Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common type of cancer in the world and also known for
its rapid clinical progression and poor prognosis (1,2). The
mortality is mainly caused by locoregional recurrence and cervical
lymph node metastasis and occasionally by distant metastasis
(3). Notably, regional and distant
metastases in HNSCC correspond to an extremely poor prognosis with
limited treatment options. The treatment resistance and tumor
recurrence are important clinical problems in the management of
HNSCC. To improve the therapeutic outcome of HNSCC, more effective
treatment strategy is urgently needed.
Epithelial-mesenchymal transition (EMT) is a
critical process in tumor progression that causes epithelial cells
to acquire a migratory mesenchymal phenotype (4,5). EMT
is thought to be a crucial step in the induction of cell invasion
and tumor metastasis (4).
Furthermore, it has also been shown that cells with an EMT
phenotype are more resistant to chemoradiotherapy in HNSCC
(6).
Snail, a zinc-finger transcription factor, plays an
important role in EMT by directly repressing epithelial marker such
as E-cadherin and by upregulating mesenchymal markers (7–12).
Several studies have shown that Snail-related transcription factors
play a transcriptional and regulatory role in invasion, metastasis,
and poor outcome for different type of malignancies, including
HNSCC (13,14).
It has been suggested in recent reports that
Snail-induced EMT causes the cancer stem cell (CSC)-like properties
in different type of malignant tumors and that both EMT and
CSC-like phenotype are associated with treatment resistance
(11,15,16).
Prince et al showed that the purified CD44+
population of HNSCC cells possesses the self renewing properties of
CSCs (17). Aldehyde dehydrogenase
1 (ALDH1) has also been shown to be a putative marker of CSC in
HNSCC (18–20). Furthermore, Chen et al
showed that CD44+/ALDH1+ cells resist
radiotherapy and may serve as a reservoir for developing tumors and
metastasis (21). These findings
suggested that Snail expression may regulate the CSC-like
properties in HNSCC via EMT. On the contrary, there is also a
report that Snail expression did not correlate with prognosis
(22).
The key role of Snail in HNSCC has not been fully
elucidated. In this study, we demonstrate that introduction of
Snail in HNSCC cells confers EMT properties such as increased cell
motility and invasiveness in vitro. In addition, we report
that Snail-induced EMT gains HNSCC cell CSC-like phenotype and are
associated with chemoresistance.
Materials and methods
Cell lines and culture
Human HNSCC cells, SAS and HSC-4, were employed in
this study. SAS cells and HSC-4 cells, obtained from the Japanese
Cancer Research Resource Bank (Tokyo, Japan), were cultured in DMEM
medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS, Invitrogen), 100 U/ml
penicillin, and 100 mg/ml streptomycin (Gibco, Grand Island, NY,
USA) at 37°C in 5% CO2.
Transfection with Snail in SAS and HSC-4
cells
cDNA fragment encoding human Snail (NM_005985.2) was
inserted into pCR 3.1 mammalian expression vector (Invitrogen). SAS
and HSC-4 cells (1.5×105 cells) were plated into 6-well
culture plates and allowed to adhere for 12 h. Then, SAS and HSC-4
cells were transfected with 2 μg of either pCR 3.1-Snail or
pCR 3.1-vector (without insert DNA) with Lipofectamine 2000 reagent
(Invitrogen) according to the manufacturer’s instructions. We
established SAS-Snail and HSC-4-Snail as transiently
Snail-expressing cell lines, and their respective control cell
lines. All assays were performed 24 h after transfection.
Immunoblot analysis and antibodies
Snail, E-cadherin, CD44 and ALDH1 signaling on SAS
and HSC-4 cells after the transfection with or without Snail were
evaluated with western blot analysis. Cells were collected and
frozen in 100 μl RIPA buffer, and stored at −30°C. Briefly,
total protein extracts were prepared according to the
freeze-thawing lysis method and protein concentrations were
measured with Bovine Serum Albumin (BSA) Protein Assay. Sample of
extract containing 20 μg of protein were then separated by
sodium dodecylsulfate-polyacrylamid gel electrophoresis (SDS-PAGE)
and transferred to polyvinylidene difluoride membranes, after
washing with phosphate-buffered saline with Tween-20 (PBST), the
membranes were incubated first with rabbit anti-Snail, rabbit
anti-E-cadherin (Cell Signaling Technology, Danvers, MA, USA;
diluted 1:1,000), rabbit anti-CD44 and goat anti-ALDH1 (Abcom,
Cambridge, MA, USA; diluted 1:2,000 and 1:500, respectively) at 4°C
overnight and then with peroxidase-conjugated secondary anti-rabbit
or goat immunoglobulin G (IgG) (Cell Signaling Technology; diluted
1:1,000) for 1 h. After rinsing in PBST (4 times, 5 min each),
immunodetection was accomplished using an ECL western blot analysis
detection reagent and analysis system. The membranes were
subsequently exposed to X-ray film as described previously
(23).
