Introduction
Various environmental stresses such as oxidation,
nutrient deprivation, radiation, and stress to the endoplasmic
reticulum induce the integrated stress response in which the
elevated phosphorylation level of eukaryotic translation initiation
factor 2α (eIF2α) may stimulate cellular apoptosis (1,2). In
response to mild stresses, the phosphorylation of eIF2α attenuates
translational efficiency and activates the pro-survival signaling
(3). However, in response to
severe stress, the phosphorylated eIF2α (eIF2α-p) promotes
apoptosis (3,4). Salubrinal, a synthetic chemical agent
known to elevate the level of eIF2α-p, is considered as a
cytoprotective agent as well as an agent to stimulate apoptosis
depending on the stress environments and cell types (5). For instance, it is reported that
salubrinal could protect against tunicamycin induced cardiomyocyte
apoptosis (6). On the contrary,
administration of salubrinal to leukemic and chondrosarcoma cells
is reported to stimulate apoptosis (7,8).
Nonetheless, few studies have been conducted to examine the effect
of salubrinal on breast cancer cells.
Breast cancer accounts for ∼25% of all cancers in
women and its therapeutic strategy heavily depends on the
expression levels of three marker genes such as estrogen receptor
(ER), progesterone receptor (PgR) and human epidermal growth factor
receptor-2 (HER2). When cancer cells exhibit a high expression
level of ER and/or PgR, hormonal treatments would be a viable
option (9). For cancer cells with
an overexpressed level of HER2, treatments with HER2-targeted drugs
such as trastuzumab and lapatinib are potentially effective
(10). A lack of expression of all
three gene products, however, defines a triple negative breast
cancer (TNBC) (11–13) that presents a challenge in
prognosis and requires a novel treatment option.
In this study, we addressed the question: does
administration of salubrinal attenuate the malignant in
vitro phenotype of TNBCs? If yes, what is the mechanism of
salubrinal’s action and does its administration to mice injected
with TNBCs suppress in vivo tumor growth? In response to
administration of salubrinal, we examined in vitro phenotype
of 4T1 mammary tumor cells and MDA-MB-231 human breast cancer cells
(14–16). We also employed guanabenz, another
synthetic drug known to elevate eIF2α-p by inhibiting
de-phosphorylation of eIF2α-p (17). In order to examine the involvement
of eIF2α, silencing by RNA interference was conducted using siRNA
specific to eIF2α. Furthermore, the potential linkage between
regulation of eIF2α and Rac1 GTPase in cell invasion and motility
was evaluated using siRNA specific to Rac1 GTPase. We also employed
a fluorescence resonance energy transfer (FRET) technique and
evaluated Rac1 activity in response to salubrinal. To test the
effects of salubrinal in vivo, 4T1 mammary tumor cancer
cells were injected to mice and suppression of tumor growth was
analyzed.
Materials and methods
Cell culture
4T1 mouse mammary tumor cells and MDA-MB-231 human
breast cancer cells were cultured in DMEM containing 10% fetal
bovine serum and antibiotics (50 U/ml penicillin and 50
μg/ml streptomycin; Life Technologies, Grand Island, NY,
USA). Cells were maintained at 37°C and 5% CO2 in a
humidified incubator. Responses to administration of 10–50
μM salubrinal (18) or 5–50
μM guanabenz acetate (Tocris Bioscience, Ellisville, MO,
USA) were evaluated using assays for MTT, adhesion, invasion and
motility.
MTT assay
Cells (5×102/well) were seeded in 96-well
plates, and the reduction of MTT to formazan was evaluated by
measuring the absorbance at 570 nm with a plate reader (EL800,
BioTek, Winooski, VT, USA).
Cell adhesion assay
Ninety-six well plates were coated with
poly-L-lysine, fibronectin, laminin (Sigma-Aldrich, St. Louis, MO,
USA) or type I collagen (BD Biosciences, Bedford, MA, USA) for 2 h.
The plates were then incubated with non-fat dry milk, followed by
washing with PBS and serum-free culture medium. Cells
(1×104/well) were added on the plate, and after 30 min
and 3 h the attached cells were stained with 0.04% crystal violet
(Sigma-Aldrich) for 10 min at room temperature. The wells were
washed with PBS, and DMSO was added. Absorbance at 550 nm was
measured using the plate reader.
In vitro invasion assay
An invasion assay was performed with a Boyden
chamber as described previously (19), with minor modifications. In brief,
Matrigel (BD Biosciences) was diluted with ice-cold PBS (100
μg/ml). Matrigel (600 μl) was added to each filter
(polyethylene terephthalate membrane, 8-μm pore size, 23.1
mm in diameter, Falcon) and left to polymerize overnight. Prior to
assembling the chamber unit, the lower chamber (6-well plate,
Falcon) was filled with culture medium consisting of salubrinal or
guanabenz. Cells (1–4×105/well) were added to the
culture medium with salubrinal or guanabenz in the upper chamber
and incubated for 24 h. The cells on the filter surface were
stained with Giemsa (Sigma-Aldrich) and the number of cells was
counted under a microscope.
Two-dimensional motility assay
To evaluate 2-dimensional motility, a wound healing
scratch motility assay was carried out as described previously
(20). In brief, cells were plated
in 12-well plates or 6-cm dishes (Falcon) and on the next day,
scratching was performed using a plastic tip. The areas newly
occupied with cells in the scratched zone were determined every 3 h
up to 24 h using images obtained by a microscope, which were
scanned with Adobe Photoshop (CS2, Adobe Systems, San Jose, CA,
USA) and quantified with ImageJ.
Western blot analysis
Cells were lysed in a radioimmunoprecipitation assay
(RIPA) buffer containing protease inhibitors (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and phosphatase inhibitors
(Calbiochem, Billerica, MA, USA). Isolated proteins were
fractionated using 10–15% SDS gels and electro-transferred to
Immobilon-P membranes (Millipore, Billerica, MA, USA). The membrane
was incubated for 1 h with primary antibodies followed by 45 min
incubation with goat anti-rabbit or anti-mouse IgG conjugated with
horse-radish peroxidase (Cell Signaling, Danvers, MA, USA). We used
antibodies against eIF2α, caspase 3, cleaved caspase (Cell
Signaling), Rac1 (Millipore), and β-actin (Sigma). Protein levels
were assayed using a SuperSignal west femto maximum sensitivity
substrate (Thermo Scientific, Waltham, MA, USA), and signal
intensities were quantified with a luminescent image analyzer
(LAS-3000, Fuji Film, Tokyo, Japan).
