Introduction
Breast cancer is the most frequently diagnosed and
the leading cause of cancer related death in women (1). A variety of drugs have been developed
to treat breast cancer, however, drug resistance often occurs, and
unexpected side-effects are common (2). Thus, novel chemotherapies that
overcome drug resistance and improve patient outcomes are urgently
needed. Using compounds from natural plants as potential cancer
preventive and/or therapeutic agents has become a fascinating
strategy (3,4). Identification and investigation of
active components from natural plants are important for assessing
their potential for clinical use. A large number of components
purified from herbs have been used to treat various cancers
including breast cancer. For example, paclitaxel (Taxol), a natural
chemotherapeutic drug isolated from the bark of the pacific yew, is
currently used widely for treating breast cancer (5). Therefore, development of new
therapeutic agents from natural source has great promise for breast
cancer treatment.
We have purified and identified four kinds of
triterpenoids derivatives from Clematis ganpiniana. They
showed cytotoxicity against breast cancer cells (6). One of them was α-hederin, which
belonged to triterpenoid saponins. Triterpenoid saponins are an
important class of natural products and distributed widely in the
plant kingdom (7,8). Several excellent studies provided an
overview of the triterpenoids as potential agents for
chemoprevention and therapy of breast cancer (9,10).
α-hederin, a monodesmosidic triterpenoid saponin
distributed in Hedera or Nigella species displays
many biological activities such as anti-viral activity (11); anti-inflammatory activity (12); anti-oxidant activity (13); anti-leishmanial activity (14) and anti-spasmodic activity (15). Moreover, α-hederin is increasingly
investigated for its promising anticancer potential since it
revealed cytotoxicity against various cancer cell lines such as
lung carcinoma, larynx epidermoid carcinoma, colon adenocarcinoma
and pancreas carcinoma (16–20)
and in vivo tumors (21–23).
It has been suggested that α-hederin exerted its cytotoxic activity
by promoting apoptosis and/or membrane alterations (24,25),
however, the molecular and cellular mechanisms are far from being
fully elucidated. Moreover, reports on the anti-breast cancer
acivity of α-hederin are scarce, most of which focus on biological
activity, while the mechanisms have not been widely reported
yet.
α-hederin have been previously reported to inhibit
growth and induce apoptosis of breast cancer cells (6), however, further effects and
mechanisms of α-hederin on breast cancer is currently unavailable.
In this study, we evaluated effects of α-hederin on growth and
apoptosis of various human breast cancer cell lines, and explored
the underlying mechanisms.
Materials and methods
Drug preparations
Protocols of the collection, storage, extraction of
the plant material of Clematis ganpiniana, the methods of
the purification and analysis of the α-hederin were described in a
previous study (6).
Cell culture
The human breast cancer cell lines MCF-7 and
MDA-MB-231 were obtained from American Type Culture Collection
(Manassas, VA, USA) and incubated in a humidified atmosphere of 5%
CO2 in air at 37°C and fed with the culture medium of
high glucose Dulbecco’s modified Eagle’s medium, supplemented with
10% fetal bovine serum, 1% penicillin-streptomycin solution. For
routine passages, cultures were split 1:3 when they reached 80–90%
confluence generally every 2–3 days. All experiments were performed
on exponentially growing cells. MCF-7 and MDA-MB-231 cells are
widely used in studies on human breast cancer. In this study, we
used these two cell lines to evaluate the growth inhibition and
explore the underlying molecular mechanisms of α-hederin.
MTT assay
The MTT assay was used to measure the inhibition of
growth by α-hederin in breast cancer cell lines. Briefly,
5×103 cells were seeded into a 96-well plate in
triplicate and 8 h later α-hederin was added into the wells at the
indicated final concentrations (0.08, 0.4, 2 and 10 μg/ml), while
cells cultured in medium with 0.05% DMSO as a negative control.
After incubation with α-hederin for 12, 24 and 48 h, the medium in
each well was replaced with 20 μl of MTT at 5 mg/ml final
concentration, and 4 h later 150 μl DMSO/well was added to dissolve
the formed violet formazan crystals within metabolically viable
cells. The plates were incubated at room temperature for 15 min and
then read at 490 nm with a microplate reader (Tecan, Grödig,
Austria). The percentage of growth inhibition was calculated as (OD
of the control - OD of the experiment samples)/OD of the control ×
100.
Apoptosis analysis by flow cytometry
After exposure to 2 μg/ml α-hederin for 6, 12 and 24
h, breast cancer cells were washed twice with PBS at 4°C,
resuspended in stain containing Annexin V-FITC and propidium iodide
(PI) for 15 min incubation on ice, and analyzed with FACSAria flow
cytometer (Becton-Dickinson, San Jose, CA, USA) using FACSDiva
software. Approximately 105 cells were analyzed for each
treatment.
Measurement of the mitochondrial membrane
potential (ΔΨm) with JC-1
The mitochondrial membrane potential was measured
according to the manufacturer’s instruction with JC-1. After
exposure to 2 μg/ml α-hederin for 6, 12 and 24 h, cells were washed
twice with PBS, incubated in the working solution of 2 μg/ml JC-1
for 30 min at 37°C in 5% CO2 atmosphere, and observed
with Zeiss LSM 5 Live confocal microscope (Carl Zeiss, Jena,
Germany). The fluorescence was measured at an excitation:emission
of 485/538 for green monomers and at an excitation:emission of
485/590 for red aggregates. Valinomycin was used at a concentration
of 0.1 μM as a positive control for depolarization of the ΔΨm.
