Introduction
Head and neck cancer includes a large group of
epithelial malignancies, originated from the oral, nasal, larynx
and pharynx areas, and is mostly described as squamous cell
carcinoma. This type of cancer is frequently common in regions with
high consumption of tobacco and alcohol, e.g., Western and Southern
Europe, Southern Africa and South-Central Asia (1). More than 400,000 deaths from this
disease occur each year; 80% of them in developing countries
(2). Numerous gene-expression
profiling studies of head and neck cancer have revealed a variety
of gene-expression alterations among normal squamous cells and
cancerous tumor cells, in metastatic and non-metastatic lesions,
and among both young and older patients. In most tested cases,
gene-expression changes were correlated with cell cycle regulation
and cell proliferation (3). Recent
reports on cancer and the carcinogenesis pathway refer to the
disease as a polyepigenetic process, apart from its polygenetic
properties (4). In addition, DNA
hypermethylation of tumor-suppressor genes, which are responsible
for gene silencing, was reported to be the main recurrent
epigenetic event detected in cancer cells (5). Currently, a number of DNA methylation
inhibitors are undergoing clinical trials for treatment of a wide
variety of cancer types, including head and neck. Zebularine was
originally synthesized as a cytidine deaminase inhibitor (6), however, a growing number of studies
have reported its behavior as a cytidine analogue, behavior that is
responsible for its inhibition of DNA methylation. Zebularine
application to cancer cells elicited gene response that was
correlated with cancer-related antigens (7) and was involved in apoptosis induction
(8), which suggests that
zebularine might exhibit antitumor potential in cancer cells.
Unlike its analogues, 5-Aza-CR and 5-Aza-CdR, which are cytotoxic
to treated cells, zebularine is more tolerated by the cells and
exhibits only reduced toxicity when administered. Other studies
reported cell cycle arrest and apoptosis induced by zebularine in
numerous types of cancers, e.g. breast cancer (9), bladder cancer (10), gastric cancer (11), cervical cancer (12) and oral squamous carcinoma (13–15).
However, to date, only limited data have been published regarding
the course of action of zebularine in head and neck cancer cells.
In the present study we addressed the hypothesis that zebularine
plays an important role in induction of apoptosis in head and neck
cancer cells; the aim was to evaluate our hypothesis regarding
whether and how zebularine is capable of inhibiting proliferation
and inducing apoptosis in head and neck cancer cells.
Materials and methods
Cell lines and culture
Head and neck cancer cell lines SCC-9 and SCC-25
(obtained from ATTC, Bethesda, MD, USA) were grown in 25 or
75-cm2 sterile flasks and maintained in a humidified
atmosphere of 5% CO2 at 37°C in MEM: F12 supplemented
with 5% fetal bovine serum (Biological Industries, Beit-Haemek,
Israel), and 2.5 mM L-glutamine, hydrocortisone at 400 ng/ml,
penicillin at 100 U/ml, and streptomycin at 100 μg/ml
(Biological Industries). Cells were seeded at a density of 50–60%
for initiation of each experiment and after 24 h the medium was
changed to media containing various concentrations of zebularine,
for another 24 to 96 h.
Cell proliferation assays
The cells were treated with 50–1,000 μM of
zebularin for 24 to 96 h. Cell viability was assayed with the XTT
Cell Proliferation Kit (Biological Industries), according to the
manufacturer’s protocol. Results were calculated as percentages of
the proliferation in the vehicle control.
DNA synthesis
BrdU was detected immunochemically with a
5-Bromo-2′-deoxy-uridine Labeling and Detection Kit (Roche,
Mannheim, Germany), which assesses BrdU levels incorporated into
newly synthesized DNA. Zebularine-treated cells were incubated with
10 μM BrdU for 4 h, and incorporated BrdU was detected with
the monoclonal anti-BrdU-POD and Fab fragments according to the
manufacturer’s protocol.
Flow cytometry
Cells were plated at a density of 60% in 10-cm
culture dishes and after 24 h were treated with 350 μM
zebularine for 24 to 120 h, after which they were trypsinized and
washed with PBS and then fixed with 70% EtOH for 1 h. They were
then incubated with 0.1% NP-40 for 5 min at 4°C, washed once more
with PBS, and incubated with RNase at 100 μg/ml and PI at 50
μg/ml (Sigma-Aldrich Ltd, Rehovot, Israel) for 20 min.
Finally, the cells were analyzed with a FACSCalibur flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Cytotoxicity assay
A Cytotoxicity Detection Kit (Roche) was used to
quantify cytotoxicity/cytolysis levels following zebularine
treatment. This method is based on measurement of LDH activity
released from damaged cells using 96-well plate. Cells were
cultured overnight in a 96-well plate and then treated with
zebularine at 50–1,000 μg. Twenty-four hours post-treatment
LDH activity levels were measured at a wavelength of 492 nm.
TUNEL assay for apoptosis detection
Apoptotic cells were detected with an In Situ Cell
Death Detection Kit (Roche) according to the manufacturer’s
protocol. In brief, cells were grown overnight in an 8-chamber
slide and then were treated with zebularine; after a further 96 h
they were fixed and permeabilized, and were exposed to TUNEL
reaction mixture for 60 min at 37°C in the dark. Samples were
analyzed under a fluorescence microscope (515–565 nm).
Methyl-specific PCR (MSP)
Genomic DNA was extracted from cells with the High
Pure PCR Template Preparation kit (Roche), and the DNA was
subjected to bisulfite treatment with the EpiTec Bisulfite kit
(Qiagen, Valencia, CA, USA). The methylation status of p21
and CHK1 genes were determined by MSP assay with the EpiTect
MSP kit (Qiagen) according to the manufacturer’s protocol,
supplemented with specific primers for methylated and unmethylated
forms, in accordance with previous reports (16,17).
The amplified samples underwent electrophoresis on 2% agarose gel
and were visualized with an XRS Molecular Imager (Bio-Rad
Laboratories Ltd, Rishon Le Zion, Israel).
Real-time PCR
The real-time PCR method was used for
gene-expression quantification experiments. Relative quantitation
of target genes was compared with that of an internal standard gene
(β-actin). In summary, 1 μl of suspension containing 50
μg of cDNA was mixed with SYBR Master mix (Applied
Biosystems, Foster City, CA, USA) and with the following primers:
p21, forward 5′-GGCAGACCAGCATG ACAGATT-3′; and reverse
5′-TCCTGTGGGCGGATTAGG-3′; CHK1, forward 5′-CCCGCACAGGTCTTTCCTT-3′;
and reverse 5′-GGCGGGAAAAGCTGATCC-3′. Results were calculated as RQ
and analyzed with Step-One software (Applied Biosystems).
