Introduction
Chondrosarcoma, the production of cartilage-like
matrix by tumor cells, is the second most common type of primary
malignant bone tumor after osteosarcoma and a common form of tumor
in patients aged more than 20 years (1,2).
Chondrosarcoma has been found to be relatively chemo- and
radio-therapy resistant for their extracellular matrix, low
percentage of dividing cells, and poor vascularity (3,4).
Chemo- and radio-therapy have not been tested for efficacy, but in
clinical routine they are not considered as active for the therapy
of this tumor and surgical resection still prevails as the primary
mode of therapy for chondrosarcoma. Since chondrosarcoma is a type
of highly malignant tumor with a potent capacity for distant
metastasis and local invasion (5),
the 10-year survival rate of this tumor being unchanged over the
past 40 years and ranging from 29% to 83% depends on the
chondrosarcoma subtype and grade (6,7).
Development of better strategies of improving chondrosarcoma
clinical management is therefore a challenging problem, and novel
therapeutic approaches are needed. Recently, an increasing number
of reports have described a new class of small regulatory RNA
molecules termed microRNAs (miRNAs) that are implicated in
chondrosarcoma progression (8).
miRNAs are a class of small non-coding RNAs that
have been identified as post-transcriptional regulators of gene and
play important roles in maintaining normal cellular functions
(9). The miRNAs mainly bind to the
3′ untranslated regions (UTRs) of target messenger RNAs (mRNAs),
leading to the blockade of mRNA translation or mRNA degradation
(10). Increasing evidence shows
that miRNAs have significant roles in diverse biological changes
and processes (11,12). Deregulation of miRNAs expression
leads to diverse human disease types, including cancer (13). In human cancer, miRNAs can function
as oncogenes or tumor suppressor genes during tumor progression and
development (14).
Recently, multiple new chemotherapeutic agents have
been developed and some are in clinical trials. Although some of
them have produced promising results, their therapeutic spectrum is
narrow along with toxicity. This toxicity problem at therapeutic
concentration has led to search for anticancer compounds derived
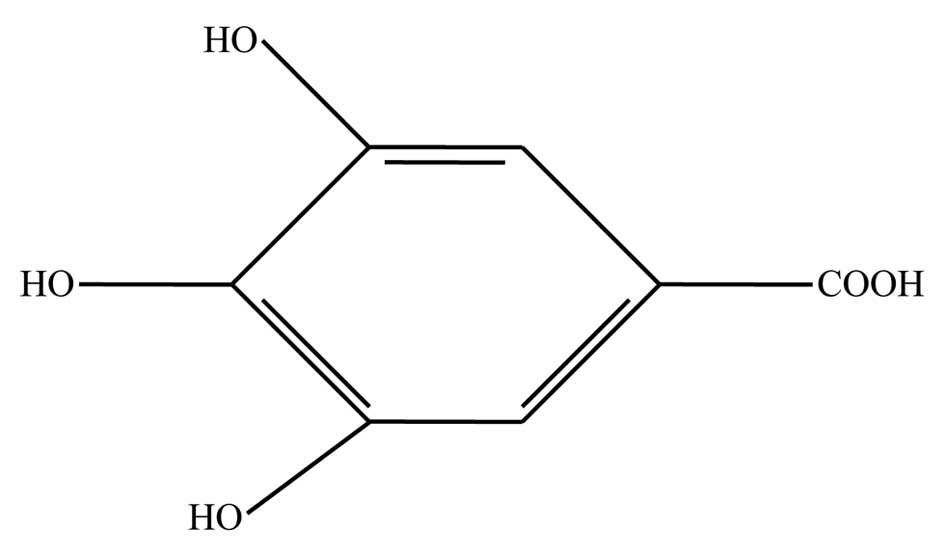
from nature. Gallic acid (GA; 3,4,5-triphydroxylbenzoic acid,
Fig. 1), a natural polyhydroxyl
phenolic compound, is widely distributed in various plants and
fruits (15,16). GA possesses various pharmacological
activities including anti-inflammatory, antimicrobial, antioxidant
and anticancer activities in various cancer cells (17,18),
and the toxicity is reported as an LD50 dose of 5 g/kg
body weight in rats (19).
In the present study, the aim was to explore the
anticancer property of GA on SW1353 human chondrosarcoma cells. We
investigated the changes of cell viability, morphology, wound
healing, apoptosis, Bcl-2, Bax, caspase-3, caspase-9, and miRNAs
expression in SW1353 cells treatment with GA. The results show that
GA induces apoptosis and inhibits cell migration by upregulating
miR-518b in SW1353 cells. It is of great importance to further
explore the biological functions, clinical significance, and target
genes of miRNAs in human chondrosarcoma.
Materials and methods
Cell culture
SW1353 cells, a human chondrosarcoma cell line, were
obtained from Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s
modified Eagle’s medium (DMEM) (Hyclone, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Hyclone), 100
μg/ml streptomycin and 100 U/ml penicillin. The cells were
maintained under standard culture conditions of 37°C, 5%
CO2 and 95% humidified air. Stock solutions were
prepared by dissolving the GA (Sigma Chemical Co., MO, USA) powder
in dimethyl sulfoxide (DMSO) to a concentration of 10 mg/ml, and
the final concentration of DMSO in the medium was no more than
0.5%.
Cell viability by MTT analysis
The cells were seeded at a density of 2000 cells per
well of 96-well plate for 24 h, and subsequently treated with
various concentration of GA (0, 5, 10, 20, 30, 40, 60 μg/ml)
for 48 h or with 30 μg/ml of GA for 6, 12, 24, 36, 48 and 72
h. At the end of treatment times, 20 μl of MTT stock
solution (5 mg/ml) was added to each well, and cells were incubated
at 37°C for 4 h. Thereafter, media were aspirated from the wells,
followed by addition of 200 μl of DMSO, and the cells were
shaken for 10 min. The color formed was determined by measuring the
OD at 550 nm using an ELISA plate reader (BioTek, Model EXL 800,
USA).
