Introduction
Cancer still ranks first as the leading cause of
death. After development of cancer, the cancer cells start to
invade the vascular and lymphatic channels, and then spread to
farther regional nodes and distant organs. Therefore, much
attention has been paid to the control of cancer invasion and
metastasis. In the progression of cancer invasion and metastasis,
growth factors and cytokines play an important role. Especially,
epidermal growth factor (EGF) is reported to promote tumor cell
motility and invasion and to be implicated in tumor progression
(1–3), and the overexpression of EGF receptor
(EGFR) has shown the poor clinical outcome (4, 5). In
the process of invasion, matrix matalloproteinases (MMPs) play a
critical role in degradation of extracellular matrix (ECM). In
addition to this degradation, epithelial cancer cells need to
change to metastasize to other organs. Epithelial-mesenchymal
transition (EMT) is one of the hypothetical processes for
epithelial cancer cells to metasta-size; it causes cancer cells to
lose epithelial characteristics and acquire invasive properties and
stem cell-like features (6).
Vitis coignetiae Pulliat (Meoru in Korea) has
been used as a Korean folk medicine for the treatment of
inflammatory disorders and cancer. Its fruit has an intense dark
red hue, reflecting an abundance of anthocyanins. The anthocyanins
reportedly have inhibitory effect on tyrosine kinase of EGFR
(7). We reported that anthocyanins
isolated from Meoru (AIMs) have anticancer property by inhibiting
Akt activity (8). However, it is
still unknown whether the inhibitory effects of AIMs on Akt was
derived from the anti-EGFR effects of AIMs. Moreover, few studies
have been conducted regarding the effects of anthocyanins on EMT.
Therefore, we investigated the effects of AIMs on cellular
responses and molecular changes in EGF-treated human lung cancer
cells in terms of cancer invasion and EMT.
Materials and methods
Cell culture and chemicals
A549 human lung cancer cells from the American Type
Culture collection (Rockville, MD, USA) were cultured in RPMI-1640
medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v)
fetal bovine serum (FBS) (Gibco-BRL, Grand Island, NY, USA), 1 mM
L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin
at 37°C in a humidified atmosphere of 95% air and 5% CO2
incubator. Molecular mass markers for proteins were obtained from
Pharmacia Biotech (Saclay, France). Antibodies against phospho-Akt
serine (Ser)473, Akt 1/2/3 (H-136), COX-2 (29), cyclin D1 (M-20), c-Myc,
extracellular-regulated kinase (ERK), phospho-ERK (E-4), E-cadherin
(6F9), MMP-2, MMP-9, phospho-p70S6 kinase α (Thr389), β-catenin
(H-102), XIAP, Bcl-2, were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Antibodies against phospho -IKB-α
(Ser32/36), vimentin (D21H3), Snail (C15D3), N-cadherin,
phosphorylated (p)-GSK-3β (Ser9), phospho-Akt (Thr308) were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Antibodies against EGFR, and phospho-EGFR were purchased from
Upstate Biotech (Waltham, MA, USA). Antibody against β-actin was
from Sigma (Beverly, MA, USA). Peroxidase-labeled donkey
anti-rabbit and sheep anti-mouse immunoglobulin, and an enhanced
chemiluminescence (ECL) kit were purchased from Amersham (Arlington
Heights, IL, USA). All other chemicals not specifically cited here
were purchased from Sigma Chemical (St. Louis, MO, USA).
Preparation of anthocyanins
Fruits of Meoru were collected in the middle of
September 2008 at Jiri Mountain, Korea and freeze-dried and stored
in dark glass containers at −20°C until required for analysis.
Anthocyanin pigments were isolated as previously described
(9). The composition of
anthocyanins isolated from Meoru (AIMs) was as follows:
delphinidin-3,5-diglucoside:
cyanidin-3,5-diglucoside:petunidin-3,5-diglucoside:delphinidin-3-glucoside:malvdin-3,5-diglucoside:peonidin-3,5-diglucoside:
cyanidin-3-glucoside:petunidin-3-glucoside:peonidin-3-glucoside:
malvidin-3-glucoside =
3.5:3.4:7.1:23.9:8.0:9.6:9.1:16.1:5.7:13.4.
Cell proliferation assays
For the cell viability assay, A549 cells were seeded
onto 24-well plates at a concentration of 5×104 cells/ml
and treated with AIMs for 24 h and the number of surviving cells
was counted using trypan blue exclusion methods.
Cell invasion assay
For the cell invasion assays, A549 cells were
cultured in serum-free media overnight. Cells (5×104)
were loaded onto pre-coated Matrigel 24-well invasion chambers (BD
Biosciences, San Jose, CA, USA) with and without AIMs. Then 0.5 ml
of medium containing 20% FBS was added to the wells of the plate to
serve as a chemo-attractant and incubated for 24 h at 37°C in 5%
CO2. After removing non-migrated or non-invaded cells,
cells on the bottom filter surface were fixed with 10% formalin,
stained with DAPI and counted.
Gelatin zymography
The gelatinolytic activities for MMP-2 and MMP-9 in
the culture medium were assayed by electrophoresis on 10%
polyacrylamide gels containing 1 mg/ml gelatin at 4°C.
Polyacrylamide gels were run at 120 V, washed in 2.5% Triton X-100
for 1 h, and then incubated for 16 h at 37°C in activation buffer
(50 mM Tris-HCl, pH 7.5, 10 mM CaCl2). After staining
with Coomassie Blue (10% glacial acetic acid, 30% methanol and 1.5%
Coomassie brilliant Blue) for 2–3 h, the gel was washed with a
solution of 10% glacial acetic acid and 30% methanol without
Coomassie Blue for 1 h. White lysis zones indicating gelatin
degradation were revealed by staining with Coomassie brilliant
Blue.
Wound healing assay
A549 cells were grown on 30 mm dish plate to 100%
confluent monolayer and then scratched to form a 100-μm
‘wound’ using sterile pipette tips. The cells were then cultured in
the presence or absence of AIMs (400 μg/ml) in serum-free
media for 24 h. The images were recorded at 12 and 24 h after
scratch using an Olympus photomicroscope.
Western blot analysis
The concentrations of cell lysate proteins were
determined by means of the Bradford protein assay (Bio-Rad
Laboratories, Richmond, CA, USA) with bovine serum albumin as a
standard. For the western blot analysis, 30 μg of proteins
were resolved by electrophoresis, eletrotransferred to a
polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA),
and then incubated with primary antibodies followed by secondary
antibody conjugated to peroxidase. Blots were developed with an ECL
detection system.
Statistical analysis
Each experiment was performed in triplicate. The
results were expressed as means ± SD. Significant differences were
determined using the one-way analysis of variance (ANOVA) with
Neuman-Keuls post hoc test in the cases at least three treatment
groups and Student’s t-test for two group comparison. Statistical
significance was defined as P<0.05.