Immunofluorescence staining
Cells were cultured in Labtech chamber slide system
(Thermo Scientific, Waltham, MA, USA), and then fixed with 4%
paraformaldehyde for 20 min at room temperature. After rinsing with
phosphate-buffered saline (PBS), the cells were permeabilized with
0.1% Triton X-100 in PBS for 30 min. Then, they were blocked with
1% BSA and 0.1% Tween-20 in PBS for 1 h at room temperature and
incubated overnight at 4°C with rabbit anti-E-cadherin antibody
(diluted 1:200). After rinsing with 0.1% Tween-20 in PBS, chamber
slides were incubated with fluorescent-labeled secondary antibody
(goat anti-rabbit-IgG-Alexa Fluor 594; Invitrogen; diluted 1:1,000)
for 1 h at room temperature in the dark. The slides were then
mounted with Prolong gold antifade Reagent with
4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). The fluorescent
images were visualized by confocal image microscopy (Keyence,
Osaka, Japan).
Wound healing migration assay
Equal number of cells was plated onto 24-well
culture plates in DMEM medium and cultured for 24 h. The cell
monolayer was scraped with a 200-μl pipette tip, washed with
PBS and changed to fresh medium. The wound closure was photographed
at 24 h after wounding with control at 0 h under phase contrast
microscope. The wound width was measured in three points per image.
This experiment was repeated at least three times on each cell
line. The cell migration potency was determined by calculating a
difference between wound width at 0 and 24 h.
