Introduction
Esophageal squamous carcinoma (ESC) is the sixth
most common cancer among males and the ninth most common cancer
among females worldwide (1,2).
Many environmental influences and genetic factors have been
implicated in ESC development. Because of the high rate of
metastasis and invasion of surrounding tissues in the early stages
of ESC, the overall 5-year survival rate of this cancer is less
than 14% (1,3). Although ESC arises from the
alteration of gene function, the molecular events required for
tumorigenesis have not been clearly elucidated.
p63, a member of the p53 family, plays a role in the
processes of cell proliferation, survival and differentiation
(4). p63 is a transcription factor
that is known to be a key regulator of epidermis development and
epithelial cell differentiation (4,5). In
the esophagus, p63 plays an important role in differentiation and
morphogenesis (4). The roles of
p63 in the formation of epithelial structures during development
have been reported in several studies (6–8); for
example, p63 knockout mice exhibit striking developmental defects
including abnormal formation of stratified epithelium of the
esophagus and skin (6–8). In particular, the esophageal lining
of these mice shows a pseudo-stratified columnar appearance that
indicates a critical role for p63 in the regulation of esophagus
formation (7). Since the human p63
gene was cloned, two isoforms of p63 that either contain an
N-terminal transactivation domain (TAp63) or lack this domain
(ΔNp63) have been identified (4,9).
ΔNp63 is overexpressed in a variety of human cancers, including
head and neck, lung, breast, esophagus and bladder tumors (10,11).
The TAp63 and ΔNp63 isoforms perform different functions. It is
clear that the ΔNp63 isoform promotes survival of cells and the
TAp63 isoform induces cell death; however, the function of p63 in
esophageal cancer is still controversial.
The PI3K/Akt pathway is a key regulator of
differentiation in a number of cell types including esophageal
cells (12). Activation of Akt has
been found in a variety of types of cancer including esophageal
squamous carcinoma (13–15). However, there is limited
information on the relationship between p63 and Akt in cancer
progression, and especially on its role in ESC. In this study, we
examined whether p63 regulates the Akt pathway during ESC
progression. We show that the two different p63 isoforms are
expressed at variable levels in esophageal cancer cells and provide
evidence that p63 promotes ESC cell growth through regulation of
the cell cycle and Akt signaling pathway.
Materials and methods
Cell culture and reagents
Human esophageal cancer cell lines (TE-8, TE-12, BE3
and OE33) were obtained from the University of Texas M.D. Anderson
Cancer Center (Houston, TX, USA). p63 siRNA, GAPDH siRNA, and the
negative control non-target siRNA vector were obtained from
Dharmacon Thermo Scientific (Walthan, MA, USA). Oligofectamin and
Opti-MEM were obtained from Invitrogen Life Technologies (Carlsbad,
CA, USA). The esophageal cancer cell lines (TE-8, TE-12, BE3 and
OE33) were maintained in DMEM/F12 medium (Gibco Life Technologies,
Carlsbad, CA, USA) containing 10% heat-inactivated fetal bovine
serum (FBS, Gibco Life Technologies) with 100 μg/ml
penicillin and 100 μg/ml streptomycin. The cells were
cultured in a humidified atmosphere containing 5% CO2 at
37°C. Antibodies against p63, ΔNp63, TAp63, p53, p27, cyclin D1,
cyclin E1, Akt, p-Akt and GAPDH were purchased from Cell Signaling
Technology (Danvers, MA, USA).
Knockdown of endogenous p63 in TE-8 and
TE-12 cell lines
TE-8 and TE-12 cells were transfected with 100 or
200 nM of p63 siRNA in 175 μl Opti-MEM. Transfection was
carried out using 4 μl oligofectamin reagent according to
the manufacturer’s protocol.
p63 overexpression in BE3 and OE33 cell
lines
For the preparation of retroviral supernatants,
1×106 293T cells were seeded into 60-mm dishes and
transient transfections were performed with Fugene-6 (Roche, CA,
USA) following the manufacturer’s recommendations. Briefly, 12
μl of Fugene-6 was mixed with 188 μl of DMEM and
incubated at room temperature for 5 min. For retroviral
overexpression of p63 in BE3 and OE33 cell lines, Mig-DNA or
Mig-p63 vector (2 μg), and the packaging vectors pVMUC (1.8
μg), and pMD2G (0.2 μg), respectively, were mixed
with DMEM and Fugene-6. GFP-positive cells with overexpression of
p63 were sorted by flow cytometry.
MTT assay for cell proliferation
The cell proliferation assay was performed as
previously described (16).
Briefly, TE-8 and TE-12 cells were plated at a density of
5×104 cells/well in 96-well plates and maintained for 24
h at 37°C with 5% CO2. At the end of siRNA transfection,
50 μl of MTT
[3-(4,5-dimethylthiazol-2,5-diphenyl)tetrazolium bromide; 2 mg/ml]
was added to the culture medium of growing cells at the indicated
time points and the cells were incubated for a further 3 h.
Dimethylsufoxide (200 μl/well) was added and absorbance was
measured at 570 nm using a model Epoch microplate reader (Bio-Tech,
CA, USA).
Soft agar colony formation assay
The soft agar colony formation assay has been
described previously (17).
Briefly, control and p63 siRNA-transfected TE-8 and TE-12 cells
(5×104 cells) in 2 ml 0.7% agar with DMEM/F-12 were
plated on top of 1% bottom agar in 6-well plates. The covering
medium was replaced every 3 days. The cells were incubated at 37°C
in a humidified 5% CO2 atmosphere for 2 weeks. Colonies
were counted under a microscope and photographed.