Observation of morphologic changes
The cells were cultured in 35 mm Petri dish at a
concentration of 5×104 cells for 24 h, and continuously
treated with different concentration of GA for 48 h. The cell
morphology was observed using a phase-contrast microscope (Olympus,
Japan).
Wound healing analysis
SW1353 cells were plated into 6-well plate, and grow
to confluence, and then made a straight scratch (stimulating a
wound) with a pipette tip. The cells treated with or without GA
were allowed to migrate for 48 h. After scratching, images were
taken under the inverted microscope to assess the ability of the
cells to migrate into the wound area. The distance of wound closure
(compared with untreated at 48 h) was measured in three-independent
wound sites per group.
Observation of apoptosis by fluorescent
microscopy with Hoechst 33258 staining
After treatment with or without GA, the SW1353 cells
were fixed in 4% neutral formaldehyde and stained with 10 μM
Hoechst 33258 (Sigma) at 37°C for 30 min in the dark. The
photographs of cells were taken using a fluorescent microscope
(Olympus).
Detection of apoptosis by flow cytometry
analysis with Annexin V/PI staining
SW1353 cells were cultured in 35 mm Petri dish at a
concentration of 5×104 cells for 24 h, and then treated
with or without GA for 48 h. The apoptosis of SW1353 cells was
tested by flow cytometry analysis using a fluorescence-activated
cell sorting (FACS) caliber (Becton-Dickinson, CA, USA) with
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
staining. Staining was performed according to the manufacturer’s
instructions.
Real-time polymerase chain reaction (PCR)
analysis
Total RNA was isolated from SW1353 cells treated
with GA using TRIzol reagent (Invitrogen, Carlsbad, CA, USA).
Reverse transcription was performed using random primers and
Superscript™ III (Invitrogen). PCR reactions were carried out in
total volumes of 25 μl using SYBR Florescence Quantization
kit (Invitrogen) in the ABI PRISM 7700 Sequence Detection System.
The forward and reverse primers for the amplifications are as
follows: Bcl-2 forward 5′-ATG TGT GTG GAG AGC GTC AA-3′ and reverse
5′-ACA GTT CCA CAA AGG CAT CC-3′, 136 bp; Bax forward 5′-GGG GAC
GAA CTG GAC AGT AA-3′ and reverse 5′-CAG TTG AAG TTG CCG TCA GA-3′,
122 bp. Amplification of glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) (forward 5′-CAG CCT CAA GAT CAT CAG CA-3′ and reverse
5′-TGT GGT CAT GAG TCC TTC CA-3′, 106 bp) was used to normalize
each reaction.
Western blot analysis
Total cellular protein was extracted from SW1353
cells treated with or without GA using RIPA buffer, and protein
concentrations were examined by Bio-Rad protein assay. An equal
amount of protein was separated on SDS-PAGE, and then transferred
onto PVDF membranes (Invitrogen). Blots were incubated with
anti-Bcl-2, Bax and β-actin (Santa Cruz Biotechnology, Santa Cruz,
CA) followed by an HRP-conjugated secondary antibody.
Immunoreactive proteins were visualized by western blotting
chemiluminescence luminol reagent (Santa Cruz Biotechnology).
Protein concentrations were quantitated using the Tocan 190 protein
assay system (Bio-Rad, USA) and normalized to β-actin in the
sample.
Analysis of caspase-3 and caspase-9
activation
The activities of caspase-3 and caspase-9 were
tested by a colorimetric assay using the caspase-3 and caspase-9
activation kits (Invitrogen), according to the manufacturer’s
instructions. The treated cells were lysed with provided lysis
buffer for 30 min on ice, and extracts were quantified using the
Bio-Rad protein assay. The protein (100 μg) was incubated
with the colorimetric tetrapeptides (50 μl), Asp-Glu-Val-Asp
(DEAD)-pNA (specific substrate of caspase-3) or Leu-Glu-His-Asp
(LEHD)-p-nitroaniline (pNA) (specific substrate of caspase-9) at
37°C for 2 h, and then estimated the caspase-3 and caspase-9
activation as instructed by the manufacturer in a 96-well
microtiter plate.
MiRNA microarray hybridization
Three samples of total RNA were obtained from the
SW1353 cells treated with or without GA, and labeled using the
miRCURY Hy3™/Hy5™ Power labeling kit and hybridized on the miRCURY
LNA Array (version 16.0) (KangChen Bio-tech, Shanghai, China).
Following the washing steps the slides were scanned using the Axon
GenePix 4000B microarray scanner. Scanned images were then imported
into GenePix Pro 6.0 software (Axon) for grid alignment and data
extraction.
Statistical analysis
All data were analyzed using the SPSS package for
Windows (version 13.0). Statistical analysis of the data was
performed with Student’s t-test and ANOVA. P-values <0.05 were
considered statistically significant.