Western blot analysis
Treated cells were collected by using trypsin,
washed once and lysed with Lou’s glycerol lysis buffer on ice for
10 min, and the protein fraction was separated by centrifugation
for 15 min at 1,500 rpm. Protein samples were electrophoresed on
non-denaturing 10% sodium dodecyl sulfate-polyacrylamide gels at
120 V for 1.5 h. Then, protein samples were transferred by semi-dry
transfer to a 0.45-micrometer-pore-size nitrocellulose membrane.
The membrane was blocked with 5% BSA for 1 h at room temperature,
following incubation with primary antibodies: monoclonal rabbit
anti-caspase 3 (Abcam, Cambridge, UK); polyclonal rabbit anti-PARP
(Cell Signaling, Danvers, MA, USA); and polyclonal rabbit anti-p21,
polyclonal mouse anti-chk1, and monoclonal mouse anti-β-actin
(Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membrane was
then washed three times with TBST, each for 10 min, incubated with
secondary antibody conjugated to horseradish peroxidase (Jackson
Immune Research Laboratories and DAKO, Glostrup, Denmark) for 1 h
at room temperature, and washed three times. Antigen/antibody
complex was detected with the ECL System (Biological Industries).
Western blot analysis results were quantified by means of Quantity
One software (Bio-Rad Laboratories Ltd). β-actin levels (as
standard protein occurring naturally in these cells) were taken as
a reference.
Statistical analysis
Statistical analyses were performed with SPSS
software, and means were compared by the two-tailed Student’s
t-test, one-way analysis of variance (ANOVA), or their
non-parametric counterparts, depending on the desired number of
comparisons. Statistical significance levels were set at
*P<0.05, **P<0.01 and
***P<0.001.
Results
Zebularine inhibits human head and neck
cancer cell growth in a time-dependent manner
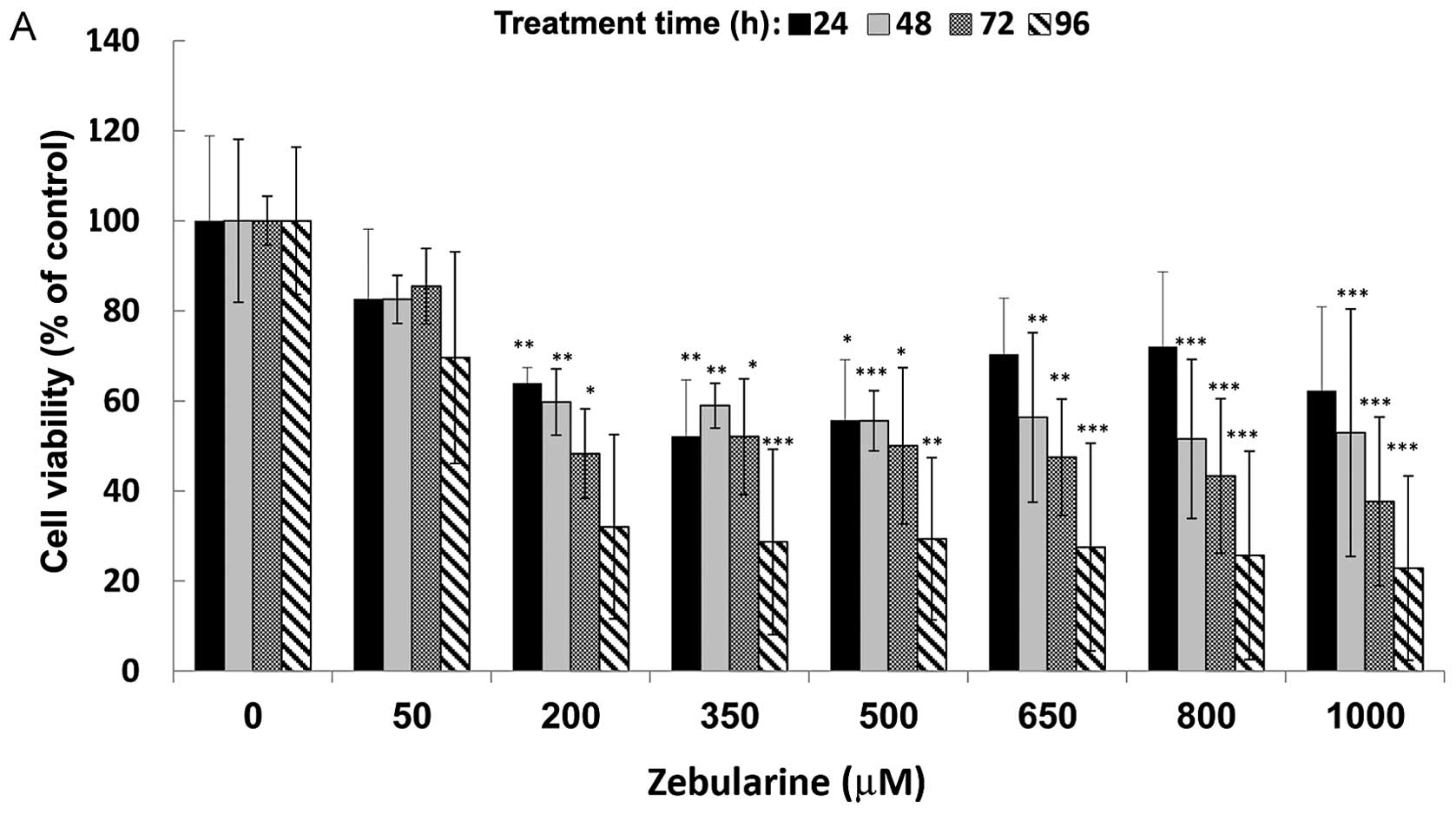
The effect of zebularine on viability of head and
neck cancer cells was measured according to the XTT method. SCC-9,
SCC-25 and normal fibroblast cells were treated with increasing
concentrations of zebularine, from 50 up to 1,000 μM. Cell
viability was measured at time points of 0 to 96 h following
treatment. Overall, 24 h of zebularine treatment did not elicit
significant changes in cell viability (Fig. 1) but at 48 h following treatment,
reduced cell viability was observed in the cell lines SCC-9 and
SCC-25 even with the smallest dose (50 μM). At this point,
we detected, on average, a 20% reduction of cell viability. In
SCC-9 cells, treatment with 200 μM zebularine for 96 h
reduced viability to 50%, and the reduced state stabilized at 70%
on average for zebularine concentrations of 200–1,000 μM,
indicating that the main controlling factor was the duration of
treatment rather than the zebularine concentration (Fig. 1A). The SCC-25 cells were highly
sensitive to zebularine treatment (Fig. 1B): cell viability was reduced
almost to 20% at 96 h post-treatment; treatment for 72 h reduced
cell viability by more than 40%. Interestingly, exposure of normal
fibroblast cells to zebularine had a minor effect on their
viability compared with its effect on cancer cells. The constant
effect of zebularine on these cells resulted in 80% cell viability
for all concentrations, regardless of treatment duration (Fig. 1C). For both SCC-9 and SCC-25,
IC50 was determined as 350 μM for 72 h of
treatment; in contrast, an IC50 value for the fibroblast
cells was indeterminable in these conditions. Furthermore, toxicity
levels of zebularine in the cells were measured with LDH activity
methods (data not shown): the results revealed low levels of
toxicity, which resembled previously published data, and further
strengthened the perception that zebularine is not toxic to human
cells, even at the highest tested concentration of 1,000
μM.
