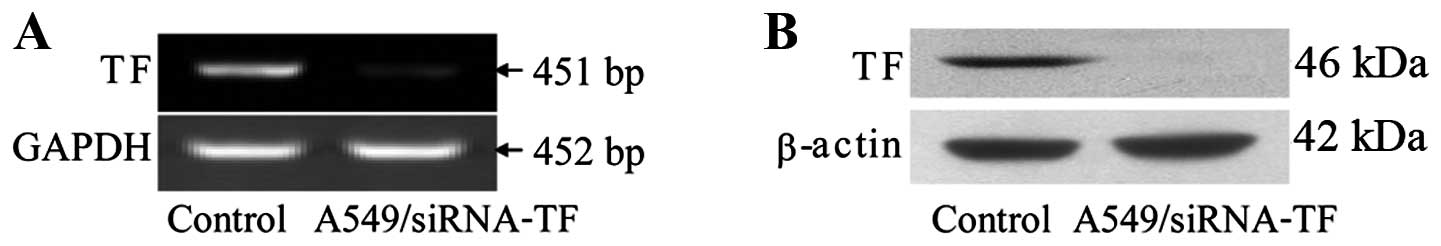Introduction
Lung cancer is one of the most common malignancies
and the development of clinical metastasis is the main cause of
morbidity and mortality (1). Tumor
invasion plays a crucial role in metastasis and involves a number
of important steps, including adhesion of tumor cells to the
basement membrane (BM); enzymatic digestion of the BM by
proteolytic enzymes followed by migration through the extracellular
matrix (ECM) with the subsequent growth and proliferation of cells
at a new site (2,3). Any agent, which can inhibit the
invasive process, may therefore become a powerful tool in the
prevention of metastasis.
LKB1 (also known as STK11) is a recently identified
tumor suppressor gene whose mutation can lead to Peutz-Jeghers
syndrome, which is characterized by gastrointestinal polyps and
cancers of different organ systems. The functional loss of LKB1 or
LKB1-inactivating mutations are known to be correlated with
one-third of sporadic lung adenocarcinomas (4,5),
indicating that LKB1 gene is related to the tumorigenesis of lung
cancer. Recent reports have suggested that LKB1 is critical for
cell migration leading to invasion and metastasis of cancer
(6). However, the molecular
mechanisms of these multiple biological events throughout the
process of tumor development and progression are largely
unknown.
To further investigate whether and how LKB1 affects
the migration and invasion of lung cancer cells, we generated an
LKB1 overexpression cell line, A549/LKB1 cells. LKB1 was examined
for its potential on lung cancer cell invasion and related
molecular mechanisms in A549 cells. Our previous studies showed
that LKB1 exerted tumor inhibitory effects on human lung carcinoma
cells in vitro (7–9). The mechanisms of these inhibitory
effects may include the upregulation or downregulation of the
different effector molecules. Here we provide evidence showing that
LKB1 suppresses tissue factor (TF) and vascular endothelial growth
factor (VEGF) expression, which is correlated well with the
inhibition of cell invasion. VEGF is the most powerful endothelial
cell specific mitogens associated with tumor neovascularization. It
has been reported that LKB1 is involved in the VEGF signaling
pathway and that the vascular defects accompanying LKB1 loss are
related with VEGF (10,11). The expression of VEGF regulated by
LKB1 is likely to play a contributory role in tumor cells invasion.
TF, an initiator of the extrinsic coagulation cascade, is also
expressed in a wide range of cancer cells. It is known that TF
promotes angiogenesis by cooperating with VEGF in several malignant
tumors (12,13). Koomagi and Volm demonstrated that
TF was related to VEGF expression and microvessel density and that
the survival times were longer in patients whose tumors were
immunologically TF-negative than in those with TF-positive tumors
(14). Given that tumor cells
sometimes mimic normal physiological systems for the purpose of
invasion and progression, TF may also contribute to its promotion
of cancer metastasis and progression. However, how LKB1 gene
regulates those different effector molecules becomes an
increasingly important question. The answer will not only shed
light on the mechanism of cancer invasion and metastasis but also
likely lead to clinical benefits.
Materials and methods
Cell culture and transfection
The LKB1-null human lung cell line A549 cells were
plated in culture plates in Dulbecco’s minimal essential medium
(DMEM, Gibco, Grand Island, NY, USA) containing 10% (v/v) fetal
bovine serum (FBS, Gibco). The cultures were maintained at 37°C in
a humidified atmosphere of 5% CO2. After culture to 70%
confluence on 6-well plates, cells were transfected with pcDNA/LKB1
or control (vehicle) pcDNA3.1 (the vectors containing Neomicin/G418
resistance gene were kindly provided by Dr Qingzhi Xu) in the
presence of Lipofectamine 2000 (Invitrogen Corp., Grand Island, NY,
USA) following the manufacturer’s protocol. Transfection medium was
replaced with growth medium containing 10% FBS after cells were
incubated with transfection reagents for 6 h. Then, day 16 after
selection with complete DMEM containing 600 μg/ml G418
(Gibco), all untransfected cells died and discrete clones were
visible in transfected cells. These clones were expanded in the
presence of 300 μg/ ml G418 to be used for the further
study. Cells transfected with pcDNA/LKB1 and pcDNA3.1 vectors were
termed A549/LKB1 and A549/vec, respectively. Both transfection and
G418 selection were conducted under sterile conditions and
duplicate plates were tested for each condition. The full-length
human Sp1 AN cDNA was made by cloning EcoRI restriction
fragments from the plasmid pBS-Sp1-AN (a kind gift from Dr J.
Marvel and Dr Y. Leverrier, France) and was inserted into
EcoRI sites of pcDNA3.1.
RT-PCR
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen) according to the procedure provided by the
manufacturer. Reverse transcription was performed from total RNA at
42°C for 60 min by M-MLV Reverse Transcriptase (RNase-free, Takara,
Shiga, Japan). PCR amplification was performed to synthesize these
gene products, and the products were analyzed by electrophoresis on
a 1.5% agarose gel. The ratio of the yield of mRNA to the yield of
the relevant internal standard [glyceraldehyde-3-phosphate
dehydrogenase (GAPDH)] was calculated and compared. The specific
primers and their annealing temperatures are listed in Table I.
 | Table I.Primers and annealing
temperatures. |
Table I.