Results
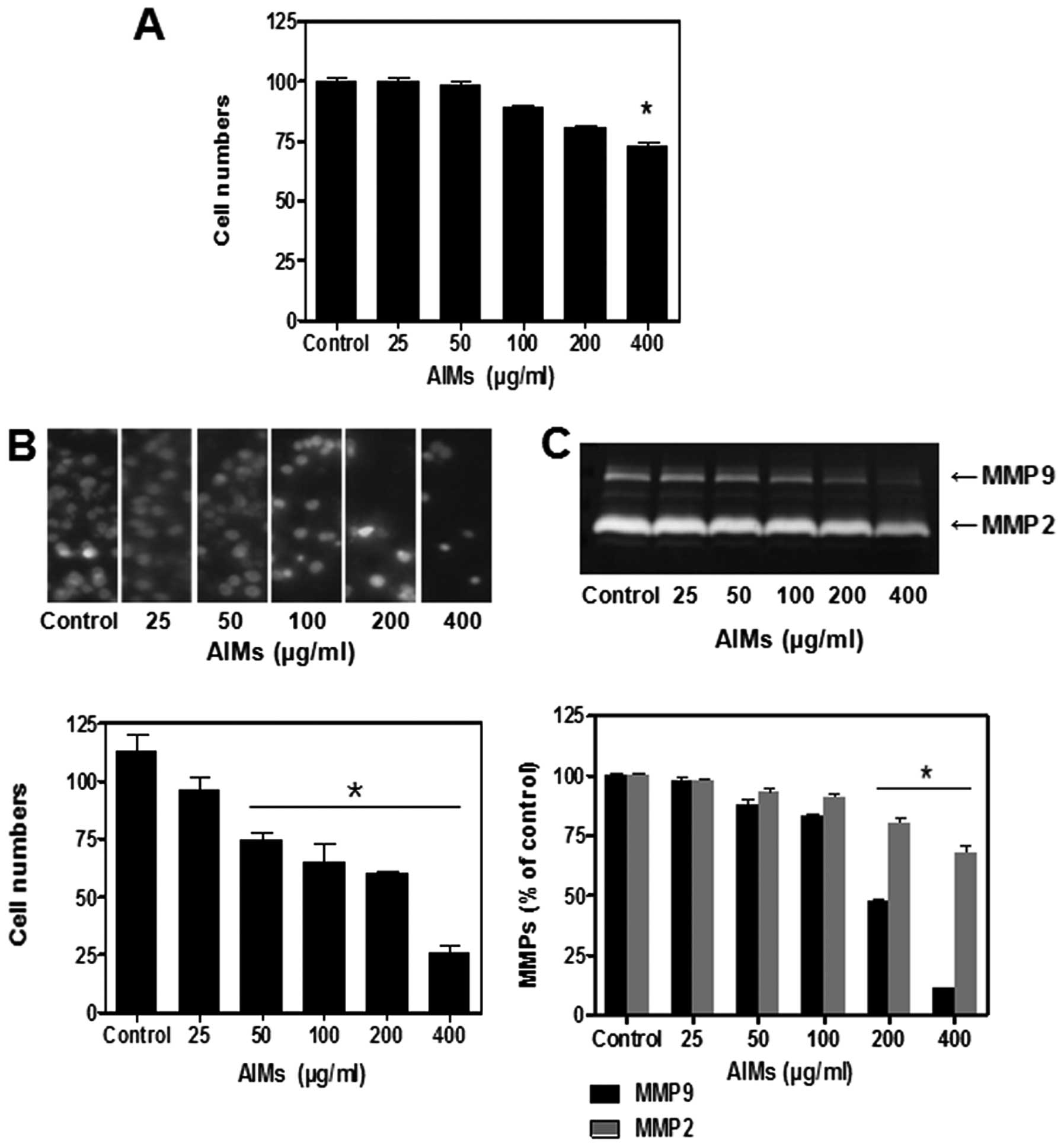
AIMs suppressed the proliferation and
invasion of A549 cells in a dose-dependent manner
At first, the growth of A549 cancer cells was
assessed by trypan blue exclusion method. It revealed that the
growth of A549 cells start to decline at the concentration of 200
μg/ml, and the inhibitory effects finally reached a
statistical significant level at the concentration of 400
μg/ml (Fig. 1A). Next, we
tested the effects of AIMs on cell invasion, because cancer cell
invasion is the first step in cancer metastasis. AIMs significantly
inhibited A549 cell invasion in a dose-dependent manner as measured
by Matrigel invasion assays, compared to the effects of AIMs on
cell growth (Fig. 1B). To verify
the molecular mechanisms, we measured the secreted MMP-2 and MMP-9
by gelatin zymographic analyses using culture media because
secreted MMPs are key molecules in degradation of the extracellular
matrix (ECM) (10,11). As indicated in Fig. 1C, AIMs have markedly suppressed the
gelatinolytic activities of secreted MMP-9 in a dose-dependent
manner, compared to the effects on MMP-2. These finding suggested
that AIMs inhibit invasion predominantly by suppressing MMP-9
secretion.
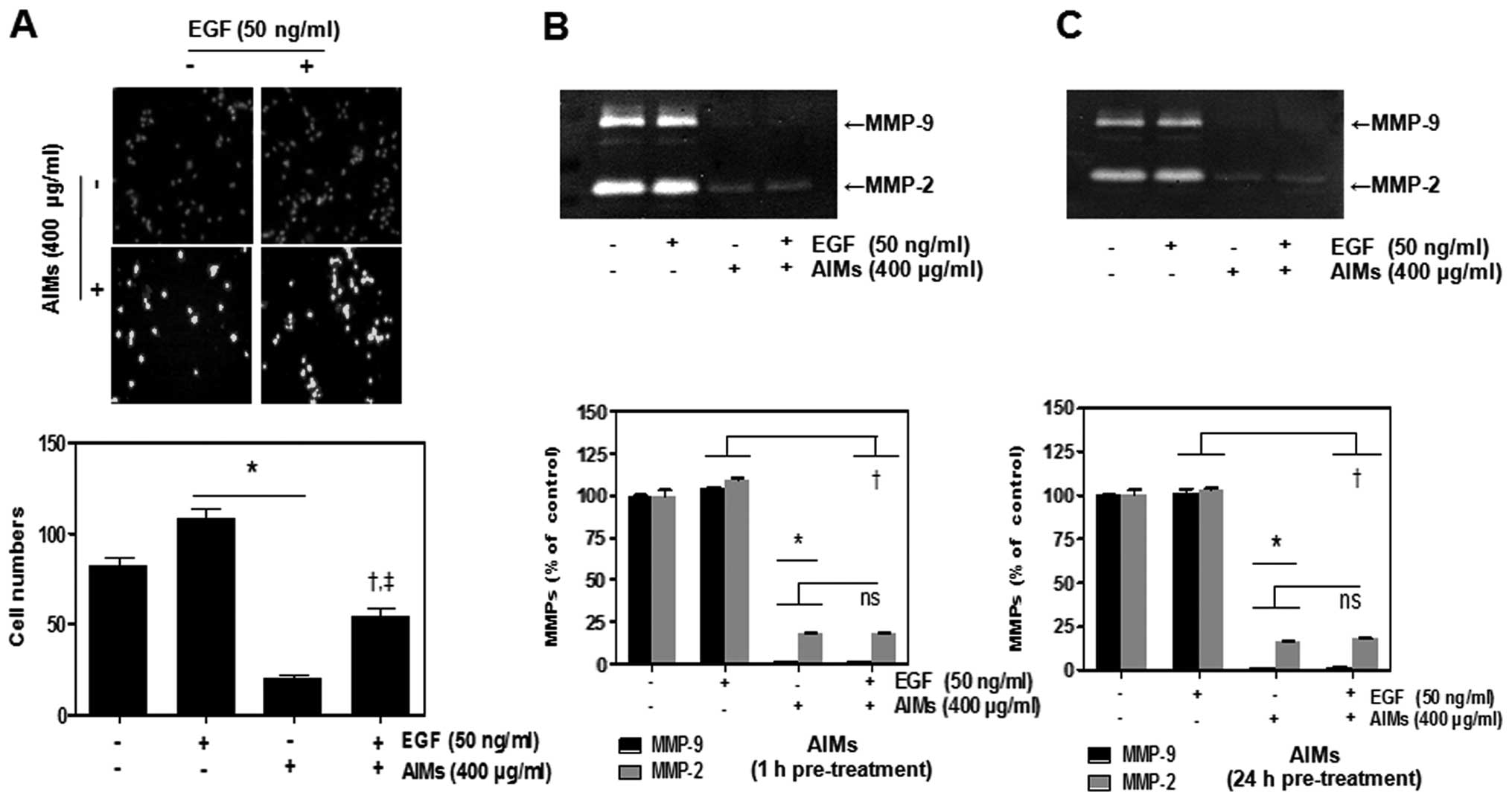
EGF reduced the inhibitory effects of
AIMs on cancer invasion other than an increase in MMP-2 and MMP-9
secretion
The human lung adenocarcinoma cell line A549 was
frequently used for EGF effects on cancer cells (12). Since EGF-induced signaling is
involved in cancer cell invasion (13,14),
we also performed Matrigel invasion test to confirm whether
EGF-promoted cell invasion. Matrigel invasion test revealed that
EGF promoted cell invasion, and that AIMs inhibited the EGF-induced
cell invasion (Fig. 2A).
Interestingly, the degree of the anti-invasive effects of AIMs on
EGF treated cells was not as strong as those on control cells. To
investigate the molecular mechanisms of the augmented effects of
EGF on cancer invasion as well as the anti-invasive effects of
AIMs, we measured the secreted MMP-2 and MMP-9 by the gelatin
zymography. Inconsistent with the results of invasion test of AIMs,
the effects of AIMs on MMP-2 and MMP-9 activities was of no
difference between control and EGF treated group (Fig. 2C). This result suggested that
EGF-augmented invasion is not caused by the increased secretion of
MMP-2 or MMP-9, but by another mechanism.