Knockdown of eIF2α and Rac1 by siRNA
Cells were treated with siRNA specific to eIF2α and
Rac1 (Life Technologies). Selected target sequences for knockdown
of eIF2α and Rac1 were: eIF2α, 5′-CGG UCA AAA UUC GAG CAG A-3′, and
Rac1, 5′-GCA UUU CCU GGA GAG UAC A -3′; and as a non-specific
control, a negative siRNA (Silencer Select no. 1, Life
Technologies) was used. Cells were transiently transfected with
siRNA for eIF2α, Rac1 or control in Opti-MEM I medium with
Lipofectamine RNAiMAX (Life Technologies). Six hours later, the
medium was replaced by regular culture medium. The efficiency of
silencing was assessed with immunoblotting 48 h after
transfection.
Fluorescence resonance energy transfer
(FRET)
To visualize Rac1 activity in response to
salubrinal, FRET imaging was conducted using a cyan fluorescent
protein (CFP)-yellow fluorescent protein (YFP) Rac1 biosensor. The
filter sets (Semrock) were chosen for CFP excitation at 438±24 nm
(center wavelength ± bandwidth), CFP emission at 483±32 nm, and YFP
emission at 542±27 nm. Time-lapse images were acquired at an
interval of 5 min using a fluorescence microscope (Nikon, Tokyo,
Japan). The level of Rac1 activity was determined by computing an
emission ratio of YFP/CFP for individual cells using NIS-Elements
software (Nikon).
In vivo tumor growth
Experimental procedures were approved by the Indiana
University Animal Care and Use Committee and were in compliance
with the Guiding Principles in the Care and Use of Animals endorsed
by the American Physiological Society. Five mice were housed per
cage, and fed with mouse chow and water ad libitum.
Thirty-five BALB/c female mice (6 weeks, Harlan Laboratories) were
used. Mice received subcutaneous injection of 4T1 mouse mammary
tumor cells (106 cells in 100 μl PBS) to the
abdomen on day 1. Salubrinal (25 μg) was administered
subcutaneously into the area of cell injection every day, while the
control animals received a vehicle. The animals were sacrificed on
day 20, and the volume and weight of tumors were determined. The
tumor volume was calculated as (long diameter) x (short
diameter)2 /2.
Statistical analysis
Three or four-independent experiments were conducted
and data were expressed as mean ± SD. For comparison among multiple
samples, ANOVA followed by post hoc tests was conducted.
Statistical significance was set at p<0.05. The single and
double asterisks in the figures indicate p<0.05 and
p<0.01.
Results
Inhibitory effects of salubrinal and
guanabenz in proliferation and survival of 4T1 cells
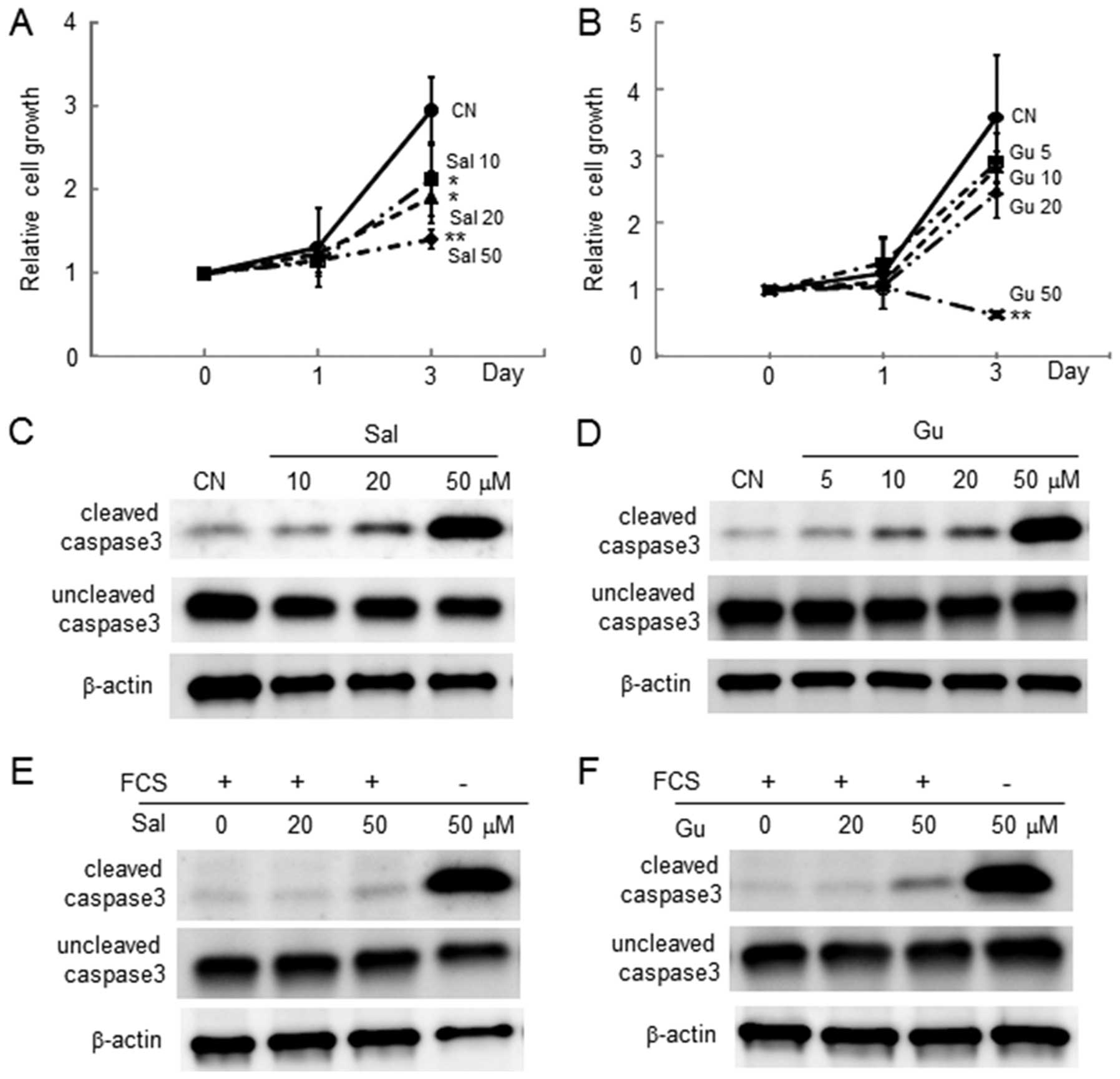
The MTT assay revealed that in response to 10, 20
and 50 μM salubrinal, the number of live 4T1 cells was
reduced in a dose-dependent manner (Fig. 1A). The number of live cells was
also decreased by 50 μM guanabenz (Fig. 1B). Consistent with the MTT results,
both salubrinal and guanabenz elevated the level of cleaved caspase
3 in the absence and presence of 10% serum in the culture medium,
respectively (Fig. 1C–F).
