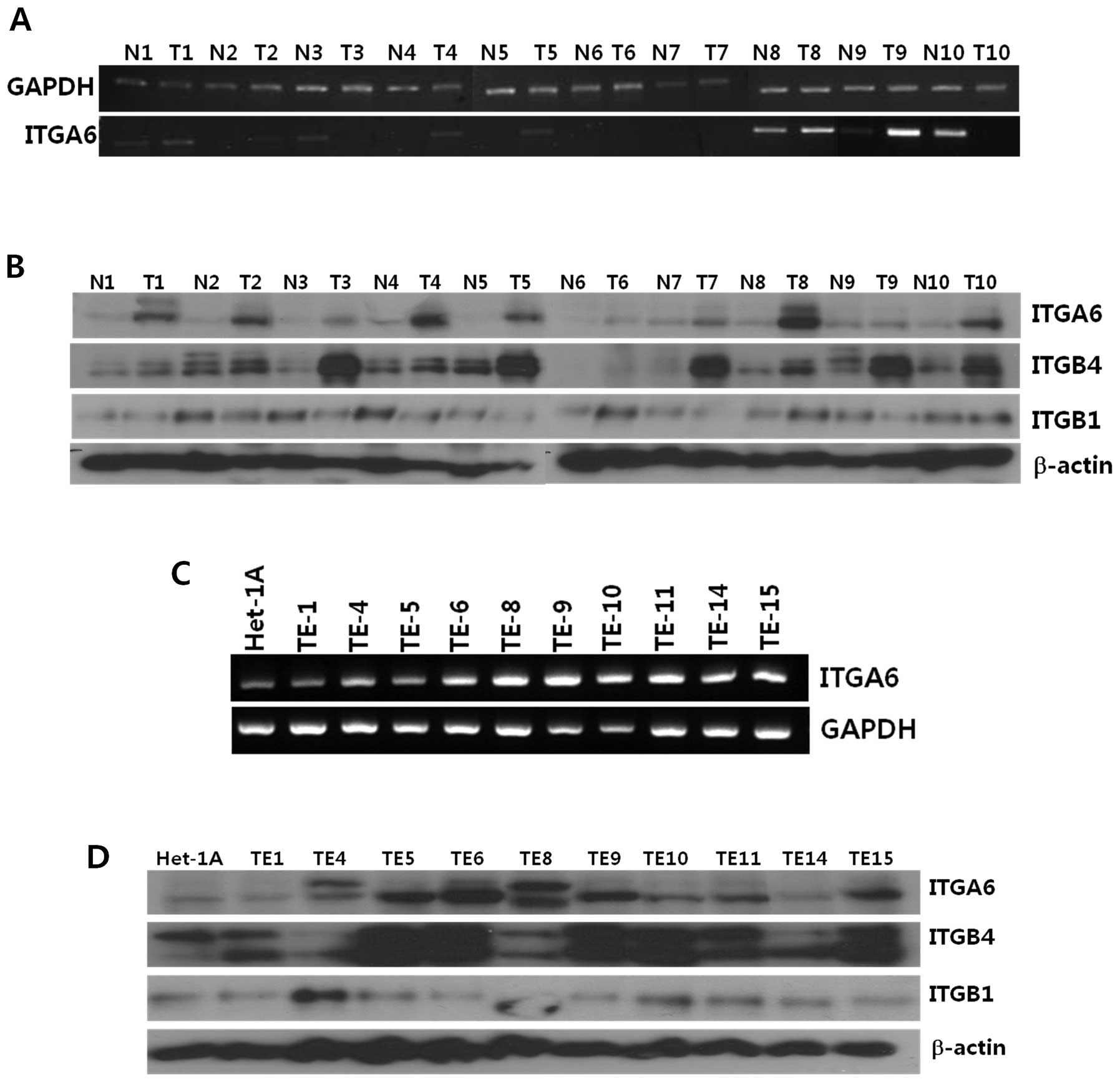
Expression levels of ITGA6 mRNA and protein in tumor
and normal tissue from 10 ESCC patients were determined by RT-PCR
and western blot analyses, respectively. ITGA6 expression was
significantly higher at both the mRNA and protein levels in ESCC
tissue than in normal esophageal epithelial tissue (Fig. 1A and B). Next, we examined ITGA6
mRNA and protein expression in 10 esophageal squamous cancer cell
lines by using RT-PCR and western blot analyses, respectively.
