Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related death in men and women (1). Up to 85% of patients are diagnosed at
an advanced stage when the tumor is unresectable because of
infiltration of local arteries or distant metastasis (2). Gemcitabine has been the standard of
care for the treatment of advanced pancreatic cancer based on a
randomized trial showing an improvement in the one-year survival
with gemcitabine compared to 5-fluorouracil (5-FU) (3). Although well-tolerated, the efficacy
of gemcitabine is modest, with reported response rates (RR) of 10%
and median survival of less than 7 months (3). The combination of gemcitabine with a
variety of cytotoxic and targeted agents has generally shown no
significant survival advantage as compared to gemcitabine alone
(4). Some studies have suggested a
significant benefit in overall survival (OS) and progression-free
survival (PFS) associated with gemcitabine-based cytotoxic
combinations in patients with good performance status (5,6). An
impressive efficacy and longer median OS of 10.2 months was first
reported in a phase II study in patients with advanced pancreatic
cancer under treatment with the combination regimen FOLFIRINOX
(oxaliplatin, irinotecan, 5-FU and leucovorin) (7). Subsequently, FOLFIRINOX was compared
to gemcitabine in a randomized phase III trial in patients with
advanced pancreatic cancer (8).
This study confirmed statistically significant and clinically
relevant improvements in OS (11.1 vs. 6.8 months), PFS (6.4 vs. 3.3
months) and RR (31.6 vs. 9.4%) with FOLFIRINOX compared to
gemcitabine alone. Because of significant higher grade 3 and 4
toxicities (mostly neutropenia, diarrhea, febrile neutropenia,
thrombocytopenia, sensory neuropathy) with FOLFIRINOX and the
limited survival time of patients with advanced pancreatic cancer,
biomarkers allowing a better patient selection are required.
Therefore, the aim of our study was to assess clinical (age, body
mass index, gender), histopathological [histology, grading, tumor
location, T-stage, levels of carboanhydrase 19.9 (CA19.9) and
carcinoembryogenic antigen (CEA), p53 and Ki67 expression]
parameters of patients with advanced pancreatic cancer treated with
FOLFIRINOX and their association with response to treatment.
Materials and methods
Study design and assessment of
response
We conducted a retrospective review of all patients
with either primarily locally advanced, metastatic or recurrent
unresectable pancreatic cancer treated with FOLFIRINOX at our
department between October, 2010 and November, 2012. We evaluated
patient and tumor characteristics such as gender, age, age adapted
body mass index (BMI), performance status, T-stage according to the
TNM classification, initial tumor localization (the head vs. the
body, tail or the papilla of the pancreas) and primary metastases
(hepatic, peritoneal, pulmonal) or localization of relapse (local
vs. visceral metastasis) based on review of the patients’ medical
records. Response was assessed by review of the patients imaging
studies according to RECIST criteria as complete (CR), partial
response (PR), stable disease (SD) or progressive disease (PD) as
well as by tumor marker response of CA19.9 (9). Thus, a decrease or increase in CA19.9
by 50% compared to baseline after treatment was classified as PR or
PD, respectively. The objective response rate was defined as the
percentage of patients with CR and PR. The disease control rate was
defined as the percentage of patients with CR, PR and SD (Table I).
 | Table I.Demographic and baseline
characteristics of all the patients. |
Table I.
Demographic and baseline
characteristics of all the patients.
| Characteristics | |
|---|
| Gender, no. (%) | |
| Male | 24 (49) |
| Female | 25 (51) |
| Age, years | |
| Median | 62 |
| Range | 42–76 |
| BMI | |
| Median | 22.3 |
| Range | 17.2–34.8 |
| ECOG performance
score 0, no. (%) | 49 (100) |
| Histology, no.