Western blot analysis
The expression of cell cycle proteins in esophageal
cancer cells was measured by western blotting. Cells were seeded in
a 6-well plate at a density of 2×105 cells/well. Cells
were washed with PBS and harvested, and the cell pellets were lysed
in ice-cold PRO-PREP™ (Intron Biotechnology, Korea). Proteins were
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to PVDF membranes (GE
Healthcare Life Sciences, Buckinghamshire, UK). Immunoblotting was
performed with primary antibodies against p63, ΔNp63, TAp63, cyclin
D1, cyclin E1, p53, p27, Akt and p-Akt. Immunoreactivity was
detected using a chemiluminescence kit (Amersham, Arlington
Heights, IL, USA).
Statistical analysis
The data were expressed as mean ± SE for MTT assay
and soft agar colony formation assay. Comparisons among the
experimental groups were performed using two-way ANOVA with the
Student’s t-test. P-values <0.05 were considered statistically
significant.
Results
Effect of p63 on survival and
proliferation of esophageal cancer cells
To investigate the role of p63 in ESC cell
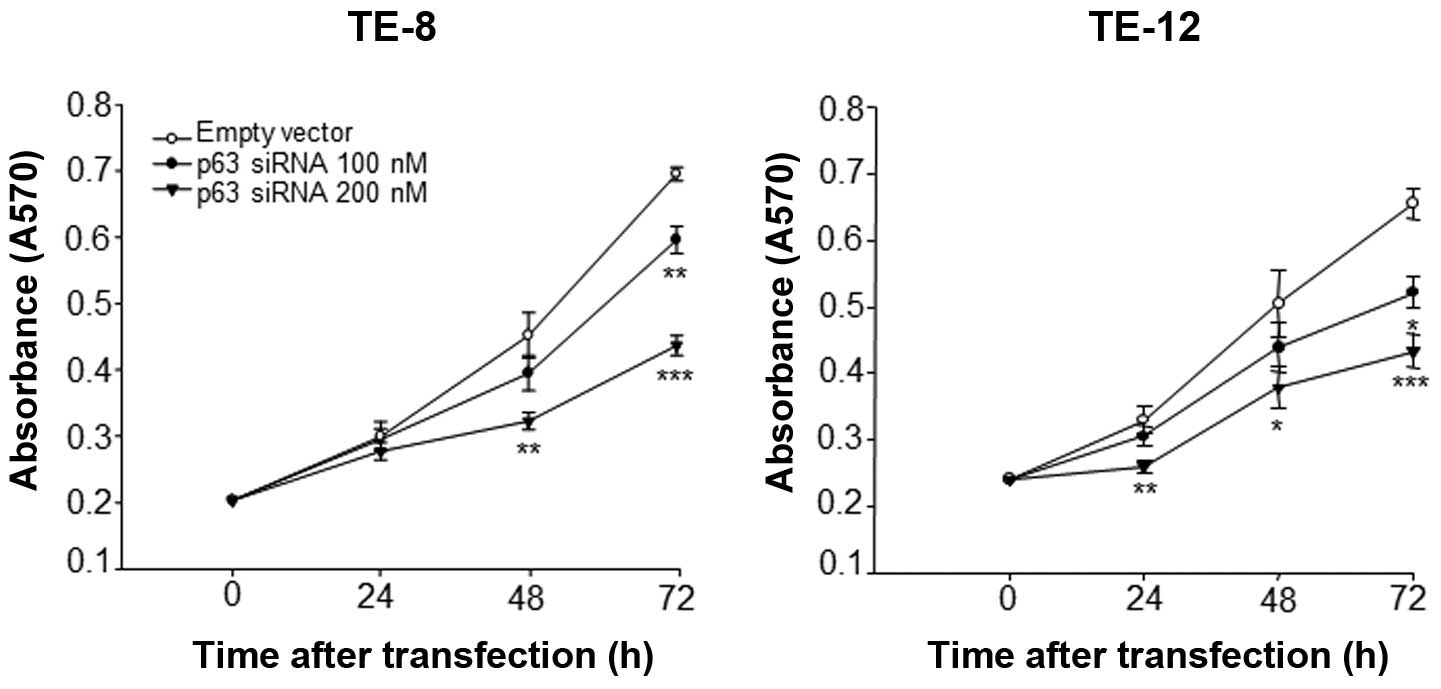
proliferation, p63 was silenced by RNA interference. As shown in
Fig. 1, the growth rates of TE-8
and TE-12 cells transfected with p63 siRNA were significantly
inhibited in a dose- and time-dependent manner compared with cells
transfected with empty vector. At 48 h, the growth of TE-8 and
TE-12 cells transfected with 200 nM p63 siRNA was significantly
lower than that of cells transfected with empty vector. At 72 h,
TE-8 and TE-12 cells transfected with 100 and 200 nM of p63 siRNA
showed even more significant inhibition of proliferation compared
with the control transfected cells. In addition, silencing of p63
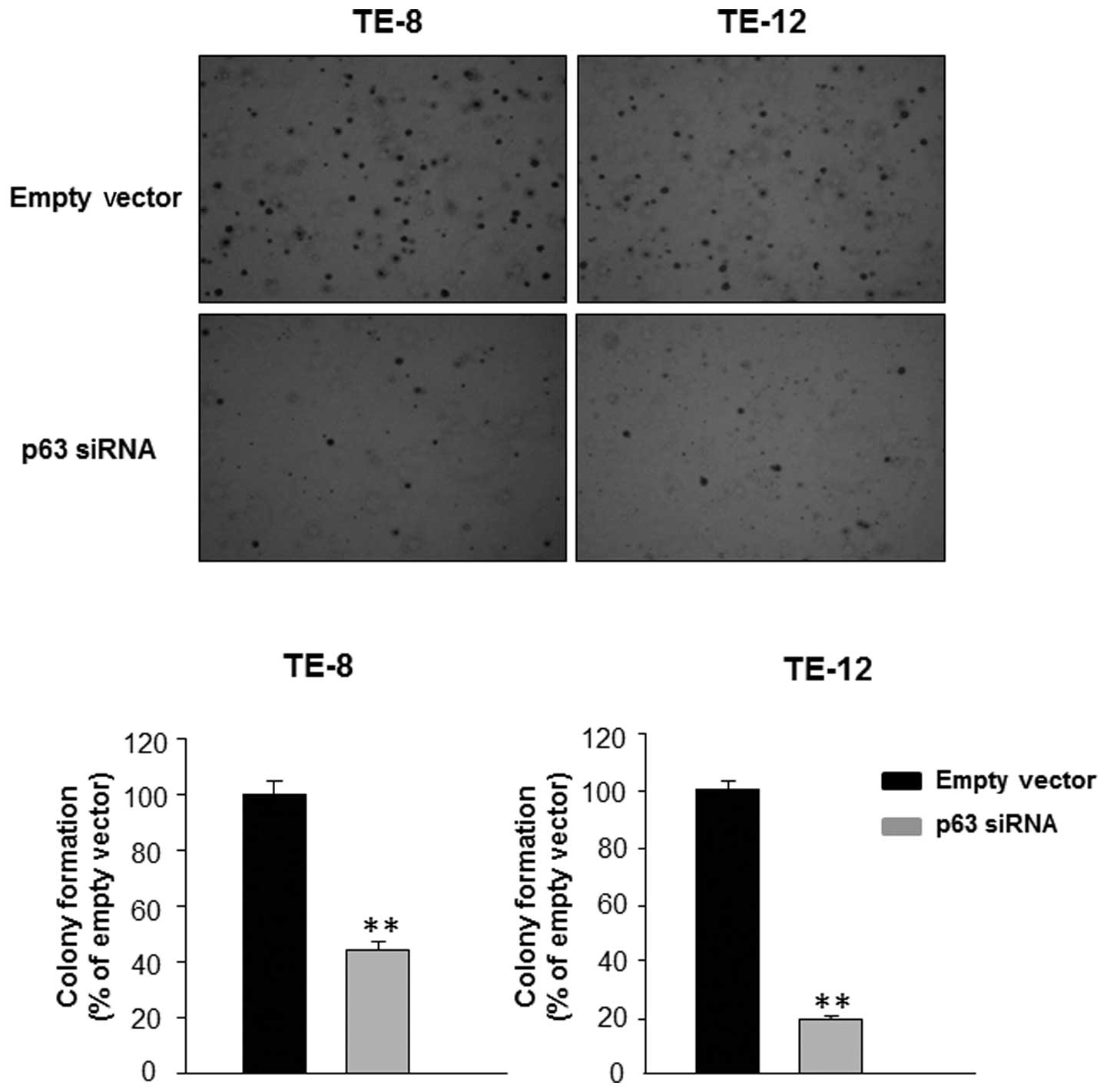
in TE-8 and TE-12 cells resulted in a significant decrease in the
number of colonies formed in soft agar compared with transfection
with empty vector (Fig. 2). Thus,
the soft agar colony formation assay confirmed that silencing of
p63 inhibited the proliferation of EAC cells.
Effect of p63 silencing on isoforms of
p63 in esophageal cancer cells