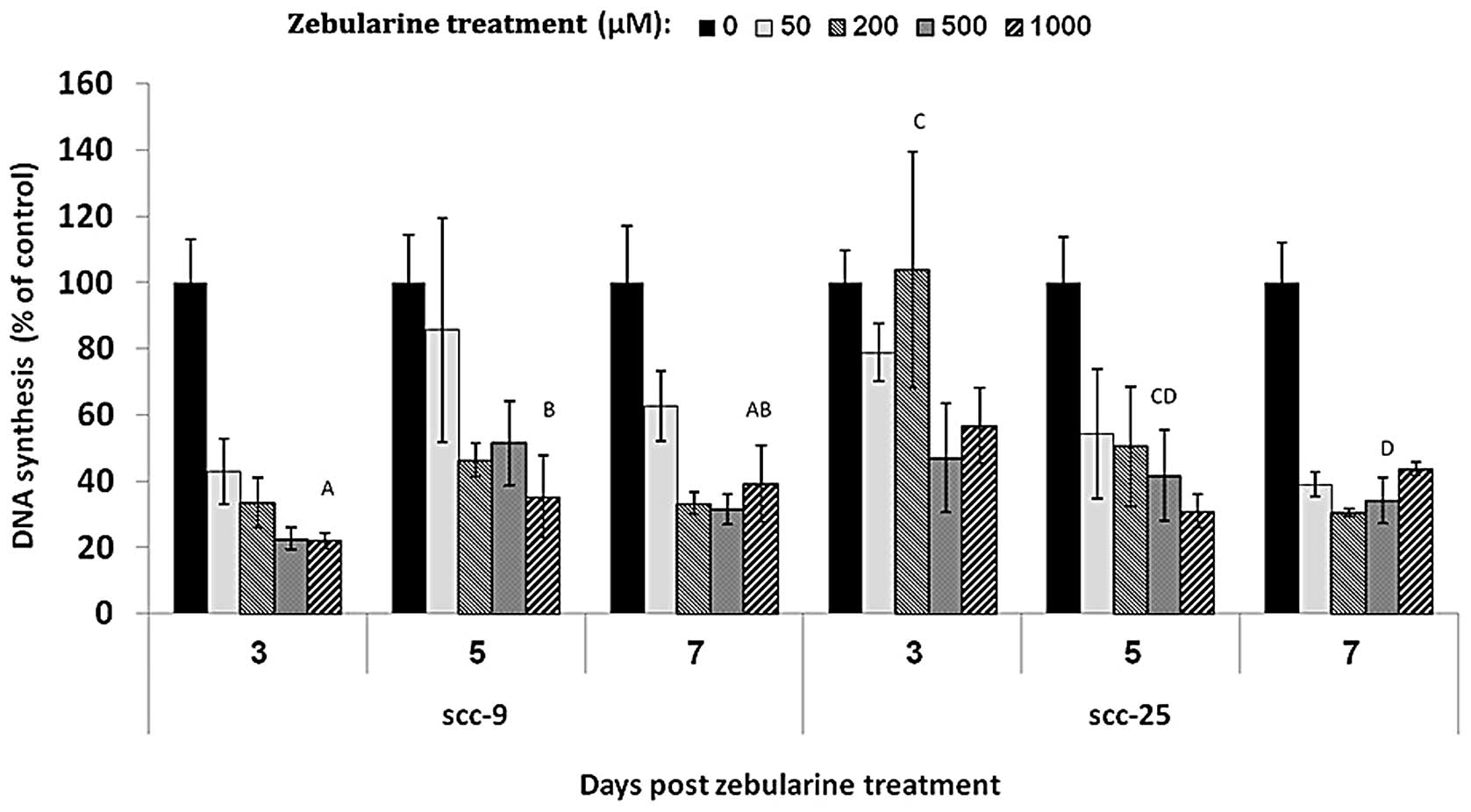
DNA synthesis levels remain depressed
after zebularine withdrawal
It is known that zebularine becomes incorporated
into DNA, and binds DNMT via a covalent link. However, the duration
of its continued influence on cell proliferation, following its
removal was previously not addressed. In order to examine the
continuing influence of zebularine subsequent to drug withdrawal,
DNA synthesis levels were evaluated by BrdU: SCC-9 and SCC-25 cells
were treated for 48 h with four concentrations of zebularine, 50,
200, 500 and 1,000 μM, and DNA synthesis was measured on
days 3, 5 and 7 following zebularine emission. In both cell lines,
zebularine was able to influence newly synthesized DNA: DNA
synthesis was reduced to less than 50% of that in the vehicle
control, and this effect was observed even 7 days after drug
removal (Fig. 2). Moreover, the
reducing effect on DNA synthesis saturated at 50 μM for
SCC-9 cells and at 200 μM for SCC-25 cell lines, and this
effect was constant for all tested times.