Measurement of cellular caspase-3 and -9
activity
Caspase-3 and -9 activity was quantified by
measuring cleavage of the colorimetric peptides RED-DEVD-FMK and
RED-LEHD-FMK, respectively (BioVision, Palo Alto, CA, USA).
Briefly, at the end of designated treatment (24 h of exposure to 2
μg/ml α-hederin), equal number of control or treated cells were
incubated with RED-DEVD-FMK and RED-LEHD-FMK, respectively (2
μg/ml) for 20 min at 37°C in 5% CO2 atmosphere, then
washed twice by PBS and analyzed with FACSAria flow cytometer. For
the caspase inhibition study, the cells were pre-incubated with
inhibitors: z-DEVD-FMK and z-LEHD-FMK (respectively, caspase-3 and
-9 inhibitors) 1 h before α-hederin treatment.
Western blot analysis
MCF-7 and MDA-MB-231 cells were seeded at
1×106 cells in 100-mm2 dishes. Cells were
treated in complete medium with α-hederin for 6, 12 and 24 h. After
treatment, adherent cells were gently scraped from the plates into
the medium containing floating cells to obtain all the cells. Cells
were then centrifuged, washed in PBS, lysed in ice-cold lysis
buffer containing phosphatase inhibitor cocktail and protease
inhibitor cocktail (Boehringer, Mannheim, Germany) to obtain total
protein. Protein concentrations were determined using the Bradford
method.
Apaf-1 and cytochrome c in mitochondrial
fraction were analyzed by isolation of mitochondrial protein using
the Cell Mitochondria Isolation kit (Beyotime Institute of
Biotechnology, Beijing, China). Briefly, after exposure, MCF-7 and
MDA-MB-231 cells were harvested and centrifuged at 800 × g at 4°C
for 10 min. The pellets were added with 20 mM
N-2-hydroxyethylpiperazine-N0-20-ethanesulfonic acid (HEPES) buffer
containing protease inhibitor cocktail and disrupted with a glass
tissue grinder. Homogenates were centrifuged at 800 × g at 4°C for
