Introduction
Ovarian cancer (OC) is the most deadly gynecological
malignancy, causing 140,200 estimated deaths worldwide every year
(1). The non-specific symptoms of
OCs, i.e., bloating and constipation, do not appear evident at the
early stages of the disease, and early detection of the disease is
also difficult, owing to the lack of specific and sensitive tests,
and 75% of diagnosed cases are at late stages (2,3). As
current therapeutic approaches result in a median overall survival
of only 2 to 4 years (3), and the
search for novel diagnostic biomarkers is still on the way, it is
of importance to deeply explore the pathogenesis and to screen
novel therapeutic targets.
The deregulated tumor growth brings tumor cells
located away from vessels under hypoxic microenvironment, which
drives cancer cells to undergo genetic and adaptive changes that
allow them to survive and even proliferate in a hypoxic environment
(4–6). Tumor hypoxia has become one of the
key issues in the study of tumor physiology and cancer treatment.
In recent years, a group of transcription factors has been reported
to be implicated in regulating a wide array of genes responsible
for the metabolic changes under hypoxia (7,8). A
pivotal component of these factors is hypoxia-inducible factor 1
(HIF-1), existing as a heterodimer composed of a constitutively
expressed HIF-1β subunit and an oxygen sensitive HIF-1α subunit.
The HIF-1α-HIF-1β dimer (9) binds
to a conserved DNA consensus on the promoters of its target genes
known as hypoxia-responsive element (10–12).
HIF induces a vast array of gene products controlling essential
cellular processes crucial for hypoxic adaptation (13). HIF-1 is a key regulator on the
vascular endothelial growth factor (VEGF) and other angiogenic
factors (14,15) which play key roles in the growth
and progression of solid tumors, including ovarian cancer (16–18).
The HIF system has also been directly implicated in the responses
of tumor cells to hypoxia (6,19).
Various cancers are characterized by enhanced HIF levels and
increased expression of hypoxia-regulated genes which correlate
both tumor aggression and patient outcome (19,20).
The role of HIF-1 has also been confirmed in upregulating ovarian
cancer invasiveness (21–23), and the HIF-1α overexpression is
associated with a poor prognosis in patients with ovarian cancer
(24). These reports suggest that
HIF-1 is important for the acquisition of aggressive behavior in
ovarian cancer cells. However, the mechanisms are not yet
clear.
MicroRNAs (miRNAs) are about 22-nt endogenous
non-coding RNAs that regulate gene expression (25) in a wide variety of organisms,
including humans, and in a broad array of cell processes in mammals
(26–28). miRNA expression signatures have
been shown to be promising biomarkers for understanding the
tumorgenesis of a wide array of human cancers (29,30),
including cervical cancer (31,32).
Recent years, numerous oncogenic microRNAs have been reported to be
associated with cervical cancer tumorigenesis. miR-214 regulates
ovarian cancer cell stemness by targeting p53/Nanog (33); miR-376c enhances ovarian cancer
cell survival by targeting activin receptor-like kinase 7 (34); and the downregulated miR-484 in
ovarian cancer attenuates its knockdown in vascular endothelial
growth factor β-subunit (VEGFβ) and stimulates tumor endothelial
cell growth and tumorigenesis (35). miR-210 is induced by hypoxia via
HIF-1α in normal cells, such as cardiomyocytes (36), keratinocyte (37) and other cells. It is significantly
upregulated in pathophysiological situlations, especially in cancer
cells, such as head and neck paragangliomas (38), lung cancer cells (39), and breast cancer cells (40). The upregulated miR-210 regulates
cancer proliferation, via its anti-apoptotic effect (41), modulating angiogenesis (42), and is not affected by the
regulation of miR-210 under hypoxia situation in ovarian cancer
cells, or the miR-210 on ovarian cancer.
In the present study, we determined the miR-210
expression in epithelial ovarian cancer (EOC) specimens, and in
ovarian cancer cell lines under hypoxia, and then determined in
detail the influence of miR-210 overexpression on the tumor cell
proliferation, and the possible mechanism of the miR-210 regulation
on the tumor growth.
Materials and methods
Human tissue specimens
Utilization of all EOC and normal tissue specimens
was approved by our hospital Internal Review Board (IRB) in Chinese
PLA General Hospital. Before treatment by radiotherapy or
chemotherapy, 48 human EOC tissue specimens were obtained by
surgical resection. Twenty-two normal human ovarian tissue
specimens were collected also by surgical resection. All tissue
specimens were stored at −70°C.
Cell culture and treatment with
reagents
The OVCAR-3 and SK-OV-3 EOC cell lines were provided
by the cell resource center of Chinese Academy of Medical Sciences
and were grown respectively in RPMI-1640 medium (Invitrogen,
Carlsbad, CA, USA) and L-15 medium (Gibco, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco, Rockville,
MD, USA), 50 μg/ml penicillin and 50 μg/ml
streptomycin. The cells were incubated at 37°C, with 5%
CO2. For hypoxia treatment, cells were placed in a
hypoxia incubator infused with a gas mixture of 5% CO2
and nitrogen to obtain 3% oxygen concentration. Oxygen
concentration was monitored continuously (Forma 3130; Thermo
Scientific, Rockford, IL, USA). siHIF-1α, siHIF-2α and siRNA
control oligos were synthesized by GenePharma Technology (Shanghai,
China) and were transfected into OVCAR-3 cells with at a
concentraction of 40 nM of lipofectamine 2000 to suppress the
HIF-1α or HIF-2α. miR-210 mimics (miR con as control) (Qiagen,
Valencia, CA, USA) and miR-210 inhibitor (Sigma-Aldrich, St. Louis,
MO, USA) were utilized to manipulate the miR-210 level. A total of
20 or 40 nM miR-210 mimics/miR con, or 10 nM miR-210 inhibitor was
transfected into EOC cells with lipofectamine 2000. 5-FU
(Sigma-Aldrich) was utilized to induce EOC apoptosis with a
concentration of 5 or 40 μg/ml.
RNA extraction and quantitative real-time
polymerase chain reaction (RT-qPCR)
Total mRNA was extracted from tissue or cell samples
by using the RNeasy mini kit (Qiagen). RT-qPCR analysis of the
HIF-1α, HIF-2α, CASP3 or CASP9 expression in mRNA level was
performed using SYBR-Green with the LightCycle 2.0 (Roche,
Mannheim, Germany). All mRNA expression levels were normalized to
GAPDH (glyceraldehyde-3-phosphate dehydrogenase). miRNA extraction
was performed using the mirVana miRNA isolation kit (Ambion,
Austin, TX, USA). Quantification of miR-210 expression was
conducted using the mirVana qRT-PCR miRNA detection kit (Ambion),
and the U6 small nuclear RNA was used as internal control. ΔΔCt
method was used for relative quantification (43). A non-radioactive northern blot
method, LED, for small RNA (about 15–40 bases) detection using
digoxigenin (DIG)-labeled oligonucleotide probes containing locked
nucleic acids (LNA) and 1-ethyl-3-(3-dimethylaminopropyl)
carbodiimide was utilized to confirm the miR-210 and U6 expression,
according to the protocol (44).
