Introduction
Ovarian cancer (OVC) is the most lethal
gynecological malignancy and ovarian papillary serous carcinoma
(OPSC) is the largest histology subgroup of ovarian cancer
(1–4). Growing clinical evidence suggests
that histologic grade has been determined to be an important
prognostic factor for ovarian serous carcinoma (5,6).
Traditionally, ovarian cancer has been graded as well, moderately
and poorly differentiated. But based on recent molecular genetic
studies with clinical and histopathologic findings, it was proposed
that OPSC tumors can be grouped into a new dualistic model based on
their distinct pathogenesis (6–8). In
this model, all ovarian surface epithelial tumors are divided into
two groups designated type I and II. Type I tumors are mainly
low-grade and have low levels of chromosomal instability. In
contrast, type II tumors are generally high grade with rapidly
growing, high aggressiveness and almost always have progressed to
advanced stage at diagnosis, where current available therapies are
seldom effective (7,9). High-grade OPSC constitutes
approximately 75% of ovarian cancer but is responsible for 90% of
ovarian cancer deaths (7). This
has important clinical implications as evidenced by the lack of
sensitivity of type II tumors to the standard cytotoxic
chemotherapy that is effective for high-grade serous carcinomas
(1,6,10,11).
Research is urgently needed on type II tumors to better recognize
the cytogenetic aberrations of this disease and design more
rational approaches to early detection and targeted therapies of
this type of patients.
MicroRNAs (miRNAs) are short non-coding RNA
molecules of 22–24 nucleotides that play important roles by
affecting various pathways related to cancer progress such as cell
cycle control, apoptosis, proliferation, differentiation,
metabolism and migration (12,13).
As a benefit from the advances in use of miRNA microarray
technologies, we can apply this approach to identify the unique
miRNAs in process of diseases. Our aim was to identify the
differences of key miRNAs and possible regulators through miRNA
microarray chip, functional target prediction, and clinical outcome
between the low and high-grade OPSC patients to find the pathogenic
basis in differentiation of ovarian cancer subtypes, and to provide
insight into clinical diagnosis and therapy for high-grade
cases.
Materials and methods
Patient and samples
Patients, who were surgically treated for ovarian
cancer at the Obstetrics and Gynecology Hospital, Dalian, China
between August, 2006 and November, 2010 were identified.
Corresponding 69 OPSC samples (type I=16; type II=53) that were
resected at the time of primary surgery from the patients were
evaluated. All pathological specimens were reviewed by two
independent pathologists with no knowledge of patients’ clinical
data. The diagnosis of the cases was based on criteria of the
International Federation of Gynecology and Obstetrics (FIGO)
staging, and the grade was assigned according to the University of
Texas M.D. Anderson Cancer Center (MDACC) grading system (5). The MDACC system is based primarily on
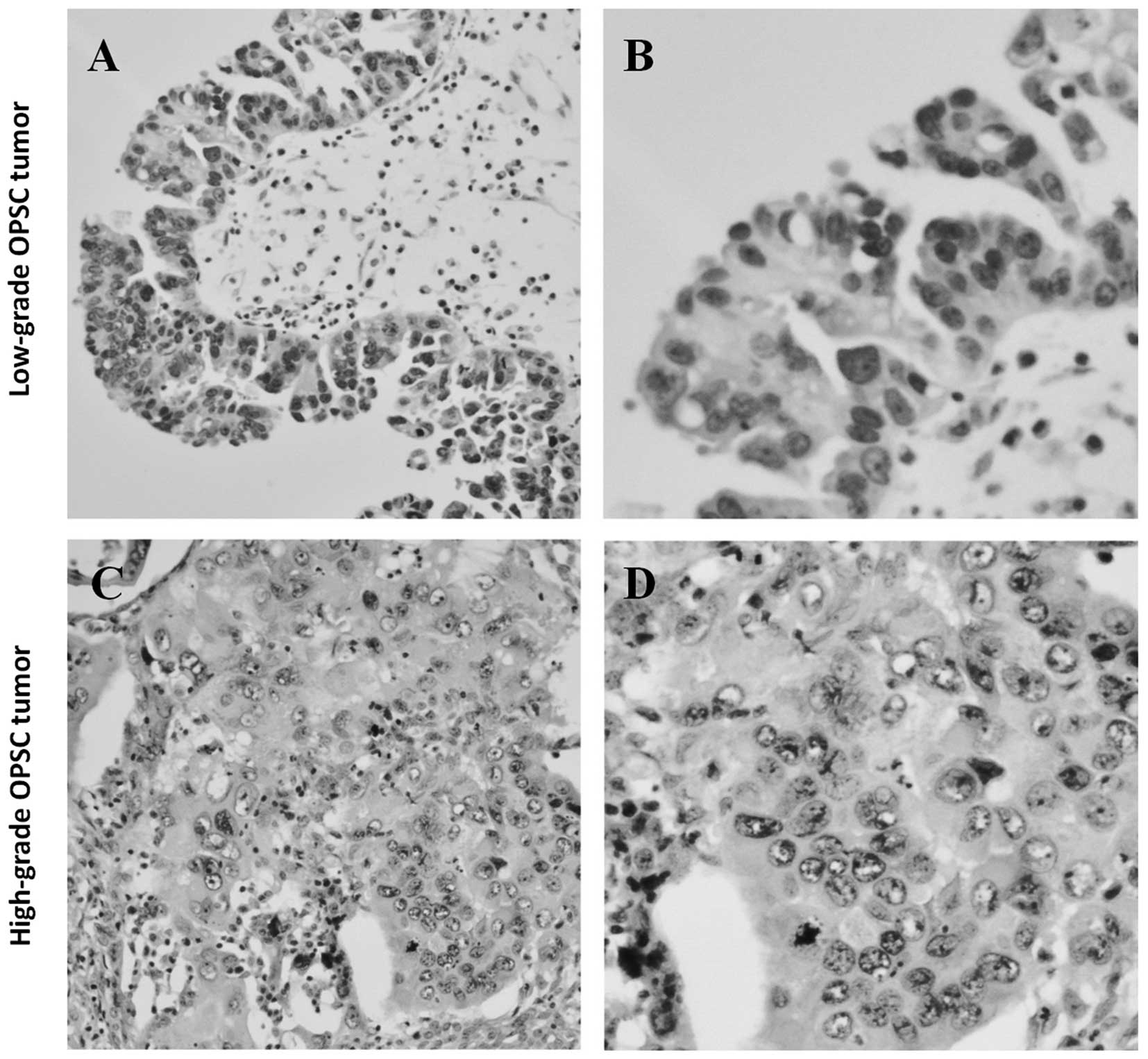
the assessment of nuclear atypia (nuclear uniformity vs.
pleomorphism) in the worst area of the tumor (5). Low-grade serous carcinoma is
characterized as the absence of marked variation of size and shape,
and their nuclei are uniformly oval or round with even or slightly
irregular chromatin (Fig. 1A and
B). On the contrary, the nuclei of high-grade serous carcinoma
have marked variation in size (≥3:1) and shape and a very irregular
chromatin pattern (Fig. 1C and D)
(5). Only serous papillary
histology was included in this investigation.
miRNA microarray and data analysis
A microarray platform optimized for the analysis of
a panel of 768 human miRNAs was used to analyze and compare the
pattern of miRNA expression between type I (n=8) and type II (n=5)
OPSC. Total RNA that was enriched for miRNAs was extracted from the
FFPE tissue by using the Ambion mirVana microRNA isolation kit
(Ambion, Austin, TX, USA). The quality of total RNA was assessed
using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara,
CA, USA). Individual real-time quantitative polymerase chain
reaction assays were formatted into a TaqMan low-density array
(TLDA; Applied Biosystems, Foster City, CA, USA), which was
performed at the Shannon McCormack Advanced Molecular Diagnostics
Laboratory Research Services, Dana Farber Cancer Institute, Harvard
Clinic and Translational Science Center. The normalized microarray
data were managed and analyzed by Statminer version 3.0
(Integromics™, Waunakee, WI, USA).