Invasion assay
Invasion assays were performed using 24-well
Matrigel-coated Transwells (BD Bioscience, Bedford, MA, USA)
(24). Cells (4×104)
were suspended in 200 μl of serum-free DMEM medium and
placed in the top chambers, and 700 μl DMEM medium
containing 10% FBS was added to the bottom chambers. After 24 h of
incubation at 37°C, non-invading cells were removed from the top of
the Matrigel with a cotton swab, while invading cells on the bottom
surface of the filter were fixed in 4% paraformaldehyde and stained
with Giemsa (Sigma-Aldrich, Dorset, UK) for 30 min. The invading
cells were then visualized at ×200 magnification and counted in
five fields for each filter.
Chemotherapy for cultured cells
In chemotherapy, cells were treated with cisplatin
(Nihonkayaku Co., Tokyo, Japan) at concentration of 1.0 or 10
μM. Chemosensitivity was assessed by Cell Counting Kit-8
(WST-8 cleavage; Dojindo, Mashikimachi, Japan) as described
previously (23). The cell
viability after the chemotherapy for the cells was evaluated with
the WST-8 cleavage. The cells were seeded in 96-well plates at an
initial density of 4×103 cells/well and incubated for 24
h. For chemotherapy, cisplatin (0–10 μM) was added to each
well. Following incubation for an additional 48 h, 10 μl of
WST-8 solution [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrop
henyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] was
added to each well, and the plate was incubated for further 2 h.
