Introduction
Gastric cancer is one of the most common
malignancies worldwide, with an estimated 21,600 new cases and
10,990 deaths reported in United States in 2013 (1). Gastric cancer is also a genetic
disease developing from a multi-step process. Single or multiple
mutations in genes related to growth control, invasion and
metastasis form the molecular genetic basis of malignant
transformation and tumor progression (2). Thus, identification of key genes or
targets related to tumorigenesis is crucial for the diagnosis and
prevention of gastric cancer.
HMGB1 is a chromosome-binding protein that also acts
as a damage-associated molecular pattern molecule. It has potent
proinflammatory effects and is one of key mediators of organ injury
(3) and the target of inflammation
controlling (4). HMGB1 is involved
in certain physiologic and pathologic conditions including cancer
and is identified to be differentially expressed in prostate and
ovarian cancers (5,6). The expression of HMGB1 has been
reported in human malignant tumors of various differentiation
levels (7), and it is upregulated
in human lymphomas (8) and
squamous cell carcinoma of head and neck (SCCHN) (9). Moreover, overexpression of HMGB1
correlates with tumor progression and poor prognosis in colorectal
carcinoma (CRC) (10), and
contributes to the malignant progression of SCCHN (11). HMGB1 enhances tumor migration by
increasing α5β1 integrin expression through the RAGE/PI3K/Akt
pathway (12), induces the
overexpression of miR-221/-222, promotes growth of thyroid cancer
cells (13), and activates TLR4
and RAGE signaling pathways inducing caspase-1 with subsequent
production of many inflammatory mediators which in turn promotes
cancer invasion and metastasis (14), suggesting that HMGB1 may be
utilized as a diagnostic and therapeutic target for cancer
(6,15). In addition, HMGB1 is released from
tumor cells after chemotherapy-induced cytotoxicity, and induces
autophagy promoting chemotherapy resistance in leukemia cells
(16), which is probably connected
with activation of the PI3K/Akt/mTORC1 pathway (17).
Only few studies indicate that HMGB1 expression is
downregulated in the lung, lymph node and spleen tumor samples
compared to their non-neoplastic counterparts (18), and the inversely associated with
the infiltration of CD45RO+ T cells and prognosis in
patients with stage IIIB colon cancer (19). HMGB1 functions as a tumor
suppressor and radiosensitizer in breast cancer (20). To further elucidate the expression
and function of HMGB1 in cancer, we examined the expression of
HMGB1 in primary cancer tissues and adjacent non-cancer tissues
derived from patients with primary GAC, and further investigated
the effects of HMGB1 knockdown by shRNA on cell proliferation and
invasion in vitro and in vivo. We hypothesized that
HMGB1 expression was increased in GAC tissues and knockdown of
HMGB1 suppressed growth and invasion of gastric adenocarcinoma
(GAC) cells, suggesting and HMGB1 may serve as a potential
therapeutic target for GAC.
Materials and methods
Materials
The GAC cell lines (SGC-7901 and AGS) used for
experiments was obtained from the Institute of Biochemistry and
Cell Biology (Shanghai, China). Lentivirus-mediated HMGB1 shRNA
vector, negative control vector, and virion-packaging elements were
purchased from Genechem (Shanghai, China). Human GAC tissues and
the corresponding adjacent non-cancer tissues (ANCT) were collected
from Department of Gastrointestinal Surgery of Shanghai Six
People’s Hospital. The tissue microarray of gastric cancer was made
by Shanghai Outdo Biotech Co. Ltd. (Shanghai, China). The antibody
of HMGB1 was purchased from Cell Signaling Technologies (Boston,
MA, USA). HMGB1 primer was synthesized by ABI (Framingham, MA,
USA).
Drugs and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were purchased from Thermo Fisher Scientific
Inc. (Waltham, MA, USA); TRIzol Reagent and Lipofectamine 2000 were
obtained from Invitrogen (Carlsbad, CA, USA); M-MLV Reverse
Transcriptase was purchased from Promega (Madison, WI, USA); SYBR
Green Master Mix was obtained from Takara (Otsu, Japan); and the
ECL Plus kit was obtained from GE Healthcare (Piscataway, NJ,
USA).
Clinical samples and data
Tissue microarray was prepared for IHC test. Human
gastric cancer tissues and the corresponding ANCT were obtained
from a biopsy in a total of 88 consecutive GAC cases admitted to
our hospital from January 2007 to December 2012. The baseline
characteristics of the patients before neo-adjuvant chemotherapy
were recorded. The study was approved by Medical Ethics Committee
of Shanghai Jiao Tong University and written informed consent was
obtained from the patients or their parents before sample
collection. Two pathologists independently reviewed all of the
cases.
Tissue microarray
The advanced tissue arrayer (ATA-100, Chemicon
International, Tamecula, CA, USA) was used to create holes in a
recipient paraffin block and to acquire cylindrical core tissue
biopsies with a diameter of 1 mm from the specific areas of the
‘donor’ block. The tissue core biopsies were transferred to the
recipient paraffin block at defined array positions. The tissue
microarrays contained tissue samples from 88 formalin-fixed
paraffin-embedded cancer specimens with known diagnosis, and
corresponding ANCT from these patients. The block was incubated in
an oven at 45°C for 20 min to allow complete embedding of the
grafted tissue cylinders in the paraffin of the recipient block,
and then stored at 4°C until microtome sectioning.
Immunohistochemical staining
Anti-HMGB1 antibody was used for IHC detection of
the expression of HMGB1 protein in tissue microarray. Tissue
microarray sections were processed for IHC analysis of KiSS1
protein as follows. Immunohistochemical examinations were carried
out on 3-mm thick sections. For anti-HMGB1 immunohistochemistry,
unmasking was performed with 10 mM sodium citrate buffer, pH 6.0,
at 90°C for 30 min. For anti-HMGB1 immunohistochemistry, antigen
unmasking was not necessary. Sections were incubated in 0.03%
hydrogen peroxide for 10 min at room temperature, to remove
endogenous peroxidase activity, and then in blocking serum (0.04%
bovine serum albumin, A2153, Sigma-Aldrich, Shanghai, China and
0.5% normal goat serum X0907, Dako Corp., Carpinteria, CA, USA, in
PBS) for 30 min at room temperature. Anti-HMGB1 antibody was used
at a dilution of 1:200. The antibody was incubated overnight at
4°C. Sections were then washed three times for 5 min in PBS.
Non-specific staining was blocked with 0.5% casein and 5% normal
serum for 30 min at room temperature. Finally, staining was
developed using diaminobenzidine substrate, and sections were
counterstained with hematoxylin. Normal serum or PBS was used to
replace anti-KiSS1 antibody in negative controls.