AIMs inhibited the phosphorylation of Akt
and EGFR, and the inhibitory effect of AIMs on Akt was derived from
the anti-EGFR activity of AIMs
It has been suggested that anthocyanins inhibit
tyrosine kinase activity of EGFR (7), and that AIMs have anti-cancer effects
by inhibiting phosphatidylinositol (PI)-3 kinase (PI3K)/Akt pathway
(8). To determine whether the
inhibitory effects of AIMs on Akt was derived from the anti-EGFR
effects of AIMs as well as to investigate the molecular mechanisms
of the anti-invasive effects of AIMs, we assessed the effects of
AIMs on phosphorylation of Akt, p70S6K (Thr389), and ERK as well as
EGFR in both EGF-treated and EGF-untreated cells. Western blot
analysis revealed that AIMs suppressed EGFR phosphorylation and the
downstream molecules [Akt, p70S6K (Thr389) and ERK]
time-dependently (Fig. 3A). Next,
we assessed the effects of EGF on these molecules. Western blot
analysis revealed that EGF induced phosphorylation of EGFR and the
downstream molecules in control cells, and also augmented EGFR and
ERK phosphorylation in AIM-treated cells (Fig. 3B). However, EGF did not augment Akt
phosphorylation (Thr308) in AIM-treated cells even though it
slightly reduced the inhibitory effects of AIMs on Akt (Ser473) and
p70S6K which is linked to mTORC1 activation in AIM-treated cells.
These findings raised the possibility that the inhibitory effect of
AIMs on Akt phosphorylation on Thr308 is not derived from anti-EGFR
effect of AIMs. Next, we tested the effects on downstream effector
molecules of EGFR involved in cell proliferation and invasion. EGF
moderately increased the gene expressions involved in cell
proliferation (COX-2, c-Myc and cyclin D1) with an increase in EGFR
activity (phosphorylation of EGFR) in either AIM-treated cells or
AIM-untreated cells (Fig. 3B and
C), but it did not significantly increase the expression of
MMP-2 and MMP-9 in AIM-treated cells (Fig. 3C). These finding suggested that
AIMs might suppress MMP-2 and MMP-9 expression by inhibiting Akt
rather than by inhibiting EGFR even though EGF-augmented invasion
may abide by EGFR activity through activation of Akt signaling as
well as ERK signaling.
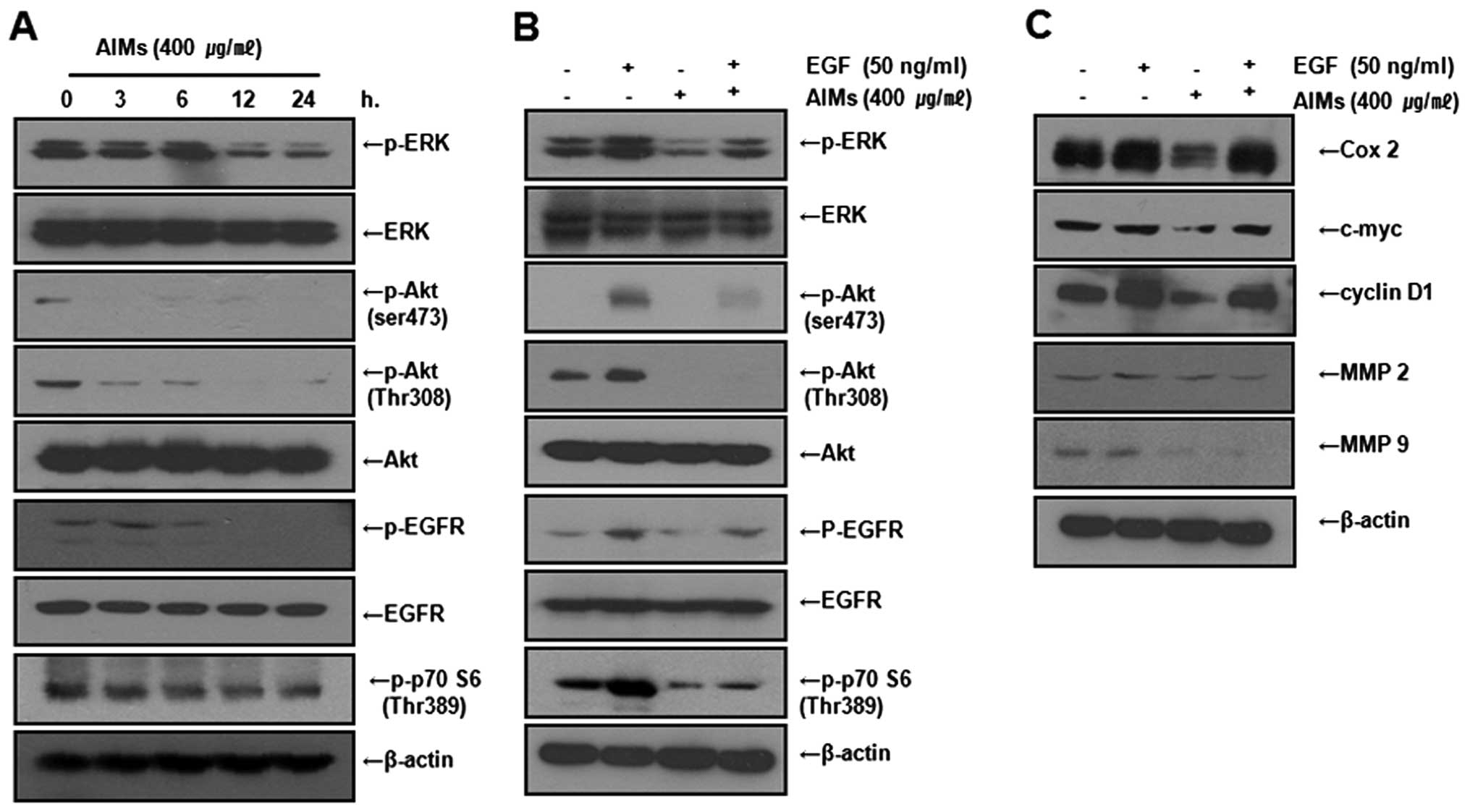 | Figure 3.The inhibitory effects of AIMs on the
phosphorylation of Akt and ERK as well as EGFR. Western blot
analysis for phospho-Akt (Thr308, and Ser473), Akt, phospho-ERK,
ERK, phospho-EGFR, and EGFR in A549 cells. (A) Cells
(5×104 cells) were pretreated with AIMs (400
μg/ml) for the indicated times and lysed. Equal amounts of
the cell lysate were separated by SDS-polyacrylamide gels and then
transferred to nitrocellulose membranes. The membranes were probed
with the indicated antibodies and detected by an ECL detection
system. (B) Cells (5×104 cells) were pretreated with
AIMs (400 μg/ml) for 1 h and then treated with EGF (50
ng/ml) for 24 h. (C) Western blot analysis for COX-2, c-Myc, cyclin
D1, MMP-2 and MMP-9 in A549 cells. Cells (5×104 cells),
either left untreated or pretreated with AIMs (400 μg/ml)
for 1 h and then were exposed to EGF (50 ng/ml) for 24 h.