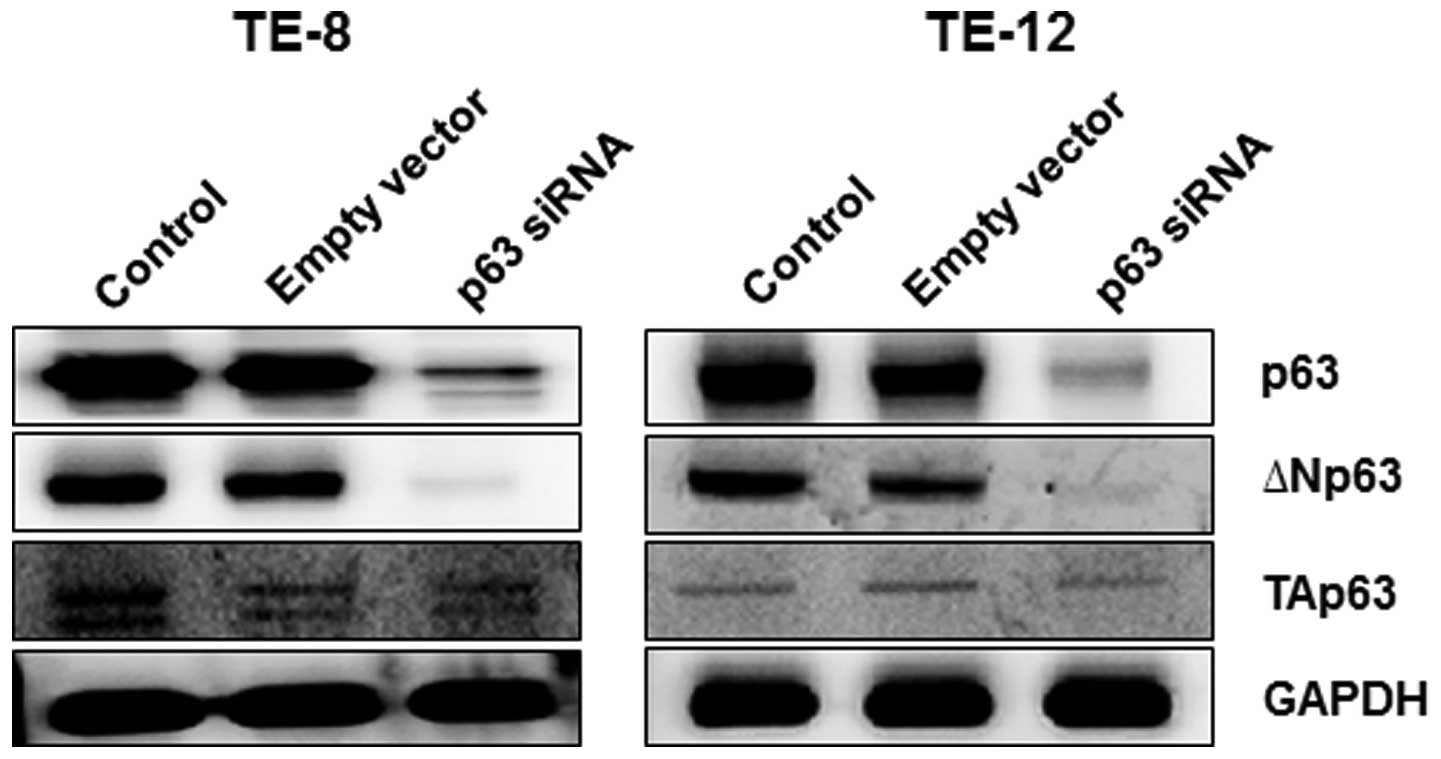
Several p63 isoforms exist. To determine which
isoforms of p63 were affected by p63 silencing, we measured protein
expression levels of ΔNp63 and TAp63 in TE-8 and TE-12 cells after
p63 silencing. Both ΔNp63 and TAp63 proteins were expressed in
non-transfected TE-8 and TE-12 cells (Fig. 3) although the ΔNp63 protein
appeared to be dominantly expressed in EAC cells. Silencing of p63
significantly decreased the expression of ΔNp63 in TE-8 and TE-12
cells, but had less effect on TAp63 protein levels.
Effect of p63 on cell cycle progression
in esophageal cancer cells
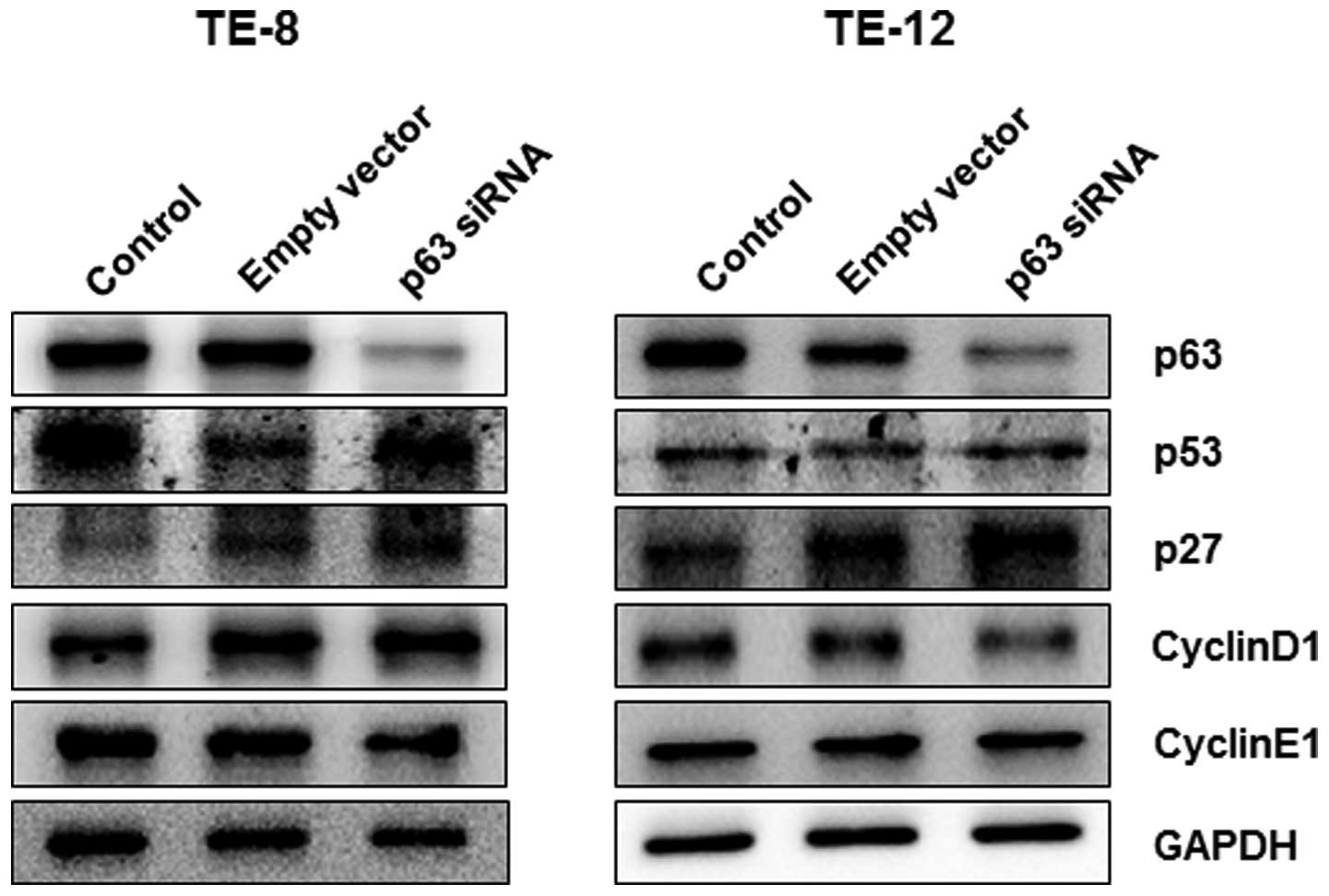
Based on the cell viability response to p63
silencing in esophageal cancer cells, we further investigated the
role of p63 in the regulation of cell cycle progression by
silencing p63 in TE-8 and TE-12 cells. At 24 h after p63 silencing,
the expression of p53 and p27 proteins was significantly increased
in TE-8 and TE-12 cells whereas expression of cyclin D1 and cyclin
E1 decreased in both cell lines (Fig.