The absorbance of each well at 450 nm (reference wave length at 620
nm) was measured by a Multiscan FC Microplate Photometer (Thermo
Scientific). The measurement was repeated at least three times for
each cell line.
Statistical analysis
Data were presented as mean ± standard error (SE).
Experimental differences between groups were assessed with the
t-test. The differences were considered to be significant at
P<0.05.
Results
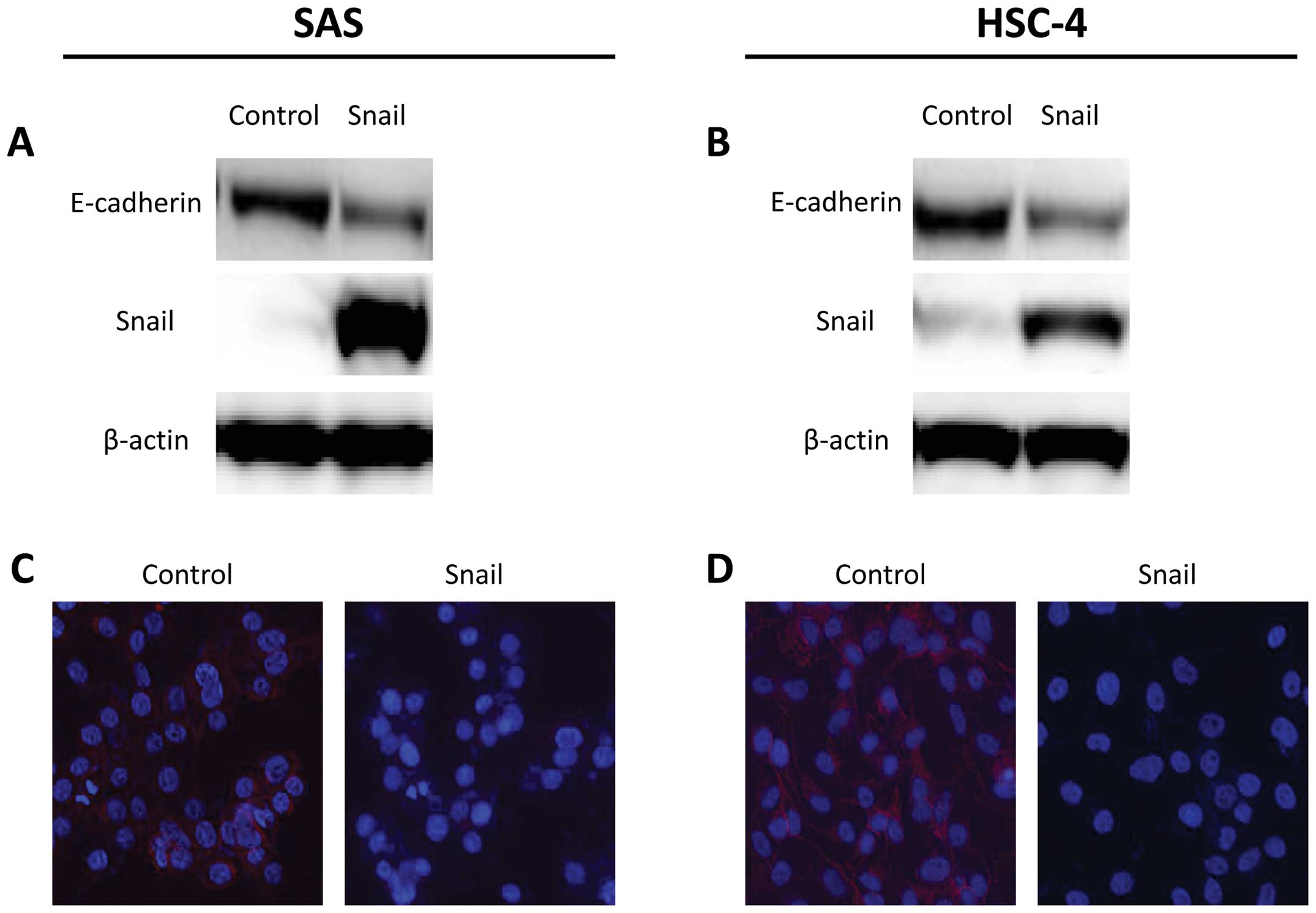
Snail regulates EMT properties in HNSCC
cells
SAS and HSC-4 cells were tranfected with Snail or
control vector. In western blot analysis, introduction of Snail
enhanced the suppression of E-cadherin protein levels in SAS and
HSC-4 cells (Fig. 1A and B). In
addition, cellular staining pattern of E-cadherin was examined by
immunofluorescence analysis. E-cadherin staining also decreased on
the cell membrane in SAS-Snail and HSC-4-Snail cells, whereas each
control cell line stained positively for E-cadherin (Fig. 1C and D).
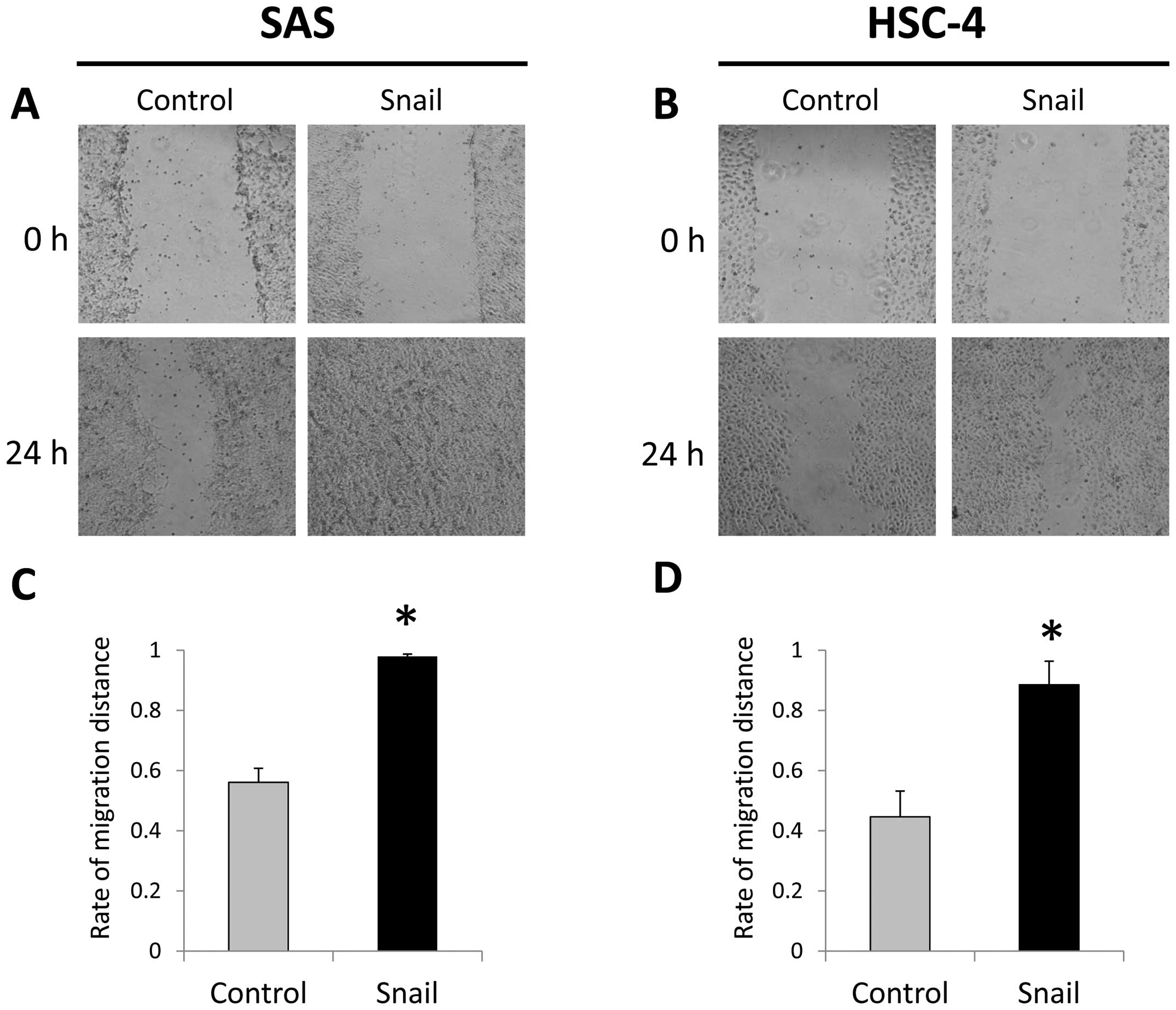
A wound healing migration assays showed that
migrating cells significantly increase in SAS-Snail and HSC-4-Snail
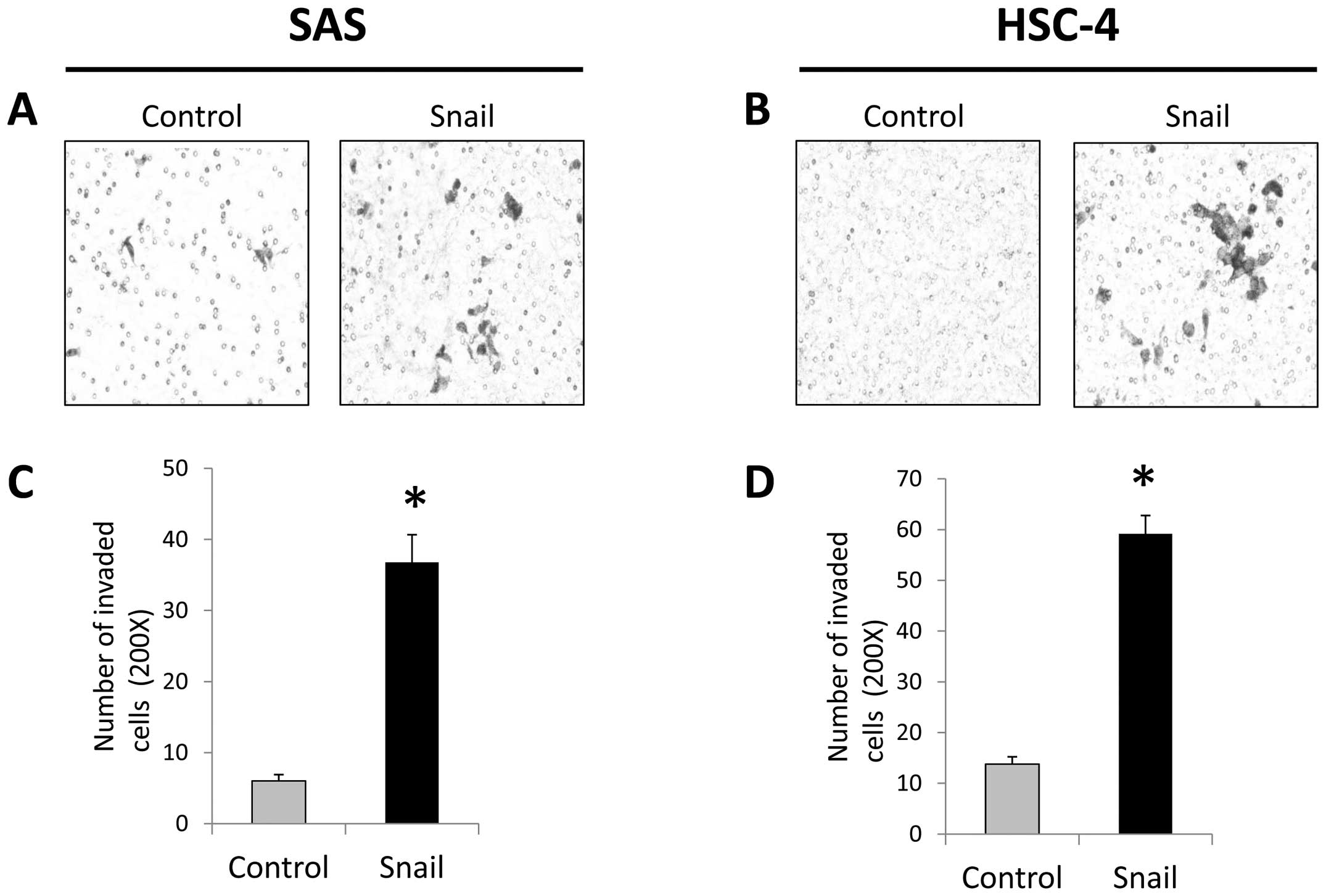
cells more than those in the control cells (Fig. 2). Furthermore, in invasion assays,
both these cell lines tranfected with Snail displayed more invasive
ability compared to the control cells with significant difference
(Fig. 3). These results suggested
that Snail was able to induce EMT in HNSCC cells.