Protein isolation and western blot
analysis
Whole cell extracts were prepared with a cell lysis
reagent (Sigma-Aldrich) according to the manual; CASP3 or CASP9
were detected by western blot analysis using anti-CASP3, anti-CASP9
or anti-GAPDH rabbit polyclonal antibody (1:500; Sigma-Aldrich).
Goat anti-rabbit IgG conjugated to horseradish peroxidase (Pierce,
Rockford, IL, USA) and ECL detection systems (Super Signal West
Femto; Pierce) were used for detection.
Cell colony formation, cell proliferation
and cell apoptosis assay
For cell colony formation assay, 5×102
cells were incubated in 12-well plates at 37°C containing 5%
CO2, and were transfected with 0 or 40 nM miR-210 mimics
or miR-210 control, 5–8 days post incubation; the cells were
stained with crystal violet (0.005%) for 20 min and recorded the
colony numbers by imaging J software. For proliferation assay, post
transfecting with miR-210 mimics or miR-210 control, cells were
incubated in CCK-8 (Dojindo, Kumamoto, Japan). The 450 nm
absorbance of each well was detected after visual color occurrence
at 24, 48 or 72 h. OVCAR-3 or SK-OV-3 cell apoptosis was examined
with an Annexin V-FITC apoptosis detection kit (Sigma-Aldrich).
Briefly, 1–5×105 cells were stained with Annexin V-FITC
and propidium iodide and detected by a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) to analyze cellular
apoptosis. The results were calculated using CellQuest™ Pro
software (BD Biosciences) and expressed as the percentage of
apoptotic cells from the total cells.
Luciferase activity assay
For luciferase reporter experiments, according to
the prediction to interact with miR-210, the 3′-UTR or the mutated
3′-UTR of PTPN1 was amplified by PCR from human genomic DNA and
inserted into the MluI and HindIII sites of pGL3
vector immediately downstream from the stop codon of luciferase.
OVCAR-3 cells were cotransfected in 12-well plates with 0.4
μg of the firefly luciferase report vector and 0.1 μg
of the control vector containing Renilla luciferase, pRLTK
(Promega, Madison, WI, USA) to eliminate differences in cell number
and transfection efficiency, as well as with or without 40 nM of
miR-210 mimics or miR-con. At 24 h post-transfection, firefly and
Renilla luciferase activities were measured consecutively
using dualluciferase assays (Promega). Experiments were carried out
in triplicate and means were determined.
Statistical analysis
Statistical analyses were performed using SPSS16.0
software (IBM SPSS, Armonk, NY, USA). Correlations between miR-210
expression and the various clinical and laboratory data of patients
with HIF-1α were analyzed using the Spearman rank correlation. The
HIF-1α, HIF-2α, CASP3 or CASP9 expression in mRNA level, the CASP3
or CASP9 expression in protein level, or miR-210 expression between
two groups were analyzed by Student’s t-test. A p-value ≤0.05 was
considered statistically significant.
Results
miR-210 is overexpressed in epithelial
ovarian cancer specimens
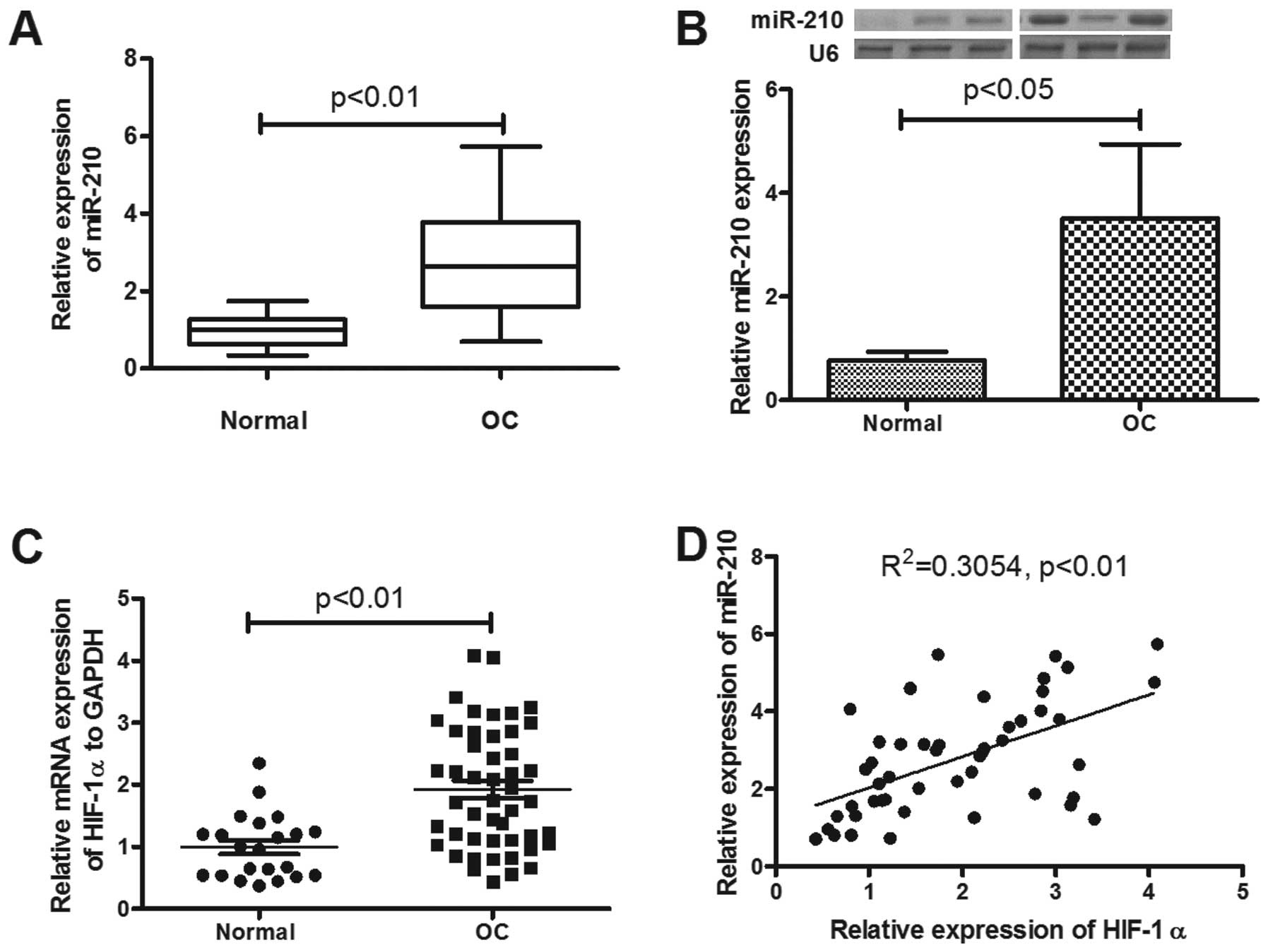
We detected the expression of miR-210 in epithelial
ovarian cancer tissues, compared to the normal ovarian tissues.