4). We further investigated the regulation of cell
cycle-related proteins in two additional esophageal cancer cell
lines that do not normally express p63, BE3 and OE33, which
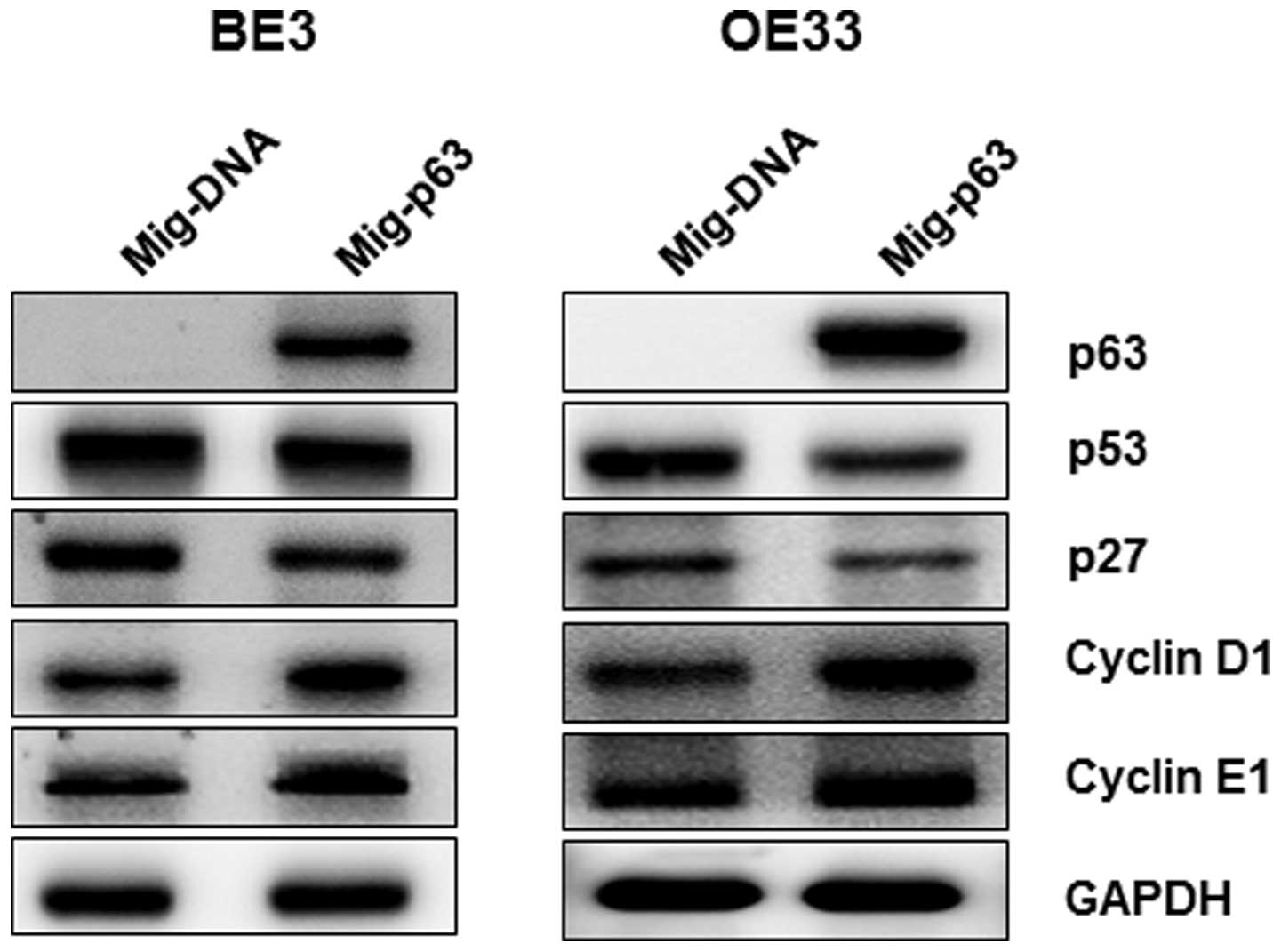
overexpressed p63. As shown in Fig.
