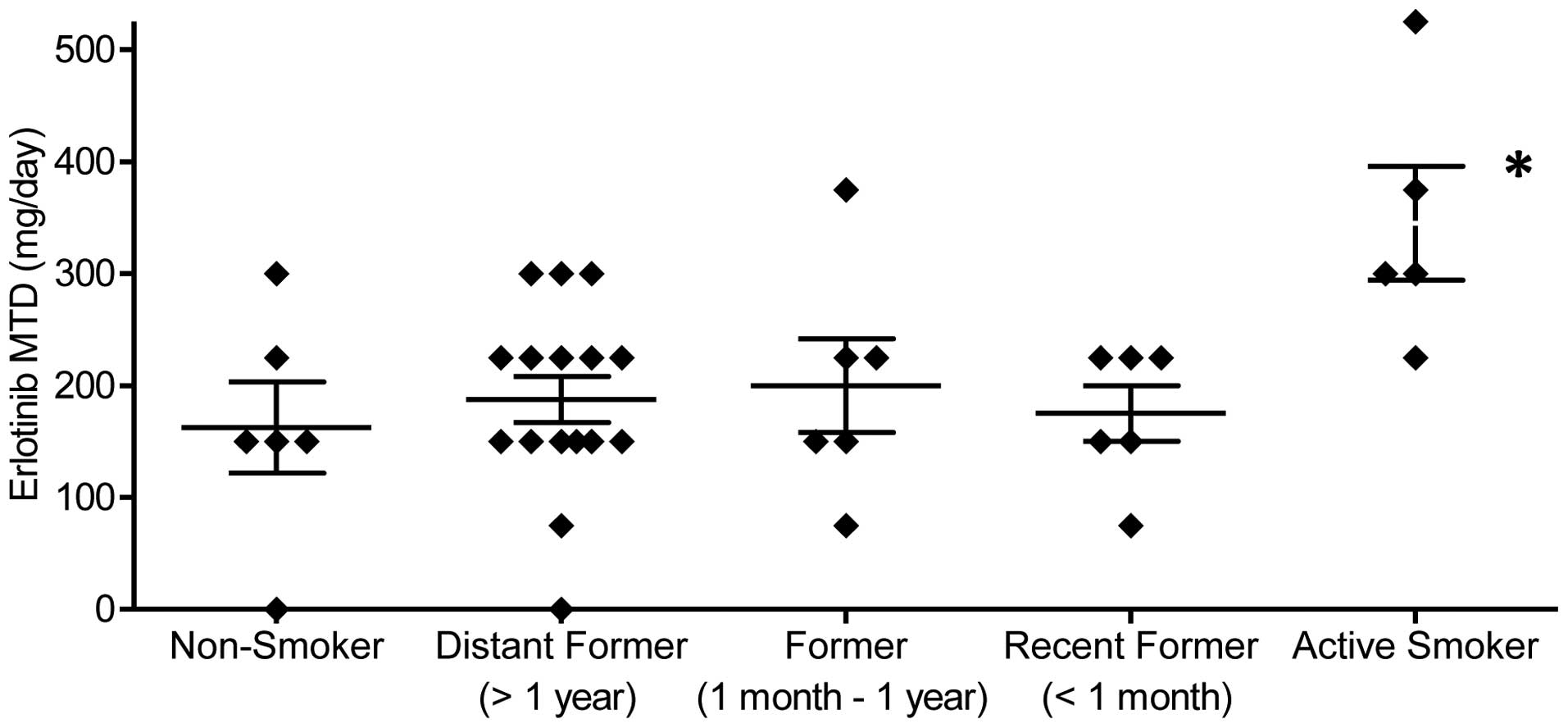
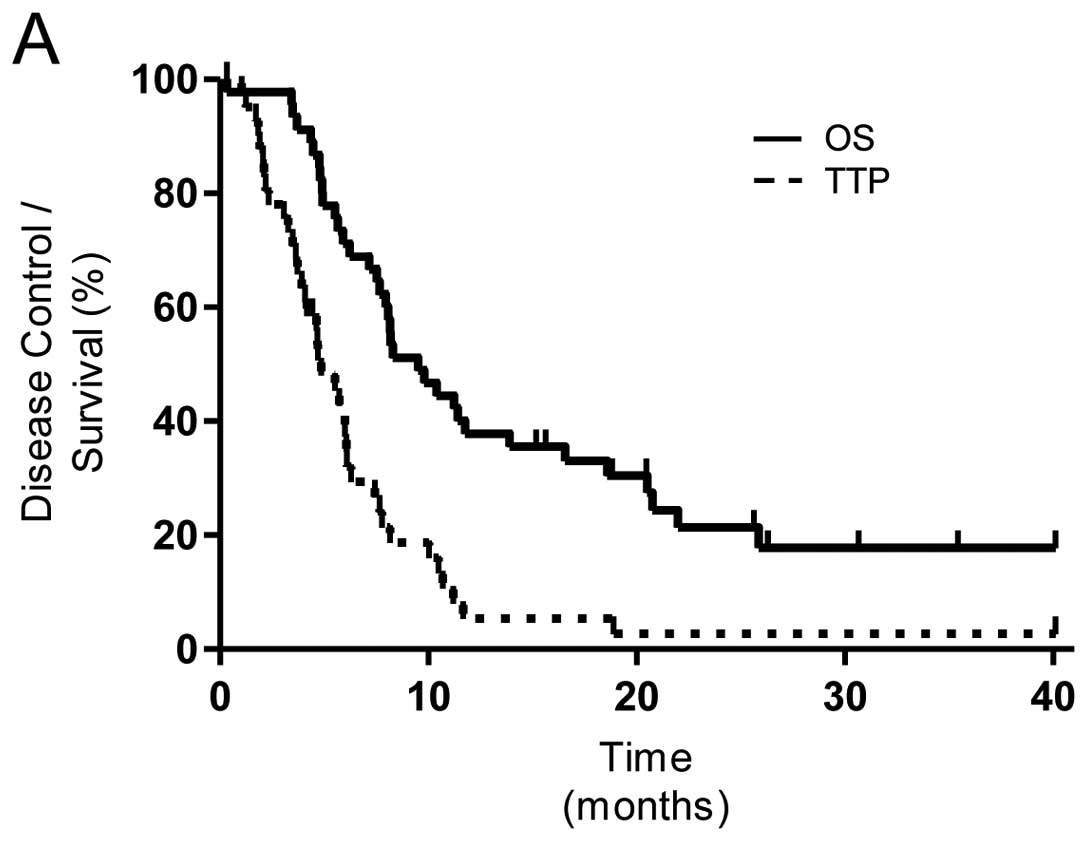
|
1.
|
Miller AA, Wang XF, Gu L, et al: Phase II
randomized study of dose-dense docetaxel and cisplatin every 2
weeks with pegfilgrastim and darbepoetin alfa with and without the
chemo-protector BNP7787 in patients with advanced non-small cell
lung cancer (CALGB 30303). J Thorac Oncol. 3:1159–1165. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shepherd FA, Rodrigues Pereira J, et al:
Erlotinib in previously treated non-small-cell lung cancer. N Engl
J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Cappuzzo F, Ciuleanu T, Stelmakh L, et al:
Erlotinib as maintenance treatment in advanced non-small-cell lung
cancer: a multicentre, randomised, placebo-controlled phase 3
study. Lancet Oncol. 11:521–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Petrelli F, Borgonovo K, Cabiddu M, et al:
Relationship between skin rash and outcome in non-small-cell lung
cancer patients treated with anti-EGFR tyrosine kinase inhibitors:
a literature-based meta-analysis of 24 trials. Lung Cancer.
