Introduction
Breast cancer is one of the most frequently
diagnosed cancers and remains the second leading cause of cancer
deaths in women (1). Understanding
the nature of breast cancer is of central importance in development
of novel therapies or improving existing therapeutics for the
treatment and prevention of this disease. Breast cancer is a
complex and heterogeneous disease, as evidenced by the diverse
histopathological features, the various responses to therapy, and
the identification of subtypes with distinct clinical outcomes by
molecular profiling (2–5). One of the common subgroups is the
ErbB2-positive subtype, which occurs in up to 30% of breast cancers
and often is associated with poor patient prognosis (6,7).
Trastuzumab (Herceptin), a humanized monoclonal antibody targeting
the extracellular domain of ErbB2/HER2, has remarkable clinical
efficacy in ErbB2-positive breast cancer (8–10).
However, the unresponsiveness to initial trastuzumab-containing
regimens (primary resistance) and development of therapeutic
resistance after continuous treatment (acquired resistance) in a
significant number of patients is a current challenge (11,12).
Several potential mechanisms of trastuzumab
resistance have been identified (13,14).
Hyperactivation of phosphatidylinositol 3-kinase (PI3K)/AKT pathway
is one of key trastuzumab resistance mechanisms (13–15).
The lipid phosphatase and tensin homolog (PTEN) is a one of the
most mutated and deleted tumor suppressors in human cancer
(16). Loss of PTEN or the loss of
its function are also common in breast cancer, which results in the
hyperactivation of PI3K/AKT pathway (17). Previous evidence suggests that loss
of PTEN conferred significant trastuzumab resistance through
enhanced PI3K/AKT signaling in ErbB2-overexpressing breast cancers
(18–20). These studies implicate that
PI3K/AKT targeting combination therapies could combat trastuzumab
resistance. In addition, several potential agents are being
evaluated intensively (21).
Previously, we reported that combining the
small-molecular PI3K/AKT inhibitor triciribine (TCN) with ErbB2/Neu
antibody effectively inhibited tumor growth and overcame
trastuzumab resistance in two distinct PTEN deficiency-mediated
trastuzumab resistance mouse models (22). One of such models was
PTEN−/−/ErbB2KI genetically engineered mice
(23,24), obtained by interbreeding with three
types of mice including ErbB2KI mice (23), MMTV-Cre mice and the flox-PTEN
mice. To avoid the major disadvantages of this model (such as long
tumor latency, high cost and time- consuming) and complement our
genetic engineering approach, in the present study, we attempted to
establish cell lines isolated from spontaneous mammary tumor that
arose in the PTEN−/−/ErbB2KI mice. We report
that one such cell line, designated MT104T, maintained the
molecular phenotype and induced a tumor with similar
histomorphology as its origin in syngeneic FVB/N mice. Then, we
found the MT104T cells conferred resistance to anti-ErbB2/Neu
antibody treatment due to PTEN loss-mediated Akt hyperactivation.
We next found that addition of Akt inhibitor triciribine (TCN)
effectively inhibited the viability and induced apoptosis of MT104T
cells, via inhibiting both PI3K/Akt and mitogen-activated protein
kinase signaling. Our results confirmed the efficacy of combination
treatment with ErbB2/Neu antibody and TCN for overcoming
trastuzumab resistance by PTEN deficiency, providing insight into
the development of more personalized therapies for
HER2/ErbB2-positive breast cancer.
Materials and methods
Cell lines and cell culture
TM15 and MT104T cells were cultured in growth medium
(DMEM/F12 with 10% FBS,5 μg/ml insulin, 10 ng/ml epidermal
growth factor (EGF), 1 μg/ml hydrocortisone, 35 μg/ml
bovine pituitary extract and 100 U penicillin/streptomycin). Cell
number was evaluated on a cell counter. TM15 cells were established
from a spontaneous ErbB2/Neu-positive-PTEN wild-type
(PTEN+/+/ErbB2KI) mammary tumor (23,25).
MT104T is an immortalized ErbB2/Neu-positive-PTEN-deficient
carcinoma cell line generated from mammary tumor of
PTEN−/−/ErbB2KI genetically engineered FVB/N
female mouse (22,24). To establish MT104T cell line, fresh
mammary tumors from PTEN−/−/ErbB2KI mice were
excised and minced with sterile scissors into approximately 1- to
2-mm3 pieces, then digested in DMEM/F12 medium
containing 10% FBS, 2 mg/ml collagenase (Sigma), 0.02 mg/ml
hyaluronidase (Sigma), and 0.01 mg/ml DNase I (Sigma) for 3 h at
37°C with gentle agitation. The supernatant was filtered through 40
μm mesh cell strainer (BD Biosciences) to remove clumps. Red
blood cells were removed by treatment with ACK buffer and washed
with cold DMEM/F12. The cells were resuspended and cultured in
DMEM/F12 containing 40% Matrigel (BD Biosciences). After 2 weeks,
cells were recovered by dispase (Sigma) treatment at room
temperature and cultured in growth medium for 24 h. Single cells,
generated by digestion with 0.05% trypsin (Invitrogen) were plated
at 1,000 cells per 10-cm dish. Pooled colonies were expanded. Cells
were then digested and resuspended in 100 μl PBS/Matrigel
(1:1) for mammary fat pad injection into the no. 2 mammary gland of
FVB/N female mice at 8 weeks of age. FVB/N mice were obtained from
the Harlan Laboratory. Twelve weeks after injection, the cells from
the resulting orthotopic tumor were explanted back into in
vitro culture as described above. The MT104T cell line was
derived from picked colonies.
