Introduction
Hepatocellular carcinoma (HCC) is one of the most
common human cancers in the world, including China (1). It ranks as the second leading cause
for cancer death worldwide. As far as great advances in the
treatment of the disease is concerned, prognosis for HCC patients
is not favorable due to the likelihood of intrahepatic and
extrahepatic recurrence, which leads to a high mortality rate
(2,3). Therefore, investigations into the
molecular mechanisms involving in HCC metastasis have major
importance to develop novel avenues for targeted therapies.
Cell motility plays an important role in tumor
invasion and metastasis. Rho GTPases regulate actin polymerization,
actomyosin contractility and microtubule dynamics controlling a
wide range of cellular processes, including cell adhesion, and
migration. They function as molecular switches in cell signaling,
alternating between inactive GDP-bound states and active GTP-bound
states. The active-GTP form of Rho is governed by a panel of
inhibitors including ARHGDIs which block activation of Rho proteins
by sequestering the GDP-bound Rho proteins in the cytosol (4). ARHGDIs includes three members, named
ARHGDIA, ARHGDIB and ARHGDIG. ARHGDIA is ubiquitously expressed and
interacts with several Rho GTPases, including RhoA, Rac1 and Cdc42
(5,6). As a regulator of Rho activity,
ARHGDIA has attracted increasing attention. There are studies
showing that ARHGDIA is aberrantly expressed in many tumors and
plays an important role in the tumor process. However, the role of
ARHGDIA in HCC remains to be unraveled. In this study, we found
that ARHGDIA was frequently downregulated in HCC and significantly
associated with prognosis of HCC patients. Loss of ARHGDIA promoted
HCC cells invasion and metastasis in vitro and in
vivo, which might be due to Rac1 and RhoA GTPase activation
induced by silencing ARHGDIA.
Materials and methods
Patients and specimens
A total of 86 patients were enrolled in the present
study. The patients did not receive any preoperative cancer
treatment and their follow-up data were available. They were
followed-up after surgical treatment until May 2011, with a median
follow-up of 29 months (range 2–73. 2 months). During the
follow-up, the patients were monitored every 2–3 months as
described previously (7). CT
scanning or MRI was performed when tumor recurrence was suspected.
The recurrent tumors were treated as described previously (8). Clinical samples were collected from
these patients after obtaining informed consent according to an
established protocol approved by the Ethics Committee of Fudan
University (Shanghai, China).
Immunohistochemical staining
Immunohistochemical staining was performed to detect
the expression of ARHGDIA in HCC and matched non-cancer tissue. The
primary antibody against ARHGDIA was obtained from Epitomics
(1:50). Intensity of staining was scored as 0 (negative), 1 (weak),
2 (moderate) or 3 (strong). The extent of staining was based on the
percentage of positive tumor cells: 0 (negative), 1 (1–25%), 2
(26–50%), 3 (51–75%) and 4 (76–100%). The final score of each
sample was assessed by summarization of the results of the
intensity and extent of staining. Therefore, each case was
considered negative if the final score was 0–1 (−) or 2–3 (±) and
positive if the final score was 4–5 (+) or 6–7 (++), respectively.
These scores were determined independently by two senior
pathologists.
Cell culture
Huh-7, SMMC-7721 and MHCC-97H cells were cultured in
DMEM medium with 10% FBS, maintained at 37°C in a humidified air
atmosphere containing 5% carbon dioxide.
Construction of plasmids, lentivirus
production and transduction
The coding sequence of human ARHGDIA was cloned into
the expression vector pCDH-CMV-MCS-EF1-Puro (System Biosciences,
Mountain View, CA, USA). The siRNA against ARHGDIA were synthesized
by Ribobio and inserted into the pLKO.1-TRC cloning vector
(Invitrogen, Carlsbad, CA, USA). All constructs were verified by
sequencing. A mixture of pCDH-ARHGDIA or pLKO.1-siARHGDIA cloning
vector, and adjuvant vectors psPAX2 and pMDG2 were transfected into
HEK293T cells using Lipofectamine 2000 reagent to generate
lentiviruses. Huh-7, SMMC-7721 and MHCC-97H cells were infected
with the recombinant lentivirus-transducing units plus 8 mg/ml
polybrene (Sigma).
Cell proliferation assay
Cell proliferation was measured with the Cell
Counting Kit-8 (CCK-8) assay kit (Dojindo Corp.); 5,000 cells were
plated into each well of a 96-well plate, in which 10 μl CCK-8 was
added to 90 μl of culture medium. The cells were subsequently
incubated for 1 h at 37°C and the attenuance was measured at 450
nm. Three independent experiments were performed.