Primers and annealing
temperatures.
| Gene | Specific primers | Annealing temperature
(°C) | Product length
(bp) |
|---|
| LKB1 |
5′>CGGCAAGGTGAAGGAA<3′ | 55 | 411 |
|
5′>ACGCCCAGGTCGGAGAT<3′ | | |
| SP1 |
5′>AACCCACAAGCCCAAACAATCACC<3′ | 60 | 428 |
|
5′>CCCCGAGCCCCTTCCTTCACT<3′ | | |
| TF |
5′>TGAAGGATGTGAAGCAGACG<3′ | 60 | 451 |
|
5′>TGAAGGATGTGAAGCAGACG<3′ | | |
| VEGF |
5′>TGGATCCATGAACTTTCTGCTGTC<3′ | 57 | 542 |
|
5′>TCACCGCCTTGGCTTGTCACAT<3′ | | 528 |
| GAPDH |
5′>ACCACAGTCCATGCCATCAC<3′ | 60 | 452 |
|
5′>TCCACCACCCTGTTGCTGTA<3′ | | |
Western blot analysis
Total cell lysates were prepared using the cell
lysis buffer (Pierce, Rockford, IL, USA) following the
manufacturer’s instructions. Total protein was estimated using BCA
analysis (Pierce). The samples were re-suspended in SDS-PAGE
loading buffer and heated at 100°C for 5 min. Equal amounts of
protein were loaded on gel and then transferred to nitrocellulose
membrane (Mini-Protean and Trans-Blot systems, Bio-Rad
Laboratories, Hercules, CA, USA). The nitrocellulose membrane was
blocked with 5% non-fat dry milk and separately incubated with
corresponding specific primary antibodies, including 1:800 rabbit
anti-LKB1 (Cell Signaling Technology, Danvers, MA, USA), 1:1,500
mouse anti-actin, 1:750 mouse anti-TF (AbD Serotec, Oxford, UK),
1:500 goat anti-VEGF (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), 1:1000 mouse anti-SP1 (Santa Cruz Biotechnology). After
washing and incubating with corresponding secondary antibodies
conjugated horseradish peroxidase (1:2,000 dilution of goat
anti-rabbit IgG antibody, 1:1,500 dilution of rabbit anti-goat IgG
antibody, 1:2,000 dilution of goat anti-mouse IgG antibody,
respectively). The proteins were detected by chemiluminescence and
exposure to light-sensitive film.
In vitro adhesion assays
Cell adhesiveness to the extracellular matrices was
measured by an adhesion assay. The 96-well culture plates were
precoated with 50 μl Matrigel (diluted 1:8 in complete DMEM
cell culture medium) and dried overnight at 4°C. The wells were
then rehydrated and blocked for 1 h at 37°C with 1% bovine serum
albumin (BSA) in serum-free DMEM cell culture medium. Cells were
seeded at a density of 1×105 cells/well on the
Matrigel-coated 96-well plates and allowed to adhere for 1 h in a
37°C humid chamber. After incubation, non-adherent cells were
carefully removed by washing gently with PBS. After stained for 4 h
at 37°C with 10 μl MTT (5 mg/ml), adherent cells were
diluted in 150 μl of DMSO and measured at 570 nm using a
microtiter plate reader (Wako, Shanghai, China). All experiments
were performed in triplicate. The adhesion index was calculated as
the percentage ratio between the number of adherent test cells and
the number of control cells.
In vitro migration assays
The Boyden chamber procedure was used to evaluate
cell migration activity in a 24-microwell chamber (Millipore,
Billerica, MA, USA). The upper and lower wells were separated by a
polyvinyl-pyrolidone-free poly-carbonate filter (8-μm pore
size). The lower wells contained DMEM with 10% FBS. Cell suspension
(2.5×104 cells in 100 μl of serum-free medium) of
A549 cells and A549/LKB1 cells (transfected with LKB1), was added
to the upper wells, respectively. The chamber was incubated at 37°C
for 24 h. Non-migrating cells on the upper surface of the filters
were removed with a cotton swab. The filters were then removed and
fixed in 95% ethanol for 10 min. Cells were stained with 0.5%
crystal violet in 20% ethanol and counted using a light microscope
in 10 random fields per each well. Migration was assayed by
measuring the number of cells moving across the filter. The number
of cells that migrated is given as the mean ± SD. Each experiment
was performed in triplicate.
In vitro invasion assays
The Matrigel invasion assay (BD Biosciences,
Heidelberg, Germany) was used to assess the invasive potential of
A549 lung cancer cells. Briefly, BioCoat Matrigel invasion chambers
were rehydrated according to the manufacturer’s instructions.
Following rehydration, the medium was carefully removed. DMEM cell
culture medium (500 μl) supplemented with 10% FBS was added
to the bottom of 24-well plates. Cells were seeded at a density of
1×105 cells/well into upper inserts and incubated at
37°C for 24 h in 5% CO2. After 24 h, the non-invading
cells were removed from the upper surface of the separating
membrane by gentle scrubbing with a cotton swab. Invading cells
were fixed in 4% paraformaldehyde for 5 min and stained with Giemsa
for 9 min. The membranes were mounted on glass slides and manually
counted in five random fields using a light microscope. All assays
were performed in triplicate.
Knockdown of TF by adenovirus mediated
shRNA
We previously constructed adenovirus mediated shRNA
against TF (Ad-shRNA-TF) and identified its silencing effect on TF
in our previous study (15). To
determine the effect of TF expression on A549 cells, we knocked
down the TF expression by using Ad-shRNA-TF. Briefly, the A549
cells were plated in 6-well plates and allowed to grow in 2% FBS
media. At 70–80% confluence, the cells were infected with
Ad-shRNA-TF or control at a MOI of 100 vp/cell. Three wells were
left uninfected to serve as a negative control. All infections were
performed in triplicate. After 12 h, the viral containing media was
removed and replaced with fresh media containing 10% FBS. The
silencing effect against TF of the recombinant adenovirus was
detected by using RT-PCR and western blot analysis as previously
described.
Chemiluminescent secreted alkaline
phosphatase assay
The chemiluminescent secreted alkaline phosphatase
(SEAP) analysis kit (Clontech, Palo Alto, CA, USA) was used to
detect the transcriptional activity of Sp1 in the transfected
cells. pTALSEAP vector (negative control), pSEAG2-SEAP vector
(positive control) and pSP-SEAP vector, in which SEAP expression is
under the control of the Sp1 E-box binding element, were used to
transfect the cells, respectively, with Lipofectamine 2000 (Gibco)
as described above, except that cells were cultured in 24-well
culture plates instead of 60-mm dishes. The culture medium was
collected at 24 h after transfection, was then subjected to the
chemiluminescent assay of SEAP activity using the great EscAPe SEAP
kit (Clontech) according to the manufacturer’s instructions. The
activity data were calculated as: (Sp1 driving vector activity -
the negative control activity)/the positive control activity. Each
vector was assayed in triplicate and three independent experiments
were performed.
Statistical analysis
Results are expressed as the mean ± SD unless
indicated otherwise. ANOVA and Student’s t-test were used to
determine the statistical significance of differences among
experimental groups. Significance was defined as p<0.05.
