Introduction
Breast cancer, the most prevalent cancer in women
worldwide and the leading cause of cancer death in women, was
projected to claim the lives of ∼39,620 women in the USA in 2013
(1). Though treatable in early
stages, once metastasis has occurred, the survival rate is
drastically reduced to a median of 2–3 years and treatment focuses
on palliative care (2).
Critical events in tumor cell invasion include cell
attachment, proteolytic degradation of the extracellular matrix
(ECM) and migration through the disrupted matrix (3). Rath and Pauling (4) proposed that nutrients such as lysine
and ascorbic acid could act as natural inhibitors of ECM
degradation, inhibiting MMP activity and strengthening the
connective tissue surrounding cancer cells, and thus potentially
modulating tumor growth and expansion. We have developed strategies
to inhibit cancer development and its spread using naturally
occurring nutrients such as lysine, proline, ascorbic acid and
green tea extract (NM). This nutrient mixture has exhibited
synergistic anticancer activity in vivo and in vitro
in a number of cancer cell lines through inhibition of cancer cell
growth, MMP secretion, invasion, metastasis and angiogenesis
(5).
A major problem in studying metastasis has been the
lack of suitable models that faithfully represent the metastatic
process as it occurs in vivo. While some human xenograft
models can approximate primary tumor growth in mice, replication of
tumor metastasis is more problematic (6–8).
Generally, human tumor cells metastasize poorly in mice and
metastases are associated with unexpected characteristics. In
contrast, murine tumor cell models often metastasize more
effectively and display metastatic characteristics more similar to
those observed in cancer patients (9). Since microenvironments and tumor-host
interactions play important roles in tumor cell behavior, this is
not surprising. When introduced orthoptopically, 4T1 is capable of
metastasis to several organs affected in breast cancer, including
lungs, liver and brain as well as bone.
In this study, our main objective was to determine
the effect of dietary supplementation with NM on the development of
tumors and metastasis to other organs challenging mice with breast
cancer 4T1 cells into the mammary pad. The 4T1 mammary carcinoma
model was chosen as it has several characteristics that make it a
suitable experimental animal model for human mammary cancer growth
and metastasis (10,11). The tumor cells are easily
transplanted into the mammary gland so that the primary tumor grows
in the anatomically correct site and, as in human breast cancer,
4T1 metastatic disease develops spontaneously from the primary
tumor. In addition, metastatic spread of 4T1 metastases to other
organs and the draining lymph nodes is similar to that of human
mammary cancer (10). In addition,
we studied the effect of NM on 4T1 cells in vitro evaluating
viability, MMP secretion, migration and invasion.
Materials and methods
Cancer cell line and culture
Murine breast cancer cell line 4T1 was obtained from
ATCC (American Type Culture Collection, Rockville, MD, USA). 4T1
cells were maintained in DMEM, supplemented with 10% fetal bovine
serum, 100 U/ml penicillin and 100 μg/ml streptomycin. The
media and sera used were obtained from ATCC, and antibiotics
(penicillin and streptomycin) were from Gibco BRL (Long Island, NY,
USA).
Composition of the nutrient
mixture
The nutrient mixture (NM) was composed of the
following in the ratio indicated: vitamin C (as ascorbic acid and
as Mg, Ca and palmitate ascorbate) 700 mg; L-lysine 1,000 mg;
L-proline 750 mg; L-arginine 500 mg; N-acetyl cysteine 200 mg;
standardized green tea extract [derived from green tea leaves, was
obtained from US Pharma Lab; the certificate of analysis indicated
the following characteristics: total polyphenol 80%, catechins 60%,
epigallocatechin gallate (EGCG) 35% and caffeine 1.0%]; 1,000 mg;
selenium 30 μg; copper 2 mg; manganese 1 mg.
In vivo studies
Animals
Female Balb/C mice, approximately five weeks of age
on arrival, were purchased from Simonsen Laboratories (Gilroy, CA,
USA) and maintained in microisolator cages under pathogen-free
conditions on a 12-h light/12-h dark schedule for a week. All
procedures were performed according to humane and customary care
and use of experimental animals and followed a protocol approved by
internal institutional animal safety review committee.
Experimental design
After housing for a week, the mice (n=14) were
inoculated with 5×105 4T1 cells in 0.2 ml PBS and 0.1 ml
Matrigel (BD Bioscience, Bedford, MA, USA) into the mammary pad.
After injection, the mice were randomly divided into two groups and
maintained for four weeks on the following diets; the control group
mice were fed regular Purina mouse chow and the NM group the
regular diet supplemented with 0.5% NM (w/w). During the study, the
mice consumed, on the average, 4 g of their respective diets per
day. Thus, the supplemented mice received ∼20 mg of NM per day.
After four weeks, the mice were sacrificed and their tumors, lungs,
livers, kidneys, hearts and spleens were excised and processed for
histology. Dimensions (length and width) of tumors were measured
using a digital caliper, and the tumor burden was calculated using
the following formula: 0.5 × length × width. Mean weight of mice at
initiation of study and termination of study did not differ
significantly between the groups.
Histology
Tissue samples were fixed in 10% buffered formalin
and sent to IDEXX Reference Laboratories for processing, blocking,
sectioning and staining with hematoxylin and eosin (H&E).
Tumors and organs from mice were evaluated using a standard light
microscope.
In vitro studies
Cell culture
Murine breast 4T1 cells were grown in DMEM,
supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and
streptomycin (100 mg/ml) in 24-well tissue culture plates (Costar,
Cambridge, MA, USA). Cells were incubated with 1 ml of media at
37°C in a tissue culture incubator equilibrated with 95% air and 5%
CO2. At near confluence, the cells were treated with the
nutrient mixture, dissolved in media and tested at 0, 10, 50, 100,
500 and 1,000 μg/ml in triplicate at each dose. Phorbol
12-myristate 13-acetate (PMA), 100 ng/ml, was added to cells to
induce MMP-9 secretion. The plates were then returned to the
incubator.
MTT assay
Cell viability was evaluated by MTT assay, a
colorimetric assay based on the ability of viable cells to reduce a
soluble yellow tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)
2,5-diphenyl tetrazolium bromide] (MTT) to a blue formazan crystal
by mitochondrial succinate dehydrogenase activity of viable cells.
This test is a good index of mitochondrial activity and thus of
cell viability. After 24-h incubation, the cells were washed with
phosphate-buffered saline (PBS) and 500 μl of MTT (Sigma no.
M-2128) 0.5 mg/ml in media was added to each well. After MTT
addition (0.5 mg/ml) the plates were covered and returned to the
37°C incubator for 2 h, the optimal time for formazan product
formation. Following incubation, the supernatant was carefully
removed from the wells, the formazan product was dissolved in 1 ml
DMSO and absorbance was measured at 570 nm in Bio Spec 1601,
Shimadzu spectrometer. The OD570 of the DMSO solution in
each well was considered to be proportional to the number of cells.
The OD570 of the control (treatment without supplement)
was considered 100%.
Gelatinase zymography
Gelatinase zymography was performed in 10% Novex
Pre-Cast SDS polyacrylamide gel (Invitrogen Corp.) in the presence
of 0.1% gelatin under non-reducing conditions. Culture media (20
μl) were mixed with sample buffer and loaded for SDS-PAGE
with tris glycine SDS buffer, as suggested by the manufacturer
(Novex). Samples were not boiled before electrophoresis. Following
electrophoresis the gels were washed twice in 2.5% Triton X-100 for
30 min at room temperature to remove SDS. The gels were then
incubated at 37°C overnight in substrate buffer containing 50 mM
Tris-HCl and 10 mM CaCl2 at pH 8.0 and stained with 0.5%
Coomassie Blue R250 in 50% methanol and 10% glacial acetic acid for
30 min and destained. Upon renaturation of the enzyme, the
gelatinases digested the gelatin in the gel, producing clear bands
against an intensely stained background. Protein standards were run
concurrently and approximate molecular weights were determined by
plotting the relative mobilities of known proteins.
Migration: scratch test
To study cell migration, a 2-mm wide single
uninterrupted scratch was made from top to bottom of culture plates
of cancer cells grown to confluence. Culture plates were washed
with PBS and incubated with NM in medium and tested at 0, 50, 100,
250, 500 and 1,000 μg/ml in triplicate at each dose for 24
h. Cells were washed with PBS, fixed and stained with H&E and
photomicrographs were taken.
Matrigel invasion
Invasion studies were conducted using Matrigel
(Becton-Dickinson) inserts in 24-well plates. Suspended in medium,
4T1 cells were supplemented with nutrients, as specified in the
design of the experiment, and seeded on the insert in the well.
