Introduction
Strong clinical and experimental evidence
demonstrates association of elevated levels of matrix
metalloproteinase MMP-9, a type IV collagenase, with cancer
progression, metastasis and shortened patient survival, as it plays
a key role in tumor cell invasion and metastasis by digesting the
basement membrane and ECM components (1–6). In
addition to proteolysis, MMP-9 has been shown to play an important
role in cell migration (7,8). A unique characteristic of MMP-9 is
the ability to be secreted in both the monomeric and a
disulfide-bonded dimeric form. Dufour et al reported that
dimerization of MMP-9 through the hemopexin domain appears
necessary for MMP-9 enhanced cell migration (7). By using mutagenesis and biochemical
approaches it was demonstrated that the MMP-9 dimer (present
usually as 10–15% of the MMP-9 population), not the monomer, is
required for this functional activity of MMP-9 (7). For example, peptides interfering with
MMP-9 dimerization abrogated MMP-9 enhanced cell migration in COS-1
(7).
Rath and Pauling (9) proposed using nutrients such as lysine
and ascorbic acid to target plasmin-mediated connective tissue
degradation as a universal approach to tumor growth and expansion.
Binding to plasminogen active sites, lysine blocks plasminogen
activation into plasmin by tissue plasminogen activator (t-PA).
Thus, it modulates the plasmin-induced MMP activation cascade
(10). Subsequent studies
confirmed this approach and led to the identification of a novel
formulation composed of lysine, ascorbic acid, proline and green
tea extract and other micronutrients (NM), which has shown
significant anticancer activity against a large number (∼40) of
cancer cell lines, blocking cancer growth, tissue invasion and MMP
expression both in vitro and in vivo (11–13).
In this study, our main objectives were to study the
relative secretion patterns of MMP-9 monomer and dimer in a variety
of carcinoma, sarcoma, adenosarcoma and leukemia cell lines and to
evaluate the effect of the NM on MMP-9 monomer and dimer secretion
by these cells.
Materials and methods
Materials
Thirty-eight different cancer cell lines were
selected on the basis of organ malignancies and included
carcinomas, sarcomas, adenosarcomas and leukemias. The cancer cell
lines and their recommended media were purchased from ATCC
(Manassas, VA, USA). Antibiotics, penicillin and fetal bovine serum
(FBS), were obtained from Gibco (BRL, Long Island, NY, USA).
Twenty-four-well tissue culture plates were obtained from Costar
(Cambridge, MA, USA). Gelatinase zymography was performed in 10%
Novex pre-cast SDS polyacrylamide gel (Invitrogen Corp.) with 0.1%
gelatin in non-reducing conditions. The nutrient mixture (NM),
prepared by VitaTech (Hayward, CA, USA) was composed of the
following ingredients in the relative amounts indicated: Vitamin C
(as ascorbic acid and as Mg, Ca, and palmitate ascorbate) 700 mg;
L-lysine 1000 mg; L-proline 750 mg; L-arginine 500 mg; N-acetyl
cysteine 200 mg; standardized green tea extract (80% polyphenol)
1000 mg; selenium 30 μg; copper 2 mg; manganese 1 mg. All
other reagents used were of high quality and were obtained from
Sigma, unless otherwise indicated.
Cell cultures
The cancer cell lines were grown in their respective
media, supplemented with 10% FBS, penicillin (100 U/ml), and
streptomycin (100 μg/ml) in 24-well tissue culture plates.
The cells were plated at a density of 1×105 cells/ml and
grown to confluency in a humidified atmosphere at 5% CO2
at 37°C. Serum-supplemented media were removed and the cell
monolayer was washed once with PBS with the recommended serum-free
media. The cells were treated with the nutrient mixture, dissolved
in media and tested at 0,10, 50, 100, 500 and 1000 μg/ml.
Parallel sets of cultures were treated with PMA (100 ng/ml) for
induction of MMP-9. Control and PMA treatments were done in
triplicates. The plates were then returned to the incubator. The
conditioned media were collected separately, pooled and centrifuged
at 4°C for 10 min at 3000 rpm to remove cells and cell debris. The
supernatant was collected and used to assess for MMP-9 monomer and
dimer by gelatinase zymography.