10 min, and the resulting supernatants were transferred to 0.5 ml
conical tubes, and further centrifuged at 10,000 × g at 4°C for 20
min. The final pellets, containing the mitochondrial fraction, were
analyzed for protein content using the Bradford method.
Cell lysates were electrophoresed through 10–12%
SDS-PAGE gel, and transferred to PVDF membranes, which were
activated in methanol. The blots were probed or reprobed with
antibodies. GAPDH was used to normalize for protein loading. The
membranes were probed using ECL and autoradiographed. The intensity
of the bands was determined using densitometric analysis. The
primary antibodies used were purified mouse anti-human apoptotic
protease activating factor-1 (Apaf-1) and cytochrome c,
purchased from BD Bioscience. β-actin was from Sigma. Anti-mouse
secondary antibodies were from Cell Signaling Technology. The
antibodies were diluted according to the manufacturer’s
instructions.
Statistical analysis
The data were analyzed using the SPSS 20.0 software.
For all the measurements, oneway ANOVA followed by Bonferroni test
was used to assess the statistical significance of difference
between control and groups-treated. A statistically significant
difference was considered at the level of p<0.05.
Results
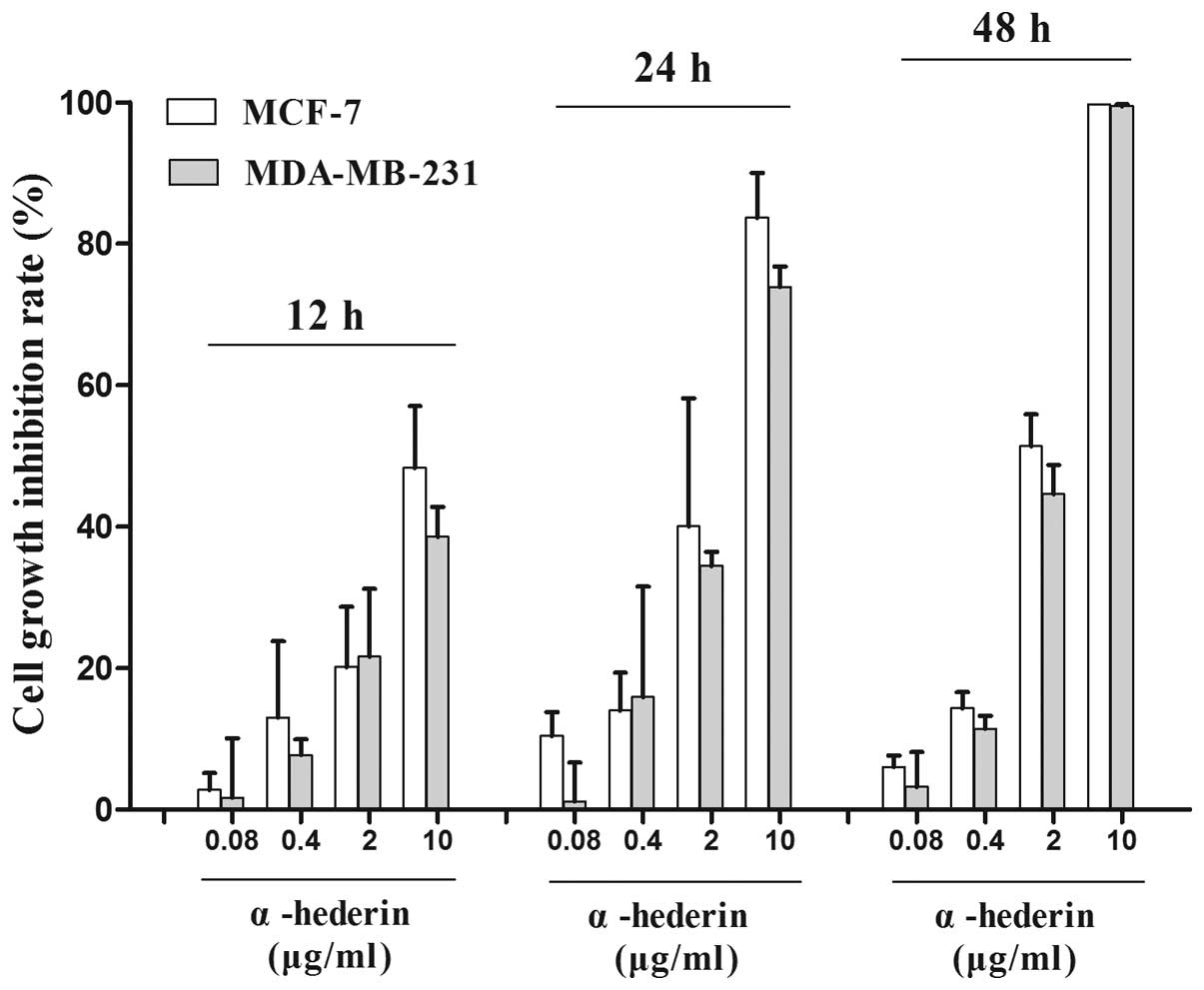
α-hederin inhibits the growth of breast
cancer cells
In this study, two breast cancer cell lines MCF-7,
MDA-MB-231, were used. The inhibitory rate of growth was determined
by MTT assay. α-hederin showed inhibition in the two breast cancer
cell lines which were statistically significant compared to the
negative control (p<0.05) (Fig.
1).
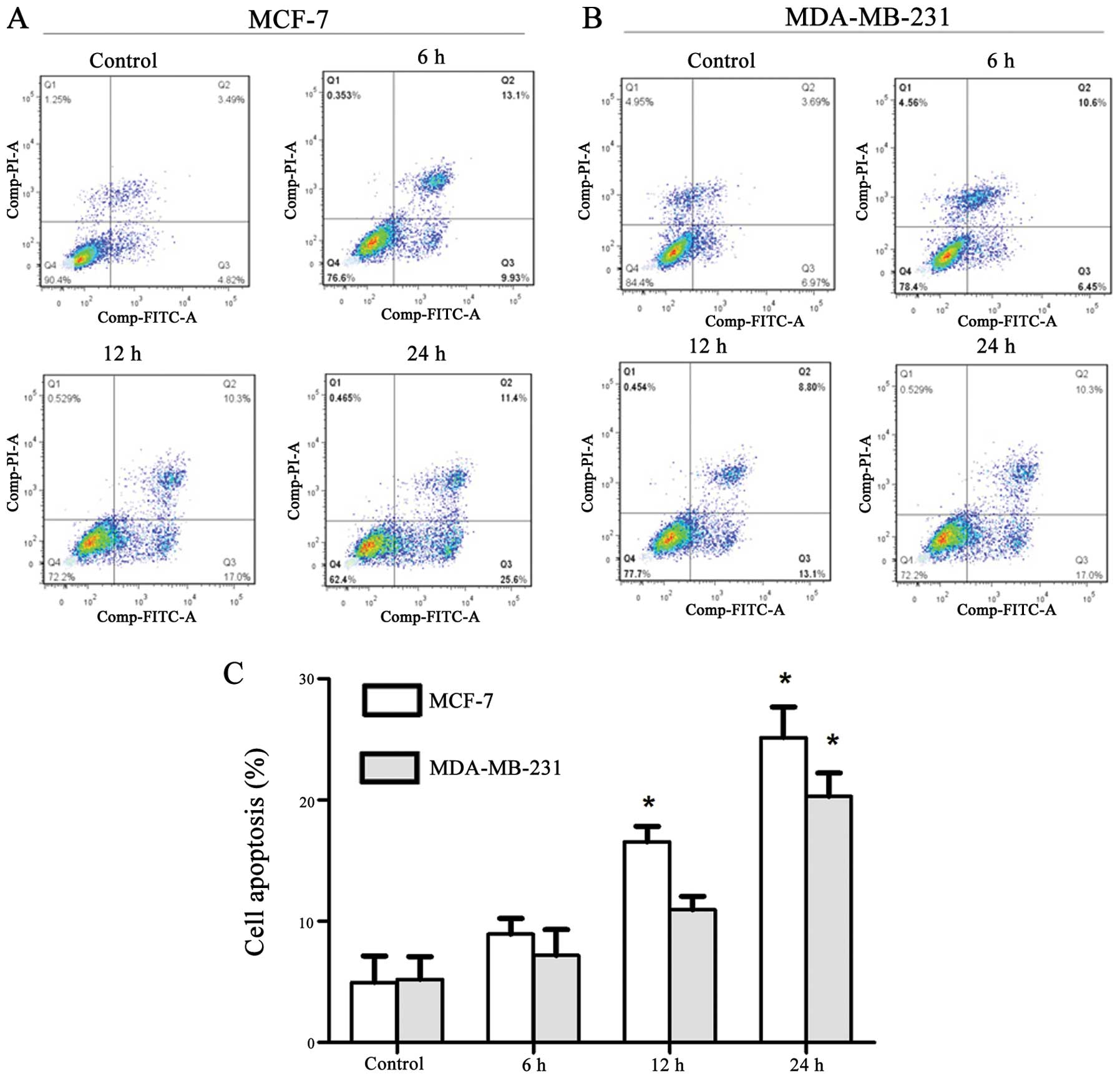
α-hederin induces apoptosis in breast
cancer cells
The apoptosis rate was measured by flow cytometry.
