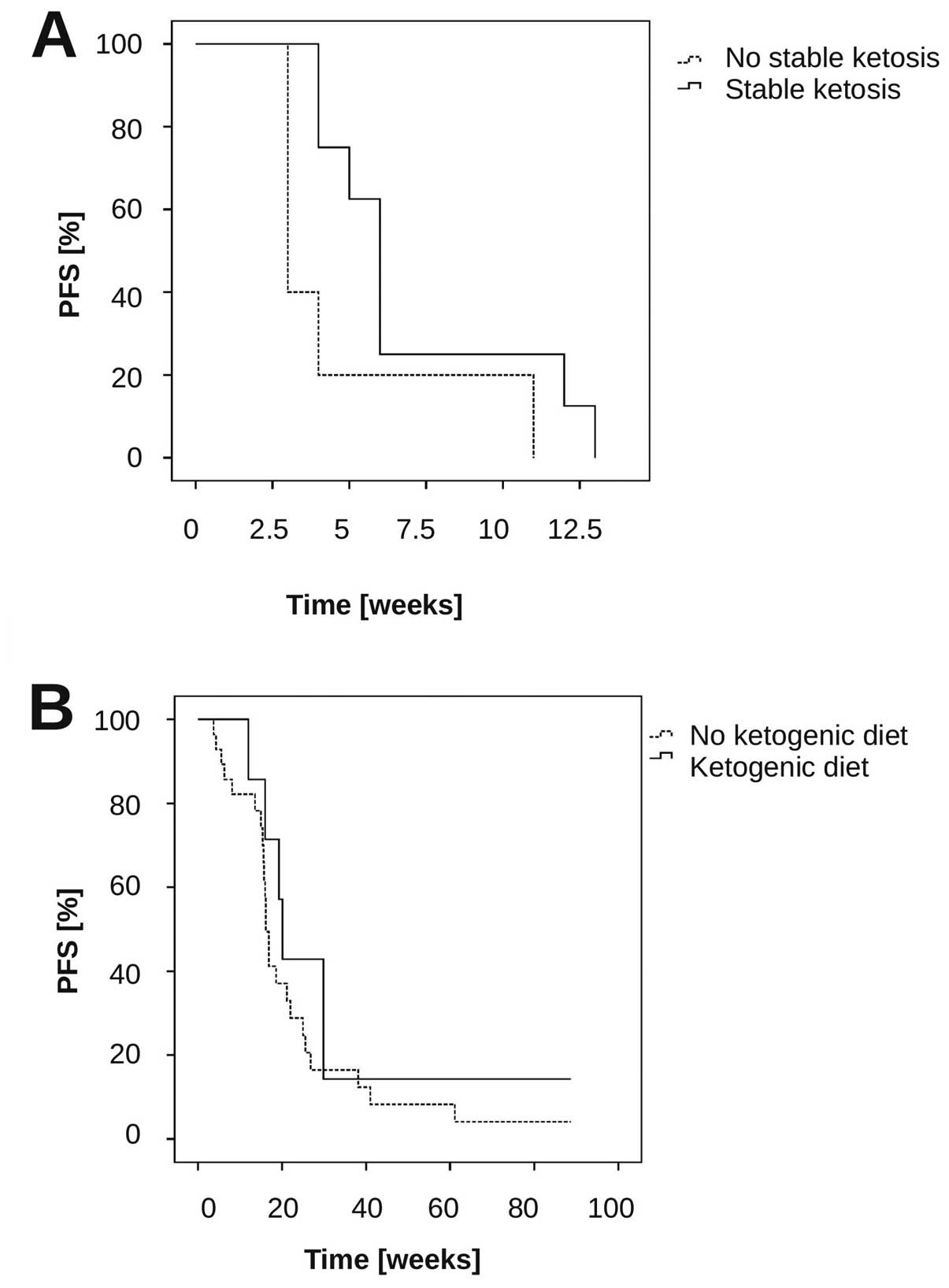
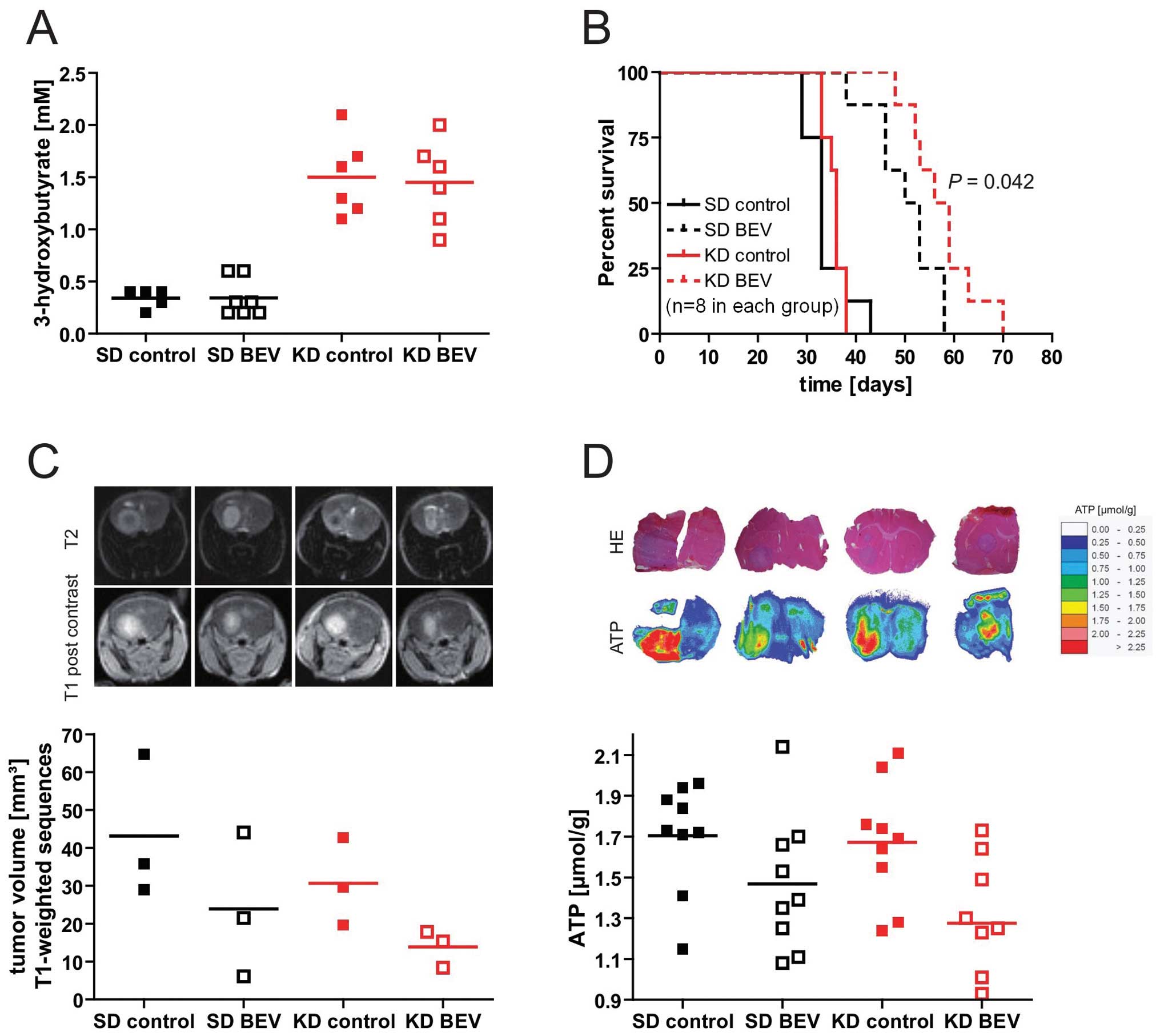
|
1.
|
Warburg O, Posener K and Negelein E: Ueber
den Stoffwechsel der Tumoren. Biochem Z. 152:319–344. 1924.(in
German).
|
|
2.
|
TCGA. Comprehensive genomic
characterization defines human glioblastoma genes and core
pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Elstrom RL, Bauer DE, Buzzai M, Karnauskas
R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM and
Thompson CB: Akt stimulates aerobic glycolysis in cancer cells.
Cancer Res. 64:3892–3899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Matoba S, Kang J, Patino WD, Wragg A,
Boehm M, Gavrilova O, Hurley PJ, Bunz F and Hwang PM: p53 regulates
mitochondrial respiration. Science. 312:1650–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wanka C, Brucker DP, Bähr O,
Ronellenfitsch M, Weller M, Steinbach JP and Rieger J: Synthesis of
cytochrome c oxidase 2: a p53-dependent metabolic regulator that
promotes respiratory function and protects glioma and colon cancer
cells from hypoxia-induced cell death. Oncogene. 31:3764–3776.
2012. View Article : Google Scholar
|
|
6.
|
Bensaad K, Tsuruta A, Selak MA, Vidal MNC,
Nakano K, Bartrons R, Gottlieb E and Vousden KH: TIGAR, a
p53-inducible regulator of glycolysis and apoptosis. Cell.