Forty-eight primary EOC patients with a mean (± SD) age of 57.2±7.1
years at diagnosis were included in the study. Of these, five were
FIGO stage I, 12 were stage II, 22 were stage III and seven were
stage IV. Ovarian tissue samples from 22 healthy age-matched
volunteers were used as controls. The mean (± SD) ΔΔCT value was
2.77±1.3 in the 48 samples from patients with EOC and 1.00±0.42 in
healthy controls (p<0.01; Fig.
1A). Thus, the miR-210 in ovarian tissue was significantly
upregulated in patients with EOC. The miR-210 overexpression was
correlated with the tumor stage or post-operative residual tumor
size (Table I; p<0.01 and
p<0.05, respectively). To reconfirm the significant miR-210
upregulation in EOC, the miRNA samples were examined by northern
blot analysis, and as shown in Fig.
1B the OC group was significantly higher than the normal group
(p<0.05).
 | Table I.Correlation of miR-210 expression
level in EOC patients with clinicopathologic parameters. |
Table I.
Correlation of miR-210 expression
level in EOC patients with clinicopathologic parameters.
| Parameters | n or mean ± SD | Relative miR-210
expression | p-value |
|---|
| No. of
patients | 48 | - | - |
| Age at first
diagnosis (years) | 57.2 (7.1) | - | - |
| Tumor stage | | | |
| FIGO I | 5 | 1.64 (0.52) | 0.01a |
| FIGO II | 12 | 2.53 (0.78) | |
| FIGO III | 22 | 2.90 (1.38) | |
| FIGO IV | 7 | 3.85 (1.66) | |
| Post-operative
residual tumor size (cm)b | | | |
| ≤2 | 25 | 2.12 (0.98) | 0.05c |
| >2 | 19 | 3.63 (1.42) | |
| Uncertain | 4 | | |
| Histological
grade | | | |
| 1 | 6 | 2.43 (0.93) | 0.3a |
| 2 | 17 | 2.68 (1.17) | |
| 3 | 25 | 2.91 (1.42) | |
| Histological
type | | | |
| Serous | 22 | 2.92 (1.24) | 0.2c |
|
Endometrioid | 5 | 2.98 (1.53) | |
| Mucinous | 8 | 2.25 (0.94) | |
| Clear cell | 4 | 1.9 (0.88) | |
| Other | 9 | 3.13 (1.67) | |
miR-210 expression correlates with a
hypoxic signature in EOC and is upregulated by hypoxia in
vitro
miR-210 is induced by hypoxia via HIF-1α (36,37).
To assess the hypoxic regulation of hsa-miR-210 in EOC specimens,
the HIF-1α mRNA expression in the EOC and normal specimens was also
determined by RT-qPCR. As shown in Fig. 1C, a significant high level of
HIF-1α mRNA was also confirmed (p<0.01). The correlation between
miR-210 and of HIF-1α mRNA expression was explored. In EOC
patients, miR-210 expression showed a positive correlation with
HIF-1α mRNA (R2=0.3054, p<0.01) (Fig. 1D). To reconfirm the correlation of
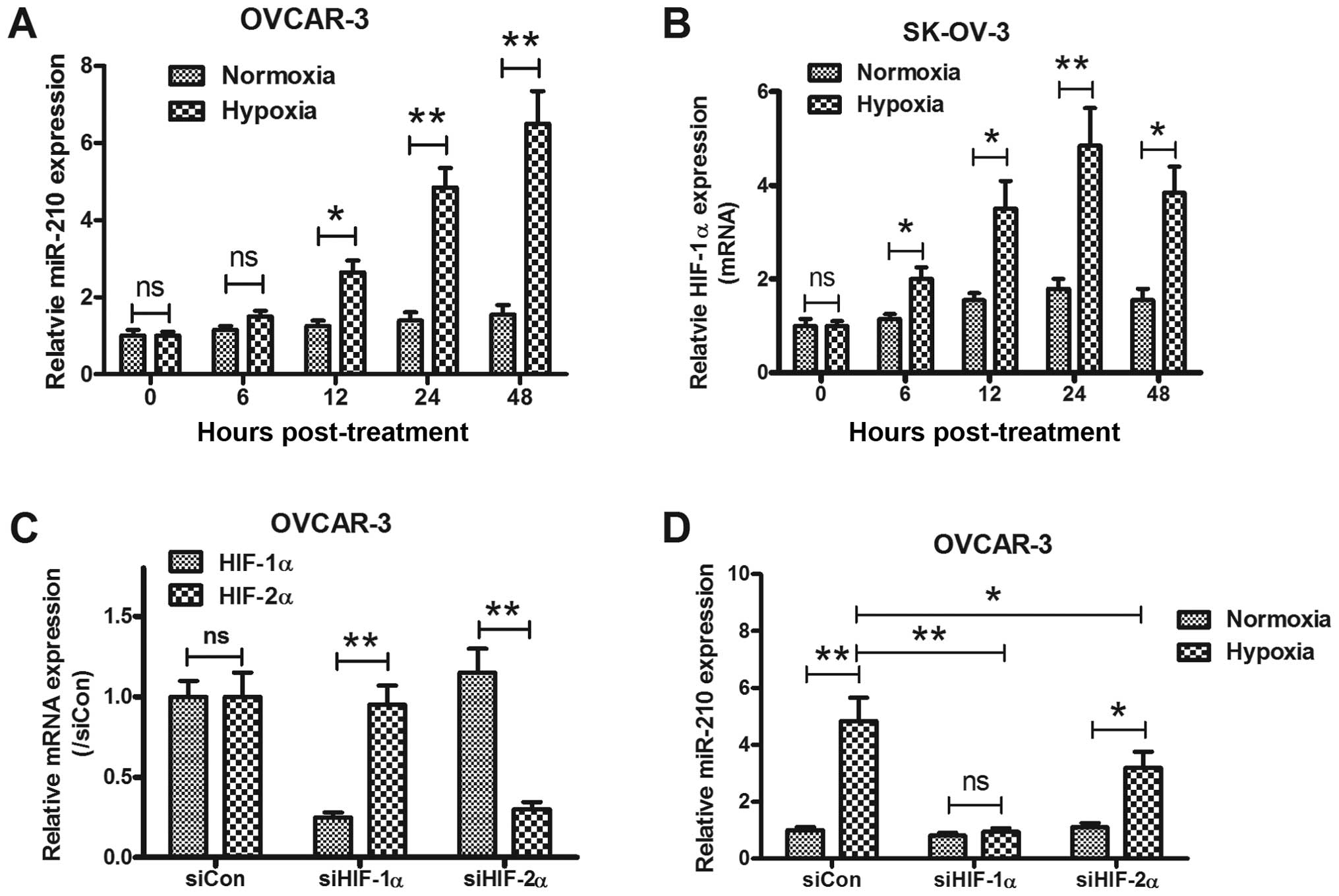
miR-210 expression with hypoxia in EOC, the miRNA samples from
OVCAR-3 and SK-OV-3 under normoxia or hypoxia, were analyzed using
quantitative-PCR (Q-PCR) too. In agreement with results of clinical
specimens, we found a substantial and significant induction of
miR-210 expression in two kinds of epithelial ovarian cancer cell
lines under hypoxia (Fig. 2A and
B). The in vitro results were also be reconfirmed by a
HIF knockdown experiment. Effective siRNAs targeting HIF-1α or
HIF-2α (Fig. 2C) abrogated the
miR-210 induction by hypoxia (p<0.01 and p<0.05,
respectively).
