Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
most common malignancy of the upper aerodigestive tract that is
characterized by a propensity for invasion and cervival lymph node
metastasis. In spite of advances in surgery, radiation, and
chemotherapy, HNSCCs continue to have poor outcomes and are still
often fatal. Therefore, new and more efficacious therapies are
needed to improve HNSCC survival (1).
The epidermal growth factor receptor (EGFR) is a
receptor tyrosine kinase that regulates crucial cellular signaling
pathways contributing to tumor progression (2,3) and
is frequently amplified and overexpressed in a high percentage of
HNSCCs (4,5). Preclinical studies have successfully
demonstrated the antitumor effects of EGFR targeting, and the Food
and Drug Administration (FDA) has approved the clinical use of EGFR
monoclonal antibody cetuximab in HNSCC (6). However, when used alone, EGFR
targeting shows insufficient suppression of HNSCCs (7,8);
hence, various combination strategies are under investigation with
an aim to improve HNSCC treatments and prognosis (9–11).
Accumulating studies have showed that EGFR
stimulation induces matrix metalloproteinase (MMP) expression,
which degrade extracellular matrix and is critical for tumor
development (12,13). Recently, extracellular matrix
metalloproteinase inducer (EMMPRIN), also known as CD147, has been
identified as a member of the immunoglobulin superfamily. In
particular, EMMPRIN has been reported to be highly expressed in
malignant tumors (14–16), including HNSCCs (17) and has been found to induce tumor
progression through MMP expression (18–20).
Although it has been reported that EGFR expression is associated
with EMMPRIN in some solid tumors (21,22),
the relationship between EMMPRIN and EGFR in oncogenicity is not
fully understood, particularly in head and neck cancers.
This study was undertaken to evaluate the
relationship between EMMPRIN and EGFR and to test the hypothesis
that EMMPRIN mediates EGFR-induced HNSCC tumorigenic behavior to
determine if combined inhibition of EMMPRIN and EGFR is a rational
treatment approach to improve prognosis in HNSCC.
Materials and methods
Cell and cell culture
The HNSCC cell line SAS, a human tongue squamous
cell carcinoma cell line, was used. Cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated
fetal bovine serum (FBS) and incubated at 37°C in the presence of
5% CO2.
Western blotting
Protein expression was detected by western blotting.
Cells were lysed in detergent, and protein levels were determined
using the Bio-Rad protein assay method. Total protein was separated
on 8% sodium dodecyl sulfatepolyacrylamide gel electrophoresis
(SDS-PAGE) gel and transferred to a nitrocellulose membrane. The
blot was incubated with each antibody and developed using the
Luminor Regent (Santa Cruz Biotechnology).
Gelatin zymography
Gelatinase expression was detected in conditioned
media by gelatin zymography. Cells were cultured for 48 h in
serum-free DMEM with or without epidermal growth factor (EGF). This
was followed by the detection of gelatinolytic activity in the
conditioned media by gelatin zymography. Using a 7.5% separating
gel containing 0.3 mg/ml gelatin, the conditioned medium was
resolved by SDS-PAGE under non-reducing conditions. The gels were
washed and then incubated for 24 h at 37°C in a reaction buffer
before being stained. After destaining, gelatinolytic activity on
the gel was detected as clear bands on a blue background of
undigested gelatin.
Small interfering RNA (siRNA) and siRNA
transfection
EGFR siGENOME siRNA, EMMPRIN siGENOME siRNA
(Dharmacon RNA Technologies, Lafayette, CO, USA) was transfected
into HNSCC cells for each protein silencing. The siGENOME
non-targeting siRNA was used as a control. The siRNA transfections
were performed using Lipofectamine 2000 (Life Technology Inc.).
Matrigel invasion and cell migration
assays
Cell invasion was evaluated in vitro using
Matrigel-coated semipermeable modified Boyden inserts with a pore
size of 8 μm (Becton-Dickinson/Biocoat, Bedford, MA, USA).
Cell migration was evaluated in vitro using semipermeable
modified Boyden inserts with a pore size of 8 μm
(Becton-Dickinson/Biocoat). For each assay, cells were plated in
duplicate at a density of 5×103 cells/well for the
invasion assay or 3×104 cells/well for the migration
assay. Plating was carried out on serum-free DMEM with 100 nM EGF
for the invasion assay, or either the control vehicle (DMSO),
AG1478 (10 μM), anti-EMMPRIN function-blocking antibody (10
μg/ml), or a combination of AG1478 and anti-EMMPRIN for the
migration assay in the inserts. The cells were plated in 96-well
plates to serve as loading controls. Both the insert and the
holding well were subjected to the same medium composition with the
exception of serum. The insert contained no serum, whereas the
lower well contained 10% FBS that served as a chemoattractant.
After 24-h treatment at 37°C in a 5% CO2 incubator, the
cells in the insert were removed by gentle wiping using a cotton
swab. Cells on the reverse side of the insert were fixed and
stained with Diff-Quik® (Sysmex, Kobe, Japan) according
to the manufacturer’s instructions. Cells plated in 24-well plates
were subjected to
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
assays, and the cell number across the groups were normalized. The
number of invading or migrating cells was adjusted accordingly.