Colony formation assay
The 500 cells were plated into 6-well culture-plates
and cultured for 14 days to allow colony formation. Colonies were
stained with 0.1% crystal violet (Amersco, Solon, OH, USA) in 50%
methanol and 10% glacial acetic acid for counting.
In vitro migration and invasion
assays
For the migration assays, 2×104 cells
were added into the upper chamber of the insert with the non-coated
membrane (Millipore, 8-mm pore size). For the invasion assays, each
well insert was layered with 50 μl of a 1:4 mixture of
Matrigel/Dulbecco’s minimal essential medium (BD Bioscience). Cells
(1×105) were added into the upper chamber of the insert.
In both assays, cells were plated in medium without serum, and
medium containing 10% FBS in the lower chamber served as
chemoattractant. After several hours of incubation, the cells that
did not migrate or invade through the pores were carefully wiped
out with cotton swab. Cells on the lower surface of the membrane
were fixed with methanol and stained with Giemsa and counted. Each
experiment was performed in triplicates.
In vivo metastasis assays
For in vivo metastasis assays, SMMC-7721
cells infected with either the ARHGDIA-siRNAs or the vector were
transplanted into nude mice (5-week-old BALB/c-nu/nu, 6 per group,
2×106 cells for each mouse) through the tail vein. After
6 weeks, mice were sacrificed. The lungs were removed, fixed in
formalin, and embedded in paraffin. Consecutive sections of the
whole lung were subjected to hematoxylin and eosin staining. All of
the metastatic foci in lung were calculated microscopically to
evaluate the development of pulmonary metastasis. The lung
metastases were calculated and evaluated independently by two
pathologists.
Western blotting
Equal amounts of protein were resolved by 10%
SDS-polyacrylamide gel electrophoresis and transblotted onto
nitrocellulose membrane (Bio-Rad). After blocking in 5% non-fat
milk, the membranes were incubated with rabbit anti-ARHGDIA
antibody (mAb; 1:1,000; Epitomics), rabbit anti-Rac1 antibody (mAb;
1:1,000; Epitomics), rabbit anti-RhoA antibody (mAb; 1:1,000;
Epitomics), rabbit anti-Cdc42 antibody (mAb; 1:1,000; Epitomics) or
rabbit anti-GAPDH mAb (1:5,000; Epitomics). The proteins were
detected with enhanced chemiluminescence reagents (Pierce).
Immunoprecipitation of active Cdc42,
RhoA, Rac1
The protocol used was based on the availability of a
mouse monoclonal antibody directed against the active form of
Cdc42, RhoA and Rac1 commercially through NewEast Biosciences
(Malvern, PA, USA). Cells were lysed in 1 ml of ice-cold lysis
buffer for 10 min. Aliquots of each cell lysate were added to two
microcentrifuge tubes, one for analysis of the active and the other
for the analysis of total potein content. Then 1 μl of anti-active
Cdc42, RhoA or Rac1 monoclonal antibody was added, as well as 20 μl
of Dynabead Protein G added, and samples were incubated overnight
with rotation at 4°C. Beads were pelleted by centrifugation for 1
min at 5,000 g, then washed three times with 0.5 ml of lysis
buffer, resuspended in 20 μl of 2× reducing SDS-PAGE sample buffer,
heated at 100°C for 5 min, then separated on 12% polyacrylamide
gels and processed for western blotting after transferring to PVDF
membranes. Rabbit polyclonal antibody against total Cdc42, RhoA and
Rac1 (mAb; 1:1,000; Epitomics) was used for western blotting.
Statistical analysis
Statistical analysis was performed with SPSS 15.0
(SPSS Inc, Chicago, IL, USA) and values are expressed as the mean ±
standard deviation. The differences between groups were analyzed
using Student’s t-test (only two groups), or one-way analysis of
variance (more than two groups were compared). P<0.05 was
considered statistically significant.