ITGA6 expression was upregulated in most of the ESCC cell lines
compared to the levels in the normal esophageal epithelial cell
line, Het-1A (Fig. 1C and D).
Knockdown of ITGA6 decreases
proliferation, invasion, and colony forming ability of ESCC
cells
To understand the role of ITGA6 in ESCC cells, we
transfected 2 different types of ITGA6 siRNAs in TE-8 cell lines.
ITGA6 expression was confirmed in ITGA6 knockdown cells by RT-PCR
and western blot analyses compared with that in control
siRNA-transfected cells. As shown in Fig. 2A, the results demonstrated that
both siRNAs effectively inhibited ITGA6 expression in TE-8 cell
lines at both the mRNA and protein levels. The effect of ITGA6
knockdown on the proliferation of ESCC cells was evaluated by MTS
assays. The results of the MTS assays showed that decrease in ITGA6
expression significantly reduced the viability of TE-8 cell lines
(p<0.0001, Fig. 2B). We next
investigated the role of ITGA6 in wound healing and invasion in
TE-8 cells. In the wound healing assay, ITGA6 siRNA-transfected
cells showed a slight delay in wound repair compared to control
siRNA-transfected cells (Fig. 2C).
In Transwell invasion assays, in the absence of serum, the invasion
of control cells was similar to that of ITGA6-knockdown cells.
However, when 10% serum was added as an attractant, invasion of
ITGA6 knockdown cells was approximately 60% lower than that in
control cells (p<0.05, Fig.
2D).
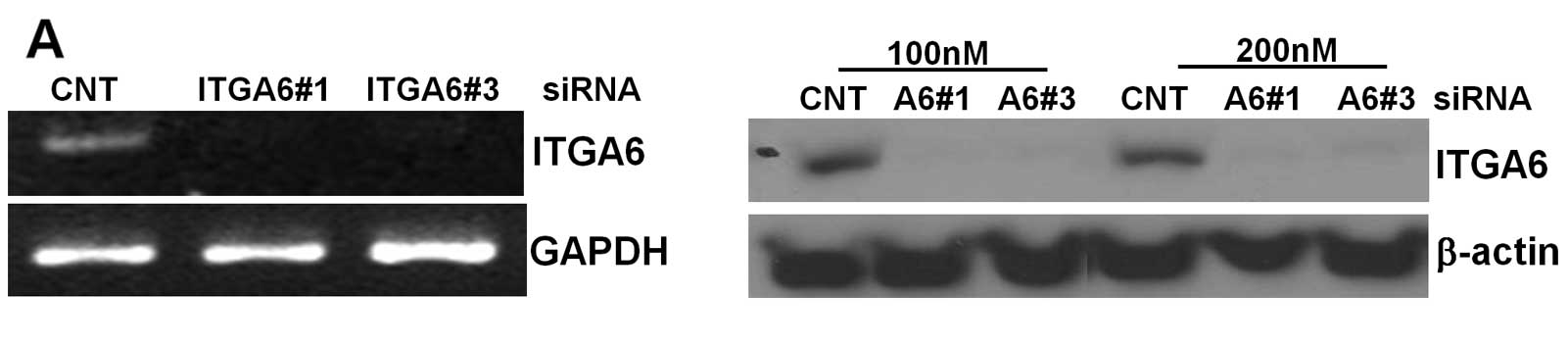 | Figure 2.Effect of ITGA6 knockdown in ESCC
cells. (A), TE-8 cells were transfected with siRNA (control, ITGA6
#1, ITGA6 #3 from Ambion) and confirmed by western blot analysis.
(B), Cell proliferation analysis was performed using MTS for 72 h.
After ITGA6 siRNA knockdown, TE-8 cell growth was lower than that
obtained with control siRNA-transfected cells. (C), TE-8 cells were
transfected with siRNA (control, ITGA6 #1, ITGA6 #3) and analyzed
for cell migration using wound healing assays. (D), Invasion of
TE-8 cells with or without ITGA6 knockdown. Cells that invaded the
Matrigel for 24 h in a Transwell system were counted. (E), Two
colony formation assay methods were used for ITGA6-knockdown in
TE-8 cells. |
The inhibitory effect of ITGA6 knockdown on ESCC
cell growth was also confirmed by 2 different types of colony
formation assays: a monolayer assay and a soft agar assay (Fig. 2E).
Collectively, our results demonstrate that
downregulation of ITGA6 suppresses ESCC cell proliferation,
invasion, and colony formation in vitro.