Quantification of protein expression
The expression of HMGB1 was semiquantitatively
estimated as the total immunostaining scores, which were calculated
as the product of a proportion score and an intensity score. The
proportion and intensity of the staining was evaluated
independently by two observers. The proportion score reflected the
fraction of positive staining cells (0, none; 1, ≤10%; 2, 10% to
≥25%; 3, >25–50%; 4, >50%), and the intensity score
represented the staining intensity (0, no staining; 1, weak; 2,
intermediate; 3, strong). Finally, a total expression score was
given ranging from 0 to 12. Based on the pre-analysis, HMGB1
expression was categorized into two groups: low-level HMGB1
expression (score 0–3) and high-level HMGB1 expression (score
4–12). The scoring was independently assessed by two
pathologists.
Cell culture and transfection
GAC cells (SGC-7901 and AGS) were cultured in DMEM
medium supplemented with 10% heat-inactivated FBS, 100 U/ml of
penicillin, and 100 μg/ml of streptomycin. Cells in this
medium were placed in a humidified atmosphere containing 5%
CO2 at 37°C. Cells were subcultured at a 1:5 dilution in
medium containing 300 μg/ml G418 (an aminoglycoside
antibody, commonly used stable transfection reagent in molecular
genetic testing). On the day of transduction, GAC cells were
replated at 5×104 cells/well in 24-well plates
containing serum-free growth medium with polybrene (5 mg/ml). At
50% confluence, cells were transfected with recombinant
experimental virus or control virus at the optimal MOI
(multiplicity of infection) of 50, and cultured at 37°C and 5%
CO2 for 4 h. Then supernatant was discarded and serum
containing growth medium was added. At 4 days of post-transduction,
transduction efficiency was measured by the frequency of green
fluorescent protein (GFP)-positive cells. Positive and stable
transfectants were selected and expanded for further study. The
HMGB1 shRNA virus vector-infected clone, the negative control
vector-infected cells, and GAC cells (SGC-7901 and AGS) were named
as Lv-shHMGB1 group and NC group, respectively.
Quantitative real-time PCR
To quantitatively determine the mRNA expression
level of HMGB1 in GAC cell lines (SGC-7901 and AGS), real-time PCR
was performed. Total RNA was extracted from each clone using TRIzol
according to the manufacturer’s protocol. Reverse transcription was
carried out using M-MLV and cDNA amplification was performed using
the SYBR Green Master Mix kit according to the manufacturer’s
guidelines. The HMGB1 gene was amplified using a specific
oligonucleotide primer and the human glyceralde-hyde-3-phosphate
dehydrogenase (GAPDH) gene was used as an endogenous control. The
PCR primer sequences were as follows: HMGB1,
5′-ATATGGCAAAAGCGGACAAG-3′ and 5′-AGGCCAGGATGTTCTCCTTT-3′; GAPDH,
5′-CAA CGAATTTGGCTACAGCA-3′ and 5′-AGGGGTCTACAT GGCAACTG-3′. Data
were analyzed using the comparative Ct method
(2−ΔΔCt). Three separate experiments were
performed for each clone.
Western blot assay
GAC cell lines (SGC-7901 and AGS) were harvested and
extracted using lysis buffer (Tris-HCl, SDS, mercaptoethanol and
glycerol). Cell extracts were boiled for 5 min in loading buffer,
and then an equal amount of cell extracts was separated on 15%
SDS-PAGE gels. Separated protein bands were transferred onto
polyvinylidene fluoride (PVDF) membranes, which were subsequently
blocked in 5% skim milk powder. Primary antibodies against HMGB1,
NF-κB p65, PCNA and MMP-9 were diluted according to the
manufacturer’s instructions and incubated overnight at 4°C.
Subsequently, horseradish peroxidase-linked secondary antibodies
were added at a dilution of 1:1,000 and incubated at room
temperature for 2 h. The membranes were washed 3 times with PBS,
and the immunoreactive bands were visualized using the ECL Plus kit
according to the manufacturer’s instructions. The relative protein
levels in different cell lines were normalized to the concentration
of GAPDH. Three separate experiments were performed for each
clone.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay.
Briefly, cells infected with KiSS1 virus were incubated in
96-well-plates at a density of 1×105 cells per well with
DMEM medium supplemented with 10% FBS. Cells were treated with 20
μl of MTT dye at 0, 24, 48 and 72 h, and subsequently
incubated with 150 μl of DMSO for 5 min. The color reaction
was measured at 570 nm using an Enzyme Immunoassay Analyzer
(Bio-Rad, Hercules, CA, USA). The proliferation activity was
calculated for each clone.
Wound-healing assay
GAC cells (SGC-7901 and AGS) were plated in each
well of a 6-well culture plate and allowed to grow to 90%
confluence. Treatment with Lv-shHMGB1 was then performed. The next
day, a wound was created using a micropipette tip. The migration of
cells towards the wound was monitored daily, and images were
captured at time intervals of 24 h.
3D-Matrigel assay
Cells were cultured on growth factor-reduced
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) using the
overlay method. Briefly, 4-chambered Lab-Tek chamber slides (Nalge
Nunc International, Rochester, NY, USA) or 24-well plates were
coated with Matrigel. Cells (2.0×104 cells/ml for
SGC-7901 and AGS) suspended in 2.5% Matrigel solution were added on
coated chamber slides and allowed to grow up to 10 days.
Subcutaneous tumor model and gene
therapy
Six-week-old female immune-deficient nude mice
(BALB/c-nu) were bred at the laboratory animal facility (Institute
of Chinese Academy of Sciences, Shanghai, China), and were housed
individually in microisolator ventilated cages with free access to
water and food. All experimental procedures were performed
according to the regulations and internal biosafety and bioethics
guidelines of Shanghai Jiaotong University and the Shanghai
Municipal Science and Technology Commission. Two mice were injected
subcutaneously with 1×108 GAC cells in 50 μl of
PBS pre-mixed with an equal volume of Matrigel matrix
(Becton-Dickinson). Mice were monitored daily and developed a
subcutaneous tumor. When the tumor size reached ∼5 mm in length,
they were surgically removed, cut into 1–2 mm3 pieces,
and re-seeded individually into other mice. When tumor size reached
∼5 mm in length, the mice were randomly assigned as NC group and
Lv-shHMGB1 group. In Lv-shHMGB1 group, 15 μl of lentivirus
was injected into subcutaneous tumors using a multi-site injection
format. Injections were repeated every other day after initial
treatment. The tumor volume every three days was measured with a
caliper, using the formula: volume = (length × width)2 /
2.
Statistical analysis
SPSS 20.0 was used for the statistical analysis.
Kruskal-Wallis H test and χ2 test were used to analyze
the expression rate in all groups. One-way analysis of variance
(ANOVA) was used to analyze the differences between groups. The LSD
method of multiple comparisons was applied when the probability for
ANOVA was statistically significant. Statistical significance was
set at P<0.05.