Proliferation assay
SAS cells were plated in triplicate at a density of
3×104 cells/well and allowed to seed overnight in a
12-well plate. Cells were then treated with either the control
vehicle (DMSO), AG1478 (10 μM), anti-EMMPRIN
function-blocking antibody (10 μg/ml), or a combination of
AG1478 and anti-EMMPRIN antibody in DMEM with 10% FBS. At selected
time-points, cells were trypsinized and stained with trypan blue,
and viable cells were counted using a hemocytometer.
Statistical analysis
The statistical significance of differences was
assessed using Wilcoxon-Mann-Whitney 2-tailed exact test.
Results
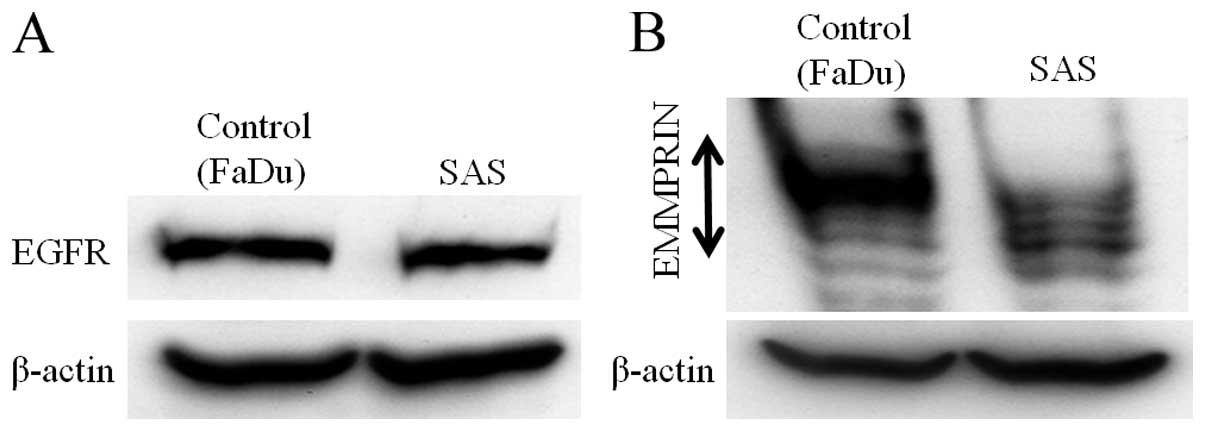
HNSCC cell line SAS expresses EGFR and
EMMPRIN
To evaluate the role of the interaction between EGFR
and EMMRPIN, both protein expressions were determined by
immunoblotting in the HNSCC cell line SAS. FaDu cells, in which the
expression of EGFR and EMMPRIN has been reported, were used as the
control (23,24) (Fig.
1).
EGF induces EMMPRIN and MMP-9
expression
Coexpression of EGFR and EMMPRIN in clinical samples
has been identified for its importance for tumor progression or
prognosis in several solid tumors (21,22);
however, the relationship between these factors have not been
studied completely. Therefore, EGF, a ligand of EGFR, was added to
the culture media at various concentrations to stimulate EGFR.
EMMPRIN expression was determined by immunoblotting and the
expression of gelatinase, MMP-2 and MMP-9, which plays an important
role in tumor invasion and metastasis through basement membrane was