All animal experiments were performed according to
the Guidelines for the Institutional Animal Care and Use Committee
of The University of Texas M.D. Anderson Cancer Center.
Polymerase chain reaction (PCR) for
genotyping
DNA was extracted by digesting with proteinase K
(Sigma) in 200 μl of lysis buffer in a 60°C incubator for 2
h, followed by heating at 95°C for 10 min to inactivate the enzyme.
After centrifugation, 1 μl of the supernatant was used for
PCR reaction. Primer sequences for NEU are:
5′-TTCCGGAACCCACATCAGGCC-3′ and 5′-GTTTCCTGCAGCAGCCTACGC-3′; for
CRE are: 5′-TGCTCTGTCCGTTTGCCG-3′ and 5′-ACTGTGTCCAGACCAGGC-3′; for
PTEN are: 5′-ACTCAAGGCAGGGATGAGC-3′ and
5′-GCCCCGATGCAATAAATATG-3′.
Western blotting
Total cell lysates were prepared using the following
lysis buffer: 1% Triton X-100, 50 mM HEPES pH 7.4, 150 mM NaCl, 1.5
mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM Na pyruvate, 1 mM
Na3VO4, 10% glycerol, with protease
inhibitors (Sigma) and phosphatase inhibitors (Roche). The protein
concentration was determined by BCA assay (Thermo Scientific), and
30 μg total protein was electrophoresed on 10% SDS-PAGE and
then transferred onto a nitrocellulose membrane (Bio-Rad). Pten,
pAkt S473, Akt, pErk and Erk antibodies were obtained from Cell
Signaling Technology. Neu and β-actin antibodies were obtained from
Santa Cruz Biotechnology and Sigma, respectively. The blots were
incubated with HRP-conjugated secondary antibodies and visualized
by ECL (Amersham). Densitometry analysis was carried out to
determine the intensity of bands by Adobe Photoshop software.
Cell viability analysis
Anti-ErbB2/neu monoclonal antibody 7.16.4 (IgG2α)
was produced in house using hybridoma obtained from Dr Mark Greene
(University of Pennsylvania). Akt inhibitor triciribine (TCN) was
purchased from Berry & Associates. Cells (5×104 in
200 μl) were plated in 96-well plates. Twenty-four hours
after seeding, cells were treated with PBS control, 2 μg/ml
7.16.4 mAb, TCN 0.25 or 0.5 μM, or mAb plus TCN combination,
respectively, for indicated time. Then 20 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (5 mg/ml in PBS; Sigma) was added and incubated at 37°C