miR-210 upregulates the viability and
growth of ovarian cancer cells in vitro
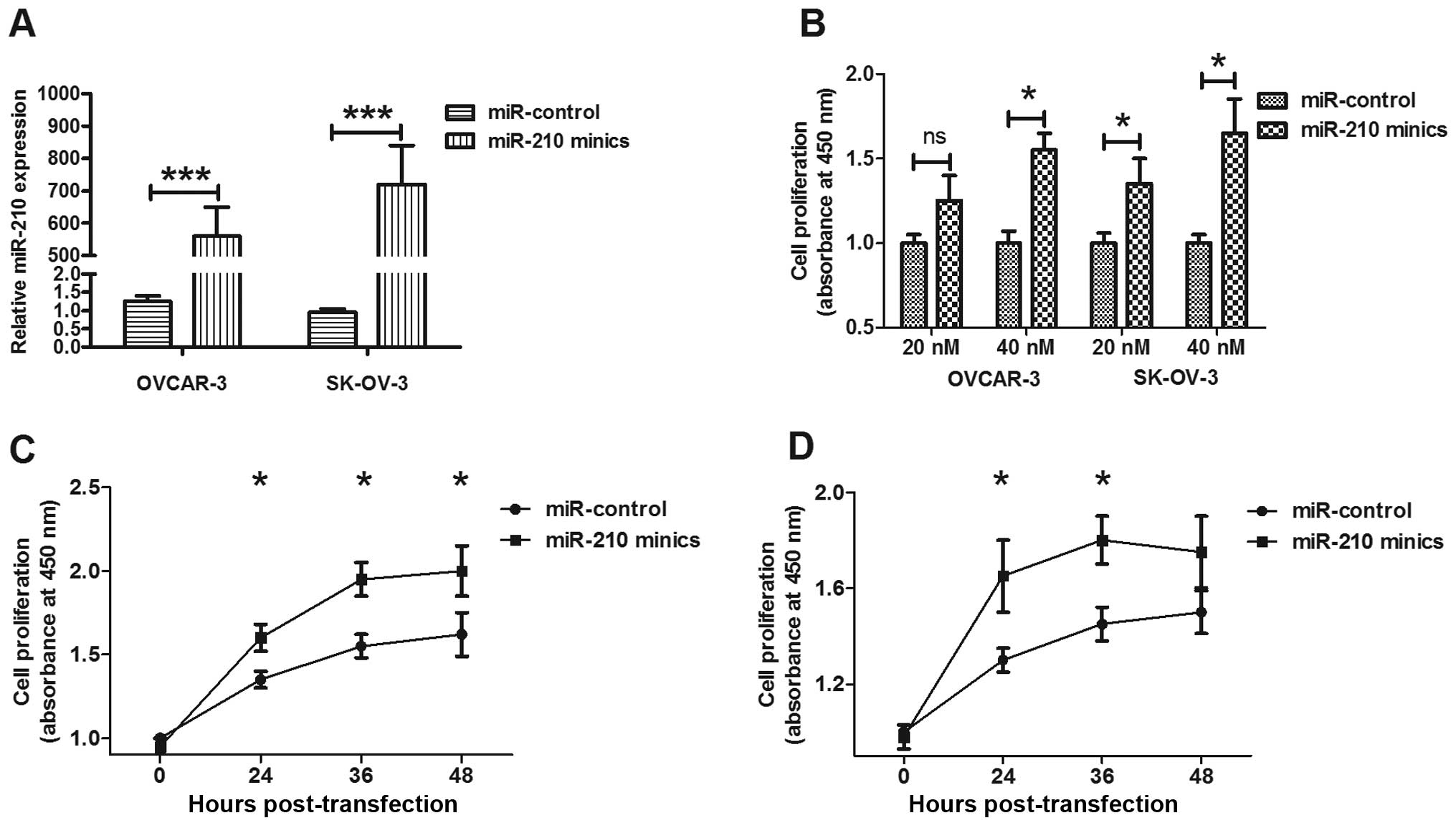
To determine the possible contribution of miR-210 to
EOC cell proliferation, we manipulated the miR-210 expression level
in OVCAR-3 and SK-OV-3 cell lines by transfecting with miR-210
mimics or miRNA control. The significant increase of miR-210 in
OVCAR-3 and SK-OV-3 cells post transfection with miR-210 mimics is
shown in Fig. 3A (both p<0.01).
Then, the proliferation of OVCAR-3 and SK-OV-3 cells post-miR-210
mimics or miRNA control was tested by CCK-8 assay. Both in OVCAR-3
and in SK-OV-3 cells, the miR-210 mimics promoted cell
proliferation rather than miRNA control time-dependently (Fig. 3B and C). We also detected the
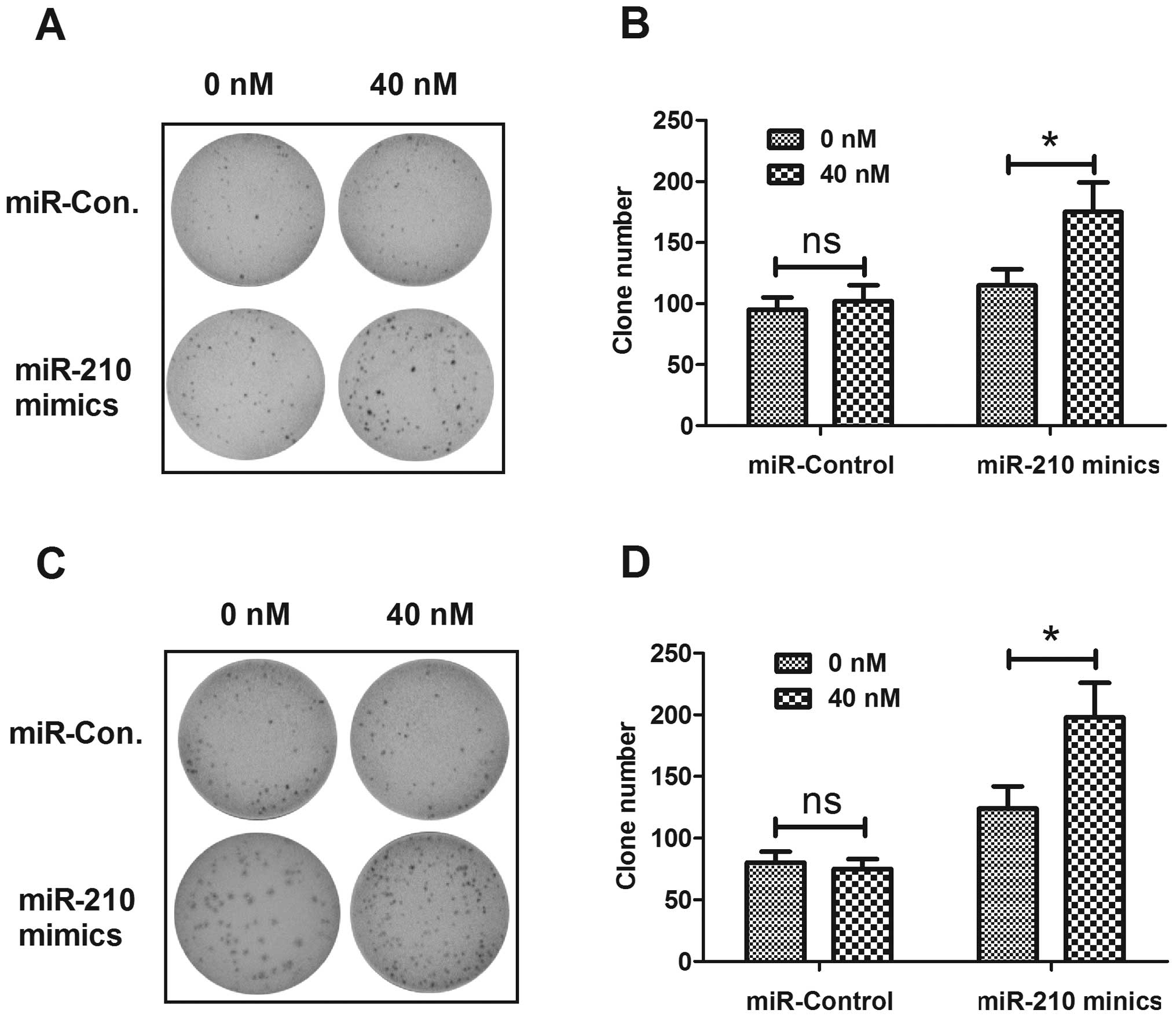
differences in colony formation of OVCAR-3 and SK-OV-3 cells
transfected with miR-210 mimics or miRNA control. Fig. 4 shows the higher capability of
colony formation for both cell lines post-transfection with miR-210
mimics than post-transfection with miRNA control. The above
findings demonstrated that upregulated miR-210 enhanced the
proliferative capability and colony formation of EOC cells in
vitro.