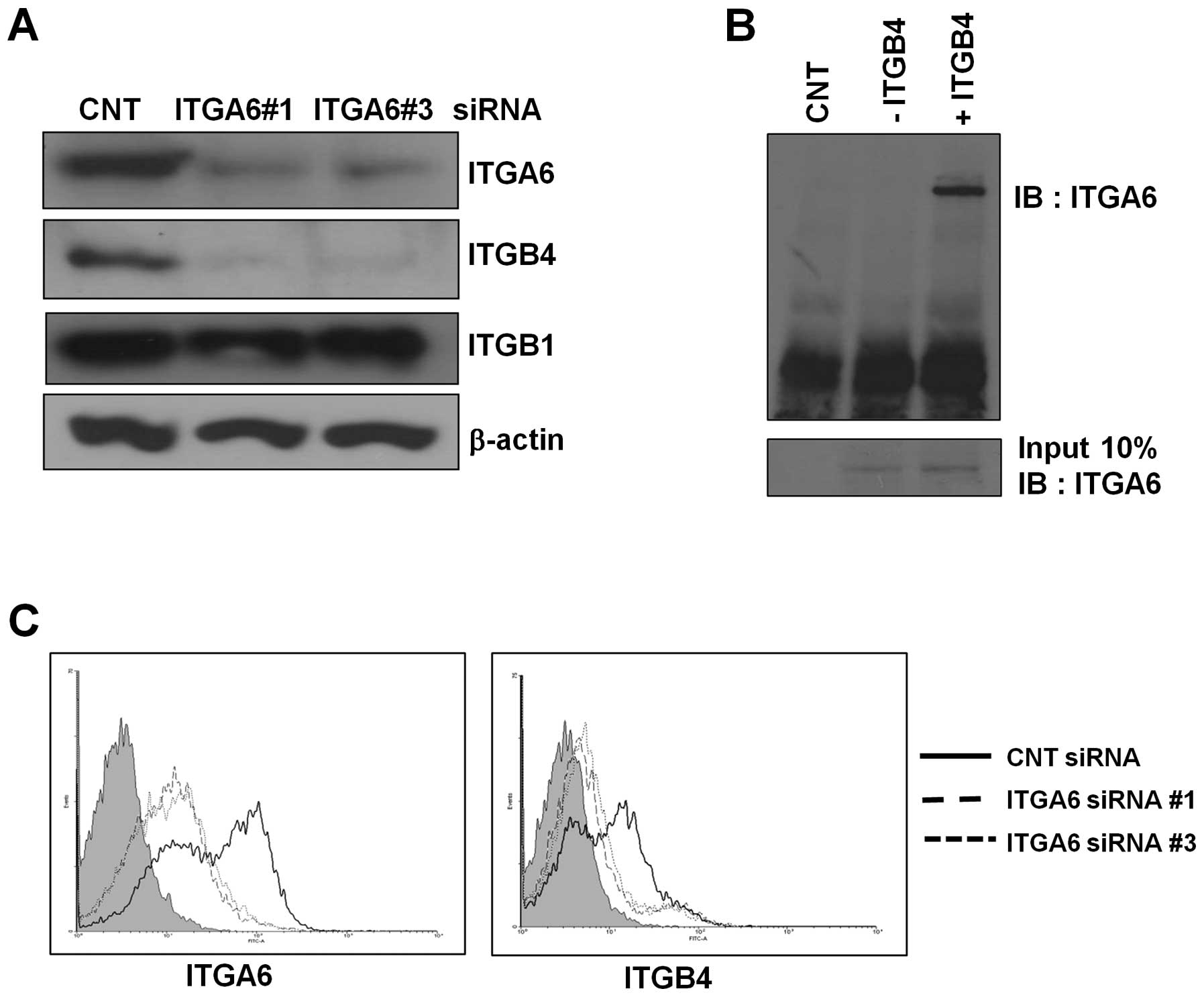
ITGA6 knockdown decreases ITGB4
expression but does not affect ITGB1
Previous studies have reported that ITGA6 forms a
complex with ITGB4 or ITGB1. To determine which integrin beta form
is the dominant complex formed with ITGA6 in ESCC, we measured
ITGB4 and ITGB1 expression levels in ESCC tissue samples and ESCC
cell lines (Fig. 1A and B). ITGB4
was upregulated in ESCC tissue and ESCC cell lines along with
ITGA6, whereas ITGB1 did not show much change. In ITGA6-knockdown
cells, ITGB4 expression decreased but ITGB1 expression was
unchanged compared to that for the control in TE-8 cell lines
(Fig. 3A). These results were
confirmed by FACS analysis. Following ITGA6 knockdown, ITGA6 cell
surface expression significantly decreased in TE-8 cells. ITGB4
surface expression levels decreased in ITGA6-knockdown cells
(Fig. 3C).