detected by gelatin zymography. EGF increased EMMPRIN expression in
a dose- (Fig. 2A) and time-
(Fig. 2B) dependent manner.
Interestingly, EGF induced MMP-9 expression, and not MMP-2
expression, in culture media in a dose-dependent manner (Fig. 2C).
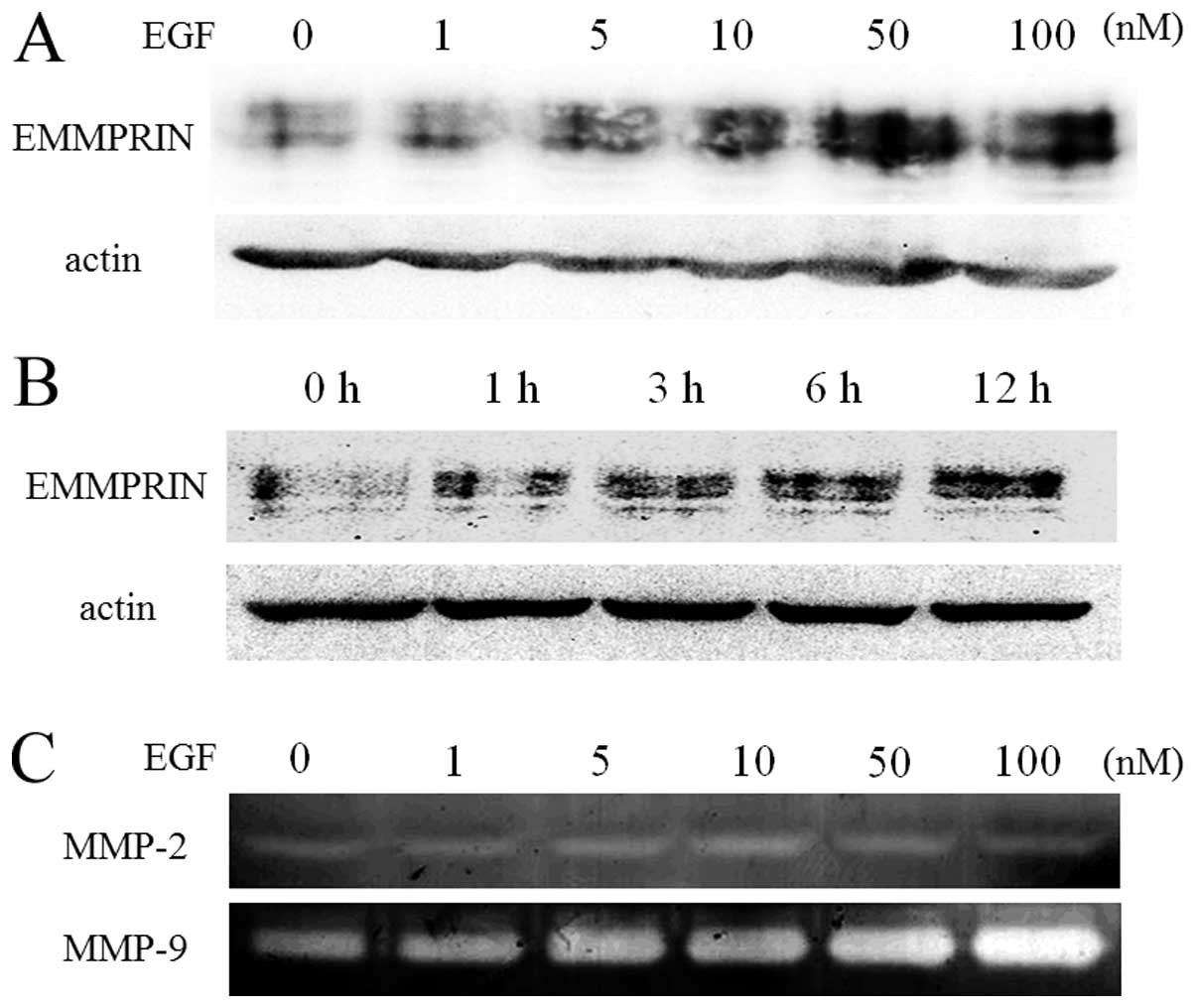 | Figure 2.EGF increases EMMPRIN and MMP-9
expression. SAS cells were plated and allowed to seed overnight in
a 6-well plate. The cells were grown in serum-free DMEM for 24 h
prior to EGF treatment. Next, the cells were treated with
serum-free DMEM containing increasing concentrations of EGF (0–100
nM) for 24 h or 10 nM for 0–12 h. The cells were lysed and
subjected to immunoblotting to determine EMMPRIN protein
expression, EGF increased EMMPRIN expression dose- (A) and a
time-dependently (B). SAS cells were also treated with 10 nM for 24
h. The culture medium was harvested followed by gelatin zymography
for the evaluation of gelatinase activity, EGF induced MMP-9
expression, but not MMP-2 expression, in the culture media in a
dose-dependent manner (C). DMEM, Dulbecco’s modified Eagle’s
medium; EGF, epidermal growth factor; EGFR, epidermal growth factor
receptor; EMMPRIN, extracellular matrix metalloproteinase inducer;
HNSCC, head and neck squamous cell carcinoma. |
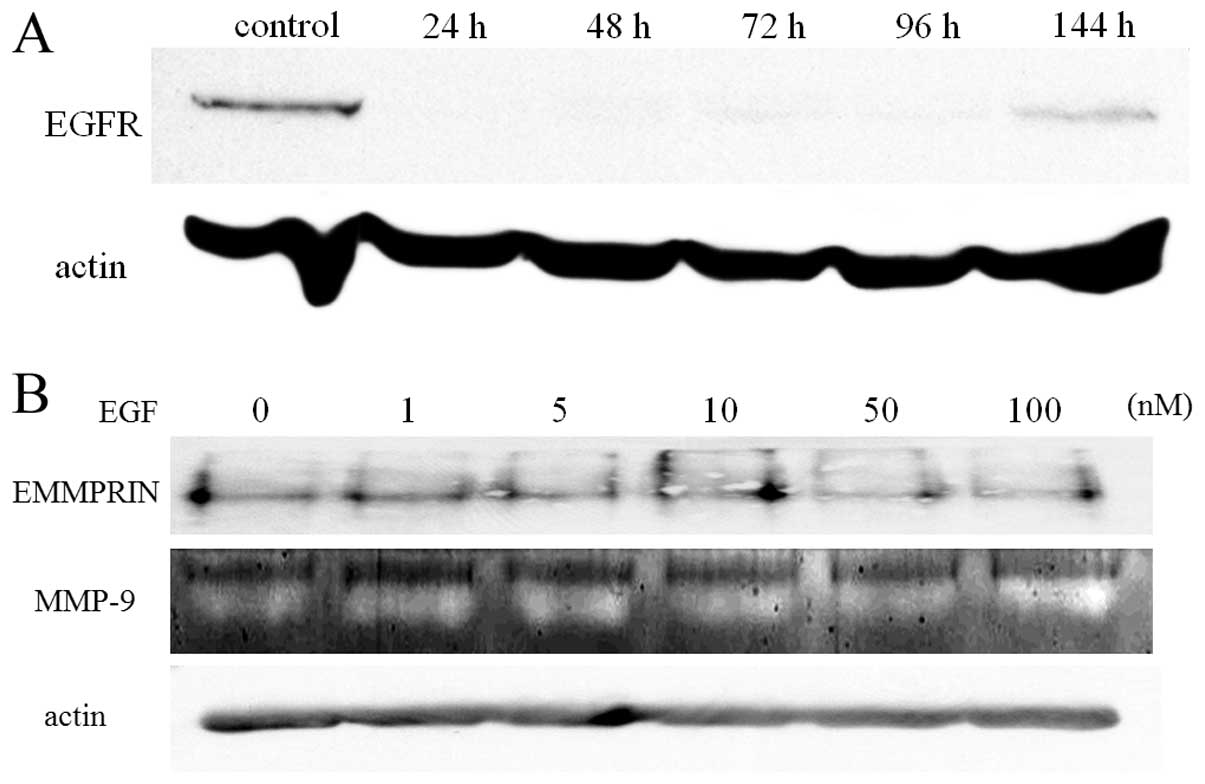
EGFR mediates EGF-induced EMMPRIN and
MMP-9 upregulation
The results shown in Fig. 2 indicate that EGF induces EMMPRIN
and MMP-9 expression in the HNSCC cell line. Although EGF is a
representative ligand for EGFR, it is unclear whether EGF induces
the phenomena mentioned in Fig. 2.