126:107–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wanka C, Steinbach JP and Rieger J:
Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects
glioma cells from starvation-induced cell death by upregulating
respiration and improving cellular redox homeostasis. J Biol Chem.
287:33436–33446. 2012. View Article : Google Scholar
|
|
8.
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
down-regulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Liu Y, Li Y, Tian R, Liu W, Fei Z, Long Q,
Wang X and Zhang X: The expression and significance of HIF-1alpha
and GLUT-3 in glioma. Brain Res. 1304:149–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Roslin M, Henriksson R, Bergström P,
Ungerstedt U and Bergenheim AT: Baseline levels of glucose
metabolites, glutamate and glycerol in malignant glioma assessed by
stereo-tactic microdialysis. J Neurooncol. 61:151–160. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Padma MV, Said S, Jacobs M, Hwang DR,
Dunigan K, Satter M, Christian B, Ruppert J, Bernstein T, Kraus G
and Mantil JC: Prediction of pathology and survival by FDG PET in
gliomas. J Neurooncol. 64:227–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hirata K, Terasaka S, Shiga T, Hattori N,
Magota K, Kobayashi H, Yamaguchi S, Houkin K, Tanaka S, Kuge Y and
Tamaki N: (18)F-Fluoromisonidazole positron emission tomography may
differentiate glioblastoma multiforme from less malignant gliomas.
Eur J Nucl Med Mol Imaging. 39:760–770. 2012. View Article : Google Scholar
|
|
14.
|
Maher EA, Marin-Valencia I, Bachoo RM,
Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C,
Madden C, Mathews D, Pascual JM, Mickey BE, Malloy CR and
Deberardinis RJ: Metabolism of [U-(13) C]glucose in human brain
tumors in vivo. NMR Biomed. 25:1234–1244. 2012.
|
|
15.
|
Marin-Valencia I, Yang C, Mashimo T, Cho
S, Baek H, Yang X, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z,
Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, Matés JM, Pascual JM,
Maher EA, Malloy CR, Deberardinis RJ and Bachoo RM: Analysis of
tumor metabolism reveals mitochondrial glucose oxidation in
genetically diverse human glioblastomas in the mouse brain in vivo.
Cell Metab. 15:827–837. 2012. View Article : Google Scholar
|
|
16.
|
Ward PS and Thompson CB: Metabolic
reprogramming: a cancer hallmark even Warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Jeon S, Chandel NS and Hay N: AMPK
regulates NADPH homeostasis to promote tumour cell survival during
energy stress. Nature. 485:661–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Goodwin PJ, Ennis M, Pritchard KI, Trudeau
ME, Koo J, Madarnas Y, Hartwick W, Hoffman B and Hood N: Fasting
insulin and outcome in early-stage breast cancer: results of a
prospective cohort study. J Clin Oncol. 20:42–51. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wolk A, Mantzoros CS, Andersson SO,
Bergström R, Signorello LB, Lagiou P, Adami HO and Trichopoulos D:
Insulin-like growth factor 1 and prostate cancer risk: a
population-based, case-control study. J Natl Cancer Inst.
90:911–915. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Allen NE, Key TJ, Appleby PN, Travis RC,
Roddam AW, Rinaldi S, Egevad L, Rohrmann S, Linseisen J, Pischon T,
Boeing H, Johnsen NF, Tjønneland A, Grønbaek H, Overvad K, Kiemeney
L, Bueno-de-Mesquita HB, Bingham S, Khaw KT, Tumino R, Berrino F,
Mattiello A, Sacerdote C, Palli D, Quirós JR, Ardanaz E, Navarro C,
Larrañaga N, Gonzalez C, Sanchez M, Trichopoulou A, Travezea C,
Trichopoulos D, Jenab M, Ferrari P, Riboli E and Kaaks R: Serum
insulin-like growth factor (IGF)-I and IGF-binding protein-3
concentrations and prostate cancer risk: results from the European
Prospective Investigation into Cancer and Nutrition. Cancer
Epidemiol Biomarkers Prev. 16:1121–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Renehan AG, Egger M, Minder C, O’Dwyer ST,
Shalet SM and Zwahlen M: IGF-I, IGF binding protein-3 and breast
cancer risk: comparison of 3 meta-analyses. Int J Cancer.
115:1006–1007; author reply, 1008, 2005.
|
|
22.