Gelatinase zymography
Gelatinase zymography was performed in 10% NOVEX
Pre-Cast SDS polyacrylamide gel (Invitrogen Corp.) in the presence
of 0.1% gelatin under non-reducing conditions. Culture media (20
μl) were mixed with sample buffer and loaded for SDS-PAGE
with Tris glycine SDS buffer as suggested by the manufacturer
(Novex). Samples were not boiled before electrophoresis. Following
electrophoresis the gels were washed twice in 2.5% Triton X-100 for
30 min at room temperature to remove SDS. The gels were then
incubated at 37°C overnight in substrate buffer containing 50 mM
Tris-HCl and 10 mM CaCl2 at pH 8.0 and stained with 0.5%
Coomassie Blue R250 in 50% methanol and 10% glacial acetic acid for
30 min and destained. Upon renaturation of the enzyme, the
gelatinases digest the gelatin in the gel and give clear bands
against an intensely stained background. Protein standards were run
concurrently and approximate molecular weights were determined by
plotting the relative mobilities of known proteins.
Gelatinase zymograms were scanned using CanoScan
9950F Canon scanner at 300 dpi. The intensity of the bands was
evaluated using the pixel-based densitometer program Un-Scan-It,
version 5.1, 32-bit, by Silk Scientific Corp. (Orem, UT, USA), at a
resolution of one scanner unit (1/100 of an inch for an image that
was scanned at 100 dpi). The pixel densitometer calculates the
optical density of each pixel (values, 0-255) using the darkly
stained background of the gel as a pixel value of 0. A logarithmic
optical density scale was used since the optical density of the
film and gels is logarithmically proportional to the concentration.
The pixel densitometer sums the optical density of each pixel to
give the band density.
Results
MMP-9 dimer secretion patterns of cancer cells fell
into different categories, as shown in Table I. MMP-9 dimer secretion was not
detected in prostate DU-145 and PC-3, testicular NTER-2,
hepatocarcinoma Hep-G2, pancreatic MIA-Pa-Ca2, colon HCT-116,
bladder T-24, head and neck FaDu, uterine MES-SA and MES-SA/Dx5,
neuroblastoma, synovial sarcoma SW-982, osteosarcoma MNNG, Ewings
sarcoma SK-ES-1, glioblastoma A-172, T-98 and LN-18 and leukemia
HL-60, Jurkat, and Raji cell lines. Cell lines, such as breast
MCF-7 and MDA-MB-231, cervical HeLa, uterine SK-UT-1, lung A-549,
tongue SC-25, melanoma A2058, osteosarcoma U-2OS, rhabdomyosarcoma,
fibrosarcoma HT-1080, chondrosarcoma SW-1350 and liposarcoma SW-872
exhibited MMP-dimer secretion only with PMA induction. Cervical
DoTc 2 4510, renal 786-0, breast Colo-824 and HCC SK-Hep-1
exhibited MMP-9 dimer without PMA treatment and increased secretion
with PMA treatment. Zymograms and densitometry analyses of
representative cell lines secreting MMP-9 dimers with and without
PMA induction are discussed below.
 | Table I.Human cancer cell lines expressing
MMP-9 and dimer without and with PMA stimulation. |
Table I.
Human cancer cell lines expressing
MMP-9 and dimer without and with PMA stimulation.