Undetectable changes in cell adhesion by
salubrinal and guanabenz in 4T1 cells
The surface was coated with poly-L-lysine, type I
collagen, fibronectin, and laminin, and the effects of salubrinal
and guanabenz on cell adhesion were examined. The absorbance
reading, which indicated the number of adherent cells on the coated
surface, did not significantly change in the presence and absence
of salubrinal and guanabenz 30 min and 3 h after cell incubation,
respectively (data not shown).
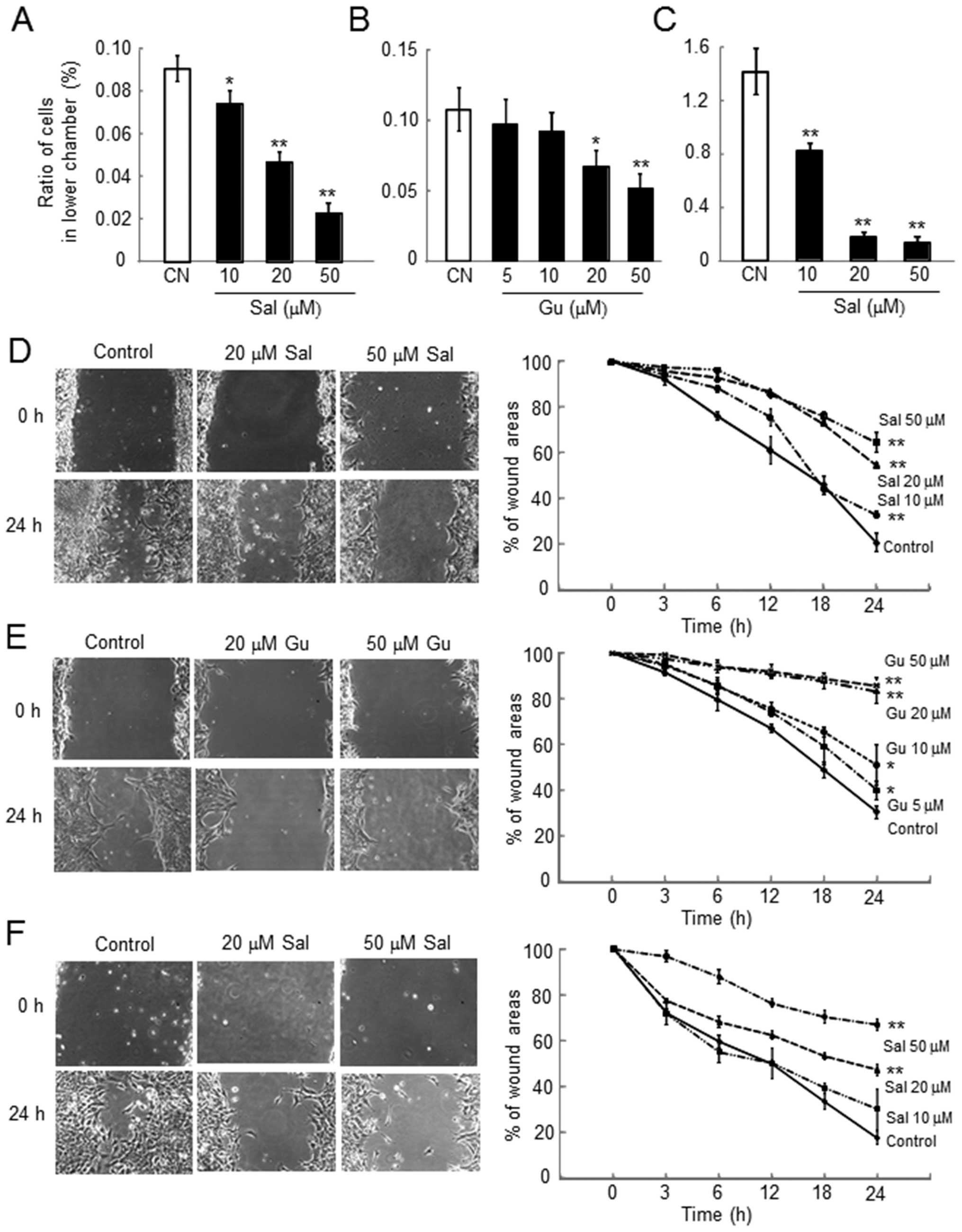
Dose-dependent reduction in cell invasion
by salubrinal and guanabenz in 4T1 cells
The number of cells invaded through the filter
coated with Matrigel was significantly reduced by administration of
salubrinal and guanabenz regardless of the presence of serum in the
medium in a dose-dependent manner (Fig. 2A–C).
Reduction in cell motility by salubrinal
and guanabenz in 4T1 cells
Using the scratch-wound assay, the wound area was
determined as an indicator of cell motility in which a reduction in
motility corresponded with a decrease in wound healing. In response
to both salubrinal and guanabenz, cell motility was reduced in a
dose-dependent manner (Fig. 2D–F).
The results with salubrinal were not affected by the presence of
serum in the culture medium.