Results
ARHGDIA is frequently downregulated in
HCC and associated with tumor invasion and metastasis
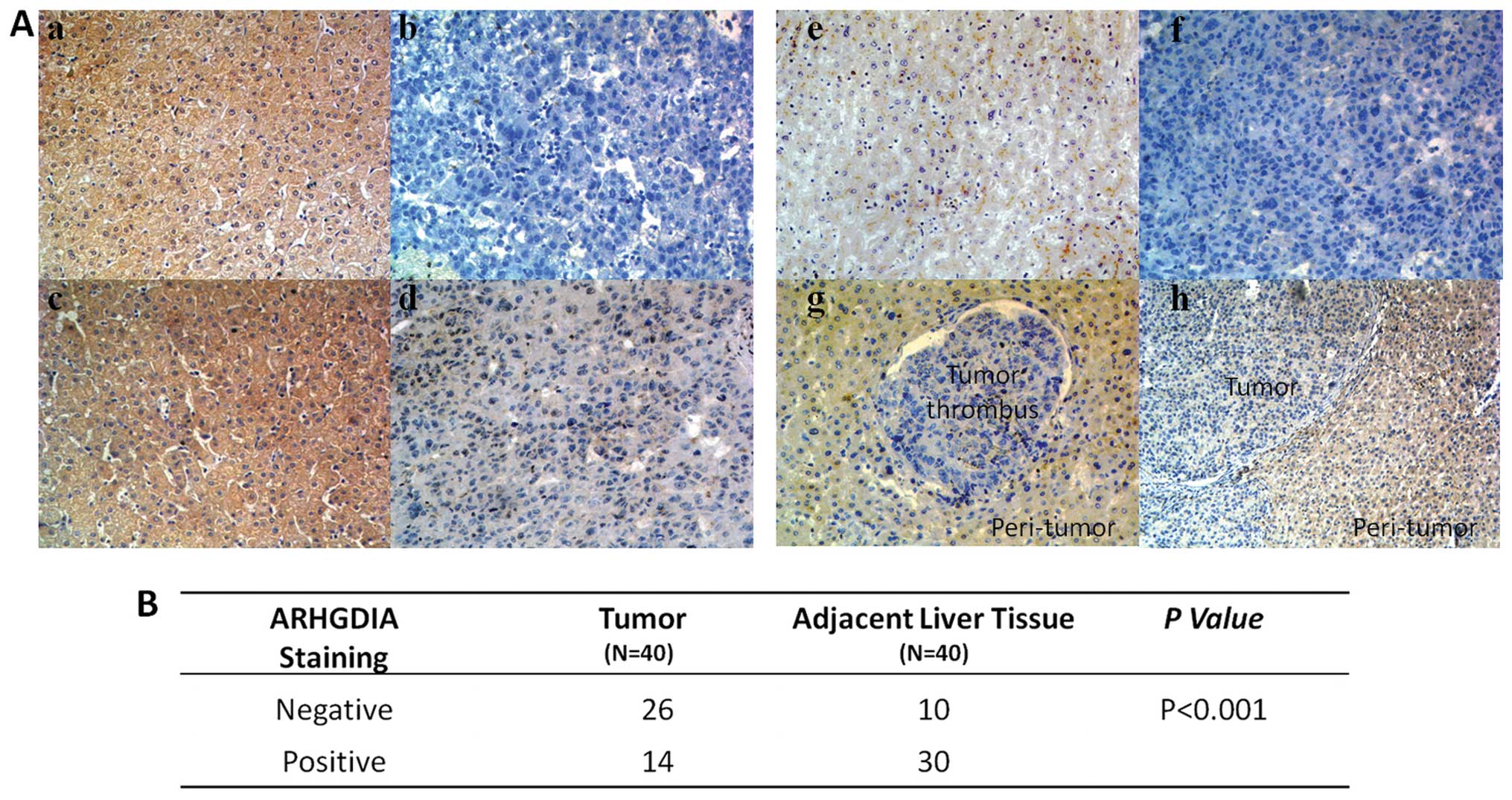
The protein levels of ARHGDIA in 86 cases of HCC
patient samples and corresponding non-cancer liver tissues (40
cases) were measured by immunohistochemical staining. Strong
staining of ARHGDIA was observed in adjacent non-cancer liver
tissue, but weaker in more than half (65%) of the HCC tissues
(Fig. 1A). The expression level is
significantly downregulated in HCC compared with non-cancer liver
tissues (P<0.001) (Fig. 1B). At
various regions of tumor within the same slide, it appeared that
ARHGDIA expression remarkably decreased at invasive cancer in
situ such as tumor embolus. Next, we analysed the relationships
between ARHGDIA and clinical pathological features of HCC.
Significant correlations were observed between ARHGDIA and vascular
invasion (tumor invasion in blood vessel or bile duct) (P=0.0216).
Low level of ARHGDIA expression was observated in 79.07% of
vascular invasion group (Table I).