Thus both the medium on the insert and in the well contained the
same supplements. The plates with the inserts were then incubated
in a culture incubator equilibrated with 95% air and 5%
CO2 for 24 h. After incubation, the media from the wells
were withdrawn. The cells on the upper surface of the inserts were
gently scrubbed away with cotton swabs. The cells that had
penetrated the Matrigel membrane and migrated onto the lower
surface of the Matrigel were stained with hematoxylin and eosin and
visually counted under a microscope.
Morphology
Morphology of cells cultured for 24 h in test
concentrations of NM were evaluated by H&E staining and
observed and photographed by microscopy.
Statistical analysis
The results are expressed as means ± SD, as
indicated in the results, for the groups. Data was analyzed by
independent sample ‘t’-test.
Results
In vivo studies
Tumor weight and burden
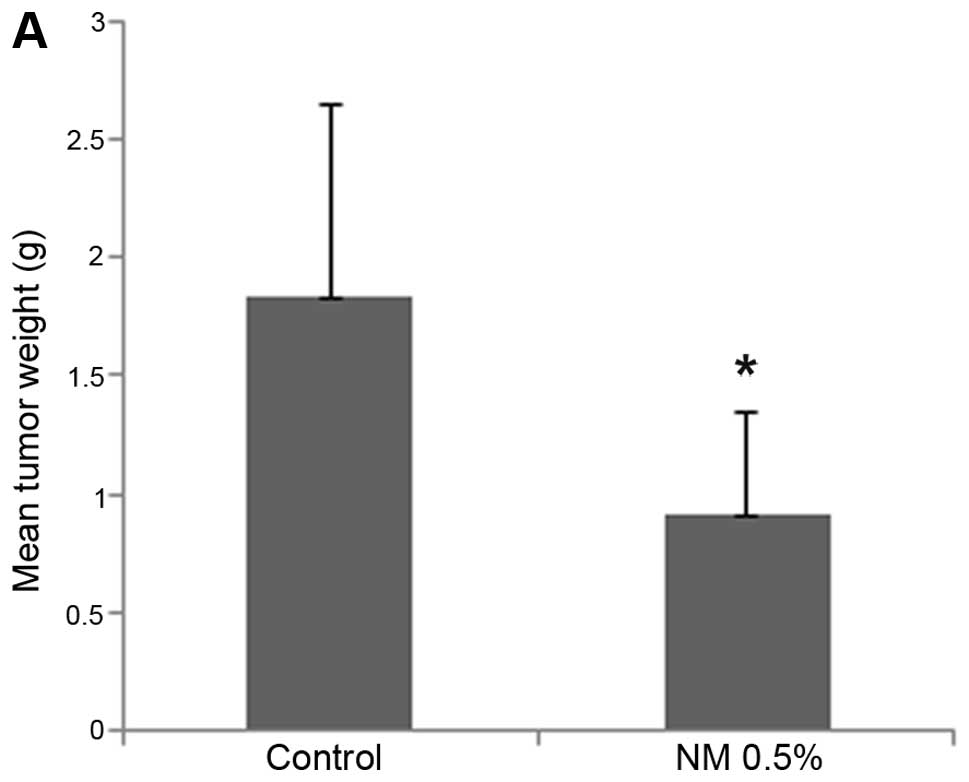
NM strongly inhibited tumor growth and burden of 4T1
tumors in female Balb/C mice. Mean tumor weight was inhibited by
50% (p=0.02) with NM 0.5% dietary supplementation, as shown in
Fig. 1A and tumor burden was
inhibited by 53.4% (p≤0.0001), as shown in Fig. 1B. Mean tumor weight of supplemented
mice was 0.91±0.43 g and that of mice on the control diet 1.83±0.81
g. Mean tumor burden of supplemented mice was 140±48 cm2
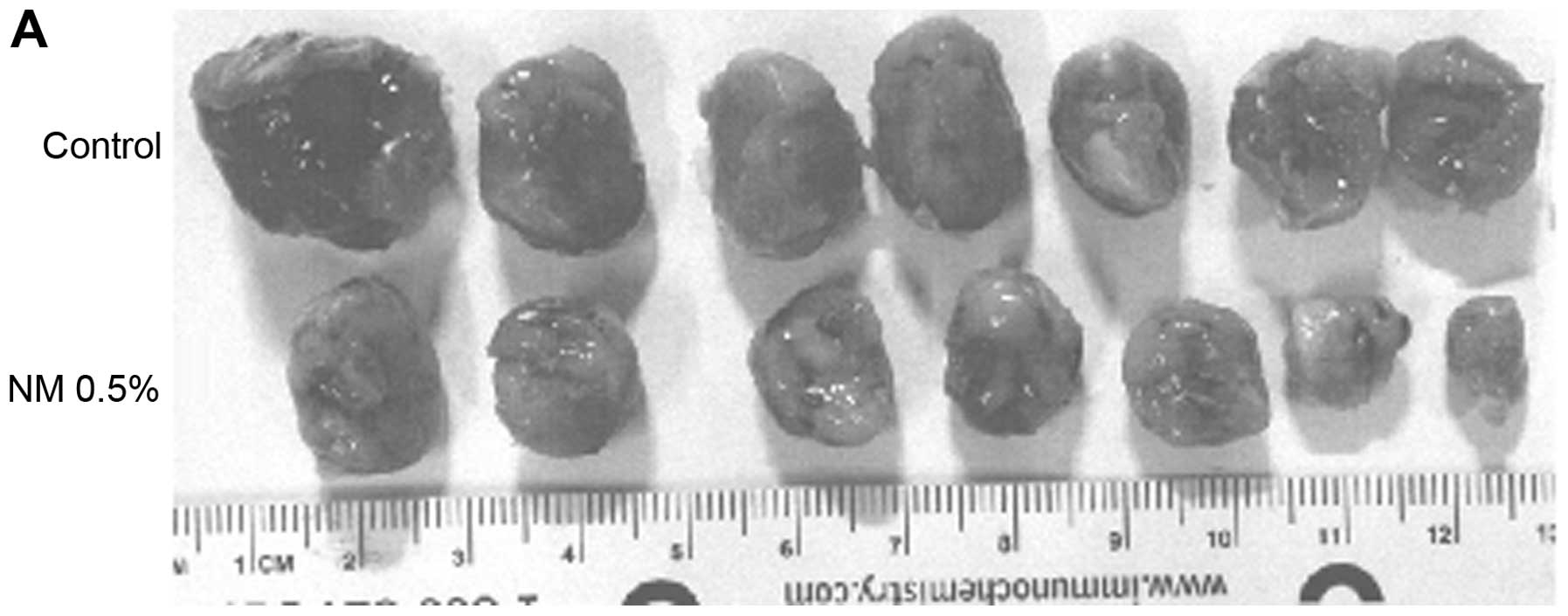
and that of mice on the control diet 300±45 cm2. Images
of mice and gross tumors from groups are shown in Fig. 2.