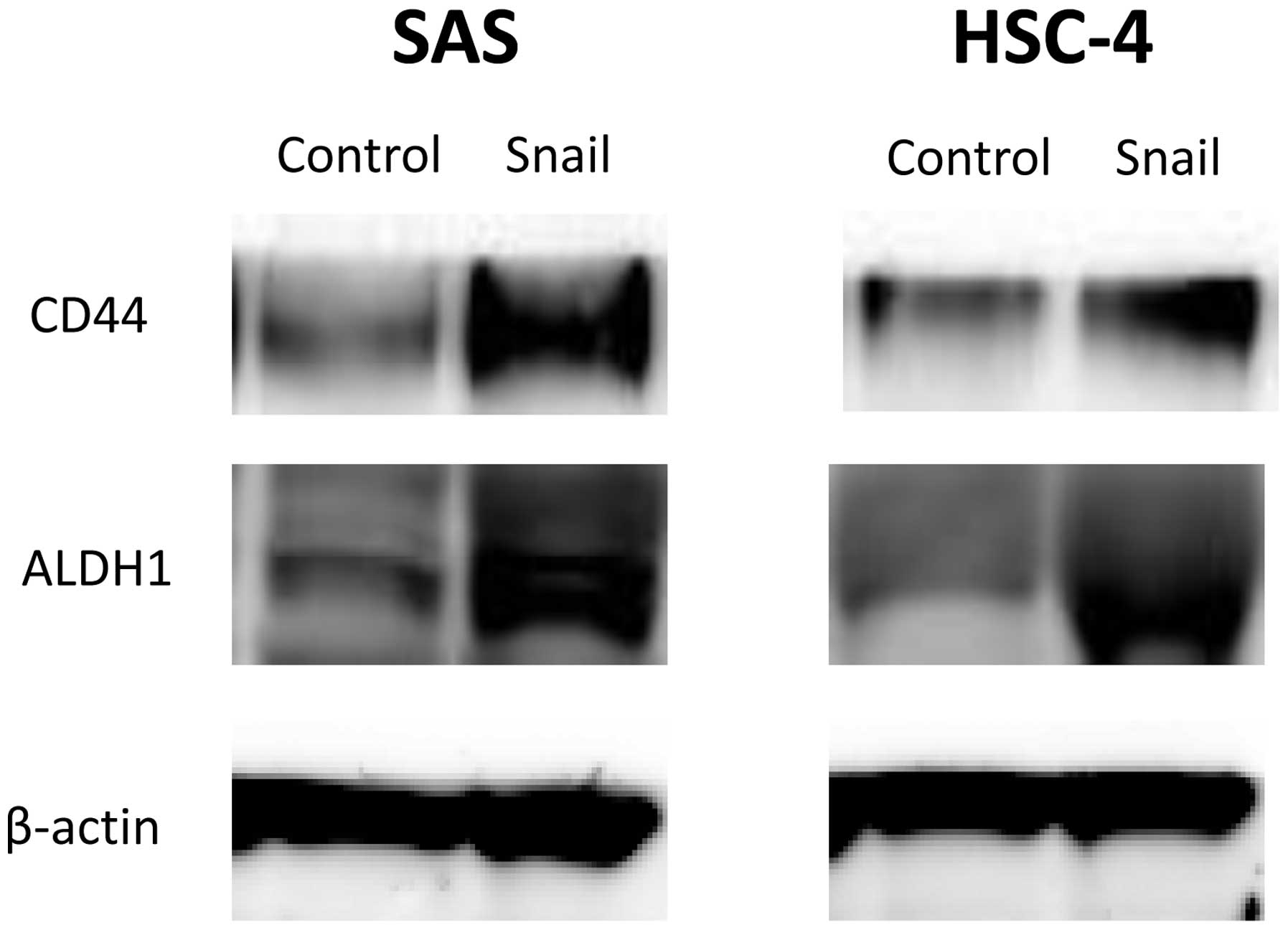
Snail expression induces CSC-like
phenotype in HNSCC cell lines
We demonstrated that EMT by Snail expression induced
a stem cell-like phenotype in HNSCC cells. The expression of CSC
surface markers in HNSCC cells was evaluated with western blot
analysis. Both CD44 and ALDH1 protein levels increased in SAS-Snail
and HSC-4-Snail cells compared with their control cells (Fig. 4). These results implied that
Snail-induced EMT could elicit a CSC-like phenotypic change as
CD44+/ALDH+ in HNSCC cells.