To confirm that ITGA6 and ITGB4 formed a complex, we
performed immunoprecipitation assays. In the presence of an ITGB4
antibody, we detected an ITGA6 band by immunoblotting (Fig. 3B). Taken together, we found that
the ITGA6B4 complex is the dominant form in ESCC and that ITGB4
expression is regulated by ITGA6 expression.
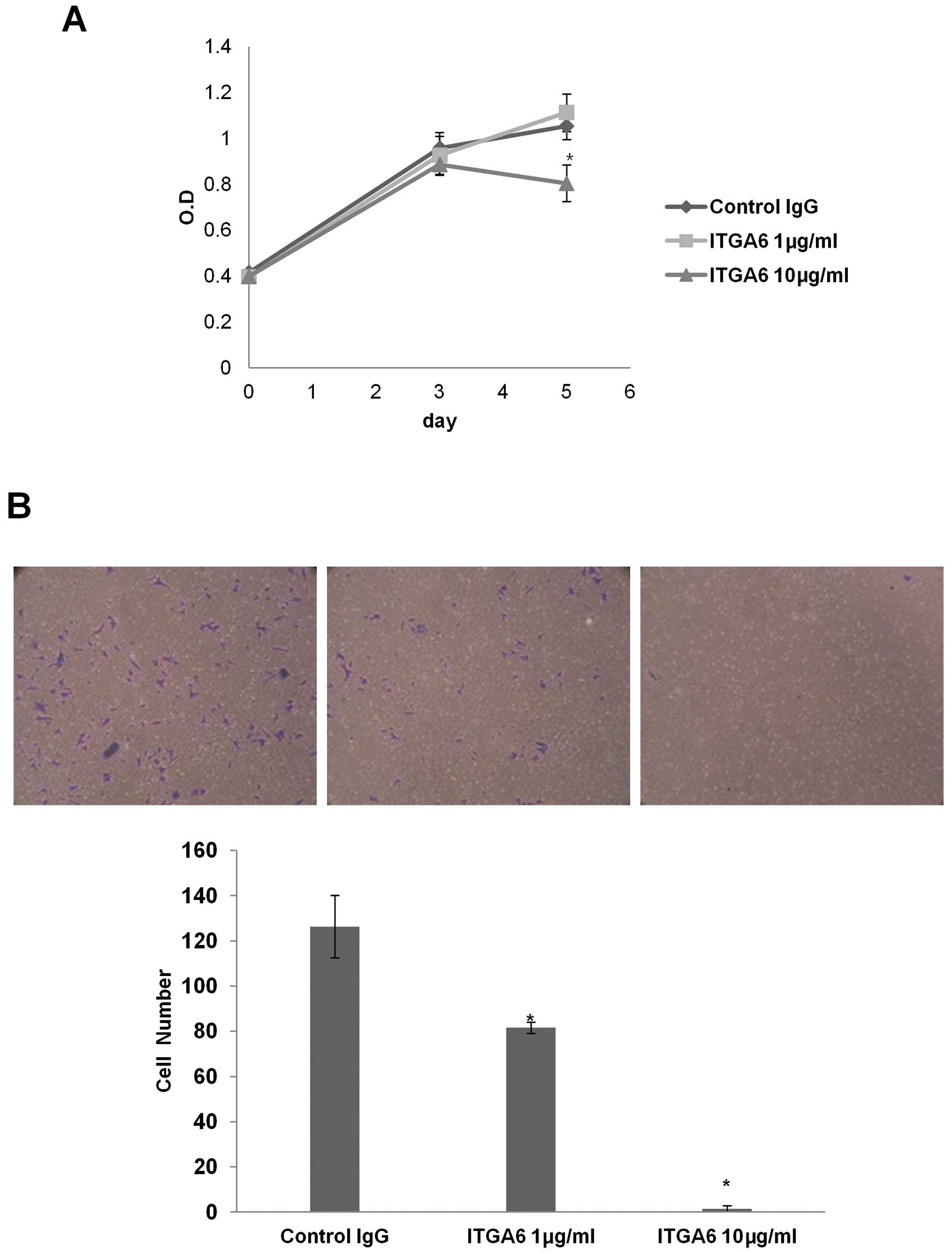
ITGA6-blocking antibody decreases
proliferation and invasion in ESCC cells
Integrin function has been disrupted with a number
of blocking antibodies and peptides, some of which have entered
clinical trials (18). To
investigate potential targeting of ITGA6, we utilized an
ITGA6-blocking antibody to evaluate ITGA6 function in ESCC cell
lines. TE-8 cell treated with 10 μg/ml of ITGA6-blocking
antibody (GoH3, Millipore) showed lower cell proliferation
(p<0.002) and invasiveness than an isotype control antibody
(Fig. 4).