Results
GA inhibits the viability of SW1353
cells
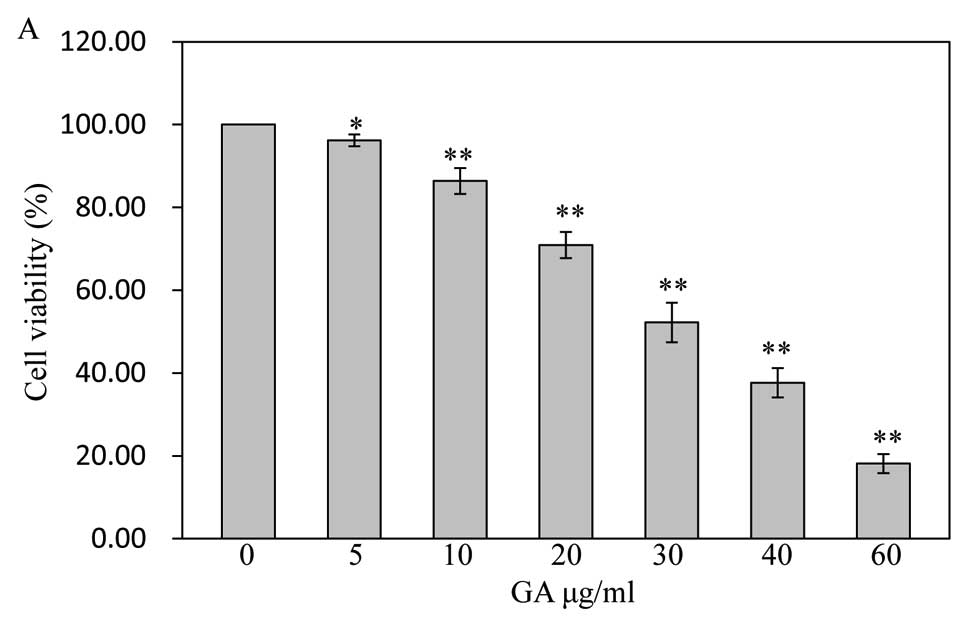
The viability of SW1353 cells treated with GA was
determined by MTT assay. As shown in Fig. 2A, the cells were treated with final
GA concentrations of 5 μg/ml (96.19±1.43%), 10 μg/ml
(86.38±3.11%), 20 μg/ml (70.94±3.16%), 30 μg/ml
(52.22±4.76%), 40 μg/ml (37.67±3.55%), and 60 μg/ml
(18.13±2.29%) for 48 h dose-dependently reduced cell viability
compared to untreated cells (100±0.00%) (P<0.05, P<0.01),
with an estimated half-maximal inhibitory concentration
(IC50) value of 30 μg/ml in this study. Treatment
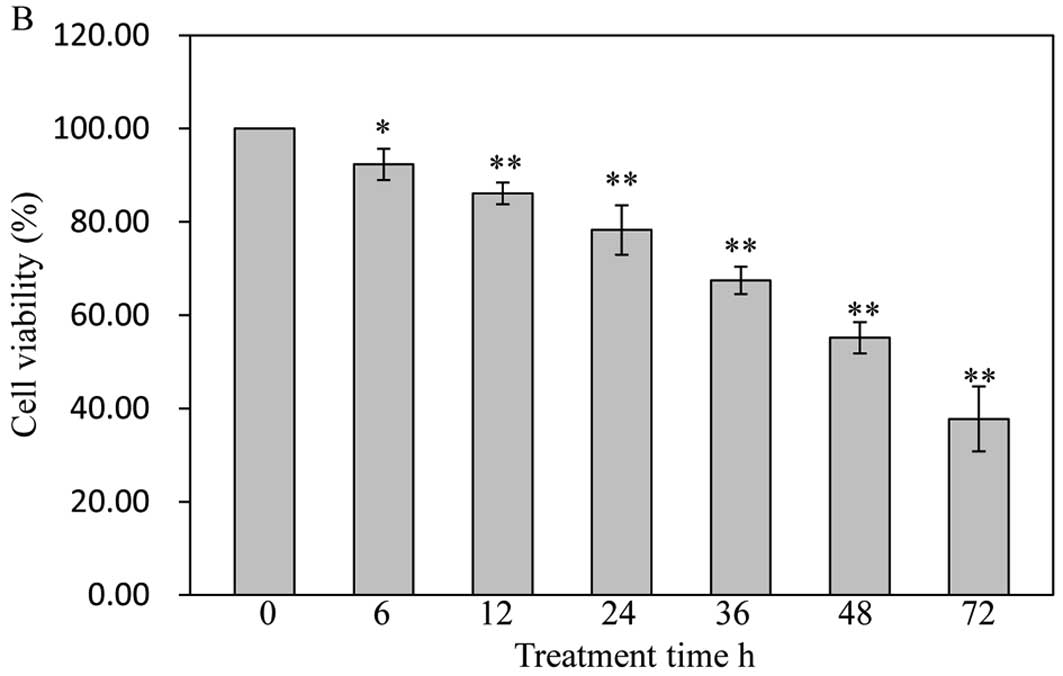
with 30 μg/ml of GA tested the effect of cell viability on
SW1353 cells for different periods of time. As shown in Fig. 2B, GA gradually decreased cell
viability with the increase of exposure time, suggesting that GA
decreases cell viability in a dose- and time-dependent manner.