for 3 h. The supernatant was removed, and 200 μl of DMSO was
added to each well. The dark-blue crystals of MTT-formazan were
dissolved by shaking the plates at room temperature for 10 min.
Spectrometric absorbance was measured on a microplate reader
(Bio-Rad) using a test wavelength of 490 nm and a reference
wavelength of 630 nm. Cell viability was normalized to the value of
control treated cells. Each experiment was done in triplicate.
Measurement of apoptosis by flow
cytometry
Both floating and adherent cells were harvested and
resuspended. Apoptosis was detected by the binding of Annexin
V-FITC (BD Biosciences) to phosphatidylserine exposed in the cell
membrane according to the manufacturer’s instructions. The samples
were run on a FACSCanto flow cytometer (BD Biosciences) and the
data were analyzed using FlowJo software. At least 15,000
events/sample were acquired.
Statistics
Statistical differences were assessed with
two-tailed Student’s t-test or one-way ANOVA as indicated. The
GraphPad Prism 5 Program (GraphPad Software) was used to perform
all statistical analyses. P-values <0.05 were considered
statistically significant.
Results
Establishment and characterization of the
MT104T cell line
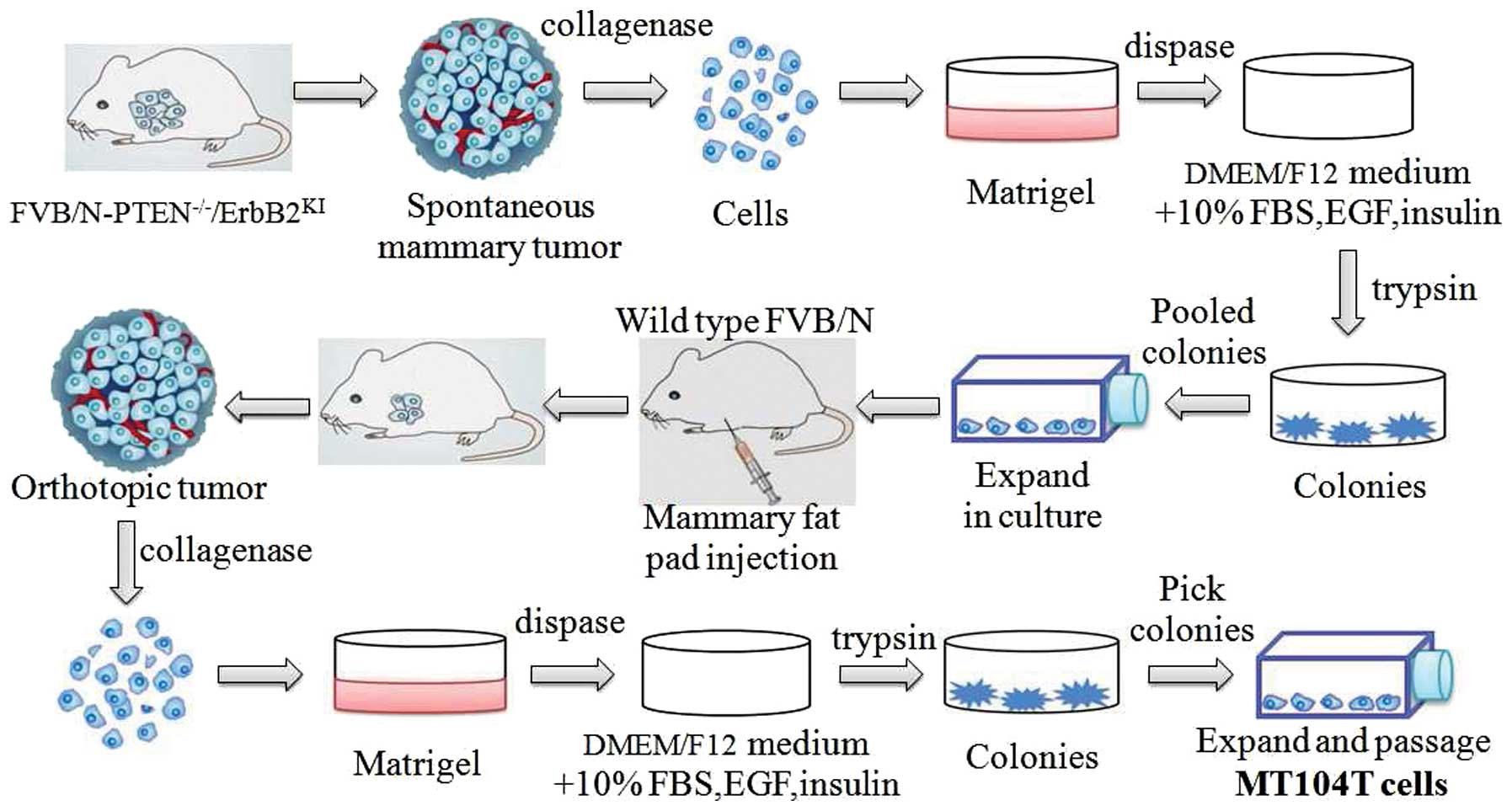
In order to be able to rapidly and stably reproduce
trastuzumab resistant model, we attempted to establish cell lines
from ErbB2/Neu-positive and PTEN-deficient
(PTEN−/−/ErbB2KI) mammary tumors. General
procedures to generate such cells are illustrated in Fig. 1. Briefly, the cells isolated from
spontaneous tumors of PTEN−/−/ErbB2KI mice
were initially established in vitro as epithelial colonies.
The pooled cells were then injected into mammary fat pad of
syngeneic FVB/N female. Next, the cells from the resulting
orthotopic tumor were explanted back into in vitro culture
as described. Then, the MT104T cell line was expanded from picked
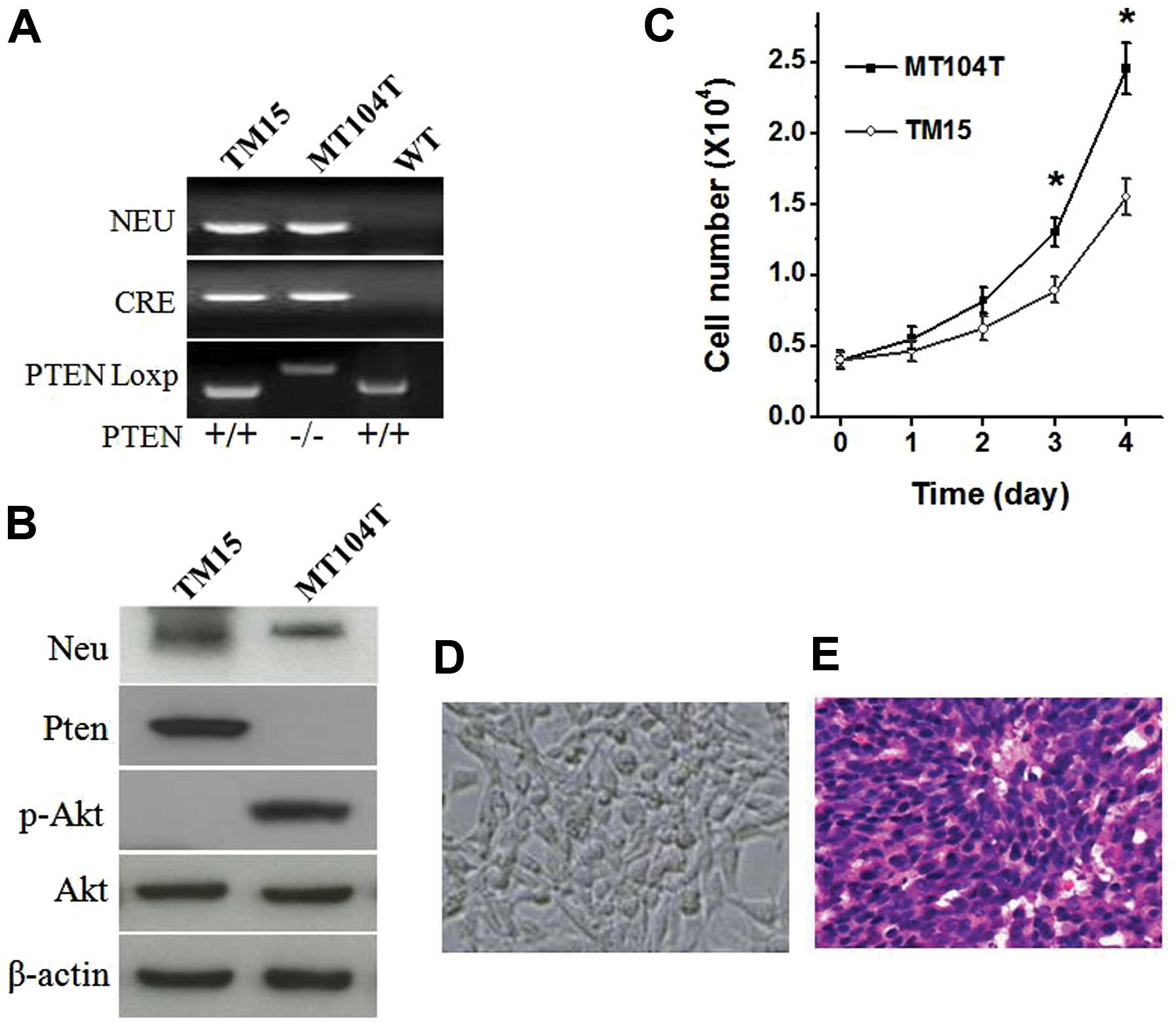
colonies. As shown in Fig. 2A and
B, the critical molecular phenotype of the cell lines were
confirmed. Compared with the PTEN wild-type TM15 cells, PTEN loss
led to hyperactivation of Akt (Fig.