Tumor histopathology
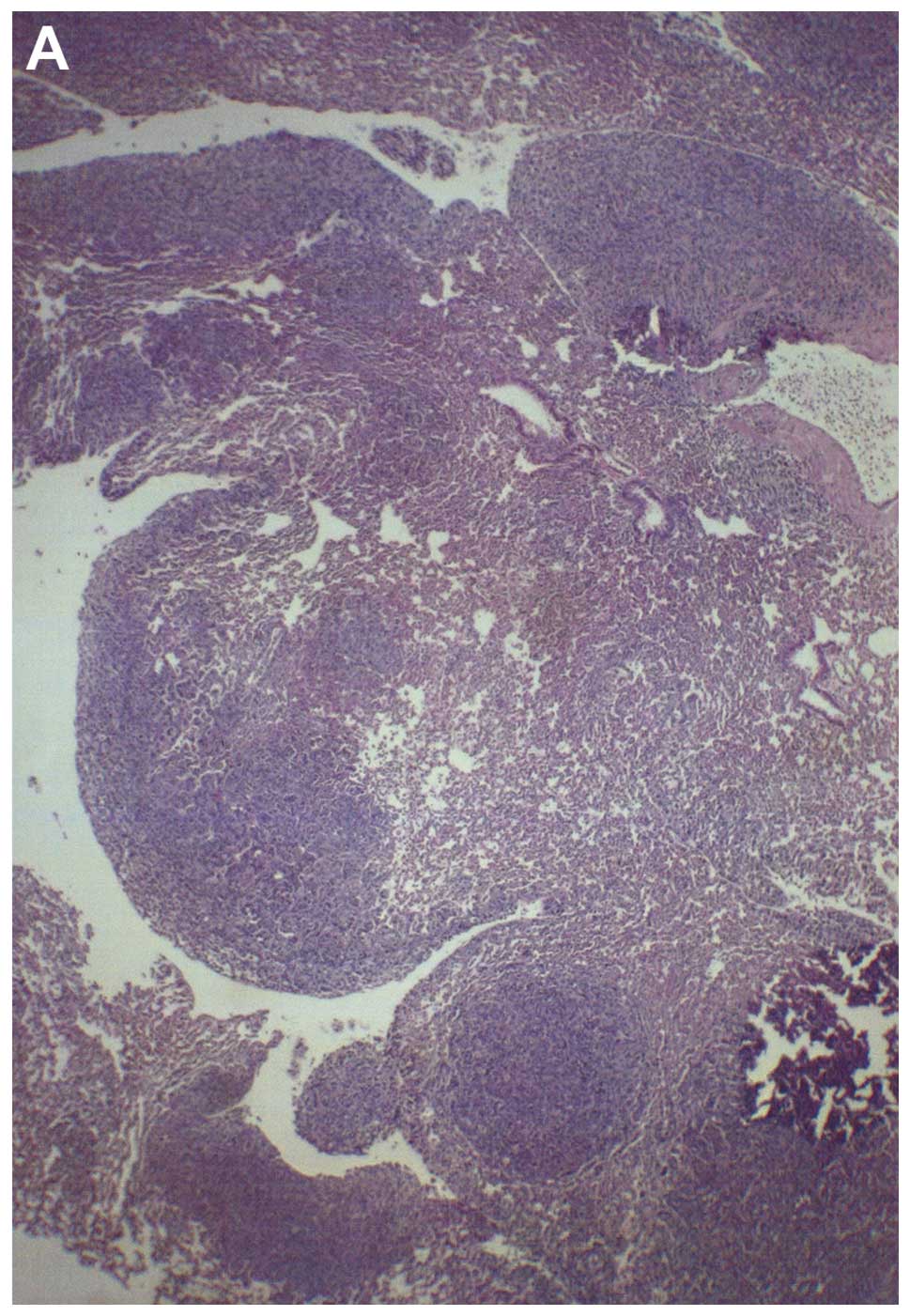
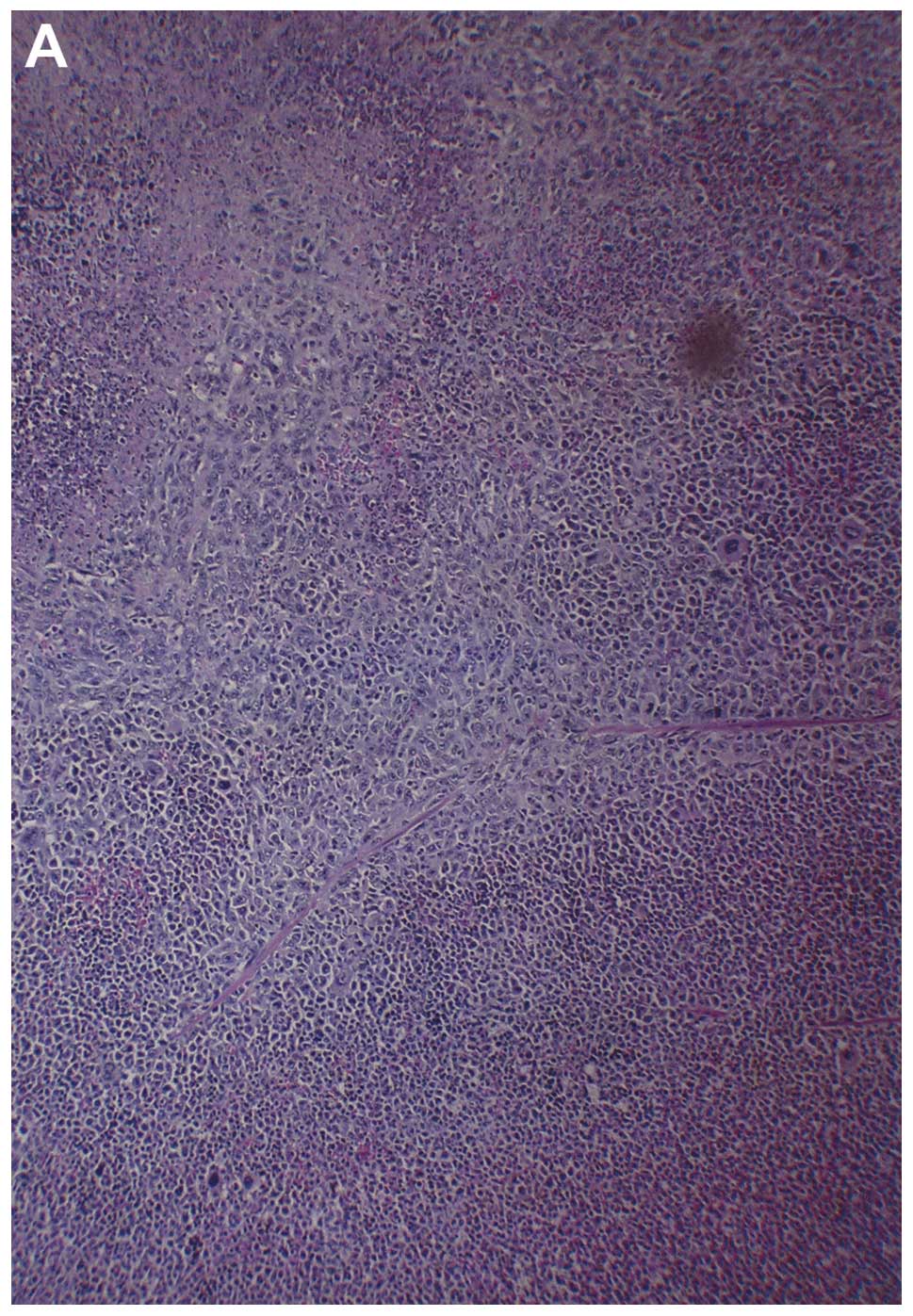
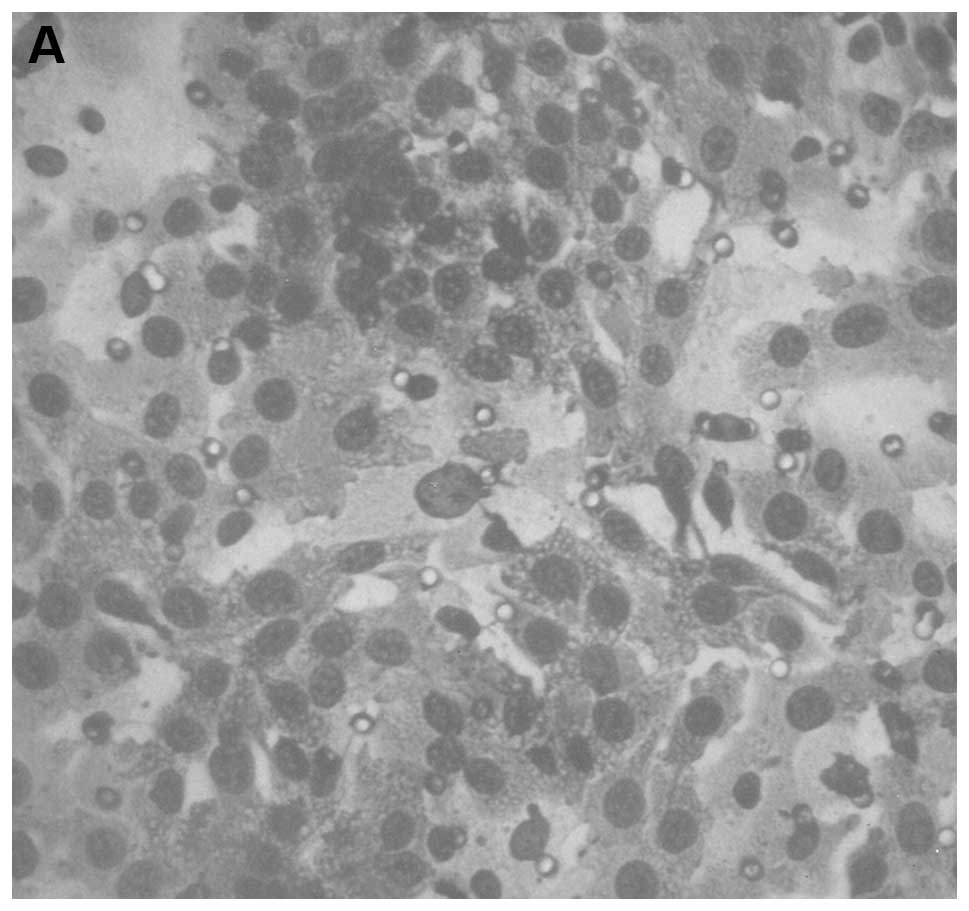
Histologically, both groups demonstrated irregularly
round subcutaneous tumors with large central areas of tumor
necrosis involving 70% of the tumor mass in the control mice and
50–70% in the supplemented mice. Viable, peripheral tumor tissue
consisted of sheaths of small irregularly-round to spindle-shaped
cells with poorly defined cytoplasm and often vesiculated nuclei.