RNA isolation and quantitative PCR
Total RNA was prepared using TRIzol reagent,
following manufacturer’s instructions. Quantitative Real-Time PCR
(qRT-PCR) was performed using the TaqMan MicroRNA Reverse
Transcription Kit (Applied Biosystems) with ABI miRNA specific
primers and primer kits on an Agilent Technologies Stratagent
M×3000P (Agilent Technologies). Specific kits used were as follows:
Hsa-miR-30a*: ABI#4373062; Hsa-miR-30e*:
ABI#4427975. The products were detected with SYBR-Green I, and U6
small nuclear RNA as the endogenous control.
miRNA target prediction and pathway
analysis
Currently, more than 900 human miRNAs are registered
in the Sanger miRBase, and hundreds of potential targets are known
for each miRNA. Several computational approaches were used to
analyze target prediction of miRNAs, including Ingenuity Systems
(Redwood City, CA, USA), MicroCosm Targets version 5 (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/),
and miRBase (http://www.mirbase.org/). Functional
analysis of these predicted targets was performed to identify
biologic pathways, according to significant gene expression. In
order to retrieve only the most relevant targets, and based on our
previous study (4), we only listed
the top 10 genes targeted by the miRNAs that showed the greatest
differential expression in the patients between type I and II
OPSC.
Semiquantitative reverse transcriptase
PCR
The procedures for reverse transcription-polymerase
chain reaction (RT-PCR) have been well established (14–16).
For semiquantitative 1-step RT-PCR analysis for ATF3 and MYC, 1
μg of total RNA was used as the template in RT-PCR with the
forward and reverse primers of vinculin in the kit (Takara, Dalian,
China) with the following program: RT at 30°C for 10 min, 42°C for
1 h, 99°C for 5 min, and 5°C for 5 min; PCR at 94°C for 2 min,
followed by 33 cycles of amplification (94°C for 1 min, 48°C for 45
sec, and 72°C for 1 min), and by extension at 72°C for 7 min. The
primers for the target and housekeeping genes (GAPDH) are
summarized in Table I. After
amplification, the samples were separated on 2% agarose gel,
visualized and quantified by Labworks-Analyst (GeneCo)
software.
 | Table I.PCR primers used in this study. |
Table I.
PCR primers used in this study.
| Sequence
(5’→3’) | Calculated size of
PCR primer product (bp) |
|---|
| ATF3 | Sense
GCTAAGCAGTCGTGGTATGGG | 227 |
| Antisense
TCCTGGAGTTGAGGCAAAGAT | |
| MYC | Sense
CCACCCATGGCAAATTCCATGGCA | 602 |
| Antisense
TCTAGACGGCAGGTCAGGTCCACC | |
| GAPDH | Sense
TGCTGCCAAGAGGGTCAAG | 1,318 |
| Antisense
GCCTCCAAGACGTTGTGAGT | |
Immunohistochemistry (IHC)
The IHC staining procedure was described previously
(4). Briefly, the sections were
incubated in 3% H2O2 for 30 min to suppress
endogenous peroxidase activity after dewaxing, and then further
blocked with a mixed solution [10% goat serum and 3% Albumin Bovine
(BSA) in PBS] for 1 h and incubated with the primary antibodies of
ATF3 (1:100 dilution; Bioworld, cat. no. BS1245); or MYC (1:100
dilution; Bioworld, cat. no. BS2261) overnight at 4°C. On the
second day, these sections were incubated with
streptavidin-peroxidase according to the manufacturer’s
instructions of the staining kit (Zhongshan Goldenbridge
Biotechnology Company, Beijing, China), then, 3,3’-diaminobenzidine
(DAB) staining was performed. For negative controls, the conditions
and the procedures were the same except that primary antibody was
eliminated.
Western blot analysis
Western blot analysis was performed as previously
described (14). Harvested cells
were lysed in lysis buffer (Beyotime Institute of Biotechnology,
Shanghai, China), and the proteins (20 μg) were separated on
10% SDS-PAGE gels and transferred to nitrocellulose membranes.
Membranes were blocked in PBS containing 0.05% Tween-20 (TBST) 5%
non-fat milk and incubated with the following antibodies: rabbit
anti-ATF3, rabbit anti-MYC and anti-GAPDH (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). After secondary antibody
incubation, the signal analysis was performed by exposure of the
blots to film, which was then scanned and band intensities were
measured with Labworks-Analyst (GeneCo) software.
Statistical analysis
The results were analyzed by SPSS 17.0 (Chicago, IL,
USA). Data are expressed as arithmetic means ± SD of the number (n)
of experiments. Samples were analyzed with repeated measures
analysis of variance, and differences in the incidences were
analyzed using ANOVA. The overall survival duration was defined as
the interval (in months) between the date of initial cytoreductive
surgery to date of last follow-up or death. The survival time
courses were studied using the Kaplan-Meier method, and groups were
compared using the log-rank test; p<0.05 was considered
statistically significant.
Results
Patient characteristics
The clinicopathological characteristics of the
patients who contributed to the OPSC samples are listed in Table II. Sixty-nine patients were
identified to fit the study criteria, including 16 type I and 53
type II OPSC patients. The percentage of patients with advanced
stage (stages III and IV) disease and the presence of positive
lymph nodes were all significantly higher in patients with type II
OPSC compared with patients with type I OPSC (p<0.001 for
all).
 | Table II.Clinicopathological characteristics
of the patients. |
Table II.
Clinicopathological characteristics
of the patients.
|
Characteristics | OPSC patients
(n=69)
| p-valueb |
|---|
| Type I grade
a (n=16) | Type II
gradea (n=53) |
|---|
| No. (%) | No. (%) |
|---|
| Age at
diagnosis | | | |
| Median | 48.2 | 59.9 | 0.041 |
| Range | (21–74) | (25–69) | |
| FIGO stage at
diagnosis | | | <0.001c |
| Stage II | 7 (43.8) | 3 (5.7) | |
| Stage III | 8 (50.0) | 37 (69.8) | |
| Stage IIIB | 5 (31.3) | 16 (30.2) | |
| Stage IIIC | 3 (18.8) | 21 (39.6) | |
| Stage IV | 1 (6.3) | 13 (24.5) | |
|
Lymphadenectomy | | | |
| Yes | 11 (68.8) | 43 (81.1) | |
| No | 4 (25.0) | 8 (15.1) | |
| Unknown | 1 (6.3) | 2 (3.8) | |
| Median no. nodes
resected | 11 | 18 | |
| Presence of
positive nodes | | | <0.001 |
| Yes | 16 (20.3) | 212 (65.2) | |
| No | 52 (65.8) | 103 (31.7) | |
| Unknown | 11 (13.9) | 10 (3.1) | |
Analysis of microarray data
To further characterize the unique miRNAs in OPSC
differentiation, specimens from the type I and type II grade OPSC
patients were initially analyzed by miRNA microarray respectively.