The result indicated that ARHGDIA might correlated with HCC
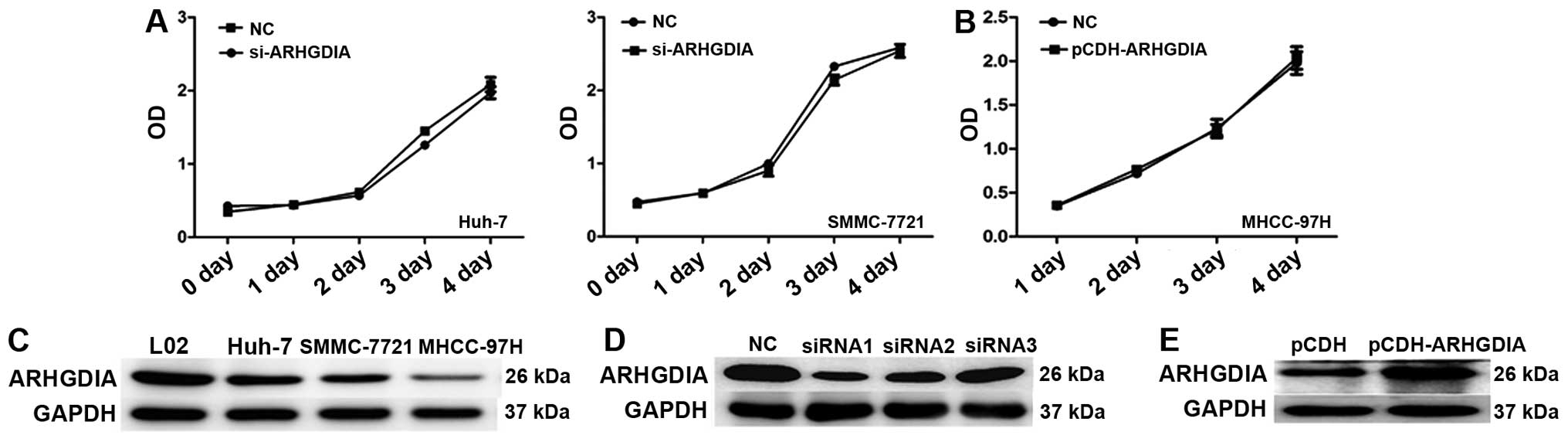
metastasis. Then, the ARHGDIA level was analyzed in a panel of
human HCC cell lines with different metastatic potential. The level
of ARHGDIA in the high-metastatic HCC cell lines (MHCC-97H) was
much lower than that in the less-metastatic HCC cell lines (Huh-7,
SMMC-7721) (Fig. 3C), indicating
that the downregulation of ARHGDIA was related to the metastatic
ability of HCC.
 | Table IExpression of ARHGDIA detected by IHC
and the clinicopathologic features of HCC patients (n=86). |
Table I
Expression of ARHGDIA detected by IHC
and the clinicopathologic features of HCC patients (n=86).
| ARHGDIA
expression | |
|---|
|
| |
|---|
| Variables | Low (n=57) | High (n=29) | P-value |
|---|
| Gender |
| Female | 6 | 4 | 0.655 |
| Male | 51 | 25 | |
| Age (years) |
| ≤51 | 23 | 13 | 0.691 |
| >51 | 34 | 16 | |
| Preoperative |
| AFP (ng/ml) |
| ≤20 | 15 | 11 | 0.268 |
| >20 | 42 | 18 | |
| HBsAg |
| Negative | 6 | 2 | 0.479 |
| Positive | 51 | 27 | |
| Liver cirrhosis |
| No | 5 | 2 | 0.764 |
| Yes | 52 | 27 | |
| ALT (U/l) |
| ≤75 | 48 | 21 | 0.194 |
| >75 | 9 | 8 | |
| Tumor size
(cm) |
| ≤5 | 18 | 13 | 0.226 |
| >5 | 39 | 16 | |
| Tumor number |
| Single | 43 | 26 | 0.118 |
| Multiple | 14 | 3 | |
| Tumor
encapsulation |
| None | 34 | 14 | 0.315 |
| Complete | 23 | 15 | |
| Vascular
invasion |
| No | 23 | 20 | 0.012 |
| Yes | 34 | 9 | |
| TNM stage |
| I | 18 | 16 | 0.063 |