Results
Stable transfection of LKB1 in A549
cells
To study the effects of restoring LKB1 expression on
A549 lung cancer cells that lack functional LKB1 expression. As
described previously, cells were transfected with a mammalian
expression vector alone (pcDNA3.1), with pcDNA/Neo carrying human
LKB1 cDNA. We selected transfected cells using the Neomicin/G418
resistance gene contained in the pcDNA/Neo expression vector.
Sixteen days after selection with G418, there was a clear reduction
in the number of colonies expressing wild-type LKB1 compared with
those carrying the empty vector. To confirm whether exogenous LKB1
is expressed in A549 transfected cells, we performed RT-PCR and
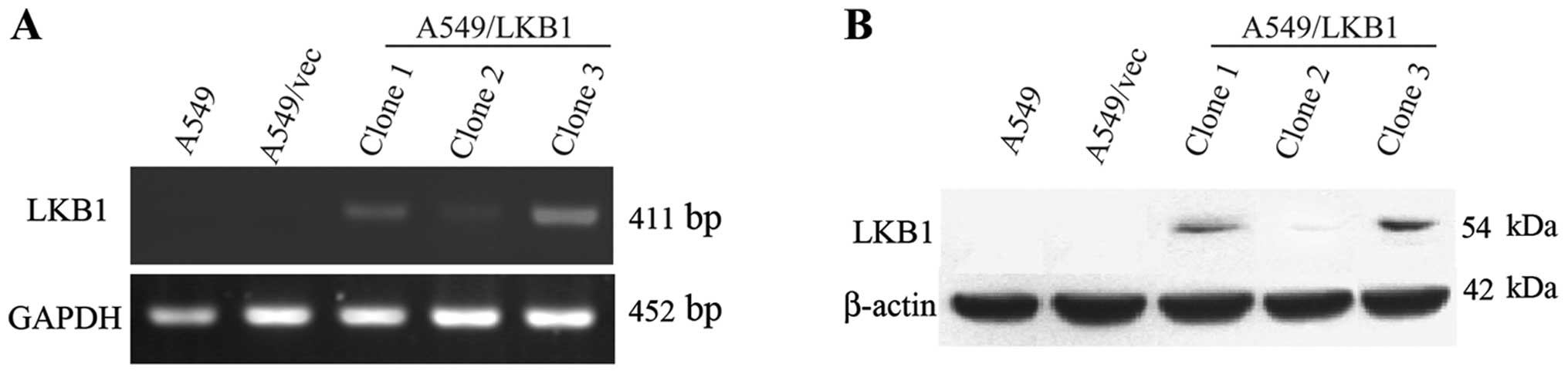
western blot analysis on these transfectants, including clones 1, 2
and 3. As shown in lane 5 in Fig.
1, the clone 3 had a higher yield of both LKB1 mRNA and
protein. Therefore, the third clone was used to make further
experiments in this study.
LKB1 inhibits cell adhesion, migration
and invasion in vitro
Compared to the controls, the LKB1 reduced adhesion
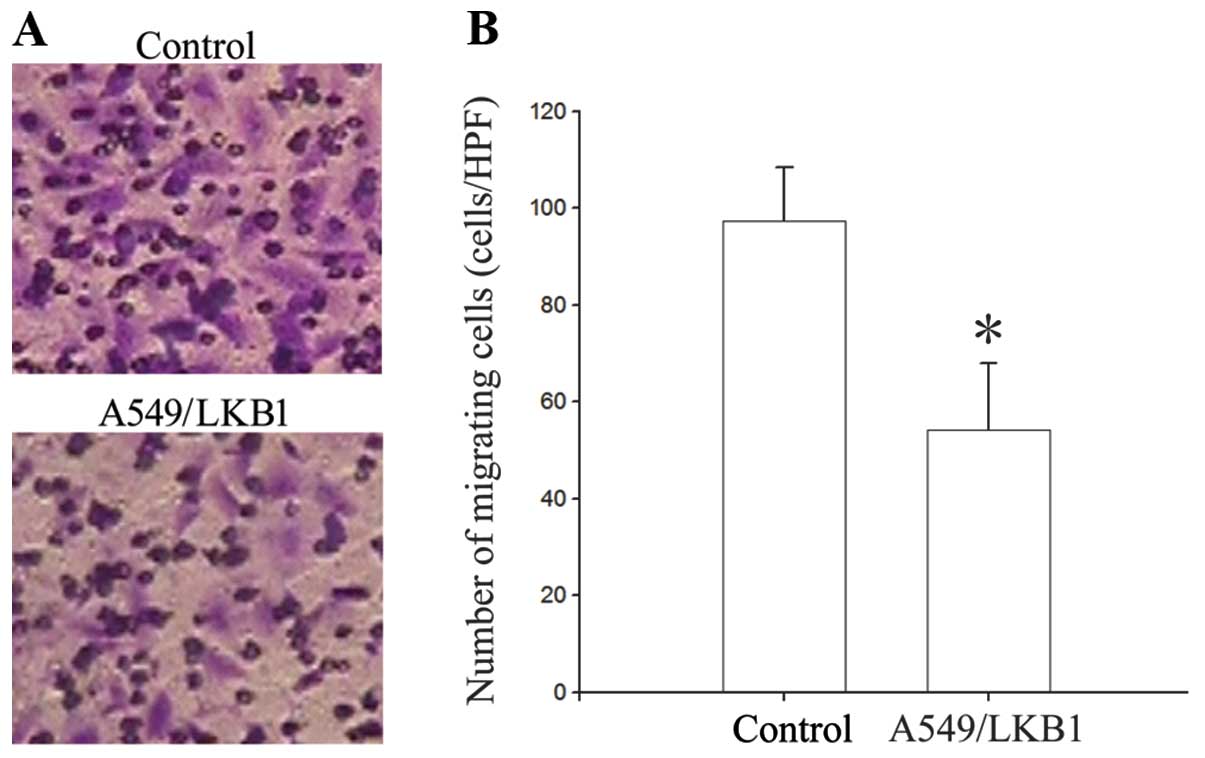
of A549 cells by 30.44% (p<0.05). The effects of LKB1 expression