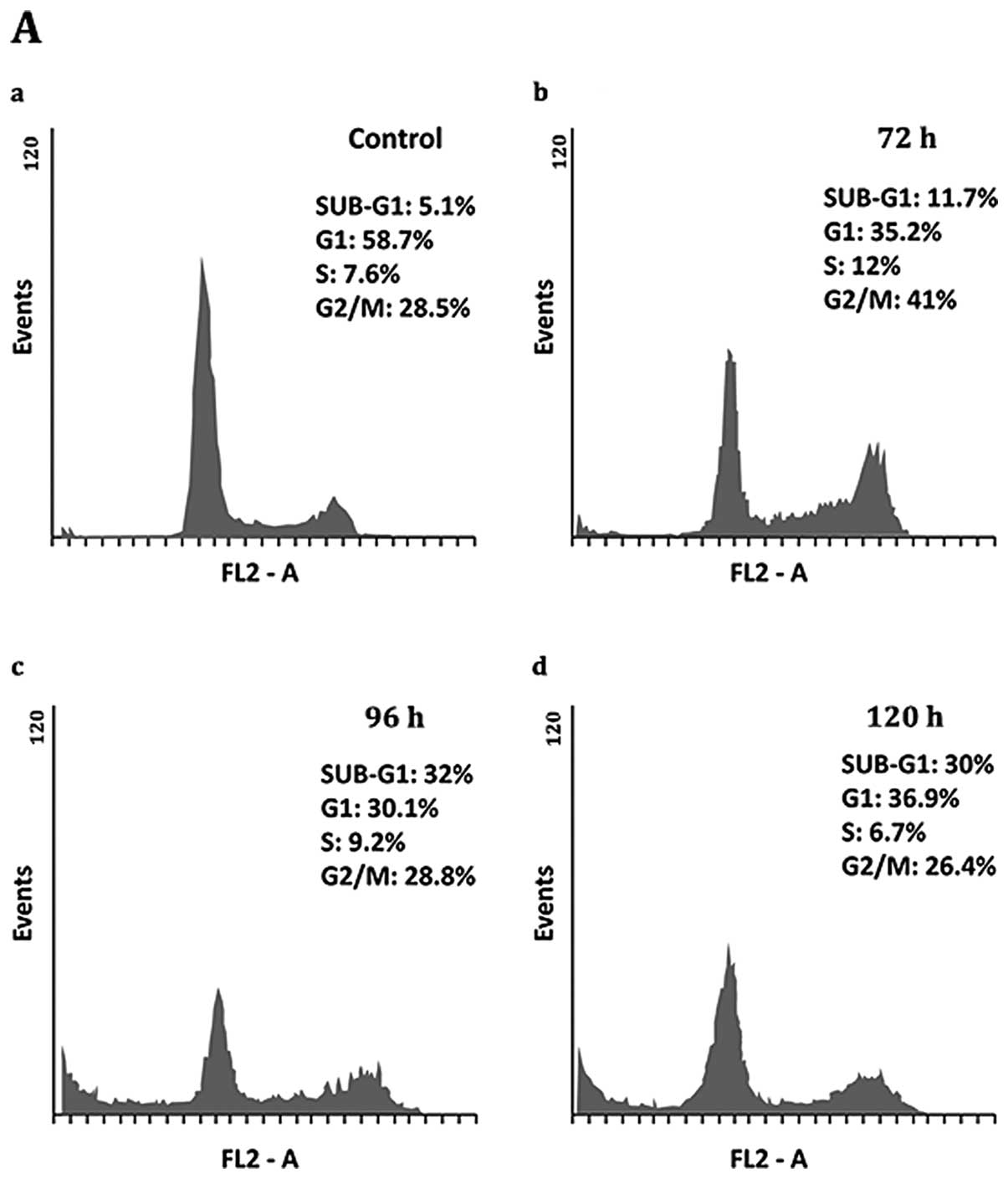
Zebularine induces sub-G1-phase
accumulation in both SCC-9 and SCC-25 cell lines
Subsequent to establishing that zebularine reduced
cell viability and DNA synthesis, the exact influence of the drug
on proliferation in the cell cycle was analyzed by flow cytometry.
Zebularine influenced the cell cycle of treated SCC-9 by causing
cell cycle arrest at G2/M after 72 h of treatment, whereas G2/M of
the control was 28.5% and rose to 41% after 72 h of zebularine
treatment. Moreover, extended periods of treatment; 96 and 120 h,
caused cells to exit from the cell cycle towards the sub-G1 phase,
indicating cell death. Sub-G1 levels at 96 and 120 h post-treatment
were 32 and 30%, respectively, whereas control sub-G1 was
approximately 5% (Fig. 3A and C).
The SCC-25 cells reacted more strongly to zebularine than the SCC-9
cells in which zebularine increased accumulation of cells in sub-G,
intensifying from 3.7% in the control up to approximately 40, 53.9,
and 50% after 72, 96 and 120 h, respectively. Furthermore, the
percentages of cells in the S and G2/M phases were unchanged as a
result of the treatment, whereas numbers of cells in the G1 phase
fell from about 54 to 20%, on average, for all treatment times. It
is noteworthy that the effect of zebularine did not increase in a
time-dependent manner, demonstrating that drug saturation was
already reached at 72 h of treatment (Fig. 3B and D).