(%) | |
| Adenocarcinoma | 42 (95.5) |
| Giant cell
carcinoma | 1 (2.3) |
| Adenosquamous
carcinoma | 1 (2.3) |
| No histology | 5 (10) |
| Grading | |
| 2 | 28 (65.1) |
| 3 | 15 (34.9) |
| Pancreatic tumor
localization, no. (%) | |
| Head | 24 (49.0) |
| Body | 7 (14.3) |
| Tail | 16 (32.7) |
| Papilla vateri | 2 (4.1) |
| T-stage | |
| 2 | 3 (6.7) |
| 3 | 26 (57.8) |
| 4 | 16 (35.6) |
| Non-metastatic
disease, no. (%) | 6 (12.2) |
| Metastatic disease,
no. (%) | 28 (57.1) |
| Metastatic sites | |
| Liver | 15 (53.6) |
|
Liver/peritoneum | 8 (28.6) |
| Lung | 4 (14.3) |
| Liver/lung | 4 (14.3) |
| Relapsed disease,
no (%) | 15 (30.6) |
| Locoregional | 1 (6.7) |
| Visceral (liver,
lung, periteonal) | 7 (46.7) |
| Locoregional and
visceral | 7 (46.7) |
Treatment
Full dose FOLFIRINOX consisted of oxaliplatin 85
mg/m2 over 3 h, followed by irinotecan 180
mg/m2 over 90 min and leucovorin 400 mg/m2
over 2 h, followed by 5-FU 400 mg/m2 as a bolus and
2,400 mg/m2 as a 46 h continuous infusion. Dose
modifications due to toxicity were made according to the discretion
of the treating physician. Treatment was discontinued in case of
unacceptable toxicity, progression of disease or pursuit of
alternative therapies including surgical resection or radiotherapy
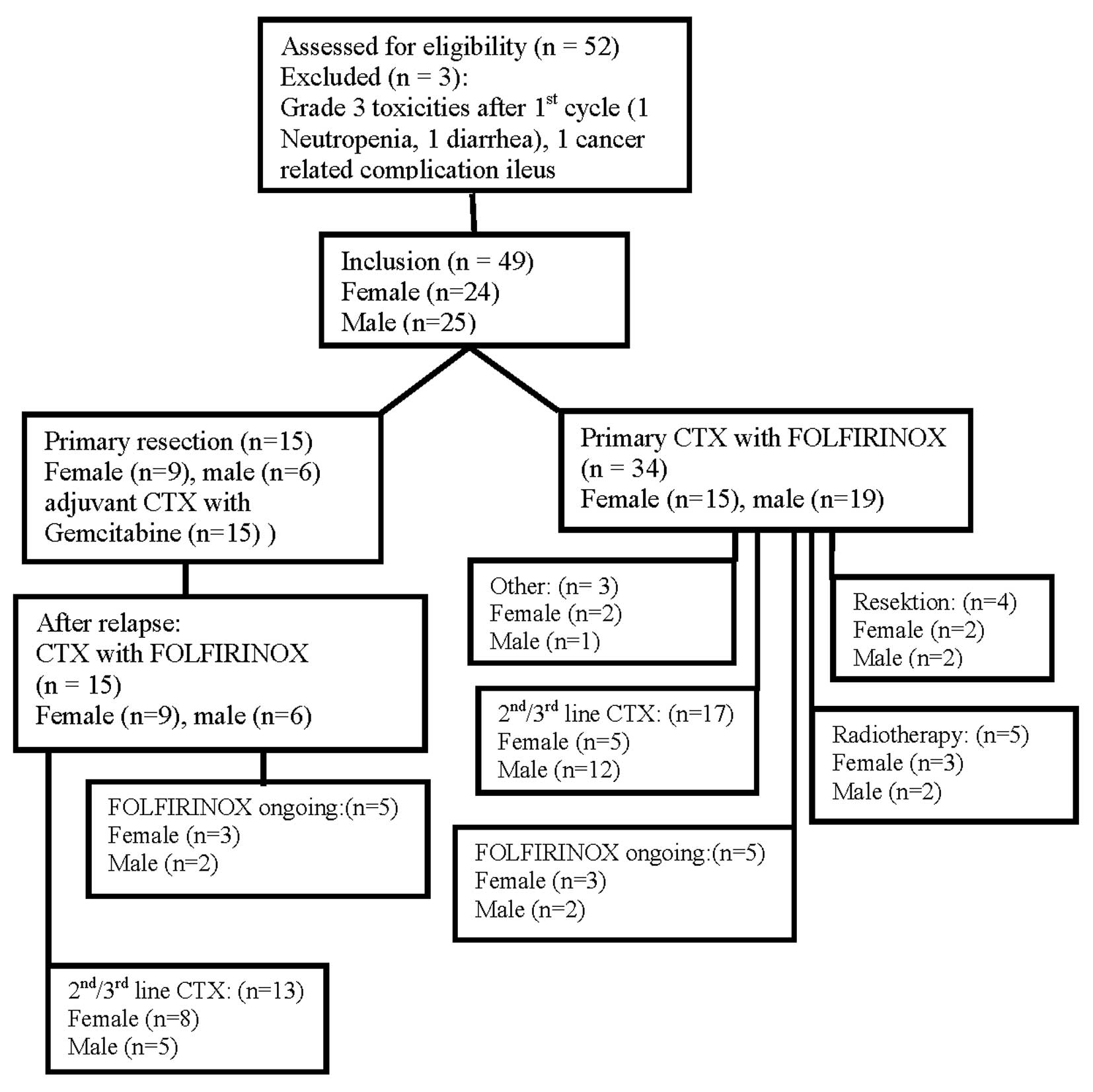
for locally advanced disease (Fig.