on cell migration in vitro are shown in Fig. 3. LKB1-expressing cells exhibited
decreased migration. The number of A549/LKB1 cells that migrated
through the filter was 54.2±13.77/HPF, which was significantly
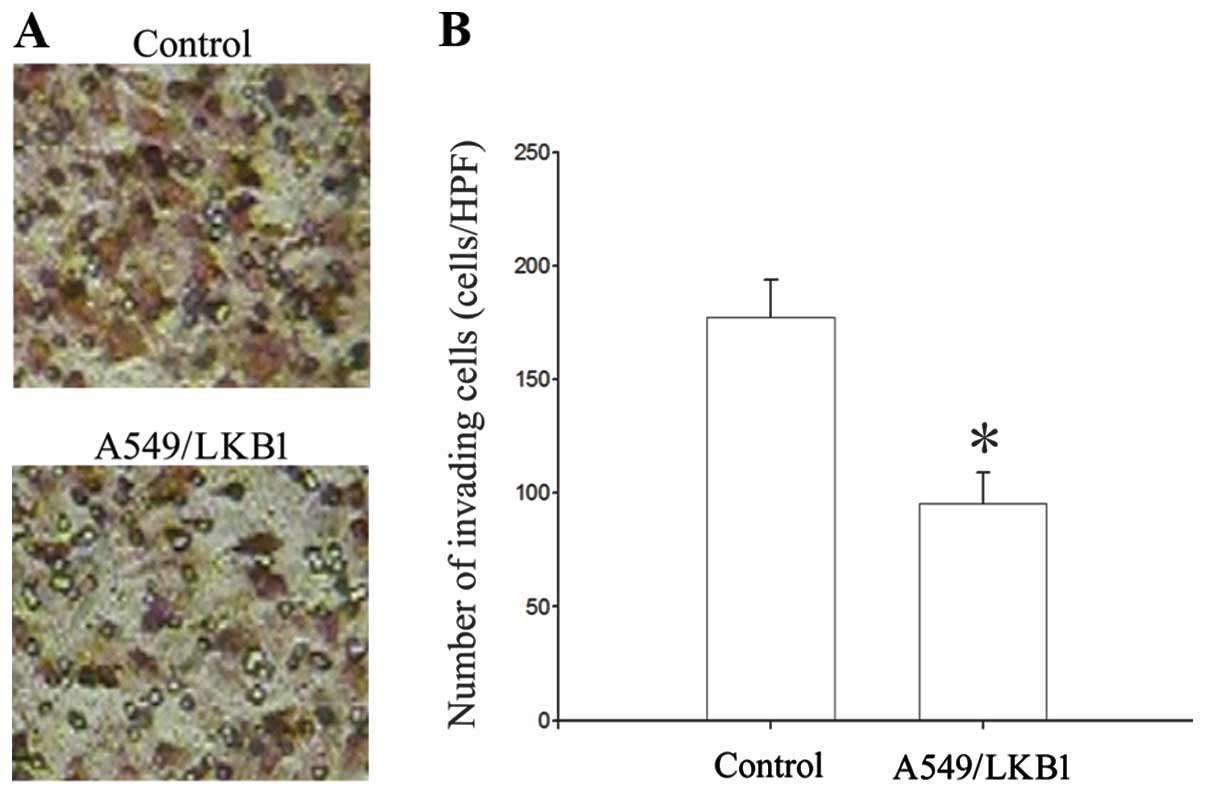
lower than that in A549 cells (97.4±10.98/HPF, p<0.05) (Fig. 2). The effect of LKB1 in regulating
the invasive ability of A549 lung cancer cell was examined by using
Matrigel invasion assays. The number of A549/LKB1 cells that
migrated through the filter was 95.4±13.68/HPF. The transfected
A549 cells with LKB1 reduced their invasive ability compared to
untreated cells (177.2±16.43/HPF, p<0.05) (Fig. 3). Analysis on the invasive capacity
of the analyzed lung cancer cells demonstrated that there was a
correlation between LKB1 and invasiveness.
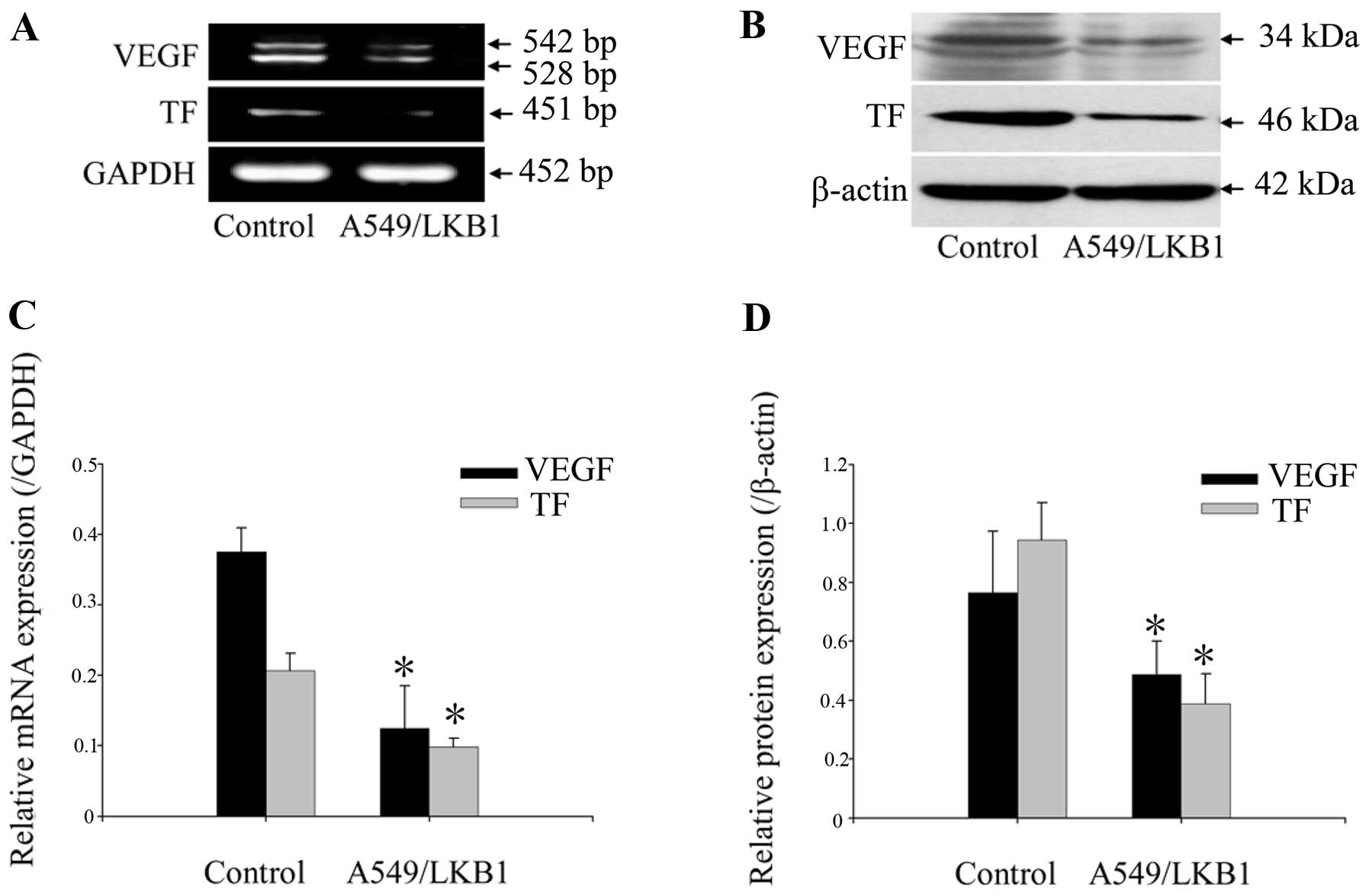
The expression of TF and VEGF can be
downregulated by LKB1
To further study the potential correlation between
LKB1 and the relevant effectors, we examined the expression of TF
and VEGF by using RT-PCR and western blot analysis. Compared with
A549 control cells, we showed that LKB1-transfected A549 cells
constitutively expressed significantly lower levels of TF and VEGF
in both mRNA and protein levels (p<0.05; Fig. 4).