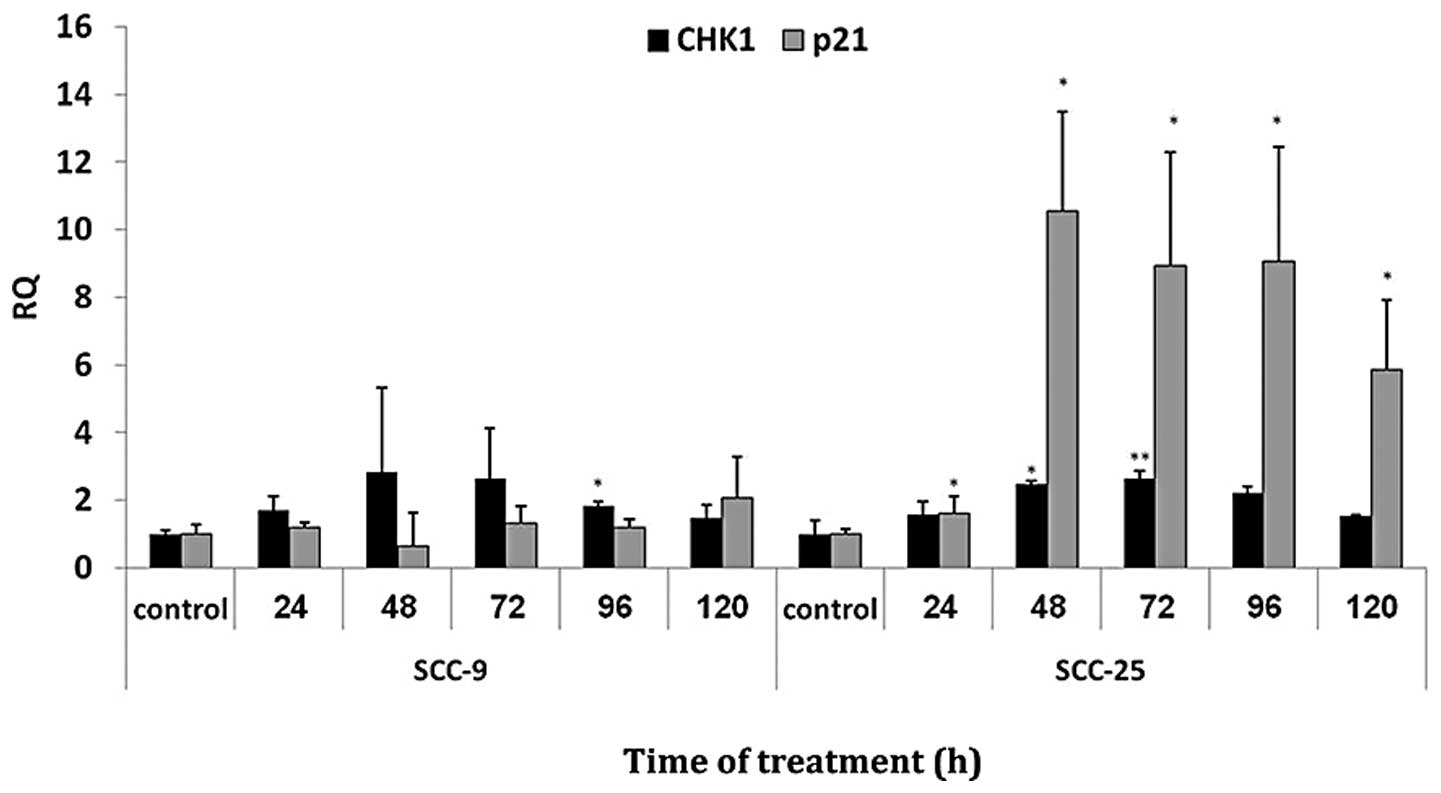
Zebularine regulates upregulation of p21
and CHK1
A total of 48 cell cycle related genes were analyzed
following zebularine treatment (data not shown), in which 2 cell
cycle-checkpoint regulated genes namely p21 and CHK1
exhibited upregulated levels (RQ>2). Real-time PCR was used to
ratify the expression levels of p21 and CHK1 mRNA in
the cell lines (Fig. 4):
p21 mRNA expression levels showed no significant change in
SCC-9, but in SCC-25 cells, p21 expression levels increased
significantly (P<0.05), starting at 48 h of treatment. For
CHK1, significant increase (P<0.05) was detected in both
SCC-9 and SCC-25 cells at 96 and 48 h of treatment, respectively.
Longer treatment times elicited no significant change in the
CHK1 expression level.
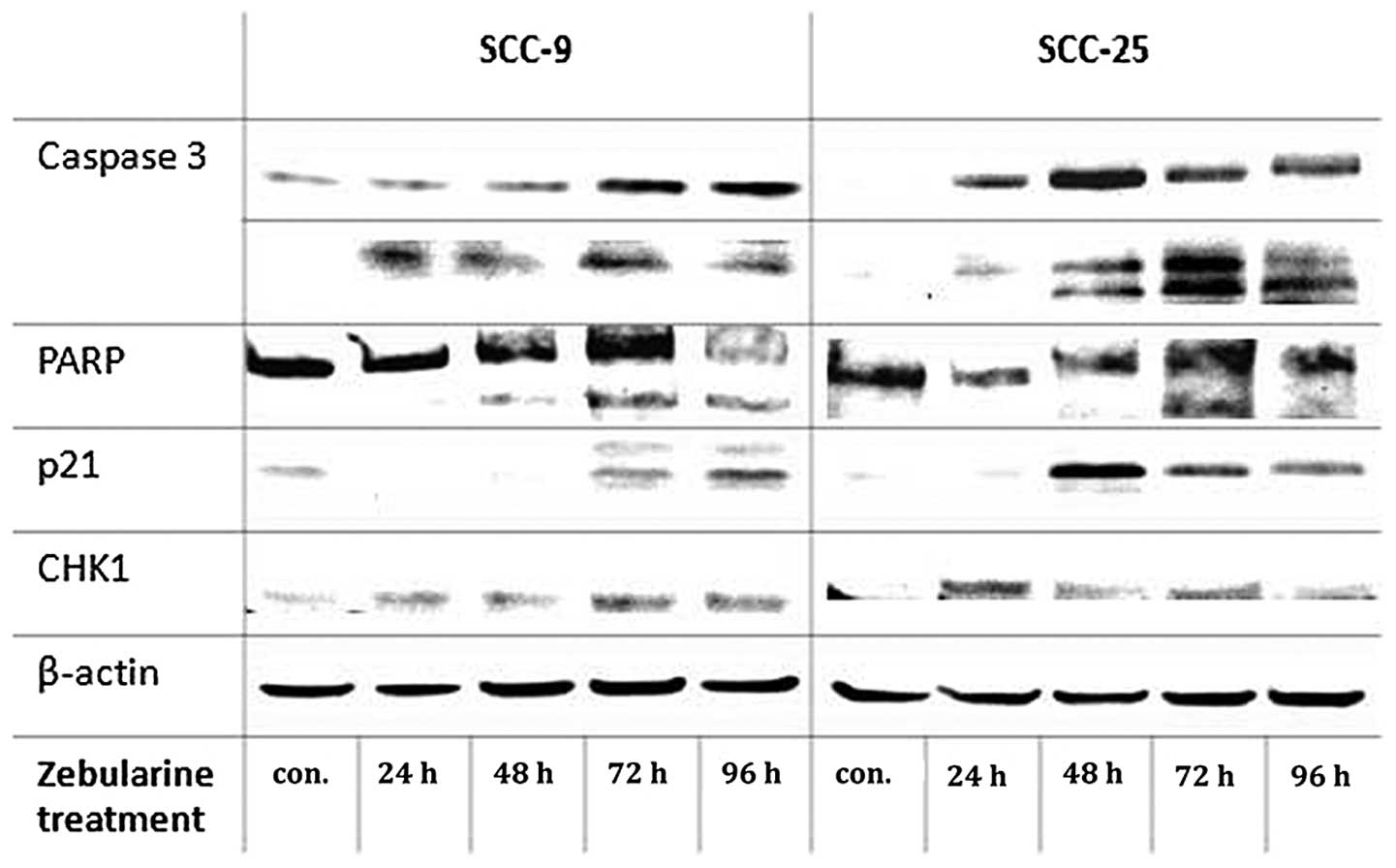
Upregulation of p21 protein following zebularine
treatment was observed in the two cell lines, for SCC-9 extreme
upregulation of the protein was observed at 96 h of treatment and
in SCC-25 cells, p21 protein showed upregulation at 48 h of
treatment. CHK1 protein exhibited basal levels in the control SCC-9
cells, and its expression level was slight increases after 72 and
96 h. The protein levels of CHK1 increased at 24 h and vaguely at
72 h of zebularine treatment in SCC-25 cells (Fig. 5).
