Introduction
The stereochemistry and synthesis of
3,7-diazabicyclo[3.3.1] nonan-9-ones (bispidines) are of interest
owing to the biological importance of these substances (1). Conformational analysis of bispidines
is also of interest from a theoretical view point and, in
particular, 2,4,6,8-tetraaryl-3,7-diazabicyclo[3.3.1]nonan-9-one
(bispidinone) constitutes an interesting case for research owing to
the presence of 4 aryl groups (2).
The preparation and use of bispidines as chelate ligands for
radioactive copper isotopes for diagnosis (64Cu) or
therapy (67Cu) have been studied (3). A bifunctional bispidine derivative
can be readily functionalized with biologically active molecules at
the pendant carboxylate groups (3). However, the biological properties of
bispidinone with regard to the molecular mechanisms of its
therapeutic effects on cancer have not been examined. Therefore, we
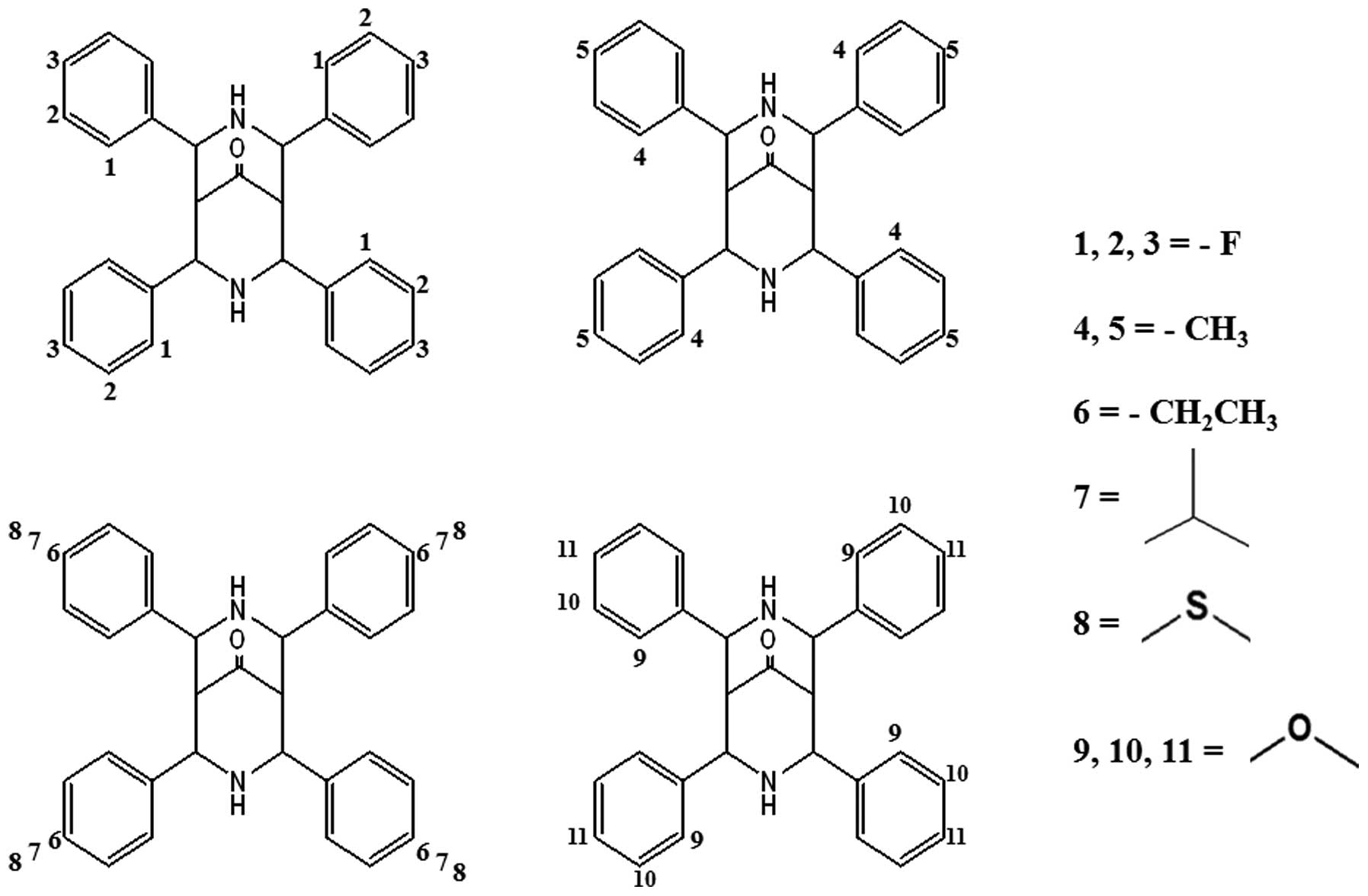
synthesized a series of 11 bispidinone analogs with
fluoro/methyl/ethyl/isopropyl/thiomethyl/methoxy substituents at
various positions (Fig. 1).
Firstly, we undertook a WST-8 assay to identify which substituents
and positions generate the strongest cytotoxic effect.
2,4,6,8-Tetrakis(2-methoxyphenyl)-3,7-diazabicyclo[3.3.1]nonan-9-one
(analog 9) was more cytotoxic in HeLa cells than the other analogs;
therefore, this study was undertaken to examine the anticancer
activity of analog 9.
Cancer is caused by the uncontrolled growth of
cells, and it has been thought to be the result of interactions
between genetic susceptibility and environmental toxins. In the
broad sense, most chemotherapeutic drugs work by impairing cell
division; however, some cause cancer cells to undergo apoptosis.
Many drugs have been developed to treat cancer, although the
principles and limitations of chemotherapy founded by the early
researchers still apply (4).
Therefore, identifying synthetic compounds with anticancer effects
is also essential for the treatment of cancer. Recently, the study
of apoptosis has been a major focus of cancer research. Apoptosis,
i.e., programmed cell death, is highly regulated through the
extrinsic and intrinsic pathways (5,6).
Apoptosis is characterized by chromatin condensation, DNA
fragmentation, membrane blebbing and cell shrinkage (7). The intrinsic pathway is induced by
many stimuli, e.g., DNA damage, cellular distress, hypoxia and
cytotoxic agents, which act inside cells (6). In recent studies, mitochondria have
been suggested to play an important role in apoptosis (8). In response to apoptosis, which is
especially related to the intrinsic pathways, some important events
occur in the mitochondria. These events can be controlled and
regulated by members of the Bcl-2 family (9), which includes anti- and proapoptotic
molecules and constitutes a critical intracellular decision point
within a common death pathway (10). The presence of the protein Bad
counters the antiapoptotic effects of Bcl-xL and Bcl-2, potentially
through direct inhibition of their activity or displacement of the
proapoptotic protein Bax (11). In
the mitochondria-mediated pathway, mitochondria release some
apoptotic proteins, including cytochrome c, into the cytosol
(12). Cytosolic cytochrome
c binds to apoptotic protease activation factor 1 (APAF1) to
produce active caspase-9 and caspase-3, thereby inducing
caspase-dependent apoptosis (13).
Caspases (cysteine-aspartic proteases or cysteine-dependent
aspartate-directed proteases) are a family of cysteine proteases
that regulate and play essential roles in apoptosis in the death
receptor- and mitochondria-mediated pathways (14). In the death receptor-mediated
pathway, caspase-8 plays an initiator role and activates the final
executioner caspase-3 (15).
Apoptosis, via the mitochondria-mediated pathway, also occurs in a
caspase-independent manner after the mitochondrial release of
apoptosis-inducing factor. It could be translocated to the nucleus
for the induction of chromatin condensation and DNA fragmentation
(16).