1).
Immunohistochemistry
Immunohistochemical staining for Ki67 and p53
protein was performed using the antibodies monoclonal mouse
anti-human Ki67 (clone MIB-1, Dako, Glostrup, Denmark) at a working
dilution of 1:500 and monoclonal mouse anti-human p53 (clone DO-7,
Dako) at a working dilution of 1:200 on routinely formalin-fixed
paraffin embedded (FFPE) tissue, using a standardized automated
platform (AutostainerPlus, Dako) in combination with Envision
polymer detection system (Dako). Archival FFPE sections (3
μm) were deparaffinized in combination with heat-induced
epitope retrieval (HIER) at 98°C for 40 min in antigen retrieval
buffer pH 9.0 (Dako) in a PT Link™-module (Dako). Endogenous
peroxidase blocking was carried out for 10 min with 3%
H2O2 in absolute methanol and normal serum
was applied. Primary antibodies and detection reagents were
incubated at RT for 30 min and after several washes detection was
performed using EnvisionFlex™ detection system, followed by
chromogenic visualisation with diaminobenzidine (DAB) for 10 min.
Nuclear counterstaining was performed with hematoxylin. Assessment
of Ki67 and p53 staining was performed according to the
recommendations of the International Ki67 in Breast Cancer working
group (10). At least three
high-power (×40 objective) fields and clear hot spots were selected
to represent the spectrum of staining seen and 100 cancer cells
were evaluated.
CA19.9 and CEA measurement
CA19.9 and CEA measurements were carried out at a
certified laboratory associated with the hospital by an
electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim,
Germany). The upper limit normally used for CA19.9 was 37 U/ml and
for CEA 3.5 μg/l. Serum levels of CA19.9 and CEA were
measured before therapy and every second week during therapy.
Statistics
Baseline and clinicopathological characteristics
were summarized as median and range for continuous variables and as
absolute and relative frequencies for categorical variables.
Comparisons in regard to gender and therapy response were either
performed with the Kruskal-Wallis, the Mann-Whitney U or the
χ2 test. P-values <0.05 were considered to indicate
statistical significance. Tumor markers were correlated with each
other using Spearman rank correlation coefficient. A multivariate
logistic regression model was performed to assess the joint effect
of gender, age and clinical variables such as tumor stage, line of
treatment (primary treatment vs. treatment in the relapsed stage)
and biomarkers such as Ki67 and p53 on the response rate. OS and
PFS were estimated using the Kaplan-Meier method together with the
log-rank test. As survival measures, median and 95% confidence
intervals are given.
Results
Characteristics of the patients
Between October 2010 and November 2012 a total of 52
patients with either locally advanced non-metastatic (n=6),
metastatic (n=31) or relapsed unresectable pancreatic cancer after
initial resection (n=15) were treated with FOLFIRINOX at our
department (Fig. 1). Two patients
discontinued CTX after the first application due to grade 3
toxicities (1 febrile neutropenia, 1 diarrhea) or cancer related
complications (1 patient with ileus) and therefore were excluded
from our analysis. Thus, a total of 49 patients could finally be
included in our study. Demographic and tumor parameters were
equally distributed between both genders (Table II). Although female patients showed
higher numbers of metastatic tumors (15 vs. 13) and no locally
advanced non-metastatic tumors before treatment compared to males
(0 vs. 6 months) (p=0.095), they were characterized by significant
higher levels of CA19.9 (p=0.037) and CEA (p=0.05).
 | Table II.Demographic and baseline
characteristics of female vs. male patients. |
Table II.
Demographic and baseline
characteristics of female vs. male patients.
|
Characteristics | Female (n=24) | Male (n=25) | P-value |
|---|
| Age, years | | | |
| Median | 61 | 63 | 0.603 |
| Range | 42–73 | 45–76 | |
| BMI | | | |
| Median | 22.3 | 22.9 | 0.379 |
| Range | 17.2–34.8 | 17.3–30-7 | |
| Level of
carbohydrate antigen 19.9 | | | |
| Median | 2494 | 185 | 0.037 |
| Range | 0–140241 | 0–32266 | |
| Level of
carcinoembryogenic antigen | | | |
| Median | 10.4 | 5.7 | 0.05 |
| Range | 2–363 | 2–306 | |
| Level of Ki-67
positive cancer cells (%) | | | |
| Median | 30 | 20 | 0.457 |
| Range | 1–80 | 1–70 | |
| Level of mutant p53
cancer cells (%) | | | |
| Median | 30 | 1 | 0.147 |
| Range | 1–100 | 0–90 | |
| Histology, no.