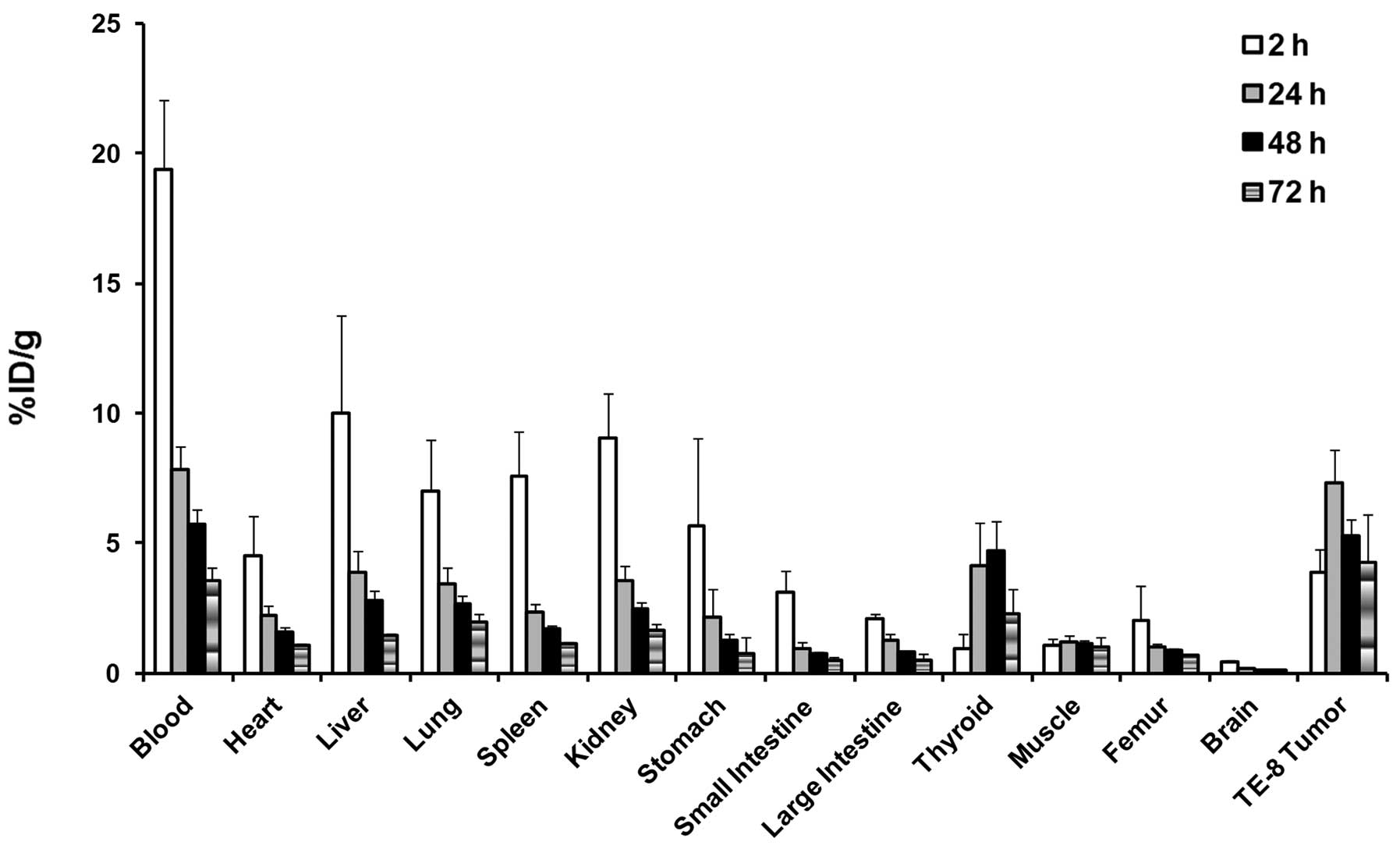
125I-labeled anti-ITGA6
targets ESCC tumors in a xenograft model
The tumor-targeting ability of the human ITGA6
antibody was studied in athymic mice bearing human esophageal
squamous cell carcinoma xenografts. The 125I-hITGA6
antibody was injected into the mouse model, and biodistribution was
examined at 2, 24, 48, and 72 h post-injection. The percentages of
the injected dose/g of tissue for tumor and normal tissues are
shown in Fig. 5. The human ITGA6
antibody showed tumor localization, peaking at 24 h, with
7.30±1.32% ID/g of tumor uptake. The uptake of radiolabeled
anti-hITGA6 antibody in TE-8 tumors was higher than those in other
tissues at 24, 48, and 72 h post-injection, except for the uptake
in blood. The tumor-to-muscle (T/M) ratio was above 4.0 at all time
points and showed a peak of 5.99±0.94 at 24 h. The tumor-to-blood
(T/B) ratio was below 1.0 at 2, 24, and 48 h; however, the T/B
ratio peaked showed a peak of 1.16±0.35 at 72 h. These uptake
patterns suggest that the anti-hITGA6 antibody specifically
targeted human integrin 6 in TE-8 tumors.