2B) and increase cell growth of MT104T cells (Fig. 2C). Gross appearance of cultured
MT104T cells displayed epithelial morphology over subsequent
passages (Fig. 2D). Furthermore,
when injected into the mammary fat pad of wild-type FVB/N female,
MT104T cells induced a tumor with similar histomorphology as its
origin (Fig. 2E) (24).
Resistance to ErbB2/Neu antibody
treatment in MT104T cells
After generation of the
ErbB2/Neu-positive-PTEN-deficient MT104T cell line, we next
examined the response of the cells to ErbB2/Neu antibody (7.16.4
mAb) treatment in vitro. 7.16.4 mAb binds rat ErbB2/Neu at
the same site as trastuzumab binds to human HER2/ErbB2/Neu
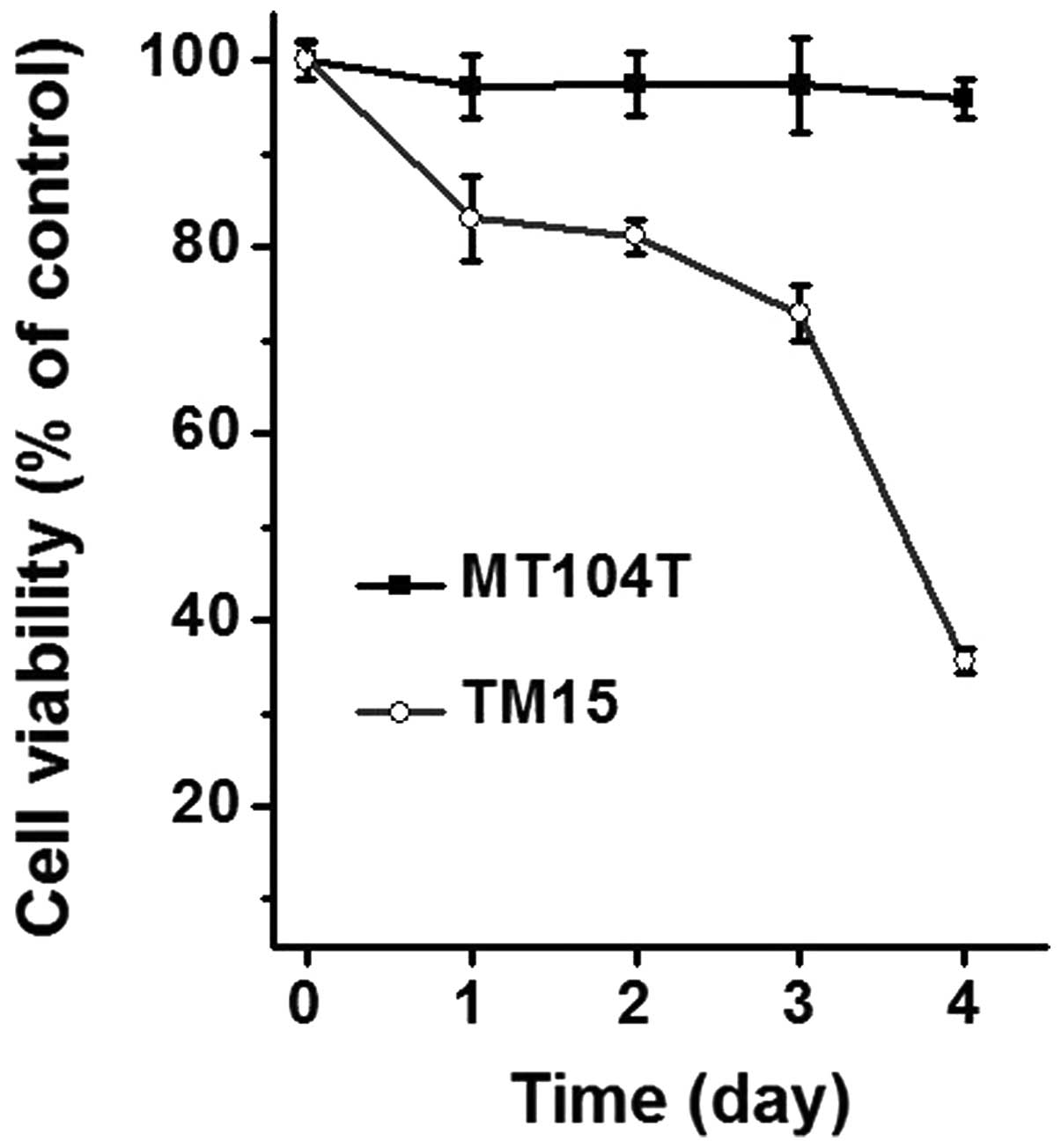
(26). As shown in Fig. 3, the cell viability was
significantly decreased in TM15 cells time-dependently, while that
was almost no change in any of the time points tested. These
results indicate that PTEN deficiency confers resistance to
ErbB2/Neu antibody treatment in mammary cancer cells in
vitro, which is consistent with prior findings in xenograft and
genetically engineered mouse models. In addition, the MT104 cells
provided a potential resource for biological study and drug
discovery for ErbB2/Neu-positive mammary tumors.
