Contents
Epidemiological evidence
Cancer stem cell niche
Niche-driven tumor progression
Cancer-associated fibroblasts and tumor
microenvironment
Tumor-driven niche development
Drug resistance
Conclusions
Epidemiological evidence
The use of electric lights means that people in the
modern era are exposed to long photoperiods throughout the year.
This disruption to the circadian system can induce a wide variety
of stresses. Abnormal circadian rhythms, including through exposure
to light at night, have been associated with an increased risk of
tumorigenesis and a poorer prognosis in carcinomas (1,2).
This suggests that the incidence of cancer may continue to
increase, in association with the stresses of modern life. The
risks of both breast and prostate cancer are high in industrialized
societies (3). Continuous hormonal
unbalances in shift workers might be caused by circadian disruption
and may contribute to the rising risk of cancer. Endocrine target
organs, such as the breast and prostate, are thought to be
susceptible to psychosocial stresses, including circadian
disruption (4). The immunological
surveillance system has also been shown to be affected, and may
thus not eliminate cancer stem cells (CSCs) effectively (5).
Circadian genes have been shown to function as
oncogenes or tumor suppressors at systemic or cellular levels
through their involvement in cell proliferation, cell cycle
regulation, apoptosis and DNA damage signaling (6,7),
indicating a direct effect of circadian effects on cancer cells.
However, the kinds of molecular and systemic mechanisms involved in
tumor growth under artificial illumination stress remain unknown,
and the importance of artificial illumination in promoting tumor
growth also needs to be established. We propose an indirect
mechanism supporting the survival of potential CSCs and discuss a
new concept of tumor niche formation induced by circadian
disruption.
Cancer stem cell niche
Clinical tumors comprise a heterogeneous cell
population including CSCs (8). The
malignant phenotype depends not only on the characteristics of the
cancer cell itself, but also on the tumor microenvironment. CSCs
have to survive for a long time in the body to generate the highly
tumorigenic cells responsible for the clinical manifestations of
cancer. During this period, the niche helps to shelter CSCs from
various insults such as the immune response, and from genotoxic
stresses such as chemotherapy (9,10).
This suggests that the niche may also play a protective role for
CSCs, and may thus contribute to the risk of cancer.
Niche-driven tumor progression
Although CSCs appear relatively frequently, they are
unable to survive in the healthy body without supportive tissues.
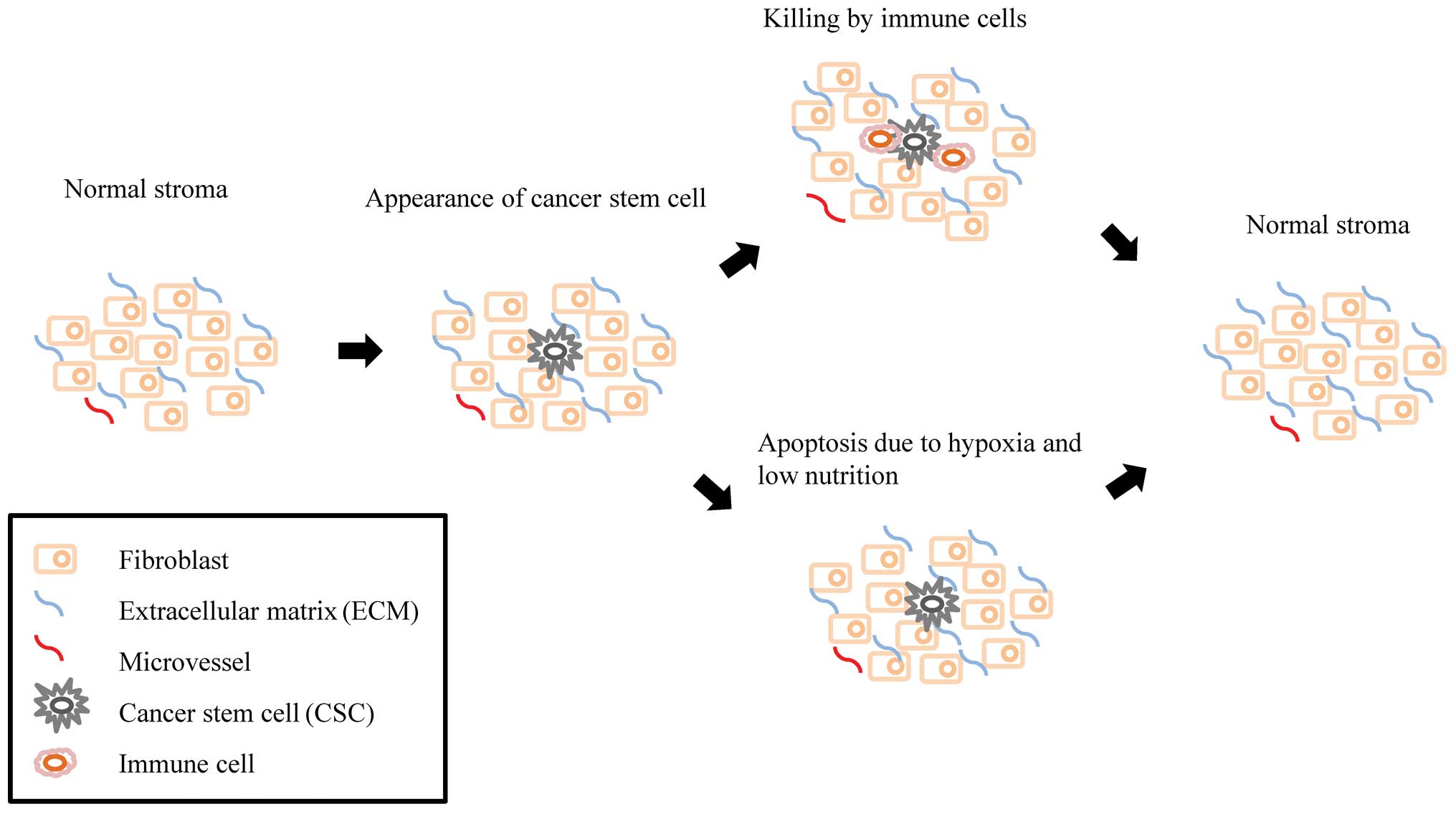
As shown in Fig. 1, CSCs are
killed by immune cells, or disappear under conditions of hypoxia
and malnutrition. In general, CSCs only survive in the primary
niche long enough to cause cancer when the microenvironment is
supportive. The primary niche is composed of fibroblasts and the
extracellular matrix (ECM) (11).