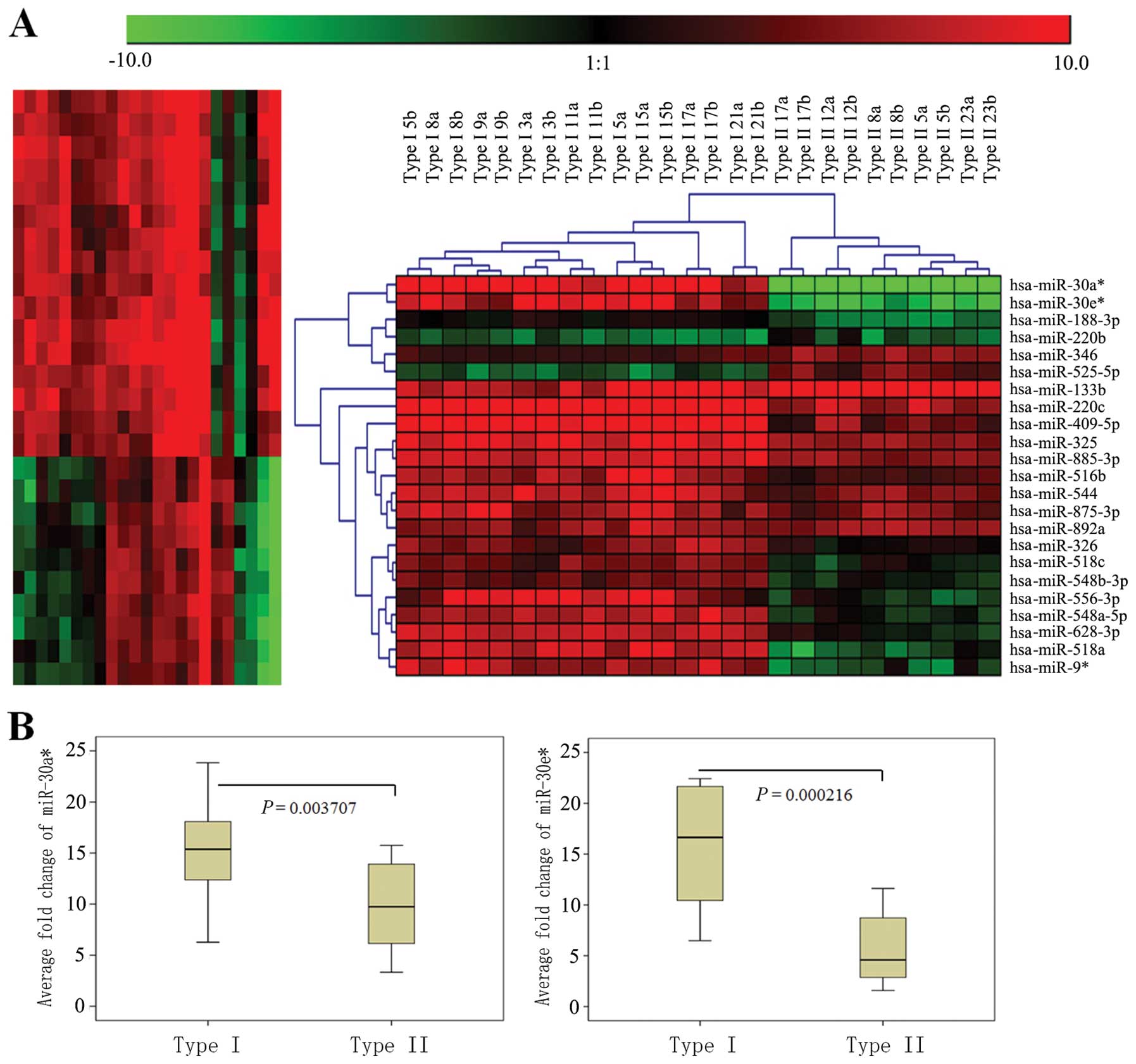
Of the 768 miRNAs analyzed by microarray, we found that 23 were
significantly different (p<0.01 for all), with at least a 2-fold
(p<0.05) change between type I and type II OPSC patients (n=8
and n=5 for the above group, respectively). The upregulation of 18
(78.3%) of these 23 key miRNAs was observed in the type I samples
and 5 (21.7%) in the type II specimens (Fig. 2A and Table III). Importantly, 8 of the 18
miRNAs with distinctly statistical significance (p<0.001) were
lowly expressed in the type II patients. In particular,
miR-30a* and miR-30e* were the top 2 miRNAs
that showed significant differential expression in OPSC patients
between the type I and type II groups and were remarkably
downregulated in the type II OPSC patients
(p=7.83×10−06, 3.52 ×10−05 and adjusted
p-value = 5.63×10−04, 1.08×10−04,
respectively; Fig. 2A and Table III).
 | Table III.Analysis of microRNA microarray data
and qRT-PCR expression of selected miRNAs. |
Table III.
Analysis of microRNA microarray data
and qRT-PCR expression of selected miRNAs.
| miRNA ID | Microarray (type I
vs. type II)
| qRT-PCRa
|
|---|
| −ΔΔCt | p-value | Adj.p-value
(q) | Type I grade | Type II grade |
|---|
| Upregulated
miRNAs | | | | | |
| hsa-miR-188-3p | 4.743210854 | 9.61E-03 | 4.66E-02 | | |
| hsa-miR-220c | 5.720880667 | 7.25E-03 | 3.95E-02 | | |
|
hsa-miR-30a* | 15.862476188 |
3.82E-05 |
4.36E-03 | 19.29
(10.01–23.88) | 7.74
(2.05–15.75) |
|
hsa-miR-30e* | 14.073272271 |
1.44E-05 |
1.06E-03 | 15.86
(6.48–22.42) | 5.62
(1.58–11.61) |
| hsa-miR-325 | 3.030099479 | 2.58E-04 | 9.88E-03 | | |
| hsa-miR-326 | 1.746044354 | 9.25E-03 | 4.59E-02 | | |
| hsa-miR-409-5p | 7.827316854 | 8.47E-03 | 4.33E-02 | | |
| hsa-miR-516b | 2.884268854 | 2.95E-04 | 9.95E-03 | | |
| hsa-miR-518a | 11.871587938 | 9.38E-03 | 4.59E-02 | | |
| hsa-miR-518c | 3.82116419 | 9.32E-03 | 4.59E-02 | | |
| hsa-miR-544 | 2.917980104 | 5.31E-03 | 3.16E-02 | | |
|
hsa-miR-548a-5p | 5.114513188 | 4.83E-03 | 3.05E-02 | | |
|
hsa-miR-548b-3p | 4.069714854 | 8.38E-03 | 4.32E-02 | | |
| hsa-miR-556-3p | 6.056558854 | 5.32E-04 | 4.36E-03 | | |
| hsa-miR-628-3p | 4.913104521 | 5.66E-04 | 8.78E-03 | | |
| hsa-miR-875-3p | 2.274845896 | 8.06E-04 | 5.05E-03 | | |
| hsa-miR-885-3p | 3.257400396 | 5.23E-04 | 1.36E-02 | | |
|
hsa-miR-9* | 7.510312854 | 2.86E-03 | 2.01E-02 | | |
| Downregulated
miRNAs | | | | | |
| hsa-miR-133b | −1.975504688 | 5.97E-03 | 3.39E-02 | | |
| hsa-miR-220b | −1.802515354 | 1.24E-03 | 1.41E-02 | | |
| hsa-miR-346 | −2.917470813 |
8.53E-05 |
6.73E-03 | | |
| hsa-miR-525-5p | −4.157091438 | 5.18E-04 | 1.36E-02 | | |
| hsa-miR-892a | −2.122343771 | 1.57E-03 | 1.41E-02 | | |
qPCR validation for microarray
results
miR-30a* and miR-30e* were
significantly downregulated in the type II OPSC when compared with
the type I OPSC by using microarray analysis. In order to confirm
microarray results, qRT-PCR validation was performed. RNA was
isolated from a new set of FFPE tissues to increase the likelihood
that the observed differences in miRNA expression profiles
represent biologically significant changes. In keeping with
microarray results, miR-30a* and miR-30e*
were all lowly expressed in type II grade OPSC with significance,
and representative analyses are shown in Fig. 2B and Table III.