5, p53 and p21 protein levels were significantly reduced by
overexpression of p63 in BE3 and OE33 cells, whereas expression of
cyclin D1 and cyclin E1 was induced, contrary to the results for
p63 knockdown cell lines. Taken together, these data confirm our
hypothesis that p63 is an important regulator of proliferation and
cell cycle progression in esophageal cancer cells.
Effect of p63 on Akt signaling in
esophageal cancer cells
To assess the function of p63 in the regulation of
Akt signaling during esophageal tumorigenesis, we examined Akt and
p-Akt protein levels after p63 silencing or overexpression in
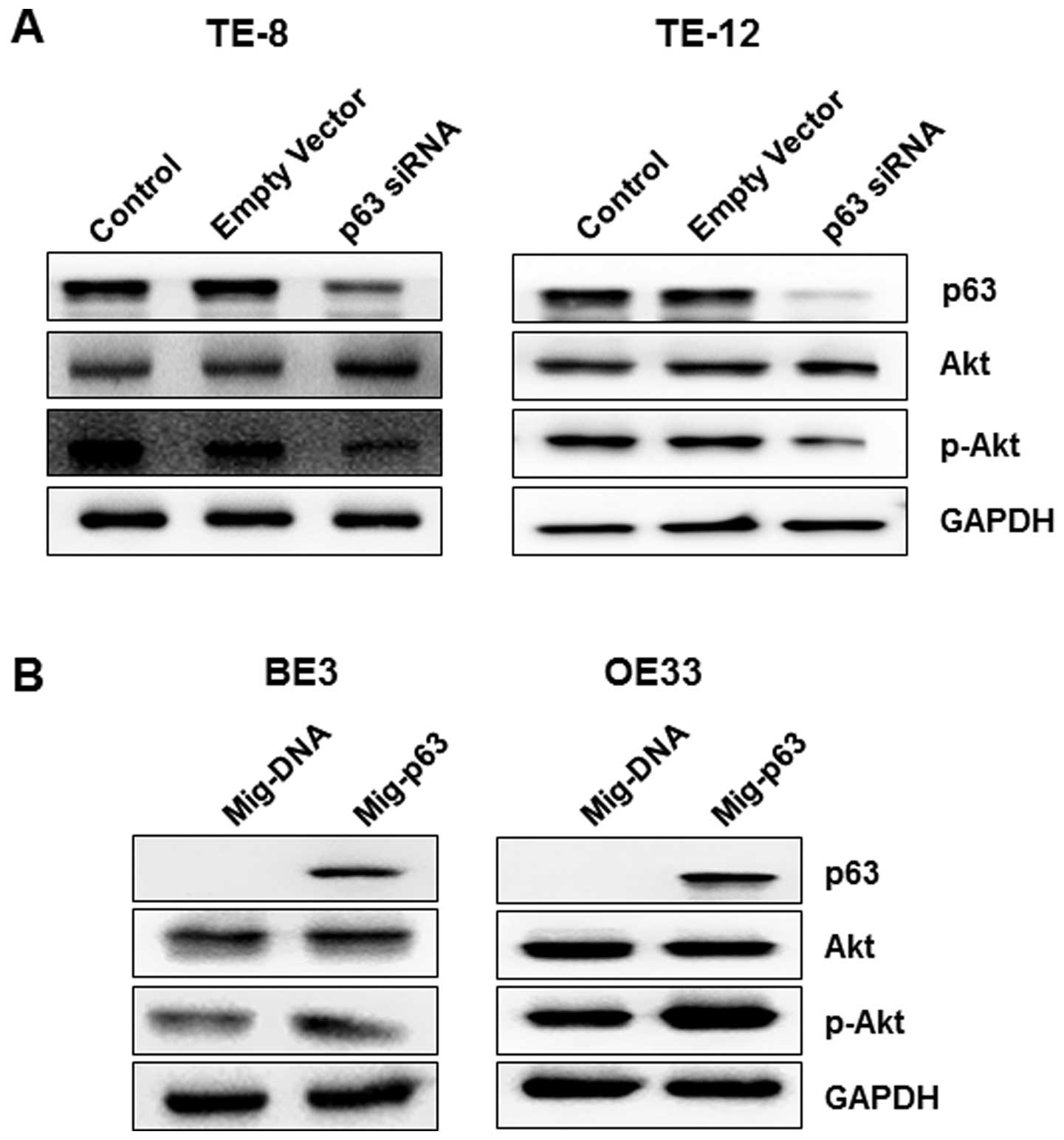
esophageal cancer cells. Silencing of p63 in TE-8 and TE-12 cells
resulted in decreased expression of p-Akt and a slight increase in
protein levels of Akt in both cell lines (Fig. 6A). Furthermore, as shown in
Fig. 6B, overexpression of p63
significantly decreased the expression of p-Akt without affecting
Akt levels, contrary to the results for p63 knock down cell
lines.
Discussion
The p63 gene encodes several protein isoforms with
homology to the tumor suppressor protein p53 and is an essential
regulator of squamous differentiation. p63 is critical for the
proliferative potential of epithelial stem cells and is frequently
overexpressed in squamous cells including esophageal cancer cells
(7,18–22).
However, the functional role of p63 in the progression of ESC has
not been clearly elucidated. In the present study we found that p63
promotes proliferation of ESC cells through regulation of the
G1 phase of the cell cycle and the Akt signaling
pathway.