Results
The expression of HMGB1 in gastric cancer
tissues
The expression of HMGB1 protein was evaluated using
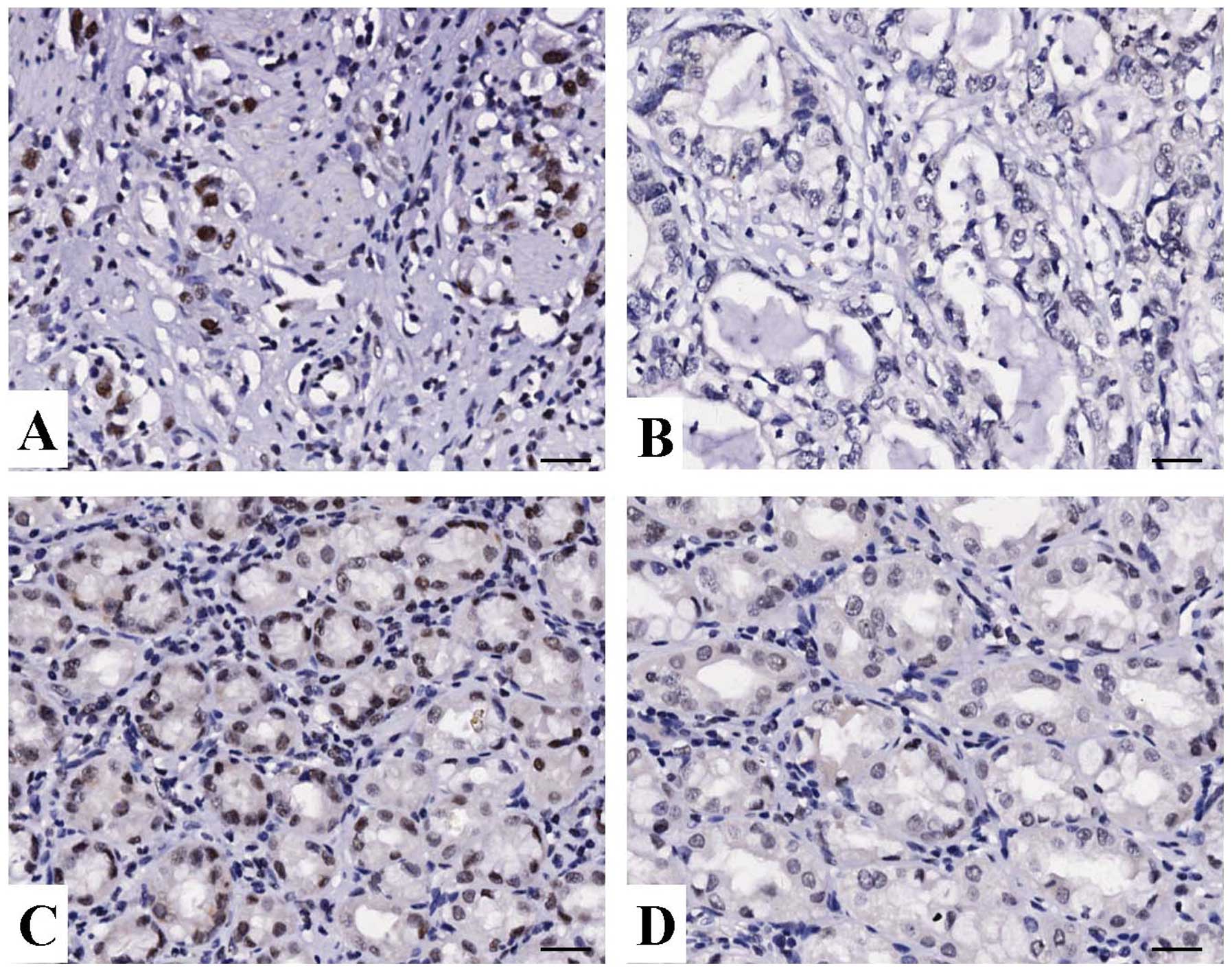
IHC staining in gastric cancer tissues. As shown in Fig. 1, different levels of positive
expression of HMGB1 protein were detected in GAC and ANCT tissues.
Positive HMGB1 immunostaining was mainly localized in the nucleus
of gastric cancer tissue cells. According to the HMGB1
immunoreactive intensity, the positive expression of HMGB1 in
gastric cancer tissues was significantly increased compared with
that of HMGB1 in ANCT (P<0.001) (Table I).
 | Table I.The expression of HMGB1 protein in
GAC tissues. |
Table I.
The expression of HMGB1 protein in
GAC tissues.
| Variables | Group | Cases (n) | Expression levels
(n)
| Positive rate
(%) | χ2 | P-value |
|---|
| − | + | ++ | +++ |
|---|
| HMGB1 | GAC | 88 | 10 | 41 | 27 | 10 | 88.6 | 13.779 | <0.001 |
| ANCT | 88 | 26 | 43 | 17 | 2 | 70.5 | | |
Correlation of HMGB1 expression with
clinicopathological parameters
According to the HMGB1 immunoreactive intensity, 51
(57.95%) patients were classified as low HMGB1 group, and 37
(42.05%) were classified as high HMGB1 group. We then analyzed the
association between HMGB1 expression and the clinicopathological
data of the patients with gastric cancer. As summarized in Table II, we found that high expression of
HMGB1 was closely correlated with the metastatic lymph node of GAC
(P=0.018), but did not correlate to the other clinicopathological
factors including age, gender, tumor size, cancer TNM stage, and
depth of invasion (each P>0.05).
 | Table II.Association between HMGB1 expression
and clinicopathologic characteristics of GAC patients. |
Table II.
Association between HMGB1 expression
and clinicopathologic characteristics of GAC patients.