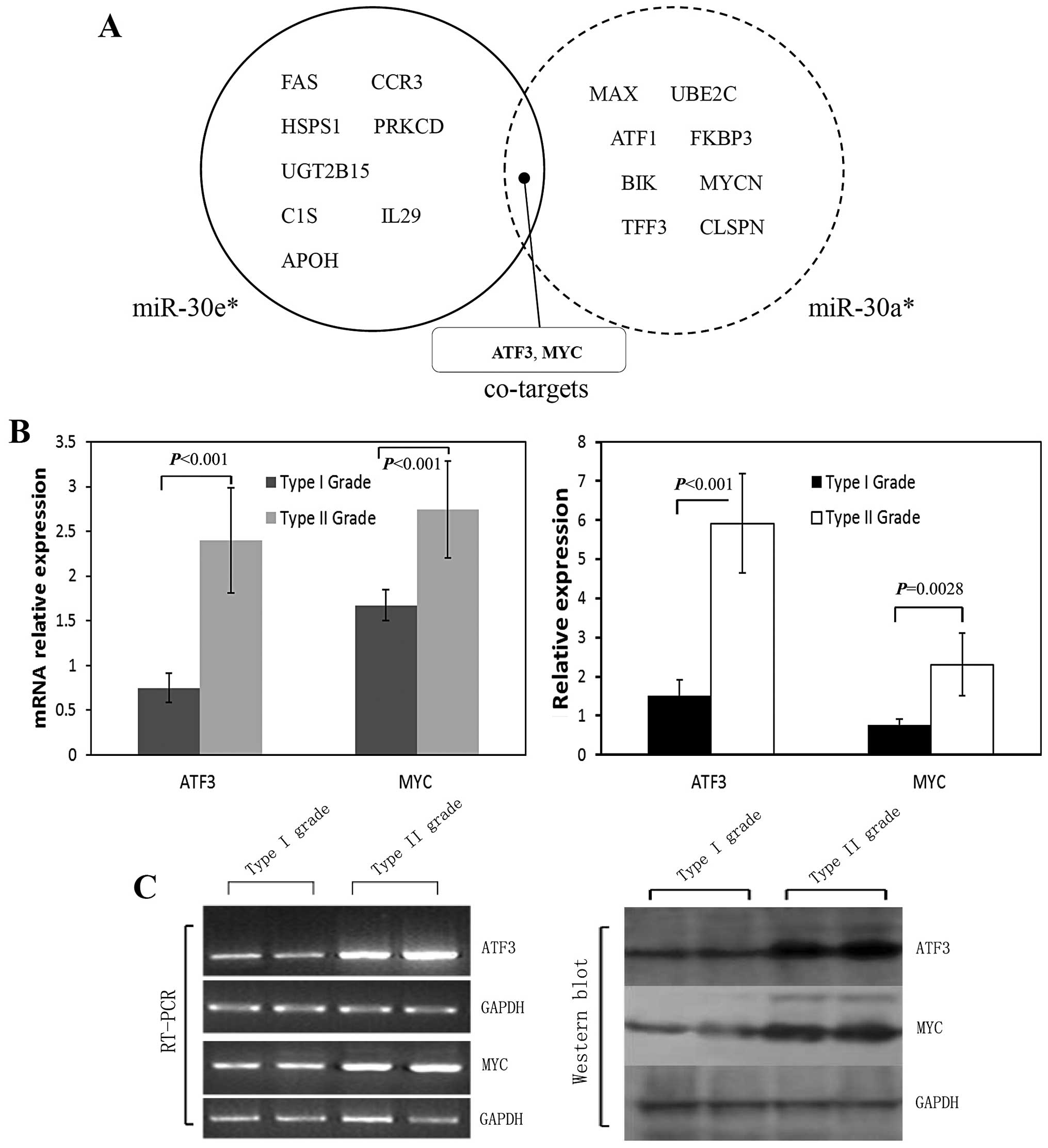
Unique miRNAs and their co-target
prediction
The analysis of miRNA predicted targets was
determined using several computational approaches, including
Ingenuity Systems, MicroCosm Targets version 5, and miRBase.
Functional analysis of these predicted gene targets can help us to
identify biologic pathways with significant involvement in gene
expression. In order to retrieve only the most relevant targets, we
listed only genes targeted by miR-30a* and
miR-30e* that were remarkably downregulated in the type
II vs. type I OPSC patients, and we found they have some targets in
common suggesting that they might play important roles in
pathogenesis in differentiation of ovarian papillary serous
carcinoma. ATF3 and MYC were indicated as potential co-targets of
both miRNAs (Fig. 3A).
Validation of miRNA target gene
predictions
Although hundreds of targets for each miRNA were
predicted using computational analysis of microarray data, the
specific binding targets that directly correlated to key miRNA
should be fully validated with standard approaches such as
polymerase chain reaction (RT-PCR), western blot analysis or
immunohistochemical assay. In target prediction analysis, ATF3 and
MYC were predicted as potential co-targets of miR-30a*
and miR-30e* (Fig. 3A),
and present as regulators in the different pathways, which include
tumorigenesis in numerous human cancers. RT-PCR analysis for ATF3
and MYC mRNA showed a significant upregulation in type II OPSC
patients compared to type I cases (p<0.001 for all, Fig. 3B and C). These target gene
predictions were all confirmed by western blot analysis results
(p-value <0.001 and = 0.0028 for ATF3 and MYC respectively,
Fig. 3B and C).
Immunohistochemical assay for the ATF3 and MYC
proteins showed a relevant upregulation in type II OPSC cells
compared to type I OPSC cells (Fig.
4). It is clear that these co-targets were all extensively
distributed in the cytoplasm of cancer cells in the tissue of type
II OPSC samples comparing with the type I OPSC sections (Fig. 4A). Through analysis with Image-Pro
plus vision 6.0, positive area and Integrate Optical Density (IOD)
per vision-field of ×400 immunohistochemistry images were detected.