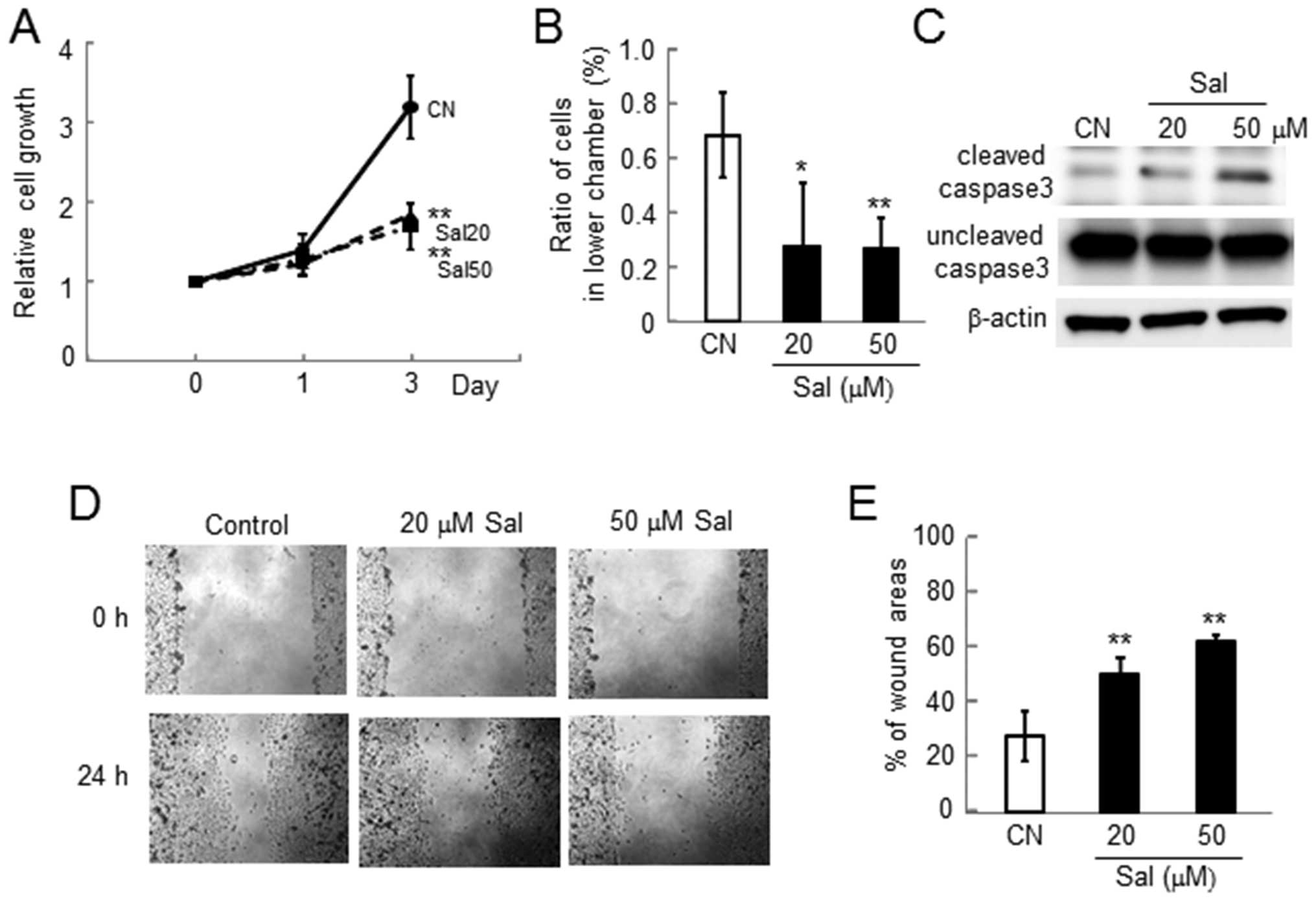
Reduction in proliferation, invasion,
survival, and motility in MDA-MB-231 cells
Consistent with the results in 4T1 mouse mammary
tumor cells, we observed reduction in proliferation, invasion,
survival and motility in MDA-MB-231 human breast cancer cells
(Fig. 3).
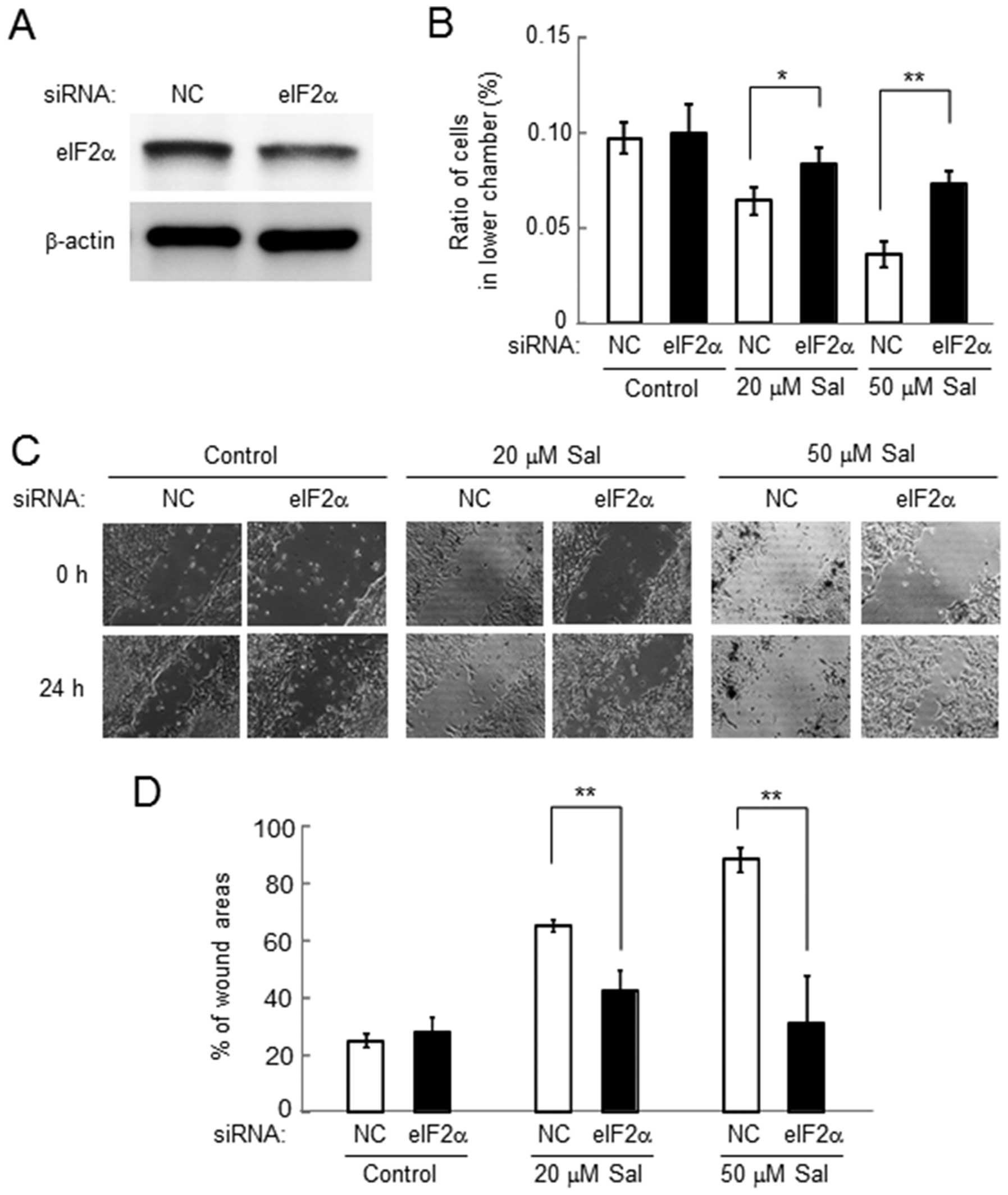
Involvement of eIF2α in salubrinal-driven
reduction in cell invasion and motility in 4T1 cells
Cell invasion and motility in response to salubrinal
were examined using the cells transiently transfected with eIF2α
siRNA (Fig. 4A). Compared to the
cells transfected with non-specific control (NC) siRNA,
salubrinal-driven reduction in cell invasion was significantly
suppressed in the cells treated with eIF2α siRNA (Fig. 4B). However, in the control cells
treated with NC siRNA, the cell invasion was reduced by salubrinal
in a dose-dependent manner. Furthermore, salubrinal-driven
reduction in cell motility was also suppressed by eIF2α siRNA
(Fig. 4C and D).