To confirm whether EGFR mediates EGF-induced EMMPRIN and MMP-9
upregulation in the cells, we used siRNA on EGFR before culturing
cells in the presence or absence of EGF. As shown in Fig. 3B, the upregulation of EMMPRIN and
MMP-9 induced by EGF, shown in Fig. 2A
and B, were not observed when EGFR was suppressed by siRNA.
This result indicates that EGF stimulates EGFR and that this
interaction resulted in the upregulation of EMMPRIN and MMP-9.
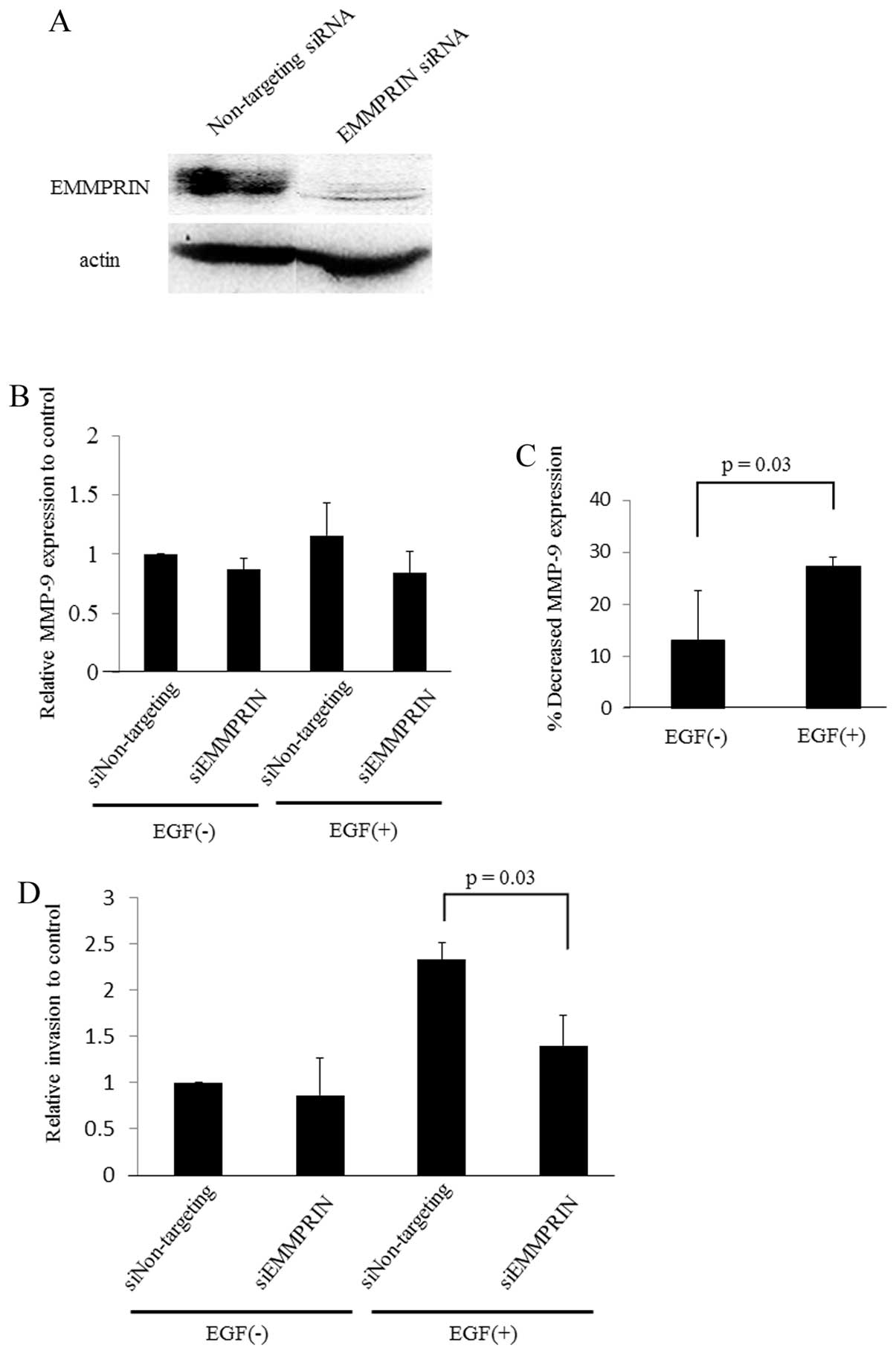
EMMPRIN plays a critical role in
EGFR-induced MMP-9 expression and cell invasion
The ability of EGFR to promote tumor progression is
well documented (2,3,25–27).