| Human cancer cell
line | MMP-9 expression | Dimer formation |
|---|
|
|
|---|
| Without PMA | With PMA | Without PMA | With PMA |
|---|
| Breast cancer | | | | |
| MDA-MB-231 | − | + | − | ++ |
| MCF-7 | − | + | − | ++ |
| Colo-824 | + | ++ | + | ++ |
| Cervical cancer | | | | |
| HeLa | − | ++ | − | ++ |
| DoTc 2 4510 | + | ++ | + | ++ |
| Uterine cancer | | | | |
| SK-UT-1 | − | ++ | − | ++ |
| MES-SA | − | + | − | − |
| MES-SA/DX5 | − | + | − | − |
| Prostate
cancer | | | | |
| Du-145 | − | + | − | − |
| PC-3 | + | + | − | − |
| Testicular | | | | |
| NTER-2 | − | + | − | − |
| Lung and
mesothelioma | | | | |
| Lung A-549 | − | + | − | + |
| MSTO-211H | + | ++ | − | − |
|
Gastrointestinal | | | | |
| SK-Hep-1
(HCC) | + | ++ | + | ++ |
| HepG2 (HCC) | − | + | − | − |
| M1A-Pa-Ca-2
(pancreas) | + | ++ | − | − |
| HCT-116
(colon) | + | −? | − | − |
| Urological | | | | |
| T-24
(bladder) | − | + | − | − |
| RCC 786-0
(renal) | + | ++ | + | ++ |
| Head and neck | | | | |
| FaDu | − | ++ | − | − |
| Tongue | + | ++ | − | ++ |
| FAHNSCC
(OHSU-973) | + | ++ | − | − |
| Glioblastoma | | | | |
| A-172 | − | + | − | − |
| T-98 | − | + | − | − |
| LN-18 | − | + | − | − |
|
Neuroblastoma | − | + | − | − |
|
Sarcomas-Pediatric | | | | |
| Osteosarcoma
MNNG-HOS | − | + | − | − |
| SK-ES-1 | − | + | − | − |
|
Rhabdomhyosarcoma | + | ++ | − | ++ |
| Osteosarcoma
U-2OS | + | + | − | ++ |
| Sarcomas-Adult | | | | |
| HT-1080 | + | ++ | − | ++ |
|
Chondrosarcoma | + | ++ | − | ++ |
| Liposarcoma | + | ++ | − | ++ |
| Synovial
sarcoma | + | ++ | − | − |
| Hematological | | | | |
| HL-60 | − | + | − | − |
| Jurkat | − | + | − | − |
| Raji | + | ++ | − | − |
| Melanoma | | | | |
| A-2058 | − | ++ | − | ++ |
Dimer secretion with PMA
Breast MCF-7 and MDA-MB-231, cervical HeLa, uterine
SK-UT-1, lung A-549, tongue SC-25, melanoma A2058, osteosarcoma
U-2OS, rhabdomyosarcoma, fibrosarcoma HT-1080, chondrosarcoma
SW-1350 and liposarcoma SW-872 exhibited MMP-dimer secretion only
with PMA induction. See Table I
for relative secretion of MMP-9 monomer and dimer. Zymograms and
densitometry analyses of secretion patterns of MMP-9 monomer and
dimer of the representative cancer cell lines breast, MCF-7, lung
A-549, osteosarcoma U-2OS and chondrosarcoma SW1353 are presented
below.
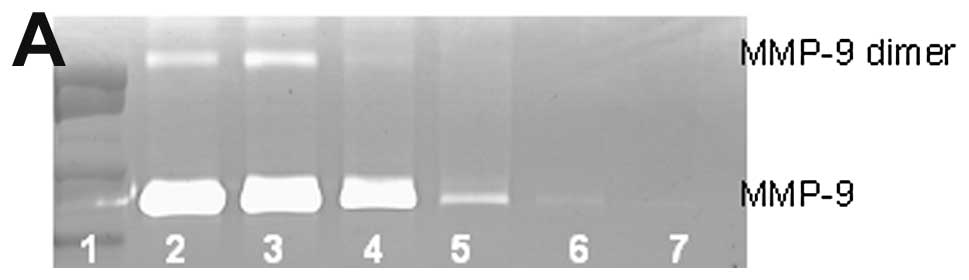
Breast cancer MCF-7 MMP-9 and dimer
secretion
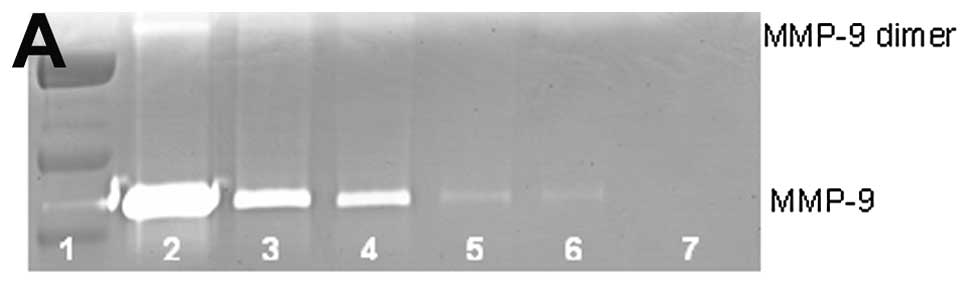
Untreated breast cancer cell line MCF-7 showed
neither MMP-9 nor MMP-9 dimer secretion. PMA (100 ng/ml) strongly
induced MMP-9 and MMP-9 dimer, as shown in Fig. 1. NM inhibited the secretion of both
in a dose-dependent manner with total block of MMP-9 dimer at 100
μg/ml (linear trend R2=0.559) and MMP-9 at 500
μg/ml (linear trend R2=0.866). Secretion of MMP-9
dimer was found to be 13% that of MMP-9.