miR-210 ameliorates the hypoxia-induced
apoptosis in ovarian cancer cells in vitro
Hypoxia induces apoptosis via HIF-1α (45–47),
in various tumor cells, including ovarian cancer (48). The most direct induction of
hypoxia-induced apoptosis is the inhibition of the electron
transport chain at the inner membrane of the mitochondria. We
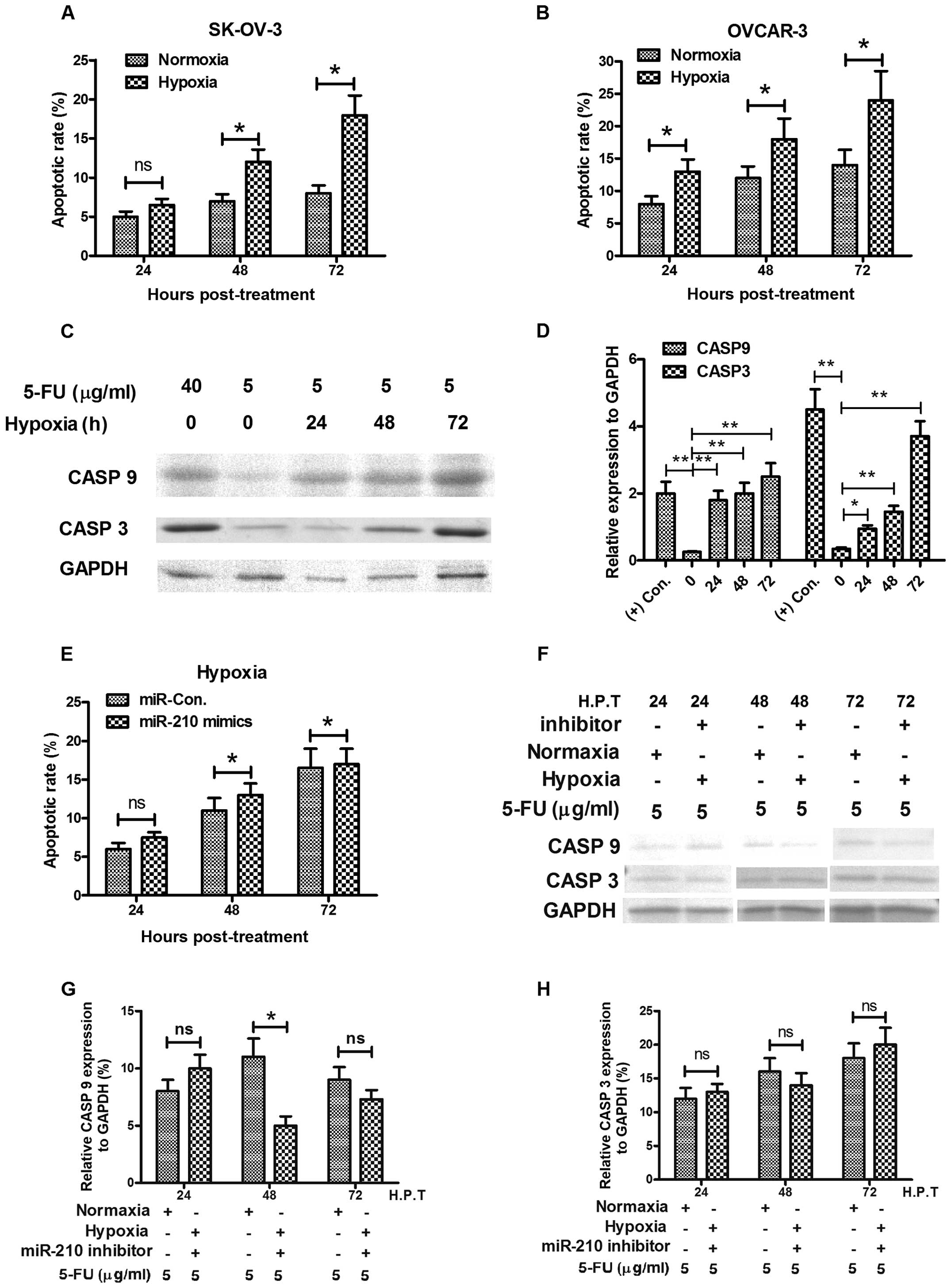
tested the sensitivity of OVCAR-3 or in SK-OV-3 ovarian cancer
cells to 5-FU. At normoxia culture conditions, the 5-FU induced low
level of apoptosis with a slight time-dependent increase, while
hypoxia costimulates higher level of apoptosis with 5-FU in both
cell types (Fig. 5A and B). We
also tested in OVCAR-3 cells, the expression of caspase 3 (CASP3)
and caspase 9 (CASP9), both of which are executional molecules in
apoptosis. The western blot analysis results demonstrated that
significantly high levels of CASP3 and CASP9 were induced by a low
dose of 5-FU under hypoxic culture condition rather than in
normoxia (Fig. 5C and D). However,
the hypoxia-induced apoptosis could be reversed by miR-210
inhibitor, the increase in apoptotic rate and the expression level
of CASP3 or CASP9 were blocked by the miR-210 inhibitor transfecion
(Fig. 5E–H).