GA induces morphological changes of
SW1353 cells
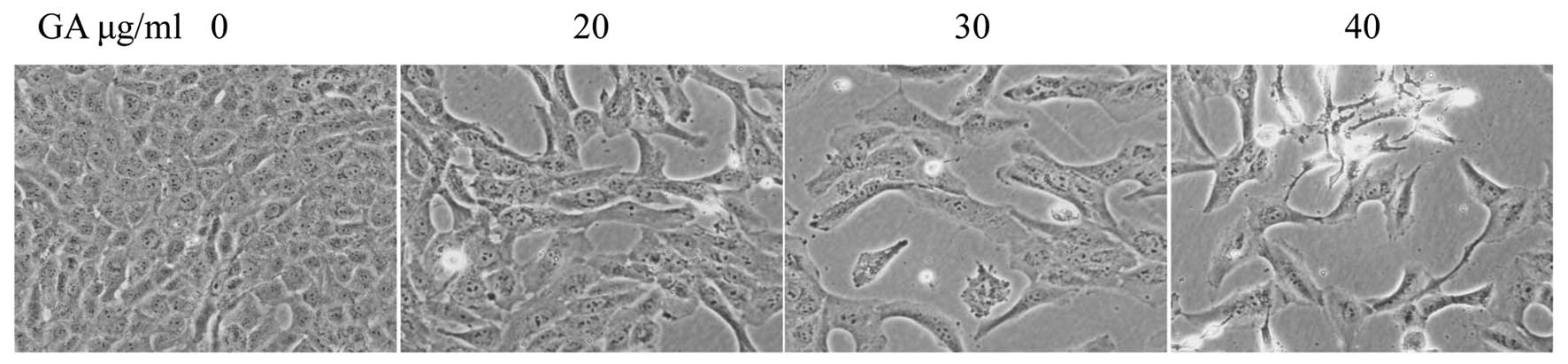
Treatment with GA resulted in morphological changes
of cells that were evaluated by phase-contrast microscopy, since
morphology of SW1353 cells in culture is an indicator of the health
status. As shown in Fig. 3,
untreated SW1353 cells showed densely disorganized multilayers,
whereas after treatment for 48 h with various concentrations of GA
many of the cells became rounded and shrunken, and detached from
each other or floated in the medium, suggesting that GA may induce
apoptosis of SW1353 cells.
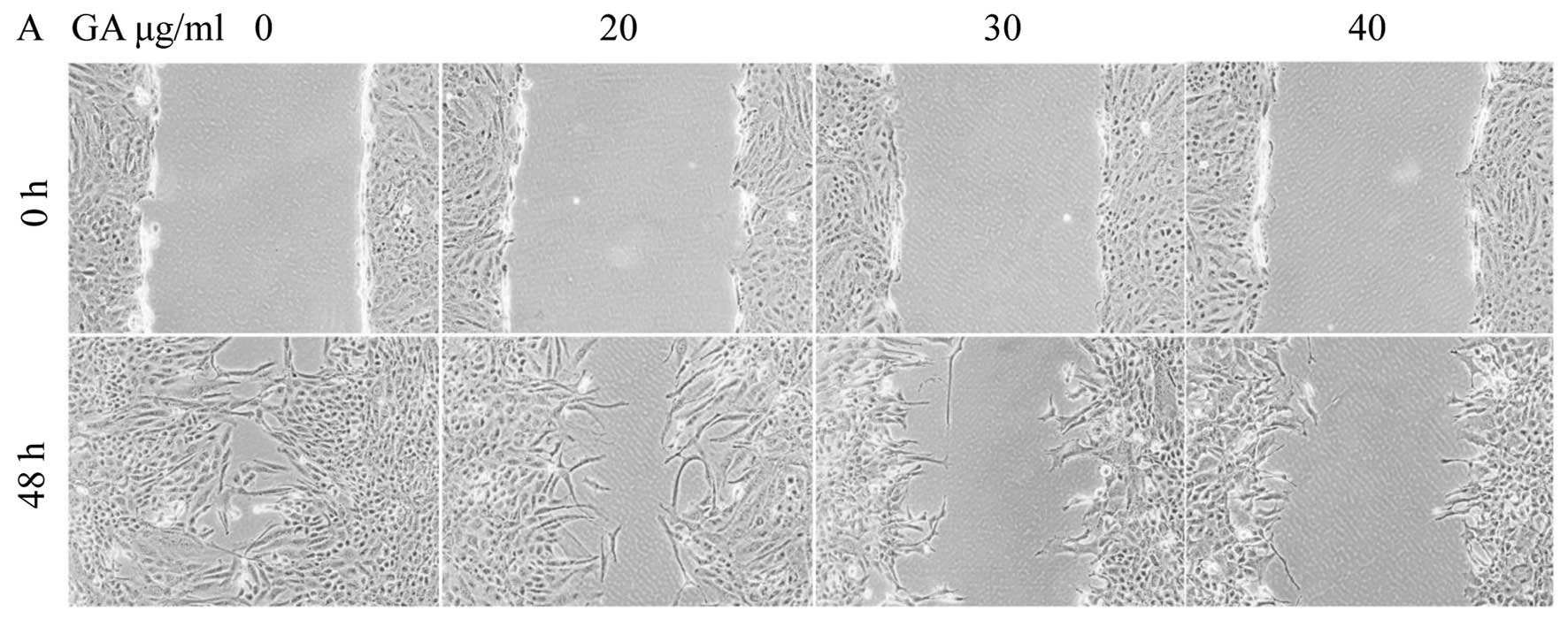
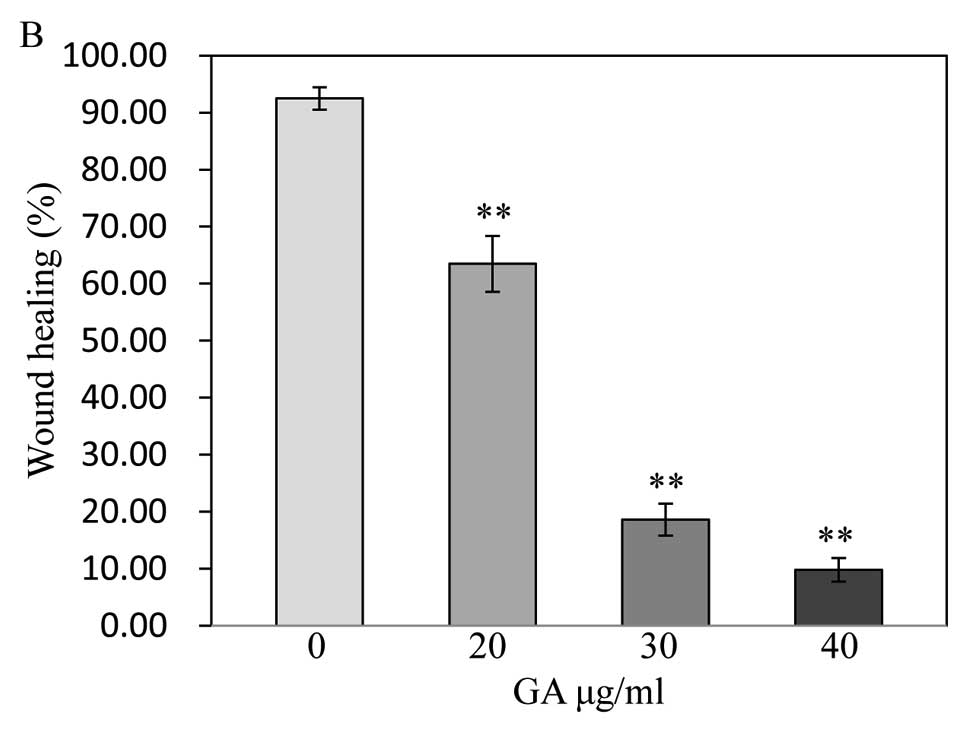
GA has antagonistic effects on the
migration of SW1353 cells
The wound healing assay showed the migratory
abilities of tumor cells, cell migration was decreased in SW1353
cells treated with GA. Treated cells closed the wound by 20
μg/ml (63.47±4.90%), 30 μg/ml (18.61±2.78%) and 40
μg/ml (9.78±2.07%) after 48 h, whereas the untreated cells
closed 92.49±1.98% of the wound during the same period (P<0.01)
(Fig. 4), indicating that GA may
inhibit cell migration by inducing apoptosis.
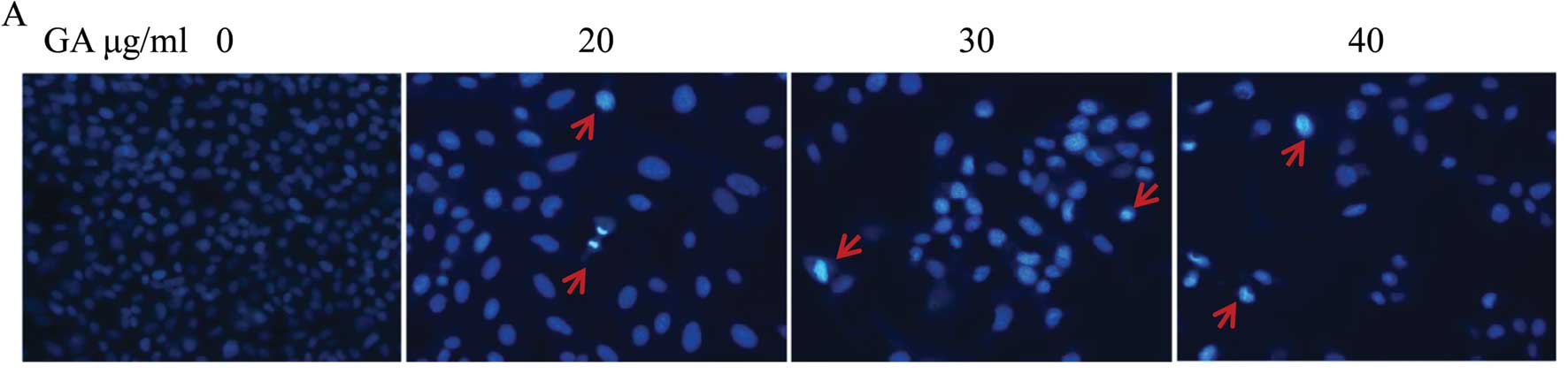
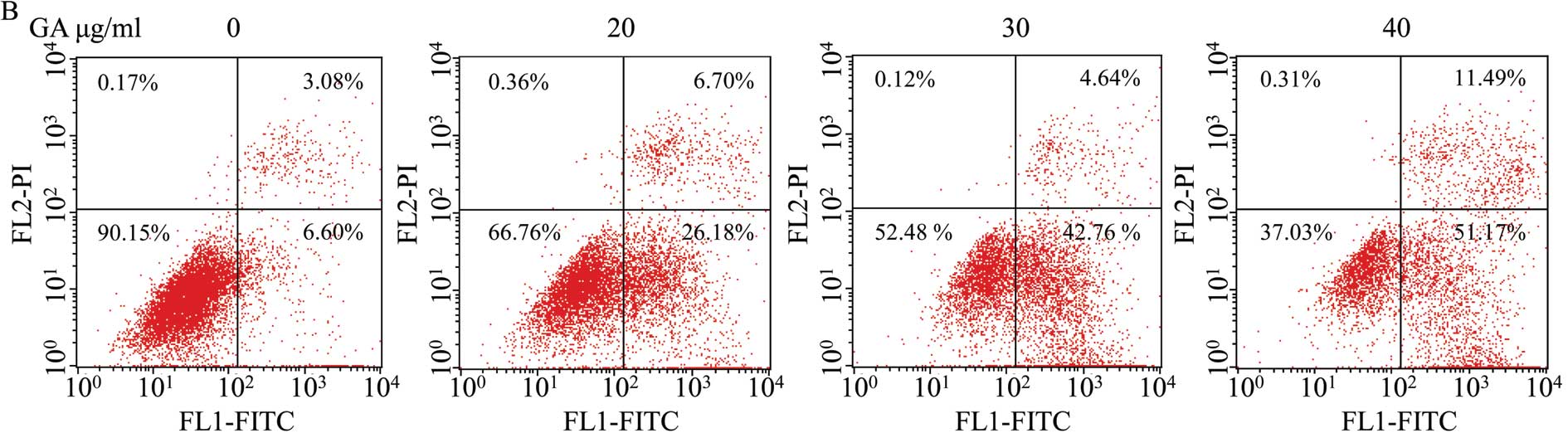
GA mediates apoptosis of SW1353
cells
To determine whether the cell growth and
cell-migration suppressive effect of GA is due to apoptosis, we
analyzed the cells in the presence of Hoechst 33258 staining, by
fluorescence microscopy. Untreated cells exhibited distribution of
the stain and round homogeneous nuclei features, whereas apoptosis
in treated cells increased gradually in a dose-dependent manner and
showed changes of typical apoptosis, including reduction of
cellular volume, staining bright and condensed or fragmented
nucleus (Fig. 5A). To further
verify that apoptosis was induced by GA, SW1353 cells were analyzed
by exposure to phosphatidylserine on the cell surface by Annexin
V/PI staining followed by FACS analysis. As shown in Fig. 5B, (LL) (Annexin V/PI double
negative population) represents viable cells; indicated as LR or UR
(Annexin V-positive/PI-negative or Annexin V/PI double-positive
population) indicates cells undergoing early or late apoptosis,
respectively. The percentage of cells undergoing apoptosis
(including the early and late apoptotic cells) with GA treatment
was significantly higher than that in untreated cells (P<0.05,
P<0.01) (Fig. 5C and D). These
data suggest that GA induces SW1353 cell apoptosis in a
dose-dependent manner.