MCF-7 and MDA-MB-231 treated with 2 μg/ml α-hederin for indicated
times (6, 12 and 24 h) were first double-stained with Annexin V and
PI, and then analyzed by flow cytometry. In cells treated with
α-hederin, we detected a major increase in the Annexin
V+/PI− fraction (regarded as early apoptotic)
subpopulations. After incubated with 2 μg/ml α-hederin for 24 h,
early apoptosis rate of MCF-7 and MDA-MB-231 cells were
significantly increased up to 25.6 and 17.0%, respectively
(Fig. 2A and B). Early apoptosis
rate of cells treated with α-hederin of three independent
experiments are shown in column statistics (Fig. 2C).
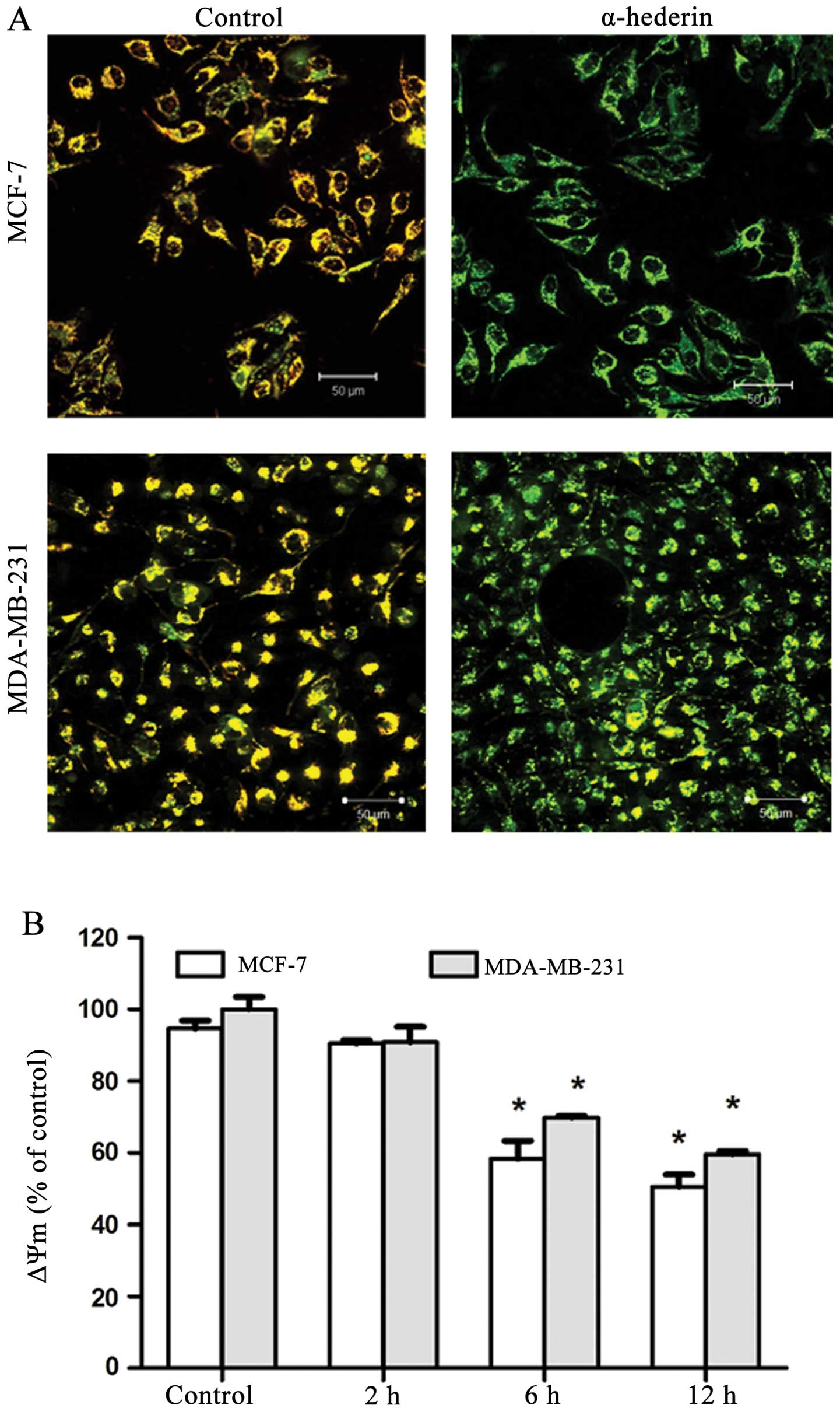
α-hederin affects the mitochondrial
membrane potential (ΔΨm) of breast cancer cells
MCF-7 and MDA-MB-231 cells were treated with 2 μg/ml
α-hederin for 6, 12 and 24 h, and then mitochondrial membrane
potential was measured. After the application of α-hederin, JC-1
fluorescence shifted from red-orange to greenish yellow, which
indicated the depolarization of mitochondrial membrane potential
(Fig. 3A). Mitochondrial membrane
potential ΔΨm of cells treated with α-hederin of three independent
experiments are shown in column statistics (Fig. 3B).