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin: response to
Farooki and Schneider. Diabetes Care. 29:1990–1991. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Derr RL, Ye X, Islas MU, Desideri S,
Saudek CD and Grossman SA: Association between hyperglycemia and
survival in patients with newly diagnosed glioblastoma. J Clin
Oncol. 27:1082–1086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zhou W, Mukherjee P, Kiebish MA, Markis
WT, Mantis JG and Seyfried TN: The calorically restricted ketogenic
diet, an effective alternative therapy for malignant brain cancer.
Nutr Metab (Lond). 4:52007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Marsh J, Mukherjee P and Seyfried TN:
Akt-dependent proapoptotic effects of dietary restriction on
late-stage management of a phosphatase and tensin
homologue/tuberous sclerosis complex 2-deficient mouse astrocytoma.
Clin Cancer Res. 14:7751–7762. 2008. View Article : Google Scholar
|
|
26.
|
Stafford P, Abdelwahab MG, Kim DY, Preul
MC, Rho JM and Scheck AC: The ketogenic diet reverses gene
expression patterns and reduces reactive oxygen species levels when
used as an adjuvant therapy for glioma. Nutr Metab (Lond).
7:74:2010
|
|
27.
|
Kossoff EH, Rowley H, Sinha SR and Vining
EPG: A prospective study of the modified Atkins diet for
intractable epilepsy in adults. Epilepsia. 49:316–319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shai I, Schwarzfuchs D, Henkin Y, Shahar
DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H,
Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z,
Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M,
Stumvoll M and Stampfer MJ: Weight loss with a low-carbohydrate,
Mediterranean, or low-fat diet. N Engl J Med. 359:229–241. 2008.
View Article : Google Scholar
|
|
29.
|
Fraser DA, Thoen J, Bondhus S, Haugen M,
Reseland JE, Djøseland O, Førre O and Kjeldsen-Kragh J: Reduction
in serum leptin and IGF-1 but preserved T-lymphocyte numbers and
activation after a ketogenic diet in rheumatoid arthritis patients.
Clin Exp Rheumatol. 18:209–214. 2000.
|
|
30.
|
Accurso A, Bernstein RK, Dahlqvist A,
Draznin B, Feinman RD, Fine EJ, Gleed A, Jacobs DB, Larson G,
Lustig RH, Manninen AH, McFarlane SI, Morrison K, Nielsen JV,
Ravnskov U, Roth KS, Silvestre R, Sowers JR, Sundberg R, Volek JS,
Westman EC, Wood RJ, Wortman J and Vernon MC: Dietary carbohydrate
restriction in type 2 diabetes mellitus and metabolic syndrome:
time for a critical appraisal. Nutr Metab (Lond). 5:92008.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Nebeling LC, Miraldi F, Shurin SB and
Lerner E: Effects of a ketogenic diet on tumor metabolism and
nutritional status in pediatric oncology patients: two case
reports. J Am Coll Nutr. 14:202–208. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Schmidt M, Pfetzer N, Schwab M, Strauss I
and Kämmerer U: Effects of a ketogenic diet on the quality of life
in 16 patients with advanced cancer: A pilot trial. Nutr Metab
(Lond). 8:542011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Fine EJ, Segal-Isaacson CJ, Feinman RD,
Herszkopf S, Romano MC, Tomuta N, Bontempo AF, Negassa A and
Sparano JA: Targeting insulin inhibition as a metabolic therapy in
advanced cancer: a pilot safety and feasibility dietary trial in 10
patients. Nutrition. 28:1028–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Macdonald DR, Cascino TL, Schold SCJ and
Cairncross JG: Response criteria for phase II studies of
supratentorial malignant glioma. J Clin Oncol. 8:1277–1280.
1990.PubMed/NCBI
|
|
35.
|
Mueller-Klieser W and Walenta S:
Geographical mapping of metabolites in biological tissue with
quantitative bioluminescence and single photon imaging. Histochem
J. 25:407–420. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Maurer GD, Brucker DP, Bähr O, Harter PN,
Hattingen E, Walenta S, Mueller-Klieser W, Steinbach JP and Rieger
J: Differential utilization of ketone bodies by neurons and glioma
cell lines: a rationale for ketogenic diet as experimental glioma
therapy. BMC Cancer. 11:3152011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chang HT, Olson LK and Schwartz KA:
Ketolytic and glycolytic enzymatic expression profiles in malignant
gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond).
10:472013. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Steinbach JP, Wolburg H, Klumpp A, Probst
H and Weller M: Hypoxia-induced cell death in human malignant
glioma cells: energy deprivation promotes decoupling of
mitochondrial cytochrome c release from caspase processing and
necrotic cell death. Cell Death Differ. 10:823–832. 2003.