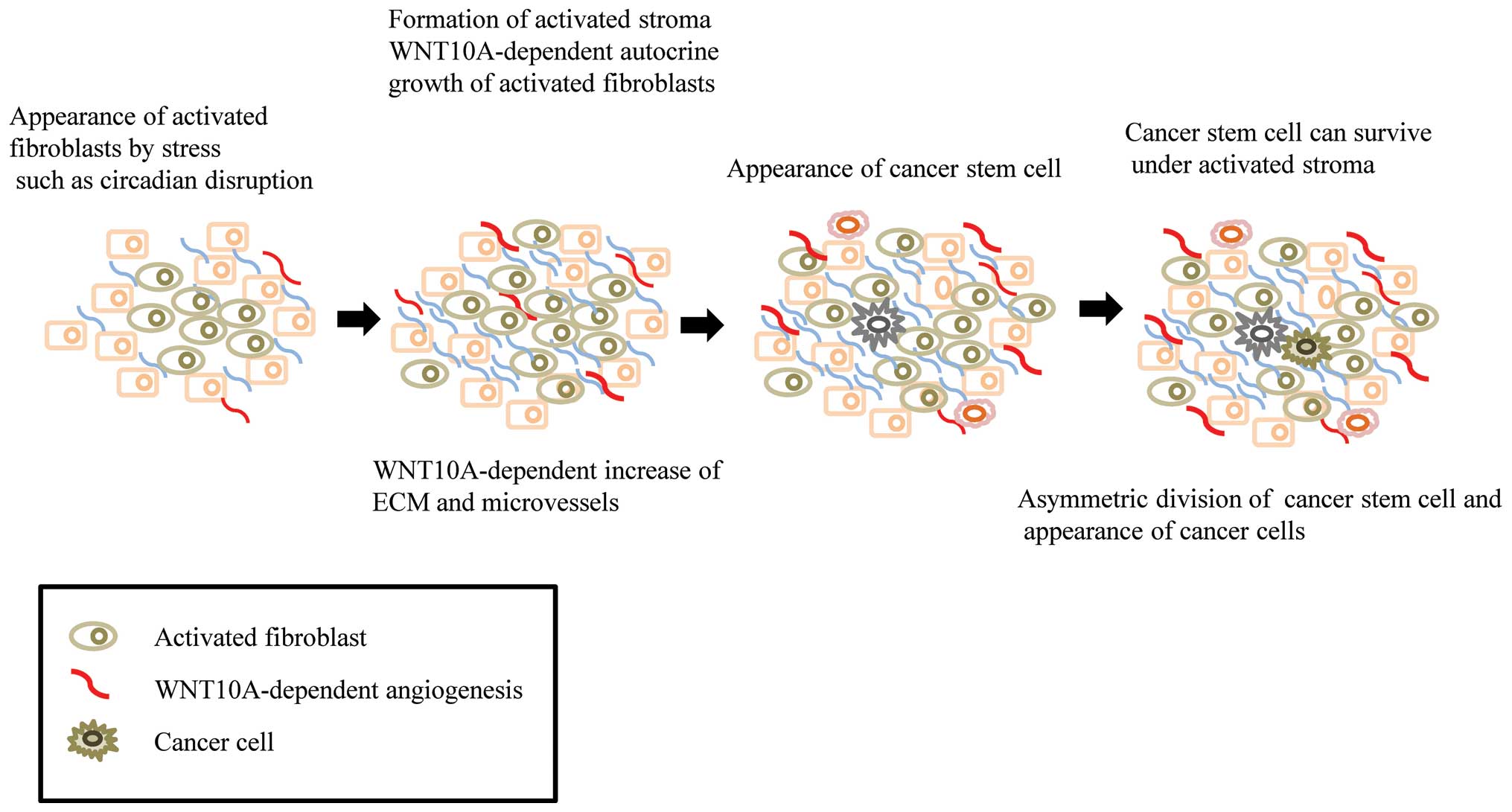
Circadian disruption activates the fibroblasts, which produce the
autocrine growth factor, WNT10a. These WNT10a-producing fibroblasts
secret ECM, and may provide the beneficial conditions required to
form an initial tumor niche or microenvironment for CSCs (Fig. 2). It is possible that this process
may contribute to the maturation of CSCs, but not their
differentiation. Psychosocial stress might activate resident
fibroblasts in the body, which together with the ECM, may provide
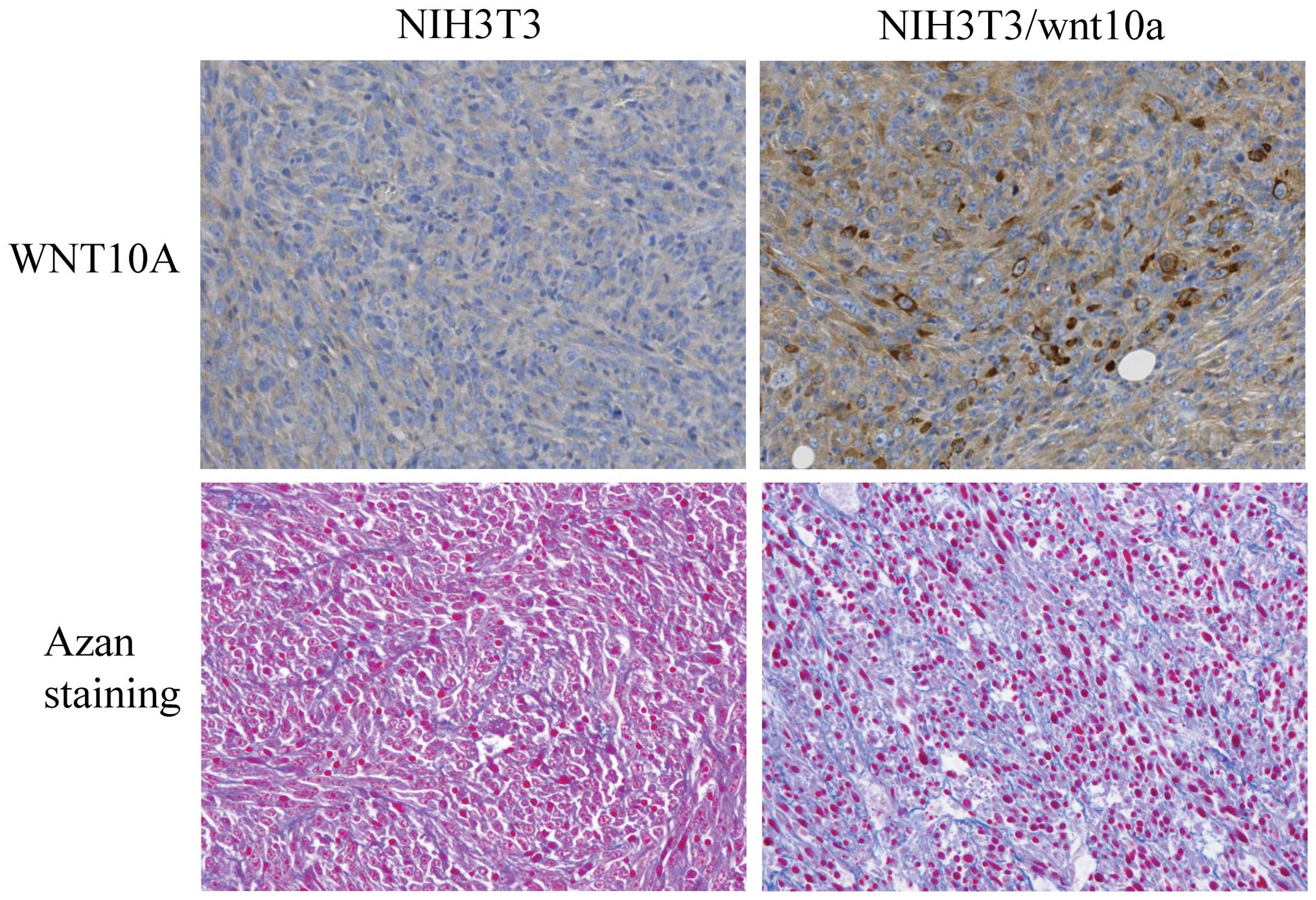
the niche required for CSCs in the preclinical phase. Mouse NIH3T3
cells overexpressing WNT10a can grow rapidly and form tumors in
nude mice. As shown in Fig. 3,
WNT10a-producing cells promote their own growth and secrete ECM.
Interestingly, it has been reported that increased collagen density
promotes mammary tumor initiation and progression (12).
It has been proposed that metastasis requires the
existence of a metastatic niche to allow the invading cancer cells
to survive, colonize and expand to form macrometastases (13). It has been reported that both
cancer cells and cancer-associated fibroblasts (CAFs) produce
angiogenic factors, such as vascular endothelial growth factor and
WNT10a, respectively, while it is well known that cancer cells
produce reactive oxygen species (ROS) that induce these angiogenic
factors (14). Rapid tumor growth
with angiogenesis induces extracellular acidosis, which in turn
leads to activation of metalloproteinases that destroy the
structure of the tumor niche (15). Hypoxic glycolysis is activated and
produces acid metabolites, and the subsequent decrease in
intracellular pH has been shown to reduce DNA repair activity,
resulting in the accumulation of spontaneous mutations following
the malignant progression of cancer cells. Thus, solid tumors
finally disrupt the niche through degradation of the ECM, and
acquire the ability to invade tissues and to metastasize during the
clinical phase. The tumor microenvironment is thus crucial for
solid tumor development (16), and
dysregulation of the pH and disruption of the tumor niche is
closely involved in the cancer hallmarks of invasion and
metastasis. These observations also suggest that circadian
disruption in cancer patients might be related to poor
prognosis.