(%) | | | |
|
Adenocarcinoma | 22 (100) | 20 (90.9) | 0.351 |
| Giant cell
carcinoma | 0 (0) | 1 (4.5) | |
| Adenosquamous
carcinoma | 0 (0) | 1 (4.5) | |
| Grading | | | |
| 2 | 14 (63.6) | 14 (66.7) | 0.835 |
| 3 | 8 (36.4) | 7 (33.3) | |
| Pancreatic tumor
location, no. (%) | | | |
| Head | 12 (50) | 12 (48.0) | 0.946 |
| Body | 4 (16.7) | 3 (12.0) | |
| Tail | 7 (29.2) | 9 (36.0) | |
| Papilla
Vateri | 1 (4.2) | 1 (4.0) | |
| T-stage | | | |
| 2 | 1 (4.8) | 2 (8.3) | 0.063 |
| 3 | 16 (76.2) | 10 (41.7) | |
| 4 | 4 (19) | 12 (50.0) | |
| Non-metastatic
disease, no. (%) | 0 (0) | 6 (24.0) | |
| Metastatic disease,
no. (%) | 15 (62.5) | 13 (52.0) | |
| Metastatic sites,
no. (%) | | | |
| Liver | 9 (60.0) | 6 (46.2) | 0.466 |
|
Liver/peritoneum | 4 (26.7) | 4 (30.7) | |
| Lung | 2 (13.3) | 2 (15.4) | |
| Liver/lung | 3 (20.0) | 1 (7.7) | |
| Relapsed disease,
no (%) | 9 (37.5) | 6 (24.0) | 0.629 |
| Locoregional | 1 (11.1) | 0 (0.0) | |
| Visceral (liver,
lung, periteonal) | 4 (44.4) | 3 (50.0) | |
|
Locoregional/visceral | 4 (44.4) | 3 (50.0) | |
Efficacy of FOLFIRINOX in the whole study
population and according to gender
Based on the imaging studies of the patients as well
as tumor marker response we confirmed a PR and SD in 27 (55.1%) and
7 (14.3%) patients, respectively. There were no complete remissions
(CR). Fifteen out of 49 patients (30.6%) patients did not respond
to a CTX with FOLFIRINOX and were classified as PD (Table III). When response rates were
analyzed according to gender, a significantly higher objective
response rate, defined as patients with either CR or PR, of 75 vs.
36% (p=0.004) as well as a higher disease control rate defined as
patients with either PR or SD of 91.7 vs. 48% (p=0.001) was seen in
female patients compared to male patients (Table III). The predictive effect of
gender on the response rate remained statistically significant
(p=0.041) in the multivariate logistic regression analysis
adjusting for age, tumor stage, line of treatment, Ki67 and p53.
Two female patients with metastatic disease as well as 2 male
patients with locally advanced disease were able to undergo
resection (Fig. 1).
 | Table III.Response rates in all patients and in
patients allocated according to gender (female vs. male). |
Table III.
Response rates in all patients and in
patients allocated according to gender (female vs. male).
| Variable | Total (n=49) | | |
|---|
| Response, no.
(%) | | | |
| CR | 0 (0) | | |
| PR | 27 (55.1) | | |
| SD | 7 (14.3) | | |
| PD | 15 (30.6) | | |
| Rate of disease
control (PR + SD) | | | |
| No. (%) | 34 (69.4) | | |
|
| Variable | Female (n=24) | Male (n=25) | P-value |
|
| Response, no.
(%) | | | |
| PR | 18 (75) | 9 (36) | 0.004 |
| SD | 4 (16.7) | 3 (12) | |
| PD | 2 (8.3) | 15 (52) | |
| Rate of disease
control (PR + SD) | | | |
| No. (%) | 22 (91.7) | 12 (48) | 0.001 |
Efficacy of FOLFIRINOX in primarily
treated and relapsed pancreatic carcinomas
Disease control rates in the whole study population
did not differ significantly when RRs were assessed in untreated
patients compared to patients after surgical resection and
treatment in the relapsed stage (70.6 vs. 66.7%). However, when
patients were analyzed according to gender and line of treatment,
in women FOLFIRINOX caused a disease control rate of 100% in the
first-line setting compared to 77.8% in the relapsed stage
(p=0.057). Male patients showed a significantly lower disease
control rate of 47.4% after first-line treatment than females
(p=0.001). Their response rates in first-line and treatment in the
relapsed stage (50%) did not differ (p=0.670) (Table IV).
 | Table IV.Efficacy of FOLFIRINOX in primary and
relapsed pancreatic carcinomas. |
Table IV.
Efficacy of FOLFIRINOX in primary and
relapsed pancreatic carcinomas.
| Primary
treatment | All patients
(n=34) | | P-value |
|---|
| Response, no.