Whole-cell extracts were prepared, and 30 μg of the
whole-cell lysate was analyzed by western blot analysis. The
results are representative of two independent experiments. |
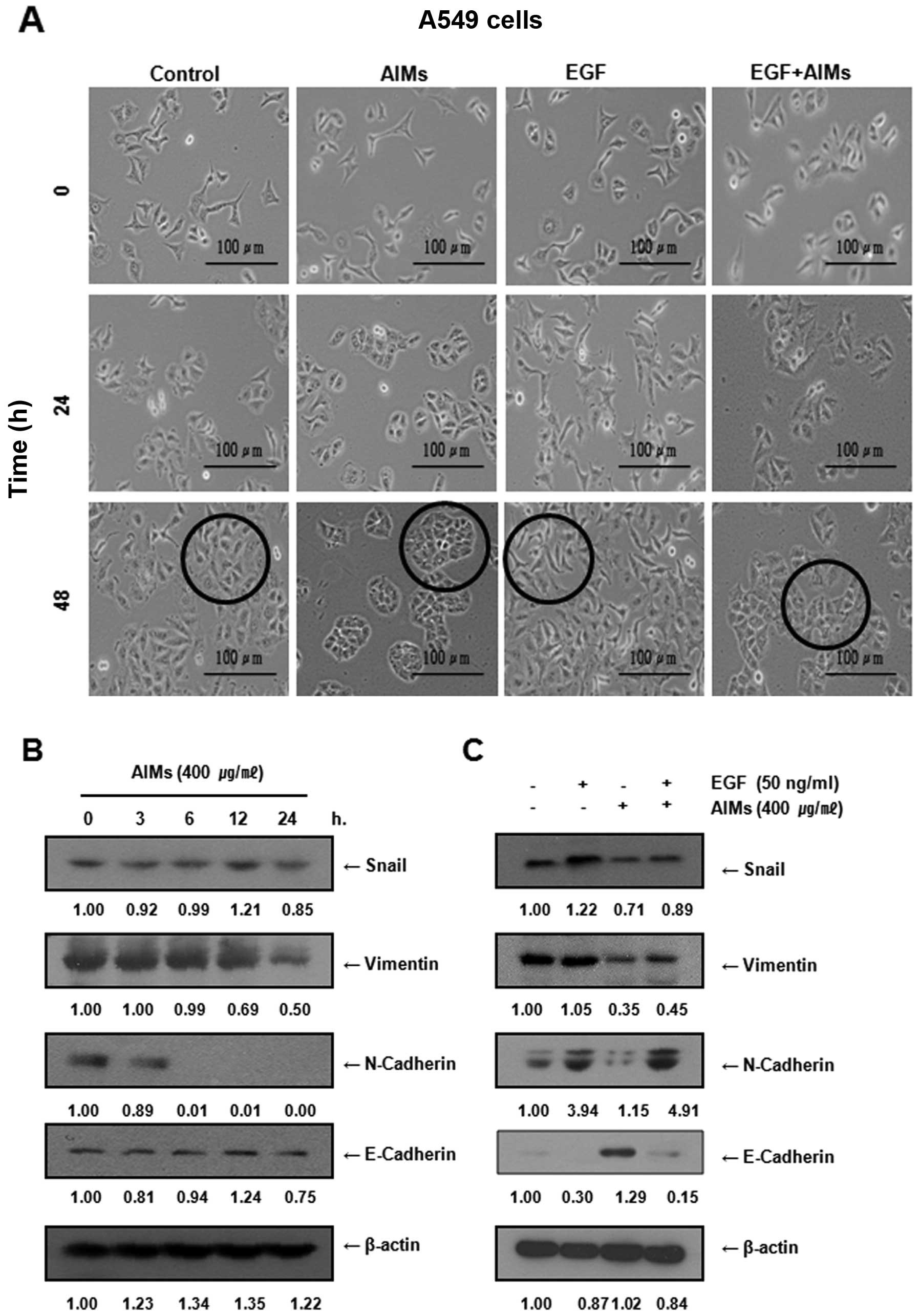
AIMs inhibited epithelial-mesenchymal
transition (EMT) of A549 cells, but not completely in EGF-treated
cells
To determine whether EGF induced EMT of A549 cells,
and whether AIMs inhibit EGF-induced EMT, we observed the cell
morphology of A549 cells for 24 and 48 h after the treatment of EGF
alone or in combination with AIMs. The cell morphology revealed
that EGF induced morphological changes of elongation of A549 cells,
and that AIMs could not completely prevent the morphological
changes (Fig. 4A). To confirm this
finding in the molecular level, we next assessed changes in EMT
biomarkers after the treatment. Western blot analysis revealed that
AIMs suppressed mesenchymal markers such as Snail, vimentin,
N-cadherin and induced E-cadherin, an epithelial marker in either
EGF-treated or untreated cells (Fig.
4B and C). Consistent with the morphology results, AIMs could
not completely suppress the EGF effects on the expression of EMT
markers (Fig. 4C) while EGF
increased EGFR activity in A549 cells (Fig. 3B). These results indicated that EGF
reduced the inhibitory effect of AIMs on EMT with an increase in
EGFR activity although AIMs inhibited EMT in either EGF-treated or
untreated cells. These findings suggest that EGF-induced EMT may
also abide by EGFR activity.
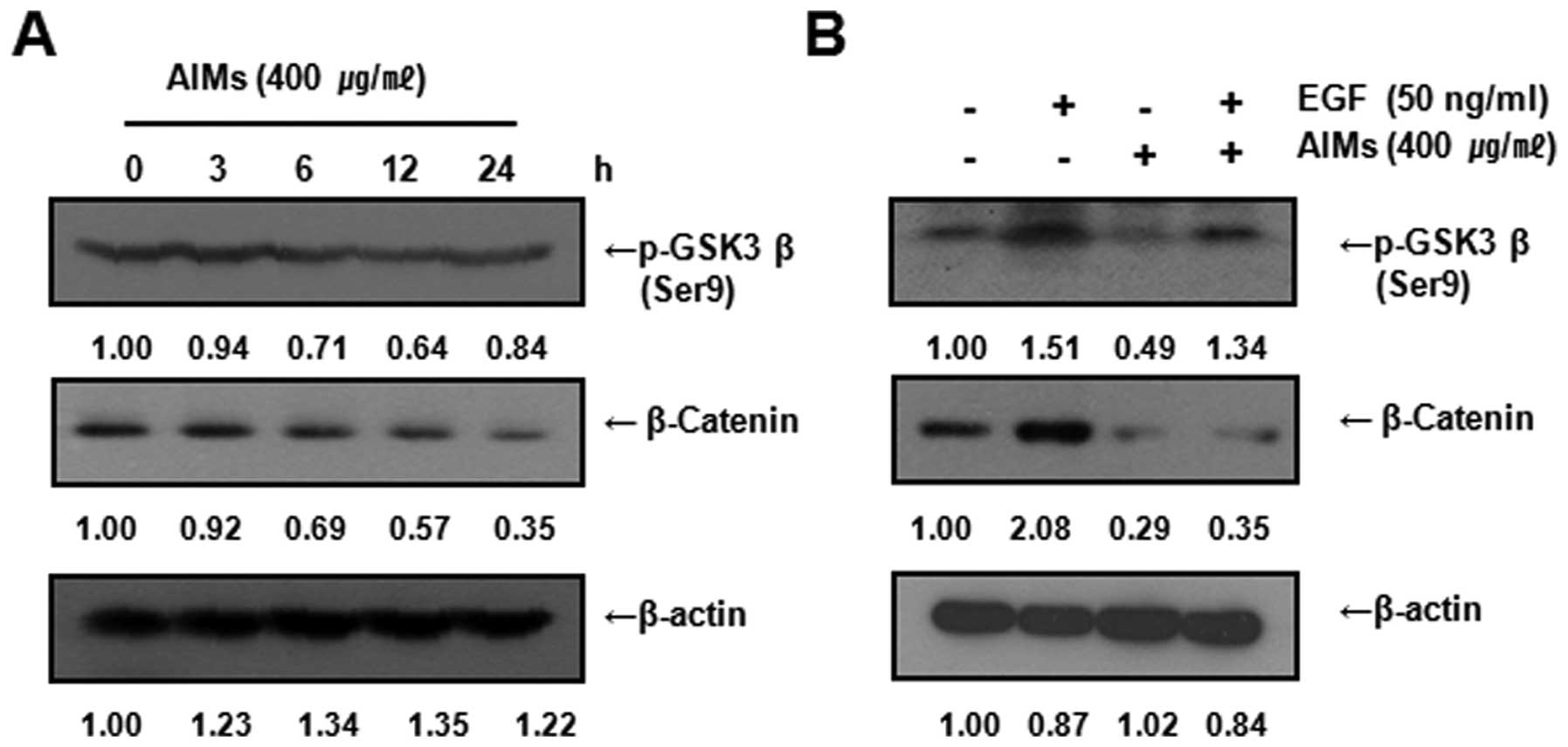
AIMs inhibited EMT at least in part by
inhibiting the expression of GSK-3β phosphorylation (Ser9) and
β-catenin
β-catenin also plays an important role in EMT in
cancer (15,16). In the degradation of β-catenin,
when Wnt signaling is not activated, it is degraded by
GSK-3-induced phosphorylation, and the enzymatic activity of GSK-3
is regulated by phosphorylation of certain GSK-3 residues.