Cell cycle regulation is closely linked to cell
proliferation, and one of the most notable features of a tumor is
abnormal cell cycle management (17). The G1 phase or post-mitotic phase
is a period in the cell cycle during interphase, before the S
phase. In many cells, this phase represents the main period of cell
growth. During this stage, new organelles are synthesized so that
the cells require the production of structural proteins and
enzymes, resulting in a high level of protein synthesis and a high
rate of metabolism (18).
Emerging evidence has characterized the induction of
apoptosis to treat cancer (19–21).
The aim of this study was to determine whether analog 9 can exert
its activity on cancer cells. In this report, we investigated the
anticancer effect of analog 9 to identify whether a novel compound
can be an appropriate candidate for an antitumor agent.
Materials and methods
Chemicals
The propidium iodide (PI)/RNase staining buffer and
Annexin V-FITC kit for apoptosis were from BD Biosciences
Pharmingen (San Diego, CA, USA). Dimethyl sulfoxide (DMSO) and
phosphate-buffered saline (PBS, pH 7.4) were purchased from
Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Eagle’s minimum
essential medium (EMEM), fetal bovine serum (FBS),
penicillin-streptomycin, and trypsin-EDTA were obtained from
HyClone Laboratories, Inc. (Logan, UT, USA). Cell Counting Kit-8
(CCK-8) was purchased from Dojindo Molecular Technologies, Inc.
(Osaka, Japan). All other chemicals were of analytical reagent
grade. Rhodamine-123 was purchased from Molecular Probes (Eugene,
OR, USA).
Cell lines
HeLa cells obtained from the American Type Culture
Collection (Manassas, VA, USA) were cultured in EMEM supplemented
with 10% FBS and 1% penicillin-streptomycin at 37°C (5%
CO2) in a humidified atmosphere.
Preparation of bispidinone and its
analogs
Bispidinone and its analogs were obtained from the
laboratory of D.H.P. and synthesized by P.P. The stock solutions
were prepared in DMSO and kept at 4°C. Further dilutions were made
immediately prior to each experiment.
Cell viability and proliferation
assays
The effect of the bispidinone analogs on cell
viability was investigated in HeLa cells according to the method
reported by Tominaga et al (22). We plated 5.0×103 cells
into each well of a 96-well microplate. After 24 h, the medium
replaced with fresh medium containing the bispidinone analogs at
various concentrations (15, 30 and 60 μM). The plate was
incubated for an additional 48 h. The CCK-8 reagent (10 μl)
was then added to each well and incubated for 2 h. Cell viability
was assessed using
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
(WST-8), an indicator that is reduced by the dehydrogenases in
cells to give an orange colored product (formazan), which is
soluble in cell culture medium. The optical density of living cells
was read at 450 nm using a multimicroplate reader (Synergy HT;
BioTek, Winooski, VT, USA). Wells containing cells and an
appropriate volume of vehicle (DMSO) served as the control. Wells
containing only culture medium and the CCK-8 reagent served as the
blank. The percentage cytotoxicity of analog 9 was calculated
according to the following equation: % cytotoxicity = [(Ac − A)/(Ac
− Ab)] ×100, where Ac, Ab and A represent the mean optical density
of the vehicle control, blank and treated groups, respectively. In
order to determine the effect of analog 9 on cell proliferation,
5.0×103 cells/ml medium were seeded in 96-well plates
and treated with or without analog 9 (15 μM) for various
durations. Each experiment was repeated at least 3 times.
Cell cycle arrest analysis
The cells (3.0×105 cells in a 60-mm dish)
treated with or without analog 9 were collected by trypsinization
and washed with ice-cold PBS by centrifugation. The cells were
suspended in PBS and fixed with 70% ethanol (v/v). The samples were
then washed with ice-cold PBS and stained with PI/RNase staining
buffer for 15 min at room temperature (23). The cells in different phases of the
cell cycle were analyzed using a FACScan flow cytometer, and 20,000
events were analyzed for each sample. The percentage of cells in
the different phases of the cell cycle was determined using ModFit
software (Becton-Dickinson Instruments, Franklin Lakes, NJ,
USA).
Annexin V-FITC/PI apoptotic analysis
Cells (3.0×105 cells in a 60-mm dish)
treated with or without analog 9 were collected by trypsinization
and washed with ice-cold PBS via centrifugation. Then,
1.0×105 cells were resuspended in 100 μl binding
buffer and stained with 5 μl Annexin V-FITC and 10 μl
PI (50 μg/ml) for 15 min at room temperature in the dark
(23). Analysis was performed
using a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA,
USA) with 10,000 events each time. The data were analyzed using
Cell Quest Pro software (Becton-Dickinson Instruments).
Measurement of apoptotic cell
morphology
HeLa cells were distributed (1.0×105
cells/well) into a 24-well plate and allowed to adhere overnight.
The cells were treated with analog 9 (15 μM) for 24 and 48
h. Non-treated wells received an equivalent volume of DMSO
(<0.1%) as the control. Optic phase-contrast photographs were
taken using a Nikon Phase Contrast-2, ELWD 0.3 inverted
microscope.
[3H]-thymidine incorporation
assay
The [3H]-thymidine incorporation assay
was performed as described previously (24). Briefly, HeLa cells were cultured in
12-well plates in growth medium (EMEM + 10% FBS + 1%
penicillin-streptomycin). After the cells had grown to 70–80%
confluence, they were rendered quiescent by incubation for 24 h in
EMEM containing 2% FBS. Analog 9 (15 μM) in EMEM (or DMSO)
supplemented with 10% FBS was added to the cells and the cultures
were incubated for 21 and 45 h. [3H]-thymidine was added
at 1 μCi/ml (1 μCi = 37 kBq) and incubated for 3 h.
Incorporated [3H]-thymidine was extracted in a cell
lysis buffer and measured using a liquid scintillation analyzer
(Tri-Carb 2910TR; Perkin-Elmer Inc., Waltham, MA, USA).
Measurement of mitochondrial-membrane
potential disruption
Changes in mitochondrial-membrane potential (ΔΨm)
were measured using flow cytometry (FACSCalibur; BD Biosciences
Pharmingen). HeLa cells treated with analog 9 and untreated control
cells were stained with 10 μg/ml rhoda-mine-123 (Rh-123;
Molecular Probes) and incubated at 37°C for 30 min. Analysis was
performed using a FACSCalibur flow cytometer (Becton-Dickinson)
with 10,000 events per sample. The data were analyzed using Cell
Quest Pro software (Becton-Dickinson Instruments).
Western blot analysis assay
After the cells were treated with or without analog
9, total cell lysates and cytosolic fractions were prepared as
described previously (25). The
protein content of the lysates was determined by the Bradford
protein assay (Bio-Rad, Hercules, CA, USA). Proteins (25 μg)
were resolved by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto nitrocellulose membranes
(Schleicher & Schuell, Keene, NH, USA) by western blot analysis
(26). The following primary
polyclonal antibodies were used: Bcl-2, procaspase-8, procaspase-9
and β-actin (1:1,000; Cell Signaling Technology, Danvers, MA, USA),
procaspase-3 and p53 (1:300; Santa Cruz Biotechnology, Santa Cruz,
CA, USA), and Bax (1:1,000; BD Biosciences Pharmingen).
Statistical analysis
Each experiment was repeated at least 3 times. Data
are expressed as the mean ± SD values of 3 independent experiments.
We performed statistical analysis by one-way analysis of variance.
The criterion for significance was set at P<0.05. Microsoft
Excel 2007 (Roselle, IL, USA) was used for the statistical and
graphical evaluations.