GA regulates the expression of
anti-apoptotic Bcl-2 and proapoptotic Bax
Bcl-2 family proteins such as anti-apoptotic Bcl-2
and pro-apoptotic Bax, central in mitochondrion-mediated apoptosis
regulation, also determines the fate of cells. To further study the
mechanism of GA, the mRNA and protein expression of Bcl-2 and Bax
in treated cells were examined by real-time PCR and western blot
analysis, respectively. The results of real-time PCR assay showed
that GA treatment profoundly decreased Bcl-2 mRNA and increased Bax
mRNA expression in SW1353 cells compared to that in untreated cells
(P<0.01) (Fig. 6A and B), and
the protein levels of Bcl-2 and Bax were similar to their
respective mRNA expressions (Fig.
6C–E), suggesting GA induces mitochondrion-dependent apoptosis
in SW1353 cells by the regulation of Bcl-2 family proteins.
GA enhances the activation of caspase-3
and caspase-9
To investigate the downstream effectors in the
mitochondrion-dependent apoptotic pathway, the activation of
caspase-3 and caspase-9 was detected by colorimetric assay. As
shown in Fig. 7, GA treatment
significantly promoted the activation of caspase-3 and caspase-9 in
SW1353 cells compared to that in untreated cells (P<0.01). Taken
together, these results suggest that GA enhances cell apoptosis by
the mitochondrion-dependent pathway.
GA upregulates miR-518b in SW1353
cells
miRNAs, small (<22 nt) and non-coding RNA
molecules, regulate gene expression post-transcription through base
pairing with mRNAs to mediate their degradation and translational
repression that play an important role in many biological
processes. To explore the mechanisms of GA on apoptosis in SW1353
cells, we used the miRCURY LNA expression array to analyze the
changes of microRNA expression. In order to select the most
significant candidates, miRNAs altered by at least 1.5-fold in all
three pairs of the samples were selected. Under these strict
criteria, there were 7 statistically significant miRNAs between
treated and untreated cells, 6 genes were downregulated in treated
cells, while 1 gene was upregulated in treated cells (Fig. 8 and Table I). miR-518b has been identified to
suppress cell proliferation by inducing apoptosis in tumor cells
(20). Our results imply that GA
induces apoptosis and inhibits cell migration by upregulating
miR-518b in SW1353 cells.
 | Table I.Up and downregulated miRNAs in SW1353
treated with GA. |
Table I.
Up and downregulated miRNAs in SW1353
treated with GA.
| ID | miRNAs | Fold change | P-value |
|---|
| 148641 | hsa-miR-518b | 1.51 | 0.00 |
| 145996 | hsa-miR-205-3p | 0.67 | 0.02 |
| 147942 | hsa-miR-4268 | 0.58 | 0.03 |
| 147947 | hsa-miR-4308 | 0.39 | 0.05 |
| 17327 | hsa-miR-630 | 0.39 | 0.02 |
| 13133 |
hsa-miR-520a-5p | 0.43 | 0.05 |
| 148038 |
hsa-miR-3679-3p | 0.68 | 0.03 |
Discussion
The characteristics of the tumor cells are a
reduction in cell apoptosis and/or an unregulated increase in cell
proliferation (21). Moreover,
disrupted apoptosis play a crucial role in drug-resistance of tumor
cells, and it has become a significant obstacle for the successful
management of patients with chondrosarcoma (22). Since current chemotherapy regimens
have limited success in improving metastasis-free survival and
limited by the severe toxicity of conventional agents, the
therapeutic bottleneck of chondrosarcoma still remains unconquered
(23,24). Natural products are important to
discover new drugs. These compounds can be used as antioxidants and
in tumor therapy drugs or prevention. Therefore, plant-derived
natural products are worthy of further exploration. GA, one of
natural products, has been associated with selective induction of
cell death and antiproliferative effects, predominantly through an
apoptotic mechanism, in many tumor cell lines (25,26).