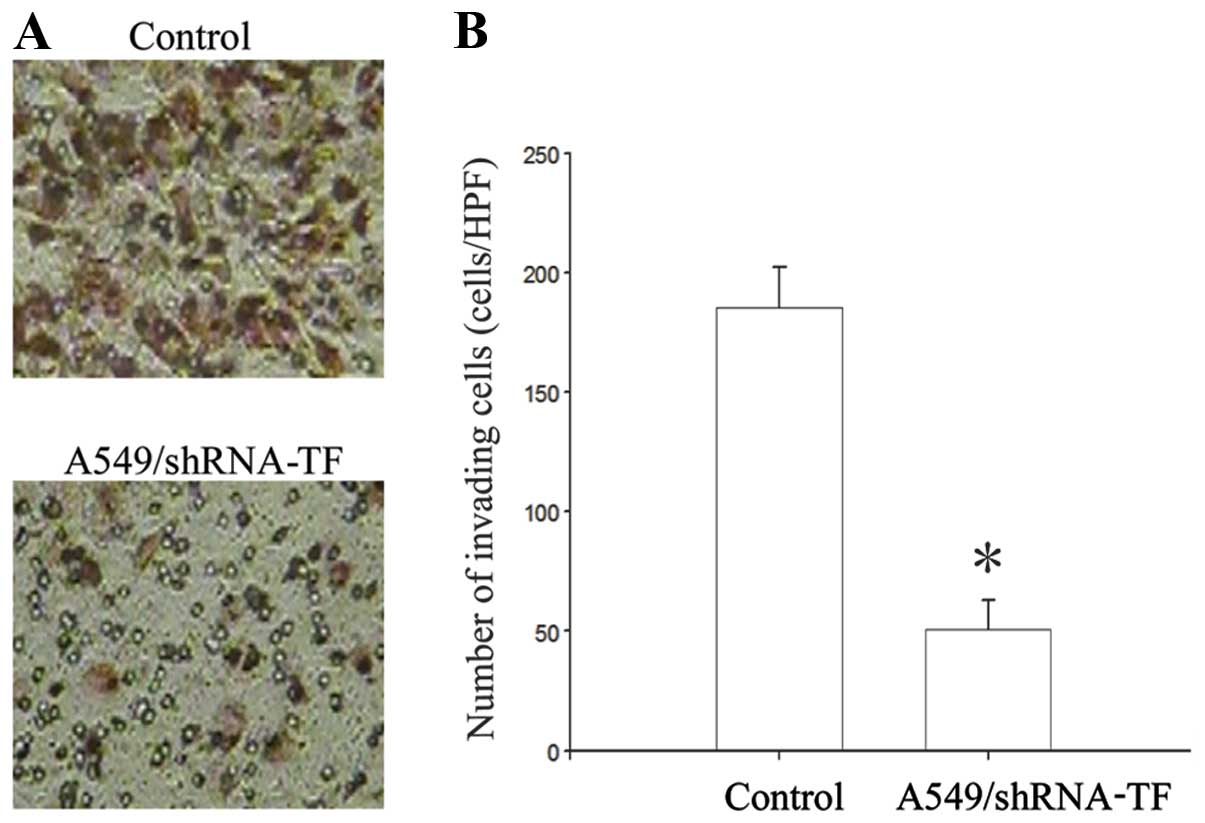
Adenovirus-mediated shRNA against TF
inhibits the expression of TF and results in impaired invasion in
A549 cells
Specific gene inhibition in mammalian cells can be
achieved by the use of small interfering RNA molecules (siRNA). The
silencing effect against TF of the recombinant adenovirus
pAd-shRNA-TF was detected by using RT-PCR and western blot
analysis. Our results showed that specific reduction in the target
protein level was observed after adenoviral infection, and that the
reduction in the protein level was correlated with a specific
reduction in the mRNA level (Fig.
5). We show that siRNA delivery significantly decreased levels
of TF in the lung cancer cell line A549. After transfected with
adenoviral vector expressing siRNA against TF, A549 cells showed
significantly decreased invasion activity through Matrigel
(Fig. 6).