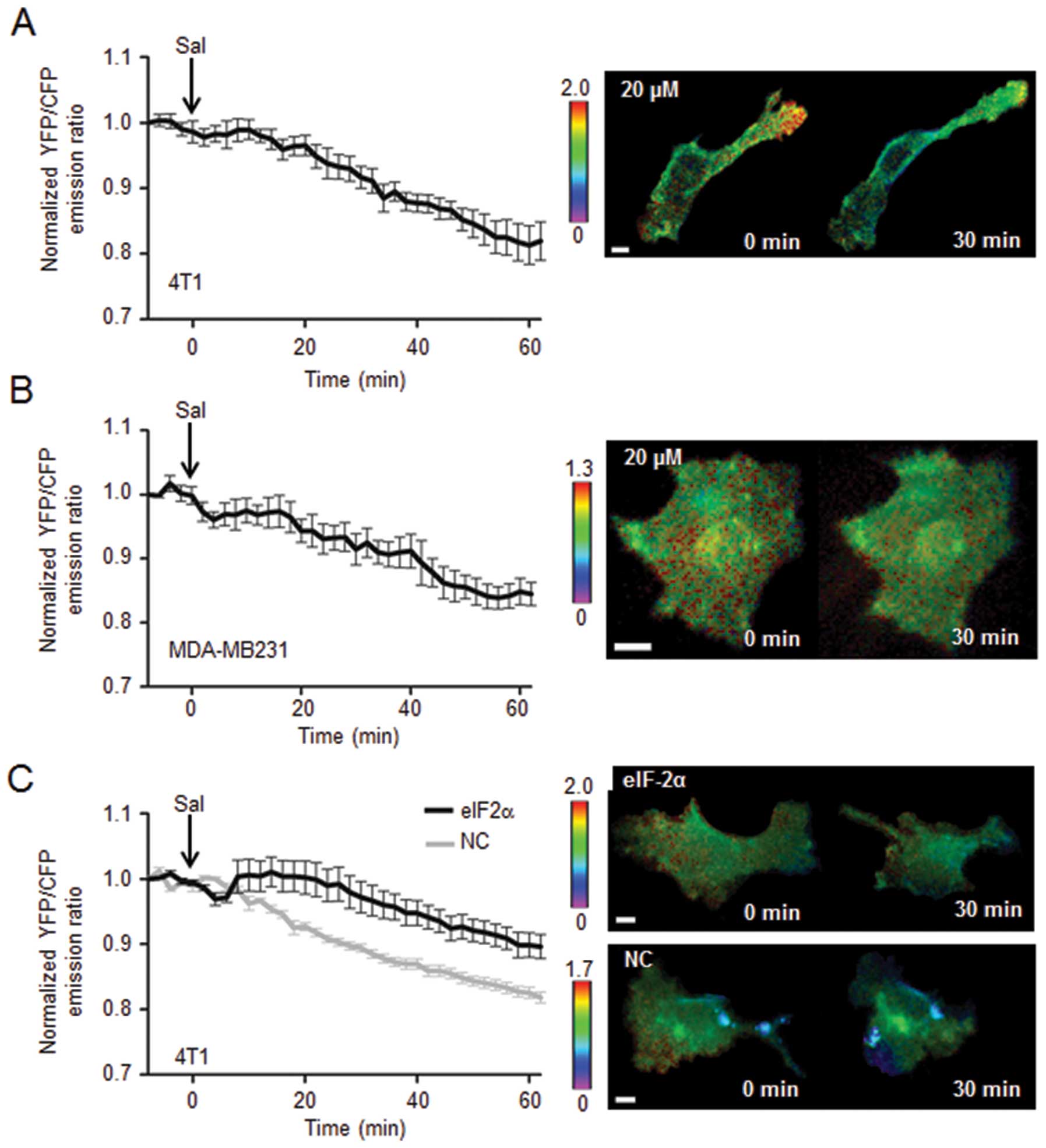
Inactivation of Rac1 GTPase by 20
μM salubrinal in 4T1 cells and MDA-MB-231 cells
To evaluate a potential involvement of Rac1 through
eIF2α-mediated signaling, the effect of salubrinal on activity of
Rac1 GTPase was examined using FRET-based single cell imaging. In
response to 20 μM salubrinal, the emission ratio of YFP/CFP
was decreased in 4T1 cells as well as MDA-MB-231 cells, indicating
that administration of salubrinal reduced the activity level of
Rac1 (Fig. 5A and B). The FRET
analysis was also conducted using 4T1 cells transfected with eIF2α
siRNA, in which salubrinal-driven reduction in Rac1 activity was
significantly suppressed (Fig.
5C).