These results also support the conclusions of the
immunohistochemical assay (Fig.
4B–C).
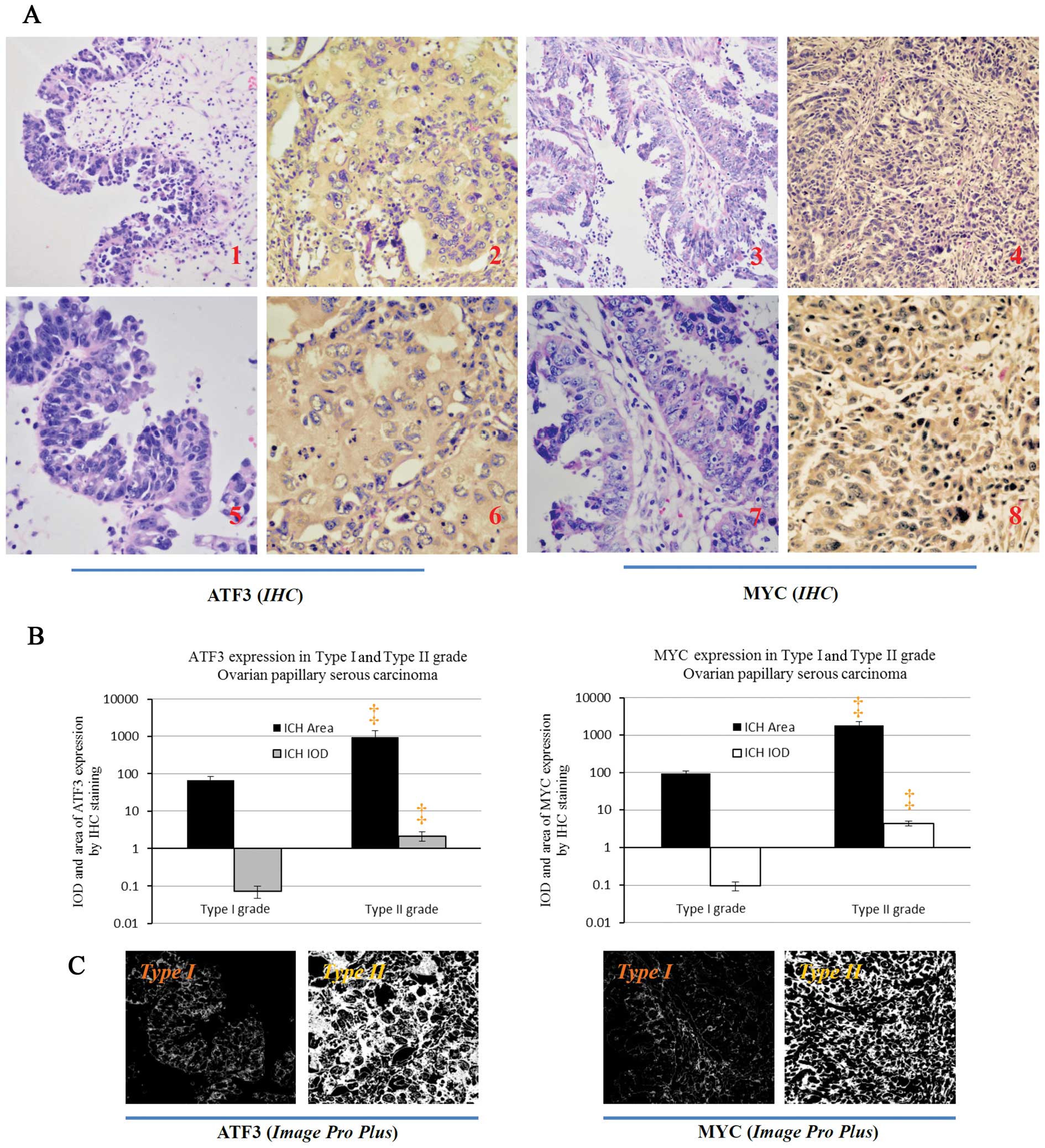 | Figure 4.ATF3 and MYC protein expressions in
paraffin-embedded tissues in the immunohistochemical assay of type
I and type II OPSC patients. (A) Identification of the different
expression of ATF3 and MYC protein between the type I grade and
type II grade OPSC cells by immunohistochemistry (IHC) with counter
staining of hematoxylin. ATF3 and MYC, are obviously expressed in
the cytoplasm of type II grade OPSC tumor cells (A2, 4, 6 and 8)
and poorly expressed in type I grade OPSC tissues (A1, 3, 5 and 7).
A1, 2, 3 and 4, magnification, ×200; and A5, 6, 7 and 8,
magnification, ×400, respectively. (B) The different expressions
for positive area and integrate optical density (IOD) of ATF3 and
MYC (per vision-field of ×400 photograph dealt with Image-Pro plus
vision 6.0) between the type I grade and type II grade OPSC tumor
through immunohistochemistry staining, respectively. Images reveal
(×400) the positive staining area of the above proteins with
Image-Pro plus vision 6.0, respectively. Compared with the type I
patients, ‡p<0.01. |
Prediction of histological grade and
survival rate in OPSC patients using miR-30a* and
miR-30e* expression patterns
To understand the significance of
miR-30a*and miR-30e* expression patterns in
epithelial ovarian cancer (EOC) differentiation, we performed a
retrospective study to check the relationship between
miR-30a*, miR-30e* expression and histologic
grade in OPSC patients. A 50-sample training set (type I, 16; type
II, 34) was used to identify miR-30a* or
miR-30e* expression pattern that could predict the
histological differentiation through the analysis of leave-one-out
cross predictions. Using a cutoff of 9.550466 and 11.82391 with the
highest Youden’s index for miR-30a* and
miR-30e*, respectively, we accurately predicted the
grade, and the accuracy, sensitivity and specificity are 80.0, 85.4
and 79.5% for miR-30a* and 86.0, 79.2 and 92.2% for
miR-30e*, respectively (Fig. 5A and B). Mann-Whitney U tests for
statistical significance (p<0.001 for all groups) demonstrated
the capacity of the predictor to distinguish type I OPSC patients
from type II OPSC patients. The mean AUC values (area under ROC
curve) of miR-30a* and miR-30e* are 0.889 and
0.895, respectively (Fig. 5C and
D).