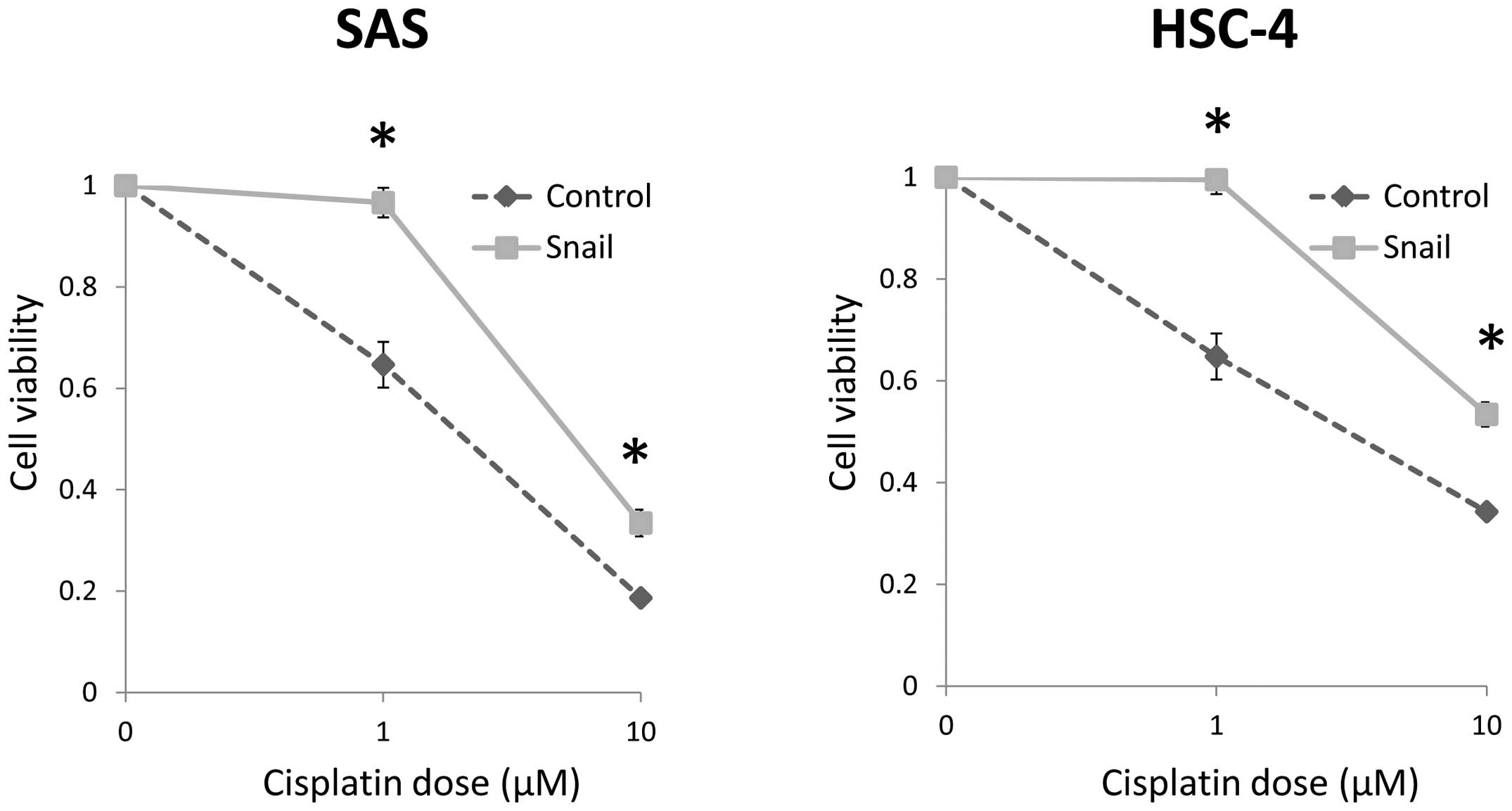
Snail expression enhances
chemoresistance
The cells transfected with Snail showed
significantly low chemosensitivity at 1.0 and 10 μM, as
compared with the control cells (Fig.
5). Thus, these results implied that the acquisition of
CSC-like phenotype caused by EMT results in enhancement of
chemo-resistance in HNSCC cells.
Discussion
In a variety of solid human tumors, the capacity to
initiate and maintain cancer growth and recurrence has been found
to reside in the small populations of cells within tumors, termed
cancer stem cells (CSCs). CSCs have the ability to undergo
self-renewal and produce differentiated progeny. These
characteristics allow CSCs to maintain a pluripotent phenotype,
while also producing a tumor composed of a heterogeneous cell
population (25,26). Several studies have shown that CSC
is implicated in tumor invasion and metastasis, and that tumor
recurrence after therapy is correlated with therapeutic resistance
of CSCs (27–30). CSC populations in HNSCC were first
identified using CD44, which has been used as a marker of CSC in
different type of malignant tumors including HNSCC (17,31,32).
However, HNSCC in CSCs are not precisely defined by CD44 expression
alone (33). Recently, ALDH1 has
been shown to be a marker of CSC. ALDH1 has also been used to
identify the CSCs (34). In
addition, Kirshnamurthy et al found that the combination of
CD44 and ALDH1 is more selective for CSC populations than either
marker used alone (35).
Epithelial tumor cells that undergo EMT lose
cell-cell adhesion properties and acquire more mesenchymal
properties, including invasiveness, motility and increase
resistance to apoptosis. EMT is an important biological process
that plays a critical role in tumor cell invasion, metastasis and
recurrence, and is commonly observed in tumor samples from HNSCC
patients (36,37). Moreover, the connection between CSC
and EMT has become more evident. It has been described that
induction of EMT results in cells gaining CSC-like properties and
treatment resistance (16,38–40).
Therefore, the importance of EMT in treatment resistance has
recently been targeted for investigation of CSCs in different type
of cancers, including HNSCC.
Snail is one of the master regulators that promotes
EMT by repressing epithelial markers and upregulating mesenchymal
markers and that mediates invasiveness as well as metastasis in
many different types of malignant tumors including HNSCC (41). Furthermore, it has been reported
that Snail expression may regulate the treatment resistance and
CSC-like properties of HNSCC (34,42).