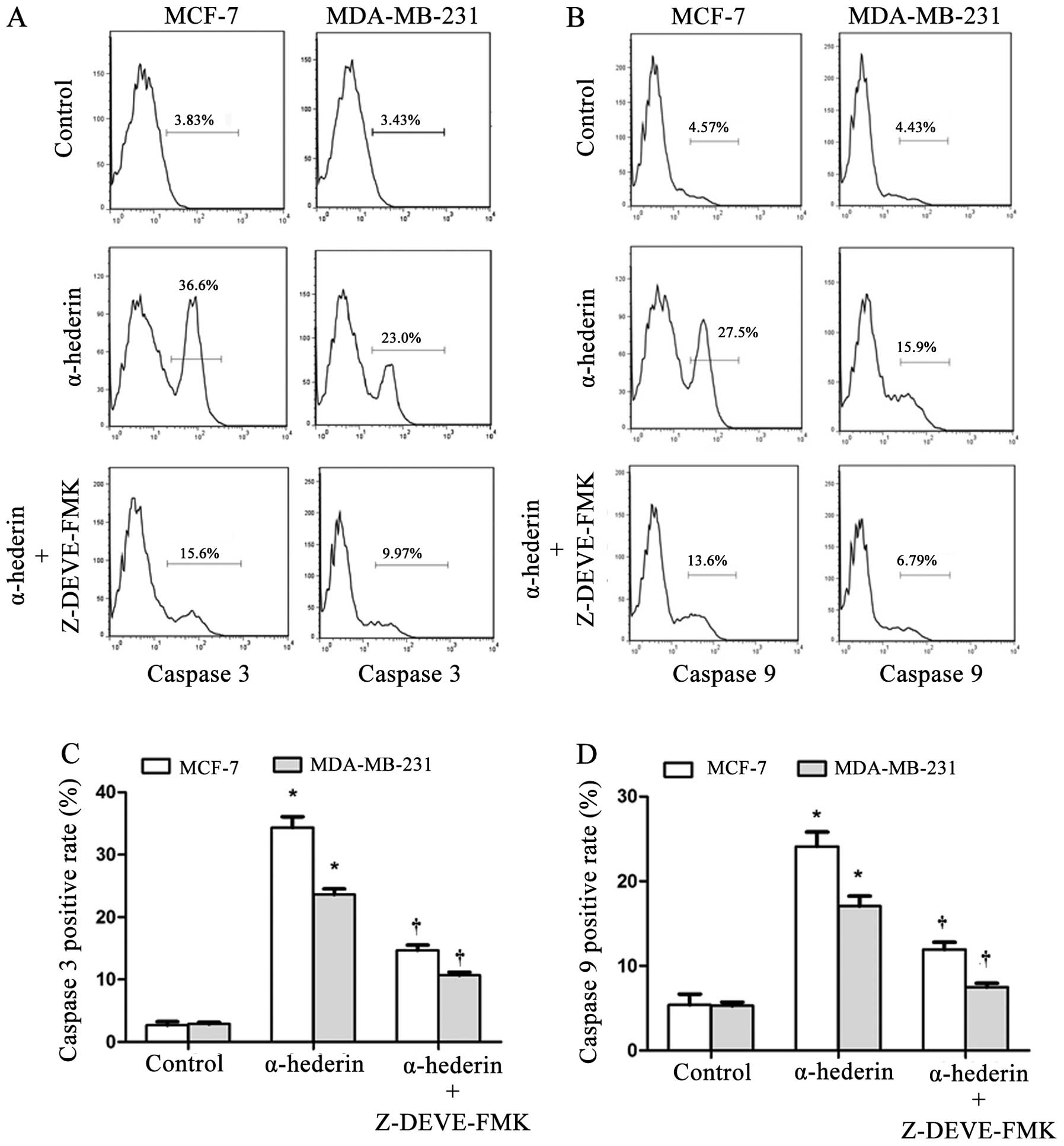
α-hederin regulates caspase-3 and
caspase-9 activation
After exposure to α-hederin (2 μg/ml) for 24 h,
activity of caspase-3 and caspase-9 was increased in both MCF-7 and
MDA-MB-231 cells. This activation could be reversed by the caspase
inhibitors (Fig. 4A and B).