Zebularine regulated apoptosis-related
proteins; caspase 3 and PARP in head and neck cancer cells
Accumulation of cells in the sub-G1 phase indicates
passage of cells through an apoptotic or necrotic process. In
studying this behavior we conducted a series of apoptosis-detecting
assays, starting with the TUNEL assay and proceeding through
western blot analysis, in which we assessed the expression of
apoptosis- and cell cycle-related proteins. Western blot analysis
of two key apoptosis-associated proteins, PARP and caspase 3,
demonstrated PARP cleavage and induction levels of an activated
form of caspase 3 (Fig. 5). The
maximum levels of the activated proteins were achieved at 72 h of
treatment, after which there was a slight reduction. The cell lines
differed regarding the increasing levels of caspase 3: after 72 h
of treatment, the active form (85 kDa) showed high levels in SCC-9
cells, 10 times more those in the controls, whereas in SCC-25 cells
there were only 4 times more, at most, after the same time
interval.
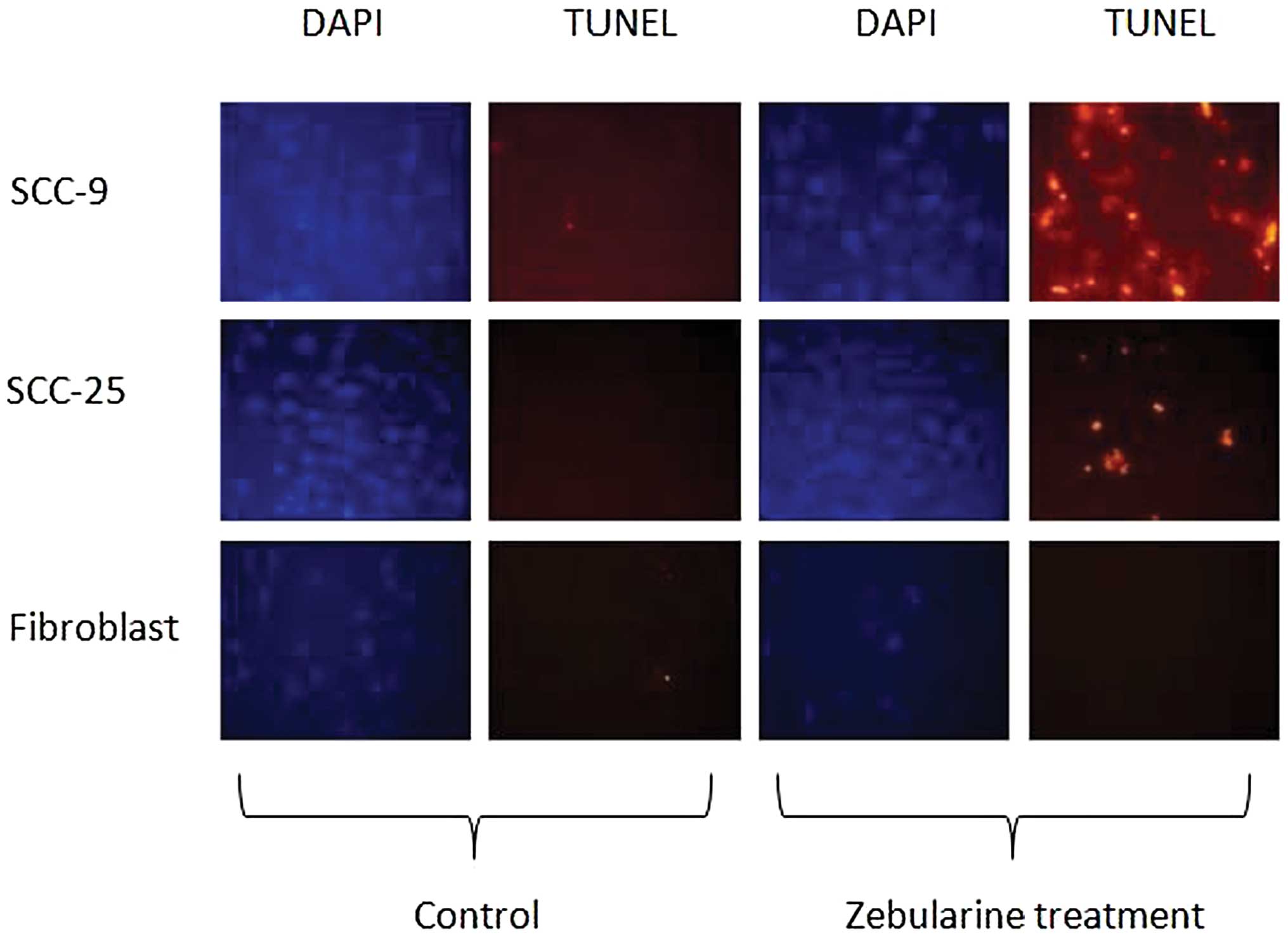
The TUNEL assay results demonstrated the presence of
apoptotic cells among both SCC-9 and SCC-25 cells (Fig. 6). Expectedly, the fibroblast cells
did not show any signs of apoptosis following treatment with
zebularine, a finding that supported the XTT results.