(%) | | | |
| PR+SD | 24 (70.6) | |
| PD | 10 (29.4) | |
|
| Female (n=15) | Male (n=19) | |
|
| Response, no.
(%) | | | |
| PR+SD | 15 (100) | 9 (47.4) | 0.001 |
| PD | 0 (0) | 10 (52.6) | |
|
| Treatment relapsed
stage | All patients
(n=15) | | P-value |
|
| Response, no.
(%) | | | |
| PR+SD | 10 (66.7) | |
| PD | 5 (33.3) | |
|
| Female (n=9) | Male (n=6) | |
|
| Response, no.
(%) | | | |
| PR+SD | 7 (77.8) | 3 (50) | 0.064 |
| PD | 2 (22.2) | 3 (50) | |
Progression-free and overall
survival
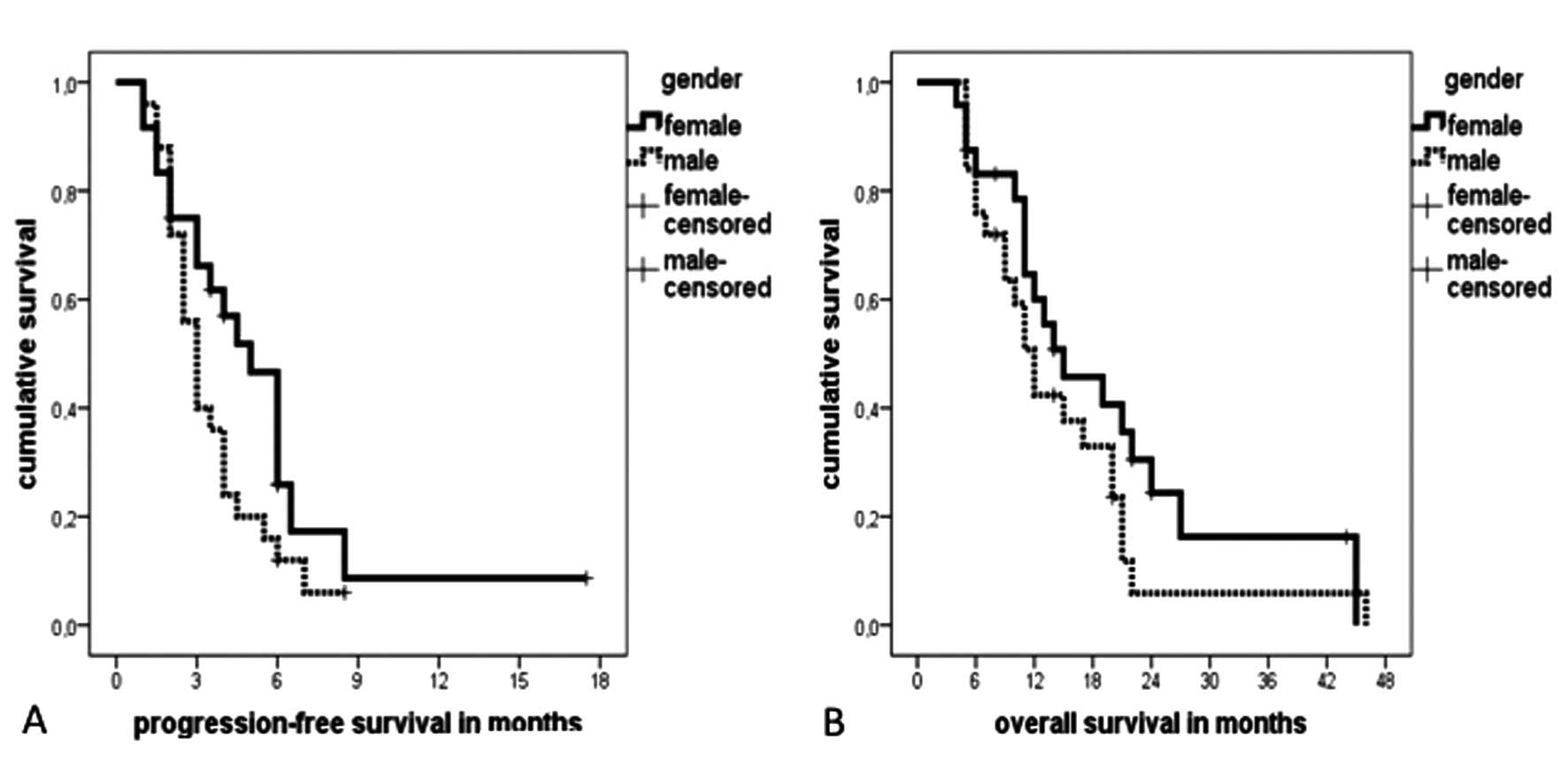
Survival data are shown in Table V and Fig. 2. For the entire cohort of patients
the median PFS was 3.5 months (95% CI, 2.7–4.3 months) with a
median OS of 13 months (95% CI, 9.4–16.6 months). Despite
significantly higher response rates, female patients showed only a
tendency towards a longer median PFS of 5 months compared to 3
months in male patients (p=0.09) (Fig.