PTPN1 is downregulated during the
miR-210-mediated anti-apoptosis
To unveil the anti-apoptosis effect of miR-210, we
predicted the possible targets of miR-210 by miRanda. PTPN1 is one
of the screened targets, and has not been determined to play roles
in ovarian cancer, though it is well known to induce apoptosis
(49–52). To evaluate the possible targeting
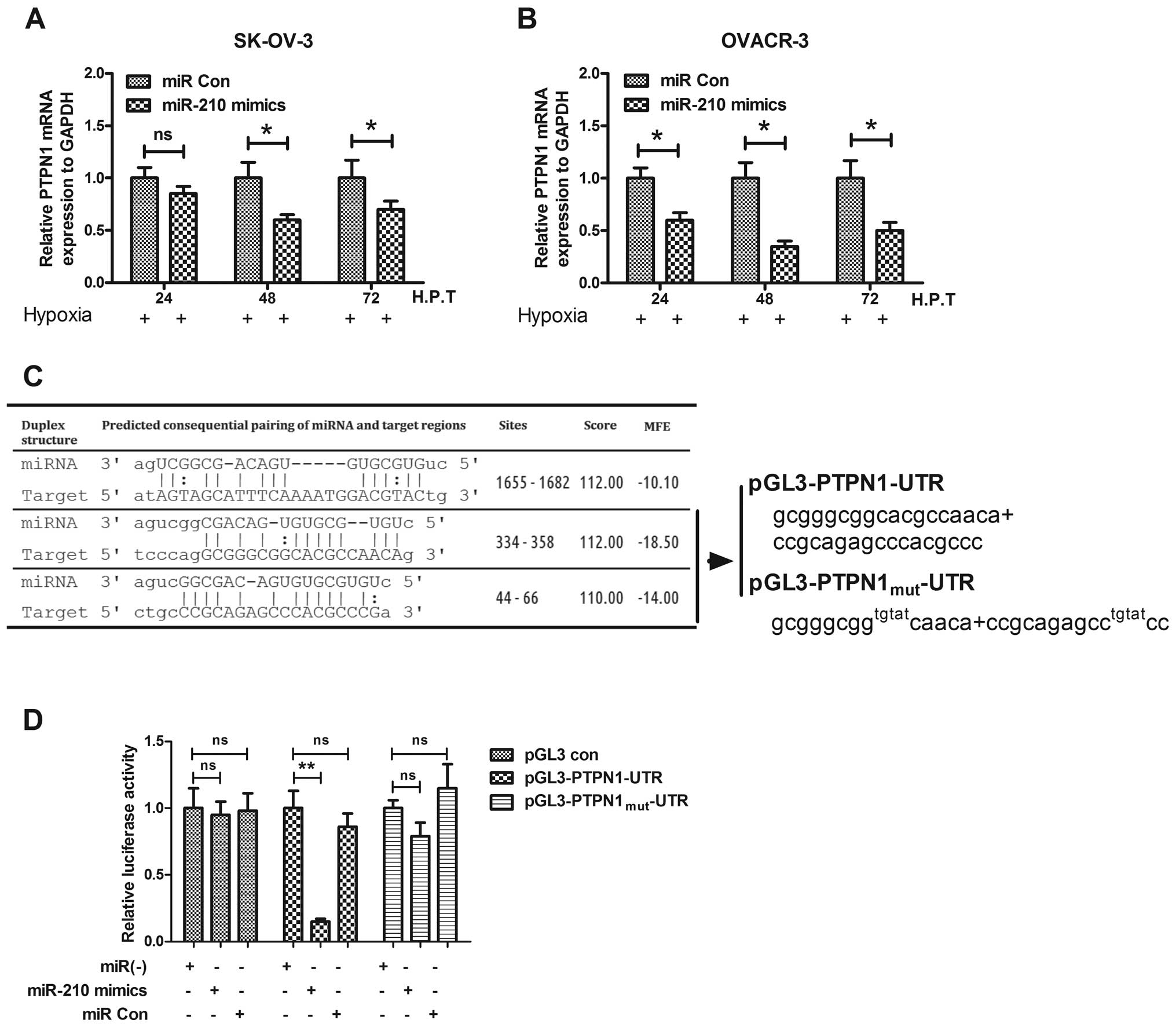
of PTPN1 by miR-210 in ovarian cancer cells, the PTPN1 expression
in mRNA and protein levels was determined with the miR-210 mimics
or miRNA control transfection in OVCAR-3 or SK-OV-3 cells. It was
shown that the proapoptotic protein was downregulated after
transfection with miR-210 mimics while there was no effect for the
control transfection (Fig. 6A and
B). Fig. 6C shows that PTPN1
had a consequential pairing with the miR-210. To reconfirm the
PTPN1 downregulation by miR-210, we construct a luciferase reporter
constructs containing the miR-210 recognition sequence of PTPN1
mRNA (Fig. 6C). As shown in
Fig. 6D, transfection with the
miR-210 mimics suppressed, while the miRNA control did not regulate
the activity of pGL3-PTPN1 reporter in OVCAR-3 cells; and the
miR-210 mimics had no suppression effect on the luciferase activity
of pGL3-PTPN1mut reporter. These results agree with the
fact that miR-210 regulates PTPN1 by targeting the 3′-UTR and
inducing translation repression of the gene. Therefore, suppression
of the particular gene contributes to the improvement of ovarian
cancer by inhibiting apoptosis of ovarian cancer cells under
hypoxia.
Discussion
In the present study, we showed that expression of
miR-210 is upregulated with an association to HIF-1α overexpression
in clinical EOC specimens as well as EOC cell lines. The
upregulated miR-210 in EOC specimens correlated with tumor stage
and the post-operative residual tumor size. Results also
demonstrated that the upregulated miR-210 promoted tumor cell
proliferation and clone generation in vitro via targeting
PTPN1 and inhibiting apoptosis. Therefore, our results indicate
that the level of miR-210 expression may serve as a clinical maker
for the degree of tumor growth in vivo. The deregulated
solid tumor growth brings a hypoxic microenvironment, which drives
tumor cells to undergo genetic and adaptive changes that allow them
to adapt to the hypoxia in their milieu. The present study provides
evidence that miR-210 is involved in a key hypoxia responsive
network in ovarian cancer. In order to expand our understanding of
the regulatory networks involved in hypoxia response, we determined
the possible miR-210 target PTPN1 effect of the blockage. The in
vitro results in EOC cell lines showed manipulated
over-expression of miR-210 significantly silenced the PTPN1, and
blocked its proapoptotic effect. Therefore, our finding add to the
knowledge on the mechanism of ovarian cancer adaptation to
hypoxia.