Mitotic figures averaged 1–2 per high-power field (Fig. 3).
Metastasis to lungs
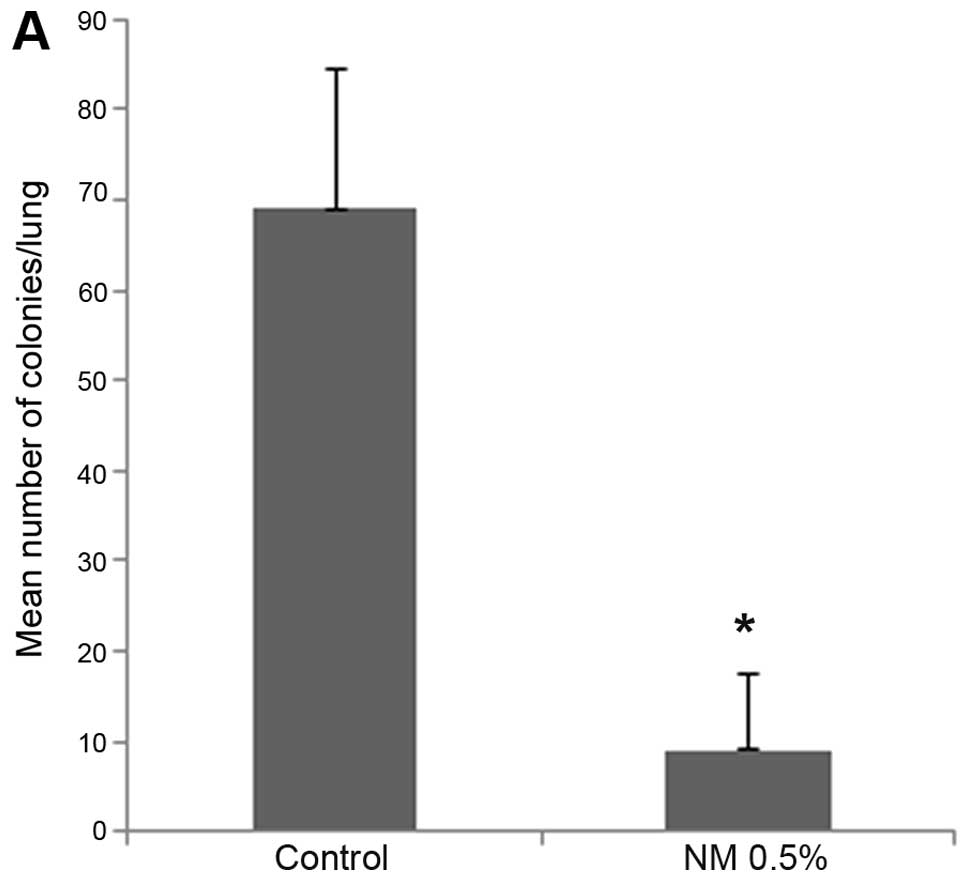
Mice supplemented with NM 0.5% showed profoundly
reduced number of colonies in the lungs as contrasted to the lungs
of control mice. Mean number of colonies in the lungs of
supplemented mice (9±8.4) were 13% (p<0.0001) of the mean number
of colonies in the lungs of control mice (69±15.6), as shown in
Fig. 4A. Furthermore, mean weight
of lungs of supplemented mice (0.24±0.05 g) were 40.7% (p=0.0001)
of the mean weight of lungs of control mice (0.59±0.16 g), as shown
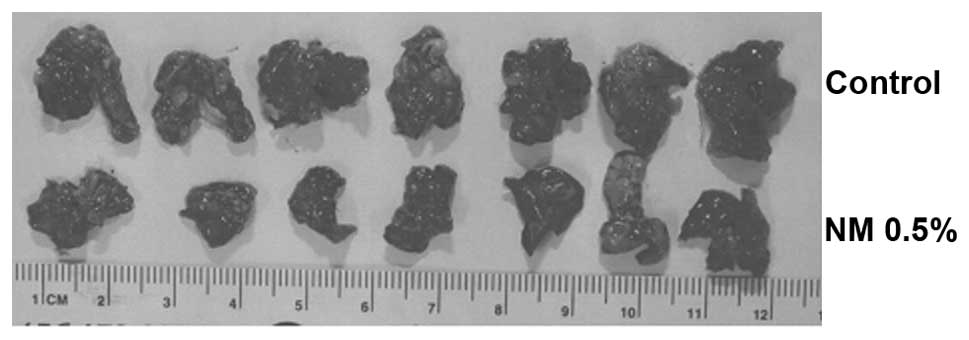
in Fig. 4B. Images of gross lungs
from groups are shown in Fig.
5.
Lung histopathology
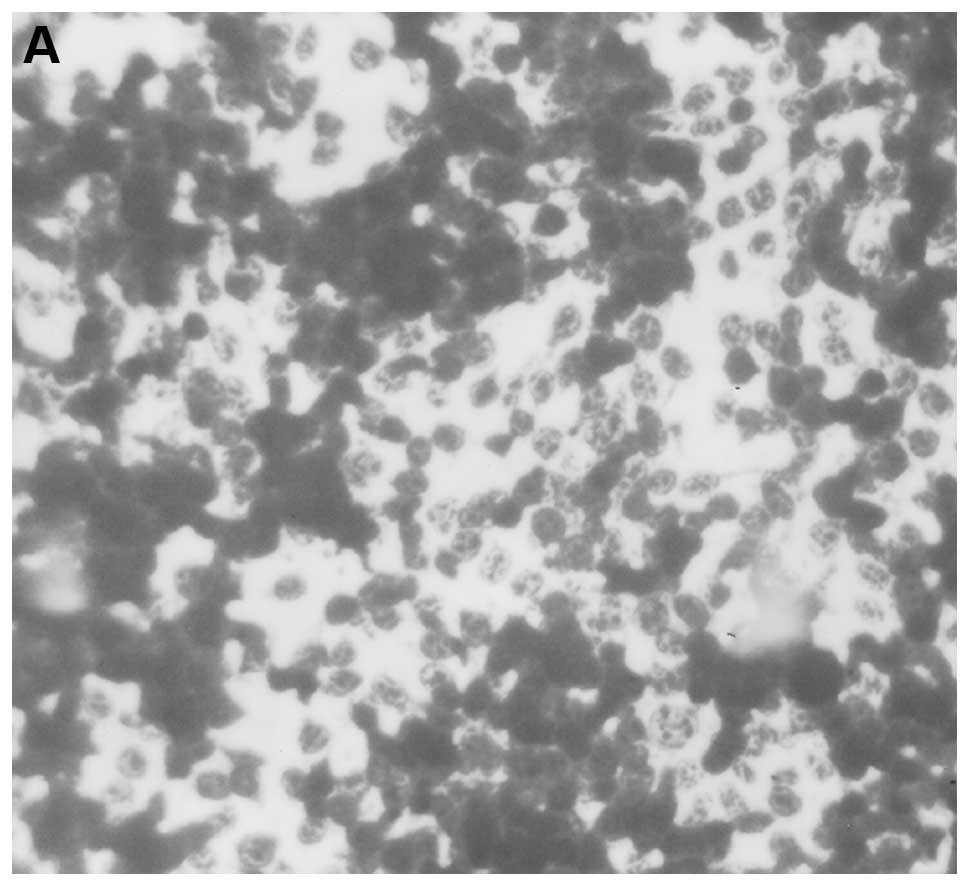
Multiple metastases were observed in the lungs of
control mice in contrast to few, small metastatic lesions in lungs
of NM supplemented mice. Neoplastic cells were large, irregularly
round, with prominent large, irregularly round nuclei and scant
cytoplasm (Fig. 6).