Cancer-associated fibroblasts and tumor
microenvironment
Fibroblasts are an abundant cell type in connective
tissues. They produce ECM components and various cytokines, and
contribute to the formation of a structural network through tissue
remodeling (17). The tumor
environment includes structural and cellular components. The
cellular components are the so-called stromal cells, including both
resident and circulating cells such as macrophages, inflammatory
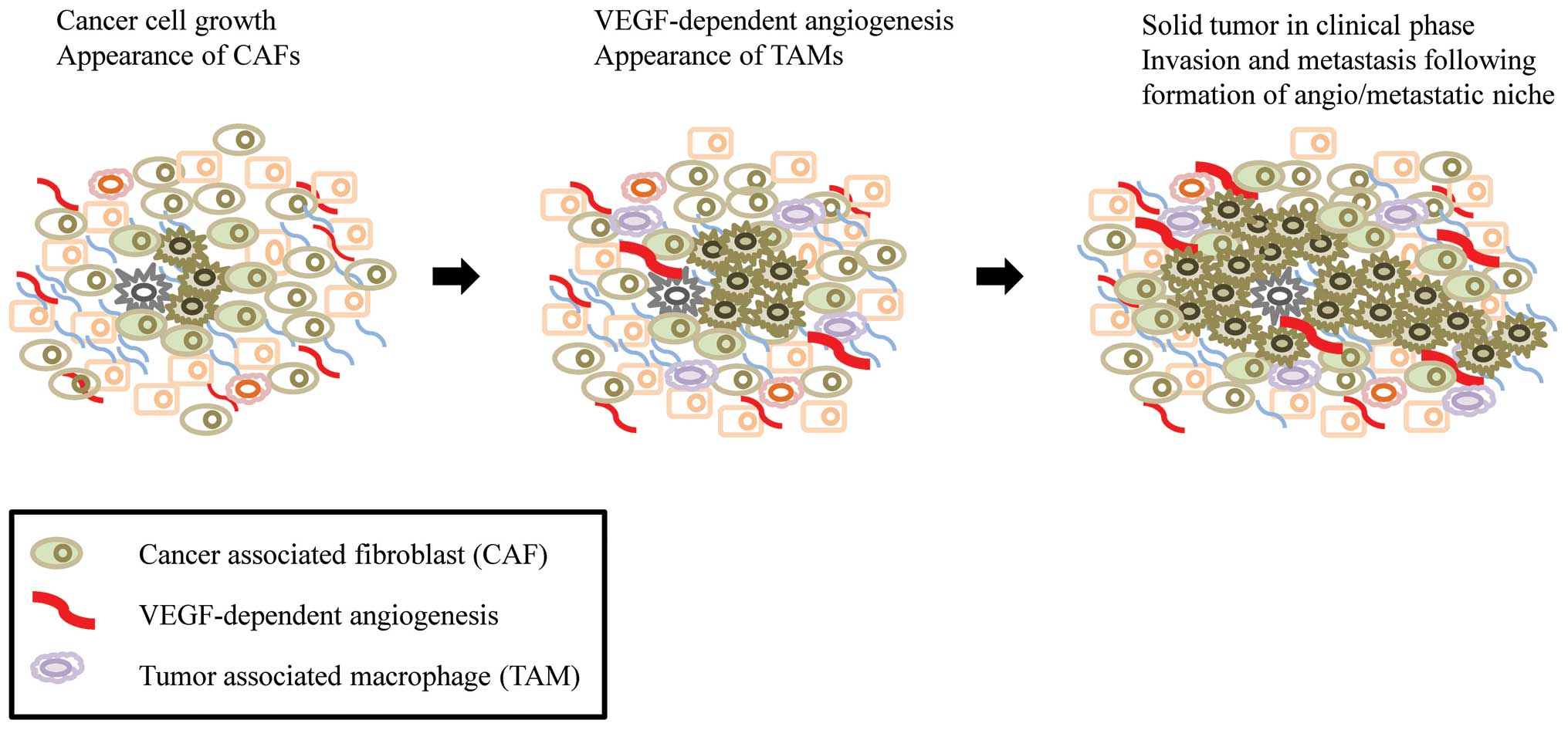
cells, endothelial cells, myofibroblasts and CAFs (18). Increasing evidence indicates that
CAFs are a main player in the hallmarks of cancer such as
angiogenesis, invasion, metastasis and inflammation, which are
critical factors for the development of solid tumors (Fig. 4). However, the origin of CAFs
remains unknown (19). Mesenchymal
stem cells contribute to the formation of tumor-associated stroma
containing cellular components such as myofibroblasts and
fibroblasts (20–23). Furthermore, the detailed roles of
CAFs are still unclear, and not all the cytokines produced by CAFs
have yet been identified. It is possible that the properties of
CAFs important during the early stage of tumor development differ
from those involved in the late stage. CAFs may be activated by
factors in the microenvironment, such as hypoxia (24), and by physiological conditions such
as circadian rhythms. We demonstrated that disruption of circadian
rhythms can promote tumor growth through WNT10A-dependent
angio/stromagenesis, associated with increased levels of oxidative
stress (25). Both endothelial
cells and stromal cells may be activated by WNT10A signals from
non-tumor cells such as CAFs. WNT signaling has been classified
into ‘canonical’ and ‘non-canonical’ pathways. In addition,
β-catenin expression has been observed in endothelial cells in
newly-formed tumor vessels, suggesting that WNT/β-catenin signaling
plays a role in tumor angiogenesis. WNT signaling is also known to
play an important role in cancer and stem cell biology (26), indicating that WNT10A might affect
not only the tumor microenvironment, but also the CSCs
themselves.