Caspase-3, and caspase-9 positive rate of cells treated with
α-hederin with/without caspase inhibitors of three independent
experiments are shown in column statistics (Fig. 4C and D).
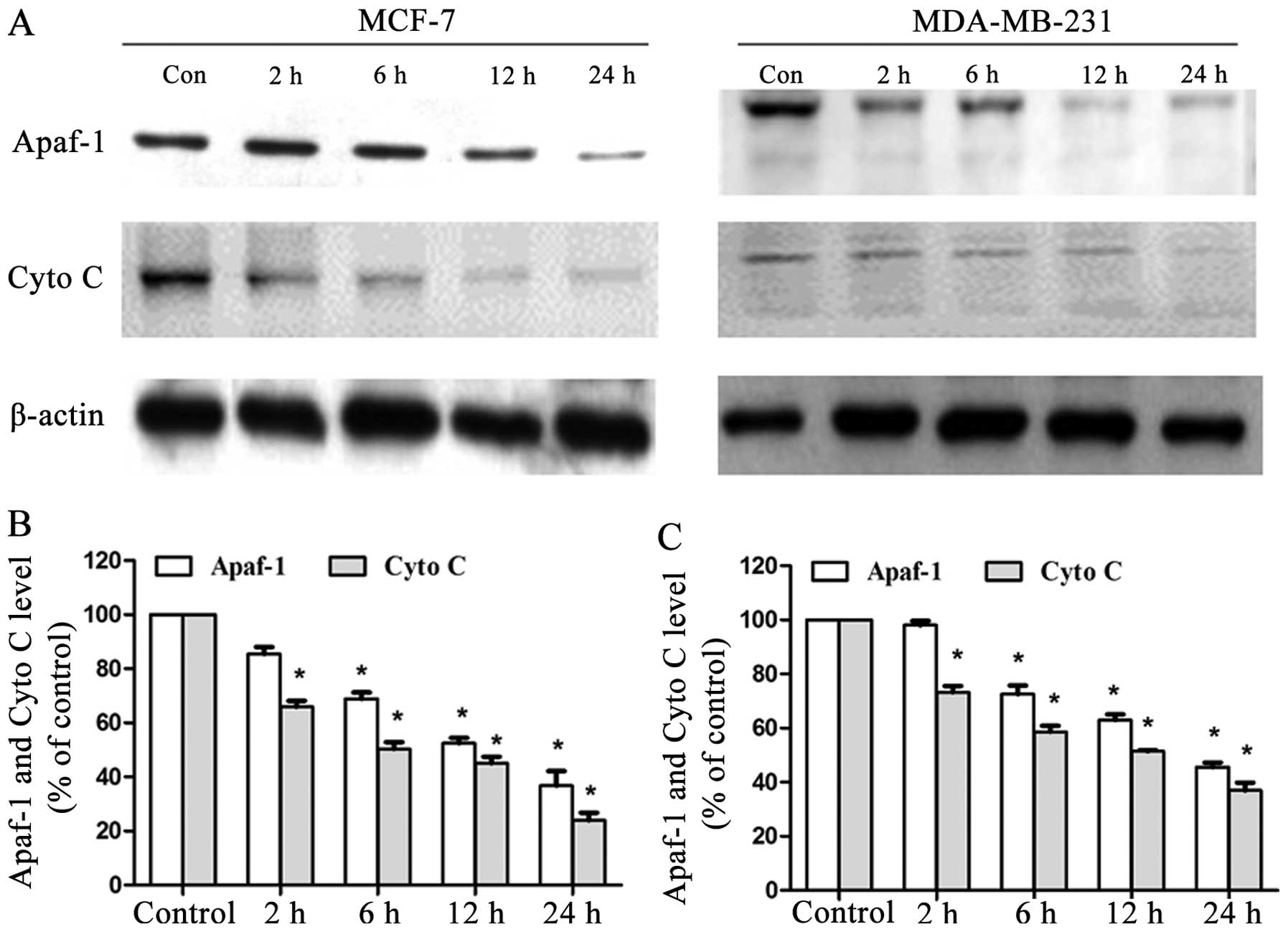
α-hederin regulates the Apaf-1 and
cytochrome c release
MCF-7 and MDA-MB-231 cells were treated for 2, 6, 12
or 24 h with α-hederin (2 μg/ml) and both mitochondrial Apaf-1 and
cytochrome c level were detected by western blot analysis.
DRβ-H decreased both mitochondrial Apaf-1 and cytochrome c
expressions in a time-dependent manner (Fig. 5A). Expressions of mitochondrial
Apaf-1 and cytochrome c of cells treated with α-hederin of
three independent experiments are shown in column statistics
(Fig. 5B and C).