78:8–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee SM, Khan I, Upadhyay S, et al:
First-line erlotinib in patients with advanced non-small-cell lung
cancer unsuitable for chemotherapy (TOPICAL): a double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 13:1161–1170.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hughes AN, O'Brien ME, Petty WJ, et al:
Overcoming CYP1A1/1A2 mediated induction of metabolism by
escalating erlotinib dose in current smokers. J Clin Oncol.
27:1220–1226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Jänne PA, Wang X, Socinski MA, et al:
Randomized phase II trial of erlotinib alone or with carboplatin
and paclitaxel in patients who were never or light former smokers
with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol.
30:2063–2069. 2012.
|
|
8.
|
Fukuoka M, Wu YL, Thongprasert S, et al:
Biomarker analyses and final overall survival results from a phase
III, randomized, open-label, first-line study of gefitinib versus
carboplatin/paclitaxel in clinically selected patients with
advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol.
29:2866–2874. 2011. View Article : Google Scholar
|
|
9.
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ling YH, Li T, Yuan Z, et al: Erlotinib,
an effective epidermal growth factor receptor tyrosine kinase
inhibitor, induces p27KIP1 up-regulation and nuclear translocation
in association with cell growth inhibition and G1/S phase arrest in
human non-small-cell lung cancer cell lines. Mol Pharmacol.
72:248–258. 2007. View Article : Google Scholar
|
|
11.
|
Petty WJ, Dragnev KH, Memoli VA, et al:
Epidermal growth factor receptor tyrosine kinase inhibition
represses cyclin D1 in aerodigestive tract cancers. Clin Cancer
Res. 10:7547–7554. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kobayashi S, Shimamura T, Monti S, et al:
Transcriptional profiling identifies cyclin D1 as a critical
downstream effector of mutant epidermal growth factor receptor
signaling. Cancer Res. 66:11389–11398. 2006. View Article : Google Scholar
|
|
13.
|
Dragnev KH, Petty WJ, Shah S, et al:
Bexarotene and erlotinib for aerodigestive tract cancer. J Clin
Oncol. 23:8757–8764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kim ES, Herbst RS, Wistuba II, et al: The
BATTLE trial: personalizing therapy for lung cancer. Cancer Discov.
1:44–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Petty WJ, Voelzke WR, Urbanic JJ, et al:
High cyclin D3 expression confers erlotinib resistance in
aerodigestive tract cancer. Lung Cancer. 74:384–391. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced non-small
cell lung cancer. N Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
18.
|
Roche trials database: A study of Tarceva
(erlotinib) to compare two different doses in currently smoking
patients with advanced or metastatic non-small cell lung cancer
(CURRENTS). <http://www.roche-trials.com/trialDetailsGet.action?studyNumber=M022162>.
|
|
19.
|
Parsons A, Daley A, Begh R, et al:
Influence of smoking cessation after diagnosis of early stage lung
cancer on prognosis: systematic review of observational studies
with meta-analysis. BMJ. 340:b55692010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Waller LL, Weaver KE, Petty WJ, et al:
Effects of continued tobacco use during treatment of lung cancer.
Expert Rev Anticancer Ther. 10:1569–1575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Brugger W, Triller N, Blasinska-Morawiec
M, et al: Prospective molecular marker analyses of EGFR and KRAS
from a randomized, placebo-controlled study of erlotinib
maintenance therapy in advanced non-small-cell lung cancer. J Clin
Oncol. 29:4113–4120. 2011. View Article : Google Scholar
|
|
22.
|
Addison CL, Ding K and Zhao H: Plasma
transforming growth factor alpha and amphiregulin protein levels in
NCIC Clinical Trials Group BR.21. J Clin Oncol. 28:5247–5256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Witta SE, Jotte RM, Konduri K, et al:
Randomized phase II trial of erlotinib with and without
entinostatin patients with advanced non-small-cell lung cancer who
progressed on prior chemotherapy. J Clin Oncol. 30:2248–2255. 2012.
View Article : Google Scholar : PubMed/NCBI
|