Results
Effects of analog 9 on the viability and
proliferation of HeLa cells
Initially, we evaluated the cytotoxicity of
different concentrations of bispidinone and its analogs against
HeLa cells. The cytotoxic effects of bispidinone compounds were
examined in a concentration-dependent manner in HeLa cells using
the WST-8 assay (Table I). After
incubation of the cells with increasing concentrations (15, 30, and
60 μM) of bispidinone and 11 analog compounds for 48 h,
analog 9 showed a significant cytotoxic effect in a
concentration-dependent manner when compared with the control. The
other compounds did not show a clear cytotoxic effect. Therefore,
we selected analog 9 for further study. The IC50 value
of analog 9 was approximately 13 μM (Fig. 2A). When HeLa cells were treated
with 15 μM analog 9 for 0, 12, 24, 36, 48 and 60 h, their
proliferation increased gradually until 48 h and began to decrease
at 60 h, whereas the untreated cells maintained an exponential
proliferation state. A significant difference in the absorbance
from the control was observed at 60 h, showing that analog 9
inhibited HeLa cell proliferation after 60-h incubation (Fig. 2B).
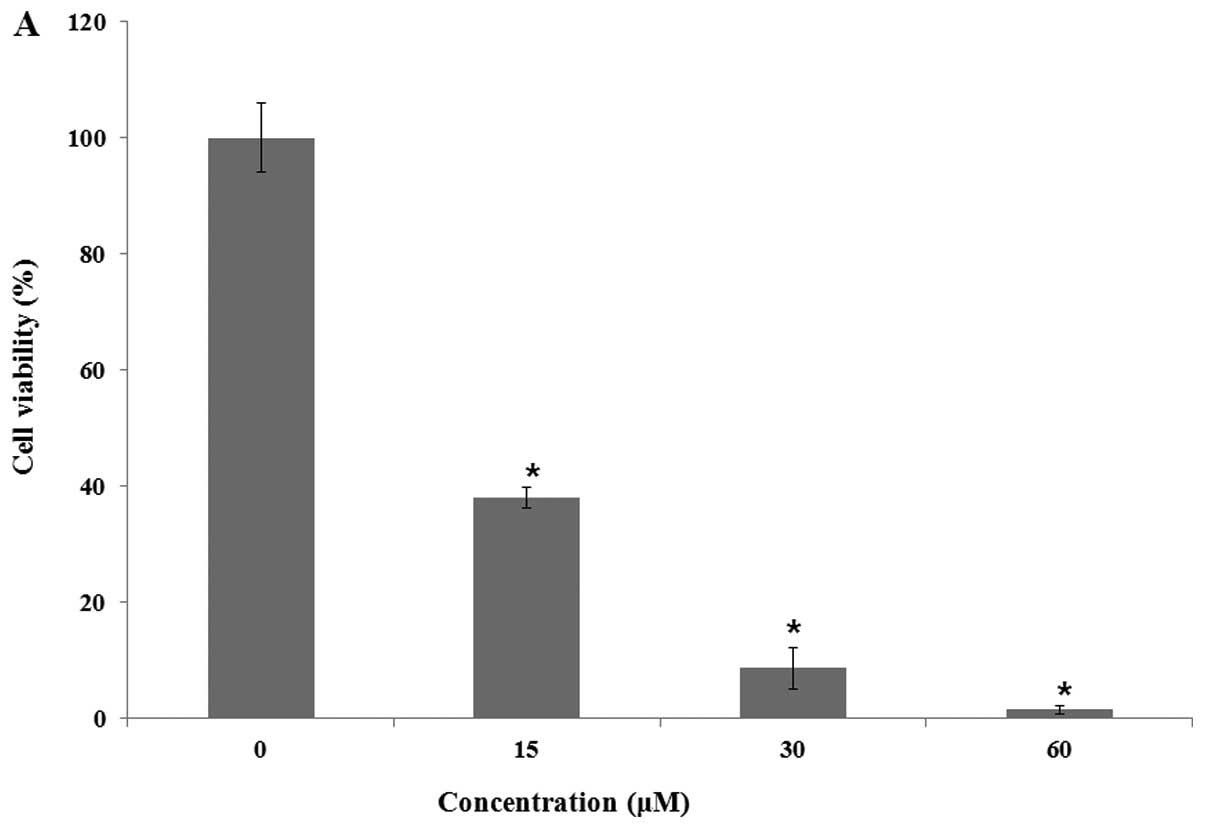 | Figure 2.Analog 9 had effects on HeLa cell
viability and proliferation. (A) HeLa cells were treated with 0,
15, 30 and 60 μM of analog 9, respectively, for 48 h. (B)
HeLa cells were treated with either vehicle alone or 15 μM
of analog 9 for 0, 12, 24, 36, 48 and 60 h, respectively. Data were
obtained by WST-8 assay at 450 nm. Results are the mean ± SD, n=3.
*P<0.05, denotes statistically significant difference
vs. the control at the same level. |
 | Table I.Evaluation of cytotoxicity of
bispidinone and 11 analogs on HeLa cells. |
Table I.
Evaluation of cytotoxicity of
bispidinone and 11 analogs on HeLa cells.
| Compound | IC50
value |
|---|
| Mother compound
(MC) | >100 |
| Analog 1 | 84.48 |
| Analog 2 | 44.84 |
| Analog 3 | 93.20 |
| Analog 4 | >100 |
| Analog 5 | >100 |
| Analog 6 | >100 |
| Analog 7 | >100 |
| Analog 8 | >100 |
| Analog 9 | 12.33 |
| Analog 10 | >100 |
| Analog 11 | 99.23 |
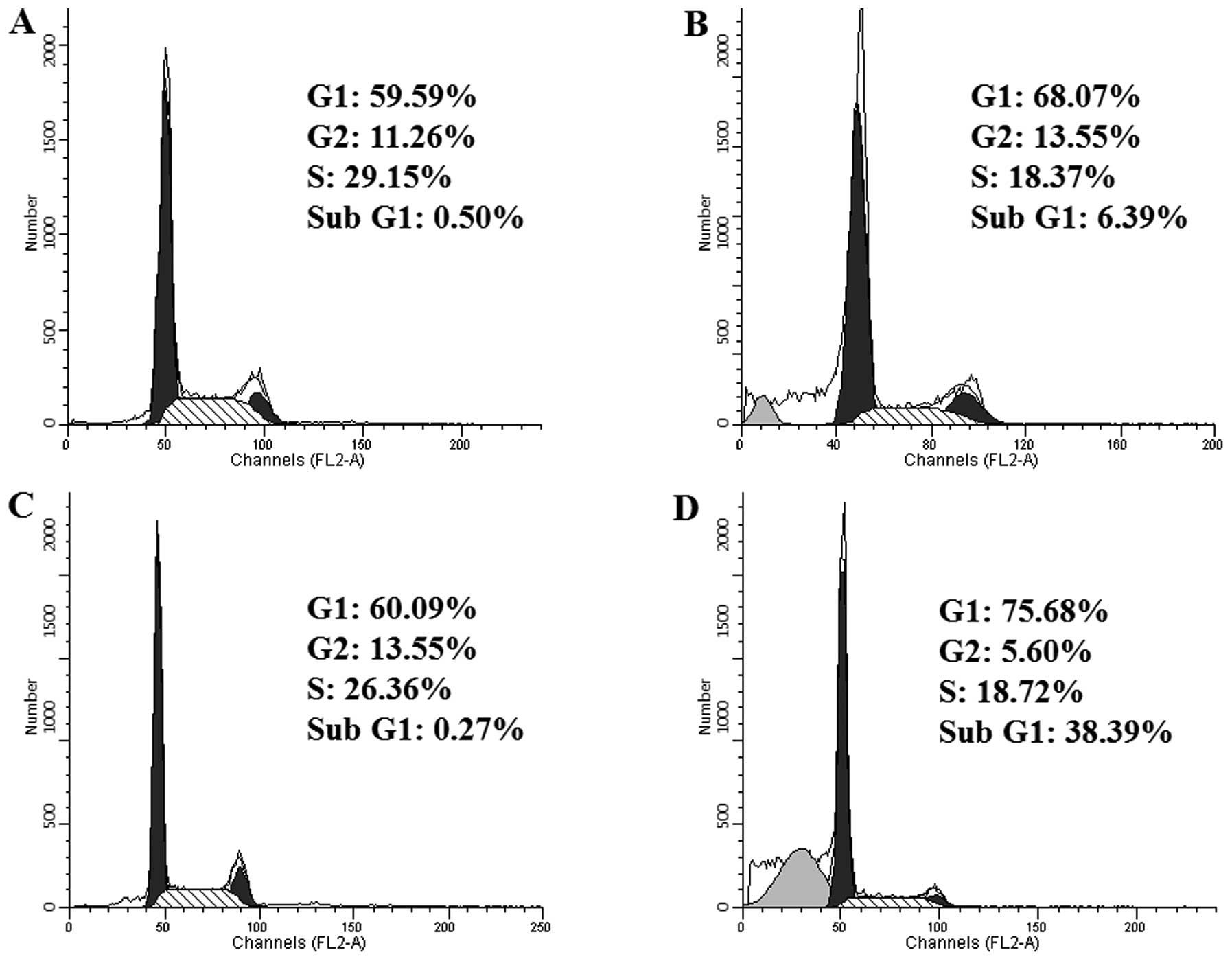
Analog 9 induces arrest at the sub-G1 and
G1 phases of the cell cycle
The growth and inhibition of cells are mediated by
the cell cycle (27). The abnormal
regulation of the cell cycle could be an event that initiates
apoptosis (28–30). To assess the effect of analog 9 on
cell cycle progression, HeLa cells were treated with or without 15
μM analog 9 for 24 and 48 h. Treatment with analog 9
(Fig. 3B and D) increased the
number of cells in the sub-G1 and G1 phases compared to the
untreated cells (Fig. 3A and C).