Discussion
Our data indicate that α-hederin from Clematis
ganpiniana had strong inhibitory activity on different breast
cancer cells. α-hederin was found to induce apoptosis in both the
ER+ human breast cancer cell line MCF-7 and
ER− breast cancer cell line MDA-MB-231. Disruption of
mitochondrial membrane potential, release of Apaf-1 and cytochrome
c, and subsequent activation of caspase-9 and caspase-3 was
detected in α-hederin-treated cells.
The abstract of Clematis ganpiniana was
traditionally used as a diuretic agent and an anti-inflammatory
remedy by the Naxi people in China. α-hederin extracted from
Clematis ganpiniana showed cytotoxicity on breast cancer
cells. Importantly, α-hederin was found to induce apoptosis in
various breast cancer cells. Apoptosis is required for proper
tissue homeostasis. Defects in apoptosis signaling pathways
contribute to carcinogenesis and chemoresistance. Most cancer
therapeutic approaches inhibit tumors by triggering cancer cell
apoptosis (26).
JC-1 staining was used to detect the membrane
potential of mitochondria. The membrane potential of mitochondria
in breast cancer cells was greatly reduced by α-hederin. Apaf-1 and
cytochrome c were released from the mitochondria to the
cytoplasm. In α-hederin-induced apoptosis, caspase-3 and caspase-9
were involved. The activation of caspase family members is a
critical component of the apoptotic machinery. The caspases
generally consist of the upstream initiator caspases, such as
caspase-2, -8, -9 and -10, and the downstream effect of caspases,
such as caspase-3, -6 and -7 (27). The results suggested that the
caspase-dependent pathway mediated α-hederin-induced apoptosis in
breast cancer cells through the mitochondrial pathway.
Mitochondria play a central role in cancer survival
and are one of the main targets for developing anticancer drugs
(28). Both the extrinsic and the
intrinsic pathway can converge at the mitochondrial level and
trigger mitochondrial membrane permeabilization (29). Mitochondrial apoptotic pathway was
reported widely for the actions of triterpenoid saponins in other
human cancers including liver cancer (30–32),
gastric cancer (33), esophageal
cancer (34), and colorectal
cancer (35). It was reported that
α-hederin from Nigella sativa induced apoptosis via
mitochondrial perturbations in murine leukemia P388 cells (24). We first reported mitochondrial
apoptotic activity of α-hederin in breast cancer cells.
In conclusion, we showed α-hederin effectively
inhibited the growth and induced apoptosis of breast cancer cells.
α-hederin reduced the mitochondrial membrane potential and
decreased mitochondrial Apaf-1 and cytochrome c expressions
of breast cancer cells. Moreover, α-hederin increased the activity
of caspase-3 and caspase-9 remarkably in breast cancer cells.
Consistent with these results, α-hederin induced
mitochondria-mediated apoptosis of MCF-7 and MDA-MB-231 cells. This
is the first report on both chemotherapeutic effects and the
mechanism of α-hederin on human breast cancer cells, which may
provide a potential option for the drug development and treatment
of breast cancer. Oriental medicinal herbs are rich sources of
potential cancer chemopreventive and therapeutic agents. Rigorous
and systematic pre-clinical evaluations in vitro was
exemplified in the current study to transform traditional herbal
practices into evidence-based medicine.
Acknowledgements
This study was financially supported by Natural
Science Foundation of China (81272916, 81202077 and 81372828), the
Natural Science Foundation of Jiangsu Province (BK2011855), the key
projects of Jiangsu Provincial Health Office (H201110), the Project
of Jiangsu Province Traditional Chinese Medicine Bureau (LZ11084),
the Six Talents Peak projects of Jiangsu Province (to Q.D.), a
project Funded by the Priority Academic Program Development of
Jiangsu higher Education Institutions (PAPD) and the Talent
Foundation of The First Affiliated Yijishan Hospital of Wannan
Medical College (YR201305).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Ribeiro JT, Macedo LT, Curigliano G, et
al: Cytotoxic drugs for patients with breast cancer in the era of
targeted treatment: back to the future? Ann Oncol. 23:547–555.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karikas GA: Anticancer and chemopreventing
natural products: some biochemical and therapeutic aspects. J BUON.
15:627–638. 2010.PubMed/NCBI
|
|
4
|
Cragg GM and Newman DJ: Natural products:
a continuing source of novel drug leads. Biochim Biophys Acta.
6:182013.