2A). The median OS did not significantly differ between female
(15 months, 95% CI, 6.5–23.5 months) and male patients (12 months,
95% CI, 9.7–14.3 months) (p=0.20) (Fig. 2B).
 | Table V.Median progression-free and overall
survival. |
Table V.
Median progression-free and overall
survival.
| Progression-free
survival median, months (95% CI) | Overall survival
median, months (95% CI) |
|---|
| Overall study
population | | |
| All patients
(n=49) | 3.5 (2.7–4.3) | 13 (9.4–16.6) |
| Female
(n=25) | 5.0 (3.6–6.4) | 15 (6.5–23.5) |
| Male (n=24) | 3.0 (2.4–3.6) | 12 (9.7–14.3) |
| Primary
treatment | | |
| All patients
(n=34) | 4.0 (2.7–5.3) | 11 (9.9–12.0) |
| Female
(n=15) | 5.0 (3.5–6.5) | 11 (9.3–12.7) |
| Male (n=19) | 3.0 (2.3–3.7) | 11 (1.3–8.4) |
| Treatment relapsed
stage | | |
| All patients
(n=15) | 3.0 (1.6–4.4) | 21 (19.1–22.9) |
| Female (n=9) | 3.5 (2.0–5.0) | 22 (19.1–24.9) |
| Male (n=6) | 2.0 (n.a.) | 20 (12.8–27.2) |
Clinicopathological characteristics and
their association with response to treatment with FOLFIRINOX
There was no significant associations of either age,
BMI, serum levels of CA19.9 and CEA or expression of p53 and Ki67
in pancreatic tumors with response to FOLFIRINOX (Table VI). However, tumors of patients
with PR to FOLFIRINOX showed a tendency towards a higher expression
of Ki67 and p53 as well as higher levels of CA19.9 compared to
resistant tumors. In univariate analysis CA19.9 levels were
significantly associated with the expression of Ki67 (p=0.017,
r=0.39) (data not shown).
 | Table VI.Clinicopathological characteristics
and their association with response to treatment with
FOLFIRINOX. |
Table VI.
Clinicopathological characteristics
and their association with response to treatment with
FOLFIRINOX.
| Variable | PR | PD | P-value |
|---|
| Ki-67 expression
(%) | | | |
| Median | 25 | 17.5 | 0.328 |
| Range | 1–80 | 1–70 | |
| Mutant p53
expression (%) | | | |
| Median | 20 | 5.5 | 0.422 |
| Range | 0–100 | 1–90 | |
| Level of
carbohydrate antigen 19.9 | | | |
| Median | 1612 | 1322 | 0.529 |
| Range | 1–140241 | 0–32266 | |
| Level of
carcino-embroygenic antigen | | | |
| Median | 7 | 7.45 | 0.752 |
| Range | 2–363 | 2–306 | |
| Age, years | | | |
| Median | 61 | 63 | 0.783 |
| Range | 42–73 | 46–69 | |
| Body mass
index | | | |
| Median | 21.8 | 23 | 0.312 |
| Range | 17.2–30.7 | 18.2–30.7 | |
Discussion
Most patients with pancreatic cancer are either
unresectable at diagnosis or will suffer from a relapse after
initial surgery, resulting in a 5-year OS of 6% (11). The median OS of patients with
advanced or metastatic pancreatic cancer is 6 months with most
chemotherapeutic options (4).
Treatment with FOLFIRINOX has offered a new option for patients
with advanced pancreatic cancer and a good performance status
(8). However, it was reported that
a considerable percentage of patients, up to 50%, treated with
FOLFIRINOX experienced grade 3 and 4 toxicities (7,8).
Understanding the features that determine response or resistance to
this chemotherapy regimen could permit the selection of the most
suitable patients for treatment with FOLFIRINOX. Thus, in our
study, we evaluated clinical and histological markers and their
associations with response to FOLFIRINOX.
The objective response rate, defined as the
percentage of patients with CR and PR, was slightly higher (55.1%)
in our overall study population compared to the 32% reported by
Conroy et al (8). This
might be explained by the fact that in our highly selected study
population all patients (100%) exhibited an ECOG performance status
(PS) 0 whereas the majority (61%) of patients in the historical
group of Conroy et al had an ECOG PS 1. Higher response
rates of 45% compared to the historical data of Conroy et al
were also obtained in another retrospective analysis performed by
Gunturu et al (12). This
study population also consisted of a higher percentage of patients
(69%) with an ECOG PS 0. The percentage of patients with SD
reported by Conroy et al was higher compared to our study
population (39 vs. 14.3%). Therefore, a similar disease control
rate, defined as percentage of patients with CR, PR or SD, could be
seen in our study compared to historical data (69.4 vs. 70%)
(8).