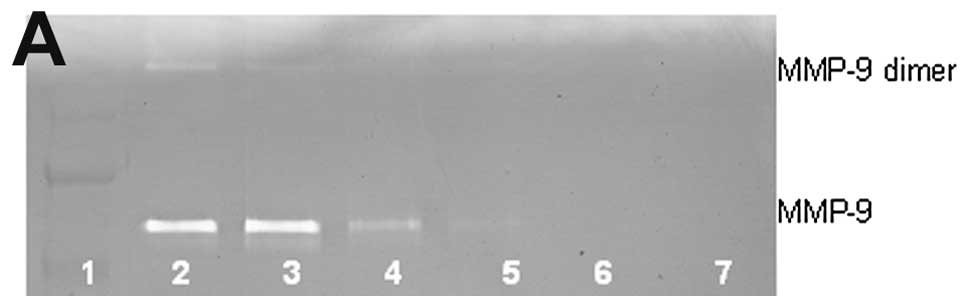
Lung cancer A-549 MMP-9 and dimer
secretion
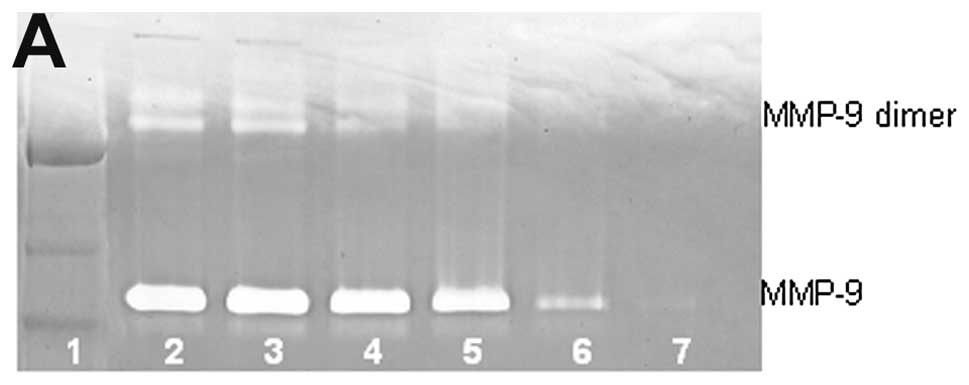
Untreated lung cancer cell line A-549 showed neither
MMP-9 nor MMP-9 dimer secretion. PMA (100 ng/ml) strongly induced
MMP-9 and MMP-9 dimer, as shown in Fig. 2. NM inhibited the secretion of both
in a dose-dependent manner with total block of MMP-9 dimer at 100
μg/ml (linear trend R2=0.560) and MMP-9 at 500
μg/ml (linear trend R2=0.722). Secretion of MMP-9
dimer was found to be 5% that of MMP-9.
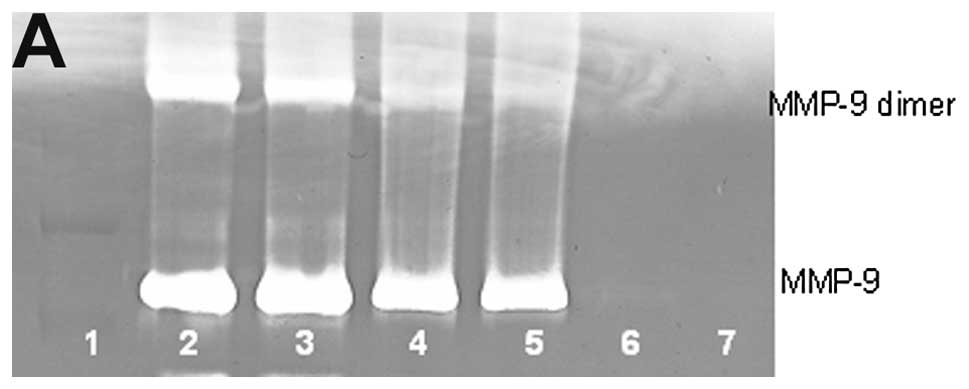
Osteosarcoma U-2OS MMP-9 and dimer
secretion
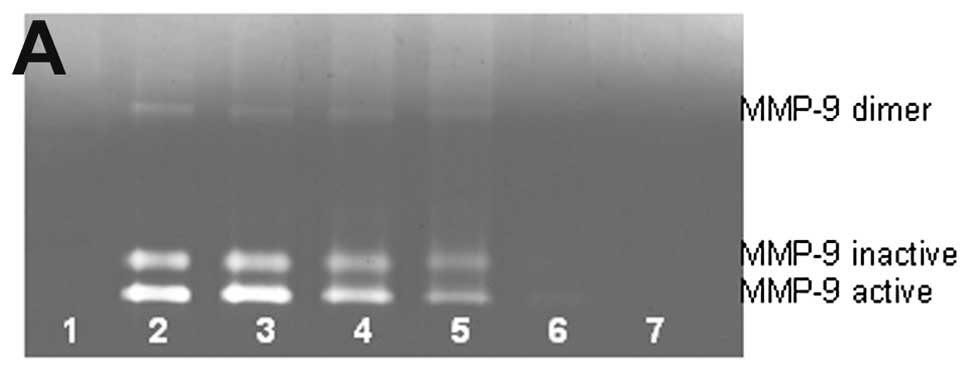
Untreated osteosarcoma cell line U-2OS showed slight
MMP-9 and no MMP-9 dimer secretion. PMA (100 ng/ml) strongly
induced MMP-9 and MMP-9 dimer, as shown in Fig. 3. NM inhibited the secretion of both
in a dose-dependent manner with total block of MMP-9 dimer at 500
μg/ml (linear trend R2=0.780) and MMP-9 at 500
μg/ml (linear trend R2=0.768). Secretion of MMP-9
dimer was found to be 54% that of MMP-9.
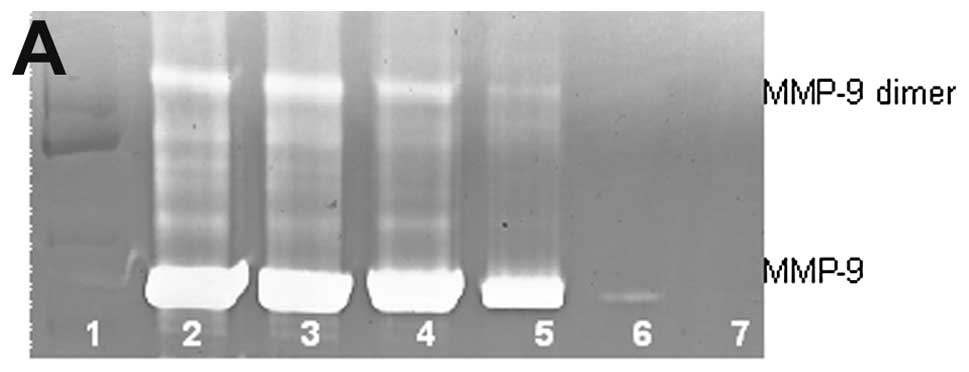
Chondrosarcoma SW-1353 MMP-9 and dimer