Discussion
ESCC is known to have the worst prognosis in the
malignant tumors of the digestive tract. Despite existing therapies
for ESCC such as surgery, chemotherapy, and radiotherapy, the
clinical outcome for patients with ESCC remains poor, with an
overall 5-year survival rate of 15–34% (2–5).
Hence, extensive research on more reliable and effective
therapeutic strategies for ESCC is urgently required, particularly
with respect to the aspects of efficacy, minimal toxicity, and
predictive response to treatment.
Drug development for cancer has been transformed
with the identification of and ability to direct treatment at
specific molecular targets (19).
Novel targeted treatments for esophageal cancer are in early
developmental stages, and encouraging results have been reported
with antibodies directed against the EGFR and VEGF ligands, as well
as tyrosine-kinase inhibitors (20,21).
To date, the HER2, EGFR, and VEGF pathways have been most
extensively studied in esophageal cancers. However, despite a
strong preclinical rationale and evidence of activity of these
targets in other cancers, the efficacy of these drugs has been
controversial when studied in the metastatic setting or in
combination with chemoradiation.
In our previous microarray analysis, we observed
that the level of ITGA6 expression increases greatly in ESCC
(17). Furthermore, since ITGA6 is
expressed on the cell surface, it is a potential target for
antibody therapy. Our present results showed that ITGA6 siRNA could
effectively downregulate ITGA6 expression and that this
downregulation of ITGA6 resulted in decreased cell proliferation,
and invasion by colony formation in ESCC cell lines. Consistent
with these findings, enhancement of cell proliferation and invasion
by ITGA6 (integrin α6β1 and α6β4) has been shown in pancreatic
carcinoma and breast cancer (22,23).
Unlike ITGA5, which functions as both a tumor suppressor and
oncogene, ITGA6 has been implicated only in promoting tumor
progression (12,24). Recent studies have demonstrated
that ITGA6 is necessary for the tumorigenicity of a stem cell-like
subpopulation within the MCF7 breast cancer cell line and that
targeting ITGA6 in cancer stem cells inhibits self-renewal,
proliferation, and tumor-formation capacity (18,25).
These results suggest that ITGA6 plays an important role in
tumorigenesis.
Changes in ITGA5 expression have been observed in
several cancerous tissues (26).
Furthermore, a previous study indicated that overexpression of
ITGA5B1 inhibits the proliferation of human HT29 colon carcinoma
cells in vitro and reduces the formation of lung colonies
and cutaneous metastases in vivo (27). However, we did not find any
differences in the ITGA5 expression patterns in ESCC tumor tissues
compared with those in normal tissues (data not shown).
Additionally, we found that ITGB4 expression
increases greatly in ESCC tissues and cell lines. Expression of the
ITGA6/ITGB4 complex promoted tumor progression and metastasis of
various cancer cells, including breast, colorectal, and thyroid
carcinomas (22,28–30).
Although some studies have reported that ITGA6B1 can facilitate
survival of breast carcinoma cells, especially in response to
environmental stress (24), we did
not find any difference in the expression patterns of ITGB1 in ESCC
tumor tissue and cell lines. In addition, we only detected
decreased expression of ITGB4, but not of ITGB1, in ITGA6-knockdown
cell lines using western blot and FACS analyses. These findings
indicated that the ITGA6B4 complex might be a major form that
controls cell survival in ESCC.