However, the molecular mechanism of GA inducing apoptosis of tumor
cells remains unclear. Our results showed that GA inhibited cell
viability and the migratory abilities of SW1353 cells dose- and
time-dependently. GA induced apoptosis by downregulating the
expression of anti-apoptotic protein Bcl-2, and upregulating the
expression of pro-apoptotic protein Bax, and the activation of
caspase-3 and caspase-9. It was also observed that miR-518b gene
was upregulated in treated cells, suggesting GA was able to induce
apoptosis and inhibit cell migration by upregulating miR-518b in
SW1353 cells.
To determine the inhibitory concentration of GA on
SW1353 cells, cell viability was examined. It was tested that
exposure to GA for 48 h, cell viability was inhibited as shown by
MTT assay. Our results show that GA dose- and time-dependently
inhibited the cell viability as compared to the untreated cells.
The morphological changes of cells imply that cells undergo
apoptosis at 48 h after incubation with the concentration of GA
chosen based on the MTT assays. To study the migration of SW1353
cells further, wound healing assay was carried out. This indicated
that GA may inhibit cell migration by inducing apoptosis and
thereby acts as an anticancer drug. This finding corroborated well
with the change of cell viability. Further Hoechst 33258 staining
assay and Annexin V/PI staining assay were performed to study
apoptosis induction by GA. We found that GA induced SW1353 cells
apoptosis.
Apoptosis (programmed cell death), a pathway of cell
death characterized by many biochemical and morphological events
(27), is initiated by two
different signals, the intracellular and extracellular, and by two
main pathways, the death receptor- and mitochondria-mediated
pathways (28). Mitochondria
mediated apoptosis is commonly involved in death stimuli by the
intrinsic pathway, which is the main mechanism of apoptosis in
various mammalian cells. This pathway of apoptosis results in an
increase of mitochondrial permeability, and the release of
pro-apoptotic molecules from the intermembrane space of the
mitochondria into the cytosol, such as cytochrome c, Smac/DIABLO
and apoptosis-inducing factor, and then activating the
caspase-cascade system (29,30).
The members of Bcl-2 family regulate the mitochondrion-dependent
apoptosis, such as Bax, one of pro-apoptotic Bcl-2 family proteins,
control the formation of pores in the mitochondria, whereas Bcl-2,
one of anti-apoptotic Bcl-2 family proteins, can prevent cell death
by interfering with the activation of Bax (31,32).
Therefore, the ratio of Bax to Bcl-2 is critical for determining
the release of mitochondrial cytochrome c which activates
caspase-9, and then subsequently induces the activation of effector
caspases, such as caspase-3 (33,34).
To further explore the molecular mechanism involved
in GA-induced apoptosis, the expressions of Bcl-2, Bax, caspase-3
and caspase-9 were assessed in SW1353 cells. Our results showed
that GA could upregulate Bax expression and down-regulate Bcl-2
expression in SW1353 cells, suggesting GA induces apoptosis by
affecting the ratio of Bax/Bcl-2. Caspase activity was quantified
by colorimetric assay. We evaluated both caspase-3 and caspase-9,
our results have shown a clear increase in the caspase-3 and
caspase-9 activities, indicating that GA induced apoptosis in
SW1353 cells is by the activation of the intrinsic pathway.
To gain insight into the molecular mechanism
involved in GA-induced apoptosis by mitochondria mediated pathway,
the expression of miRNA was assessed in SW1353 cells. There were 7
statistically significant miRNAs between treated and untreated
cells, including 6 genes downregulated in treated cells, while 1
gene was upregulated in treated cells. Considering the function of
miR-518b in invasion and metastasis, it will be interesting to
explore molecular mechanisms mediating miR-518b indirectly or
directly affecting cell progression in chondrosarcoma. However,
there are opposite observations in the expression of miR-518b in
other tumors, such as miR-518b upregulated in extranodal marginal
zone lymphomas compared to gastritis and in hepatocellular
carcinoma compared to non-cancerous tissue (20,35).
As each miRNA can control the expression of hundreds of different
target genes containing tumor suppressor genes and oncogenes, the
function for the pro-tumor or antitumor roles of a miRNA was
determined by the competition among its target genes in specific
tumor types (36–38). Our results showed that miR-518b
markedly increased in SW1353 cells treated with GA. In the future
experiments, it is very important to validate targets of miR-518b
by further functional assays.
In conclusion, our data demonstrate that GA induces
mitochondrion-dependent apoptosis and inhibits cell migration by
upregulating miR-518b in SW1353 cells. These results indicate that
GA may be a potential novel antitumor agent for the treatment of
chondrosarcoma. Further study on GA treatment of tumors, is
required especially given the potential for cross-reactivity and
unintended consequences when taken with other antitumor agents.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant nos. 81202645
and 81173203), the Natural Science Foundation of Fujian Province
(grant no. 2011J05076).
References
|
1.
|
Schrage YM, Briaire-De Bruijn IH, De
Miranda NF, et al: Kinome profiling of chondrosarcoma reveals
SRC-pathway activity and dasatinib as option for treatment. Cancer
Res. 69:6216–6222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Li X, Ye H, Cai L, et al: Millimeter wave
radiation induces apoptosis via affecting the ratio of Bax/Bcl-2 in
SW1353 human chondrosarcoma cells. Oncol Rep. 27:664–672.
2012.PubMed/NCBI
|
|
3.
|
Dickey ID, Rose PS, Fuchs B, et al:
Dedifferentiated chondrosarcoma: the role of chemotherapy with
updated outcomes. J Bone Joint Surg Am. 86:2412–2418.