Furthermore, analog 9 increased the number of cells in the G1 phase
in a time-dependent manner. Thus, analog 9 induced the arrest of
HeLa cells in the sub-G1 and G1 phases of the cell cycle.
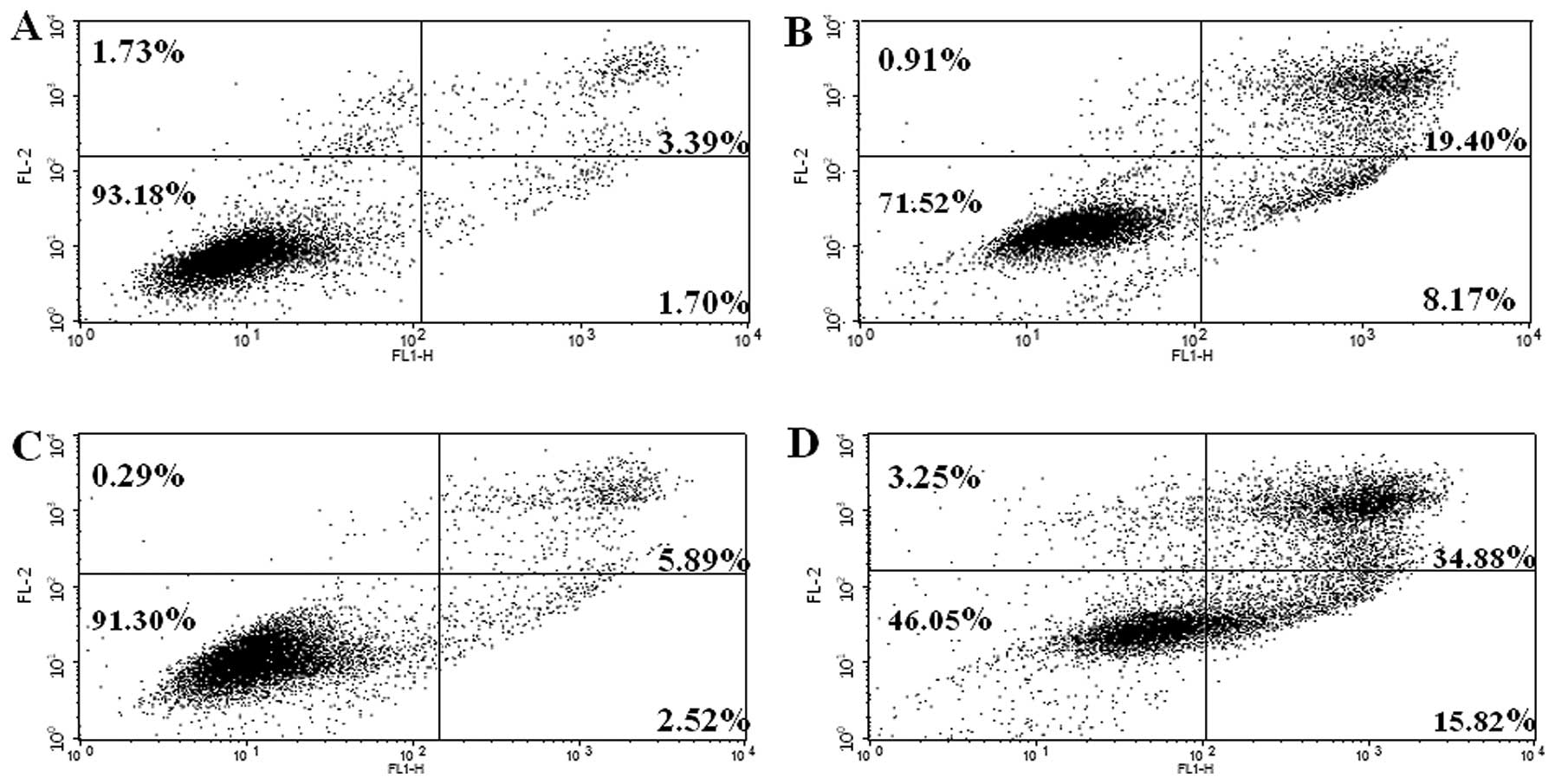
Analog 9 induces apoptosis in HeLa
cells
An Annexin V-FITC and PI double-staining assay was
performed to assess the induction of apoptosis. To evaluate whether
analog 9 induced apoptosis, HeLa cells were treated with or without
15 μM analog 9 for 24 and 48 h and then stained with Annexin
V-FITC and PI, followed by analysis using flow cytometry. This
double-staining assay allows live non-apoptotic cells (Annexin
V−/PI−) to be distinguished from early
apoptotic cells (Annexin V+/PI−) and late
apoptotic cells (Annexin V+/PI+) (31,32).
As shown in Fig. 4, treatment with
analog 9 increased the number of cells undergoing apoptosis
(Fig. 4B and D) compared with the
untreated cells (Fig. 4A and C).
Therefore, these results show that analog 9 induced apoptosis in
HeLa cells in a time-dependent manner.