secretion
Untreated chondrosarcoma cell line SW-1353 showed
moderate MMP-9 and no MMP-9 dimer secretion. PMA (100 ng/ml)
strongly induced MMP-9 and MMP-9 dimer, as shown in Fig. 4. NM inhibited the secretion of both
in a dose-dependent manner with total block of MMP-9 dimer at 250
μg/ml (linear trend R2=0.843) and MMP-9 at 500
μg/ml (linear trend R2=0.729). Secretion of MMP-9
dimer was found to be 25% that of MMP-9.
Dimer secretion with and without PMA
Cervical DoTc 2 4510, renal 786-0, breast Colo-824
and HCC SK-Hep-1 exhibited MMP-9 dimer secretion without PMA
treatment and increased secretion with PMA treatment. See Table I for relative secretion of MMP-9
monomer and dimer. Zymograms and densitometry analyses of secretion
patterns of MMP-9 monomer and dimer of representative cancer cell
lines cervical DoTc 2 4510, hepatocellular carcinoma Sk-Hep-1 and
leiomyosarcoma SK-UT-1 are presented below.
Cervical cancer DoTc 2 4510 MMP-9 and
dimer secretion
Untreated cervical cancer cell line DoTc 2 4510
showed MMP-9 and MMP-9 dimer secretion which was strongly induced
with PMA (100 ng/ml), as shown in Fig.