2004.PubMed/NCBI
|
|
4.
|
Kalinski T, Sel S, Kouznetsova I, Röpke M
and Roessner A: Heterogeneity of angiogenesis and blood vessel
maturation in cartilage tumors. Pathol Res Pract. 205:339–345.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yuan J, Dutton CM and Scully SP: RNAi
mediated MMP-1 silencing inhibits human chondrosarcoma invasion. J
Orthop Res. 23:1467–1474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gelderblom H, Hogendoorn PC, Dijkstra SD,
et al: The clinical approach towards chondrosarcoma. Oncologist.
13:320–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bovée JV, Cleton-Jansen AM, Taminiau AH
and Hogendoorn PC: Emerging pathways in the development of
chondrosarcoma of bone and implications for targeted treatment.
Lancet Oncol. 6:599–607. 2005.PubMed/NCBI
|
|
8.
|
Zuntini M, Salvatore M, Pedrini E, et al:
MicroRNA profiling of multiple osteochondromas: identification of
disease-specific and normal cartilage signatures. Clin Genet.
78:507–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang W, Zhao LJ, Tan YX, Ren H and Qi ZT:
MiR-138 induces cell cycle arrest by targeting cyclin D3 in
hepatocellular carcinoma. Carcinogenesis. 33:1113–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
He L and Hannon GJ: MicroRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Anglicheau D, Muthukumar T and
Suthanthiran M: MicroRNAs: small RNAs with big effects.
Transplantation. 90:105–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
15.
|
Locatelli C, Filippin-Monteiro FB and
Creczynski-Pasa TB: Alkyl esters of gallic acid as anticancer
agents: a review. Eur J Med Chem. 60:233–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Verma S, Singh A and Mishra A: Gallic
acid: molecular rival of cancer. Environ Toxicol Pharmacol.
35:473–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kim YJ: Antimelanogenic and antioxidant
properties of gallic acid. Biol Pharm Bull. 30:1052–1055. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Panich U, Onkoksoong T, Limsaengurai S,
Akarasereenont P and Wongkajornsilp A: UVA-induced melanogenesis
and modulation of glutathione redox system in different melanoma
cell lines: the protective effect of gallic acid. J Photochem
Photobiol B. 108:16–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Shahrzad S, Aoyagi K, Winter A, Koyama A
and Bitsch I: Pharmacokinetics of gallic acid and its relative
bioavailability from tea in healthy humans. J Nutr. 131:1207–1210.
2001.PubMed/NCBI
|
|
20.
|
Zhang M, Zhou S, Zhang L, et al: miR-518b
is down-regulated, and involved in cell proliferation and invasion
by targeting Rap1b in esophageal squamous cell carcinoma. FEBS
Lett. 586:3508–3521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Liang W, Li X, Li C, et al:
Quercetin-mediated apoptosis via activation of the
mitochondrial-dependent pathway in MG-63 osteosarcoma cells. Mol
Med Rep. 4:1017–1023. 2011.PubMed/NCBI
|
|
22.
|
Kim HJ, Lee SG, Kim YJ, et al:
Cytoprotective role of autophagy during paclitaxel-induced
apoptosis in Saos-2 osteosarcoma cells. Int J Oncol. 42:1985–1992.
2013.PubMed/NCBI
|
|
23.
|
Kim SY and Helman LJ: Strategies to
explore new approaches in the investigation and treatment of
osteosarcoma. Cancer Treat Res. 152:517–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Russinoff S, Miran S, Gowda AL and Lucas
PA: Osteosarcoma cells differentiate into phenotypes from all three
dermal layers. Clin Orthop Relat Res. 469:2895–2904. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Liang CZ, Zhang X, Li H, et al: Gallic
acid induces the apoptosis of human osteosarcoma cells in vitro and
in vivo via the regulation of mitogen-activated protein kinase
pathways. Cancer Biother Radiopharm. 27:701–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Liao CL, Lai KC, Huang AC, et al: Gallic
acid inhibits migration and invasion in human osteosarcoma U-2 OS
cells through suppressing the matrix metalloproteinase-2/-9,
protein kinase B (PKB) and PKC signaling pathways. Food Chem
Toxicol. 50:1734–1740. 2012. View Article : Google Scholar
|
|
27.
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Low IC, Kang J and Pervaiz S: Bcl-2: a
prime regulator of mitochondrial redox metabolism in cancer cells.
Antioxid Redox Signal. 15:2975–2987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Del Gaizo Moore V and Letai A: BH3
profiling-measuring integrated function of the mitochondrial
apoptotic pathway to predict cell fate decisions. Cancer Lett.
332:202–205. 2013.PubMed/NCBI
|
|
33.
|
Fernández-Luna JL: Apoptosis regulators as
targets for cancer therapy. Clin Transl Oncol. 9:555–562. 2007.
|
|
34.
|
Lalier L, Cartron PF, Juin P, et al: Bax
activation and mitochondrial insertion during apoptosis. Apoptosis.
12:887–896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Thorns C, Kuba J, Bernard V, et al:
Deregulation of a distinct set of microRNAs is associated with
transformation of gastritis into MALT lymphoma. Virchows Arch.
460:371–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Lima RT, Busacca S, Almeida GM, Gaudino G,
Fennell DA and Vasconcelos MH: MicroRNA regulation of core
apoptosis pathways in cancer. Eur J Cancer. 47:163–174. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Creighton CJ, Fountain MD, Yu Z, et al:
Molecular profiling uncovers a p53-associated role for microRNA-31
in inhibiting the proliferation of serous ovarian carcinomas and
other cancers. Cancer Res. 70:1906–1915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cai CK, Zhao GY, Tian LY, et al: miR-15a
and miR-16-1 down-regulate CCND1 and induce apoptosis and cell
cycle arrest in osteosarcoma. Oncol Rep. 28:1764–1770.
2012.PubMed/NCBI
|