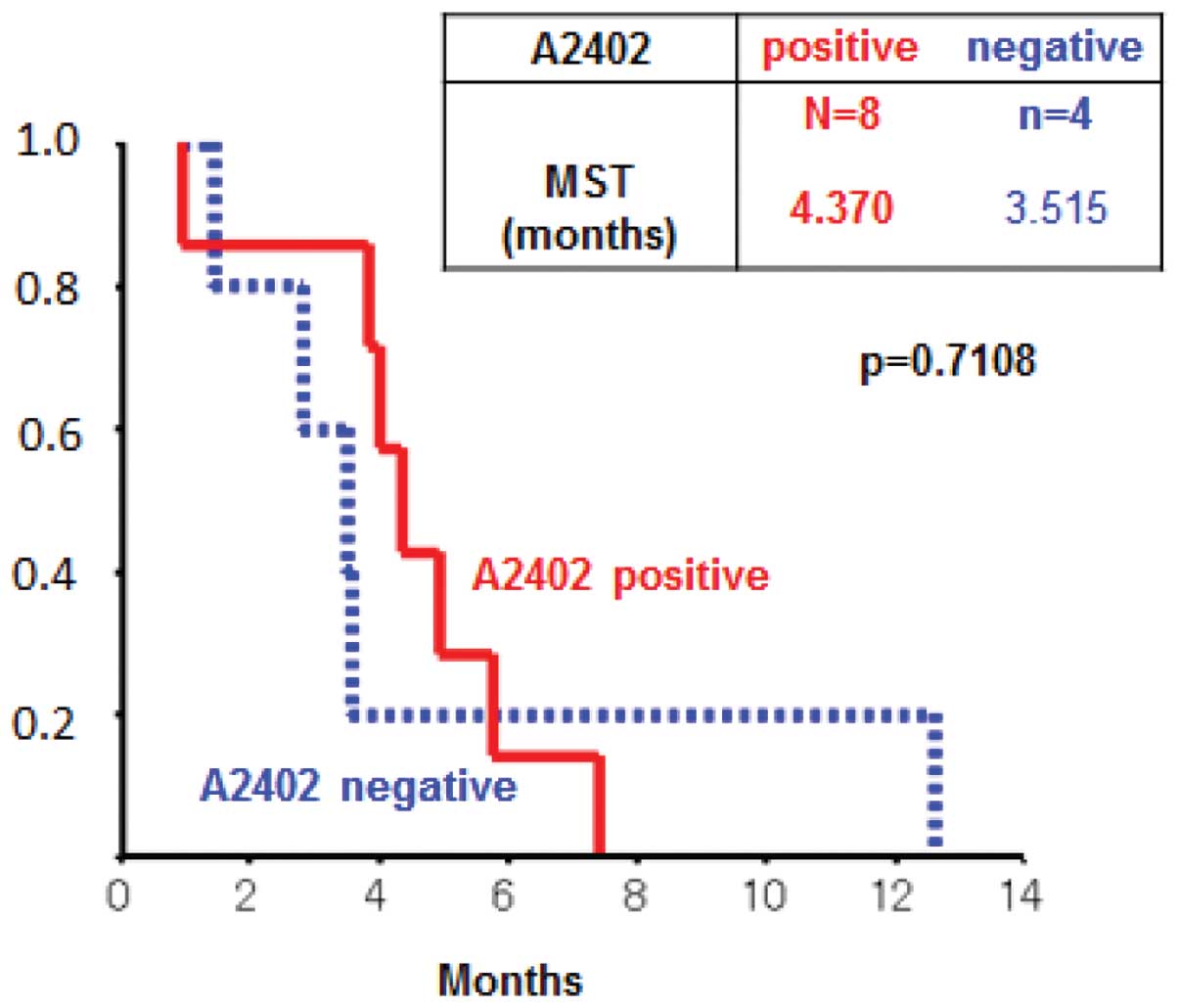
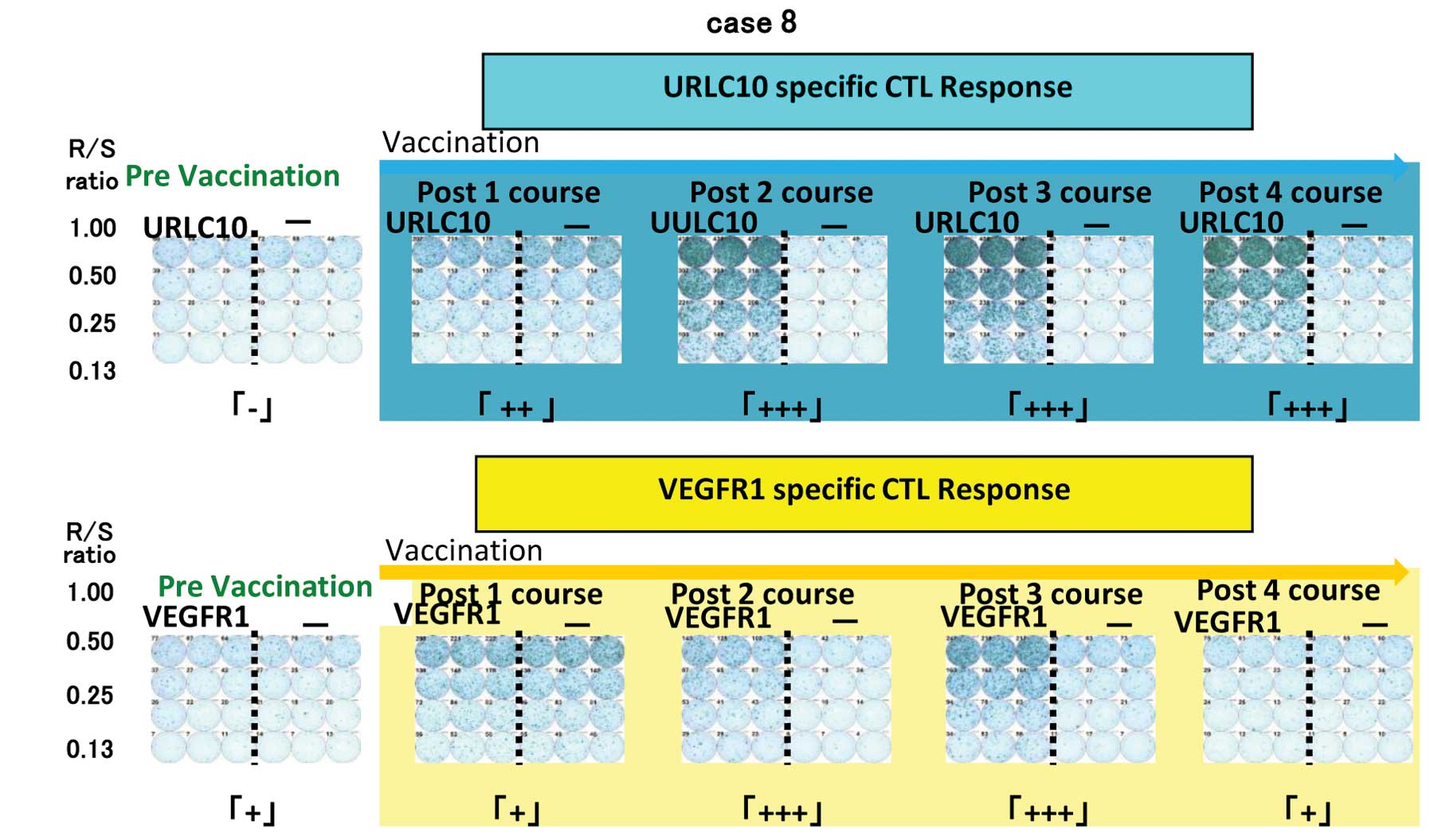
|
1.
|
Noguchi M, Mine T, Komatsu N, Suekane S,
Moriya F, Matsuoka K, Yutani S, et al: Assessment of immunological
biomarkers in patients with advanced cancer treated by personalized
peptide vaccination. Cancer Biol Ther. 10:1266–1279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordic F, et al: Trastuzumab in
combination with chemotherapy versus chemotherapy alone for
treatment of HER2-positive advanced gastric or gastro-esophageal
junction cancer (ToGA): a phase 3, open-label, randomized control
trial. Lancet. 376:687–697. 2010. View Article : Google Scholar
|
|
3.
|
Cheever MA and Higano CS: PROVENGE
(Sipuleusel-T) in prostate cancer: the first FDA-approved
therapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kono K, Mizukami Y, Daigo Y, Takano A,
Masuda K, Yoshida K, Tsunoda T, et al: Vaccination with multiple
peptides derived from novel cancer-testis antigens can induce
specific T-cell esophageal cancer. Cancer Sci. 100:1502–1509. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Miyazawa M, Ohsawa R, Tsunoda T, Hirono S,
Kawai M, Tani M, Nakamura Y, et al: Phase I clinical trial using
peptide vaccine for human vascular endothelial growth factor
receptor 2 in combination with gemcitabine for patients with
advanced pancreatic cancer. Cancer Sci. 101:433–439. 2010.
View Article : Google Scholar
|
|
6.
|
Kato J, Nagahara A, Kotani T, Higashihara
Y, Matsumura Y, Osada T, Yoshizawa T, et al: Phase I clinical trial
of peptide vaccination with KIF20 and VEGFR1 epitope peptides in
patients with advanced pancreatic cancer. Pancreatic Dis Ther.
2:1022012. View Article : Google Scholar
|
|
7.
|
Kawada J, Wada H, Isobe M, Gnjatic S,
Nishikawa H, Jungbluth AA, Okazaki N, et al: Heteroclitic
serological response in esophageal and prostate cancer patient
after NY-ESO-1 protein vaccination. Int J Cancer. 130:584–592.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Watanabe T, Suda T, Tsunoda T, Uchida N,
Ura K, Kato T, Hasegawa S, et al: Identification of immunoglobulin
super-family 11 (IGSF11) as a novel target for cancer immunotherapy
of gastrointestinal and hepatocellular carcinoma. Cancer Sci.