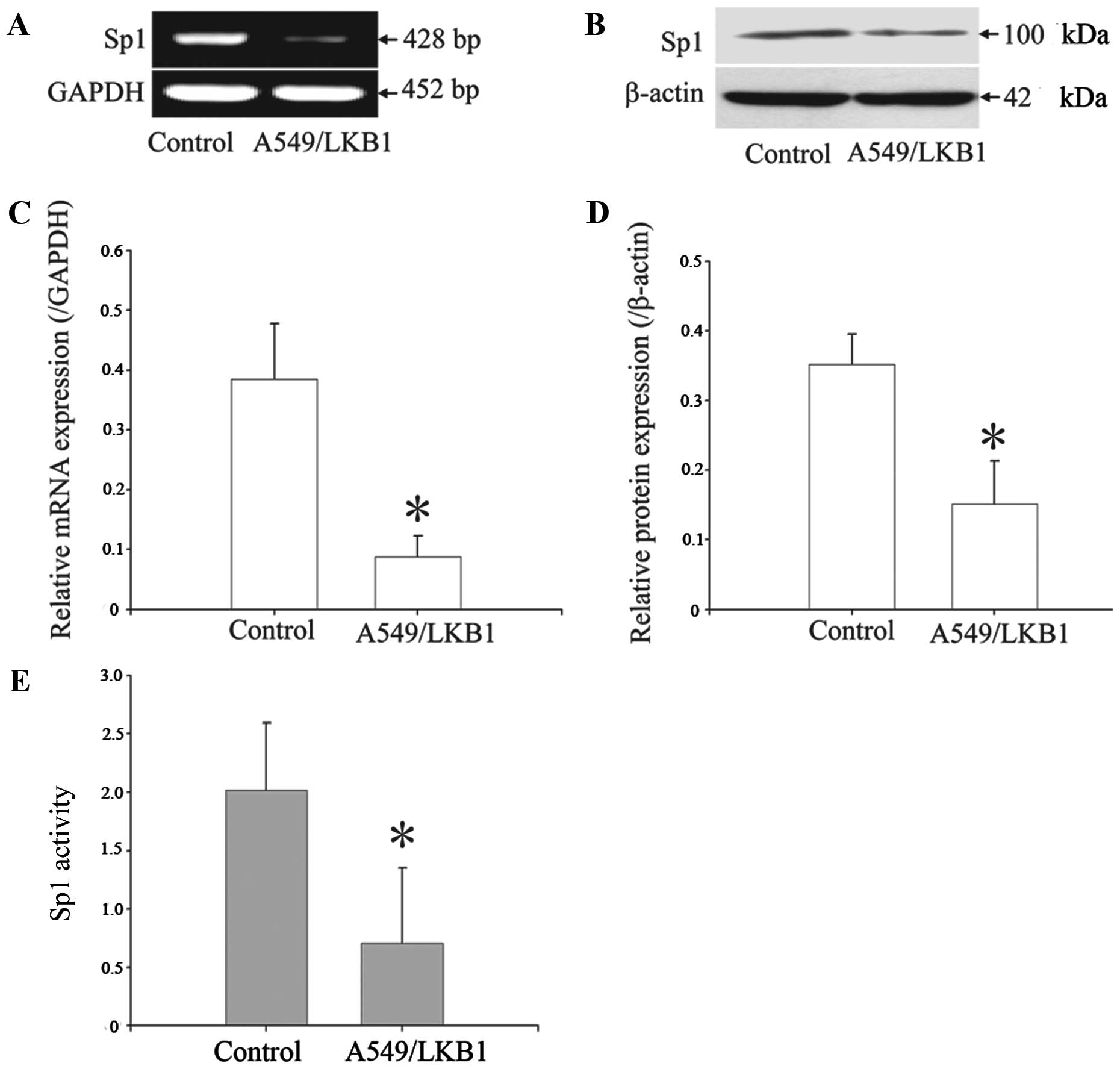
Overexpression of LKB1 inhibits the
expression and activity of Sp1
Sp1 is an essential transcription factor for many
genes that are key to the regulation of multiple aspects of tumor
cell growth and metastasis. To determine whether LKB1 represses Sp1
expression, we examined the effects of LKB1 overexpression on Sp1
expression by using RT-PCR and western blot analysis. We found that
overexpressing LKB1 mediated transcriptional repression of Sp1 gene
expression at both the mRNA and protein levels (Fig. 7A and B). To verify this decreased
expression of Sp1 protein after overexpressing LKB1, we then
investigated the transcriptional activity of Sp1 by means of Sp1
driving the SEAP reporter expression strategy. In this reporter
system, expression of SEAP (a secreted form of human placental
alkaline phosphatase) in the pSP-SEAP vector is under the control
of the E-box enhancer, an Sp1 binding element. Thus, the level of
SEAP can well reflect the Sp1 activity in the tested cells. The
culture medium was collected at 24 h after transfecting the cells
with the pSP-SEAP vector or the control vectors, and the activities
of SEAP in the medium were detected. As shown in Fig. 7E, the transcriptional activity of
Sp1 was significantly decreased in the cells overexpressed
LKB1.
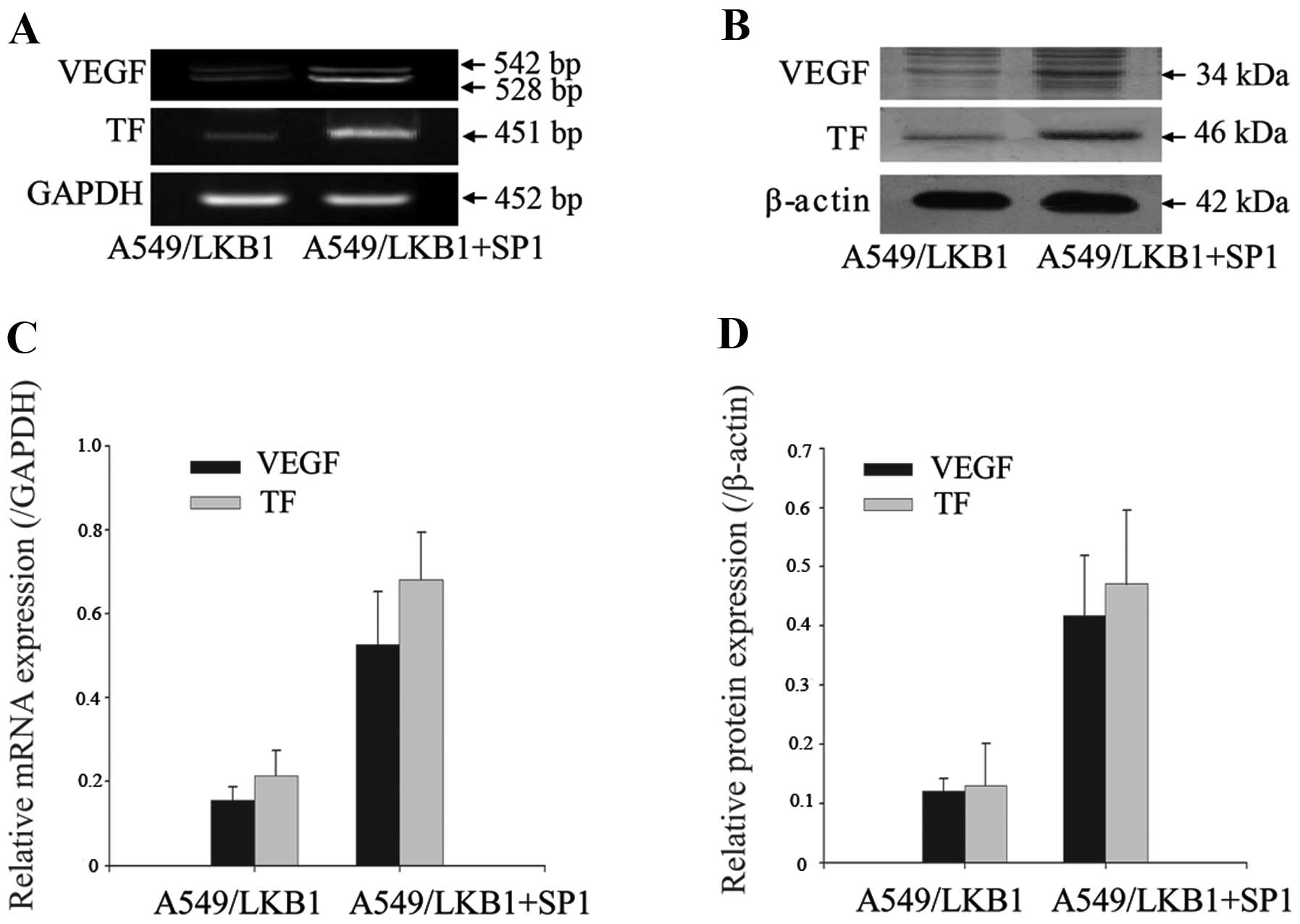
Sp1 functions as a positive regulator for
TF and VEGF expression
We transfected increasing amounts of Sp1 recombinant
plasmid into A549/LKB1 cells and measured the expression of mRNA
and protein levels of TF and VEGF gene. Increasing expression of
Sp1 enhanced the TF and VEGF mRNA and protein levels in A549/LKB1
cells (Fig. 8). These results
supported the positive role of Sp1 in the regulation of TF and
VEGF.