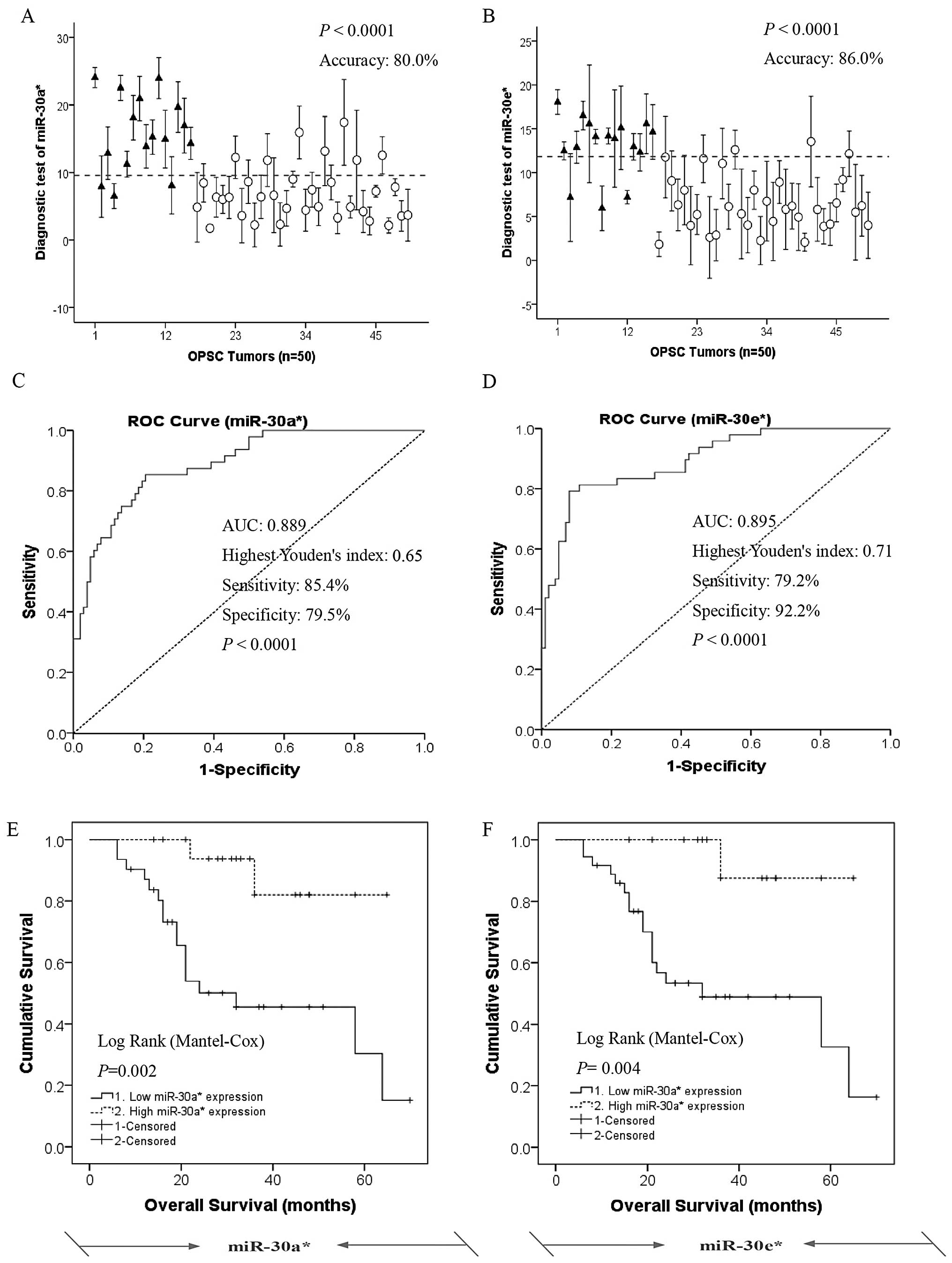 | Figure 5.miR-30a* and
miR-30e* serve as novel prediction and prognostic
biomarkers for the grade and survival of OPSC patients. (A and B)
Leave-one-out cross predictions of OPSC differentiation (black
triangle, type I OPSC patients; white cycle, type II OPSC patients)
with (A) miR-30a* and (B) miR-30e*,
respectively, n=50 for all; cutoffs are 9.550466 and 11.82391 with
the highest Youden’s index for each; accuracy, sensitivity and
specificity are 80.0, 85.4 and 79.5% for miR-30a* and
86.0, 79.2 and 92.2% for miR-30e*, respectively. (C and
D) Receiver operating characteristic (ROC) curves of the
predictions of OPSC differentiation according to (C)
miR-30a* and (D) miR-30e*, respectively, n=50
for all. AUC values are 0.889 and 0.895 for miR-30a* and
miR-30e*, respectively. (E and F) miR-30a*
and miR-30e* serve as novel prediction and prognostic
biomarkers for the response to survival of OPSC patients. The
overall survival result of type I and II OPSC patients divided into
two sub-sets according to high and low expression level of (E)
miR-30a* and (F) miR-30e*, respectively.
Diagnosis is based on the analysis of above ROC curves.
Kaplan-Meier survival analysis indicated that low expression of
miR-30a* and miR-30e* were all significantly
associated with shorter overall survival of the OPSC patients,
p<0.01 for all). |
To further validate the predictive value of
prognostic biomarkers for miR-30a* and
miR-30e* expressions in clinical outcome, we performed
Kaplan-Meier survival analysis for OPSC patients who were divided
into two sub-sets according to high and low expression level of the
miR-30a* and miR-30e* (Fig. 5E and F). Diagnosis is based on the
analysis of above ROC curves. Kaplan-Meier survival analysis
indicated that low expression of miR-30a* and
miR-30e* was significantly associated with shorter
overall survival of the OPSC patients as compared with the high
miR-30a* and miR-30e* expression groups,
respectively, log-rank p<0.01 for all.
Taken together, these results indicate that the
expression levels of miR-30a* and miR-30e*
could serve as novel predictors and prognostic biomarkers for OPSC
patient response to histologic grade and survival.
Discussion
Histological grade has already been recognized as a
very important prognostic factor for ovarian serous carcinoma
(5,6). However, there are still several
unresolved issues concerning the grading of this disease in which
there is no agreement regarding the designation with well,
moderate, poor differentiation vs. I, II, III vs. 1,2,3,4 vs. low
and high-grade (5,6). Based on the recent
clinicopathological and molecular studies, some researchers
proposed a model for grading serous ovarian cancer. In this model,
surface epithelial tumors are divided into a two-tier system for
histologic grade that is based primarily on the assessment of
nuclear atypia (uniformity vs. pleomorphism) in the worst area of
the tumor (1,5,7).
Type I tumors are mainly low-grade and relatively genetically
stable. In contrast, high grade serous carcinoma is by far the most
common type II ovarian cancer and highly aggressive. Type I tumors
are associated with distinct molecular changes that are rarely
found in type II tumors. Low-grade serous carcinomas typically
pursue an indolent course that may last more than 20 years
(1,17,18).
This contrasts with conventional high-grade serous carcinoma that
accounts for approximately 75% of ovarian cancer but is responsible
for most of the deaths (6,19) and almost always have progressed to
advanced stage at diagnosis, when current available therapies are
seldom curative (7,9). Women in our study had 77% high-grade,
whereas only 23% had low-grade. This is in line with the other
studies examining survival of ovarian serous carcinomas using the
two-tier system (6). In our
investigation, we found that women with high-grade had a
significantly increased risk of ovarian cancer compared with women
with low-grade (Table II). Similar
results were found in other studies (5,6).
Through miRNA microarray and targets prediction
analysis, 23 miRNAs were found to be highly differentially
expressed in tumors from type I vs. type II OPSC patients.
miR-30a* and miR-30e* were the top 2 miRNAs
that showed significant differential expression in OPSC patients
between the type I and II, and were remarkably downregulated in the
latter group, suggesting that these miRNAs are involved in
differentiation of serous ovarian cancer. To further understand the
mechanisms and to investigate the functions of miRNAs in the
development of ovarian pathological differentiation, miRNA target
prediction and pathway analysis were done. ATF3 and MYC were
indicated as potential co-targets of both miRNAs, and they were
validated as significantly upregulated in type II OPSC
patients.