5. NM inhibited the secretion of both in a dose-dependent
manner with total block of MMP-9 dimer at 50 μg/ml (linear
trend R2=0.429) and MMP-9 at 500 μg/ml (linear
trend R2=0.769). Secretion of MMP-9 dimer was found to
be 21% that of MMP-9.
Hepatocellular carcinoma SK-Hep-1 MMP-9
and dimer secretion
Untreated and PMA (100 ng/ml)-treated hepato
cellular carcinoma cell line SK-Hep-1 showed both MMP-9 and MMP-9
dimer secretion. NM inhibited the secretion of both in a
dose-dependent manner with total block of MMP-9 dimer at 250
μg/ml (linear trend R2=0.824) and MMP-9 at 1000
μg/ml (linear trend R2=0.746), as shown in
Fig. 6. Secretion of MMP-9 dimer
was found to be 14% that of MMP-9.
Uterine leiomyosarcoma SK-UT-1 MMP-9 and
dimer secretion
PMA (100 ng/ml)-treated leiomyosarcoma cell line
SK-UT-1 showed both MMP-9 and MMP-9 dimer secretion. NM inhibited
the secretion of both in a dose-dependent manner with total block
of MMP-9 dimer at 250 μg/ml (linear trend
R2=0.713) and MMP-9 active and inactive at 500
μg/ml (linear trend R2=0.884 and 0.994,
respectively), as shown in Fig. 7.
Secretion of MMP-9 dimer was found to be 4% that of MMP-9
active.
Discussion
Tumor cell invasion requires the critical steps of
cell attachment, degradation of the ECM and migration through the
disrupted matrix. Matrix metalloproteinases, especially MMP-2 and
MMP-9, play pivotal roles in tumor cell invasion and metastasis due
to their ability to degrade type IV collagen, a major component of
the ECM. In our study, MMP-9 dimer secretion patterns of cancer
cells fell into different categories. We observed no MMP-9 dimer in
prostate DU-145 and PC-3, pancreatic MIA-Pa-Ca2, colon HCT-116,
bladder T-24, head and neck FaDu, glioblastoma A-172, T-98 and
LN-18 and leukemia HL-60, Jurkat, and Raji cell lines. MMP-dimer
secretion only with PMA induction was seen in breast MCF-7 and
MDA-MB-231, uterine SK-UT-1, lung A-549, tongue SC-25, melanoma
A2058, osteosarcoma U-2OS, rhabdomyosarcoma, fibrosarcoma HT-1080,
chondrosarcoma SW-1350 and liposarcoma SW-872.
Cervical HeLa and DoTc 2 4510, renal 786-0 and HCC
SK-Hep-1 exhibited MMP-9 dimer without PMA treatment and increased
secretion with PMA treatment. Sarcomas had the highest levels of
MMP-9 monomer and dimer combined with and without PMA among these
cancer cell lines. In addition, osteosarcoma showed the highest
MMP-9 dimer to MMP-9 ratio, indicating a very aggressive cancer.
Cervical, uterine, and male breast cancer cell lines showed the
next highest levels of combined MMP-9, followed by breast cancer
cell lines. Melanoma, renal, lung, head and neck and HCC showed
lower levels and prostate, glioblastoma, bladder and leukemia cell
lines the lowest. The NM showed dose-dependent inhibition of MMP-9
monomer and dimer in all cell lines tested.
In contrast to the associated toxicity and limited
efficacy of standard cancer chemotherapy and radiation therapy, the
efficacy and safety of dietary and botanical natural compounds in
cancer prevention has been extensively documented (11). The critical aspect in cancer
invasion, metastasis and angiogenesis is stability and integrity of
connective tissue which is compromised by its excessive enzymatic
digestion (MMPs and uPA) and insufficient production due to
deficiency of critical nutrients in cancer patients (5,6,10).
Therefore, our nutrient mixture was formulated by selecting
nutrients that act on critical physiological targets in cancer
progression and metastasis, as documented in both clinical and
experimental studies.
Optimal ECM structure depends upon adequate supplies
of ascorbic acid and the amino acids lysine and proline to ensure
proper synthesis and hydroxylation of collagen fibers. In addition,
lysine contributes to ECM stability as a natural inhibitor of
plasmin-induced proteolysis (9,13).
Manganese and copper are also essential for collagen formation.