In addition, accumulating studies have reported that EMMPRIN
induces malignant phenotypes such as invasion, proliferation, and
MMP expression in some solid tumors including HNSCCs (28–31).
Based on these reports and our findings shown in Fig. 3, we hypothesized that EGFR
stimulation induces EMMPRIN expression, which induces malignant
phenotypes in SAS. To investigate the importance of EMMPRIN in the
EGFR-induced tumorigenic process, we analyzed MMP-9 expression and
cell invasion in the presence or absence of siRNA-targeting
EMMPRIN, with or without EGF in the cell culture media.
The results showed a trend for SAS to decrease MMP-9
expression when EMMPRIN was knocked-down in either the presence or
absence of EGF (Fig. 4B). However,
the percent decrease in MMP-9 was significantly higher in the
presence of EGF (Fig. 4C). This
suggests that EMMPRIN plays an important role in MMP-9 expression
induced by EGFR stimulation and that both EGFR and EMMPRIN are
crucial for MMP-9 expression in SAS. In addition, cell invasiveness
was significantly decreased when EMMPRIN was silenced by siRNA
under EGFR stimulation by EGF (Fig.
4D), indicating that EMMPRIN has a key role in EGFR-induced
HNSCC cell invasion.
Combined inhibition of EGFR and EMMPRIN
prevents the growth and migration of HNSCC cells
The aforementioned results indicate that EGFR
induces HNSCC malignant phenotypes through EMMPRIN. Indeed, it is
well known that EGFR induces oncogenicity (32) and that its targeting is effective
in managing various solid tumors (33). In addition, both EMMPRIN
tumorigenicity and the antitumor effect of EMMPRIN inhibition have
been investigated previously (34,35).
Hence, we examined the combined effect of EGFR and EMMPRIN in HNSCC
cell proliferation and migration.
To inhibit EGFR and EMMPRIN function, cells were
treated with or without the EGFR antibody AG1478 and/or the EMMPRIN
function-blocking antibody. As shown in Fig. 5B and C, cell proliferation and
migration were significantly decreased by both AG1478 and EMMPRIN
function-blocking antibody. Furthermore, combined inhibition showed
a more marked and significant reduction in proliferation and
migration when compared with single inhibition of EGFR and
EMMPRIN.
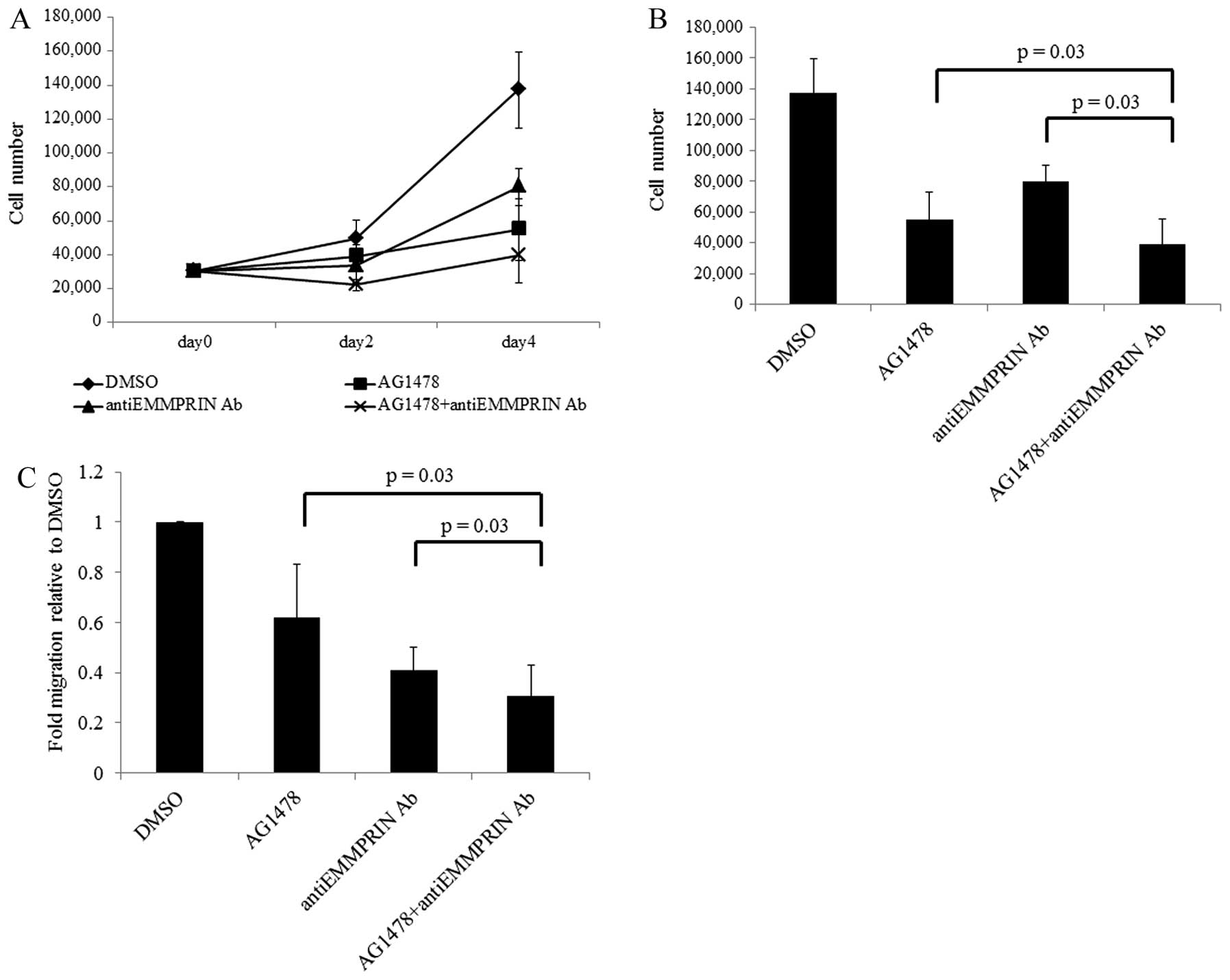 | Figure 5.Combined inhibition of EMMPRIN and
EGFR inhibits growth and migration of SAS cells. (A) A
proliferation assay of SAS HNSCC cells treated with AG1478 and/or
anti-EMMPRIN function-blocking antibody. After 24 h, cells were
plated in 12-well plates in DMEM with 10% FBS, and the growth media
was replaced with media containing each agent. Cells were harvested
and counted by vital dye exclusion. Cell counts on days 2 and 4
from 3 independent experiments are presented. (B) Cell numbers on
day 4 in each condition are presented. (C) For migration
experiments, SAS cells were plated in inserts and cultured with
each agent in DMEM with 10% FBS, After 48-h incubation, migrating
cells on the reverse side of the insert were counted using light
microscopy. The experiment was repeated 3 times with similar
results. Proliferation and invasion of SAS cells treated with the
combination of AG1478 and anti-EMMPRIN antibody decreased to 29.0%
(±12.6%) and 30.6% (±12.5%), respectively, of the control vehicle.
This was lower than AG1478 alone [41.1±17.7% (P=0.03) and
61.9±21.3% (P=0.03), respectively] or anti-EMMPRIN antibody alone
[60.0±15.3% (P=0.03) and 40,9±9,1% (P=0.03), respectively] using
the Wilcoxon-Mann-Whitney 2-tailed exact test. EGF, epidermal