ATF3 is a member of the ATF/CREB family of
transcription factors (4), as an
oncogene, ATF3 contributes to the successful propagation of human
cancer (4,21–24),
and promotes metastasis, cell adhesion and invasion, cell
proliferation, apoptosis and cell differentiation (25–27)
(Fig. 6). ATF3 plays a complex
role in tumor progression, and it is possible that some of the
apparent contradictions in terms of the function of the ATF3 gene
arise at least in part due to the difference in the cellular
context (25). Similarly to ATF3,
MYC levels can be activated in a wide variety of human
hematological malignancies and solid tumors (28). Deregulation of MYC contributes to
the genesis of a wide spectrum of human tumors through many
biological activities including cell growth and division, cell
cycling, cell differentiation, apoptosis and cell adhesion and
motility (28) (Fig. 6). The most common MYC aberration in
solid tumors is gene amplification. In ovarian cancer, MYC
over-expression is observed with a frequency of around 40%
(28). Importantly, MYC
inactivation resulted in tumor regression and/or differentiation in
several experimental systems (28–31).
Jain et al found that even transient inactivation of MYC was
sufficient to achieve tumor regression and cell differentiation
(32). The above-mentioned studies
revealed that ATF3 and MYC are often associated with aggressive
behavior and poor differentiation, especially in human cancers
(28). These results are in good
agreement with our findings and point toward a regulating
differentiation function of the miR-30a* and
miR-30e* genes.
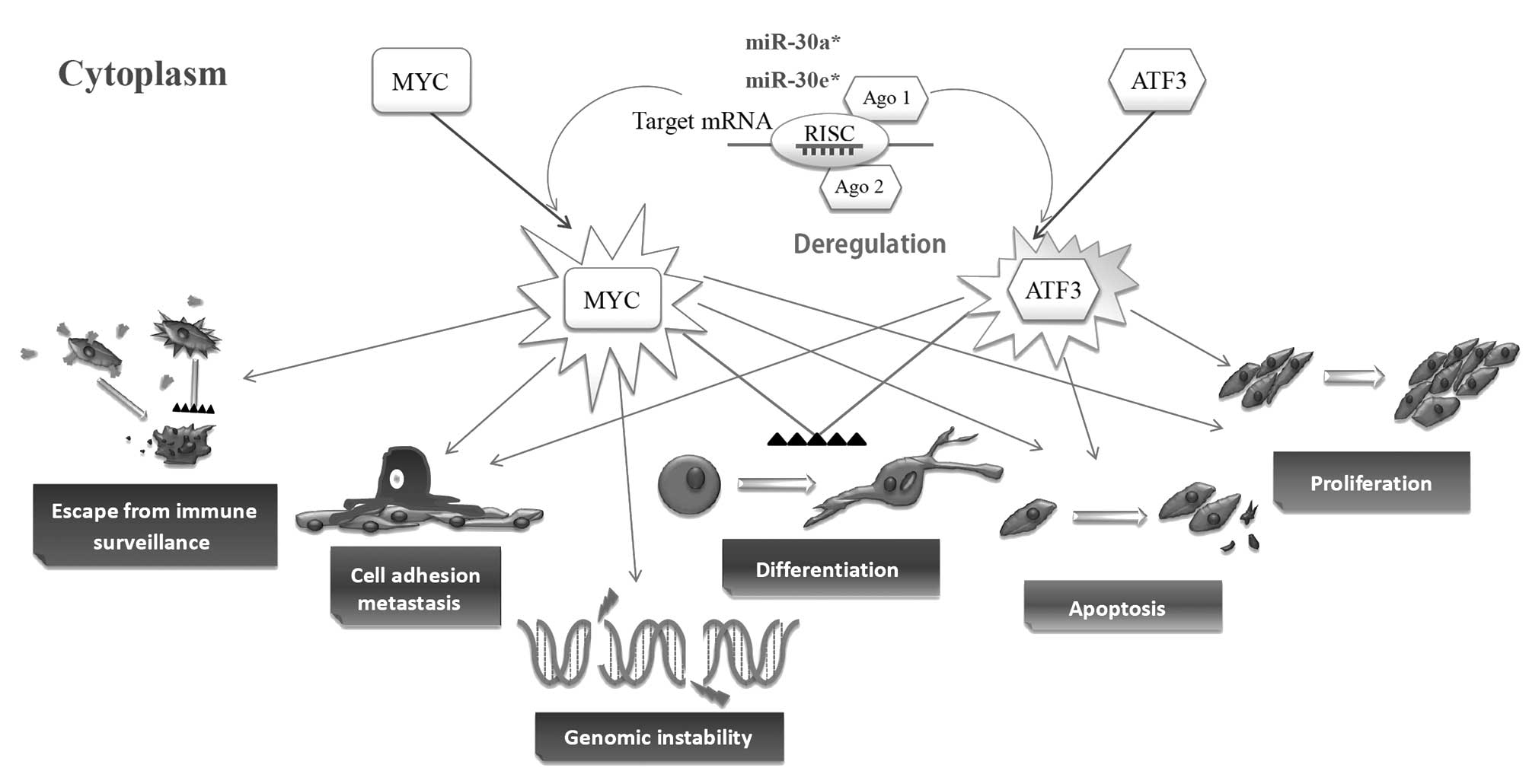 | Figure 6.Cellular processes regulated by ATF3
and MYC during normal conditions and tumorigenesis. ATF3 and MYC
are hallmarks of many cancers and occur as a consequence of
activation of many signaling pathways that induce their expression
and function as a regulator of gene transcription. As an oncogene,
ATF3 contributes to the successful propagation of human cancer
(4,21–24),
and promote metastasis, cell adhesion and invasion, cell
proliferation, apoptosis and cell differentiation (25–27).
MYC is also a key regulator of many biological activities including
cell growth and division, cell cycling, cell differentiation,
apoptosis, and cell adhesion and motility (28). Arrows indicate promotion, and black
triangle bars (▴▴▴▴) indicate suppression. |
To further validate this hypothesis, we investigated
the leave-one-out cross predictions of OPSC histological grade
stratified by expression levels of miR-30a* and
miR-30e*, respectively. By checking and following-up on
the patient’s medical records, we unexpectedly found that
miR-30a* and miR-30e* can predict
histological grade with accuracy of up to 80.0 and 86.0%, and AUC
values are 0.889 and 0.895, respectively. Kaplan-Meier survival
analysis indicated that low expression of miR-30a* and
miR-30e* were all significantly associated with shorter
overall survival of the OPSC patients. These findings strongly
suggested that miR-30a* and miR-30e* can be
used as biomarkers to tailor histological grade before starting the
regimen, and they have important roles in ovarian cancer
differentiation resulting in poorer prognosis.
To our knowledge, this study is the first to
indicate and validate the roles and significance of
miR-30a* and miR-30e* in histologic
differentiation of ovarian papillary serous carcinoma. As most
current cancer therapies are highly toxic and often non-specific,
recently, a potentially less toxic approach, termed
‘differentiation therapy’, to treating this prevalent disease is
raised. This approach employs agents to modify cancer cell
differentiation, which on appropriate treatment, results in tumor
re-programming and a concomitant loss in proliferative capacity and
induction of terminal differentiation or apoptosis (33). We hope our research can improve
understanding of molecular mechanisms of EOC development and
progression, especially in histologic differentiation, so that we
can provide improved diagnostic, prognostic and therapeutic
approaches to individual patients, especially in differentiation
therapy to high-grade OPSC cases.