96:498–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Komori H, Nakahara T, Senju S, Yoshitake
Y, Motomura Y, Ikuta Y, Fukuma D, et al: Identification of HLA-A2-
or HLA-A24-restricted CTL epitopes possibly useful for
glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin
Cancer Res. 12:2689–2697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Imai K, Hirata S, Irie A, Senju S, Ikuta
Y, Yokomine K, Harao M, et al: Identification of a novel
tumor-associated antigen, cadherin 3/P-cadherin, as a possible
target for immunotherapy of pancreatic, gastric, and colorectal
cancer. Clin Cancer Res. 14:6487–6495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nakatsura T, Komori H, Kubo T, Yoshitake
Y, Senju S, Kataguri T, Fukukawa Y, et al: Mouse homologue of a
novel human oncofetal antigen, glypican-3, evokes T-cell-mediated
tumor rejection without autoimmune reactions in mice. Clin Cancer
Res. 10:8630–8640. 2004. View Article : Google Scholar
|
|
12.
|
Ishizaki H, Tsynoda T, Wada S, Yamauchi M,
Shibuya M and Tahara H: Inhibition of tumor growth with
antiangiogenic cancer vaccine using epitope peptides derived from
human vascular endothelial growth factor receptor 1. Clin Cancer
Res. 12:5841–5849. 2006. View Article : Google Scholar
|
|
13.
|
Mizukami Y, Kono K, Daigo Y, Takano A,
Tsunoda T, Kawaguchi Y, Nakamura Y, et al: Detection of novel
cancer-testis antigen-specific T-cell responses in TIL, regional
lymph nodes, and PBL in patients with esophageal squamous cell
carcinoma. Cancer Sci. 99:1448–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kono K, Iinuma H, Akutsu Y, Tanaka H,
Hayashi N, Uchikado Y, Noguchi T, et al: Multicenter, phase II
clinical trial of cancer vaccination for advanced esophageal cancer
with three peptides derived from novel cancer-testis antigens. J
Transl Med. 10:1412012. View Article : Google Scholar
|
|
15.
|
Suda T, Tsunoda T, Daigo Y, Nakamura Y and
Tahara H: Identification of human leukocyte antigen-A24-restricted
epitope peptides derived from gene products upregulated in lung and
esophageal cancer as novel targets for immunotherapy. Cancer Sci.
98:1803–1808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Fujiwara Y, Kishi K, Yano M, Mototsugu M,
Ishikawa O, Okada K, Masuzawa T, et al: Peptide vaccination for
gastric cancer. J Gastrointest Res. 20:120–127. 2012.
|
|
17.
|
Van der Brunggen P, Traversari C, Chomez
P, Lurquin C, De Plaen E, Van den Eynde, Kunth A, et al: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991.
|
|
18.
|
Correale P, Cusi MG, Tsang KY, Del Vecchio
MT, Marsili S, Placa ML, Intrivici C, et al: Chemo-immunotherapy of
metastatic colorectal carcinoma with gemcitabine plus FOLFOX4
followed by subcutaneous granulocyte macrophage colony-stimulating
factor and interleukin-2 induces strong immunologic and antitumor
activity in metastatic colon cancer patients. J Clin Oncol.
23:8950–8958. 2005.
|
|
19.
|
Hattori T, Mine T, Komatsu N, Yamada A,
Itoh K, Shiozaki H and Okumo K: Immunological evaluation of
personalized peptide vaccination in combination with UFT and UZEL
for metastatic colorectal carcinoma patients. Cancer Immunol
Immunother. 58:1843–1852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Shimokawa T, Matsushima S, Tsunoda T,
Tahara H, Nakamura Y and Furukawa Y: Identification of TOMMO34,
which shows elevated expression in the majority of human colon
cancer, as a novel drug target. Int J Oncol. 29:381–386.
2006.PubMed/NCBI
|
|
21.
|
Masuzawa T, Fujiwara Y, Okada K, Nakamura
A, Takiguchi S, Nakajima K, Miyata H, et al: Phase I/II study of
S-1 plus cisplatin combined with peptide vaccines for human
vascular endothelial growth factor receptor 1 and 2 in patients
with advanced gastric cancer. Int J Oncol. 41:1297–1304. 2012.
|
|
22.
|
Sato Y, Shomura H, Maeda Y, Mine T, Ueno
Y, Akasaka Y, Kondo M, et al: Immunological evaluation of peptide
vaccination for patients with gastric cancer based on pre-existing
cellular response to peptide. Cancer Sci. 94:802–808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Miyagi Y, Imai N, Sasatomi T, Yamada A,
Mine T, Katagiri K, Nakagawa M, et al: Induction of cellular immune
responses to tumor cells and peptides in colorectal cancer patients
by vaccination with SART3 peptides. Clin Cancer Res. 7:3950–3962.
2001.PubMed/NCBI
|
|
24.
|
Koizumi W, Nakahara H, Hara T, Takigane A,
Akiya T, Takagi M, Miyashita K, et al: S-1 plus cisplatin versus
S-1 alone for first-line treatment of advanced gastric cancer
(SPIRITS trial): a phase III trial. Lancet Oncol. 9:215–221. 2008.
View Article : Google Scholar : PubMed/NCBI
|