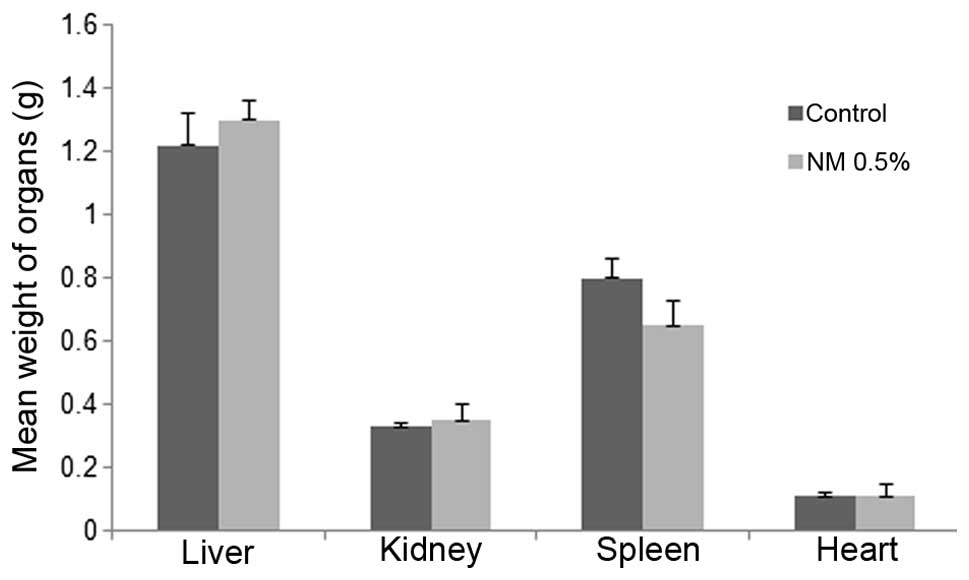
Mean weights of livers, kidneys,
spleens and hearts
No significant differences were found between
control and NM supplemented mean organ weights, as shown in
Fig. 7.
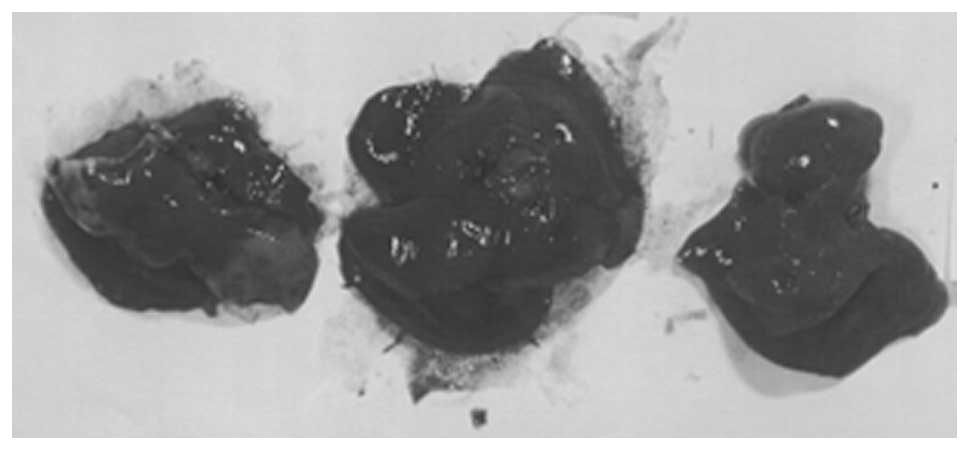
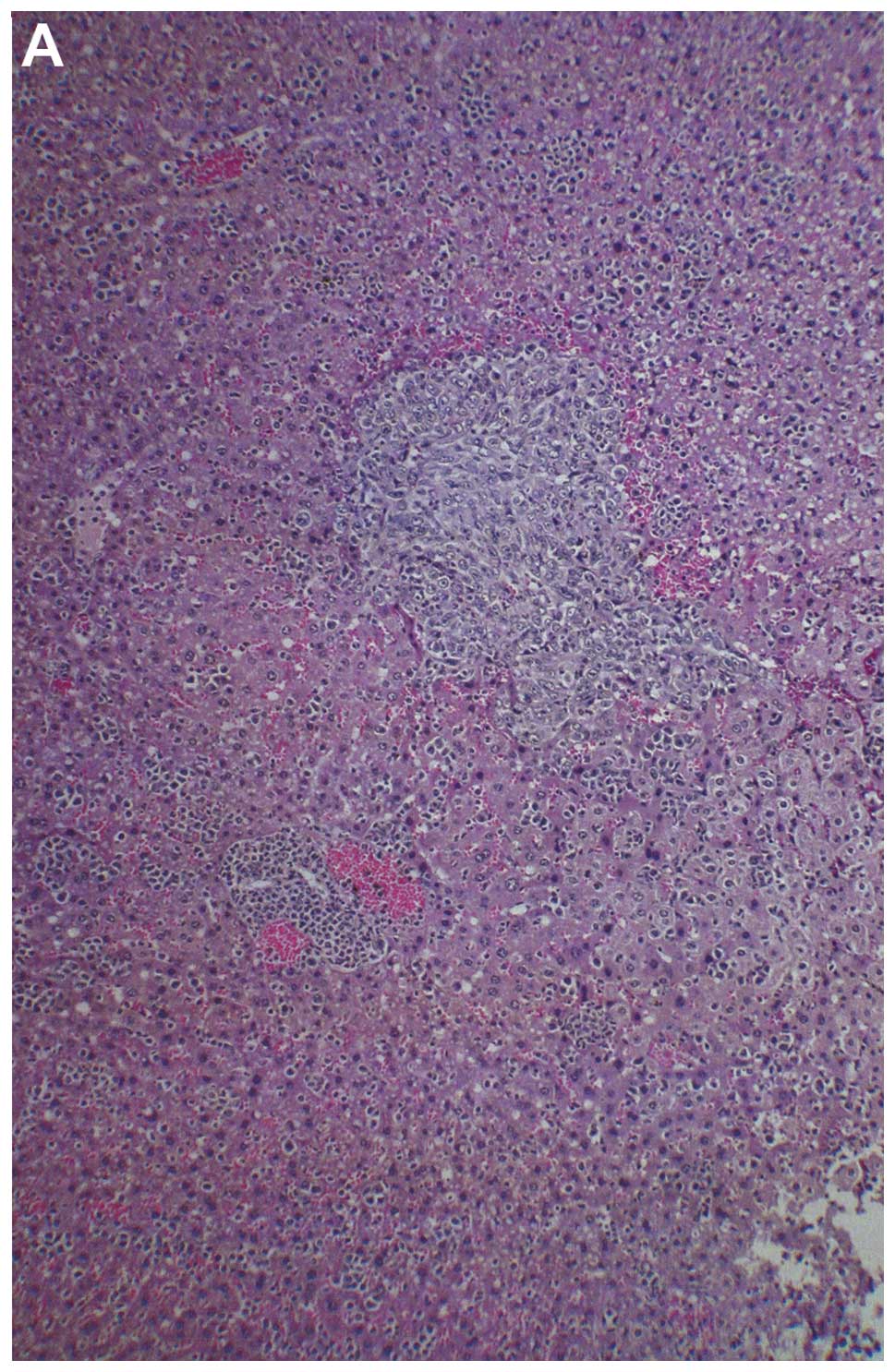
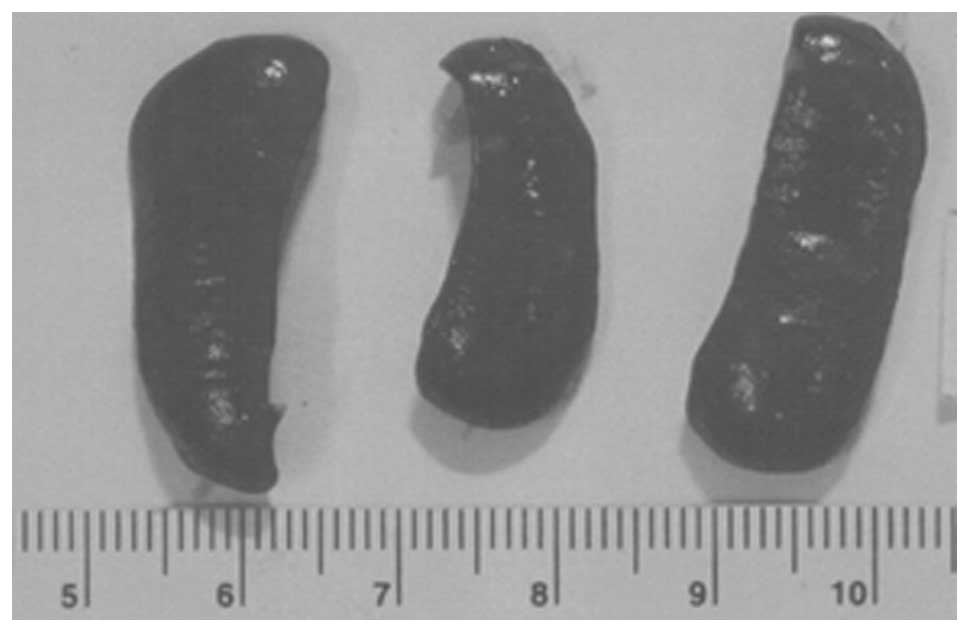
Metastasis to liver
Two of three liver sections examined from control
group livers showed 2–3 small, metastatic lesions associated with
severe, perivascular and sinusoidal neutrophilic infiltration. The
third section had no evidence of metastasis or severe neutrophilic
infiltration, but did have multifocal areas of severe, acute liver
necrosis. All four sections of liver examined in NM 0.5% fed mice,
showed no definite metastatic lesions. Many vessels were severely
cuffed with neutrophils. A few questionable cells presented in
sinusoids, but these most likely were myeloid in origin. Gross
images of control livers are shown in Fig. 8 and histopathology of livers from
control and supplemented mice is shown in Fig. 9.