Addition of Akt inhibitor triciribine
effectively inhibits the MT104T cells
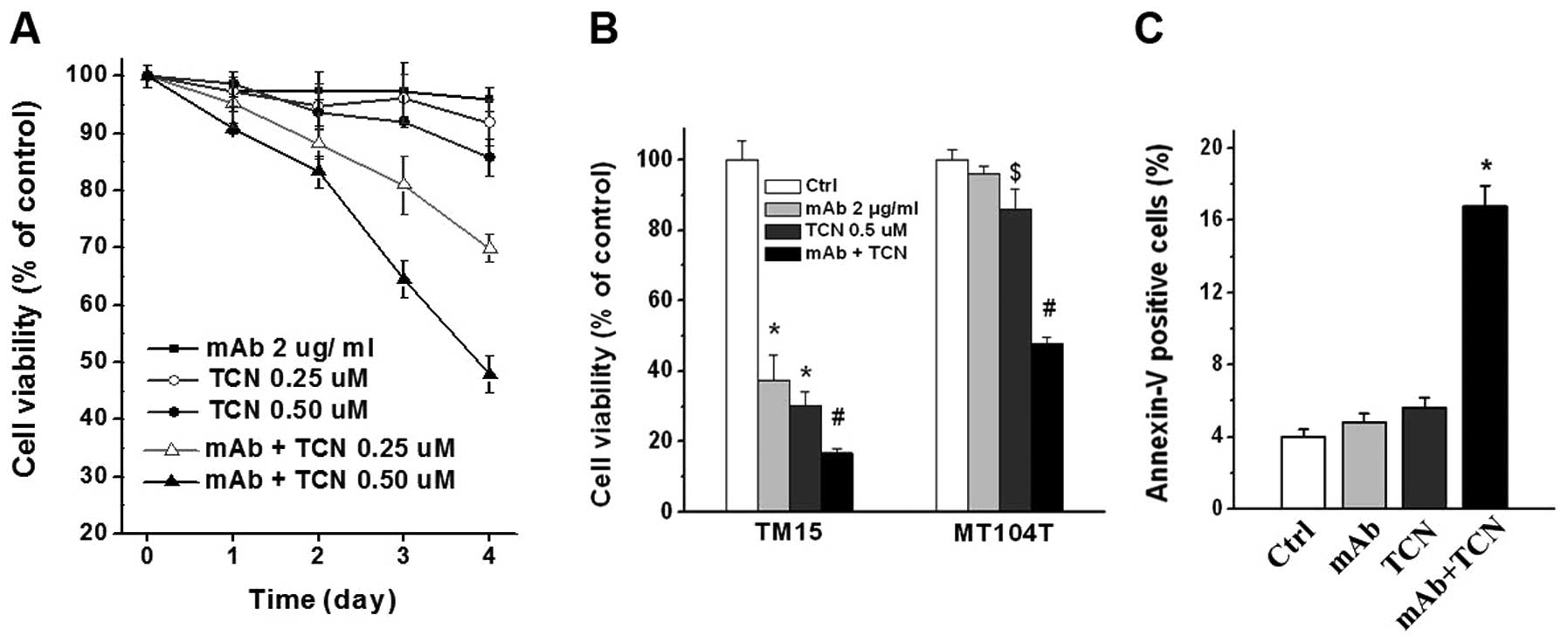
We tested whether addition of Akt inhibitor
triciribine (TCN) could overcome ErbB2/Neu antibody resistance
in vitro. Combination treatment with antibody and TCN
decreased cell viability of MT104T cells time-dependently.
Increasing TCN doses resulted in greater effect, while TCN alone
had no significant (or modest) reduction on cell viability
(Fig. 4A). Single and combination
treatment for 4 days significantly inhibited TM15 cells (P<0.01,
Fig. 4B). Notably, 4 days of
combination treatment effectively suppressed MT104T cells compared
with control or antibody or TCN single treatment (P<0.01), but
the reduction level of cell viability in MT104T cells was not as
dramatic as TM15 cells (Fig. 4B).
The cell viability of MT104T cells reduced very modestly in 0.5
μM TCN treatment group, although with statistical difference
compared with control or antibody treatment (P<0.05, Fig. 4B). Moreover, measurement of
apoptosis by flow cytometry after Annexin V-FITC staining showed
great increase of apoptotic cells in combination treatment group of
MT104T cells, comparing with antibody or TCN single treatment
(P<0.01, Fig. 4C). These data
indicate that combination treatment with ErbB2/Neu antibody and Akt
inhibitor TCN was able to effectively inhibit antibody resistant
MT104T cells.