According to previous studies, integrins represent
ideal pharmacological targets in tumors as they are cell surface
receptors interacting with extracellular ligands and play important
roles in tumor angiogenesis and tumorigenesis (19,31).
In our present study, ITGA6 inhibitory antibody experiments
demonstrated that blocking ITGA6 decreased cell proliferation and
invasion in ESCC. The results for biodistribution analysis
involving 125I-labeled anti-ITGA6 showed that the
ITGA6-targeting antibody could rapidly and specifically localize to
esophageal tumors. These results showed that new combination
strategies targeting integrins, especially ITGA6, with
anti-angiogenesis or tyrosine kinase-inhibitor agents could be
effective therapeutic tools for ESCC. Therefore, ITGA6 represents a
potential therapeutic antibody target in ESCC, although highly
effective humanized ITGA6 antibody development is required to
maximize therapeutic efficiency.
In conclusion, our research demonstrated that the
ECM receptor ITGA6 is overexpressed in ESCC tumor tissue and cell
lines. Using ITGA6 knockdown, we found that ITGA6 plays an
important role in the proliferation, and invasion by colony
formation by ESCC cells. We found that ITGA6 was associated with
ITGB4 and that this heterodimer complex was upregulated in both
ESCC tissue and cell lines. We also found that the ITGA6 inhibitory
antibody has the same effect as siRNA in ESCC cell lines. Moreover,
the results for biodistribution analysis in an ESCC xenograft model
suggested that ITGA6 could be used as a target of antibody-related
therapy in ESCC. Taken together, our results indicate that ITGA6
plays an important role in tumorigenesis and has potential as a
therapeutic target in ESCC.
Acknowledgements
This work was supported by grants from
the National R&D Program for Cancer Control (1120260 to
J.H.P.), Ministry of Health and Welfare, Republic of Korea, and
from the Radiological Translational Research Program (RTR), Korea
Institute of Radiological & Medical Sciences (50455-2012).
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Darling G: The role of lymphadenectomy in
esophageal cancer. J Surg Oncol. 99:189–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kranzfelder M, Schuster T, Geinitz H,
Friess H and Buchler P: Meta-analysis of neoadjuvant treatment
modalities and definitive non-surgical therapy for oesophageal
squamous cell cancer. Br J Surg. 98:768–783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chiu PW, Chan AC, Leung SF, et al:
Multicenter prospective randomized trial comparing standard
esophagectomy with chemoradiotherapy for treatment of squamous
esophageal cancer: early results from the chinese university
research group for esophageal cancer (CURE). J Gastrointest Surg.
9:794–802. 2005. View Article : Google Scholar
|
|
5.
|
Xu XH, Peng XH, Yu P, Xu XY, Cai EH, Guo P
and Li K: Neoadjuvant chemotherapy for resectable esophageal
carcinoma: a meta-analysis of randomized clinical trials. Asian Pac
J Cancer Prev. 13:103–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hynes RO: Integrins: versatility,
modulation, and signaling in cell adhesion. Cell. 69:11–25. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Giancotti FG and Tarone G: Positional
control of cell fate through joint integrin/receptor protein kinase
signaling. Annu Rev Cell Dev Biol. 19:173–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Avraamides CJ, Garmy-Susini B and Varner
JA: Integrins in angio genesis and lymphangiogenesis. Nat Rev
Cancer. 8:604–617. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mizejewski GJ: Role of integrins in
cancer: survey of expression patterns. Proc Soc Exp Biol Med.
222:124–138. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hwang R and Varner J: The role of
integrins in tumor angiogenesis. Hematol Oncol Clin North Am.
18:991–1006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Friedrichs K, Ruiz P, Franke F, Gille I,
Terpe HJ and Imhof BA: High expression level of alpha 6 integrin in
human breast carcinoma is correlated with reduced survival. Cancer
Res. 55:901–906. 1995.PubMed/NCBI
|
|
12.
|
Mercurio AM and Rabinovitz I: Towards a
mechanistic understanding of tumor invasion - lessons from the
alpha6beta 4 integrin. Semin Cancer Biol. 11:129–141. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mukhopadhyay R, Theriault RL and Price JE:
Increased levels of alpha6 integrins are associated with the
metastatic phenotype of human breast cancer cells. Clin Exp
Metastasis. 17:325–332. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wewer UM, Shaw LM, Albrechtsen R and
Mercurio AM: The integrin alpha 6 beta 1 promotes the survival of
metastatic human breast carcinoma cells in mice. Am J Pathol.