| II | 17 | 8 | |
| III | 22 | 5 | |
| Tumor
differentiation |
| I–II | 44 | 13 | 0.321 |
| III–IV | 25 | 4 | |
The association of ARHGDIA with prognosis
of HCC patients
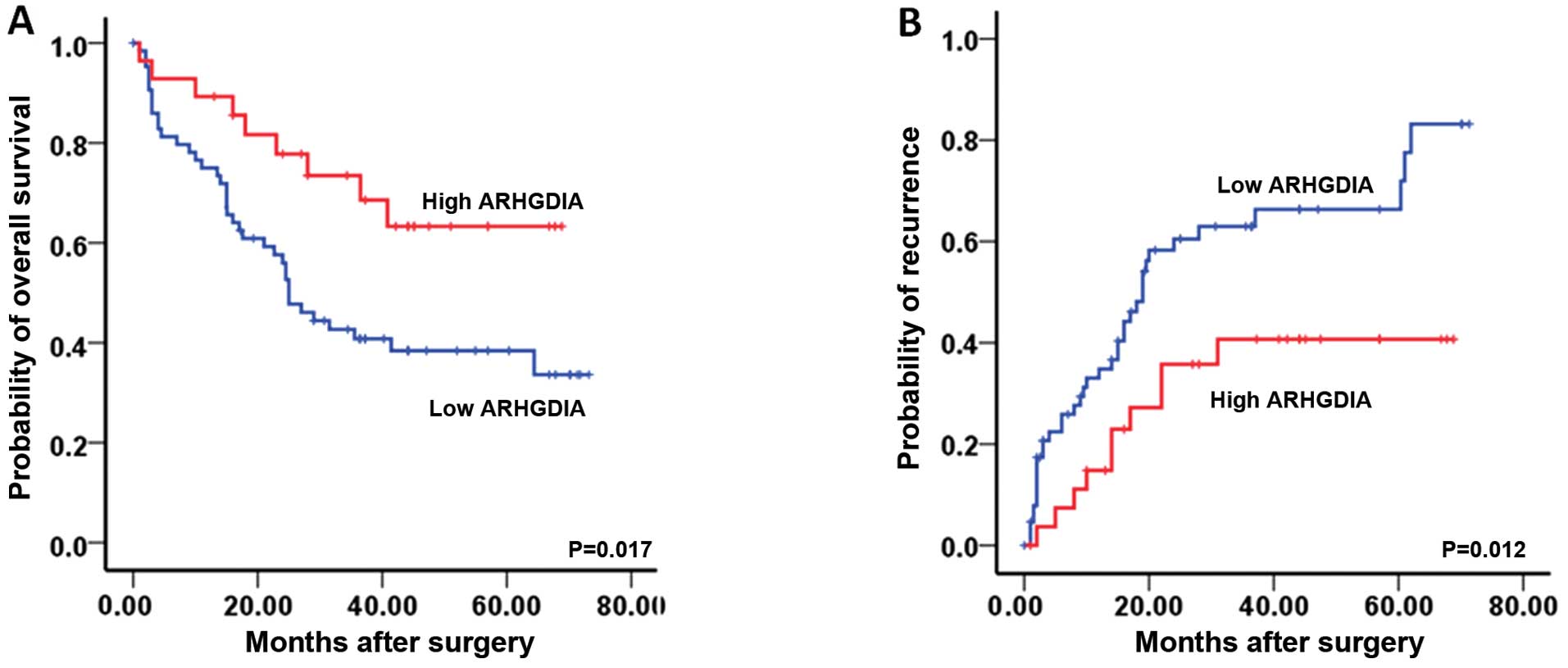
In the Kaplan-Meier analyses, the expression level
of ARHGDIA was significantly associated with OS and TTR. The
patients with low level of ARHGDIA exhibited a decreased
postoperative OS and a shorter TTR compared those with high level
(Fig. 2). The 1-, 3- and 5-year OS
rates of the patients with low level were 74.8, 42.2 and 36.6%,
respectively, which were significantly lower than those with high
level group (86.9, 65.4 and 51.5%, respectively; P=0.017). The 1-,
3- and 5-year cumulative recurrence rates of low level group were
37.8, 63.9 and 75.6%, respectively, which were significantly higher
than those of the high level group (20.5, 47.2 and 60.4%,
respectively; P=0.012).
ARHGDIA has no effect on HCC cell
proliferation or the colony formation ability
To explore the functions of ARHGDIA in HCC, specific
siRNAs against ARHGDIA were exploited to knockdown expression in
SMMC-7721, and Huh-7 cell lines. As shown in Fig. 3D, siRNA significantly reduced the
expression of ARHGDIA protein. We also constructed a lentivirus
vector expressing ARHGDIA and established the stable cell line
MHCC-97H, which has low basal levels of ARHGDIA (Fig. 3E). In cell proliferation assays,
knocking down ARHGDIA showed no obvious impact on the proliferation
of SMMC-7721 and Huh-7 cells (Fig.