Zebularine regulates p21 and CHK1
independently of promoter methylation
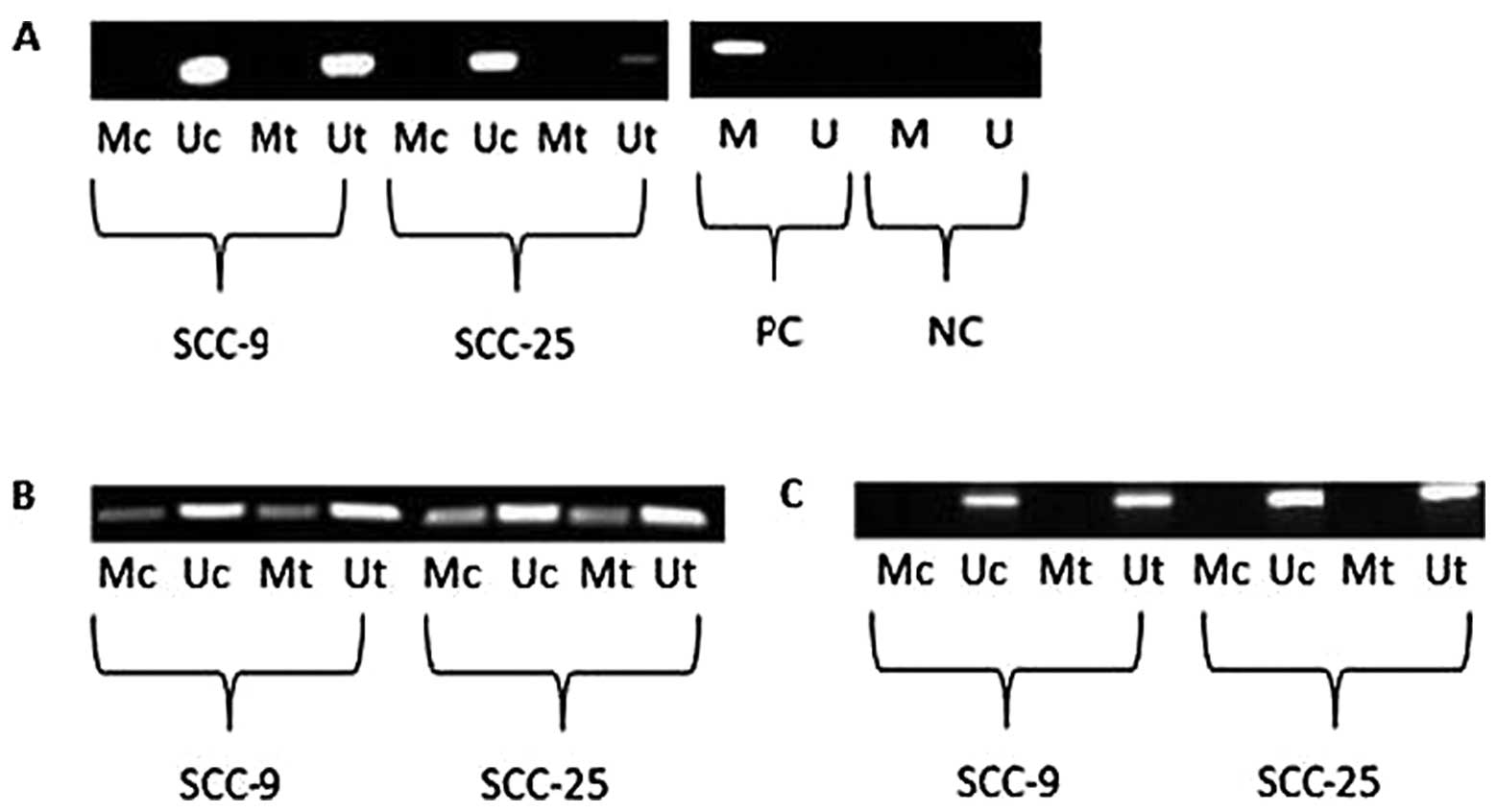
Effects of aberrant methylation of the p21
and CHK1 genes in the cell lines were tested following their
regulation. In the case of p21, one region of approximately
300 bp in the promoter region was analyzed, whereas for
CHK1, two regions in the promoter area, each of 300 bp, were
analyzed. The results of p21 promoter methylation analysis
revealed merely amplification of the unmethylated region in both
cell lines following 72 h of zebularine treatment (Fig. 7A). However, the two sections of the
CHK1 promoter showed differing results (Fig. 7B and C). In one section, both
methylated and unmethylated primers elicited amplification in
treated cells as well as in control cells (Fig. 7B), and the ratio between the bands
was unchanged following zebularine treatment. In the second section
(Fig. 7C), only the unmethylated
primers were capable of amplifying bands, indicating that
methylation caused no modification in that segment. Since the
effects of the CHK1 promoter in the first segment were
ambiguous with respect to methylation status, a more sensitive
method, bisulfite genomic sequencing (BGS), was used for
methylation analysis. Methylation patterns were similar for both
SCC-9 and SCC-25 cells, in which none of the tested CG positions
were methylated, either in the control or the treated cells (data
not shown).
Discussion
The present results indicate that zebularine reduced
cell viability of human head and neck cancer cells in vitro.
This effect was found to be mediated through induction of
apoptosis, as confirmed by TUNEL assay and activation of
pre-apoptotic genes. Interestingly, zebularine had no effect on
normal human fibroblast cells. Thus, the overall effect of
zebularine was reduction of cell viability in a time-dependent
rather than dose-dependent manner, as reported recently also by You
and Park (12). The present
findings match those of previous studies of the effects of
zebularine on numerous cancer types, e.g., breast, bladder,
cervical, and head and neck cancers (9,10,12-15).
A study by Cheng et al (18) also supported these results in which
they showed that continuous treatment with zebularine substantially
retarded the growth of human cancer cell lines, with little affect
on growth of normal human fibroblasts.
Current studies concerning zebularine and other
methyltransferase inhibitors are focusing mainly on combinations of
these inhibitors with established chemotherapeutic drugs in order
to sensitize treated tumors. However, remethylation and resilencing
of tumor-suppressor genes is a common problem with
methylation-inhibitor drugs, and it potentially can cause
complications in clinical application, which requires continuous
drug administration. To date, the full duration of zebularine
influence on cancer cell proliferation following its emission was
not approached, but in the present study, we were able to show that
despite withdrawal of zebularine from the medium, cells exhibited
continuous reduction of DNA synthesis several days after its
removal. This finding indicates that zebularine should be preferred
to existing analogous drugs, and these results could promote the
development of new administration and management strategies in the
treatment of head and neck cancer, with daily administration of
zebularine being replaced by weekly application.
The cell cycle findings that SCC-9 cells were
arrested in G2/M following zebularine treatment, whereas SCC-25
cells were arrested at the sub-G1 phase, show that even cells from
the same origin (in this case, tongue carcinoma) respond
differently to zebularine administration. The results of Suzuki
et al (13–15) concerning the effect of zebularine
on HSC-3 cells originating from tongue squamous cell carcinoma also
showed increased percentages of cells arrested at the G2/M phase of
the cell cycle for 48 h following each of the tested doses; 120 and
220 μM. The disparity between the results of Suzuki et
al and our present findings are not considered to indicate any
conflict, because our cell cycle assay used different treatment
durations from theirs; 72-120 h and 48 h, respectively. Moreover,
the two assays were conducted with differing tongue cancer cell
lines, which could account for the differences in the FACS results.