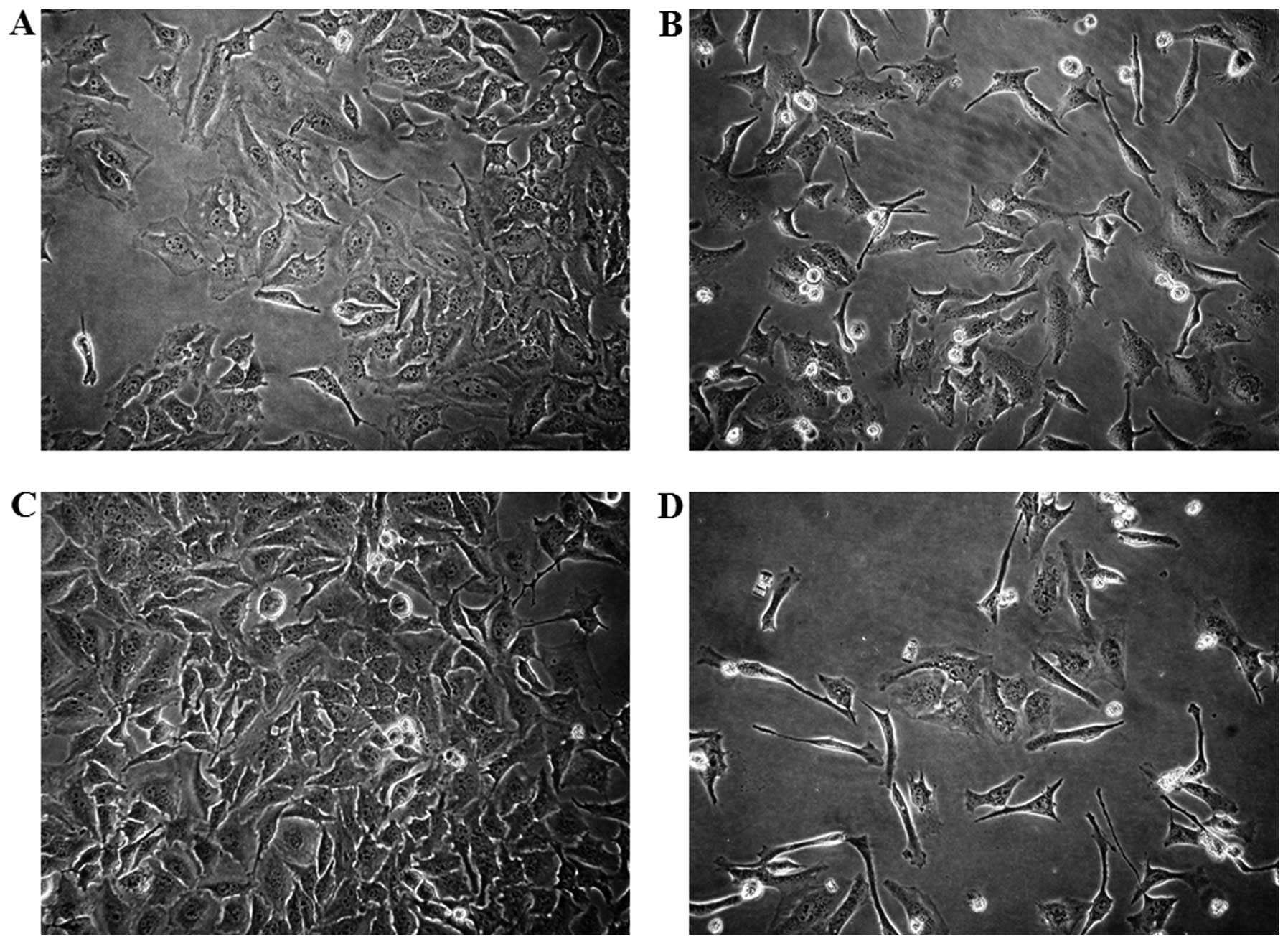
Analog 9 induces morphological changes in
HeLa cells
Cell morphological observation reveals important
characteristics of apoptotic cells. Morphological changes in the
cells treated with various times of analog 9 using light microscopy
revealed the presence of apoptosis. Under light microscopy,
non-treated analog 9-HeLa cells spread regularly in the culture
plates and grew to near confluence (Fig. 5A and C). After treatment with 15
μM analog 9 for 24 and 48 h, a significant number of HeLa
cells detached from the plate. The detached cells and most of the
remnant attached cells showed significant morphological changes
that were indicative of apoptosis, e.g., cellular shrinkage and
disruption (Fig. 5B and D).
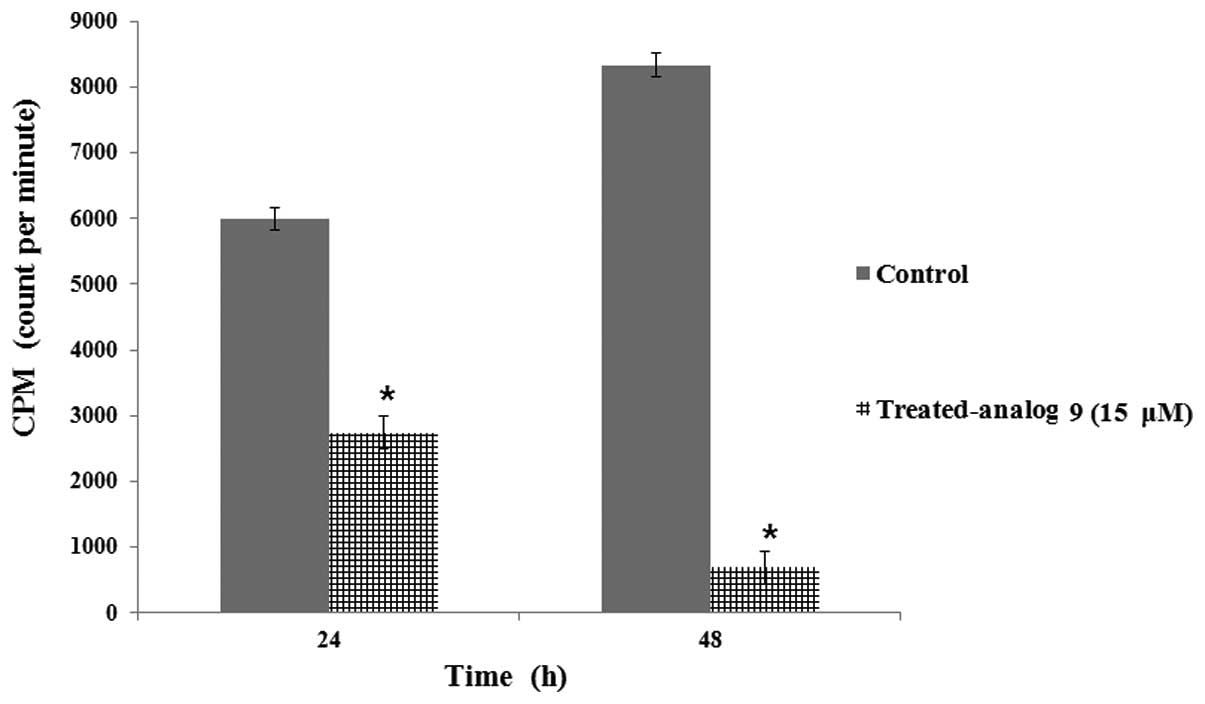
Analog 9 inhibits DNA replication in HeLa
cells
To confirm that analog 9 can influence HeLa cells at
the DNA level, we examined DNA replication in HeLa cells treated
with or without analog 9 by using a [3H]-thymidine
incorporation assay. The reduction of [3H]-thymidine
incorporation in HeLa cells treated with analog 9 indicates that
DNA replication was inhibited (Fig.
6) in a time-dependent manner; therefore, we concluded that
analog 9 had an effect on DNA replication of the cells.