View Article : Google Scholar
|
|
39.
|
Rieger J, Bähr O, Müller K, Franz K,
Steinbach J and Hattingen E: Bevacizumab-induced
diffusion-restricted lesions in malignant glioma patients. J
Neurooncol. 99:49–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
de Groot JF, Fuller G, Kumar AJ, Piao Y,
Eterovic K, Ji Y and Conrad CA: Tumor invasion after treatment of
glioblastoma with bevacizumab: radiographic and pathologic
correlation in humans and mice. Neuro Oncol. 12:233–242.
2010.PubMed/NCBI
|
|
41.
|
DeLay M, Jahangiri A, Carbonell WS, Hu Y,
Tsao S, Tom MW, Paquette J, Tokuyasu TA and Aghi MK: Microarray
analysis verifies two distinct phenotypes of glioblastomas
resistant to antiangiogenic therapy. Clin Cancer Res. 18:2930–2942.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Hattingen E, Jurcoane A, Bähr O, Rieger J,
Magerkurth J, Anti S, Steinbach JP and Pilatus U: Bevacizumab
impairs oxidative energy metabolism and shows antitumoral effects
in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic
resonance imaging study. Neuro Oncol. 13:1349–1363. 2011.
View Article : Google Scholar
|
|
43.
|
Tamura H, Hatazawa J, Toyoshima H,
Shimosegawa E and Okudera T: Detection of deoxygenation-related
signal change in acute ischemic stroke patients by
T2*-weighted magnetic resonance imaging. Stroke.
33:967–971. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Seiler A, Jurcoane A, Magerkurth J, Wagner
M, Hattingen E, Deichmann R, Neumann-Haefelin T and Singer OC: T2’
imaging within perfusion-restricted tissue in high-grade occlusive
carotid disease. Stroke. 43:1831–1836. 2012.
|
|
45.
|
Otto C, Kaemmerer U, Illert B, Muehling B,
Pfetzer N, Wittig R, Voelker HU, Thiede A and Coy JF: Growth of
human gastric cancer cells in nude mice is delayed by a ketogenic
diet supplemented with omega-3 fatty acids and medium-chain
triglycerides. BMC Cancer. 8:1222008. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Liu AG, Most MM, Brashear MM, Johnson WD,
Cefalu WT and Greenway FL: Reducing the glycemic index or
carbohydrate content of mixed meals reduces postprandial glycemia
and insulinemia over the entire day but does not affect satiety.
Diabetes Care. 35:1633–1637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Mukherjee P, Mulrooney TJ, Marsh J, Blair
D, Chiles TC and Seyfried TN: Differential effects of energy stress
on AMPK phosphorylation and apoptosis in experimental brain tumor
and normal brain. Mol Cancer. 7:372008. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Sarkar NH, Fernandes G, Telang NT,
Kourides IA and Good RA: Low-calorie diet prevents the development
of mammary tumors in C3H mice and reduces circulating prolactin
level, murine mammary tumor virus expression, and proliferation of
mammary alveolar cells. Proc Natl Acad Sci USA. 79:7758–7762. 1982.
View Article : Google Scholar
|
|
49.
|
Giovanella BC, Shepard RC, Stehlin JS,
Venditti JM and Abbott BJ: Calorie restriction: effect on growth of
human tumors heterotransplanted in nude mice. J Natl Cancer Inst.
68:249–257. 1982.PubMed/NCBI
|
|
50.
|
Kalaany NY and Sabatini DM: Tumours with
PI3K activation are resistant to dietary restriction. Nature.
458:725–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Lee C, Raffaghello L, Brandhorst S, Safdie
FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S,
Merlino A, Emionite L, de Cabo R and Longo VD: Fasting cycles
retard growth of tumors and sensitize a range of cancer cell types
to chemotherapy. Sci Transl Med. 4:124ra272012.PubMed/NCBI
|
|
52.
|
Abdelwahab MG, Fenton KE, Preul MC, Rho
JM, Lynch A, Stafford P and Scheck AC: The ketogenic diet is an
effective adjuvant to radiation therapy for the treatment of
malignant glioma. PLoS ONE. 7:e361972012. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Zuccoli G, Marcello N, Pisanello A,
Servadei F, Vaccaro S, Mukherjee P and Seyfried TN: Metabolic
management of glioblastoma multiforme using standard therapy
together with a restricted ketogenic diet: case report. Nutr Metab
(Lond). 7:332010. View Article : Google Scholar : PubMed/NCBI
|