Phosphorylation of GSK-3β on Ser9 is induced by Akt activation, and
results in inhibition of GSK-3 (17). Therefore, we assessed the changes
in the expression of β-catenin, and p-GSK-3β (Ser9) after AIM
treatment. Western blot analysis revealed that AIMs suppressed
β-catenin and Ser9 time-dependently (Fig. 5A). Next to determine whether the
inhibitory effect of AIMs on β-catenin and GSK-3β are influenced by
EGF, we assessed the expression of β-catenin and Ser9 after EGF
treatment in either AIM-treated or untreated cells. Western blot
analysis revealed that the inhibitory effects of AIMs on Ser9 and
β-catenin attenuated by EGF while AIMs clearly inhibited the
expression of β-catenin and Ser9 (Fig.
5B). These results suggest that AIMs inhibited EMT, at least in
part, by inhibiting the expression of GSK-3β phosphorylation (Ser9)
and β-catenin.
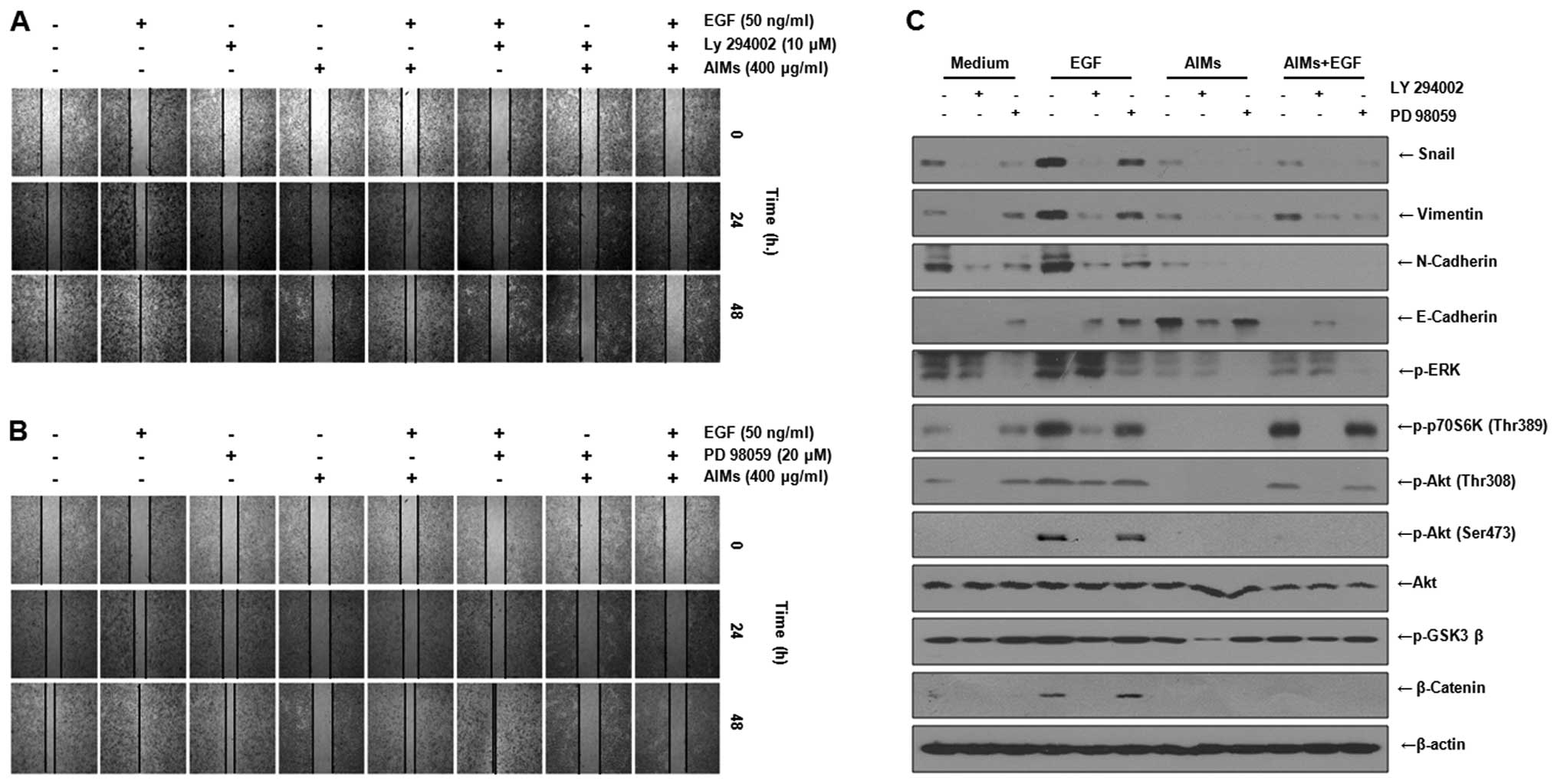
AIMs suppress EMT and invasion by
suppressing Akt activity as well as EGFR activity, but the
inhibitory effects of AIMs on Akt are not derived from EGFR
To give more convincing evidence that AIMs inhibit
EMT by inhibiting Akt activity as well as EGFR activity, we
assessed the effects of inhibition of EGFR downstream pathways (ERK
pathway and PI3K/Akt pathway) on cell migration with the
EGF-stimulated cells. Wound healing tests reveled that AIMs
partially inhibited the EGF-augmented cell migration just as AIMs
inhibited EGF-augmented EMT. The PI3K inhibitor Ly 294002 and the
ERK inhibitor PD 98059 both augmented the inhibitory effects of
AIMs on migration of A549 cells treated with EGF (Fig. 6A and B), suggesting each of the two
downstream pathways of EGFR (ERK pathway and PI3K/Akt pathway) are
contributing to EGF-augmented cancer cell migration. Next, using
the PI3K inhibitor and the ERK inhibitor, we also assessed the
effects of AIMs and/or EGFR on EMT by western blot analysis. It
revealed that EGF activated the two downstream pathways in both
AIM-treated and AIM-untreated cells, and that the ERK inhibitor PD
98059 or the PI3K/Akt inhibitor LY 294002 inhibited EMT in
EGF-treated cells in both AIM-treated and AIM-untreated cells
(Fig. 6C). These finding indicated
that EGF cannot induce the Akt phosphorylation (Ser473), even
though EGF treatment can partially reverse the inhibitory effect of
AIMs on EGFR and re-induce Akt phosphorylation (Thr308) and
p-p70S6K (Thr389) in AIM-treated cells. These findings suggested
that and AIMs might suppress Akt activity by inhibiting Akt
phosphorylation on Ser473 apart from inhibiting EGFR activity.
Taken together, these data also support the finding
that cancer migration and EMT in EGF-treated cells abide by EGFR
activity through the two EGFR downstream pathways (ERK pathway and
PI3K/Akt pathway), and that the inhibitory effect of AIMs on Akt
activity may be independent of anti-EGFR activity.
AIMs inhibit transforming growth factor
(TGF-β)-induced EMT in A549 cells, indicating the inhibitory effect
of AIMs on Akt activity is independent of EGFR activity
To confirm that the inhibitory effects of AIMs on
Akt pathway is independent of EGFR, we investigated the effects of
AIMs on TGF-β-induced invasion and EMT along with assessing the
changes of EMT biomarkers, because TGF-β is a well known cytokine
involved in invasion and EMT, as well as in augmenting Akt activity
independent of EGFR activity. Firstly, to confirm whether TGF-β
also induces EMT of A549 cells and augments cancer cell invasion,
we observed morphological changes and assessed the invasive
activity of A549 cells after TGF-β treament; TGF-β induced
elongation of A549 cells (Fig. 7A)
and augemented invasion of the cancer cells (Fig. 7B). AIMs inhibited the morphological
changes to EMT and the invasive activity even in TGF-β-treated or
TGF-β-untreated cells, but TGF-β also reduced the effects of AIMs
on EMT and invasive activity similarly to EGF (Fig. 7A and B). We also measured the
expression of the MMPs in AIMs-treated A549 cells by the gelatin
zymography analysis. TGF-β augmented the expression of MMP-2 and
MMP-9 in both AIM-treated and untreated cells, and the pattern of
the inhibition of MMP-2 and MMP-9 by AIMs was similar to those
shown by EGF; the inhibitory effect of AIMs on MMP-2 and MMP-9 was
not influenced by TGF-β treatment (Fig. 7C). The protein expression of MMP-2
and MMP-9 revealed by western blot analysis showed similar pattern
as the gelatinolytic activity (Fig.