| Variables | Cases (n) | HMGB1 expression
(n)
| χ2 | P-value |
|---|
| Low | High |
|---|
| Age | | | | | |
| ≤60 | 26 | 16 | 10 | | |
| >60 | 62 | 35 | 27 | 0.195 | 0.659 |
| Gender | | | | | |
| Male | 65 | 36 | 29 | | |
| Female | 23 | 15 | 8 | 0.674 | 0.412 |
| Tumor size
(cm) | | | | | |
| ≤3.5 | 17 | 9 | 8 | | |
| >3.5 | 71 | 42 | 29 | 0.217 | 0.641 |
| Cancer TNM
grade | | | | | |
| I+II | 24 | 11 | 13 | | |
| III+IV | 64 | 40 | 24 | 1.990 | 0.158 |
| Depth of
invasion | | | | | |
|
Mucosa/submucosa | 4 | 2 | 2 | | |
| Muscularis | 8 | 6 | 2 | | |
| Serosa | 59 | 33 | 26 | | |
| Serosal outer
layer | 17 | 10 | 7 | 1.162 | 0.762 |
| Metastatic lymph
node | | | | | |
| Negative | 29 | 22 | 7 | | |
| Positive | 59 | 29 | 30 | 5.628 | 0.018 |
| T stage | | | | | |
| T1+ T2 | 11 | 7 | 4 | | |
| T3+ T4 | 77 | 44 | 33 | 0.167 | 0.683 |
| N stage | | | | | |
| N0+N1 | 36 | 18 | 18 | | |
| N2+N3 | 52 | 33 | 19 | 1.582 | 0.208 |
The effect of HMGB1 knockdown on NF-κB
expression
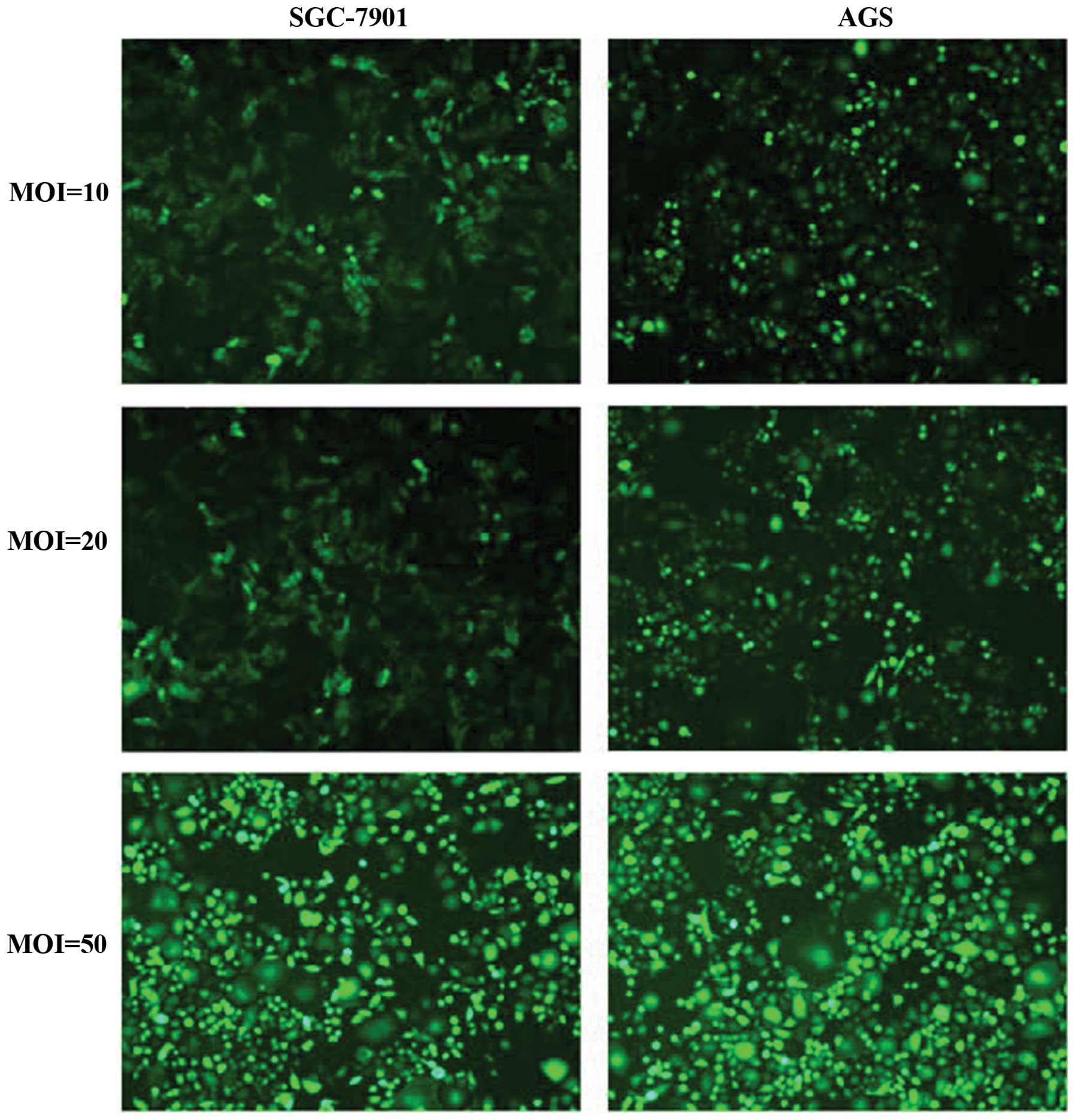
First, lentiviruses of different multiplicity of
infection (MOI) were used to infect GAC cell lines (SGC-7901 and
AGS), and the infection efficiency was observed by fluorescence