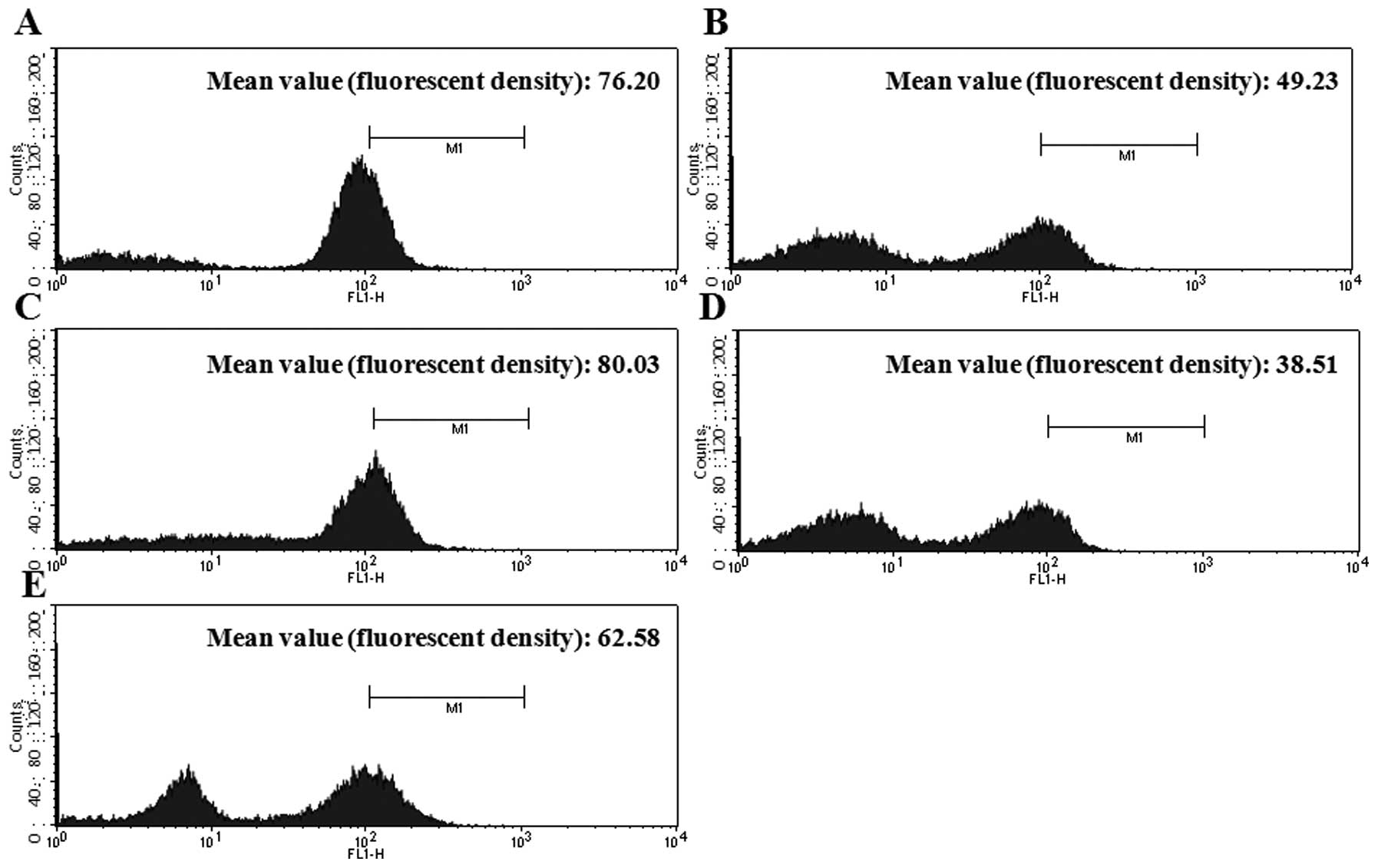
Analog 9 decreases intracellular
ΔΨm
The cohesion of mitochondrial membranes in HeLa
cells was examined by Rh-123 staining, where a decrease in Rh-123
fluorescence density reflects a decrease in intracellular
ΔΨm. The cells were treated with or without analog 9 for
12 and 24 h. Untreated cells had similar fluorescence between 12
and 24 h (Fig. 7A and C); however,
cells treated with analog 9 exhibited a time-dependent decrease in
intracellular ΔΨm (Fig. 7B
and D). Fig. 7E shows the data
for cells treated with H2O2, which were used
as a positive control to confirm these data.
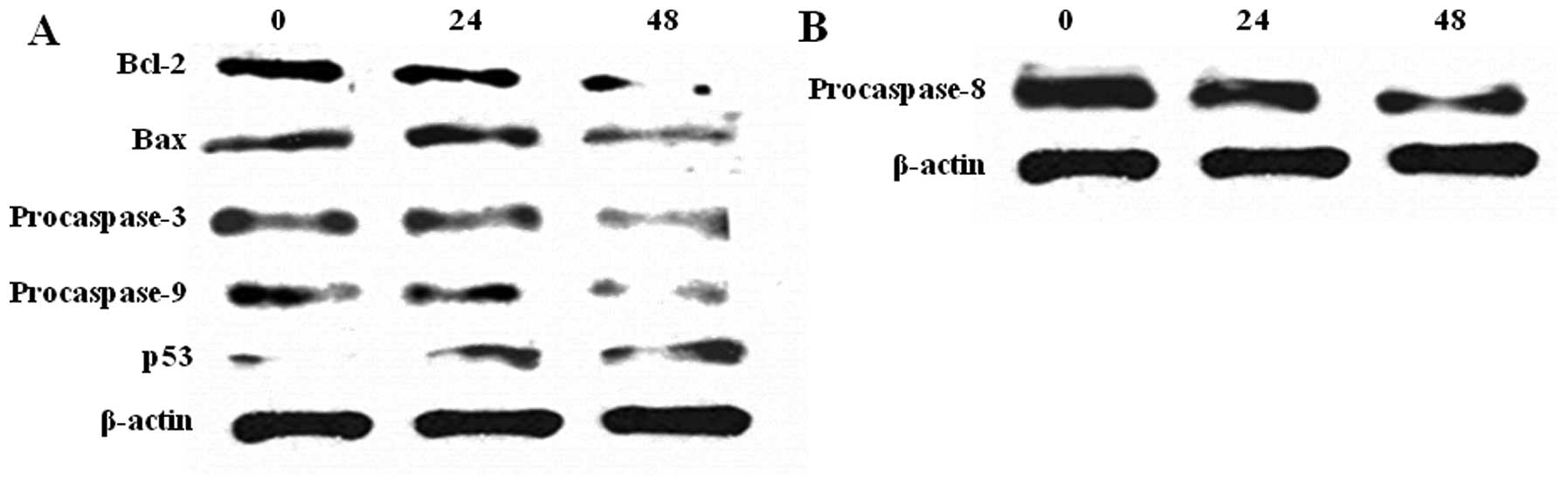
Analog 9 induces the
mitochondria-mediated apoptosis pathway in HeLa cells
Caspases play a central role in apoptosis, once
activated, they can activate other procaspases, allowing the
initiation of a protease cascade. In order to determine the
molecular mechanism of analog 9-induced apoptosis in HeLa cells, we
evaluated whether the caspase-dependent signaling pathway and
mitochondria-mediated pathway were involved in analog 9-induced
apoptosis. HeLa cells were treated with 15 μM analog 9
(according to the cytotoxicity data). The members of the Bcl-2
family play essential roles in initiating the mitochondria-mediated
pathway (33). Bcl-2 is known as
an antiapoptotic protein, and its levels were decreased in cells
treated with analog 9 in a time-dependent manner. Conversely, Bax
levels were increased in cells treated with analog 9 because it is
a proapoptotic protein (Fig. 8A).
Change in the levels of Bcl-2 family proteins results in the
release of cytochrome c from the mitochondria, which then
activates the downstream caspase program (21). When the levels of procaspases
decrease, the levels of caspases increase to initiate apoptosis. As
shown in Fig. 8A, the levels of
procaspase-3 and -9 decreased following treatment with analog 9;
therefore, we concluded that the levels of caspase-3 and -9 were
increased by analog 9. The levels of p53, a tumor suppressor
protein, showed a tendency to increase following treatment with
analog 9; therefore, we concluded that analog 9 suppressed the
levels of a tumor growth factor.