When RRs were assessed according to gender a highly
significant difference in response to FOLFIRINOX could be seen
between the female and male treatment groups. As shown in Table III, there was a significant higher
percentage of female patients with PR (75%) compared to males (36%)
(p=0.004) leading to a significantly (p=0.001) higher disease
control rate in female patients (91.7 vs. 48.0%). To our knowledge,
this is the first study reporting different response rates to CTX
according to gender in patients with pancreatic cancer. Although we
could see a tendency towards a longer median PFS in female patients
(5 months) compared to males (3 months) (p=0.099) significantly
higher response rates did not cause a significant difference in the
median OS between female and male patients (15 vs. 12 months)
(p=0.200) (Table V, Fig. 2). This might be explained by the
small sample size as well as the fact, that there was a higher
number of locally advanced non-metastatic tumors in male patients
compared to females (6 vs. 0) (p=0.095) (Table II). Patients with locally advanced
pancreatic tumors are known to have a longer median OS of 15.7
months than patients with metastatic pancreatic tumors (9.5 months)
(7). Thus, the higher number of
locally advanced pancreatic tumors in male patients might have
nullified the benefit of higher response rates in female patients.
Retrospective analyses should be viewed with caution and causes for
the improved RR to FOLFIRINOX in the female study population remain
speculative. Demographic and baseline disease characteristics were
well-balanced and did not differ significantly between the female
and male group in our study. CA19.9 is the most extensively studied
and validated biomarker for the diagnosis of pancreatic cancer
(13). Despite its limitations as
false negative results in sialyl Lewis negative individuals and
false positive elevation in the presence of obstructive jaundice
CA19.9 serum levels as well as a reduction in CA19.9 levels during
treatment have prognostic and predictive value (13). It has been reported that patients
with pretreatment levels lower than median showed a better tumor
response to neoadjuvant radiochemotherapy with 5-FU or adjuvant CTX
with gemcitabine (14,15). There are no data so far regarding
the predictive value of pretreatment levels of CA19.9 and response
to CTX with FOLFIRINOX. In our patients serum levels of CA19.9
(p=0.037) and CEA (0.05) were significantly higher in female
patients compared to males (Table
II). There was a tendency of higher serum levels of CA19.9
(median 1,612 vs. 1,322 U/ml) in tumors of objective responders
(PR) compared to patients with PD (Table V). However, the difference did not
reach significance probably because of the limited sample size.
While an association of response to CTX and the rate of Ki67 in
breast cancer as well some gastrointestinal cancers has been
reported, data in pancreatic cancer for Ki67 as a potential
predictive marker for response CTX cancer are lacking (16–19).
The proliferation rate of tumor cells as defined by Ki67 was higher
in responders vs. non-responders (median 25.0 vs. 17.5% positive
cells) in our patients. In univariate analysis of our cohort,
CA19.9 serum levels were significantly associated with the
expression of Ki67 in univariate analysis (p=0.017, r=0.39, data
not shown).
Despite its central role in the control of
apoptosis, senescence and cell cycle arrest, the tumor suppressor
protein p53 remains an enigma for its possible role in predicting
response to chemotherapy in cancer patients. Mutant p53 proteins
generally have an increased stability and accumulate in the nucleus
of tumor cells and may be detectable by immunohistochemistry. It is
believed that this is a consequence of the inactive p53 mutant
protein not inducing the MDM2 protein required to target its own
degradation (20). The status of
p53 is associated with cancer cell resistance to various
chemotherapeutics (21,22). Wild-type p53 is thought to render
tumors more sensitive to treatment by the induction of apoptosis,
and p53 inactivation may lead to resistance to treatment. However,
because p53 is also responsible for prolonged cell cycle arrest
after chemotherapy-induced genetic damage, it is expected to
facilitate DNA repair in the absence of an apoptotic response.
Therefore tumors that inactivate p53 might be less capable of DNA
repair and more sensitive to a DNA damage-induced mitotic
catastrophe as reported in gastric cancer (23). In our cohort of patients p53
staining was increased in responders as compared to non-responders
(20.0 vs. 5.5% positive cells, Table
VI), although this difference was not statistically
significant. The role of p53 in predicting response to FOLFIRINOX
in pancreatic cancer patients will have to be studied in a larger
cohort of patients.