Our study clearly showed that loss of p63
significantly inhibited ESC cell growth in a time- and
dose-dependent manner. We also found that loss of p63 significantly
decreased colony formation in a soft agar assay, suggesting that
p63 functions as an oncogene in ESC cells. Since p63 has multiple
isoforms as a result of alternative exon splicing, we examined
which p63 isoforms were expressed in the ESC cell lines TE-8 and
TE-12. We found that both ΔNp63 and TAp63 isoforms were expressed
in these EAC cell lines, but ΔNp63 was dominantly expressed.
Silencing of p63 induced more severe knock down of ΔNp63 protein
level than that of TAp63, strongly implicating a role for ΔNp63 in
the progression of ESC. These findings are in agreement with
previous reports of the cancer-promoting activity of ΔNp63 in many
cell types (23–28). In fact, overexpression of ΔNp63 has
been found in cancers of squamous epithelial origin and has been
shown to support tumor survival (23–28).
ΔNp63 enhances the proliferative capacity of both epithelial stem
cells and cancer cells, and loss of p63 reduces the proliferative
rate of breast, bladder, pancreas, esophagus, and head and neck
cancer cells (23–28). Therefore, ΔNp63 is strongly
implicated in the survival and maintenance of ESC cells.
Because p63 is a member of the p53 family and
interacts with all p53 family members, we further investigated the
role of p63 in cell cycle regulation in ESC cells. ΔNp63 has been
shown to directly bind to the p21 promoter (29,30).
Moreover, ablation of endogenous p63 expression in human primary
keratinocytes increases p21 expression and leads to G1
cell cycle arrest (31). DeYoung
et al demonstrated that ΔNp63 is required for G1
progression in keratinocytes (32). In the present study, we found that
silencing of p63 enhanced the expression of p53 and p27 proteins
and decreased the level of cyclin D1 protein in TE-8 and TE-12
cells. The increased expression of cyclin-dependent kinase
inhibitors (p53 and p27) by p63 silencing in ESC cells is in
agreement with the results of previous studies in keratinocytes. We
confirmed these findings by overexpression of p63 in BE3 and OE33
esophageal cancer cells that do not normally express p63.
Overexpression of p63 inhibited the expression of p53 and p27
protein in both cell lines. Cyclin D1 expression was also induced
by overexpression of p63 in BE3 and OE33 cells. These effects
contrast with those observed with silencing of p63 and are
consistent with earlier studies in mouse keratinocytes, in which
reduced p63 levels resulted in decreased cyclin D1 and CDK2
expression (30,31). Together, these findings suggest
that p63 regulates the G1 phase of the cell cycle in
esophageal cancer cells and underscore the importance of p63 in
tumor progression in esophageal cancer.
The PI3K pathway is an important regulator of growth
in many cell types, including esophageal cells (12,13).
PI3K primarily affects cell growth through the Akt pathway, which
is frequently altered in esophageal cancer (15). In the present study, we found a
strong correlation between regulation of p63 and Akt activation in
esophageal cancer cells: silencing of p63 inhibited activation of
Akt and overexpression of p63 resulted in activation of Akt. These
findings are consistent with a previous study by Ha et al,
in which overexpression of p63 resulted in an increased level of
p-Akt in keratinocytes (33).
Although our results suggest that regulation of p63 leads to Akt
activation in esophageal cancer cells, the mechanism by which p63
activates Akt remains unclear and may be dependent on the
activation of PI3K or other molecules upstream of PI3K, or even on
currently unknown factors. Further studies are needed to elucidate
the mechanisms by which p63 activates the Akt signaling pathway in
esophageal cancer cells.
In conclusion, we demonstrated that p63 promotes the
proliferation of ESC cells that is mediated, at least in part,
through cell cycle regulation and the Akt pathway. Therefore, our
results suggest that p63 plays an important function in the
progression of ESC and that molecular targeted therapy against
these pathways could be an effective approach against ESC.
Acknowledgements
This research was supported by the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (20110014864 and 2012R1A1A2005729) and by
the Korean government (MSIP) (No. 2008-0062279).
References
|
1.
|
Guo W and Jiang YG: Current gene
expression studies in esophageal carcinoma. Curr Genomics.
10:534–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Scott RB, Harrison J, Boulton C, et al:
Global attentional-executive sequelae following surgical lesions to
globus pallidus interna. Brain. 125:562–574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bergholz J and Xiao ZX: Role of p63 in
development, tumorigenesis and cancer progression. Cancer
Microenviron. 5:311–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wu N, Rollin J, Masse I, Lamartine J and
Gidrol X: p63 regulates human keratinocyte proliferation via
MYC-regulated gene network and differentiation commitment through
cell adhesion-related gene network. J Biol Chem. 287:5627–5638.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Celli J, Duijf P, Hamel BC, et al:
Heterozygous germline mutations in the p53 homolog p63 are the
cause of EEC syndrome. Cell. 99:143–153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mills AA, Zheng B, Wang XJ, Vogel H, Roop
DR and Bradley A: p63 is a p53 homologue required for limb and
epidermal morphogenesis. Nature. 398:708–713. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yang A, Schweitzer R, Sun D, et al: p63 is
essential for regenerative proliferation in limb, craniofacial and
epithelial development. Nature. 398:714–718. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Higashikawa K, Yoneda S, Tobiume K, Taki
M, Shigeishi H and Kamata N: Snail-induced down-regulation of
DeltaNp63alpha acquires invasive phenotype of human squamous cell
carcinoma. Cancer Res. 67:9207–9213. 2007. View Article : Google Scholar
|
|
10.
|
Deyoung MP and Ellisen LW: p63 and p73 in
human cancer: defining the network. Oncogene. 26:5169–5183. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Danilov AV, Neupane D, Nagaraja AS, et al:
DeltaNp63alpha-mediated induction of epidermal growth factor
receptor promotes pancreatic cancer cell growth and
chemoresistance. PloS One. 6:e268152011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lee HH, Ye S, Li XJ, Lee KB, Park MH and
Kim SM: Combination treatment with paclitaxel and doxorubicin
inhibits growth of human esophageal squamous cancer cells by
inactivation of Akt. Oncol Rep. 31:183–188. 2014.PubMed/NCBI
|
|
13.