microscopy. As shown in Fig. 2,
the infection efficiency of Lv-shHMGB1 (MOI 50) was the highest,
reaching >90%. After Lv-shHMGB1 (MOI 50) transfection into GAC
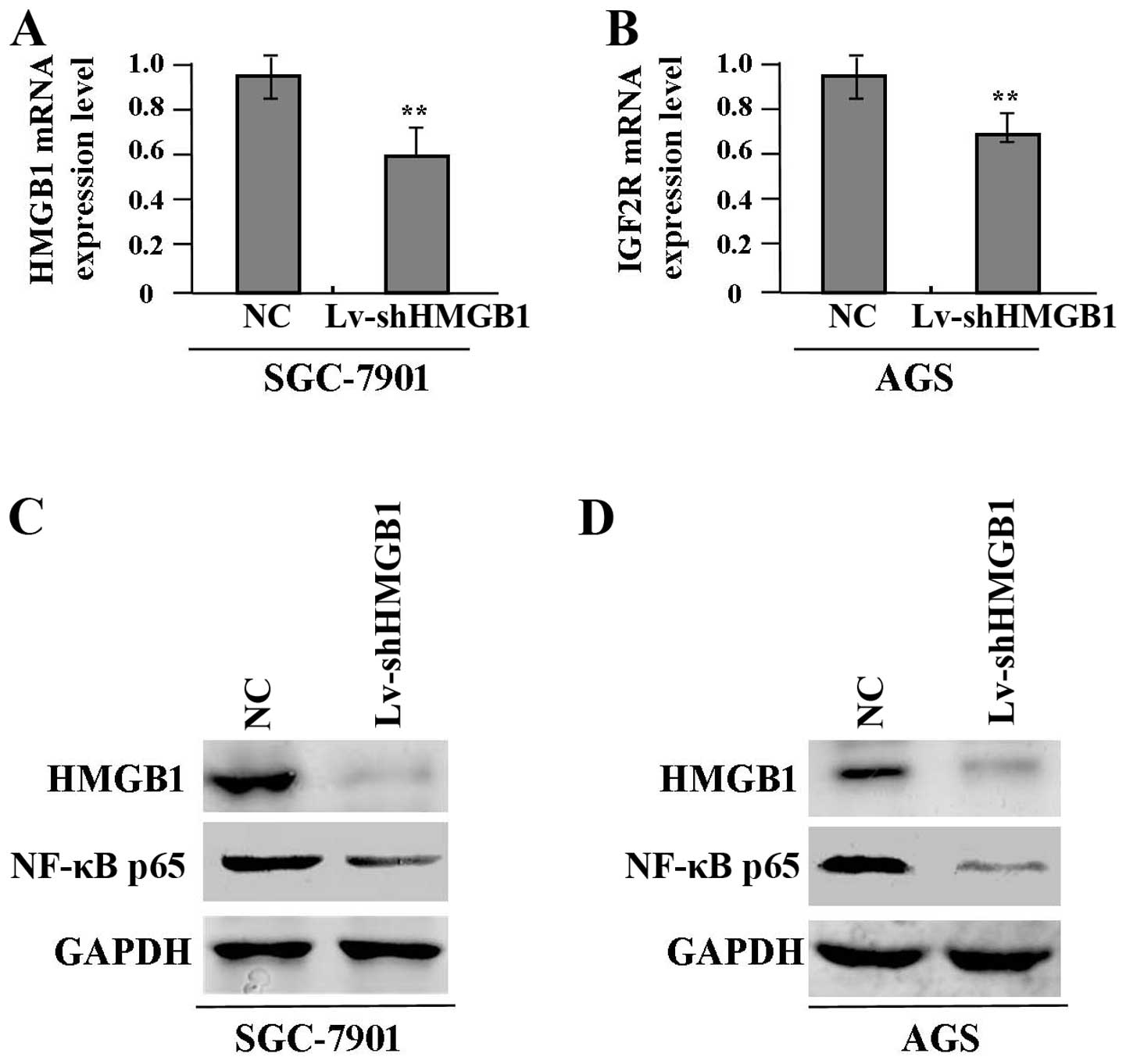
cells (SGC-7901 and AGS) for 24 h, the expression levels of HMGB1
mRNA (Fig. 3A and B) and protein
(Fig. 3C and D) and NF-κB p65
protein (Fig. 3C and D) were
detected by real-time PCR and western blot assays, indicating clear
inhibition of HMGB1 and NF-κB p65 expression in Lv-shHMGB1 group
compared with the NC group.
The effect of HMGB1 knockdown on cell
proliferation
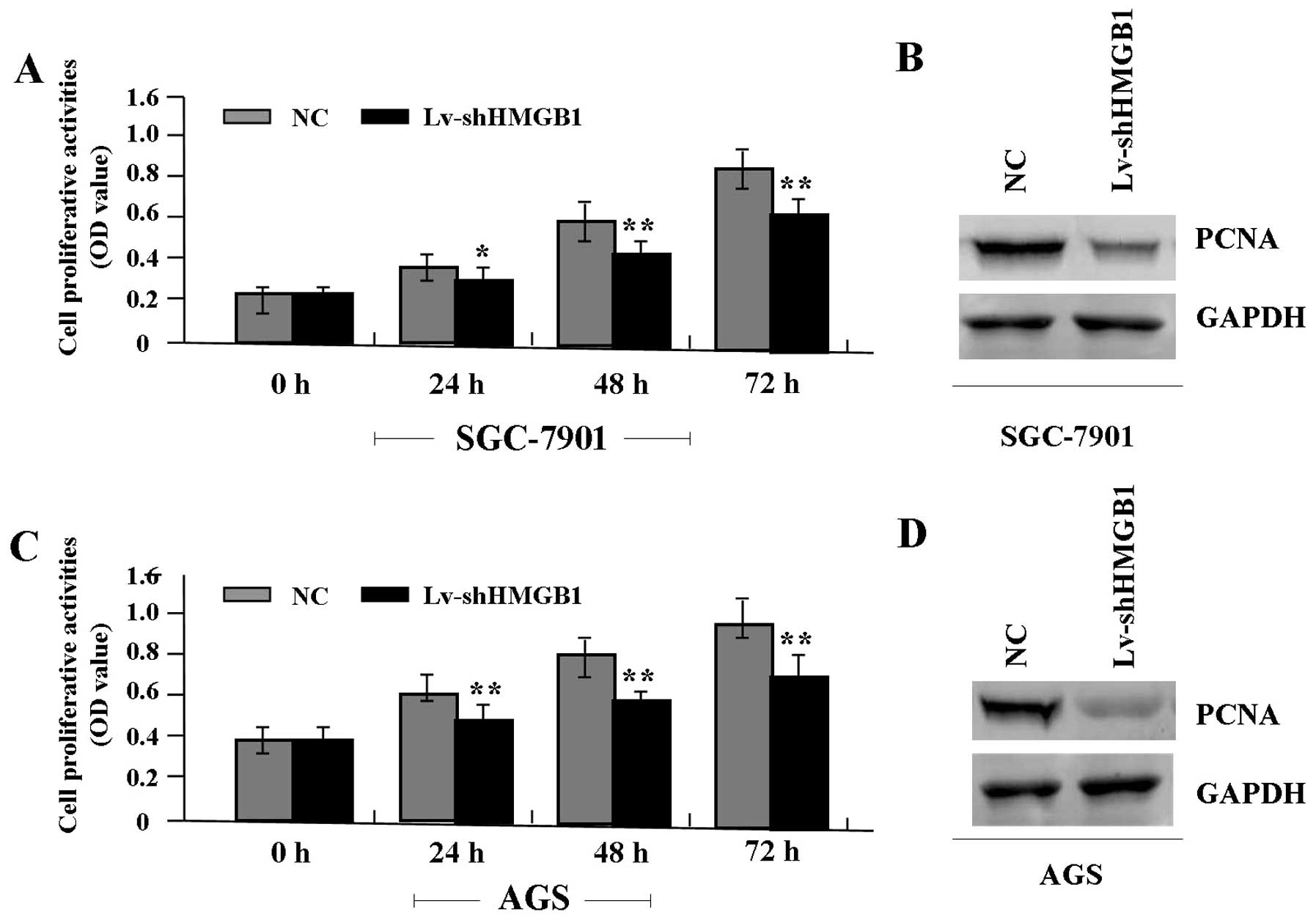
Deregulated cell proliferation is a hallmark of
cancer (21). To verify the effect
of HMGB1 knockdown on tumor growth in GAC cells (SGC-7901 and AGS),
we examined cell proliferative activities by MTT assay. It was
found that the knockdown of HMGB1 markedly diminished the
proliferative activities of GAC cells in a time-dependent manner
compared to the NC group (Fig. 4A and
C). In addition, the expression level of PCNA protein, examined
by western blot assay (Fig. 4B and
D), was found significantly downregulated in Lv-shHMGB1 group
compared with the NC group.