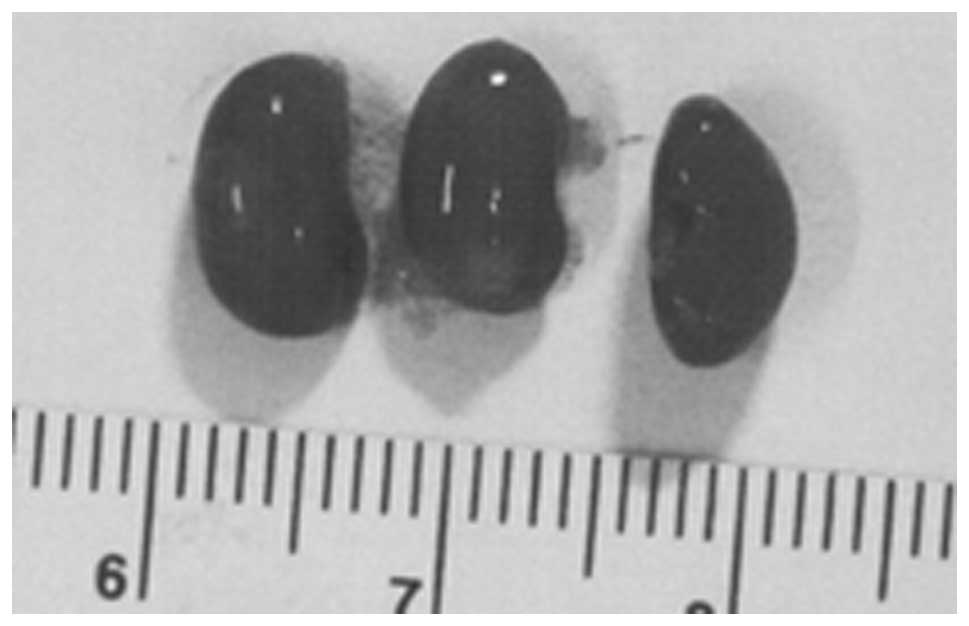
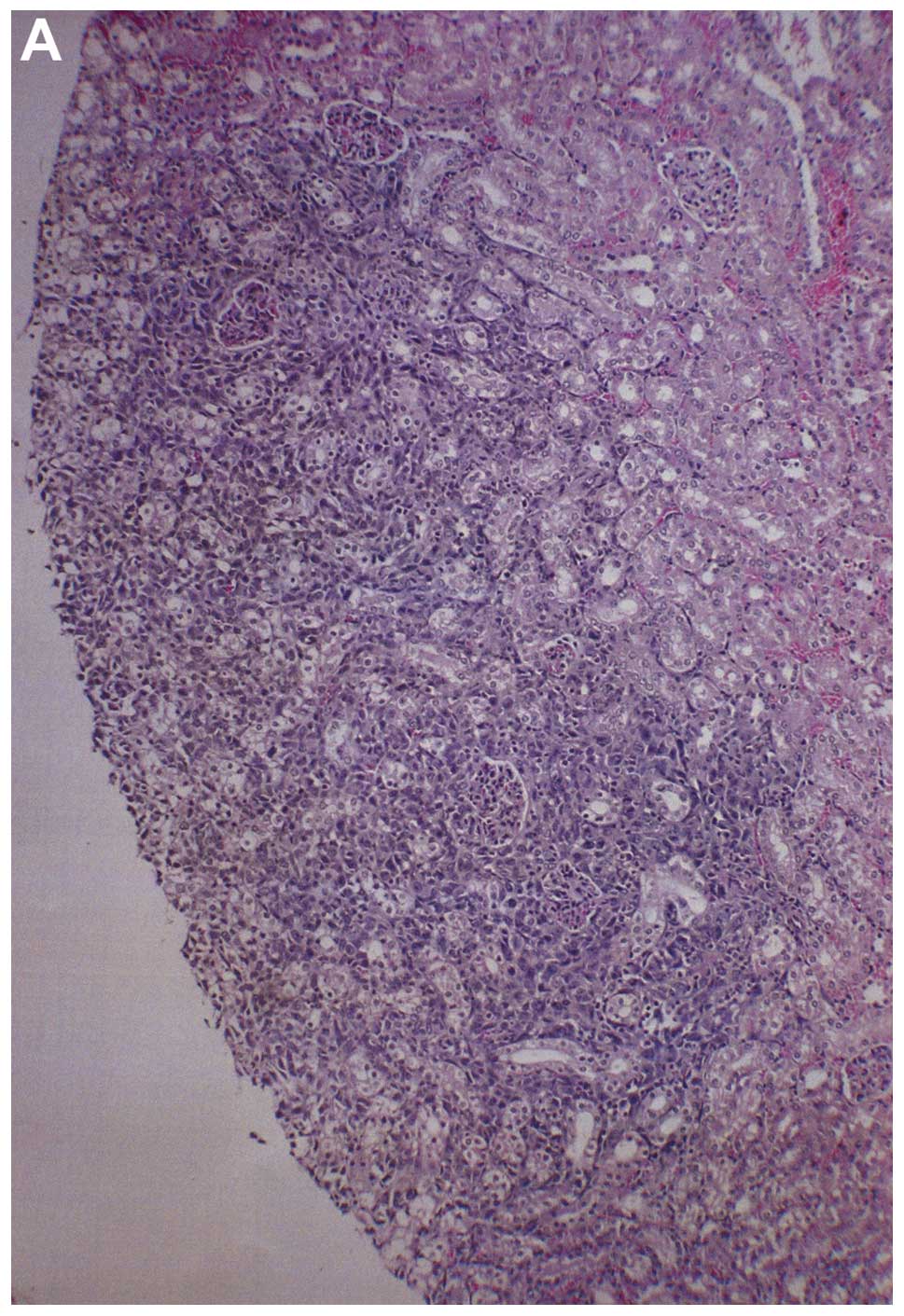
Metastasis to kidney
Three partial kidney sections of control mice
presented subscapsular, metastatic lesions and one section areas of
acute infarction. Of three sections of NM kidney examined, no
metastases or specific changes were noted. Gross images of control
kidneys are shown in Fig. 10 and
histopathology of kidneys from control and supplemented mice is
shown in Fig. 11.
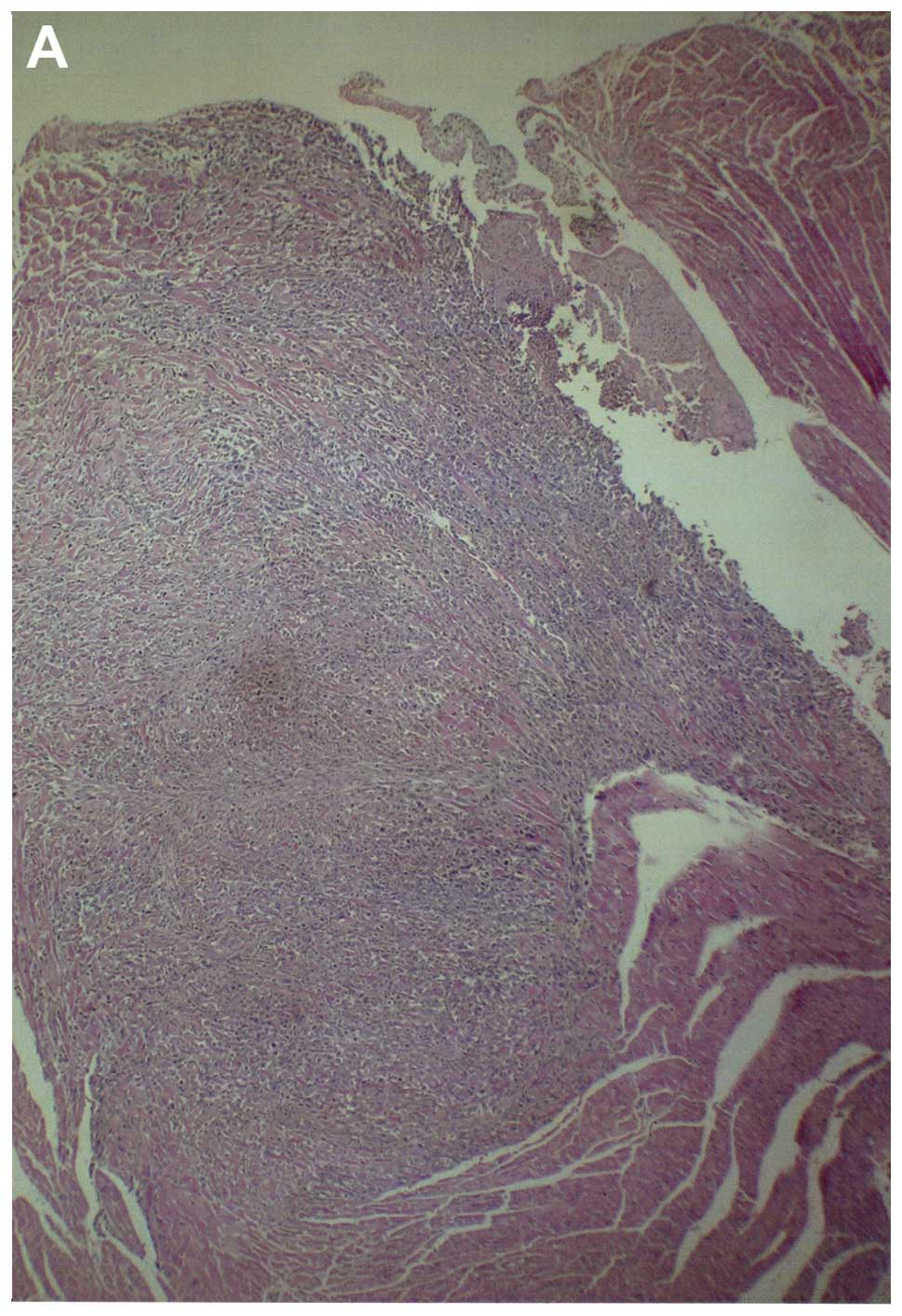
Metastasis to heart
Among the control group heart sections examined,
four of five showed myocardial metastatic lesions, one large and
three smaller metastases. In the NM heart sections examined, two of
four sections each had a metastatic lesion near the base of the
heart. Gross images of hearts from both groups are shown in
Fig. 12 and histopathology of
hearts from control and supplemented mice is shown in Fig. 13.