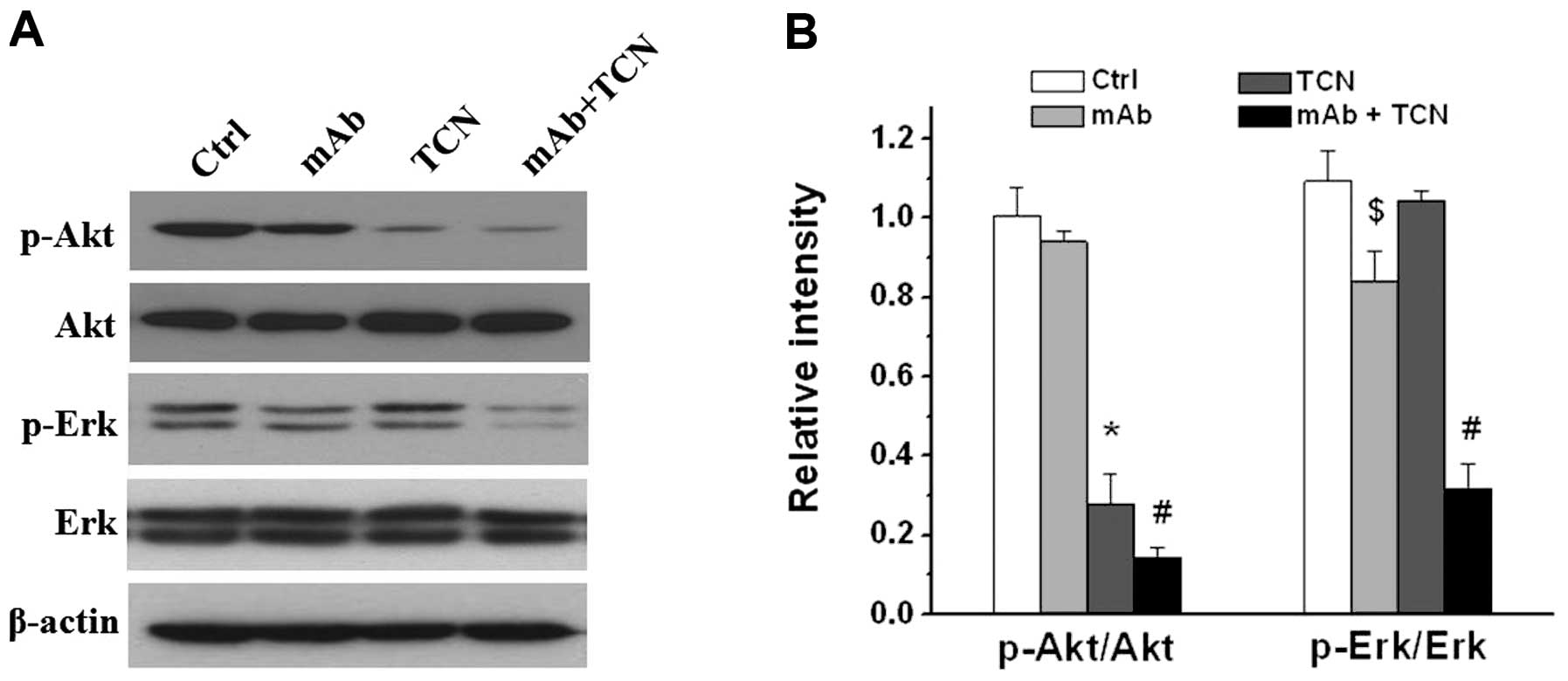
Suppression of Akt and Erk activities
after TCN and antibody combination treatment
As a member of receptor tyrosine kinases (RTKs),
HER2/ErbB2/Neu promotes cell survival and proliferation by
activation of several pathways, and the PI3K/AKT pathway and the
mitogen-activated protein kinase (MAPK) pathway are two critical
ones. We next examined the effects of antibody and TCN on PI3K/AKT
and MAPK signaling on MT104T cells. Western blot analysis showed
single antibody treatment modestly decreased pErk but not pAkt, TCN
alone decreased pAkt but had no effect on pErk, whereas combination
treatment markedly attenuated both Akt and Erk activities
(P<0.01, Fig. 5).
Discussion
A growing number of genetically engineered mice and
tumor xenograft models of breast cancers have been developed
(27–29), which have led to substantial
progress in our understanding of the biology and in developing
novel therapeutics of breast cancer. Breast cancer is a collection
of complex diseases that have distinct histopathological features,
genetic and genomic heterogeneity, and diverse clinical outcomes.
The FVB/N-PTEN−/−/ErbB2KI model develops
mammary tumors with features of both basal-like and HER2/ErbB2
human breast cancer (24,30). Despite the histological and
molecular relevance to human breast cancer, the
PTEN−/−/ErbB2KI model has major disadvantages
of heterogeneity with regard to frequency, latency, and growth.
Briefly, the PTEN−/−/ErbB2KI mice were
obtained by interbreeding with three types of mice including
ErbB2KI mice (23),
MMTV-Cre mice and the flox-PTEN mice. ErbB2KI model has
a knocked in allele of the ErbB2 endogenous promoter with a floxed
stop codon conditionally controlling activated Neu expression
(23). When the ErbB2KI
mice are crossed with the MMTV-Cre mice, the stop codon is removed
allowing the endogenous promoter to drive activated Neu expression
at the physiological levels of human ErbB2 expression. Then, these
mice are crossed with the flox-PTEN mice, PTEN is disrupted,
resulting in PTEN−/−/ErbB2KI mice, which
finally develop ErbB2/Neu-positive-PTEN loss mammary tumors. In
this study, our initial aim was to establish an in vitro
cell line from PTEN−/−/ErbB2KI tumors. Such a
cell line, designated MT104T, retained the key molecular
characteristic and formed tumors in immune-competent syngeneic
hosts with highly similar phenotype to its origin. With the
development of the syngeneic xenograft model, the contribution of
immune system to tumor progression and therapeutic response could
be better investigated. Moreover, the effects of other host factors
to tumor cells could also be studied using corresponding types of
genetically engineered mice on the FVB/N background. The generation
and development of the MT104T cell line model provides an
alternative resource for future preclinical study, possessing
advantages of easy use, relatively inexpensive and reproducible as
well as the potential for in vitro manipulation.