Considering the slight response to chemotherapy of
EOC, we have attempted to identify novel biomarkers for therapeutic
response and molecular targets to increase sensitivity to
treatment. miRs, a class of gene regulators, have been proven by
accumulated evidence to be effective in regulating tumorigenesis,
tumor growth, migration and even metastasis (53). Data on ovarian cancer thus far
indicate that the miR network is very important to the
understanding of ovarian cancer biology and resistance to therapy
(54,55). The hypoxia microenvironment posing
for almost all tumors has become one of the key issues in the study
of tumor physiology, and focused our attention on miR-210, which
has been proved upregulated in various cancers (38–40).
Giannakakis et al confirmed the miR-210 upregulation in
ovarian cancer lines under hypoxia condition in vitro but
surprisingly not in clinical ovarian cancer specimens (56). Further determination by them showed
that there was a remarkably high frequency of miR-210 gene copy
deletions in ovarian cancer patients. However, in the present
study, we found there was a miR-210 upregulation in Chinese EOC
specimens by both test methods. Interestingly, the miR-210
upregulation correlated with a higher tumor stage or larger
post-operative residual tumor size, the miR-210 expression was not
significantly upregulated in the specimens of cancer with lower
stage or postoperative residual tumor size. Therefore, the
hypoxia-induced miR-210 upregulation might correlate with other
parameters and needs to be further determined.
miR-210 has been strongly linked with the hypoxia
condition and is upregulated by hypoxia-inducible factors (57). It is also overexpressed in cells
affected by cardiac disease and tumors (58). miR-210 in particular, has been well
studied for its effects in rescuing cardiac function after
myocardial infarcts via the upregulation of angiogenesis and
inhibition of cardiomyocyte apoptosis (59). Accumulated data show that it is
significantly upregulated in various kinds of tumors, such as head
and neck paragangliomas (38),
lung cancer cells (39) and breast
cancer cells (40). The
upregulated miR-210 regulates cancer proliferation, via its
anti-apoptotic effect (41),
modulating angiogenesis (60). The
anti-apoptotic effect of miR-210 was achieved by inhibiting the
target genes, such as EFNA3 (61),
ISCU (62), E2F3 (63) and FGFRL1 (57). More recently, it was shown that
miR-210 could target the tyrosine-protein phosphatase non-receptor
type 1 (PTPN1), also known as protein tyrosine phosphatase-1B
(PTP1B) protein and regulates the susceptibility of tumor cells to
lysis by cytotoxic T cells (64).
The PTPN1 is known to induce apoptosis through the downregulation
of pro-survival RTK signaling (49,50),
the enhancement of ER stress signaling (51), or the facilitation of caspase 8/9
activities (52). However, up to
now, little was known of the regulation of miR-210 under hypoxia
situation in ovarian cancer cells and about actions of miR-210 on
ovarian cancer modulating. It is not clear whether miR-210 targets
PTPN1 and inhibits apoptosis in ovarian cancer or other tumor
cells. The present study firstly demonstrated that the upregulated
miR-210 in EOCs promoted tumor cell proliferation and clone
generation in vitro via targeting PTPN1 and inhibiting
apoptosis. It implied that the EOCs with a higher level of miR-210
may be more aggresive in vivo.
In summary, miR-210 expression is upregulated, in
response to a hypoxia condition, and with an association to HIF-1α
overexpression in clinical EOC specimens as well as EOC cell lines.
The upregulated miR-210 promoted the tumor growth in vitro
via targeting PTPN1 and inhibiting apoptosis. Therefore, our
finding provide new information on the mechanism of ovarian cancer
adaptation to hypoxia.
Acknowledgements
This study was supported by a grant
from Chinese PLA General Hospital.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Colombo N, Van Gorp T, Parma G, et al:
Ovarian cancer. Crit Rev Oncol Hematol. 60:159–179. 2006.
View Article : Google Scholar
|
|
3.
|
Moss C and Kaye SB: Ovarian cancer:
progress and continuing controversies in management. Eur J Cancer.
38:1701–1707. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hockel M and Vaupel P: Tumor hypoxia:
definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Dang CV and Semenza GL: Oncogenic
alterations of metabolism. Trends Biochem Sci. 24:68–72. 1999.
View Article : Google Scholar
|
|
6.
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cummins EP and Taylor CT:
Hypoxia-responsive transcription factors. Pflugers Arch.
450:363–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Licausi F, Weits DA, Pant BD, Scheible WR,
Geigenberger P and van Dongen JT: Hypoxia responsive gene
expression is mediated by various subsets of transcription factors
and miRNAs that are determined by the actual oxygen availability.
New Phytol. 190:442–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
10.
|
Christofk HR, Vander Heiden MG, Harris MH,
et al: The M2 splice isoform of pyruvate kinase is important for
cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Pouyssegur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: the central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Blancher C, Moore JW, Talks KL, Houlbrook
S and Harris AL: Relationship of hypoxia-inducible factor
(HIF)-1alpha and HIF-2alpha expression to vascular endothelial
growth factor induction and hypoxia survival in human breast cancer
cell lines. Cancer Res. 60:7106–7113. 2000.
|
|
15.
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
16.
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Pralhad T, Madhusudan S and Rajendrakumar
K: Concept, mechanisms and therapeutics of angiogenesis in cancer
and other diseases. J Pharm Pharmacol. 55:1045–1053. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Semenza GL: HIF-1 and tumor progression:
pathophysiology and therapeutics. Trends Mol Med. 8:S62–S67. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chi JT, Wang Z, Nuyten DS, et al: Gene
expression programs in response to hypoxia: cell type specificity
and prognostic significance in human cancers. PLoS Med. 3:e472006.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Imai T, Horiuchi A, Wang C, et al: Hypoxia
attenuates the expression of E-cadherin via up-regulation of SNAIL
in ovarian carcinoma cells. Am J Pathol. 163:1437–1447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Horiuchi A, Imai T, Shimizu M, et al:
Hypoxia-induced changes in the expression of VEGF, HIF-1 alpha and
cell cycle-related molecules in ovarian cancer cells. Anticancer
Res. 22:2697–2702. 2002.PubMed/NCBI
|
|
23.
|
Horiuchi A, Hayashi T, Kikuchi N, et al:
Hypoxia upregulates ovarian cancer invasiveness via the binding of
HIF-1alpha to a hypoxia-induced, methylation-free hypoxia response
element of S100A4 gene. Int J Cancer. 131:1755–1767. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Osada R, Horiuchi A, Kikuchi N, et al:
Expression of hypoxia-inducible factor 1alpha, hypoxia-inducible
factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian
neoplasms and allelic loss of von Hippel-Lindau gene: nuclear
expression of hypoxia-inducible factor 1alpha is an independent
prognostic factor in ovarian carcinoma. Hum Pathol. 38:1310–1320.