There is considerable documentation of the potency of green tea
extract in modulating cancer cell growth, metastasis, angiogenesis,
and other aspects of cancer progression (14–20).
N-acetyl cysteine and selenium have demonstrated inhibition of
tumor cell MMP-9 and invasive activities, as well as migration of
endothelial cells through ECM (21–23).
Ascorbic acid demonstrates cytotoxic and antimetastatic actions on
malignant cell lines (24–29) and cancer patients have been found
to have low levels of ascorbic acid (30,31).
Low levels of arginine, a precursor of nitric oxide (NO), can limit
the production of NO, which has been shown to predominantly act as
an inducer of apoptosis (32).
In conclusion, high MMP-9 and dimer secretion levels
correlated with the most aggressive cancer cell lines. NM was
effective in inhibiting MMP-9 and dimer secretion in all cell lines
tested, suggesting its therapeutic potential as an antimetastatic
agent.
Acknowledgements
This study was funded by Dr. Rath
Health Foundation (Santa Clara, CA, USA), a non-profit
organization. Mr. Monterrey provided assistance in scanning the
gels.
References
|
1.
|
Kleiner DL and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43(Suppl): S42–S51. 1999. View Article : Google Scholar
|
|
2.
|
Yurchenko PD and Schitny JC: Molecular
architecture of basement membranes. FASEB J. 4:1577–1590.
1990.PubMed/NCBI
|
|
3.
|
Barsky SH, Siegel GP, Jannotta F and
Liotta LA: Loss of basement membrane components by invasive tumors
but not by their benign counterparts. Lab Invest. 49:140–147.
1983.PubMed/NCBI
|
|
4.
|
Liotta LA, Tryggvason K, Garbisa A, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
6.
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dufour A, Zucker S, Sampson NS, Kuscu C
and Cao J: Role of matrix metalloproteinase-9 dimers in cell
migration: design of inhibitory pepides. J Biol Chem.
285:35944–35956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bjorland M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
9.
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. J Orthomol Med. 7:17–23. 1992.
|
|
10.
|
Andreasen PA, Kjøller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: a review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Amin ARMR, Kucek O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of high-affinity lysine binding
sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochem Biophys Acta.
1596:182–192. 2002.PubMed/NCBI
|
|
14.
|
Kemberling JK, Hampton JA, Keck RW, Gomez
MA and Selman SH: Inhibition of bladder tumor growth by the green
tea derivative epigallocatechin-3-gallate. J Urol. 170:773–776.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sato D and Matsushima M: Preventive
effects of urinary bladder tumors induced by
N-butyl-N-(4-hydroxybutyl)-nitrosamine in rat by green tea leaves.
Int J Urol. 10:160–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Valcic S, Timmermann BN, Alberts DS,
Wachter GA, Krutzsch M, Wymer J and Guillen JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Mukhtar H and Ahmed N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71(Suppl 6): S1698–S1704. 2000.PubMed/NCBI
|
|
18.
|
Yang GY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimikawa R: Effect of (-) epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hara Y: Green Tea: Health Benefits and
Applications. Marcel Dekker; New York, Basel: 2001, View Article : Google Scholar
|
|
21.
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP 9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
22.
|
Morini M, Cai T, Aluigi MG, Noonan DM,
Masiello L, De Floro S, D’Agostinin F, Albini A and Fassima G: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
23.
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT 1080 tumor cells. J Biol
Chem. 276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Naidu KA, Karl RC and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: The utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH and Park CH:
Differential effects and transport kinetics of ascorbate
derivatives in leukemic cell lines. Anticancer Res. 8:2487–2493.
1998.PubMed/NCBI
|
|
28.
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Harakeh S, Diab-Assaf M, Khalife JC,
Abu-el-Ardat KA, Baydoun E, Niedzwiecki A, El-Sabban ME and Rath M:
Ascorbic acid induces apoptosis in adult T-cell leukemia.
Anticancer Res. 27:289–298. 2007.PubMed/NCBI
|
|
30.
|
Núñez Martín C and Ortiz de Apodaca y Ruiz
A: Ascorbic acid in the plasma and blood cells of women with breast
cancer. The effect of consumption of food with an elevated content
of this vitamin. Nutr Hosp. 10:368–372. 1995.(In Spanish).
|
|
31.
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineo-plastic activity of doxorubicin, cisplatin and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|