3A). Similarly, overexpression of ARHGDIA did not affect
MHCC-97H cell growth either (Fig.
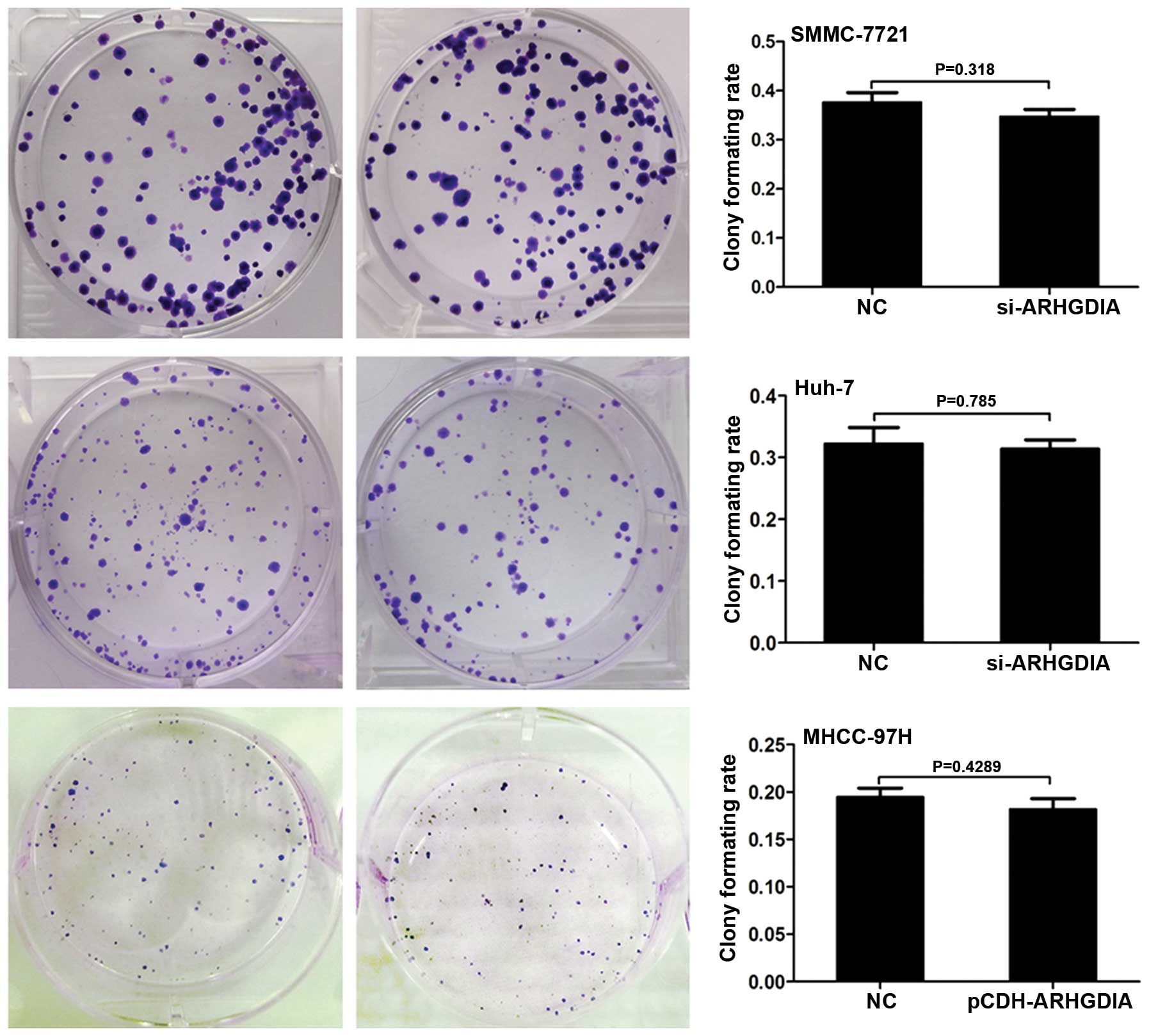
3B). Next, the colony formation assays were performed to
observe the effects of ARHGDIA on the anchoring growth ability of
HCC cells. No obvious effects were observed on the colony formation
ability of HCC cells after infection with ARHGDIA-siRNAs or
lenti-ARHGDIA (Fig. 4).