Tumor-driven niche development
The generation of CSCs and cancer progression
involve a long-term and complicated series of processes.
Accumulating evidence suggests that psychosocial stress can
influence cancer cell growth via many processes. Cancer cells
induce inflammation (27). Both
tumor-associated macrophages and CAFs are critical for cancer
progression (28). In addition,
both cancer cells and activated stromal cells produce high levels
of ROS and cytokines. ROS induce not only DNA damage following
malignant transformation, but also CAF activation. These tumor-host
interactions alter the local tumor environment and contribute to
tumor growth. Activated CAFs produce ECM around cancer cells, and
CAF-dependent ECM production may support the formation of a new
niche allowing the development of local micrometastases around the
primary tumor. Finally, the development of an angiogenic niche
around the main tumor supports invasion and metastasis. Fig. 5 shows a typical case that supports
this idea. Tumor-associated connective tissue is often observed in
front of the main gastric tumor. Both azan staining and
immunohistochemical studies showed that this region was rich in
collagen and microvessels (Fig. 5B and
C), and the vascular smooth muscle cells in the microvessels
and the fibroblastic cells observed in the collagen deposits were
positive for WNT10A (Fig. 5D).
WNT10A may thus contribute to the formation of the
angio/stromagenic niche. We also suggest that the tumor-associated
connective tissue functions as an angio/stromagenic niche around
the primary tumor. Bateman (29)
also proposed that cancer cells modify the stroma of remote organs
and create a premetastatic niche.
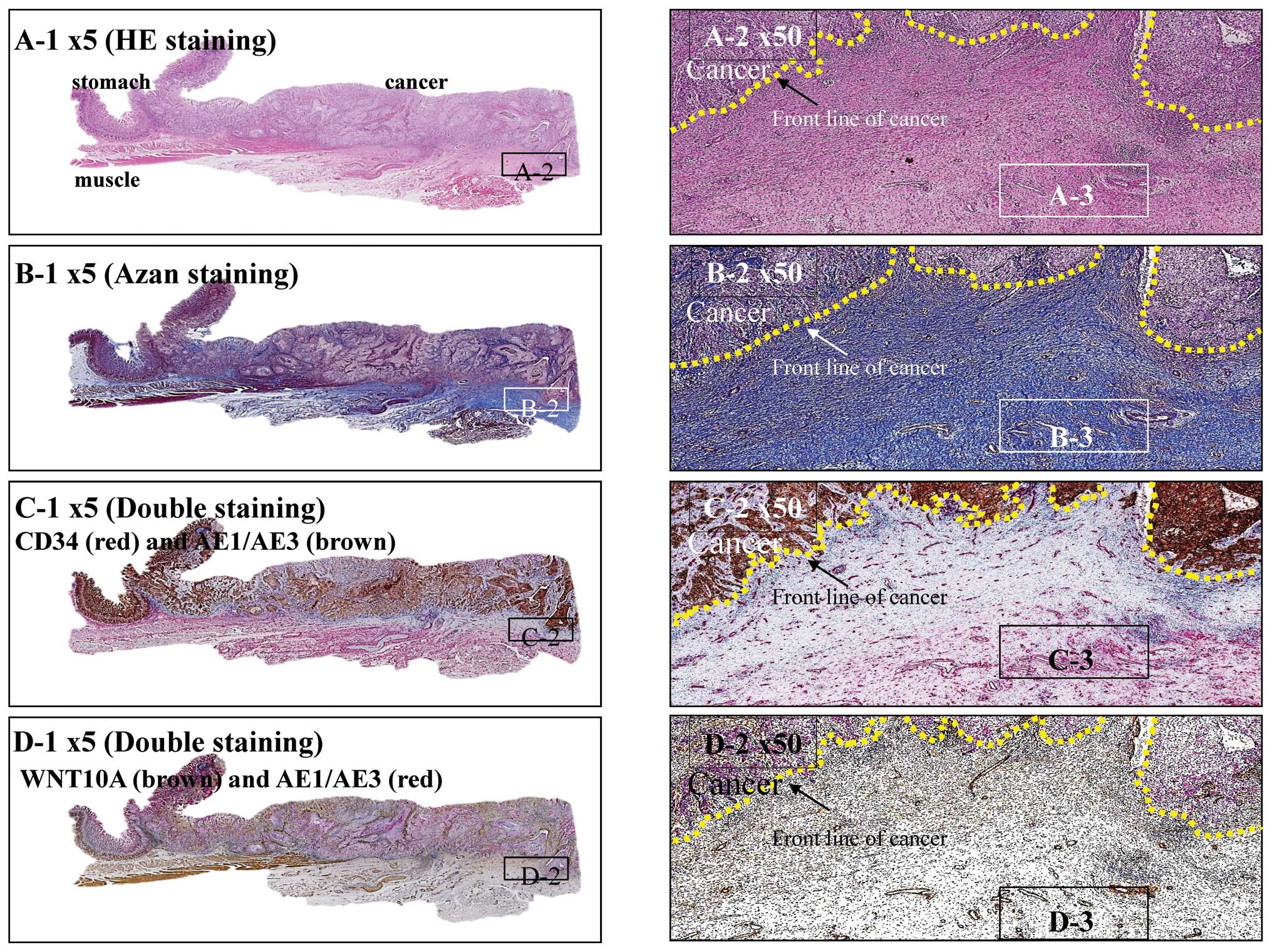 | Figure 5.Stomach cancer with remodeling of
connective tissue as an angio/stromagenic niche. (A) Hematoxylin
and eosin stained squamous cell carcinoma at the gastro-esophageal
junction. Tumor penetrates the serosa (A-1). Carcinoma arranged in
sheet-like nests or irregular cords confined to the serosa. Yellow
dotted line indicates the front line of the cancer partitioned from
the stroma (A-2). Scar-like stromal reaction, in which peripheral
desmoplasia with proliferating spindle-shaped cells and numerous
well-developed blood capillaries, is found throughout the front
line of carcinoma progression (A-3). (B) Azan staining. In
desmoplastic stromal areas, spindle-shaped cells and blood
capillaries were counterstained red (B-1 and 2), as were nuclei,
fibrin and epithelial hyaline bodies. Collagen fibers and mucus
were counterstained blue, together with the basal lamina of the
blood capillaries (B-3). (C) CD34 (red) - AE1/AE3 (brown) double
staining method. Immunohistochemically, carcinoma cells were
positive for AE1/AE3 (brown) and angioendothelial cells were
positive for CD34 (red) (C-1 and 2). In stromal areas,
spindle-shaped cells and the basal lamina of blood capillaries were
stained red (C-3). (D) AE1/AE3 (red) - WNT10 (brown) double
staining method. Immunohistochemically, carcinoma cells were
positive for AE1/AE3 (red) and the stromal area was positive for
WNT10 (brown) (D-1 and 2). In the stromal area, spindle-shaped
cells and the basal lamina of blood capillaries were stained brown
(D-3). |
WNT10A mutations are associated with autosomal
recessive ectodermal dysplasia (30). In addition, the expression of WNT