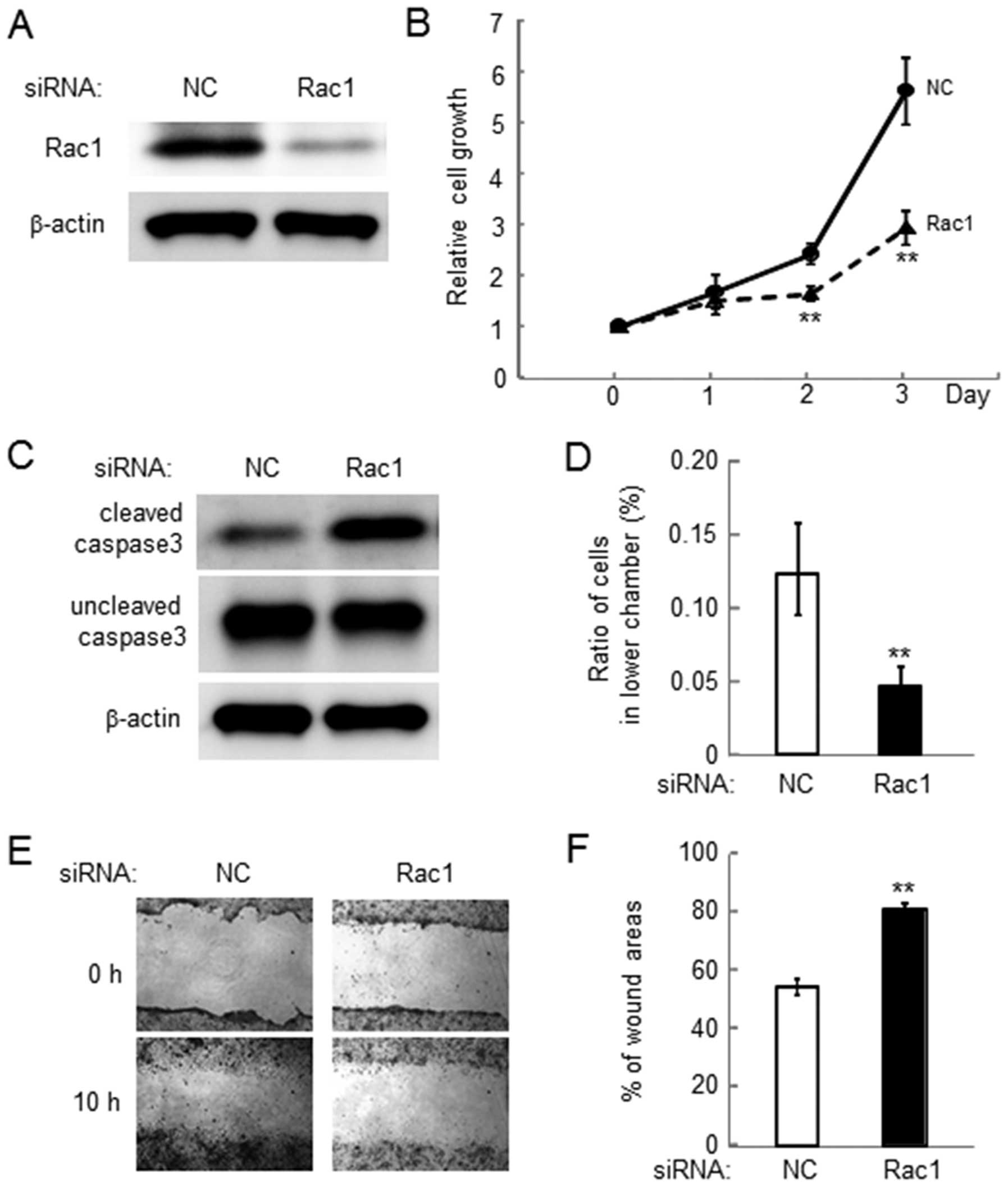
Reduction in cell growth, invasion and
motility by Rac1 siRNA in 4T1 cells
In order to examine the role of Rac1 in
salubrinal-driven suppression of malignant phenotypes, cell growth,
invasion and motility was examined using siRNA specific to Rac1
(Fig. 6A). The result revealed
that reduction in the expression level of Rac1 lowered cell growth
and elevated the level of cleaved caspase 3 (Fig. 6B and C). Furthermore, silencing
Rac1 decreased cell invasion as well as cell motility (Fig. 6D and F).
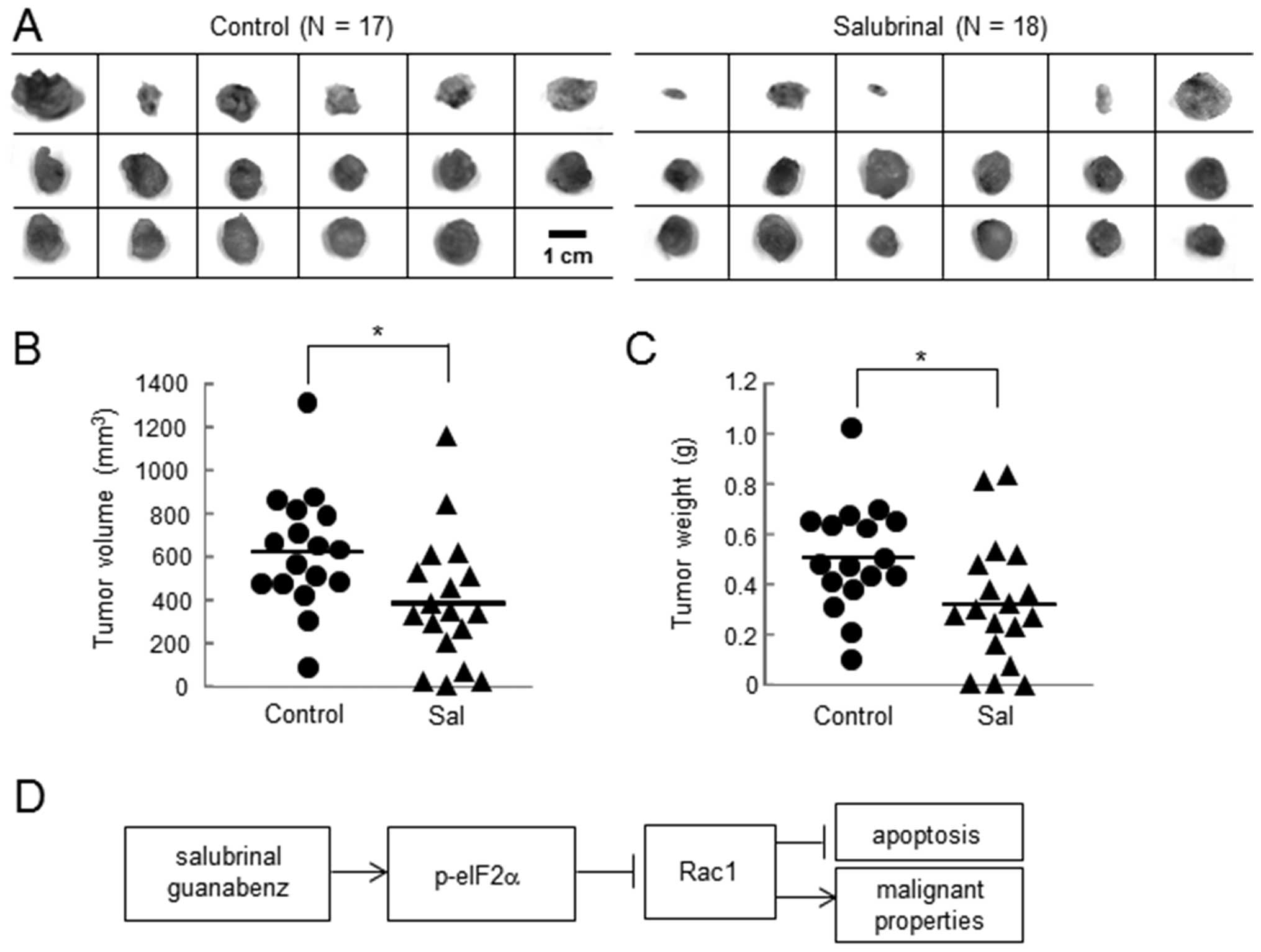
Inhibitory effects of salubrinal in the
volume and weight of tumors
Using the in vivo mouse model, the effects of
salubrinal on tumor growth were evaluated. A comparison of the
tumors isolated from the control mice (N=17) and the
salubrinal-treated mice (N=18) revealed that the tumor volume
(control, 620.1±271.1 mm3; and salubrinal, 384.5±248.1
mm3) and weight (control, 0.51±0.21 g; and salubrinal,
0.32±0.24 g) were significantly larger in the control group than
the salubrinal-treated group (Fig. 7A
and B).