Another study indicated that zebularine induced S phase arrest of
the cell cycle in lung cancer cells (19). Therefore, it is possible to
postulate that the effect of zebularine on cell cycle progression
is tissue specific.
We postulated that apoptosis resulting from
zebularine treatment occurs in a caspase 3- and
PARP-cleavage-dependent manner in head and neck cancer cell lines.
This notion is reinforced by a previous study on breast cancer
(9), in which zebularine was able
to increase activated levels of caspase 3, with enhanced cleavage
of PARP, in MDA-MB-231 cell lines. Nonetheless, the MCF-7 cells,
which are known for their lack of caspase 3 expression, did not
show significant upregulation of PARP cleavage, but were able to
undergo apoptosis via a different path.
Interestingly, no methylation was observed in the
promoter region of the upregulated p21 and CHK1
genes. However, previous studies concerning methylation of
p21 promoter reported discrepancies among results, in some
of the tested cells and tumors methylation and silencing of the
p21 gene were obtained, whereas in other studies no
methylation was observed in the promoter region of p21. In
squamous cells originating from lung cancer, aberrant methylation
of p21 was reported, especially for NSCLC, but not in MPM
cells, and consequently p21 protein was frequently lost from NSCLC
and SCLC (20). However, we found
no reports of methylation of p21 in oral squamous cells. For
the CHK1 gene, a report from Tort et al (17) showed low levels of CHK1 expression
in human lymphoid neoplasm, which was suspected to be methylated
but, nonetheless, methylation analysis revealed no methylation at
the CpG positions of the CHK1 promoter. In conclusion, we
can state that alteration of p21 and CHK1 following zebularine
administration was not due to inhibition of methylation of their
promoter.
In summary, our results confirm that zebularine
induced apoptosis in squamous cell carcinoma SCC-9 and SCC-25
cells, in a caspase 3- and PARP-dependent manner, and its
anti-proliferative activity continued to be effective for several
days following its withdrawal. Nevertheless, the observed
upregulation of p21 and CHK1 were not due to inhibition of promoter
methylation by zebularine. The issue of apoptotic pathway activated
by zebularine need to be further studied in cancer cells. This may
lead to improved strategies for combating drug resistance in head
and neck cancer, by integrating zebularine into existing
therapies.
References
|
1.
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar
|
|
2.
|
Pisani P, Parkin DM and Ferlay J:
Estimates of the worldwide mortality from eighteen major cancers in
1985. implications for prevention and projections of future burden.
Int J Cancer. 55:891–903. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Perez-Ordonez B, Beauchemin M and Jordan
RC: Molecular biology of squamous cell carcinoma of the head and
neck. J Clin Pathol. 59:445–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Brait M and Sidransky D: Cancer
epigenetics: Above and beyond. Toxicol Mech Methods. 21:275–288.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar
|
|
6.
|
Laliberte J, Marquez VE and Momparler RL:
Potent inhibitors for the deamination of cytosine arabinoside and
5-aza-2′-deoxycytidine by human cytidine deaminase. Cancer
Chemother Pharmacol. 30:7–11. 1992.PubMed/NCBI
|
|
7.
|
Cheng JC, Matsen CB, Gonzales FA, et al:
Inhibition of DNA methylation and reactivation of silenced genes by
zebularine. J Natl Cancer Inst. 95:399–409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pompeia C, Hodge DR, Plass C, Wu YZ,
Marquez VE, Kelley JA and Farrar WL: Microarray analysis of
epigenetic silencing of gene expression in the KAS-6/1 multiple
myeloma cell line. Cancer Res. 64:3465–3473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Billam M, Sobolewski MD and Davidson NE:
Effects of a novel DNA methyltransferase inhibitor zebularine on
human breast cancer cells. Breast Cancer Res Treat. 120:581–592.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ben-Kasus T, Ben-Zvi Z, Marquez VE, Kelley
JA and Agbaria R: Metabolic activation of zebularine, a novel DNA
methylation inhibitor, in human bladder carcinoma cells. Biochem
Pharmacol. 70:121–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tan W, Zhou W, Yu HG, Luo HS and Shen L:
The DNA methyltransferase inhibitor zebularine induces
mitochondria-mediated apoptosis in gastric cancer cells in vitro
and in vivo. Biochem Biophys Res Commun. 430:250–255. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
You BR and Park WH: Zebularine inhibits
the growth of HeLa cervical cancer cells via cell cycle arrest and
caspase-dependent apoptosis. Mol Biol Rep. 39:9723–9731. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Suzuki M, Shinohara F, Nishimura K, Echigo
S and Rikiishi H: Epigenetic regulation of chemosensitivity to
5-fluorouracil and cisplatin by zebularine in oral squamous cell
carcinoma. Int J Oncol. 31:1449–1456. 2007.PubMed/NCBI
|
|
14.
|
Suzuki M, Shinohara F and Rikiishi H:
Zebularine-induced reduction in VEGF secretion by HIF-1alpha
degradation in oral squamous cell carcinoma. Mol Med Rep.
1:465–471. 2008.PubMed/NCBI
|
|
15.
|
Suzuki M, Shinohara F, Endo M, Sugazaki M,
Echigo S and Rikiishi H: Zebularine suppresses the apoptotic
potential of 5-fluorouracil via cAMP/PKA/CREB pathway against human
oral squamous cell carcinoma cells. Cancer Chemother Pharmacol.
64:223–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Brakensiek K, Langer F, Kreipe H and
Lehmann U: Absence of p21(CIP 1), p27(KIP 1) and p 57(KIP 2)
methylation in MDS and AML. Leuk Res. 29:1357–1360. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tort F, Hernandez S, Bea S, et al:
Checkpoint kinase 1 (CHK1) protein and mRNA expression is
downregulated in aggressive variants of human lymphoid neoplasms.
Leukemia. 19:112–117. 2005.PubMed/NCBI
|
|
18.
|
Cheng JC, Yoo CB, Weisenberger DJ, et al:
Preferential response of cancer cells to zebularine. Cancer Cell.
6:151–158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
You BR and Park WH: Zebularine-induced
apoptosis in calu-6 lung cancer cells is influenced by ROS and GSH
level changes. Tumour Biol. 34:1145–1153. 2013. View Article : Google Scholar
|
|
20.
|
Teramen H, Tsukuda K, Tanaka N, et al:
Aberrant methylation of p21 gene in lung cancer and malignant
pleural mesothelioma. Acta Med Okayama. 65:179–184. 2011.PubMed/NCBI
|