signaling antagonists has been shown to be downregulated in
fibroblasts in keloids, which are an aggressive type of
wound-healing tissue (31). These
previous reports indicate that WNT signaling is involved in both
tissue repair and wound healing. An earlier hypothesis suggests
that cancer results from uncontrolled wound-healing (32). This is supported by the observation
that WNT10A expression was markedly increased in fibroblastic cells
in the hyperplastic stroma of keloid tissue, suggesting its
function as an angio/stromagenesis gene in tumor progression.
There are some limitations associated with
experiments using mice. We cannot exclude the possibility that
other physiological and/or hormonal factors, such as melatonin, may
affect the growth of implanted cancer cells in mouse models.
Melatonin suppression through exposure to artificial light at night
leads to carcinogenesis of target endocrine organs (33). However, serum melatonin cannot be
measured in mice because the pineal gland is too small. Thus
although the subcutaneous injection of rapidly growing human cancer
cells into nude mice provides a setting in which tumor growth can
be assessed in a relatively short time span, an orthotopic model
that more accurately reproduces the interactions between tumor
cells and their microenvironment is required to confirm these
results.
Drug resistance
The molecular mechanisms responsible for the
cellular sensitivity to anticancer agents have been extensively
studied in cancer cell lines (16). However, drug resistance is also
influenced indirectly by the tumor microenvironment. Cisplatin
resistance is affected by several factors that influence
intracellular drug accumulation, including the levels of cellular
thiols and DNA repair activity (34). Our own studies showed that
glutathione biosynthesis was upregulated by activating
transcription factor 4 (ATF4), which is also regulated by the
circadian transcription system (35,36).
ATF4 expression was induced by oxidative stress through the Nrf2
transcription factor (37). The
role of histone acetyltransferase (HAT) gene expression in the
development of drug resistance has not been extensively studied,
though it has been shown that HAT genes such as CLOCK and TIP60 are
overexpressed, and are involved in glutathione biosynthesis and DNA
repair (38), indicating that the
system protecting against various stresses might be regulated
periodically. These results indicate that similar mechanisms to
those observed in CAFs may contribute to niche-dependent drug
resistance.
The circadian transcription system thus drives the
expression of genes that regulate the cell cycle, DNA repair, and
thiol production, which are involved in drug sensitivity,
indicating that the circadian rhythm may contribute to the efficacy
of chemotherapy in cancer patients, with implications for
side-effects and patient outcomes, including prognosis (39). New methods are required to
understand the status of the circadian rhythm in an individual
patient. WNT16B expression has recently been reported to be
upregulated in fibroblasts by chemotherapy, and to promote
epithelial-mesenchymal transition in neoplastic prostate epithelium
through paracrine signaling (40).
They also showed that WNT16B promoted the survival of cancer cells
after chemotherapy, suggesting that the tumor environment functions
as a stromal chemoresistant niche.