Metastasis to spleen
Of the three sections of control group spleens
examined, all showed severe, extramedullary hematopoiesis and 2–3
small metastases. Sections of NM spleen showed severe,
extramedullary hematopoietic activity and a small, metastatic
lesion in one section. Gross images of control spleens are shown in
Fig. 14 and histopathology of
spleens from control and supplemented mice is shown in Fig. 15.
In vitro
Cell proliferation: MTT assay
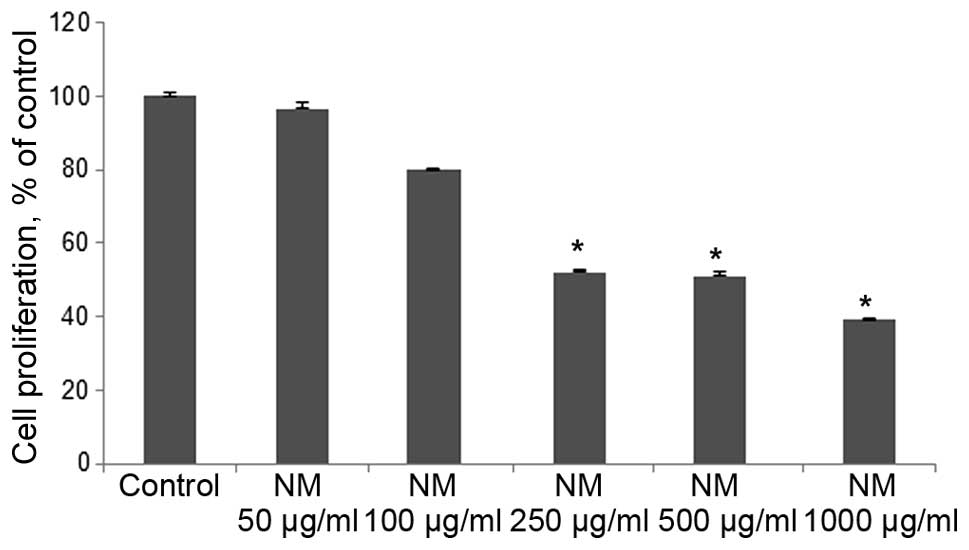
NM exhibited dose-dependent inhibition of 4T1 cell
growth with 50% (p<0.0001) antiproliferative effect at 250 and
500 μg/ml and 60% (p<0.0001) at 1,000 μg/ml
compared to control, as shown in Fig.
16.
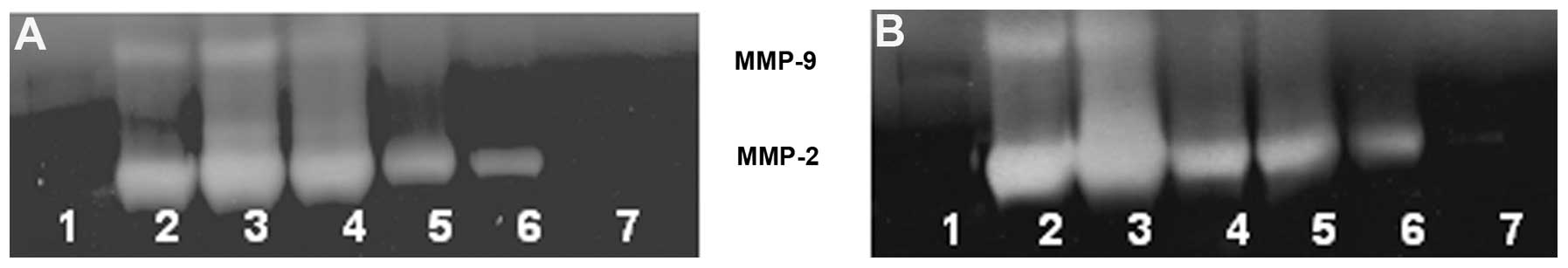
Gelatinase zymography
Zymography demonstrated MMP-2 and MMP-9 secretion by
untreated and PMA-treated 4T1 cells. NM inhibited secretion of both
MMPs in a dose-dependent manner with virtual total inhibition of
both at 1,000 μg/ml, as shown in Fig. 17.
Migration: scratch test
NM reduced breast cancer 4T1 cell migration in a
dose-dependent manner, with total inhibition at 1,000 μg/ml,
as shown in Fig. 18.
Matrigel invasion
NM significantly inhibited 4T1 invasion through
Matrigel in a dose-dependent manner, with total block at 250
μg/ml, as shown in Fig.
19.
Morphology: H&E staining
H&E staining showed no morphological changes
even at higher concentrations of NM, as shown in Fig. 20.
Discussion
The results of the in vivo study of murine
4T1 cells injected into the mammary pads of Balb/C mice
demonstrated significant suppression of tumor growth (50% reduction
in tumor weight and 53% in tumor burden) with NM dietary
supplementation. Lung metastasis was profoundly inhibited by NM
supplementation: mean number of colonies was reduced by 87% and
mean weight of lungs by 60% compared to control mice. Metastasis to
liver, spleen, kidney and heart was significantly reduced with NM
supplementation. The results from the in vitro studies
support the in vivo findings. In vitro, NM inhibited
cell proliferation by 50% at 250 and 500 μg/ml
concentrations compared to the control. Zymography demonstrated
MMP-2 and MMP-9 secretion which was inhibited by NM in a
dose-dependent manner, with virtual total inhibition of both at
1,000 μg/ml. Migration by scratch test and invasion through
Matrigel were inhibited dose-dependently with total block of
invasion at 250 and of migration at 1,000 μg/ml.
Tao et al reported that metastasis of 4T1
tumors was associated with extensive necrosis and inflammation
within the primary tumor and hematopoiesis in several mouse organs
including spleen and liver (10).
In our study, both groups demonstrated irregularly round
subcutaneous tumors with large central areas of tumor necrosis
involving 70% of the tumor mass in the control mice and 50–70% in
the supplemented mice. As discussed earlier, we observed multiple
metastases in the lungs of control mice in contrast to few small
metastatic lesions in lungs of NM supplemented mice. Splenic
metastasis showed severe, extramedullary hematopoiesis and 2–3
small metastases in the spleen sections of control mice and a small
metastatic lesion and extramedullary hematopoietic activity in the
sections of supplemented mice. Liver metastasis in our study showed
several small metastatic lesions associated with severe,
perivascular and sinusoidal neutrophilic infiltration in the
control sections and absence of metastatic lesions but presence of
neutrophils in supplemented sections.