Discussion
We demonstrated in this study that salubrinal has
inhibitory effects on the malignant phenotypes of 4T1 mammary tumor
cells and MDA-MB-231 breast cancer cells that hold a triple
negative phenotype. Salubrinal significantly reduced cellular
proliferation, invasion, and migration, although it did not alter
cellular adhesion to surfaces coated with poly-L-lysine, type I
collagen, fibronectin or laminin. The inhibitory effects were
commonly observed in response to salubrinal and guanabenz, both of
which elevate the level of p-eIF2α. RNA silencing with eIF2α siRNA
abolished salubrinal driven reduction in Rac1 and RNA silencing
with Rac1 siRNA attenuated malignant phenotypes as seen in the
responses to salubrinal. Furthermore, in vivo tumor size and
weight in 4T1 cells injected mice were significantly reduced by
daily administration of salubrinal (Fig. 7D).
The elevated level of cleaved caspase 3 indicates
that cellular apoptosis is stimulated by salubrinal. An increase in
apoptotic death was more significant in the absence of FBS in the
medium than that with FBS, suggesting that potency of salubrinal is
enhanced in a nutrient poor environment. Previous studies reported
that salubrinal could induce either stimulatory or inhibitory
effects on cellular death through modulation of the level of
p-eIF2α (6,7). Our data are consistent with a notion
that in an abnormal growth condition such as in a solid tumor
without well-developed vasculature, the elevation of p-eIF2α leads
to a pro-apoptotic pathway.
Rac1 GTPase is a regulator of various cellular
processes, including cell cycle, motility, invasion and cell-cell
adhesion (21). It has been known
to play a substantial role in the development of various cancers
including breast cancer and pancreatic cancer (22,23).
It is reported that the Rac-guanine nucleotide exchange factor
(GEF) P-Rex1 is an essential stimulator of Rac1 activation, and
P-Rex1 is activated by the phosphatidylinositide 3-kinases (PI3K)
pathway (24,25). Furthermore, Rac1 can be activated
through integrins, tyrosin-kinase receptors, and various stress
factors including mechanical stimulation, and the stress to the
endoplasmic reticulum (21). Since
salubrinal could relieve the stress to the endoplasmic reticulum,
there is a possibility that eIF2α-mediated Rac1 suppression is
linked to modulation of stress responses in 4T1 mammary tumor
cells. In inflammatory cartilage and chondrocytes, it is also
reported that the activity of Rac1 is reduced by treatment with
salubrinal (26). Further analysis
is necessary to identify the mechanistic role of eIF2α in
salubrinal-driven regulation of Rac1.
Since 4T1 and MDA-MB-231 cells are considered to be
triple negative, the action of salubrinal is indubitably different
from that of selective estrogen receptor modulators (SERMs) which
target the estrogen receptor. Tamoxifen, for instance, is thought
to act as an agonist at the bone and uterus and an antagonist at
the breast (9). Unlike salubrinal,
however, tamoxifen is not effective for estrogen receptor-negative
breast cancer cells. Regarding the involvement of Rac1 in response
to salubrinal, it is reported that inhibition of Rac1 using a
pharmacological agent (EHT1864) decreases estrogen receptor levels
and proliferation of both tamoxifen-sensitive and resistant cells
(27). Although the reported study
suggests that inhibition of Rac1 could be a therapeutic strategy
for estrogen receptor-positive cells, our study indicates that
salubrinal-driven reduction of Rac1 is also effective in
attenuating malignant phenotypes of estrogen receptor-negative
cells. In this context, MDA-MB-231 cells used in this study have
K-Ras mutation and are dependent on Rac1 for growth factor driven
invasion and migration (28).
Therefore, Rac1 activity may potentially serve as a biomarker of
response to salubrinal.
Approximately 20% of breast cancer patients are
likely to develop metastatic tumors in distant organs such as the
lungs, liver, brain and bone (29). In particular, bone is the most
common site for metastasis of breast cancer. The current
chemotherapy for the treatment of bone metastasis includes
administration of SERMs such as tamoxifen, bisphosphonates (e.g.,
zoledronate, pamidronate and alendronate), and anti-receptor
activator of nuclear factor κ-B ligand (RANKL) antibody (Denosumab)
(30,31). Bisphosphonates are the most
commonly used medication to reduce bone resorption, bone
destruction and tumor growth. However, it does not stimulate bone
formation and it often exhibits side effects such as joint
inflammation and avascular osteonecrosis of the jaw (32). Denosumab is reported to reduce bone
metastasis, but it effects on tumor growth have to be elucidated.
Salubrinal and guanabenz, which inhibit the de-phosphorylation of
eIF2α could both stimulate osteoblastgenesis through upregulation
of ATF4 and attenuate osteoclastogenesis through down-regulation of
nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1)
(33,34). Since salubrinal was shown to
stimulate the growth of new bone and enhance the healing of bone
wound, examining its effects on tumor growth would have a
significant impact on treatment of breast cancer and bone
metastasis.
In conclusion, we demonstrated that an inhibitory
agent of dephosphorylation of eIF2α potentially offers a novel
therapeutic strategy for attenuating malignant phenotypes of triple
negative breast cancer cells. It was shown to downregulate the
activity of Rac1 through eIF2α mediated signaling. It may prevent
not only tumor growth but also bone resorption associated with
metastasis to bone.
Acknowledgements
The authors thank H. Nakshatri for the
provision of MDA-MB-231 cells and a critical review of this study,
and M. Hamamura for technical support. This study was supported by
the Indiana University - Purdue University Indianapolis Research
Support Funds Grant and the Japan Society for the Promotion of
Science Core-to-Core Program, 23003.
References
|
1.
|
Ron D: Translational control in the
endoplasmic reticulum stress response. J Clin Invest.
110:1383–1388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hamamura K and Yokota H: Stress to
endoplasmic reticulum of mouse osteoblasts induces apoptosis and
transcriptional activation for bone remodeling. FEBS Lett.
581:1769–1774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hamamura K, Goldring MB and Yokota H:
Involvement of p38 MAPK in regulation of MMP13 mRNA in chondrocytes
in response to surviving stress to endoplasmic reticulum. Arch Oral
Biol. 54:279–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Boyce M, Bryant KF, Jousse C, et al: A
selective inhibitor of eIF2α dephosphorylation protects cells from
ER stress. Science. 307:935–939. 2005.
|
|
6.
|
Liu CL, Li X, Hu GL, et al: Salubrinal
protects against tunic-amycin and hypoxia induced cardiomyocyte
apoptosis via the PERK-eIF2α signaling pathway. J Geriatr Cardiol.