growth factor; EGFR, epidermal growth factor receptor; EMMPRIN,
extracellular matrix metalloproteinase inducer; FBS, fetal bovine
serum; HNSCC, head and neck squamous cell carcinoma; MMP-9, matrix
metallopeptidase 9; siRNA, small interfering RNA. |
Discussion
This study demonstrated that in HNSCC, EMMPRIN
partly promotes increased MMP-9 expression and cell invasion
induced by EGF stimulation through EGFR. Furthermore, combined
inhibition of EMMPRIN and EGFR shows effective suppression of HNSCC
proliferation and migration. These results suggest the need for
further investigation into the mechanism of combined EMMPRIN and
EGFR targeting in cancers that express increased levels of these
proteins.
HNSCC is the most common malignancy of the upper
aerodigestive tract (36), and
despite advancements in conventional treatment, the prognosis has
remained unchanged over the past several decades with 30% patient
deaths every year (37).
Therefore, new and more effective therapeutic approaches are needed
to improve HNSCC survival (1).
Recently, molecular targeting has been introduced for the treatment
of malignancy, including HNSCC (38). EGFR is one such target that is
thought to be an ideal therapeutic target (39) because it is frequently amplified
and overexpressed in high percentage of HNSCCs (4). However, the use of EGFR inhibitors as
monotherapy have yielded low response rates in clinical practice
(8). Although it has been reported
that an EGFR variant is a possible reason for resistance to EGFR
targeting in HNSCCs (37,40), the tumor features that contribute
to EGFR targeting resistance are incompletely understood. A
promising solution to improve the clinical response rate may be
through the combination of EGFR inhibitors with other treatment
modalities. Some reports have showed promising antitumor efficacy
by blocking EGFR with other tumorigenic factors, such as c-Met
(11), Src (10), signal transducer and activator of
transcription 3 (STAT3) (9) and
G-protein-coupled receptor (GPCR) (41).
The importance of EMMPRIN in tumor progression and
its association with poor prognosis is widely known in solid tumors
including HNSCC. In addition, there are accumulating reports
suggesting that EMMPRIN is a negative prognostic factor in
malignant tumors (42–44). We previously reported that the
EMMPRIN-EMMPRIN hemophilic interaction or EMMPRIN-cyclophilin A
interaction play an important role in MMP expression and
activation, as well as in invasion and migration (24,28).
Recently, several studies have showed that EMMPRIN blocking or
silencing is efficient for cancer suppression (35,45),
particularly in HNSCCs, where there have been several reports
showing the possibility of EMMPRIN targeting therapy for tumor
suppression in vivo. Hence, it appears that there is a more
detailed mechanism underlying EMMPRIN-mediated cancer progression
and the relationship with other oncogenic factors that contribute
to an improvement in HNSCC progression.
The relationship between EMMPRIN, MMP, and EGFR have
been reported in colon (21) and
breast (22) cancers. In these
studies, increased EGFR expression correlated with MMP-9 and
EMMPRIN expression in clinical samples, but details of the
interaction mechanisms between EGFR, MMP-9, and EMMPRIN expression
were not described. Here we showed that EGFR induces EMMPRIN
expression, with EMMPRIN mediating EGFR-induced MMP-9 expression
and HNSCC invasion. Our results may account for the expression
mechanisms of EGFR, MMP-9, and EMMPRIN in previous studies.