Analog 9 has the potential to induce the
Fas signaling apoptosis pathway
Caspase-8 is known for its role in the Fas signaling
apoptosis pathway. We analyzed procaspase-8 to assess whether
analog 9 could also trigger the extrinsic pathway. At the first
step of this pathway, Fas ligand binds to Fas protein, inducing Fas
to decrease procaspase-8 levels. When the levels of procaspase-8
are decreased, caspase-8 levels are increased. Activated caspase-8
can trigger other caspase proteins, especially caspase-3 (15). Therefore, analog 9 may trigger Fas
signaling apoptosis pathway by decreasing the levels of
procaspase-8 (Fig. 8B).
Discussion
Various chemotherapy drugs have been used to treat
cancer and many novel compounds are still being developed. Our
experimental findings showed that analog 9 induced cytotoxicity in
HeLa cells and inhibited their proliferation significantly after 36
h. As analog 9 had the strongest cytotoxic effect on HeLa cells
compared to the other compounds, we suggest that the methoxy group
of bispidinone compounds is important for cell cytotoxicity. Analog
9 inhibited the proliferation of HeLa cells at a low concentration;
therefore, we wanted to examine the underlying mechanism of cell
death.
Many methods have been developed to monitor the
different steps of apoptotic pathways (34,35).
We initially assessed apoptosis in HeLa cells by observing
morphological changes. The abnormal morphology of cells treated
with analog 9 showed an apoptotic pattern, including a reduction in
the volume, number, and fragmentation of HeLa cells. The Annexin
V-FITC/PI double staining assay suggested that analog 9 induced
early apoptosis in HeLa cells time-dependently. These data
indicated that analog 9 inhibited the proliferation of HeLa cells
and induced cytotoxicity by triggering apoptosis. However, the
enzyme activity of apoptotic and cell cycle regulators should be
characterized to clarify the mechanisms by which analog 9 induced
apoptosis. Since the activation and execution of apoptosis are
regulated by complex molecular mechanisms, numerous points of
interaction exist between the regulatory pathways of the cell cycle
and apoptosis (36). We observed
the accumulation of HeLa cells at the G1 phase at 24 h following
treatment with analog 9 (30,37).
On the basis of these results, we concluded that analog 9 induced
G1 phase arrest.
The [3H]-thymidine incorporation assay
suggested that analog 9 inhibited DNA replication; therefore, we
obtained evidence that analog 9 influenced the DNA levels in HeLa
cells. Through the process of apoptosis, any cell that is severely
damaged beyond the capacity of the DNA repair system or cannot be
repaired, is eliminated. Therefore, analog 9 induced DNA strand
breaks and inhibited the incorporation of [3H]-thymidine
before any cell cycle change had occurred. In conclusion, this
study provides experimental evidence that analog 9 induced
apoptosis in HeLa cells that was accompanied by the inhibition of
DNA replication.
To identify the underlying apoptotic pathway that
was induced by analog 9, we measured ΔΨm using Rh-123
staining and western blot analysis. Rh-123 staining indicated that
analog 9 disrupted ΔΨm. Western blot analysis was
performed to confirm this finding. The Bcl-2 family proteins Bax
and Bcl-2 play important roles in initiating the
mitochondria-mediated apoptotic pathway (33). Proapoptotic Bax translocates to the
mitochondria and integrates into the outer mitochondrial membrane,
where it promotes the release of cytochrome c into the
cytosol. In contrast, antiapoptotic Bcl-2 prevents this process by
preserving mitochondrial integrity. Thus, the ratio of Bax to Bcl-2
is crucial to the sustenance of drug-induced apoptosis in the
mitochondria-dependent apoptotic pathway (38). A recent study showed that analog 9
upregulated the expression of Bax and downregulated the expression
of Bcl-2. The release of mitochondrial cytochrome c
facilitates the formation of apoptosome complexes consisting of
APAF-1 and caspase-9, which subsequently activate caspases, e.g.,
caspase-3, resulting in apoptosis (21). In the present study, the activation
of caspase-3 and -9 was detected. In addition, the levels of p53, a
tumor suppressor protein, increased significantly. These results
showed that analog 9 induced apoptosis via the caspase- and
mitochondria-mediated pathways. In addition, we suggest the
possibility that analog 9 induced the extrinsic apoptotic
pathway.
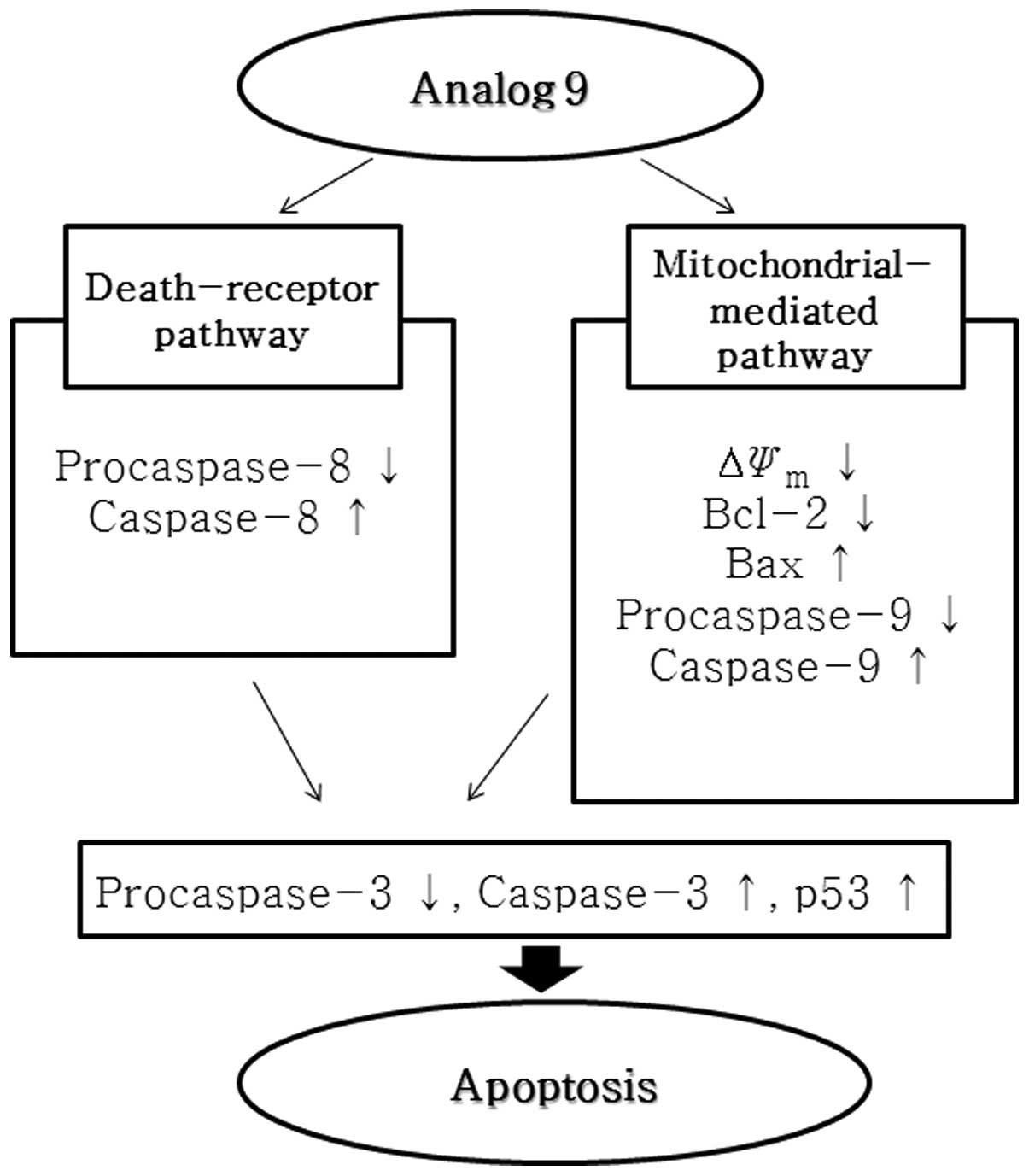
In conclusion, this study provides experimental
evidence that analog 9 induces apoptosis in HeLa cells via the
mitochondria- and caspase-mediated pathways, and may induce the
extrinsic pathway. Analog 9 also inhibits DNA replication in HeLa
cells and induces sub-G1 and G1 phase arrest in the HeLa cell
cycle. Finally, analog 9 also disrupts ΔΨm (Fig. 9).