The results of our study indicate, that female
gender could positively predict response to FOLFIRINOX in patients
with advanced pancreatic cancer. The predictive value of serum
levels of CA19.9 and expression of p53 and Ki67 in advanced
pancreatic tumors require further substantiation.
Abbreviations:
|
CTX
|
chemotherapy
|
|
BMI
|
body mass index
|
|
PR
|
partial remission
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
APC
|
advanced pancreatic cancer
|
|
CEA
|
carcinoembryogenic antigen
|
|
CA19.9
|
carboanhydrase 19.9
|
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Lefebvre AC, Maurel J, Boutreux S, et al:
Pancreatic cancer: incidence, treatment and survival trends - 1175
cases in Calvados (France) from 1978 to 2002. Gastroenterol Clin
Biol. 33:1045–1051. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Burris HA III, Moore MJ, Andersen J, et
al: Improvements in survival and clinical benefit with gemcitabine
as first-line therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
4.
|
Di Marco M, Di Cicilia R, Macchini M, et
al: Metastatic pancreatic cancer: is gemcitabine still the best
standard treatment? (Review). Oncol Rep. 23:1183–1192.
2010.PubMed/NCBI
|
|
5.
|
Cunningham D, Chau I, Stocken DD, et al:
Phase III randomized comparison of gemcitabine versus gemcitabine
plus capecitabine in patients with advanced pancreatic cancer. J
Clin Oncol. 27:5513–5518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
7.
|
Conroy T, Paillot B, Francois E, et al:
Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil
in advanced pancreatic cancer - a Groupe Tumeurs Digestives of the
Federation Nationale des Centres de Lutte Contre le Cancer study. J
Clin Oncol. 23:1228–1236. 2005. View Article : Google Scholar
|
|
8.
|
Conroy T, Desseigne F, Ychou M, et al:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
10.
|
Dowsett M, Nielsen TO, A’Hern R, et al:
Assessment of Ki67 in breast cancer: recommendations from the
International Ki67 in Breast Cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sant M, Allemani C, Santaquilani M, Knijn
A, Marchesi F and Capocaccia R; EUROCARE-4: Survival of cancer
patients diagnosed in 1995–1999 Results and commentary. Eur J
Cancer. 45:931–991. 2009.
|
|
12.
|
Gunturu KS, Yao X, Cong X, et al:
FOLFIRINOX for locally advanced and metastatic pancreatic cancer:
single institution retrospective review of efficacy and toxicity.
Med Oncol. 30:3612013. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ballehaninna UK and Chamberlain RS: The
clinical utility of serum CA 19-9 in the diagnosis, prognosis and
management of pancreatic adenocarcinoma: An evidence based
appraisal. J Gastrointest Oncol. 3:105–119. 2012.PubMed/NCBI
|
|
14.
|
Micke O, Bruns F, Kurowski R, et al:
Predictive value of carbohydrate antigen 19-9 in pancreatic cancer
treated with radiochemotherapy. Int J Radiat Oncol Biol Phys.
57:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Humphris JL, Chang DK, Johns AL, et al:
The prognostic and predictive value of serum CA19.9 in pancreatic
cancer. Ann Oncol. 23:1713–1722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Fasching PA, Heusinger K, Haeberle L, et
al: Ki67, chemo-therapy response, and prognosis in breast cancer
patients receiving neoadjuvant treatment. BMC Cancer. 11:4862011.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhang GC, Qian XK, Guo ZB, et al:
Pre-treatment hormonal receptor status and Ki67 index predict
pathologic complete response to neoadjuvant trastuzumab/taxanes but
not disease-free survival in HER2-positive breast cancer patients.
Med Oncol. 29:3222–3231. 2012. View Article : Google Scholar
|
|
18.
|
Carlomagno C, Pepe S, D’Armiento FP, et
al: Predictive factors of complete response to neoadjuvant
chemoradiotherapy in patients with rectal cancer. Oncology.
78:369–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ressiot E, Dahan L, Liprandi A, et al:
Predictive factors of the response to chemoradiotherapy in
esophageal cancer. Gastroenterol Clin Biol. 32:567–577. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Soussi T: p53 alterations in human cancer:
more questions than answers. Oncogene. 26:2145–2156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sax JK and El-Deiry WS: p53 downstream
targets and chemo-sensitivity. Cell Death Differ. 10:413–417. 2003.
View Article : Google Scholar
|
|
22.
|
Bertheau P, Espie M, Turpin E, et al: TP53
status and response to chemotherapy in breast cancer. Pathobiology.
75:132–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bataille F, Rummele P, Dietmaier W, et al:
Alterations in p53 predict response to preoperative high dose
chemotherapy in patients with gastric cancer. Mol Pathol.
56:286–292. 2003. View Article : Google Scholar : PubMed/NCBI
|