Conclusions
WNT10a is a key molecule in the development of the
tumor niche. WNT10a-dependent activation of the tumor niche not
only supports the emerging links between the circadian rhythm,
oxidative stress and tumor progression at the molecular level, but
also alerts us to the potentially adverse effects of artificial
light. Further studies are needed to clarify whether
WNT10A-Frizzled binding mediates cell proliferation in both
endothelial cells and stromal cells. Examining WNT10A receptors and
their associated signal transduction pathways may provide valuable
insights into the role of circadian rhythms in tumor progression. A
greater understanding of the complexity of the tumor
microenvironment and the role of the tumor niche will lead to
further advances in cancer treatment.
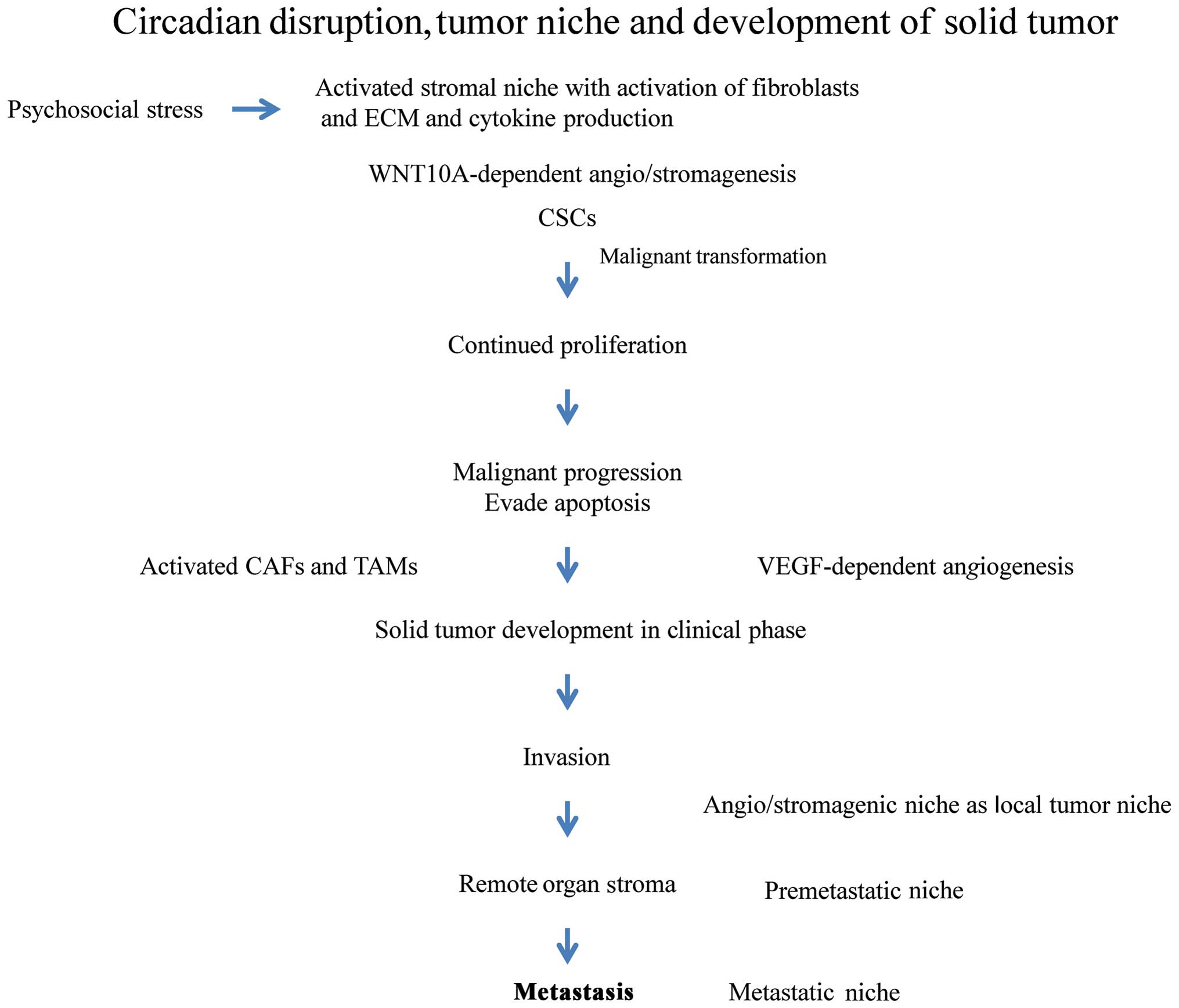
Long-term disturbance and disruption of the
circadian rhythm contributes to the development of cancer from the
preclinical to clinical phases through the evolution of a highly
specialized tumor niche (Fig. 6).
As expected, WNT10A expression is controlled by a clock gene
(41). These results suggest that
researchers should consider the relevance of chronobiology based on
the results of occupational science related to shift work. Improved
understanding of the circadian rhythm will also allow the further
development of chronotherapy in cancer patients.