Medelsohn et al have recently reported that Snail is an
independent marker of tumor metastasis in patients with HNSCC
(43).
In this study, we showed that induction of Snail
could suppress E-cadherin expression and increase motility and
invasiveness of HNSCC cells. These results suggested that Snail
could promote EMT and mediate tumor invasion. In addition, we
demonstrated that induction of EMT via Snail could lead HNSCC cells
to adopt CSC-like phenotype and chemoresistance for cisplatin.
Previous data imply that CSCs also rely on a microenvironment,
called the CSC niche, which controls their differentiation and
proliferation (44–47). The CSC niche has a complex
anatomical unit and is composed of diverse stromal cells, such as a
vascular network, mesenchymal and immune cells, extracellular
matrix (ECM), and soluble factors derived from niche cells
(47–50). It has been suggested that
interactions of CSC with CSC niche could induce tumor invasion and
treatment resistance. The detail of interactions between CSCs and
their niche are still unknown, and so the precise mechanisms should
be verified in further investigation. However, understanding the
interactions between the CSCs by Snail-induced EMT and their niche
microenvironments, which contribute to treatment resistance, may
pave the way for the development of novel strategies for treating
cancer including HNSCC.
In summary, we obtained EMT properties by the
over-expression of Snail in HNSCC cells. Moreover, these data
suggest that Snail also acquires CSC-like phenotype via EMT and
enhances treatment resistance. This Snail-induced EMT is considered
to play an essential role in tumor progression and treatment
resistance of HNSCC. Although the precise involvement of EMT and
CSC by Snail remains to be elucidated, they could be involved in
the latent effect. The critical mechanisms still need to be further
investigated. However, the strategy targeting EMT-regulating Snail
could be useful for cancer treatments, as the inhibition of EMT may
serve to block not only cancer invasion and metastasis but also the
formation of CSC.
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma;
|
|
EMT
|
epithelial-mesenchymal transition;
|
|
ALDH1
|
aldehyde dehydrogenase 1;
|
|
DAPI
|
4′,6-diamidino-2-phenylindole;
|
|
CSC
|
cancer stem cell
|
Acknowledgements
This study was supported in part by
Grants-in-Aids for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology of Japan.
References
|
1.
|
Ang KK, Trotti A, Brown BW, et al:
Randomized trial addressing risk features and time factors of
surgery plus radio-therapy in advanced head-and-neck cancer. Int J
Radiat Oncol Biol Phys. 51:571–578. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ozer E, Grecula JC, Agrawal A, et al:
Long-term results of a multimodal intensification regimen for
previously untreated advanced resectable squamous cell cancer of
the oral cavity, oropharynx, or hypopharynx. Laryngoscope.
116:607–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lo WL, Kao SY, Chi LY, et al: Outcomes of
oral squamous cell carcinoma in Taiwan after surgical therapy:
factors affecting survival. J Oral Maxillofac Surg. 61:751–758.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hsu DS, Lan HY, Huang CH, et al:
Regulation of excision repair cross-complementation group 1 by
Snail contributes to cisplatin resistance in head and neck cancer.
Clin Cancer Res. 16:4561–4571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moody SE, Perez D, Pan TC, et al: The
transcriptional repressor Snail promotes mammary tumor recurrence.
Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Debies MT, Gestl SA, Mathers JL, et al:
Tumor escape in a Wnt1-dependent mouse breast cancer model is
enabled by p19Arf/p53 pathway lesions but not p16 Ink4a loss. J
Clin Invest. 118:51–63. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kudo-Saito C, Shirako H, Takeuchi T, et
al: Cancer metastasis is accelerated through immunosuppression
during Snail-induced EMT of cancer cells. Cancer Cell. 15:195–206.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Thiery JP, Acloque H, Huang RY, et al:
Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Vincent T, Neve EP, Johnson JR, et al: A
SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta
mediated epithelial-mesenchymal transition. Nat Cell Biol.
11:943–950. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yokoyama K, Kamata N, Hayashi E, et al:
Reverse correlation of E-cadherin and snail expression in oral
squamous cell carcinoma cells in vitro. Oral Oncol. 37:65–71. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Peinado H, Ballestar E, Esteller M, et al:
Snail mediates E-cadherin repression by the recruitment of the
Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol.
24:306–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Prince ME, Sivanandan R, Kaczorowski A, et
al: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sheridan C, Kishimoto H, Fuchs RK, et al:
CD44+/CD24− breast cancer cells exhibit
enhanced invasive properties: an early step necessary for
metastasis. Breast Cancer Res. 8:R592006. View Article : Google Scholar
|
|
19.
|
Visus C, Ito D, Amoscato A, et al:
Identification of human aldehyde dehydrogenase 1 family member A1
as a novel CD8+ T-cell-defined tumor antigen in squamous
cell carcinoma of the head and neck. Cancer Res. 67:10538–10545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mack B and Gires O: CD44s and CD44v6
expression in head and neck epithelia. PLoS One. 3:e33602008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Chen YC, Chang CJ, Hsu HS, et al:
Inhibition of tumorigenicity and enhancement of
radiochemosensitivity in head and neck squamous cell cancer-derived
ALDH1-positive cells by knockdown of Bmi-1. Oral Oncol. 46:158–165.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hayry V, Makinen LK, Atula T, et al: Bmi-1
expression predicts prognosis in squamous cell carcinoma of the
tongue. Br J Cancer. 102:892–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hayashi K, Motoyama S, Sugiyama T, et al:
REG Ialpha is a reliable marker of chemoradiosensitivity in
squamous cell esophageal cancer patients. Ann Surg Oncol.