Discussion
Tumor development and progression require cell
growth, apoptosis resistance, cell invasion and angiogenesis. In
our previous study, we observed a suggested tumor-suppressive
function for LKB1 in human lung cancer cells proliferation
(9). In the present study, we
provide evidence that LKB1 inhibits the migration and invasion of
the highly metastatic lung cancer A549 cells. Some studies
demonstrated that LKB1 is a critical barrier to pulmonary
tumorigenesis, controlling initiation, differentiation and
metastasis (16). Consistent with
our observation, the anti-invasive property of LKB1 has been
demonstrated on human lung carcinoma cells.
In our study, we also showed that TF and VEGF
expressions in both mRNA and protein level were significantly
downregulated in LKB1-transfected A549 cells in vitro, which
may contribute to the inhibition of tumor growth and metastasis. TF
plays a major role in promoting tumor metastasis and overexpression
of TF has been shown to be associated with the progression and
invasion of tumors (17). Its
abnormal overexpression has been reported in non-small cell lung
cancer and a direct correlation between elevated TF expression and
advanced stages of malignancy has been confirmed (18). The present study revealed that
knockdown of endogenous TF could suppress the invasiveness of a
lung adenocarcinoma cell line in vitro, suggesting that TF
plays an important role in tumor invasion. Downregulation of TF
expression might lead to the suppression of not only tumor
invasiveness but also angiogenesis. Angiogenesis is essential for
the growth and metastasis of solid tumors (19). Among the many reported angiogenic
factors, VEGF is the most powerful endothelial cell specific
mitogens associated with tumor neovascularization. Without
vascularization most tumours will not expand and remain
non-invasive and do not metastasise (20,21).
Various studies indicate that TF plays an essential role in cancer
by its ability to upregulate the VEGF gene and thereby improve
tumor angiogenesis, which is crucial to tumor growth and metastasis
(22,23). The pro-angiogenic properties of TF
is supported by a significant association between TF expression and
a high MVD in various tumors including lung cancer (23,24).
The expression of TF contributes to tumor-derived procoagulant
activity and the well-known clinical association of malignancy and
thrombosis. Such activation may lead to an encapsulation of tumor
cells in a platelet-fibrin clot, and arrest in the
microcirculation, which is a critically important mechanism in
dissemination of tumor cells and may lead to metastasis (25). These findings support the
hypothesis that TF expression is positively involved in the
mechanisms of human lung cancer development. This study thus
provides a mechanistic link between LKB1 tumor suppressor and TF
gene expression.
Invasive potential of LKB1-null cells is, at least
in part, mediated by SP1 because most cells with downregulation of
SP1 levels showed decreased cellular migration and invasion.
Because Sp1 is an essential transcription factor for many genes
that are key to the regulation of multiple aspects of tumor cell
survival, growth, and angiogenesis, abnormal Sp1 expression and
activation may contribute to lung cancer development and
progression (26,27). Enforced LKB1 expression repressed
Sp1 expression at the promoter activity, mRNA, and protein levels.
Sp1 promoter contains putative Sp1 binding sites, Sp1
overexpression may result from autotransactivation of its own
promoter. A region within the proximal Sp1 promoter was identified
to have overlapping LKB1 and Sp1-binding sites, to which LKB1 and
Sp1 compete for binding (28). Sp1
positively regulated its own promoter, whereas LKB1 did the
opposite. Our data suggests that disruption of LKB1-mediated
negative regulation contributes to the molecular events of Sp1
overexpression and to the development and progression of human lung
cancer. Furthermore, constitutive activation of the transcription
factor Sp1 plays a critical role in VEGF and TF expression
(29,30). We also proved that Sp1 plays an
important role in the regulation of VEGF and TF expression which is
essential for the invasion and metastasis of lung cancer. We
transfected exogenous Sp1 into A549/LKB1 cells and found that
increasing expression of Sp1 enhanced the TF and VEGF mRNA and
protein levels in A549/LKB1 cells. Therefore, the LKB1-reduced
invasiveness of human lung cancer cells was found to correlate with
down-regulation of TF and VEGF expression and was thought to be
regulated by blocking the SP1 activation and/or its expression.
In conclusion, we identified that overexpression of
LKB1 inhibited lung cancer invasion in human lung cancer A549
cells, and the antitumor activity of LKB1 gene is correlated with
suppression of TF and VEGF, which partly depend on the activation
of the transcription factor Sp1. To our knowledge the present study
is the first to demonstrate the role of LKB1 in regulating
TF-mediated invasion on A549 cells. TF may be a viable therapeutic
target for human cancers with LKB1 mutations. These results suggest
that LKB1 represents a potential anti-metastatic gene therapy and
this new beneficial effect may expand future research on anticancer
properties of LKB1 in vitro and in vivo. Further
validation of these results will require studies in more cell
lines, in vivo studies, and additional observations on LKB1
downstream effects. Collectively, our data provide a novel
molecular mechanism for the antitumor activity of LKB1 and may help
further improve its effectiveness in controlling lung cancer growth
and metastasis.
Acknowledgements
This study was supported by National
Natural Science Foundation of China (no. 81101777). We thank Dr
Qingzhi Xu from the Academy of Military Medical Sciences of China
(Beijing, RPB, China) for providing us with pcDNA 3.1 expression
vector. We thank Dr J. Marvel and Dr Y. Leverrier from Inserm of
France (Lyon, France) for providing us with pBSK-SP1-AN
plasmid.
References
|
1.
|
Bennett A and White J: Improving care and
quality of life for patients with lung cancer. Nursing Stand.
28:50–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Gutierrez-Fernandez A, Fueyo A, Folgueras
AR, et al: Matrix metalloproteinase-8 functions as a metastasis
suppressor through modulation of tumor cell adhesion and invasion.