References
|
1.
|
Antoni MH, Lutgendorf SK, Cole SW, et al:
The influence of bio-behavioural factors on tumour biology:
pathways and mechanisms. Nat Rev Cancer. 6:240–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lutgendorf SK, Sood AK and Antoni MH: Host
factors and cancer progression: biobehavioral signaling pathways
and interventions. J Clin Oncol. 28:4094–4099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Costa G, Haus E and Stevens R: Shift work
and cancer-considerations on rationale, mechanisms, and
epidemiology. Scand J Work Environ Health. 36:163–179. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kolstad HA: Nightshift work and risk of
breast cancer and other cancers - a critical review of the
epidemiologic evidence. Scand J Work Environ Health. 34:5–22. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
McGregor BA and Antoni MH: Psychological
intervention and health outcomes among women treated for breast
cancer: a review of stress pathways and biological mediators. Brain
Behav Immun. 23:159–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fu L and Lee CC: The circadian clock:
pacemaker and tumour suppressor. Nat Rev Cancer. 3:350–361. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yu EA and Weaver DR: Disrupting the
circadian clock: gene-specific effects on aging, cancer, and other
phenotypes. Aging (Albany, NY). 3:479–493. 2011.PubMed/NCBI
|
|
8.
|
Borovski T, De Sousa E, Melo F, Vermeulen
L and Medema JP: Cancer stem cell niche: the place to be. Cancer
Res. 71:634–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li L and Xie T: Stem cell niche: structure
and function. Annu Rev Cell Dev Biol. 21:605–631. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
McAllister SS and Weinberg RA: Tumor-host
interactions: a far-reaching relationship. J Clin Oncol.
28:4022–4028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: a dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Provenzano PP, Inman DR, Eliceiri KW, et
al: Collagen density promotes mammary tumor initiation and
progression. BMC Med. 6:112008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Coghlin C and Murray GI: Current and
emerging concepts in tumour metastasis. J Pathol. 222:1–15. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhou Y, Yan H, Guo M, et al: Reactive
oxygen species in vascular formation and development. Oxid Med Cell
Longev. 2013:3749632013. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kohno K, Uchiumi T, Niina I, et al:
Transcription factors and drug resistance. Eur J Cancer.
41:2577–2586. 2005. View Article : Google Scholar
|
|
17.
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
18.
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: a diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mishra PJ, Mishra PJ, Humeniuk R, et al:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Quante M, Tu SP, Tomita H, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu S, Ginestier C, Ou SJ, et al: Breast
cancer stem cells are regulated by mesenchymal stem cells through
cytokine networks. Cancer Res. 71:614–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Cabarcas SM, Mathews LA and Farrar WL: The
cancer stem cell niche - there goes the neighborhood? Int J Cancer.
129:2315–2327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yasuniwa Y, Izumi H, Wang KY, et al:
Circadian disruption accelerates tumor growth and
angio/stromagenesis through a Wnt signaling pathway. PLoS One.
5:e153302010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Fodde R and Brabletz T: Wnt/beta-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Pagès F, Galon J, Dieu-Nosjean MC, et al:
Immune infiltration in human tumors: a prognostic factor that
should not be ignored. Oncogene. 29:1093–1102. 2010.PubMed/NCBI
|
|
28.
|
Mantovani A, Germano G, Marchesi F, et al:
Cancer-promoting tumor-associated macrophages: new vistas and open
questions. Eur J Immunol. 41:2522–2525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Bateman A: Growing a tumor stroma: a role
for granulin and the bone marrow. J Clin Invest. 121:516–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Bohring A, Stamm T, Spaich C, et al:
WNT10A mutations are a frequent cause of a broad spectrum of
ectodermal dysplasias with sex-biased manifestation pattern in
heterozygotes. Am J Hum Genet. 85:97–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Russell SB, Russell JD, Trupin KM, et al:
Epigenetically altered wound healing in keloid fibroblasts. J
Invest Dermatol. 130:2489–2496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Schäfer M and Werner S: Cancer as an
overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell
Biol. 9:628–638. 2008.PubMed/NCBI
|
|
33.
|
Mediavilla MD, Sanchez-Barcelo EJ, Tan DX,
Manchester L and Reiter RJ: Basic mechanisms involved in the
anti-cancer effects of melatonin. Curr Med Chem. 17:4462–4481.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Torigoe T, Izumi H, Ishiguchi H, et al:
Cisplatin resistance and transcription factors. Curr Med Chem
Anticancer Agents. 5:15–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Tanabe M, Izumi H, Ise T, et al:
Activating transcription factor 4 increases the cisplatin
resistance of human cancer cell lines. Cancer Res. 63:8592–8595.
2003.PubMed/NCBI
|
|
36.
|
Igarashi T, Izumi H, Uchiumi T, et al:
Clock and ATF4 transcription system regulates drug resistance in
human cancer cell lines. Oncogene. 26:4749–4760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Miyamoto N, Izumi H, Miyamoto R, et al:
Transcriptional regulation of activating transcription factor 4
under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16
cells. Invest Ophthalmol Vis Sci. 52:1226–1234. 2011. View Article : Google Scholar
|
|
38.
|
Miyamoto N, Izumi H, Noguchi T, et al:
Tip60 is regulated by circadian transcription factor clock and is
involved in cisplatin resistance. J Biol Chem. 283:18218–18226.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lévi F, Okyar A, Dulong S, Innominato PF
and Clairambault J: Circadian timing in cancer treatments. Annu Rev
Pharmacol Toxicol. 50:377–421. 2010.
|
|
40.
|
Sun Y, Campisi J, Higano C, et al:
Treatment-induced damage to the tumor microenvironment promotes
prostate cancer therapy resistance through WNT16B. Nat Med.
18:1359–1368. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Guo B, Chatterjee S, Li L, et al: The
clock gene, brain and muscle Arnt-like 1, regulates adipogenesis
via Wnt signaling pathway. FASEB J. 26:3453–3463. 2012. View Article : Google Scholar : PubMed/NCBI
|