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal-regulating kinase 1. Mol Cell Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhang HB, Lu P, Guo QY, Zhang ZH and Meng
XY: Baicalein induces apoptosis in esophageal squamous cell
carcinoma cells through modulation of the PI3K/Akt pathway. Oncol
Lett. 5:722–728. 2013.PubMed/NCBI
|
|
15.
|
Lin ML, Lu YC, Chen HY, Lee CC, Chung JG
and Chen SS: Suppressing the formation of lipid raft-associated
Rac1/PI3K/Akt signaling complexes by curcumin inhibits
SDF-1α-induced invasion of human esophageal carcinoma cells. Mol
Carcinog. 53:360–379. 2014.PubMed/NCBI
|
|
16.
|
Li XJ, Park ES, Park MH and Kim SM:
3,3′-Diindolylmethane suppresses the growth of gastric cancer cells
via activation of the Hippo signaling pathway. Oncol Rep.
30:2419–2426. 2013.
|
|
17.
|
Li XJ, Leem SH, Park MH and Kim SM:
Regulation of YAP through an Akt-dependent process by
3,3′-diindolylmethane in human colon cancer cells. Int J Oncol.
43:1992–1998. 2013.PubMed/NCBI
|
|
18.
|
Yang A, Kaghad M, Wang Y, et al: p63, a
p53 homolog at 3q27–29, encodes multiple products with
transactivating, death-inducing, and dominant-negative activities.
Mol Cell. 2:305–316. 1998.
|
|
19.
|
Di Como CJ, Urist MJ, Babayan I, et al:
p63 expression profiles in human normal and tumor tissues. Clin
Cancer Res. 8:494–501. 2002.PubMed/NCBI
|
|
20.
|
Parsa R, Yang A, McKeon F and Green H:
Association of p63 with proliferative potential in normal and
neoplastic human keratinocytes. J Invest Dermatol. 113:1099–1105.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Leonard MK, Kommagani R, Payal V, Mayo LD,
Shamma HN and Kadakia MP: DeltaNp63alpha regulates keratinocyte
proliferation by controlling PTEN expression and localization. Cell
Death Differ. 18:1924–1933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hara T, Kijima H, Yamamoto S, et al:
Ubiquitous p63 expression in human esophageal squamous cell
carcinoma. Int J Mol Med. 14:169–173. 2004.PubMed/NCBI
|
|
23.
|
Perou CM, Sorlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Matos I, Dufloth R, Alvarenga M, Zeferino
LC and Schmitt F: p63, cytokeratin 5, and P-cadherin: three
molecular markers to distinguish basal phenotype in breast
carcinomas. Virchows Arch. 447:688–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hu H, Xia SH, Li AD, et al: Elevated
expression of p63 protein in human esophageal squamous cell
carcinomas. Int J Cancer. 102:580–583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Sniezek JC, Matheny KE, Westfall MD and
Pietenpol JA: Dominant negative p63 isoform expression in head and
neck squamous cell carcinoma. Laryngoscope. 114:2063–2072. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Massion PP, Taflan PM, Jamshedur Rahman
SM, et al: Significance of p63 amplification and overexpression in
lung cancer development and prognosis. Cancer Res. 63:7113–7121.
2003.PubMed/NCBI
|
|
28.
|
Weber A, Bellmann U, Bootz F, Wittekind C
and Tannapfel A: Expression of p53 and its homologues in primary
and recurrent squamous cell carcinomas of the head and neck. Int J
Cancer. 99:22–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chatterjee A, Chang X, Sen T, Ravi R, Bedi
A and Sidransky D: Regulation of p53 family member isoform
DeltaNp63alpha by the nuclear factor-kappaB targeting kinase
IkappaB kinase beta. Cancer Res. 70:1419–1429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Westfall MD, Mays DJ, Sniezek JC and
Pietenpol JA: The Delta Np63 alpha phosphoprotein binds the p21 and
14-3-3 sigma promoters in vivo and has transcriptional repressor
activity that is reduced by Hay-Wells syndrome-derived mutations.
Mol Cell Biol. 23:2264–2276. 2003. View Article : Google Scholar
|
|
31.
|
Truong AB, Kretz M, Ridky TW, Kimmel R and
Khavari PA: p63 regulates proliferation and differentiation of
developmentally mature keratinocytes. Genes Dev. 20:3185–3197.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
DeYoung MP, Johannessen CM, Leong CO,
Faquin W, Rocco JW and Ellisen LW: Tumor-specific p73 up-regulation
mediates p63 dependence in squamous cell carcinoma. Cancer Res.
66:9362–9368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Ha L, Ponnamperuma RM, Jay S, Ricci MS and
Weinberg WC: Dysregulated DeltaNp63alpha inhibits expression of
Ink4a/arf, blocks senescence, and promotes malignant conversion of
keratinocytes. PloS One. 6:e218772011. View Article : Google Scholar : PubMed/NCBI
|