The effect of HMGB1 knockdown on cell
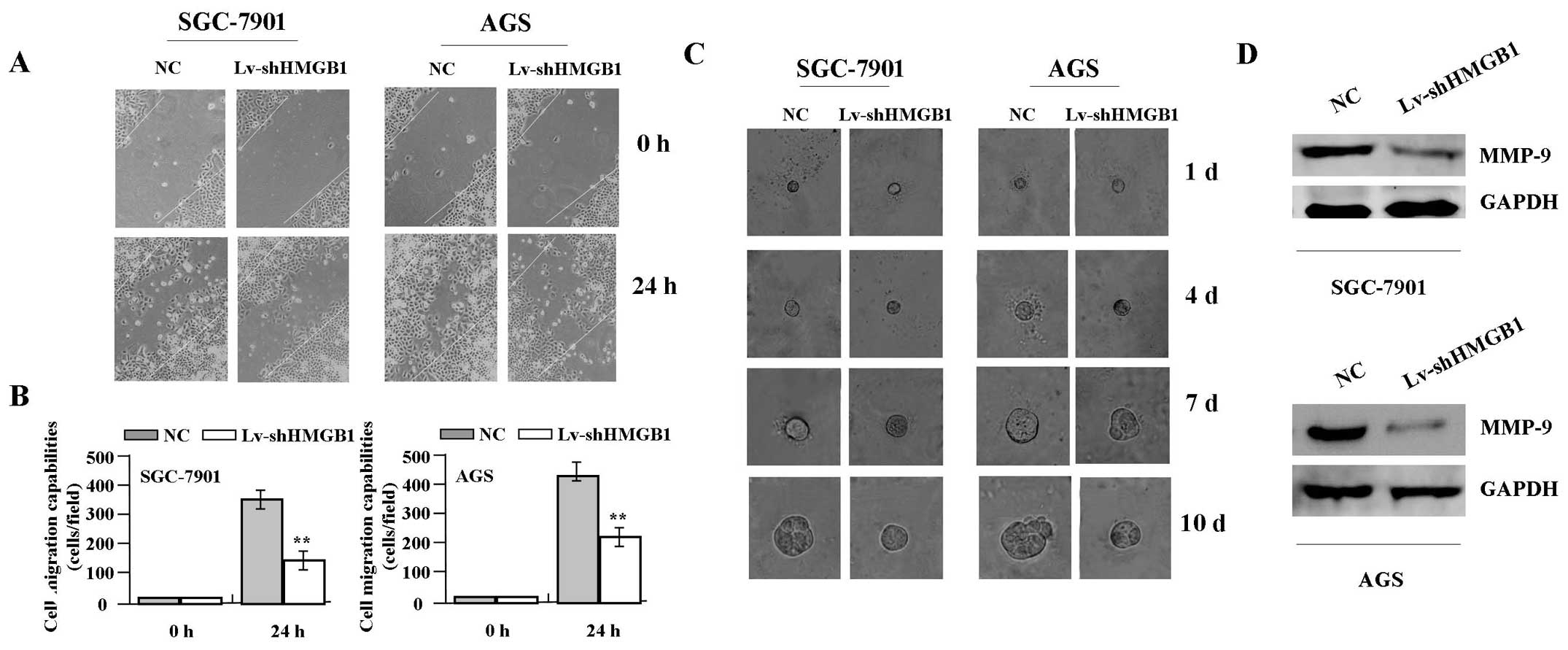
migration and invasion
To determine the effect of HMGB1 knockdown on cell
migration and invasion, wound-healing and 3D-Matrigel assays were
performed. The results indicated that, the migration capabilities
of GAC cells (SGC-7901 and AGS) in Lv-shHMGB1 group were markedly
weakened at 24 h compared with those in NC group (Fig. 5A and B). GAC cells grew very slowly
in 3D environments and therefore were allowed to grow up to 10
days. In Matrigel, the cells formed small mostly round masses, and
Lv-shHMGB1 treatment induced no consistent changes in cell size and
morphology of the cells. In contrast, the average size of GAC
masses was much smaller in Lv-shHMGB1 group (25.50±7.90 μm)
than that of NC group (45.30±6.41 μm) at day 10 (P<0.01,
Fig. 5C). In addition, the
expression level of MMP-9 protein, examined by western blot assay
(Fig. 5D), was found significantly
downregulated in Lv-shHMGB1 group compared with the NC group.
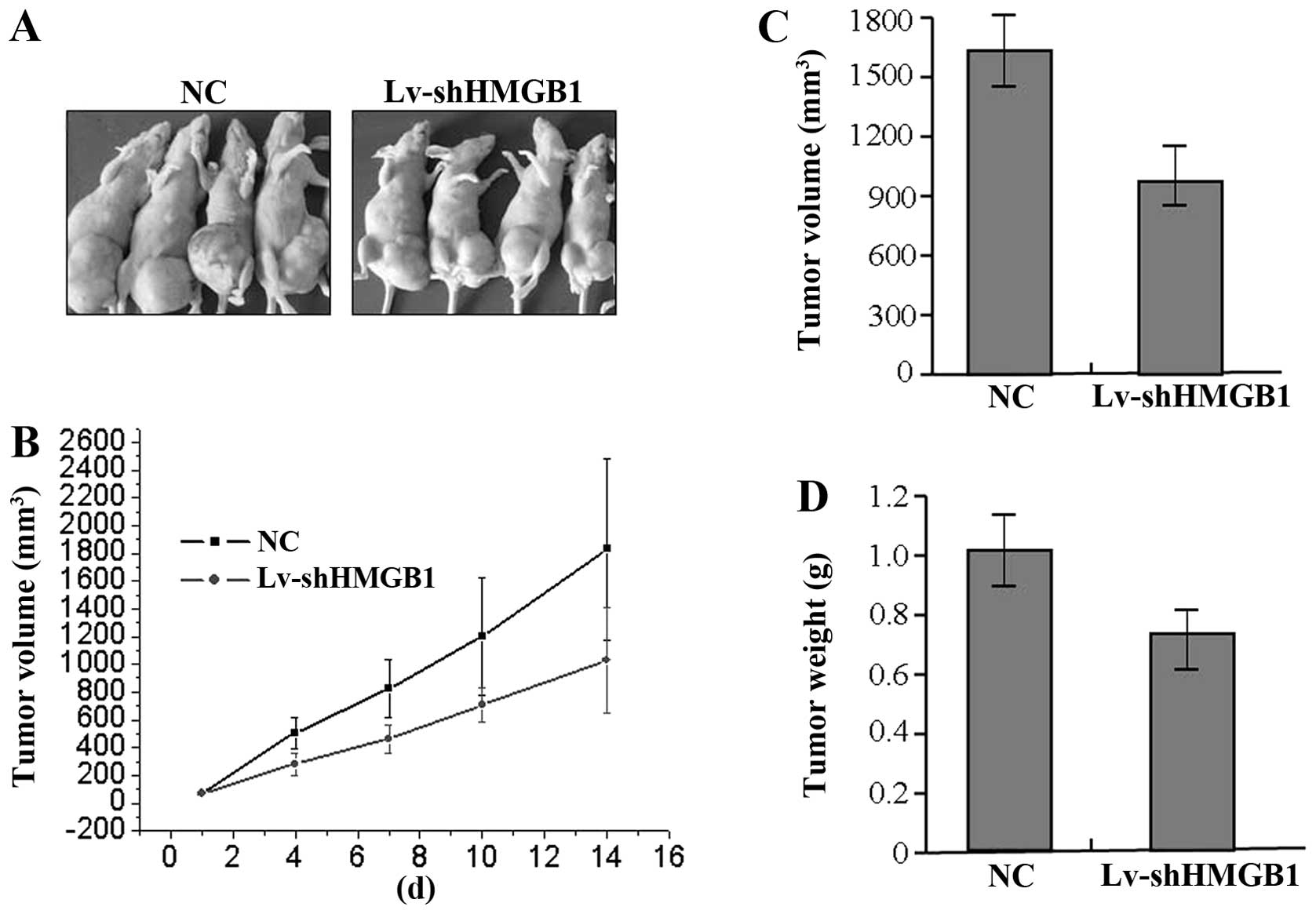
The effect of HMGB1 knockdown on
xenograft tumor growth
Our in vitro experiments demonstrated the
inhibitory effect of HMGB1 knockdown on tumor growth of GAC cells.