Loss of ARHGDIA promotes HCC cell
invasion and metastasis in vitro and in vivo
Given that expression of ARHGDIA is highly
associated with the metastatic property of HCC, we wondered whether
ARHGDIA could play an important role in HCC cell invasion and
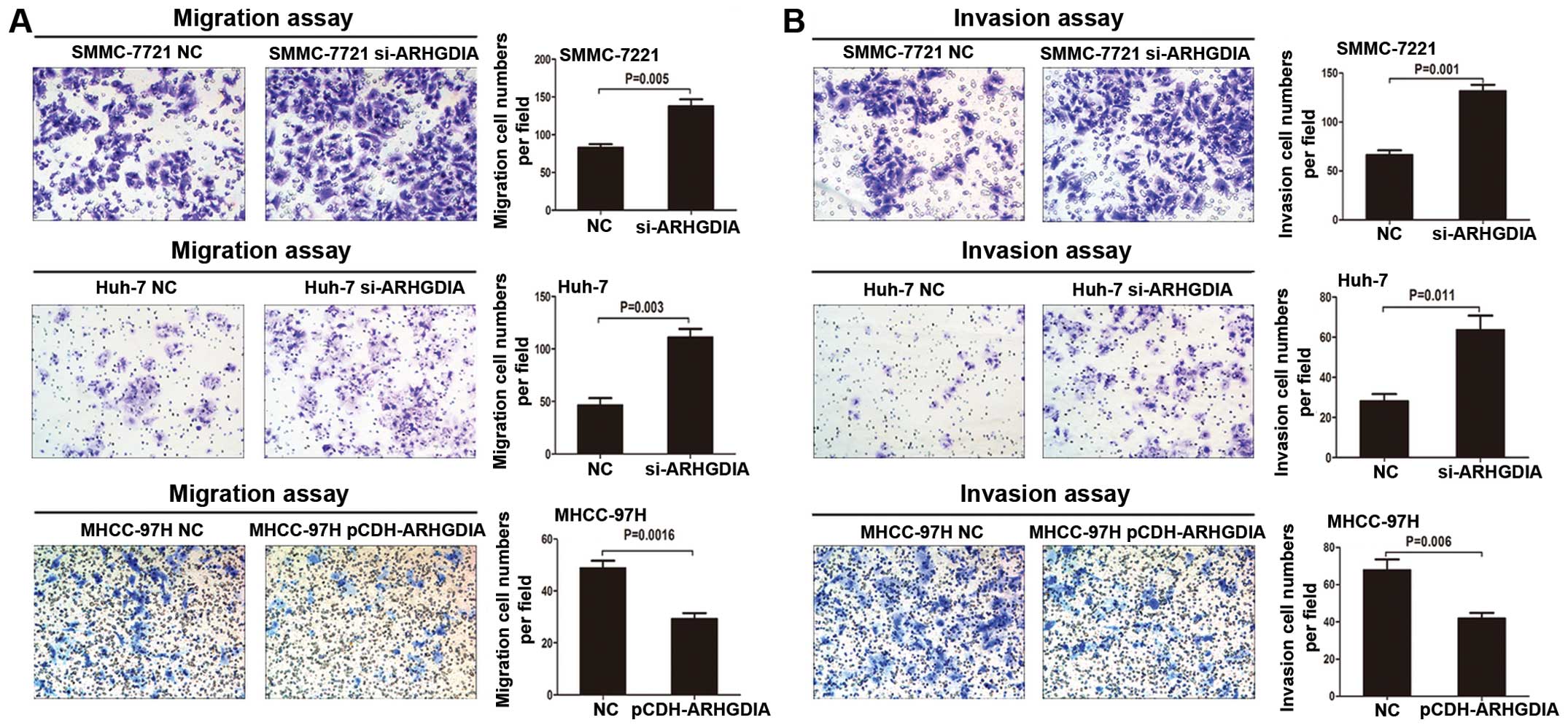
metastasis. Transwell assays without Matrigel demonstrated that
downregulation of ARHGDIA could significantly promote migration of
Huh-7 and SMMC-7721 cells when compared with vector groups
(Fig. 5A). Transwell assays with
Matrigel showed that the invasive capacities were dramatically
enhanced in these two stable cell lines when compared with the
control cells (Fig. 5B). However,
the migration and invasion of MHCC-97H cells decreased when ARHGDIA
was upregulated (Fig. 5). These
results indicated that loss of ARHGDIA could significantly enhance