7D). Additionally, to confirm TGF-β-driven EMT was not
associated with EGFR activity, but rather with Akt activity, we
assessed the effects of AIMs on phosphorylation of Akt and ERK as
well as EGFR. It revealed that TGF-β induced Akt phosphorylation,
but not EGFR phosphorylation, and that AIMs suppressed the Akt
phosphorylation (Ser473) even in TGF-β-treated cells (Fig. 7E). TGF-β re-induced Akt
phosphorylation (Thr308) and the downstream molecule p70S6K, which
is linked to mTORC1 activation in AIM-treated cells. These findings
indicated that AIMs have an inhibitory effect on Akt, independent
of anti-EGFR effects of AIMs. We also assessed the changes in the
molecules involved in EMT. Western blot analysis revealed that AIMs
suppressed mesenchymal markers such as Snail, vimentin, N-cadherin
and induced E-cadherin, which is an epithelial marker in control
cells (Fig. 7F). However, TGF-β
increased mesenchymal biomarkers as well as GSK-3β phosphorylation
and β-catenin expression in both AIM-treated and AIM-untreated
cells. These results confirmed that the inhibitory effect of AIMs
on Akt activity is independent of EGFR activity, and AIMs still
suppressed Akt phosphorylation on Ser473 even in TGF-β-treated
cells whereas the inhibitory effects of AIMs on TGF-β-induced EMT
was limited.
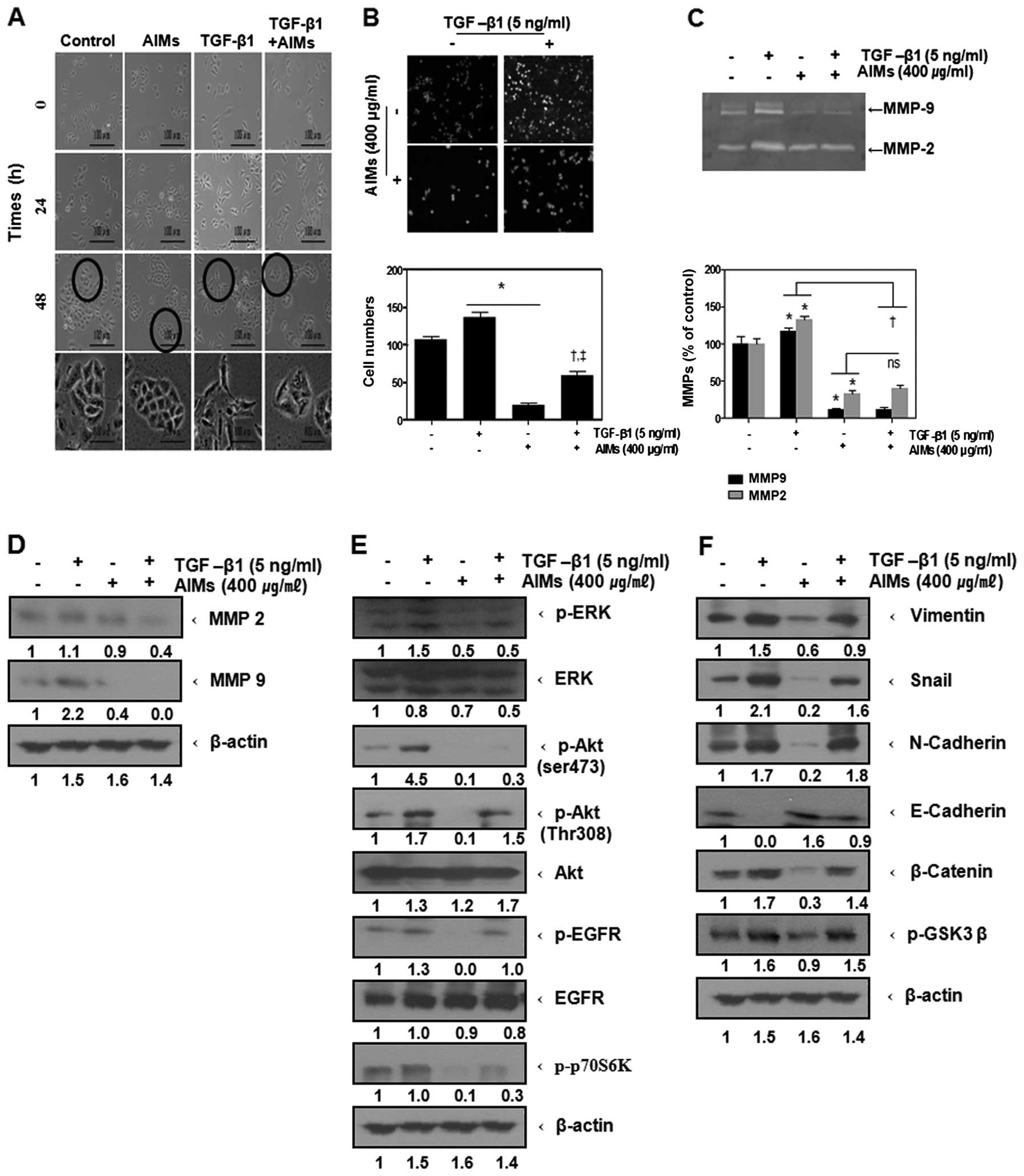 | Figure 7.Inhibitory effects of AIMs on
TGF-β-induced invasion and EMT in A549 cells. (A) Cells were
pretreated with AIMs (400 μg/ml) for 1 h and then treated
with TGF-β (5 ng/ml) for 24 h. Data are representative of three
independent experiments. (B) Cells were serum-starved for 24 h with
or without AIMs (400 μg/ml). Cells (5×104 cells)
were loaded onto pre-coated Matrigel 24-well invasion chambers in
the presence or absence of TGF-β (5 ng/ml). The Matrigel invasion
chambers were incubated for 24 h. *P<0.05 vs.
control, †P<0.05 vs. AIMs. (C) MMP-2 and MMP-9
protein levels were measured by gelatin zymography. Cells were
incubated for 48 h without or with AIMs. Values represent means ±
SD from three independent experiments. *P<0.05 vs.
control. (D–F) Cells (5×104 cells), either left
untreated or pretreated with AIMs (400 μg/ml) for 24 h and
then were exposed to TGF-β (5 ng/ml) for indicated times.
Whole-cell extracts were prepared, and 30 μg of the
whole-cell lysate was analyzed by western blot analysis for (D)
MMP-2 and MMP-9, (E) phospho-Akt (Thr308 and Ser473), Akt,
phospho-ERK, ERK, phospho-EGFR and EGFR, and (F) Snail, vimentin,
N-cadherin and E-cadherin in A549 cells. The results are
representative of two independent experiments. The expression of
the indicated proteins were measured by densitometry and expressed
as relative ratio. |
Discussion
This study was designed to answer the question
whether the inhibitory effects of AIMs on Akt activity were
associated with anti-EGFR effects on cancer invasion, and whether
AIMs have anti-EMT effects. Here, we investigated the effects of
AIMs on cellular responses and molecular changes involved in cancer
invasion and EMT in human lung cancer cells treated with EGF or
TGF-β. We demonstrated that AIMs suppressed PI3k/Akt and EGFR
pathway independently in a dual suppression mode, and that AIMs
suppressed invasion and migration at least in part by suppressing
EMT. A previous report showed that anthocyanins have anti-EGFR
(7,18) and anti-Akt activities (19). Here, we firstly demonstrated that
the anti-Akt activity was independent of anti-EGFR activity of
anthocyanins in cancer invasion and EMT by using inhibitors for
each EGFR downstream pathway (ERK pathway and PI3K/Akt pathway),
EGF or TGF-β stimulation.