Bonfil et al reported that the distribution
of necrosis within a primary tumor was responsible in part for the
development of metastases (12),
and that tumor necrosis was an important source of gelatinase/type
IV collagenase, mainly in its 92-kDa form, and thus played a major
role in tumor invasion (13). In a
previous study, we found that among gulo KO mice injected with
mammary 4T1 cells, those mice deprived of ascorbate developed large
tumors with dark cores, showing more necrosis, and poorly defined
borders, while gulo KO mice supplemented with ascorbate hosted
smaller tumors with smaller, lighter cores, less necrosis and
enhanced collagen encapsulation, signifying less metastatic
potential (14).
High MMP-2 and MMP-9 levels have been found to
correlate with aggressiveness of cancers, as exemplified by breast
cancer (15,16). Our present in vitro study
showed dose-dependent inhibition of MMP-2 and MMP-9 secretion, cell
migration and cell invasion through Matrigel with treatment of 4T1
cells with NM.
A causal relationship between inflammation and 4T1
metastasis was proposed by Connolly et al based on
observation of an inhibitory effect of the COX-2 inhibitor sC-236
on metastasis of 4T1 after primary tumor excision (17). Inflammation has been noted to have
a positive effect on metastasis in several systems (18,19).
The NM has been shown to have an inhibitory effect on inflammatory
mediators as COX-2 in prior studies (5,20,21).
Optimal ECM structure depends upon adequate supplies
of ascorbic acid and the amino acids lysine and proline to ensure
proper synthesis and hydroxylation of collagen fibers. In addition,
lysine contributes to ECM stability as a natural inhibitor of
plasmin-induced proteolysis (4,22).
Manganese and copper are also essential for collagen formation.
There is considerable documentation of the potency of green tea
extract in modulating cancer cell growth, metastasis, angiogenesis,
and other aspects of cancer progression (23–27).
N-acetyl cysteine and selenium have demonstrated inhibition of
tumor cell MMP-9 and invasive activities, as well as migration of
endothelial cells through ECM (28–30).
Ascorbic acid demonstrates cytotoxic and antimetastatic actions on
malignant cell lines (14,31–35)
and cancer patients have been found to have low levels of ascorbic
acid (36,37). Low levels of arginine, a precursor
of nitric oxide (NO), can limit the production of NO, which has
been shown to predominantly act as an inducer of apoptosis
(38).
In conclusion, the results of the in vivo
study of murine 4T1 cells injected into the mammary pads of Balb/C
mice confirmed the validity of this model to study breast cancer
metastasis since metastasis was observed in lung, liver, spleen,
kidney and heart, as in human breast cancer. The present study also
demonstrated significant suppression of tumor growth and metastasis
to all of these organs with NM dietary supplementation, which
indicates NM inhibition of metastasis is based on targeting common
parameters as MMPs and the collagen barrier. Furthermore, the in
vivo results were supported by in vitro findings of NM
suppression of cell proliferation, secretion of MMP-2 and MMP-9,
migration and Matrigel invasion by 4T1 cells. These results suggest
that NM has therapeutic potential in treatment of breast
cancer.
Acknowledgements
The tissues were processed by IDEXX
Reference Laboratories Inc. and consulting pathologist Dr Alexander
DePaoli, provided the histology slides and analysis. The research
was funded by Dr Rath Health Foundation (Santa Clara, CA, USA), a
non-profit organization.
References
|
1.
|
American Cancer Society: Breast cancer:
what are the key statistics about breast cancer? http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics).
Accessed 10/9/13. Last Medical Review: 09/11/2013. Last Revised:
10/01/2013.
|
|
2.
|
Ali SM, Harvey HA and Lipton A: Metastatic
breast cancer: overview of treatment. Clin Orthop. 414(Suppl):
132–137. 2003. View Article : Google Scholar
|
|
3.
|
Fidler IJ: Molecular biology of cancer:
invasion and metastasis. Cancer: Principles and Practice of
Oncology. 5th edition. De Vita VT, Hellman S and Rosenberg SA:
Lippincott-Raven; Philadelphia, PA: pp. 135–152. 1997
|
|
4.
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. Orthomolecular Med. 7:17–23. 1992.
|
|
5.
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Eccles SA, Box G, Court W, Sandle J and
Dean CJ: Preclinical models for the evaluation of targeted
therapies of metastatic disease. Cell Biophys. 24–25:279–291.
1994.
|
|
8.
|
Hoffman RM: Orthotopic metastatic mouse
models for anti-cancer drug discovery and evaluation: a bridge to
the clinic. Invest New Drugs. 17:343–359. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Vernon E, Bakewell SJ and Chodash LA:
Deciphering the molecular basis of breast cancer metastasis with
mouse models. Rev Endocr Metab Disord. 8:199–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tao K, Fang M, Aloy J and Sahagian GG:
Imagable 4T1 model for the study of late stage breast cancer. BMC
Cancer. 8:2282008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Aslakson CJ and Miller FR: Selective
events in the metastatic process defined by analysis of the
sequential dissemination of subpopulations of a mouse mammary
tumor. Cancer Res. 52:1399–1405. 1992.PubMed/NCBI
|
|
12.
|
Bonfil RD, Bustuabad OD, Ruggiero RA,
Meiss RP and Pasqualini CD: Tumor necrosis can facilitate the
appearance of metastases. Clin Exp Metastasis. 6:121–129. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bonfil RD, Medina PA, Gómez DE, Farías E,
Lazarowski A, Lucero Gritti MF, Meiss RP and Bustuabad OD:
Expression of gelatinase/type IV collagenase in tumor necrosis
correlates with cell detachment and tumor invasion. Clin Exp
Metastasis. 10:211–220. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cha J, Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Ascorbate supplementation inhibits growth
and metastasis of B16FO melanoma and 4T1 breast cancer cells in
vitamin C deficient mice. Int J Oncol. 42:55–64. 2013.PubMed/NCBI
|
|
15.
|
Bachmeier BE, Nerlich AG, Lichtinghagen R
and Sommerhoff CP: Matrix metalloproteinases (MMPs) in breast
cancer cell lines of different tumorigenicity. Anticancer Res.
6A:3821–3828. 2001.PubMed/NCBI
|
|
16.
|
Pellikainen JM, Ropponen KM, Kataja VV,
Kellokoski JK, Eskelinen MJ and Kosma VM: Expression of matrix
metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special
reference to activator protein-2, HER-2, and prognosis. Clin Cancer
Res. 10:7621–7628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Connolly EM, Harmey JH, O'Grady T, Foley
D, Roche-Nagle G, Kay E and Bouchier-Hayes DJ: Cyclo-oxygenase
inhibition reduces tumour growth and metastasis in an orthotopic
model of breast cancer. Br J Cancer. 87:231–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Denardo DG, Johansson M and Coussans LM:
Immune cells as mediators of solid tumor metastasis. Cancer
Metastasis Rev. 27:11–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
van Kempen LC, de Visser KE and Coussens
LM: Inflammation, proteases and cancer. Eur J Cancer. 42:728–734.
2006.PubMed/NCBI
|
|
20.
|
Roomi MW, Kalinovsky T, Roomi NW, Rath M
and Niedzwiecki A: Inhibition of growth and expression of
inflammation mediators in human leukemic cell line. Exp Oncol.
35:180–186. 2013.PubMed/NCBI
|
|
21.
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Pleiotropic effects of a micronutrient mixture on critical
parameters of bladder cancer. Bladder Cancer Etymology, Diagnosis
and Treatments. Nilsson WE: Nova Science Publishers, Inc.; New
York: 2009
|
|
22.
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of high-affinity lysine binding
sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochem Biophys Acta.
1596:182–192. 2002.PubMed/NCBI
|
|
23.
|
Valcic S, Timmermann BN, Alberts DS,
Wachter GA, Krutzsch M, Wymer J and Guillen JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Mukhtar H and Ahmed N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71:S1698–S1702. 2000.PubMed/NCBI
|
|
25.
|
Yang GY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimikawa R: Effect of (−) epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
|
|
27.
|
Hara Y: Green tea: Health Benefits and
Applications. Marcel Dekker; New York, Basel: 2001, View Article : Google Scholar
|
|
28.
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP 9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
29.
|
Morini M, Cai T, Aluigi MG, Noonan DM,
Masiello L, De Floro S, D’Agostinin F, Albini A and Fassima G: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
30.
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT 1080 tumor cells. J Biol
Chem. 276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Naidu KA, Karl RC and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: the utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH and Park CH:
Differential effects and transport kinetics of ascorbate
derivatives in leukemic cell lines. Anticancer Res. 8:2487–2493.
1998.PubMed/NCBI
|
|
35.
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Nunez C, Ortiz de Apodaca Y and Ruiz A:
Ascorbic acid in the plasma and blood cells of women with breast
cancer. The effect of consumption of food with an elevated content
of this vitamin. Nutr Hosp. 10:368–372. 1995.PubMed/NCBI
|
|
37.
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineoplastic activity of doxorubicin, cisplatin and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|