Therefore, it is necessary to further investigate the effect of
HMGB1 knockdown on xenograft tumor growth in vivo. The mean
volume of tumors in the experimental mice before treatment was
58.68±28.22 mm3. During the whole tumor growth period,
the tumor growth activity was measured. The tumors treated with
Lv-shHMGB1 grew substantially slower compared to the NC group
(Fig. 6A and B). When the tumors
were harvested, the average weight and volume of the tumors in
Lv-shHMGB1 group were significantly smaller than those of the NC
group (Fig. 6C and D), suggesting
that the knockdown of HMGB1 suppressed the growth of GAC cells.
Discussion
HMGB1 plays an important role in a number of
clinical conditions, such as autoimmunity, cardiovascular disease
and cancer. HMGB1 promotes proliferation and invasion of lung
cancer by activation of the Erk1/2 and p38MAPK pathways (22). HMGB1 is correlated with
angiogenesis, acts as a key regulator in the progression of
carcinoma, and serves as a potential diagnostic and therapeutic
target (23,24). HMGB1 is overexpressed in tumor
cells and promotes activity of regulatory T cells in patients with
head and neck cancer (25).
Expression of HMGB1 is also significantly associated with
malignancy and clinical stage of T-cell lymphomas, and may be an
important biomarker for the development and progression of T-cell
lymphoma (26). The expression of
HMGB1 is closely correlated with pathological grade and distant
metastases of liver cancer, and knockdown of HMGB1 inhibits liver
cancer growth and metastasis, suggesting that HMGB1 may represent a
potential therapeutic target for the aggressiveness of this
malignancy (27).
Importantly, the expression of HMGB1 is increased in
gastric cancer with the intestinal type compared to that in the
diffuse type, and is linked to the degree of macrophage
infiltration in the tumor microenvironment (28). In the present study, we also found
that the positive expression of HMGB1 was significantly increased
in the nucleus of gastric cancer tissues compared with the adjacent
non-cancer tissues. Similar with other studies (10,26–28),
HMGB1 was found correlated with metastatic lymph nodes in gastric
cancer, suggesting that nuclear accumulation of HMGB1 might be
involved in the development and progression.
Furthermore, the focus of research should be on the
function of HMGB1 in cancer. In this study, a loss of function
experiment was performed to clarify the function of HMGB1 in GAC
cells, showing that knockdown of HMGB1 gene by shRNA suppressed
cell proliferative activities and invasive potential, and slowed
xenograft tumor growth. Taken into account the correlation of HMGB1
with lymph node metastasis of GAC, our findings indicated that
HMGB1 might play an important role in promoting tumorigenesis of
GAC. Increased expression of HMGB1 is also associated with distant
metastases of liver cancer, and knockdown of HMGB1 inhibits liver
cancer growth and metastasis (27). The HMGB1/RAGE inflammatory pathway
directly promotes pancreatic tumor growth by regulating
mitochondrial bioenergetics (29).
Importantly, ethyl pyruvate, a potent inhibitor of HMGB1 has been
shown to inhibit tumor growth and metastasis and have a therapeutic
role in the treatment of cancer in conjunction with other
therapeutic agents (30,31). Though few studies indicate HMGB1
not to be the principal mediator of inflammation in malignant
epidermal tumors (32), most
studies, including ours, support HMGB1 functions as a
tumor-promoting gene, and may serve as a potential therapeutic
target for treatment of cancer.
PCNA is a nuclear protein that is expressed in
proliferating cells and may be required for maintaining cell
proliferation, used as a marker for cell proliferation of gastric
cancer (33). MMP-9 is thought to
be a key enzyme involved in the degradation of type IV collagen and
high level of MMP-9 in tissues is associated with tumor growth and
invasion (34). It has been
reported that blockade of NF-κB pathway by RNAI inhibits growth and
metastasis of malignant tumors via regulation of PCNA and MMP-9
expression (35). In our study, it
was found that knockdown of HMGB1 downregulated the expression of
NF-κB, PCNA and MMP-9 in GAC cells, suggesting that targeted
blockade of HMGB1 pathway might inhibit growth and metastasis of
GAC cells through NF-κB-mediated regulation of PCNA and MMP-9
expression.
In conclusion, these findings suggest that increased
expression of HMGB1 is associated with tumor metastasis of GAC, and
the knockdown of HMGB1 suppresses growth and invasion of GAC cells
through the NF-κB pathway in vitro and in vivo, thus
HMGB1 may serve as a potential therapeutic target for GAC.
Acknowledgements
This study was supported by National
Nature Science Foundation of China (nos. 81302093 and
81272752).
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2.
|
Tajima Y, Yamazaki K, Makino R, et al:
Gastric and intestinal phenotypic marker expression in early
differentiated-type tumors of the stomach: clinicopathologic
significance and genetic background. Clin Cancer Res. 12:6469–6479.