References
|
1.
|
Shih IeM and Kurman RJ: Ovarian
tumorigenesis: a proposed model based on morphological and
molecular genetic analysis. Am J Pathol. 164:1511–1518. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pignata S and Vermorken JB: Ovarian cancer
in the elderly. Crit Rev Oncol Hematol. 49:77–86. 2004. View Article : Google Scholar
|
|
3.
|
Boren T, Xiong Y, Hakam A, et al:
MicroRNAs and their target messenger RNAs associated with ovarian
cancer response to chemotherapy. Gynecol Oncol. 113:249–255. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zhao H, Ding Y, Tie B, et al: miRNA
expression pattern associated with prognosis in elderly patients
with advanced OPSC and OCC. Int J Oncol. 43:839–849.
2013.PubMed/NCBI
|
|
5.
|
Malpica A, Deavers MT, Tornos C, et al:
Interobserver and intraobserver variability of a two-tier system
for grading ovarian serous carcinoma. Am J Surg Pathol.
31:1168–1174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hannibal CG, Vang R, Junge J,
Kjaerbye-Thygesen A, Kurman RJ and Kjaer SK: A binary histologic
grading system for ovarian serous carcinoma is an independent
prognostic factor: a population-based study of 4317 women diagnosed
in Denmark 1978–2006. Gynecol Oncol. 125:655–660. 2012.PubMed/NCBI
|
|
7.
|
Lu D, Kuhn E, Bristow RE, et al:
Comparison of candidate serologic markers for type I and type II
ovarian cancer. Gynecol Oncol. 122:560–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Gloss BS and Samimi G: Epigenetic
biomarkers in epithelial ovarian cancer. Cancer Lett. 342:257–263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kurman RJ, Visvanathan K, Roden R, Wu TC
and Shih IeM: Early detection and treatment of ovarian cancer:
shifting from early stage to minimal volume of disease based on a
new model of carcinogenesis. Am J Obstet Gynecol. 198:351–356.
2008.PubMed/NCBI
|
|
10.
|
Vang R, Shih I and Kurman RJ: Ovarian
low-grade and high-grade serous carcinoma: pathogenesis,
clinicopathologic and molecular biologic features, and diagnostic
problems. Adv Anat Pathol. 16:267–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kurman RJ and Shih I: Molecular
pathogenesis and extraovarian origin of epithelial ovarian
cancer-shifting the paradigm. Hum Pathol. 42:918–931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Neelakandan K, Babu P and Nair S: Emerging
roles for modulation of microRNA signatures in cancer
chemoprevention. Curr Cancer Drug Targets. 12:716–740. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Li C, Hashimi SM, Good DA, et al:
Apoptosis and microRNA aberrations in cancer. Clin Exp Pharmacol
Physiol. 39:739–746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wang Y, Wang Q, Zhao Y, et al: Protective
effects of estrogen against reperfusion arrhythmias following
severe myocardial ischemia in rats. Circ J. 74:634–643. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Li ZD, Hu XW, Wang YT and Fang J: Apigenin
inhibits proliferation of ovarian cancer A2780 cells through Id1.
FEBS Lett. 583:1999–2003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Schick B, Wemmert S, Jung V, Steudel WI,
Montenarh M and Urbschat S: Genetic heterogeneity of the MYC
oncogene in advanced juvenile angiofibromas. Cancer Genet
Cytogenet. 164:25–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Seidman JD and Kurman RJ:
Subclassification of serous borderline tumors of the ovary into
benign and malignant types. A clinicopathologic study of 65
advanced stage cases. Am J Surg Pathol. 20:1331–1345. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Smith Sehdev AE, Sehdev PS and Kurman RJ:
Noninvasive and invasive micropapillary (low-grade) serous
carcinoma of the ovary: a clinicopathologic analysis of 135 cases.
Am J Surg Pathol. 27:725–736. 2003.PubMed/NCBI
|
|
19.
|
Lalwani N, Prasad SR, Vikram R, Shanbhogue
AK, Huettner PC and Fasih N: Histologic, molecular, and cytogenetic
features of ovarian cancers: implications for diagnosis and
treatment. Radiographics. 31:625–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Malpica A, Deavers MT, Lu K, et al:
Grading ovarian serous carcinoma using a two-tier system. Am J Surg
Pathol. 28:496–504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kim MS, In SG, Park OJ, et al: Increased
expression of activating transcription factor 3 is related to the
biologic behavior of cutaneous squamous cell carcinomas. Hum
Pathol. 42:954–959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hai T and Hartman MG: The molecular
biology and nomenclature of the activating transcription
factor/cAMP responsive element binding family of transcription
factors: activating transcription factor proteins and homeostasis.
Gene. 273:1–11. 2001. View Article : Google Scholar
|
|
23.
|
Thompson MR, Xu D and Williams BR: ATF3
transcription factor and its emerging roles in immunity and cancer.
J Mol Med (Berl). 87:1053–1060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Pelzer AE, Bektic J, Haag P, et al: The
expression of transcription factor activating transcription factor
3 in the human prostate and its regulation by androgen in prostate
cancer. J Urol. 175:1517–1522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Bandyopadhyay S, Wang Y, Zhan R, et al:
The tumor metastasis suppressor gene Drg-1 down-regulates the
expression of activating transcription factor 3 in prostate cancer.
Cancer Res. 66:11983–11990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ishiguro T, Nagawa H, Naito M and Tsuruo
T: Inhibitory effect of ATF3 antisense oligonucleotide on ectopic
growth of HT29 human colon cancer cells. Jpn J Cancer Res.
91:833–836. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Jang MK, Kim CH, Seong JK and Jung MH:
ATF3 inhibits adipocyte differentiation of 3T3-L1 cells. Biochem
Biophys Res Commun. 421:38–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Vita M and Henriksson M: The Myc
oncoprotein as a therapeutic target for human cancer. Semin Cancer
Biol. 16:318–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Pelengaris S, Khan M and Evan GI:
Suppression of Myc-induced apoptosis in beta cells exposes multiple
oncogenic properties of Myc and triggers carcinogenic progression.
Cell. 109:321–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Shachaf CM, Kopelman AM, Arvanitis C, et
al: MYC inactivation uncovers pluripotent differentiation and
tumour dormancy in hepatocellular cancer. Nature. 431:1112–1117.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Yu D, Dews M, Park A, Tobias JW and
Thomas-Tikhonenko A: Inactivation of Myc in murine two-hit B
lymphomas causes dormancy with elevated levels of interleukin 10
receptor and CD20: implications for adjuvant therapies. Cancer Res.
65:5454–5461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Jain M, Arvanitis C, Chu K, et al:
Sustained loss of a neoplastic phenotype by brief inactivation of
MYC. Science. 297:102–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Leszczyniecka M, Roberts T, Dent P, Grant
S and Fisher PB: Differentiation therapy of human cancer: basic
science and clinical applications. Pharmacol Ther. 90:105–156.
2001. View Article : Google Scholar : PubMed/NCBI
|