In this study, AIMs predominantly suppressed MMP-9
rather than MMP-2, which was consistent the previous findings which
our colleagues demonstrated in HT-29 human colon cancer cells
(9). EMT has attracted a great
deal of attention as a potential mechanism for tumor cell
metastasis (20). Snail is a
well-known transcription factor that promotes EMT by repressing the
expression of E-cadherin which is important in cell adhesion. With
E-cadherin, cancer cells are tightly bound to each other and to
stromal cells. To metastasize, cancer cells need to change in order
to gain mesenchymal phenotype. Snail is degraded and exported from
the nucleus by GSK-3β (21). Here,
we demonstrated that EGF induced Snail expression and suppressed
E-cadherin expression, and that AIMs inhibited the EGF effects on
Snail and E-cadherin. These effects of AIMs on EGF- and
TGF-β-induced EMT were also confirmed in HeLa human uterine cancer
cells treated by TNF (data not shown).
However, there are still several findings that are
not clearly demonstrated. Both EGF and TGF-β enhanced cancer
invasion without any further induction of MMP-2 and MMP-9 in the
AIM-treated cells (Figs. 2A and B,
7B and C). This finding indicated
that there are other mechanisms for EGF- and TGF-β-augmented cancer
cell invasion rather than the secretion of MMPs. Here, we
demonstrated that one of the mechanisms of EGF- and TGF-β-augmented
cancer cell invasion was EMT with assessing the changes in cell
morphology and EMT biomarkers. We suggested that AIMs might
suppress the secretion of MMP-2 and MMP-9 by inhibition of Akt
signaling which is well known to regulate MMP-9 expression
(22) because of the reports
demonstrating that anthocyanins inhibit MMP-2 and MMP-9 by
suppression of PI3K/Akt (19).
However, this study also suggests that there is a possibility that
AIMs inhibit MMP-9 by another mechanism that is not influenced by
either EGFR or Akt activity because EGF and TGF-β activated Akt
activity and EGFR activity in AIM-treated cells.
The effect of AIMs on EMT were different between
EGF-treated cells and TGF-β-treated cells; AIMs partially inhibited
EGF-induced EMT, but not TGF-β-induced EMT although AIMs inhibited
Akt phosphorylation on Ser473 in both EGF- and TGF-β-treated cells.
The difference can be explained by the way TGF-β induces EMT
because TGF-β also induces EMT by SMAD-dependent signaling which
does not involve Akt activation (23–25).
EGF stimulated the MMPs only slightly compared to
those derived from TGF-β. There are some reports that EGF can
induce MMP-9 (26), but in others
show that the extent of EGF-induced MMP-9 secretion is far less
than that induced by TGF-β or tumor necrosis factor (TNF), and that
EGF can even inhibit MMP-9 expression in some cancer cells
(27,28). The latter finding is consistent
with our findings. Another merit of the finding that EGF did not
increase MMP-2 and MMP-9 expression give us more valid evidence
that EGF did augmented invasion by induction of EMT but not by
induction of MMPs.
We determined whether the inhibitory effect of AIMs
on GSK-3β was derived from that on Akt. Previous studies
demonstrated that Akt and GSK-3β signaling pathway are involved in
EMT (29), and that GSK-3β
signaling pathway is dependent on Akt pathway and other signaling
such as WNT signaling (29,30).
In addition, EGFR signaling pathway is also involved in GSK-3
pathway (31). GSK-3 is encoded by
two known genes, GSK-3α and GSK-3β. The site of serine or threonine
phosphorylation determines the activity of GSK-3; phosphorylation
of Ser9 in GSK-3β significantly decreases the activity of GSK-3.
The phosphorylation of Ser9 in GSK-3β is regulated by Akt (17). Therefore, we assessed
phosphorylation of Ser9 in GSK-3β to look at GSK-3 activity
regulated by Akt. GSK-3 activty is closely linked to β-catenin
activation which is important in EMT. In this study, we found that
AIMs suppressed p-GSK-3β (Ser9) and β-catenin, and that EGF and
TGF-β enhanced GSK-3β activity by suppressing phosphorylation of
Ser9 in GSK-3β. These results support that the inhibitory effects
of AIMs on GSK-3β activity is derived from the anti-Akt effects. Up
to now, few studies has been performed regarding the effects of
anthocyanins on GSK-3. There is a report that cyanidin-3-glucoside
upregulated p-GSK-3β (Ser9) in a neuroblastoma cell line (32). The result was opposite to ours.
However, there is another study supporting our results, which
showed that the extract of skin of Muscadine grape containing
abundant anthocyanins suppressed p-GSK-3β (Ser9) (33). In that our anthocyanins were
isolated from fruit, it is likely that the latter study would be
more similar to ours.
Although we demonstrated that EGF and TGF-β
re-induced into AIM-treated cells the p70S6K linked to mTORC-1
signaling activation with Akt phosphorylation on Ser473, but not on
Thr308, this finding is different from the rescent finding that
EGFR-mediated activation of mTORC-1 signaling is by Akt
phosphorylation on Ser473, not Akt phosphorylation on Thr308.
However, this study demonstrated that the inhibition of both mTORC1
and mTORC2 activity lead to activation of EGFR followed by mTORC-1
signaling activation through Akt phosphorylation on Thr308.
However, it does not indicate that EGF induced Akt activation
should follow the same pathway. There is one report showing exactly
the same pattern of Akt activation by EGF stimulation with or
without sirolimus (rapamycin) treatment (34).
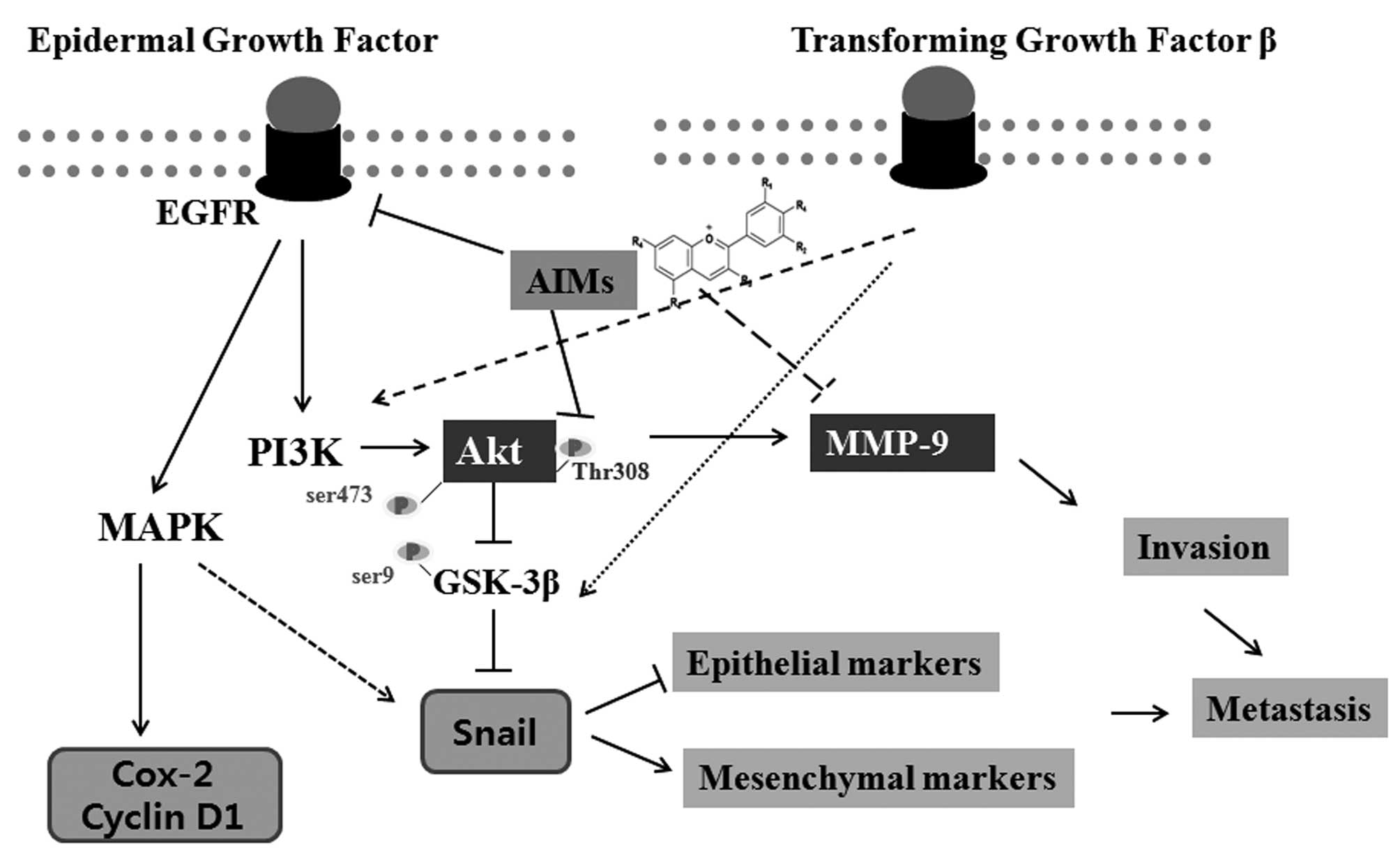
In conclusion, this study suggests that the
inhibitory effect of AIMs on Akt activity is independent of that on
EGFR, and that AIMs suppressed invasion and migration, at least in
part, by suppressing EMT by inhibiting Akt activity as well as EGFR
(Fig. 8). This study provides
evidence that AIMs have anti-metastatic effects on human lung
cancer by inhibition of both PI3k/Akt and EGFR pathways involved in
invasion and EMT.