Trastuzumab-based regimens have shown significant
clinical benefit in HER2/ErbB2 overexpressing breast cancer
patients. However, it is increasingly evident that primary and
acquired resistance has major limitations. Advances of resistance
mechanisms is critical to determine what combination of drugs will
be used to treat resistant tumors or even to prevent the occurrence
of resistance. Accumulating evidence suggests that hyperactivation
of the PI3K pathway or/and loss of PTEN function may be associated
with trastuzumab resistance. As expected, the established cell line
MT104T had high level of Akt phosphorylation and undetectable PTEN
expression. Furthermore, the molecular phenotype also conferred
cell resistance to anti-ErbB2/Neu antibody treatment in
vitro. Thus, this MT104T cell line model might be particularly
useful for elucidating the detail molecular mechanisms that
underlie therapeutic resistance and for evaluating the effect of
potential regimens to overcome resistance.
Akt is a promising target to combat trastuzumab
resistance, and novel inhibitors have been developed and assessed.
Triciribine (TCN) is a tricyclic nucleoside, inhibiting
phosphorylation of Akt1, Akt2 or Akt3. Phase I and II clinical
trials proved the safety of TCN (31–33).
Although TCN single-agent trials failed to show efficacy against
advanced breast, colon, and lung cancer even at very high doses
(31,32), recently preclinical test combined
TCN with other agent showing therapeutic activity in T-cell acute
lymphoblastic leukemia (34),
breast cancer (22,35,36)
and prostate cancer (37). The
ability of anticancer agents to inhibit cell viability in
vitro is typically evaluated as a measure of drug activity and
has been shown to have clinical predictive value for breast cancer
(38). We performed a test to
evaluate whether addition of Akt inhibitor TCN could overcome
ErbB2/Neu antibody resistance of MT104T in cell culture
circumstances. Our results showed the efficacy of the combination
treatment, and inhibition of Akt and Erk activities were associated
with therapeutic effect. These results suggested that ErbB2/Neu
antibody and TCN combination may be a potentially effective therapy
modality, particularly for PTEN loss or PI3K hyperactivation
mediated trastuzumab resistance.
Studies have shown that both autonomous (inhibition
of oncogenic signaling of tumor cells) and non-autonomous (immune
response or stroma-tumor interactions in tumor microenvironment)
mechanisms were involved in the action of trastuzumab (13,39,40).
Further studies are warranted to investigate the roles of
non-autonomous mechanisms on therapeutic response of combination
treatment, and which would be carried out using the MT104T
syngeneic xenograft model.
Many of the commonly used genetically engineered
mice developing mammary tumor have been established as useful cell
lines which may be manipulated in vitro and transplanted in
syngeneic animals. In this study, we provided a valuable resource
for elucidating the nature of ErbB2-positive breast cancer, as well
as for the experimental therapeutics studies of this disease. Our
findings also implicated that combination of Akt inhibitor
triciribine (or other alternatives) could be a potential strategy
for personalized treatment of trastuzumab-resistant breast cancer,
particularly mediated by PTEN loss or PI3K hyperactivation, which
warrants further preclinical and clinical investigation.
Acknowledgements
We thank Dr Mark Greene at the
University of Pennsylvania for generously providing the hybridoma
for 7.16.4 mAb, Dr William Muller at McGill University for kindly
providing the mouse strains and the TM15 cells, Dr Dihua Yu at the
University of Texas MD Anderson Cancer Center for her support of
this study. This study was supported by grants from National
Natural Science Foundation of China (nos. 30572117, 30972786 and
81302241), and key project of Medical Science and Technology
Development Foundation from Nanjing Department of Health
(Q.W.).
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2.
|
Network CGA: Comprehensive molecular
portraits of human breast tumours. Nature. 490:61–70. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Perou CM, Sorlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sorlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sotiriou C and Piccart MJ: Taking
gene-expression profiling to the clinic: when will molecular
signatures become relevant to patient care? Nat Rev Cancer.
7:545–553. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Romond EH, Perez EA, Bryant J, et al:
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive
breast cancer. N Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Vogel CL, Cobleigh MA, Tripathy D, et al:
Efficacy and safety of trastuzumab as a single agent in first-line
treatment of HER2-overexpressing metastatic breast cancer. J Clin
Oncol. 20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lan KH, Lu CH and Yu D: Mechanisms of
trastuzumab resistance and their clinical implications. Ann NY Acad
Sci. 1059:70–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhang S, Huang WC, Li P, et al: Combating
trastuzumab resistance by targeting SRC, a common node downstream
of multiple resistance pathways. Nat Med. 17:461–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gajria D and Chandarlapaty S:
HER2-amplified breast cancer: mechanisms of trastuzumab resistance
and novel targeted therapies. Expert Rev Anticancer Ther.
11:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nahta R, Yu D, Hung MC, Hortobagyi GN and
Esteva FJ: Mechanisms of disease: understanding resistance to
HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol.
3:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Berns K, Horlings HM, Hennessy BT, et al:
A functional genetic approach identifies the PI3K pathway as a
major determinant of trastuzumab resistance in breast cancer.