151:1191–1198. 1997.PubMed/NCBI
|
|
15.
|
Hemler ME, Crouse C and Sonnenberg A:
Association of the VLA alpha 6 subunit with a novel protein. A
possible alternative to the common VLA beta 1 subunit on certain
cell lines. J Biol Chem. 264:6529–6535. 1989.PubMed/NCBI
|
|
16.
|
Hu YC, Lam KY, Law S, Wong J and
Srivastava G: Profiling of differentially expressed cancer-related
genes in esophageal squamous cell carcinoma (ESCC) using human
cancer cDNA arrays: overexpression of oncogene MET correlates with
tumor differentiation in ESCC. Clin Cancer Res. 7:3519–3525.
2001.
|
|
17.
|
Kwon YJ, Lee SJ, Koh JS, Kim SH, Kim YJ
and Park JH: Expression patterns of aurora kinase B, heat shock
protein 47, and periostin in esophageal squamous cell carcinoma.
Oncol Res. 18:141–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lathia JD, Gallagher J, Heddleston JM, et
al: Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem
Cell. 6:421–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tew WP, Kelsen DP and Ilson DH: Targeted
therapies for esophageal cancer. Oncologist. 10:590–601. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Vanhoefer U, Tewes M, Rojo F, et al: Phase
I study of the humanized antiepidermal growth factor receptor
monoclonal antibody EMD72000 in patients with advanced solid tumors
that express the epidermal growth factor receptor. J Clin Oncol.
22:175–184. 2004. View Article : Google Scholar
|
|
21.
|
Capdevila J, Elez E, Macarulla T, Ramos
FJ, Ruiz-Echarri M and Tabernero J: Anti-epidermal growth factor
receptor monoclonal antibodies in cancer treatment. Cancer Treat
Rev. 35:354–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Cruz-Monserrate Z and O’Connor KL:
Integrin alpha 6 beta 4 promotes migration, invasion through Tiam1
upregulation, and subsequent rac activation. Neoplasia. 10:408–417.
2008.PubMed/NCBI
|
|
23.
|
Yang XH, Richardson AL, Torres-Arzayus MI,
et al: CD151 accelerates breast cancer by regulating alpha 6
integrin function, signaling, and molecular organization. Cancer
Res. 68:3204–3213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chung J and Mercurio AM: Contributions of
the alpha6 integrins to breast carcinoma survival and progression.
Mol Cells. 17:203–209. 2004.PubMed/NCBI
|
|
25.
|
Cariati M, Naderi A, Brown JP, Smalley MJ,
Pinder SE, Caldas C and Purushotham AD: Alpha-6 integrin is
necessary for the tumourigenicity of a stem cell-like subpopulation
within the MCF7 breast cancer cell line. Int J Cancer. 122:298–304.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Su JM, Gui L, Zhou YP and Zha XL:
Expression of focal adhesion kinase and alpha5 and beta1 integrins
in carcinomas and its clinical significance. World J Gastroenterol.
8:613–618. 2002.PubMed/NCBI
|
|
27.
|
Varner JA, Emerson DA and Juliano RL:
Integrin alpha 5 beta 1 expression negatively regulates cell
growth: reversal by attachment to fibronectin. Mol Biol Cell.
6:725–740. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Beaulieu JF: Integrin alpha6beta4 in
colorectal cancer. World J Gastrointest Pathophysiol. 1:3–11.
2010.PubMed/NCBI
|
|
29.
|
Noh TW, Soung YH, Kim HI, Gil HJ, Kim JM,
Lee EJ and Chung J: Effect of {beta}4 integrin knockdown by RNA
interference in anaplastic thyroid carcinoma. Anticancer Res.
30:4485–4492. 2010.
|
|
30.
|
Bon G, Folgiero V, Di Carlo S, Sacchi A
and Falcioni R: Involvement of alpha6beta4 integrin in the
mechanisms that regulate breast cancer progression. Breast Cancer
Res. 9:2032007. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lu X, Lu D, Scully M and Kakkar V: The
role of integrins in cancer and the development of anti-integrin
therapeutic agents for cancer therapy. Perspect Medicin Chem.
2:57–73. 2008.PubMed/NCBI
|