9:258–268. 2012.PubMed/NCBI
|
|
7.
|
Drexler HCA: Synergistic apoptosis
induction in leukemic cells by the phosphatase inhibitor salubrinal
and proteasome inhibitors. PLoS One. 4:e41612009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Koizumi M, Tanjung NG, Chen A, et al:
Administration of salubrinal enhances radiation induced cell death
of SW1353 chondrosarcoma cells. Anticancer Res. 32:3667–3674.
2012.PubMed/NCBI
|
|
9.
|
Osborne CK, Zhao H and Fuqua SA: Selective
estrogen receptor modulators: structure, function, and clinical
use. J Clin Oncol. 18:3172–3186. 2000.PubMed/NCBI
|
|
10.
|
De Laurentiis M, Cianniello D, Caputo R,
et al: Treatment of triple negative breast cancer (TNBC): current
options and future perspectives. Cancer Treat Rev. 36(Suppl 3):
S80–S86. 2010.
|
|
11.
|
Gluz O, Liedtke C, Gottschalk N, Pusztai
L, Nitz U and Harbeck N: Triple-negative breast cancer-current
status and future directions. Ann Oncol. 20:1913–1927. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bosch A, Eroles P, Zaragoza R, Vina JR and
Lluch A: Triple-negative breast cancer: molecular features,
pathogenesis, treatment and current lines of research. Cancer Treat
Rev. 36:206–215. 2010. View Article : Google Scholar
|
|
13.
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Prud’homme GJ, Glinka Y, Toulina A, Ace O,
Subramaniam V and Jothy S: Breast cancer stem-like cells are
inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS
One. 5:e138312010.PubMed/NCBI
|
|
15.
|
Bouquet F, Pal A, Pilones KA, et al: TGFβ1
inhibition increases the radiosensitivity of breast cancer cells in
vitro and promotes tumor control by radiation in vivo. Clin Cancer
Res. 17:6754–6765. 2011.
|
|
16.
|
Kaur P, Nagaraja GM, Zheng H, et al: A
mouse model for triple-negative breast cancer tumor-initiating
cells (TNBC-TICs) exhibits similar aggressive phenotype to the
human disease. BMC Cancer. 12:1202012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tsaytler P, Harding HP, Ron D and
Bertolotti A: Selective inhibition of a regulatory subunit of
protein phosphatase 1 restores proteostasis. Science. 332:91–94.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Niknejad N, Gorn-Hondermann I, Ma L, et
al: Lovastatin-induced apoptosis is mediated by activating
transcription factor 3 and enhanced in combination with salubrinal.
Int J Cancer. 134:268–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hamamura K, Furukawa K, Hayashi T, et al:
Ganglioside GD3 promotes cell growth and invasion through p130Cas
and paxillin in malignant melanoma cells. Proc Natl Acd Sci USA.
102:11041–11046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Shibuya H, Hamamura K, Hotta H, et al:
Enhancement of malignant properties of human osteosarcoma cells
with disialyl gangliosides GD2/GD3. Cancer Sci. 103:1656–1664.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sander EE and Collard JG: Rho-like
GTPases: Their role in epithelial cell-cell adhesion and invasion.
Eur J Cancer. 35:1302–1308. 1999. View Article : Google Scholar
|
|
22.
|
Wertheimer E, Gutierrez-Uzquiza A,
Rosemblit C, Lopez-Haber C, Sosa MS and Kazanietz MG: Rac signaling
in breast cancer: a tale of GEFs and GAPs. Cell Signal. 24:353–362.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Heid I, Lubeseder-Martellato C, Sipos B,
et al: Early requirement of Rac1 in a mouse model of pancreatic
cancer. Gastroenterology. 141:719–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Welch HCE, Coadwell WJ, Ellson CD,
Ferguson GJ, Andrews SR, Erdjument-Bromage H, et al: P-Rex1, a
Ptdlns (3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange
factor for Rac. Cell. 108:809–821. 2002.
|
|
25.
|
Yoshizawa M, Kawauchi T, Sone M, et al:
Involvement of a Rac activator, p-Rex1, in neurotrophin-derived
signaling and neuronal migration. J Neurosci. 25:4406–4419. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Shim JW, Hamamura K, Chen A, Wan Q, Na S
and Yokota H: Rac1 mediates load-driven attenuation of mRNA
expression of nerve growth factor beta in cartilage and
chondrocytes. J Musculoskelet Neuronal Interact. 13:372–379.
2013.PubMed/NCBI
|
|
27.
|
Rosenblatt AE, Garcia MI, Lyons L, et al:
Inhibition of the Rho GTPase, Rac1, decreases estrogen receptor
levels and is a novel therapeutic strategy in breast cancer. Endocr
Relat Cancer. 18:207–219. 2011.
|
|
28.
|
Yang Y, Du J, Hu Z, et al: Activation of
Rac1-PI3K/Akt is required for epidermal growth factor-induced PAK1
activfation and cell migration in MDA-MB-231 brest cancer cells. J
Biomed Res. 25:237–245. 2011. View Article : Google Scholar
|
|
29.
|
Eckhardt BL, Francis PA, Parker BS and
Anderson RL: Strategies for the discovery and development of
therapies for metastatic breast cancer. Nat Rev Drug Discov.
11:479–497. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Van Poznak CH, Temin S, Yee GC, et al:
American society of clinical oncology executive summary of the
clinical practice guideline update on the role of bone-modifying
agents in meta-static breast cancer. J Clin Oncol. 29:1221–1227.
2011.PubMed/NCBI
|
|
31.
|
Lee BL, Higgins MJ and Goss PE: Denosumab
and the current status of bone-modifying drugs in breast cancer.
Acta Oncol. 51:157–167. 2012. View Article : Google Scholar
|
|
32.
|
Marx RE: Pamidronate (Aredia) and
zoledronate (Zometa) induced avascular necrosis of the jaws: a
growing epidemic. J Oral Maxillofac Sug. 61:1115–1118. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
He L, Lee J, Jang JH, et al: Osteoporosis
regulation by salubrinal through eIF2α mediated differentiation of
osteoclast and osteoblast. Cell Signal. 25:552–560. 2013.
|
|
34.
|
Hamamura K, Tanjung N and Yokota H:
Suppression of osteoclastogenesis through phosphorylation of
eukaryotic translation initiation factor 2 alpha. J Bone Miner
Metab. 31:618–628. 2013. View Article : Google Scholar : PubMed/NCBI
|