Cancer Cell. 12:395–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000.PubMed/NCBI
|
|
17.
|
Hollander MC, Blumenthal GM and Dennis PA:
PTEN loss in the continuum of common cancers, rare syndromes and
mouse models. Nat Rev Cancer. 11:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Esteva FJ, Guo H, Zhang S, et al: PTEN,
PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab
response and survival in patients with HER2-positive metastatic
breast cancer. Am J Pathol. 177:1647–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Fujita T, Doihara H, Kawasaki K, et al:
PTEN activity could be a predictive marker of trastuzumab efficacy
in the treatment of ErbB2-overexpressing breast cancer. Br J
Cancer. 94:247–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Nagata Y, Lan KH, Zhou X, et al: PTEN
activation contributes to tumor inhibition by trastuzumab, and loss
of PTEN predicts trastuzumab resistance in patients. Cancer Cell.
6:117–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Alvarez RH, Valero V and Hortobagyi GN:
Emerging targeted therapies for breast cancer. J Clin Oncol.
28:3366–3379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wang Q, Li SH, Wang H, et al: Concomitant
targeting of tumor cells and induction of T-cell response
synergizes to effectively inhibit trastuzumab-resistant breast
cancer. Cancer Res. 72:4417–4428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Andrechek ER, Hardy WR, Siegel PM,
Rudnicki MA, Cardiff RD and Muller WJ: Amplification of the
neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc
Natl Acad Sci USA. 97:3444–3449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Dourdin N, Schade B, Lesurf R, et al:
Phosphatase and tensin homologue deleted on chromosome 10
deficiency accelerates tumor induction in a mouse model of ErbB-2
mammary tumorigenesis. Cancer Res. 68:2122–2131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ursini-Siegel J, Rajput AB, Lu H, et al:
Elevated expression of DecR1 impairs ErbB2/Neu-induced mammary
tumor development. Mol Cell Biol. 27:6361–6371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zhang H, Wang Q, Montone KT, et al: Shared
antigenic epitopes and pathobiological functions of
anti-p185(her2/neu) monoclonal antibodies. Exp Mol Pathol.
67:15–25. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Frese KK and Tuveson DA: Maximizing mouse
cancer models. Nat Rev Cancer. 7:645–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Vargo-Gogola T and Rosen JM: Modelling
breast cancer: one size does not fit all. Nat Rev Cancer.
7:659–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Gopinathan A and Tuveson DA: The use of
GEM models for experimental cancer therapeutics. Dis Model Mech.
1:83–86. 2008. View Article : Google Scholar
|
|
30.
|
Schade B, Rao T, Dourdin N, et al: PTEN
deficiency in a luminal ErbB-2 mouse model results in dramatic
acceleration of mammary tumorigenesis and metastasis. J Biol Chem.
284:19018–19026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Mittelman A, Casper ES, Godwin TA, Cassidy
C and Young CW: Phase I study of tricyclic nucleoside phosphate.
Cancer Treat Rep. 67:159–162. 1983.PubMed/NCBI
|
|
32.
|
Hoffman K, Holmes FA, Fraschini G, et al:
Phase I-II study: triciribine (tricyclic nucleoside phosphate) for
metastatic breast cancer. Cancer Chemother Pharmacol. 37:254–258.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Garrett CR, Coppola D, Wenham RM, et al:
Phase I pharmacokinetic and pharmacodynamic study of triciribine
phosphate monohydrate, a small-molecule inhibitor of AKT
phosphorylation, in adult subjects with solid tumors containing
activated AKT. Invest New Drugs. 29:1381–1389. 2011. View Article : Google Scholar
|
|
34.
|
Evangelisti C, Ricci F, Tazzari P, et al:
Preclinical testing of the Akt inhibitor triciribine in T-cell
acute lymphoblastic leukemia. J Cell Physiol. 226:822–831. 2011.
View Article : Google Scholar
|
|
35.
|
Lu CH, Wyszomierski SL, Tseng LM, et al:
Preclinical testing of clinically applicable strategies for
overcoming trastuzumab resistance caused by PTEN deficiency. Clin
Cancer Res. 13:5883–5888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Balasis ME, Forinash KD, Chen YA, et al:
Combination of farnesyltransferase and Akt inhibitors is
synergistic in breast cancer cells and causes significant breast
tumor regression in ErbB2 transgenic mice. Clin Cancer Res.
17:2852–2862. 2011. View Article : Google Scholar
|
|
37.
|
Dieterle A, Orth R, Daubrawa M, et al: The
Akt inhibitor triciribine sensitizes prostate carcinoma cells to
TRAIL-induced apoptosis. Int J Cancer. 125:932–941. 2009.
View Article : Google Scholar
|
|
38.
|
Voskoglou-Nomikos T, Pater JL and Seymour
L: Clinical predictive value of the in vitro cell line, human
xenograft, and mouse allograft preclinical cancer models. Clin
Cancer Res. 9:4227–4239. 2003.PubMed/NCBI
|
|
39.
|
Park S, Jiang Z, Mortenson ED, et al: The
therapeutic effect of anti-HER2neu antibody depends on both innate
and adaptive immunity. Cancer Cell. 18:160–170. 2010. View Article : Google Scholar
|
|
40.
|
Ferris RL, Jaffee EM and Ferrone S: Tumor
antigen-targeted, monoclonal antibody-based immunotherapy: clinical
response, cellular immunity, and immunoescape. J Clin Oncol.
28:4390–4399. 2010. View Article : Google Scholar
|