|
|
5
|
Wang TH, Wang HS and Soong YK:
Paclitaxel-induced cell death: Where the cell cycle and apoptosis
come together. Cancer. 88:2619–2628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding Q, Yang LX, Yang HW, Jiang C, Wang YF
and Wang S: Cytotoxic and antibacterial triterpenoids derivatives
from Clematis ganpiniana. J Ethnopharmacol. 126:382–385.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Podolak I, Galanty A and Sobolewska D:
Saponins as cytotoxic agents: a review. Phytochem Rev. 9:425–474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Augustin JM, Kuzina V, Andersen SB and Bak
S: Molecular activities, biosynthesis and evolution of triterpenoid
saponins. Phytochemistry. 72:435–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bishayee A, Ahmed S, Brankov N and Perloff
M: Triterpenoids as potential agents for the chemoprevention and
therapy of breast cancer. Front Biosci (Landmark Ed). 16:980–996.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patlolla JMR and Rao CV: Triterpenoids for
cancer prevention and treatment: Current status and future
prospects. Curr Pharm Biotechnol. 13:147–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calabrese AI: Letter: Antiviral activity
of hederin. J Pharm Sci. 64:VIII1975. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gepdiremen A, Mshvildadze V, Suleyman H
and Elias R: Acute anti-inflammatory activity of four saponins
isolated from ivy: alpha-hederin, hederasaponin-C,
hederacolchiside-E and hederacolchiside-F in carrageenan-induced
rat paw edema. Phytomedicine. 12:440–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gulcin I, Mshvildadze V, Gepdiremen A and
Elias R: Antioxidant activity of saponins isolated from ivy:
alpha-hederin, hederasaponin-C, hederacolchiside-E and
hederacolchiside-F. Planta Med. 70:561–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ridoux O, Di Giorgio C, Delmas F, et al:
In vitro antileishmanial activity of three saponins isolated from
ivy, alpha-hederin, beta-hederin and hederacolchiside A(1), in
association with pentamidine and amphotericin B. Phytother Res.
15:298–301. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trute A, Gross J, Mutschler E and
Nahrstedt A: In vitro antispasmodic compounds of the dry extract
obtained from Hedera helix. Planta Med. 63:125–129. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quetin-Leclercq J, Elias R, Balansard G,
Bassleer R and Angenot L: Cytotoxic activity of some triterpenoid
saponins. Planta Med. 58:279–281. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Danloy S, Quetin-Leclercq J, Coucke P, et
al: Effects of alpha-hederin, a saponin extracted from Hedera
helix, on cells cultured in vitro. Planta Med. 60:45–49. 1994.
View Article : Google Scholar
|
|
18
|
Rooney S and Ryan MF: Effects of
alpha-hederin and thymoquinone, constituents of Nigella
sativa, on human cancer cell lines. Anticancer Res.
25:2199–2204. 2005.PubMed/NCBI
|
|
19
|
Tian Z, Liu YM, Chen SB, et al:
Cytotoxicity of two triterpenoids from Nigella glandulifera.
Molecules. 11:693–699. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan LH, Xu LZ, Lin J, Yang SL and Feng YL:
Triterpenoid saponins from the stems of Clematis parviloba.
J Asian Nat Prod Res. 11:332–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumara SS and Huat BT: Extraction,
isolation and characterisation of antitumor principle,
alpha-hederin, from the seeds of Nigella sativa. Planta Med.
67:29–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feller G, Kugel A, Moonshine D, et al:
African descents are more sensitive than European descents to the
antitumor compounds alpha-hederin and kalopanaxsaponin I. Planta
Med. 76:1847–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pasi S, Aligiannis N, Pratsinis H,
Skaltsounis AL and Chinou IB: Biologically active triterpenoids
from Cephalaria ambrosioides. Planta Med. 75:163–167. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swamy SM and Huat BT: Intracellular
glutathione depletion and reactive oxygen species generation are
important in alpha-hederin-induced apoptosis of P388 cells. Mol
Cell Biochem. 245:127–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rooney S and Ryan MF: Modes of action of
alpha-hederin and thymoquinone, active constituents of Nigella
sativa, against HEp-2 cancer cells. Anticancer Res.
25:4255–4259. 2005.PubMed/NCBI
|
|
26
|
Liu JJ, Lin M, Yu JY, Liu B and Bao JK:
Targeting apoptotic and autophagic pathways for cancer
therapeutics. Cancer Lett. 300:105–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi Y: Mechanisms of caspase activation
and inhibition during apoptosis. Mol Cell. 9:459–470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dias N and Bailly C: Drugs targeting
mitochondrial functions to control tumor cell growth. Biochem
Pharmacol. 70:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suen DF, Norris KL and Youle RJ:
Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang QF, Chen JC, Hsieh SJ, Cheng CC and
Hsu SL: Regulation of Bcl-2 family molecules and activation of
caspase cascade involved in gypenosides-induced apoptosis in human
hepatoma cells. Cancer Lett. 183:169–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Zhao XZ, Qi Q, et al:
Macranthoside B, a hederagenin saponin extracted from Lonicera
macranthoides and its anti-tumor activities in vitro and in
vivo. Food Chem Toxicol. 47:1716–1721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar
|
|
33
|
Chun J, Ha IJ and Kim YS:
Antiproliferative and apoptotic activities of triterpenoid saponins
from the roots of Platycodon grandiflorum and their
structure-activity relationships. Planta Med. 79:639–645. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mo S, Xiong H, Shu G, et al:
Phaseoloideside E, a novel natural triterpenoid saponin identified
from Entada phaseoloides, induces apoptosis in Ec-109
esophageal cancer cells through reactive oxygen species generation.
J Pharmacol Sci. 122:163–175. 2013.PubMed/NCBI
|
|
35
|
Wang CZ, Li XL, Wang QF, Mehendale SR,
Fishbein AB, Han AH, Sun S and Yuan CS: The mitochondrial pathway
is involved in American ginseng-induced apoptosis of SW-480 colon
cancer cells. Oncol Rep. 21:577–584. 2009.PubMed/NCBI
|