Acknowledgements
This study was supported by a grant of
the National R&D Program for Cancer Control, Ministry for
Health, Welfare and Family Affairs, Republic of Korea
(0820050).
References
|
1.
|
Wells A: Tumor invasion: role of growth
factor-induced cell motility. Adv Cancer Res. 78:31–101. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Price JT, Wilson HM and Haites NE:
Epidermal growth factor (EGF) increases the in vitro invasion,
motility and adhesion interactions of the primary renal carcinoma
cell line, A704. Eur J Cancer. 32A:1977–1982. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK,
Schaefer E, Damsky CH, et al: FAK integrates growth-factor and
integrin signals to promote cell migration. Nat Cell Biol.
2:249–256. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gullick WJ: Prevalence of aberrant
expression of the epidermal growth factor receptor in human
cancers. Br Med Bull. 47:87–98. 1991.PubMed/NCBI
|
|
5.
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, et al: EMT and dissemination precede
pancreatic tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Meiers S, Kemeny M, Weyand U, Gastpar R,
von Angerer E and Marko D: The anthocyanidins cyanidin and
delphinidin are potent inhibitors of the epidermal growth-factor
receptor. J Agric Food Chem. 49:958–962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shin DY, Lee WS, Lu JN, Kang MH, Ryu CH,
Kim GY, et al: Induction of apoptosis in human colon cancer HCT-116
cells by anthocyanins through suppression of Akt and activation of
p38-MAPK. Int J Oncol. 35:1499–1504. 2009.PubMed/NCBI
|
|
9.
|
Yun JW, Lee WS, Kim MJ, Lu JN, Kang MH,
Kim HG, et al: Characterization of a profile of the anthocyanins
isolated from Vitis coignetiae Pulliat and their
anti-invasive activity on HT-29 human colon cancer cells. Food Chem
Toxicol. 48:903–909. 2010.PubMed/NCBI
|
|
10.
|
Vihinen P and Kahari VM: Matrix
metalloproteinases in cancer: prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
12.
|
Goldkorn T, Balaban N, Matsukuma K, Chea
V, Gould R, Last J, et al: EGF-receptor phosphorylation and
signaling are targeted by H2O2 redox stress.
Am J Respir Cell Mol Biol. 19:786–798. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Carpenter G and Cohen S: Epidermal growth
factor. J Biol Chem. 265:7709–7712. 1990.
|
|
14.
|
Margolis B, Bellot F, Honegger AM, Ullrich
A, Schlessinger J and Zilberstein A: Tyrosine kinase activity is
essential for the association of phospholipase C-gamma with the
epidermal growth factor receptor. Mol Cell Biol. 10:435–441.
1990.PubMed/NCBI
|
|
15.
|
Li Y, Welm B, Podsypanina K, Huang S,
Chamorro M, Zhang X, et al: Evidence that transgenes encoding
components of the Wnt signaling pathway preferentially induce
mammary cancers from progenitor cells. Proc Natl Acad Sci USA.
100:15853–15858. 2003. View Article : Google Scholar
|
|
16.
|
Chu EY, Hens J, Andl T, Kairo A, Yamaguchi
TP, Brisken C, et al: Canonical WNT signaling promotes mammary
placode development and is essential for initiation of mammary
gland morphogenesis. Development. 131:4819–4829. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Jope RS, Yuskaitis CJ and Beurel E:
Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and
therapeutics. Neurochem Res. 32:577–595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Teller N, Thiele W, Marczylo TH, Gescher
AJ, Boettler U, Sleeman J, et al: Suppression of the kinase
activity of receptor tyrosine kinases by anthocyanin-rich mixtures
extracted from bilberries and grapes. J Agric Food Chem.
57:3094–3101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Huang HP, Shih YW, Chang YC, Hung CN and
Wang CJ: Chemoinhibitory effect of mulberry anthocyanins on
melanoma metastasis involved in the Ras/PI3K pathway. J Agric Food
Chem. 56:9286–9293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Nurwidya F, Takahashi F, Murakami A and
Takahashi K: Epithelial mesenchymal transition in drug resistance
and metastasis of lung cancer. Cancer Res Treat. 44:151–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar
|
|
22.
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS, et al: Akt/PKB promotes cancer cell invasion via increased
motility and metalloproteinase production. FASEB J. 15:1953–1962.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Peinado H, Quintanilla M and Cano A:
Transforming growth factor beta-1 induces snail transcription
factor in epithelial cell lines: mechanisms for epithelial
mesenchymal transitions. J Biol Chem. 278:21113–21123. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lee J, Moon HJ, Lee JM and Joo CK: Smad3
regulates Rho signaling via NET1 in the transforming growth
factor-beta-induced epithelial-mesenchymal transition of human
retinal pigment epithelial cells. J Biol Chem. 285:26618–26627.
2010. View Article : Google Scholar
|
|
25.
|
Willis BC and Borok Z: TGF-beta-induced
EMT: mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Qiu Q, Yang M, Tsang BK and Gruslin A:
EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves
activation of both PI3K and MAPK signalling pathways. Reproduction.
128:355–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bouchard F, Belanger SD, Biron-Pain K and
St-Pierre Y: EGR-1 activation by EGF inhibits MMP-9 expression and
lymphoma growth. Blood. 116:759–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Stuelten CH, DaCosta Byfield S, Arany PR,
Karpova TS, Stetler-Stevenson WG and Roberts AB: Breast cancer
cells induce stromal fibroblasts to express MMP-9 via secretion of
TNF-alpha and TGF-beta. J Cell Sci. 118:2143–2153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wang H, Wang HS, Zhou BH, Li CL, Zhang F,
Wang XF, et al: Epithelial-mesenchymal transition (EMT) induced by
TNF-alpha requires Akt/GSK-3beta-mediated stabilization of snail in
colorectal cancer. PLoS One. 8:e566642013. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yan D, Avtanski D, Saxena NK and Sharma D:
Leptin-induced epithelial-mesenchymal transition in breast cancer
cells requires beta-catenin activation via Akt/GSK3- and MTA1/Wnt1
protein-dependent pathways. J Biol Chem. 287:8598–8612. 2012.
View Article : Google Scholar
|
|
31.
|
Saito Y, Vandenheede JR and Cohen P: The
mechanism by which epidermal growth factor inhibits glycogen
synthase kinase 3 in A431 cells. Biochem J. 303:27–31.
1994.PubMed/NCBI
|
|
32.
|
Chen G, Bower KA, Xu M, Ding M, Shi X, Ke
ZJ, et al: Cyanidin-3-glucoside reverses ethanol-induced inhibition
of neurite outgrowth: role of glycogen synthase kinase 3 beta.
Neurotox Res. 15:321–331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Hudson TS, Hartle DK, Hursting SD, Nunez
NP, Wang TT, Young HA, et al: Inhibition of prostate cancer growth
by muscadine grape skin extract and resveratrol through distinct
mechanisms. Cancer Res. 67:8396–8405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Galbaugh T, Cerrito MG, Jose CC and Cutler
ML: EGF-induced activation of Akt results in mTOR-dependent p70S6
kinase phosphorylation and inhibition of HC11 cell lactogenic
differentiation. BMC Cell Biol. 7:342006. View Article : Google Scholar : PubMed/NCBI
|