However, EGFR silencing did not abolish the expression of EMMPRIN
(data not shown). These results suggest that the expression of
EMMPRIN is not completely dependent on EGFR expression and that
there may be an independent EMMPRIN tumorigenic pathway involved.
Therefore, blocking of both EGFR and EMMPRIN may be necessary to
regulate HNSCC progression.
In this study, we showed that the combined
inhibition of EGFR and EMMPRIN effectively reduces HNSCC cell
proliferation and migration. Therefore, complementary blockade of
EGFR and EMMPRIN oncogenic pathways might provide a more
efficacious treatment approach. Further research into the role of
EMMPRIN, and of the apparent synergistic effect, may contribute to
improved prognoses in HNSCC.
Acknowledgements
This study was supported by JSPS
KAKENHI grant no. 24791740.
References
|
1.
|
Leon X, Hitt R, Constenla M, et al: A
retrospective analysis of the outcome of patients with recurrent
and/or metastatic squamous cell carcinoma of the head and neck
refractory to a platinum-based chemotherapy. Clin Oncol.
17:418–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Carpenter G and Cohen S: Epidermal growth
factor. J Biol Chem. 265:7709–7712. 1990.
|
|
3.
|
Mendelsohn J and Baselga J: The EGF
receptor family as targets for cancer therapy. Oncogene.
19:6550–6565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Grandis JR and Tweardy DJ: Elevated levels
of transforming growth factor alpha and epidermal growth factor
receptor messenger RNA are early markers of carcinogenesis in head
and neck cancer. Cancer Res. 53:3579–3584. 1993.PubMed/NCBI
|
|
5.
|
Santini J, Formento JL, Francoual M, et
al: Characterization, quantification, and potential clinical value
of the epidermal growth factor receptor in head and neck squamous
cell carcinomas. Head Neck. 13:132–139. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cohen EE, Rosen F, Stadler WM, et al:
Phase II trial of ZD1839 in recurrent or metastatic squamous cell
carcinoma of the head and neck. J Clin Oncol. 21:1980–1987. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Soulieres D, Senzer NN, Vokes EE, Hidalgo
M, Agarwala SS and Siu LL: Multicenter phase II study of erlotinib,
an oral epidermal growth factor receptor tyrosine kinase inhibitor,
in patients with recurrent or metastatic squamous cell cancer of
the head and neck. J Clin Oncol. 22:77–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sen M, Joyce S, Panahandeh M, et al:
Targeting Stat3 abrogates EGFR inhibitor resistance in cancer. Clin
Cancer Res. 18:4986–4996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Koppikar P, Choi SH, Egloff AM, et al:
Combined inhibition of c-Src and epidermal growth factor receptor
abrogates growth and invasion of head and neck squamous cell
carcinoma. Clin Cancer Res. 14:4284–4291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Xu H, Stabile LP, Gubish CT, Gooding WE,
Grandis JR and Siegfried JM: Dual blockade of EGFR and c-Met
abrogates redundant signaling and proliferation in head and neck
carcinoma cells. Clin Cancer Res. 17:4425–4438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Barsky SH, Siegal GP, Jannotta F and
Liotta LA: Loss of basement membrane components by invasive tumors
but not by their benign counterparts. Lab Invest. 49:140–147.
1983.PubMed/NCBI
|
|
13.
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Caudroy S, Polette M, Tournier JM, et al:
Expression of the extracellular matrix metalloproteinase inducer
(EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary
and breast lesions. J Histochem Cytochem. 47:1575–1580. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Polette M, Gilles C, Marchand V, et al:
Tumor collagenase stimulatory factor (TCSF) expression and
localization in human lung and breast cancers. J Histochem
Cytochem. 45:703–709. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Muraoka K, Nabeshima K, Murayama T, Biswas
C and Koono M: Enhanced expression of a tumor-cell-derived
collagenase-stimulatory factor in urothelial carcinoma: its
usefulness as a tumor marker for bladder cancers. Int J Cancer.
55:19–26. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Rosenthal EL, Shreenivas S, Peters GE,
Grizzle WE, Desmond R and Gladson CL: Expression of extracellular
matrix metalloprotease inducer in laryngeal squamous cell
carcinoma. Laryngoscope. 113:1406–1410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Li R, Huang L, Guo H and Toole BP: Basigin
(murine EMMPRIN) stimulates matrix metalloproteinase production by
fibroblasts. J Cell Physiol. 186:371–379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sameshima T, Nabeshima K, Toole BP, et al:
Glioma cell extracellular matrix metalloproteinase inducer
(EMMPRIN) (CD147) stimulates production of membrane-type matrix
metalloproteinases and activated gelatinase A in co-cultures with
brain-derived fibroblasts. Cancer Lett. 157:177–184. 2000.
View Article : Google Scholar
|
|
20.
|
Guo H, Zucker S, Gordon MK, Toole BP and
Biswas C: Stimulation of matrix metalloproteinase production by
recombinant extracellular matrix metalloproteinase inducer from
transfected Chinese hamster ovary cells. J Biol Chem. 272:24–27.
1997. View Article : Google Scholar
|
|
21.
|
Jin JS, Wu CY, Lin YF, et al: Higher
expression of epidermal growth factor receptor is associated with
extracellular matrix metalloprotease inducer in colorectal
adenocarcinoma: tissue microarray analysis of immunostaining score
with clinicopathological parameters. Dis Markers. 22:309–316. 2006.