To date, no study has assessed the anticancer
activity of bispidinone analogs, and this present study serves as
the first attempt to evaluate the action of analog 9 on HeLa cells.
These findings show that analog 9 should be studied as an
anticancer agent in future research.
Acknowledgements
This study was supported by the 2013
Inje University research grant.
References
|
1.
|
Balaiah V, Jeyaraman R and Chandrasekaran
L: Synthesis of 2,6-disubstituted piperidines, oxanes, and thianes.
Chem Rev. 83:3791983. View Article : Google Scholar
|
|
2.
|
Vijayakumar V and Sundaravadivelu M:
Synthesis and study on 2,4,6,8-tetraaryl-3,7-diazabicyclo[3.3.1].
Magn Reson Chem. 43:479–482. 2005.
|
|
3.
|
Juran S, Walther M, Stephan H, Bergmann R,
Steinbach J, Kraus W, Emmerling F and Comba P: Hexadentate
bispidine derivatives as versatile bifunctional chelate agents for
copper(II) radioisotopes. Bioconjug Chem. 20:347–359. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Joensuu H: Systemic chemotherapy for
cancer: from weapon to treatment. Lancet Oncol. 9:3042008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ziegler DS and Kung AL: Therapeutic
targeting of apoptosis pathways in cancer. Curr Opin Oncol.
20:97–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kurokawa M and Kornbluth S: Caspases and
kinases in a death grip. Cell. 138:838–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Desagher S and Martinou JC: Mitochondria
as the central control point of apoptosis. Trends Cell Biol.
10:369–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Cory S and Adams JM: The Bcl-2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Farrow NS and Brown R: New members of the
Bcl-2 family and their protein partners. Curr Opin Genet Develop.
6:45–49. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yang E, Zha J, Jockel J, Bosie LH,
Thompson CB and Korsmeyer SJ: Bad, a heterodimeric partner for
Bcl-xL and Bcl-2, displaces bax and promotes cell death. Cell.
80:285–291. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Verhagen AM, Ekert PG, Pakusch M, Silke J,
Connolly LM, Reid GE, Moritz RL, Simpson RJ and Vaux DL:
Identification of DIABLO, a mammalian protein that promotes
apoptosis by binding to and antagonizing IAP proteins. Cell.
102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar
|
|
15.
|
Kumar S and Vaux DL: Apoptosis. A
Cinderella caspase takes center stage. Science. 297:1290–1291.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
17.
|
Liu J, Shen M, Yue Z, Yang Z, Wang M, Li
C, Xin C, Wang Y, Mei Q and Wang Z: Triptolide inhibits
colon-rectal cancer cells proliferation by induction of G1 phase
arrest through upregulation of p21. Phytomedicine. 19:756–762.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lodish H, Berk A, Zipursky SL, Matsudaria
P, Baltimore D and Darnell J: Molecular Cell Biology. 4th edition.
W.H. Freeman; New York, NY: 2000
|
|
19.
|
Zhao J, Kang SRM, Zhang X, You S, Park JS,
Jung JH and Kim DK: Apoptotic activity of a new jasmonate analogue
is associated with its induction of DNA damage. Oncol Rep.
24:771–777. 2010.PubMed/NCBI
|
|
20.
|
Fisher DE: Apoptosis in cancer therapy:
crossing the threshold. Cell. 78:539–542. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tominaga H, Ishiyama M, Ohseto F, Sasamoto
K, Hamamoto T, Suzuki K and Watanabe M: A water-soluble tetrazolium
salt useful for colorimetric cell viability assay. Anal Commun.
36:47–50. 1999. View
Article : Google Scholar
|
|
23.
|
Zhang X, Zhao Z, Kang SRM, Yi MJ, You S,
Shin DS and Kim DK: A novel cromakalim analogue induces cell cycle
arrest and apoptosis in human cervical carcinoma HeLa cells through
the caspase- and mitochondria-dependent pathway. Int J Oncol.
39:1609–1617. 2011.
|
|
24.
|
Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH
and Lee WS: Magnolol suppresses proliferation of cultured human
colon and liver cancer cells by inhibiting DNA synthesis and
activating apoptosis. J Cell Biochem. 84:532–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Qi F, Li A, Zhao L, Xu H, Inagaki Y, Wang
D, Cui X, Gao B, Kokudo N, Nakata M and Tang W: Cinobufacini, an
aqueous extract from Bufo bufo gargarizans Cantor, induces
apoptosis through a mitochondria-mediated pathway in human
hepatocellular carcinoma cells. J Ethnopharmacol. 128:654–661.
2010.
|
|
26.
|
Zhang Y, Ahn EY, Jiang Y, Kim DK, Kang SG,
Wu C, Kang SW, Park JS, Son BW and Jung JH:
3-Chloro-2,5-dihydroxybenzyl alcohol activates human cervical
carcinoma HeLa cell apoptosis by inducing DNA damage. Int J Oncol.
31:1317–1323. 2007.PubMed/NCBI
|
|
27.
|
Sánchez I and Dynlacht BD: New insights
into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol.
16:311–321. 2005.PubMed/NCBI
|
|
28.
|
Lee S, Christakos S and Small MB:
Apoptosis and signal transduction: clues to a molecular mechanism.
Curr Opin Cell Biol. 5:286–291. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Dou QP: Putative roles of retinoblastoma
protein in apoptosis. Apoptosis. 2:5–18. 1997.PubMed/NCBI
|
|
30.
|
Smith DM, Gao G, Zhang X, Wang G and Dou
QP: Regulation of tumor cell apoptotic sensitivity during the cell
cycle (Review). Int J Mol Med. 6:503–507. 2000.PubMed/NCBI
|
|
31.
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar
|
|
32.
|
Del Bino G, Darzynkiewicz Z, Degraef C,
Mosselmans R, Fokan D and Galand P: Comparison of methods based on
annexin-V binding, DNA content or TUNEL for evaluating cell death
in HL-60 and adherent MCF-7 cells. Cell Prolif. 32:25–37.
1999.PubMed/NCBI
|
|
33.
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kiechle FL and Zhang X: Apoptosis:
biochemical aspects and clinical implications. Clin Chim Acta.
326:27–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Otsuki Y, Li Z and Shibata MA: Apoptotic
detection methods from morphology to gene. Prog Histochem Cytochem.
38:275–339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Orren DK, Petersen LN and Bohr VA:
Persistent DNA damage inhibits S-phase and G2 progression and
results in apoptosis. Mol Biol Cell. 8:1129–1142. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Qi F, Inagaki Y, Gao B, Cui X, Xu H,
Kokudo N, Li A and Tang W: Bufalin and cinobufagin induce apoptosis
of human hepatocellular carcinoma cells via Fas- and
mitochondria-mediated pathways. Cancer Sci. 102:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|