Cancer Res. 68:2755–2763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sugahara KN, Hirata T, Tanaka T, et al:
Chondroitin sulfate E fragments enhance CD44 cleavage and
CD44-dependent motility in tumor cells. Cancer Res. 68:7191–7199.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ghaffar H, Sahin F, Sanchez-Cepedes M, et
al: LKB1 protein expression in the evolution of glandular neoplasia
of the lung. Clin Cancer Res. 9:2998–3003. 2003.PubMed/NCBI
|
|
5.
|
Jimenez AI, Fernandez P, Dominguez O,
Dopazo A and Sanchez-Cespedes M: Growth and molecular profile of
lung cancer cells expressing ectopic LKB1: down-regulation of the
phosphatidylinositol 3′-phosphate kinase/PTEN pathway. Cancer Res.
63:1382–1388. 2003.PubMed/NCBI
|
|
6.
|
Marcus AI and Zhou W: LKB1 regulated
pathways in lung cancer invasion and metastasis. J Thorac oncol.
5:1883–1886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Liang X, Nan KJ and Xu QZ: Effect of small
interfering RNA-induced LKB1 gene silencing on the biological
behavior of lung carcinoma cells. Nan Fang Yi Ke Da Xue Xue Bao.
27:1303–1306. 2007.(In Chinese).
|
|
8.
|
Liang X, Nan KJ, Li CL, Yao Y, Tian T and
Wang SH: Effect of transfection of LKB1 on biological behavior of
human lung adenocarcinoma cells. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 24:1140–1142. 2008.(In Chinese).
|
|
9.
|
Liang X, Nan KJ, Li ZL and Xu QZ:
Overexpression of the LKB1 gene inhibits lung carcinoma cell
proliferation partly through degradation of c-myc protein. Oncol
Rep. 21:925–931. 2009.PubMed/NCBI
|
|
10.
|
Ylikorkala A, Rossi DJ, Korsisaari N, et
al: Vascular abnormalities and deregulation of VEGF in
Lkb1-deficient mice. Science. 293:1323–1326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Brugarolas J and Kaelin WG Jr:
Dysregulation of HIF and VEGF is a unifying feature of the familial
hamartoma syndromes. Cancer Cell. 6:7–10. 2004. View Article : Google Scholar
|
|
12.
|
Nakasaki T, Wada H, Shigemori C, et al:
Expression of tissue factor and vascular endothelial growth factor
is associated with angiogenesis in colorectal cancer. Am J Hematol.
69:247–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhang J, Ding J, Zhang X, Shao X and Hao
Z: Regulation of vascular endothelial growth factor (VEGF)
production and angiogenesis by tissue factor (TF) in SGC-7901
gastric cancer cells. Cancer Biol Ther. 4:769–772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Koomagi R and Volm M: Tissue-factor
expression in human non-small-cell lung carcinoma measured by
immunohistochemistry: correlation between tissue factor and
angiogenesis. Int J Cancer. 79:19–22. 1998. View Article : Google Scholar
|
|
15.
|
Li ZL, Xu WJ, Tian PX, et al: Recombinant
adenovirus-mediated RNA silencing of tissue factor expression in
human islet: an in vitro study. Nan Fang Yi Ke Da Xue Xue Bao.
27:1299–1302. 2007.(In Chinese).
|
|
16.
|
Ji H, Ramsey MR, Hayes DN, et al: LKB1
modulates lung cancer differentiation and metastasis. Nature.
448:807–810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Xu C, Gui Q, Chen W, et al: Small
interference RNA targeting tissue factor inhibits human lung
adenocarcinoma growth in vitro and in vivo. J Exp Clin Cancer Res.
30:632011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Minamiya Y, Matsuzaki I, Sageshima M, et
al: Expression of tissue factor mRNA and invasion of blood vessels
by tumor cells in non-small cell lung cancer. Surg Today. 34:1–5.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Mohammadi B, Haghpanah V and Larijani B: A
stochastic model of tumor angiogenesis. Comput Biol Med.
38:1007–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Vokes E, Herbst R and Sandler A:
Angiogenesis inhibition in the treatment of lung cancer. Clin Adv
Hematol Oncol. 4:1–10. 2006.PubMed/NCBI
|
|
21.
|
Wang S, Liu H, Ren L, Pan Y and Zhang Y:
Inhibiting colorectal carcinoma growth and metastasis by blocking
the expression of VEGF using RNA interference. Neoplasia.
10:399–407. 2008.PubMed/NCBI
|
|
22.
|
Rickles FR, Shoji M and Abe K: The role of
the hemostatic system in tumor growth, metastasis, and
angiogenesis: tissue factor is a bifunctional molecule capable of
inducing both fibrin deposition and angiogenesis in cancer. Int J
Hematol. 73:145–150. 2001. View Article : Google Scholar
|
|
23.
|
Regina S, Rollin J, Blechet C, Iochmann S,
Reverdiau P and Gruel Y: Tissue factor expression in non-small cell
lung cancer: relationship with vascular endothelial growth factor
expression, microvascular density, and K-ras mutation. J Thorac
Oncol. 3:689–697. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Poon RT, Lau CP, Ho JW, Yu WC, Fan ST and
Wong J: Tissue factor expression correlates with tumor angiogenesis
and invasiveness in human hepatocellular carcinoma. Clin Cancer
Res. 9:5339–5345. 2003.PubMed/NCBI
|
|
25.
|
Regina S, Valentin JB, Lachot S, Lemarie
E, Rollin J and Gruel Y: Increased tissue factor expression is
associated with reduced survival in non-small cell lung cancer and
with mutations of TP53 and PTEN. Clin Chem. 55:1834–1842. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Han S and Roman J: COX-2 inhibitors
suppress integrin alpha5 expression in human lung carcinoma cells
through activation of Erk: involvement of Sp1 and AP-1 sites. Int J
Cancer. 116:536–546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zhang J, Jia Z, Li Q, et al: Elevated
expression of vascular endothelial growth factor correlates with
increased angiogenesis and decreased progression-free survival
among patients with low-grade neuroendocrine tumors. Cancer.
109:1478–1486. 2007. View Article : Google Scholar
|
|
28.
|
Lutzner N, De-Castro AJ and Rosl F: Gene
expression of the tumour suppressor LKB1 is mediated by Sp1, NF-Y
and FOXO transcription factors. PLoS One. 7:e325902012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shi Q, Le X, Abbruzzese JL, et al:
Constitutive Sp1 activity is essential for differential
constitutive expression of vascular endothelial growth factor in
human pancreatic adenocarcinoma. Cancer Res. 61:4143–4154.
2001.PubMed/NCBI
|
|
30.
|
Ishibashi H, Nakagawa K, Onimaru M, et al:
Sp1 decoy transfected to carcinoma cells suppresses the expression
of vascular endothelial growth factor, transforming growth factor
beta1, and tissue factor and also cell growth and invasion
activities. Cancer Res. 60:6531–6536. 2000.
|