View Article : Google Scholar
|
|
22.
|
Menashi S, Serova M, Ma L, Vignot S,
Mourah S and Calvo F: Regulation of extracellular matrix
metalloproteinase inducer and matrix metalloproteinase expression
by amphiregulin in transformed human breast epithelial cells.
Cancer Res. 63:7575–7580. 2003.
|
|
23.
|
Toulany M, Dittmann K, Baumann M and
Rodemann HP: Radiosensitization of Ras-mutated human tumor cells in
vitro by the specific EGF receptor antagonist BIBX1382BS. Radiother
Oncol. 74:117–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Takahashi M, Suzuki S and Ishikawa K:
Cyclophilin A-EMMPRIN interaction induces invasion of head and neck
squamous cell carcinoma. Oncol Rep. 27:198–203. 2012.PubMed/NCBI
|
|
25.
|
Rubin Grandis J, Melhem MF, Gooding WE, et
al: Levels of TGF-alpha and EGFR protein in head and neck squamous
cell carcinoma and patient survival. J Natl Cancer Inst.
90:824–832. 1998.
|
|
26.
|
Ang KK, Berkey BA, Tu X, et al: Impact of
epidermal growth factor receptor expression on survival and pattern
of relapse in patients with advanced head and neck carcinoma.
Cancer Res. 62:7350–7356. 2004.PubMed/NCBI
|
|
27.
|
Gupta AK, McKenna WG, Weber CN, et al:
Local recurrence in head and neck cancer: relationship to radiation
resistance and signal transduction. Clin Cancer Res. 8:885–892.
2002.PubMed/NCBI
|
|
28.
|
Suzuki S, Sato M, Senoo H and Ishikawa K:
Direct cell-cell interaction enhances pro-MMP-2 production and
activation in co-culture of laryngeal cancer cells and fibroblasts:
involvement of EMMPRIN and MT1-MMP. Exp Cell Res. 293:259–266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Hanata K, Yamaguchi N, Yoshikawa K, et al:
Soluble EMMPRIN (extra-cellular matrix metalloproteinase inducer)
stimulates the migration of HEp-2 human laryngeal carcinoma cells,
accompanied by increased MMP-2 production in fibroblasts. Arch
Histol Cytol. 70:267–277. 2007. View Article : Google Scholar
|
|
30.
|
Bordador LC, Li X, Toole B, et al:
Expression of emmprin by oral squamous cell carcinoma. Int J
Cancer. 85:347–352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou
T and Rosenthal EL: CD147 and AGR2 expression promote cellular
proliferation and metastasis of head and neck squamous cell
carcinoma. Exp Cell Res. 318:1788–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37(Suppl 4): S9–S15. 2001.
View Article : Google Scholar
|
|
33.
|
Laskin JJ and Sandler AB: Epidermal growth
factor receptor: a promising target in solid tumours. Cancer Treat
Rev. 30:1–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Tang X, Guo N, Xu L, Gou X and Mi M:
CD147/EMMPRIN: an effective therapeutic target for hepatocellular
carcinoma. J Drug Target. 21:224–231. 2012. View Article : Google Scholar
|
|
35.
|
Yang X, Zhang P, Ma Q, et al: EMMPRIN
silencing inhibits proliferation and perineural invasion of human
salivary adenoid cystic carcinoma cells in vitro and in vivo.
Cancer Biol Ther. 13:85–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
37.
|
Sok JC, Coppelli FM, Thomas SM, et al:
Mutant epidermal growth factor receptor (EGFRvIII) contributes to
head and neck cancer growth and resistance to EGFR targeting. Clin
Cancer Res. 12:5064–5073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Goerner M, Seiwert TY and Sudhoff H:
Molecular targeted therapies in head and neck cancer - an update of
recent developments. Head Neck Oncol. 2:82010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Cassell A and Grandis JR: Investigational
EGFR-targeted therapy in head and neck squamous cell carcinoma.
Expert Opin Investig Drugs. 19:709–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Wheeler SE, Suzuki S, Thomas SM, et al:
Epidermal growth factor receptor variant III mediates head and neck
cancer cell invasion via STAT3 activation. Oncogene. 29:5135–5145.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Bhola NE, Thomas SM, Freilino M, et al:
Targeting GPCR-mediated p70S6K activity may improve head and neck
cancer response to cetuximab. Clin Cancer Res. 17:4996–5004. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Tian L, Zhang Y, Chen Y, Cai M, Dong H and
Xiong L: EMMPRIN is an independent negative prognostic factor for
patients with astrocytic glioma. PLoS One. 8:e580692013. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Zheng HC, Takahashi H, Murai Y, et al:
Upregulated EMMPRIN/CD147 might contribute to growth and
angiogenesis of gastric carcinoma: a good marker for local invasion
and prognosis. Br J Cancer. 95:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Reimers N, Zafrakas K, Assmann V, et al:
Expression of extracellular matrix metalloproteases inducer on
micrometastatic and primary mammary carcinoma cells. Clin Cancer
Res. 10:3422–3428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Nakamura K, Kodama J, Hongo A and
Hiramatsu Y: Role of EMMPRIN in endometrial cancer. BMC Cancer.
12:1912012. View Article : Google Scholar : PubMed/NCBI
|