2006. View Article : Google Scholar
|
|
3.
|
Asavarut P, Zhao H, Gu J and Ma D: The
role of HMGB1 in inflammation-mediated organ injury. Acta
Anaesthesiol Taiwan. 51:28–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nogueira-Machado JA and de Oliveira Volpe
CM: HMGB-1 as a target for inflammation controlling. Recent Pat
Endocr Metab Immune Drug Discov. 6:201–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Teiten MH, Gaigneaux A, Chateauvieux S, et
al: Identification of differentially expressed proteins in
curcumin-treated prostate cancer cell lines. OMICS. 16:289–300.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Chen J, Xi B, Zhao Y, et al: High-mobility
group protein B1 (HMGB1) is a novel biomarker for human ovarian
cancer. Gynecol Oncol. 126:109–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kostova N, Zlateva S, Ugrinova I and
Pasheva E: The expression of HMGB1 protein and its receptor RAGE in
human malignant tumors. Mol Cell Biochem. 337:251–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sterenczak KA, Joetzke AE, Willenbrock S,
et al: High-mobility group B1 (HMGB1) and receptor for advanced
glycation end-products (RAGE) expression in canine lymphoma.
Anticancer Res. 30:5043–5048. 2010.
|
|
9.
|
Choi J, Lee MK, Oh KH, et al: Interaction
effect between the receptor for advanced glycation end products
(RAGE) and high-mobility group box-1 (HMGB-1) for the migration of
a squamous cell carcinoma cell line. Tumori. 97:196–202.
2011.PubMed/NCBI
|
|
10.
|
Yao X, Zhao G, Yang H, et al:
Overexpression of high-mobility group box 1 correlates with tumor
progression and poor prognosis in human colorectal carcinoma. J
Cancer Res Clin Oncol. 136:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu Y, Xie C, Zhang X, et al: Elevated
expression of HMGB1 in squamous-cell carcinoma of the head and neck
and its clinical significance. Eur J Cancer. 46:3007–3015. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tang CH, Keng YT and Liu JF: HMGB-1
induces cell motility and α5β1 integrin expression in human
chondrosarcoma cells. Cancer Lett. 322:98–106. 2012.
|
|
13.
|
Mardente S, Mari E, Consorti F, et al:
HMGB1 induces the overexpression of miR-222 and miR-221 and
increases growth and motility in papillary thyroid cancer cells.
Oncol Rep. 28:2285–2289. 2012.PubMed/NCBI
|
|
14.
|
Yan W, Chang Y, Liang X, et al: High
mobility group box 1 activates caspase-1 and promotes
hepatocellular carcinoma invasiveness and metastases. Hepatology.
55:1863–1875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lee H, Song M, Shin N, et al: Diagnostic
significance of serum HMGB1 in colorectal carcinomas. PLoS One.
7:e343182012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Liu L, Yang M, Kang R, et al:
HMGB1-induced autophagy promotes chemotherapy resistance in
leukemia cells. Leukemia. 25:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yang L, Yu Y, Kang R, et al: Up-regulated
autophagy by endogenous high mobility group box-1 promotes
chemoresistance in leukemia cells. Leuk Lymphoma. 53:315–322. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sterenczak KA, Kleinschmidt S, Wefstaedt
P, et al: Quantitative PCR and immunohistochemical analyses of
HMGB1 and RAGE expression in canine disseminated histiocytic
sarcoma (malignant histiocytosis). Anticancer Res. 31:1541–1548.
2011.
|
|
19.
|
Peng RQ, Wu XJ, Ding Y, et al:
Co-expression of nuclear and cytoplasmic HMGB1 is inversely
associated with infiltration of CD45RO+ T cells and
prognosis in patients with stage IIIB colon cancer. BMC Cancer.
10:4962010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Jiao Y, Wang HC and Fan SJ: Growth
suppression and radiosensitivity increase by HMGB1 in breast
cancer. Acta Pharmacol Sin. 28:1957–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hanahan D and Weinberg RA: The hallmarks
of cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Sun KK, Ji C, Li X, et al: Overexpression
of high mobility group protein B1 correlates with the proliferation
and metastasis of lung adenocarcinoma cells. Mol Med Rep.
7:1678–1682. 2013.PubMed/NCBI
|
|
23.
|
Wang W, Jiang H, Zhu H, et al:
Overexpression of high mobility group box 1 and 2 is associated
with the progression and angiogenesis of human bladder carcinoma.
Oncol Lett. 5:884–888. 2013.
|
|
24.
|
van Beijnum JR, Nowak-Sliwinska P, van den
Boezem E, et al: Tumor angiogenesis is enforced by autocrine
regulation of high-mobility group box 1. Oncogene. 32:363–374.
2013.PubMed/NCBI
|
|
25.
|
Wild CA, Brandau S, Lotfi R, et al: HMGB1
is overexpressed in tumor cells and promotes activity of regulatory
T cells in patients with head and neck cancer. Oral Oncol.
48:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mao XJ, Wang GF, Chen ZJ, et al:
Expression of HMGB1 and its clinical significance in T-cell
lymphoma. Asian Pac J Cancer Prev. 13:5569–5571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Dong YD, Cui L, Peng CH, et al: Expression
and clinical significance of HMGB1 in human liver cancer: knockdown
inhibits tumor growth and metastasis in vitro and in
vivo. Oncol Rep. 29:87–94. 2013.PubMed/NCBI
|
|
28.
|
Akaike H, Kono K, Sugai H, et al:
Expression of high mobility group box chromosomal protein-1
(HMGB-1) in gastric cancer. Anticancer Res. 27:449–457.
2007.PubMed/NCBI
|
|
29.
|
Kang R, Tang D, Schapiro NE, et al: The
HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by
regulating mitochondrial bioenergetics. Oncogene. Jan 14–2013.Epub
ahead of print. View Article : Google Scholar
|
|
30.
|
Liang X, Chavez AR, Schapiro NE, et al:
Ethyl pyruvate administration inhibits hepatic tumor growth. J
Leukoc Biol. 86:599–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Zhang J, Zhu JS, Zhou Z, et al: Inhibitory
effects of ethyl pyruvate administration on human gastric cancer
growth via regulation of the HMGB1-RAGE and Akt pathways in
vitro and in vivo. Oncol Rep. 27:1511–1519.
2012.PubMed/NCBI
|
|
32.
|
Weng H, Deng Y, Xie Y, et al: Expression
and significance of HMGB1, TLR4 and NF-κB p65 in human epidermal
tumors. BMC Cancer. 13:3112013.
|
|
33.
|
Czyzewska J, Guzińska-Ustymowicz K, Lebelt
A, et al: Evaluation of proliferating markers Ki-67, PCNA in
gastric cancers. Rocz Akad Med Bialymst. 49:64–66. 2004.PubMed/NCBI
|
|
34.
|
Cawston TE and Wilson AJ: Understanding
the role of tissue degrading enzymes and their inhibitors in
development and disease. Best Pract Res Clin Rheumatol.
20:983–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Park SK, Hwang YS, Park KK, et al:
Kalopanaxsaponin A inhibits PMA-induced invasion by reducing matrix
metallo-proteinase-9 via PI3K/Akt- and PKCdelta-mediated signaling
in MCF-7 human breast cancer cells. Carcinogenesis. 30:1225–1233.
2009. View Article : Google Scholar
|