15:1224–1231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hayashi Y, Osanai M and Lee GH: Fascin-1
expression correlates with repression of E-cadherin expression in
hepatocellular carcinoma cells and augments their invasiveness in
combination with matrix metalloproteinases. Cancer Sci.
102:1228–1235. 2011. View Article : Google Scholar
|
|
25.
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells-perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
27.
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Li X, Lewis MT, Huang J, et al: Intrinsic
resistance of tumor-igenic breast cancer cells to chemotherapy. J
Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar
|
|
29.
|
Gupta PB, Onder TT, Jiang G, et al:
Identification of selective inhibitors of cancer stem cells by
high-throughput screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Charafe-Jauffret E, Ginestier C, Iovino F,
et al: Aldehyde dehydrogenase 1-positive cancer stem cells mediate
metastasis and poor clinical outcome in inflammatory breast cancer.
Clin Cancer Res. 16:45–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Locke M, Heywood M, Fawell S, et al:
Retention of intrinsic stem cell hierarchies in carcinoma-derived
cell lines. Cancer Res. 65:8944–8950. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Okamoto A, Chikamatsu K, Sakakura K, et
al: Expansion and characterization of cancer stem-like cells in
squamous cell carcinoma of the head and neck. Oral Oncol.
45:633–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Bhaijee F, Pepper DJ, Pitman KT, et al:
Cancer stem cells in head and neck squamous cell carcinoma: a
review of current knowledge and future applications. Head Neck.
34:894–899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Chen YC, Chen YW, Hsu HS, et al: Aldehyde
dehydrogenase 1 is a putative marker for cancer stem cells in head
and neck squamous cancer. Biochem Biophys Res Commun. 385:307–313.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Krishnamurthy S, Dong Z, Vodopyanov D, et
al: Endothelial cell-initiated signaling promotes the survival and
self-renewal of cancer stem cells. Cancer Res. 70:9969–9978. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Schipper JH, Frixen UH, Behrens J, et al:
E-cadherin expression in squamous cell carcinomas of head and neck:
inverse correlation with tumor dedifferentiation and lymph node
metastasis. Cancer Res. 51:6328–6337. 1991.PubMed/NCBI
|
|
37.
|
Chung CH, Parker JS, Ely K, et al: Gene
expression profiles identify epithelial-to-mesenchymal transition
and activation of nuclear factor-kappaB signaling as
characteristics of a high-risk head and neck squamous cell
carcinoma. Cancer Res. 66:8210–8218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Bao B, Wang Z, Ali S, et al:
Over-expression of FoxM1 leads to epithelial-mesenchymal transition
and cancer stem cell phenotype in pancreatic cancer cells. J Cell
Biochem. 112:2296–2306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Chen C, Wei Y, Hummel M, et al: Evidence
for epithelial-mesenchymal transition in cancer stem cells of head
and neck squamous cell carcinoma. PLoS One. 6:e164662011.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Fan F, Samuel S, Evans KW, et al:
Overexpression of Snail induces epithelial-mesenchymal transition
and a cancer stem cell-like phenotype in human colorectal cancer
cells. Cancer Med. 1:5–16. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Ota I, Li XY, Hu Y and Weiss SJ: Induction
of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration
program in cancer cells by Snail1. Proc Natl Acad Sci USA.
106:20318–20323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Zhu LF, Hu Y, Yang CC, et al: Snail
overexpression induces an epithelial to mesenchymal transition and
cancer stem cell-like properties in SCC9 cells. Lab Invest.
92:744–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Mendelsohn AH, Lai CK, Shintaku IP, et al:
Snail as a novel marker for regional metastasis in head and neck
squamous cell carcinoma. Am J Otolaryngol. 33:6–13. 2011.
View Article : Google Scholar
|
|
44.
|
Li L and Neaves WB: Normal stem cells and
cancer stem cells: the niche matters. Cancer Res. 66:4553–4557.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Chumsri S, Phatak P, Edelman MJ, et al:
Cancer stem cells and individualized therapy. Cancer Genomics
Proteomics. 4:165–174. 2007.
|
|
46.
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Boral D and Nie D: Cancer stem cells and
niche mircoenvironments. Front Biosci (Elite Ed). 4:2502–2514.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Calabrese C, Poppleton H, Kocak M, et al:
A perivascular niche for brain tumor stem cells. Cancer Cell.
11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Gilbertson RJ and Rich JN: Making a
tumour’s bed: glioblastoma stem cells and the vascular niche. Nat
Rev Cancer. 7:733–736. 2007.
|
|
50.
|
Yang ZJ and Wechsler-Reya RJ: Hit ‘em
where they live: targeting the cancer stem cell niche. Cancer Cell.
11:3–5. 2007.
|