2007.
|
|
25.
|
Ambros V: MicroRNA pathways in flies and
worms: growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: Bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Reinhart BJ, Slack FJ, Basson M, et al:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Jay C, Nemunaitis J, Chen P, Fulgham P and
Tong AW: miRNA profiling for diagnosis and prognosis of human
cancer. DNA Cell Biol. 26:293–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yu SL, Chen HY, Yang PC and Chen JJ:
Unique microRNA signature and clinical outcome of cancers. DNA Cell
Biology. 26:283–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Qiang R, Wang F, Shi LY, et al: Plexin-B1
is a target of miR-214 in cervical cancer and promotes the growth
and invasion of HeLa cells. Int J Biochem Cell Biol. 43:632–641.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Au Yeung CL, Tsang TY, Yau PL and Kwok TT:
Human papillomavirus type 16 E6 induces cervical cancer cell
migration through the p53/microRNA-23b/urokinase-type plasminogen
activator pathway. Oncogene. 30:2401–2410. 2011.
|
|
33.
|
Xu CX, Xu M, Tan L, et al: MicroRNA
miR-214 regulates ovarian cancer cell stemness by targeting
p53/Nanog. J Biol Chem. 287:34970–34978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Ye G, Fu G, Cui S, et al: MicroRNA 376c
enhances ovarian cancer cell survival by targeting activin
receptor-like kinase 7: implications for chemoresistance. J Cell
Sci. 124:359–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Vecchione A, Belletti B, Lovat F, et al: A
microRNA signature defines chemoresistance in ovarian cancer
through modulation of angiogenesis. Proc Natl Acad Sci USA.
110:9845–9850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Mutharasan RK, Nagpal V, Ichikawa Y and
Ardehali H: microRNA-210 is upregulated in hypoxic cardiomyocytes
through Akt- and p53-dependent pathways and exerts cyto-protective
effects. Am J Physiol Heart Circ Physiol. 301:H1519–H1530. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Biswas S, Roy S, Banerjee J, et al:
Hypoxia inducible microRNA 210 attenuates keratinocyte
proliferation and impairs closure in a murine model of ischemic
wounds. Proc Natl Acad Sci USA. 107:6976–6981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Merlo A, de Quiros SB, Secades P, et al:
Identification of a signaling axis HIF-1alpha/microRNA-210/ISCU
independent of SDH mutation that defines a subgroup of head and
neck paragangliomas. J Clin Endocrinol Metab. 97:E2194–E2200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Wang H, Bian S and Yang CS: Green tea
polyphenol EGCG suppresses lung cancer cell growth through
upregulating miR-210 expression caused by stabilizing HIF-1alpha.
Carcinogenesis. 32:1881–1889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Camps C, Buffa FM, Colella S, et al:
hsa-miR-210 is induced by hypoxia and is an independent prognostic
factor in breast cancer. Clin Cancer Res. 14:1340–1348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Gou D, Ramchandran R, Peng X, et al:
miR-210 has an anti-apoptotic effect in pulmonary artery smooth
muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol.
303:L682–L691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Fasanaro P, D’Alessandra Y, Di Stefano V,
et al: MicroRNA-210 modulates endothelial cell response to hypoxia
and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
44.
|
Kim SW, Li Z, Moore PS, et al: A sensitive
non-radioactive northern blot method to detect small RNAs. Nucleic
Acids Res. 38:e982010. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Greijer AE and van der Wall E: The role of
hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J
Clin Pathol. 57:1009–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Carmeliet P, Dor Y, Herbert JM, et al:
Role of HIF-1alpha in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Moritz W, Meier F, Stroka DM, et al:
Apoptosis in hypoxic human pancreatic islets correlates with
HIF-1alpha expression. FASEB J. 16:745–747. 2002.PubMed/NCBI
|
|
48.
|
Zhu P, Ning Y, Yao L, Chen M and Xu C: The
proliferation, apoptosis, invasion of endothelial-like epithelial
ovarian cancer cells induced by hypoxia. J Exp Clin Cancer Res.
29:1242010. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Sangwan V, Paliouras GN, Cheng A, Dube N,
Tremblay ML and Park M: Protein-tyrosine phosphatase 1B deficiency
protects against Fas-induced hepatic failure. J Biol Chem.
281:221–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Gonzalez-Rodriguez A, Escribano O, Alba J,
Rondinone CM, Benito M and Valverde AM: Levels of protein tyrosine
phosphatase 1B determine susceptibility to apoptosis in
serum-deprived hepatocytes. J Cell Physiol. 212:76–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Gu F, Nguyen DT, Stuible M, Dube N,
Tremblay ML and Chevet E: Protein-tyrosine phosphatase 1B
potentiates IRE1 signaling during endoplasmic reticulum stress. J
Biol Chem. 279:49689–49693. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Akasaki Y, Liu G, Matundan HH, et al: A
peroxisome proliferator-activated receptor-gamma agonist,
troglitazone, facilitates caspase-8 and -9 activities by increasing
the enzymatic activity of protein-tyrosine phosphatase-1B on human
glioma cells. J Biol Chem. 281:6165–6174. 2006. View Article : Google Scholar
|
|
53.
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Dahiya N and Morin PJ: MicroRNAs in
ovarian carcinomas. Endocr Relat Cancer. 17:F77–F89. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Shih KK, Qin LX, Tanner EJ, et al: A
microRNA survival signature (MiSS) for advanced ovarian cancer.
Gynecol Oncol. 121:444–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Giannakakis A, Sandaltzopoulos R, Greshock
J, et al: miR-210 links hypoxia with cell cycle regulation and is
deleted in human epithelial ovarian cancer. Cancer Biol Ther.
7:255–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Tsuchiya S, Fujiwara T, Sato F, et al:
MicroRNA-210 regulates cancer cell proliferation through targeting
fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem.
286:420–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Li T, Cao H, Zhuang J, et al:
Identification of miR-130a, miR-27b and miR-210 as serum biomarkers
for atherosclerosis obliterans. Clin Chim Acta. 412:66–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Puissegur MP, Mazure NM, Bertero T, et al:
miR-210 is overexpressed in late stages of lung cancer and mediates
mitochondrial alterations associated with modulation of HIF-1
activity. Cell Death Differ. 18:465–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Ramon LA, Braza-Boils A, Gilabert J, et
al: microRNAs related to angiogenesis are dysregulated in
endometrioid endometrial cancer. Hum Reprod. 27:3036–3045. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Chen WY, Liu WJ, Zhao YP, et al:
Induction, modulation and potential targets of miR-210 in
pancreatic cancer cells. Hepatobiliary Pancreat Dis Int.
11:319–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Favaro E, Ramachandran A, McCormick R, et
al: MicroRNA-210 regulates mitochondrial free radical response to
hypoxia and krebs cycle in cancer cells by targeting iron sulfur
cluster protein ISCU. PLoS One. 5:e103452010. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Nakada C, Tsukamoto Y, Matsuura K, et al:
Overexpression of miR-210, a downstream target of HIF1alpha, causes
centrosome amplification in renal carcinoma cells. J Pathol.
224:280–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Noman MZ, Buart S, Romero P, et al:
Hypoxia-inducible miR-210 regulates the susceptibility of tumor
cells to lysis by cytotoxic T cells. Cancer Res. 72:4629–4641.
2012. View Article : Google Scholar : PubMed/NCBI
|