HCC cell migration and invasion in vitro. To further explore
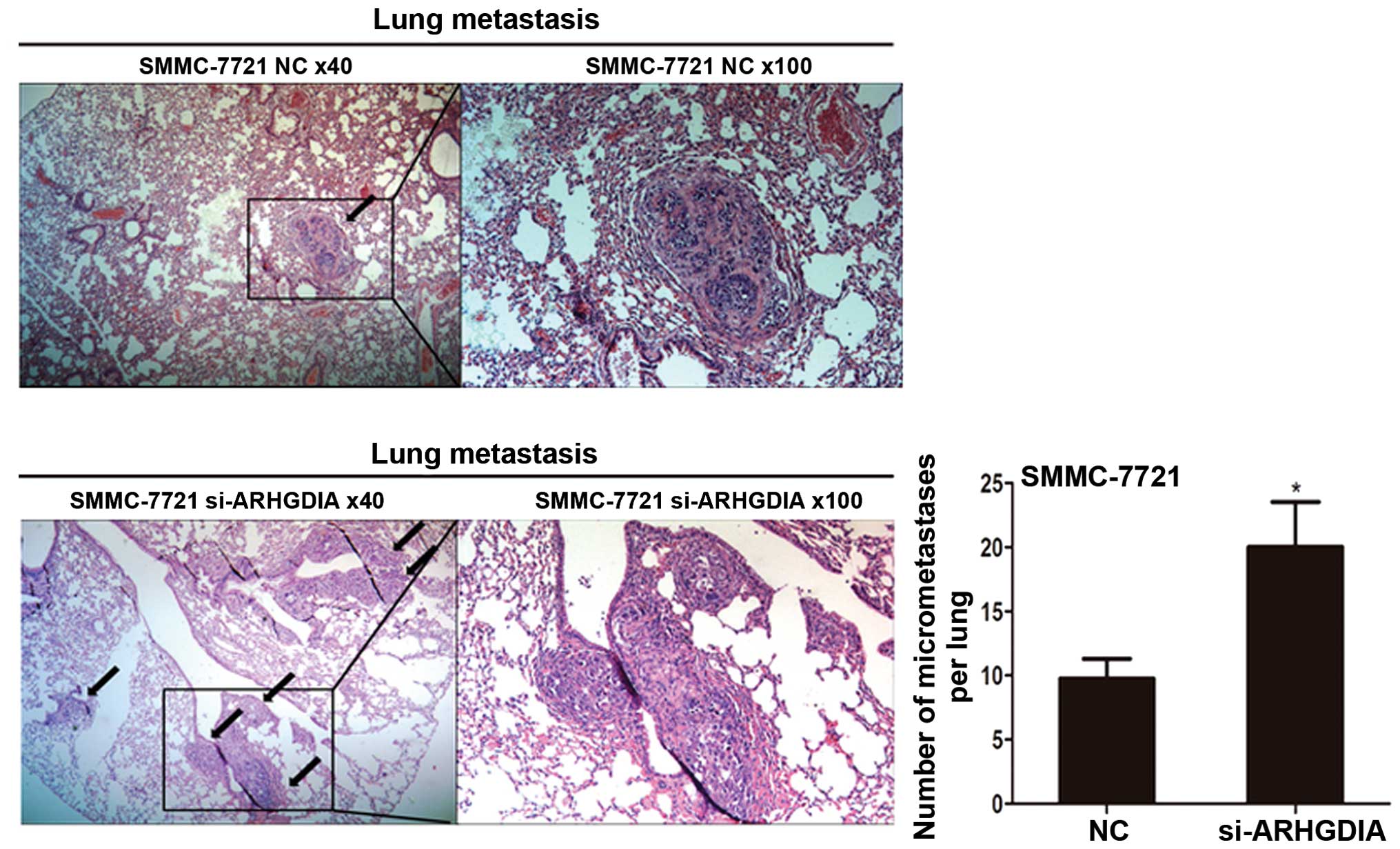
the role of ARHGDIA in tumor metastasis in vivo, SMMC-7721
cells infected with si-ARHGDIA were transplanted into nude mice
through the tail vein. Interestingly, the number of the metastatic
nodules in the lung were dramatically increased in si-ARHGDIA
groups compared with vector control (P=0.0377) (Fig. 6). Taken together, these
observations suggested that ARHGDIA is a negative metastatic
regulator for HCC.
Loss of ARHGDIA significantly increases
the activities of Rac1 and RhoA GTPases in HCC cells
Regulation of the cytosol-membrane cycling of the
Rho GTPase by ARHGDIs has a major role in controlling Rho GTPase
activity and function. Given the important role of ARHGDIA in HCC
cell migration and invasion, we conducted immunoprecipitation
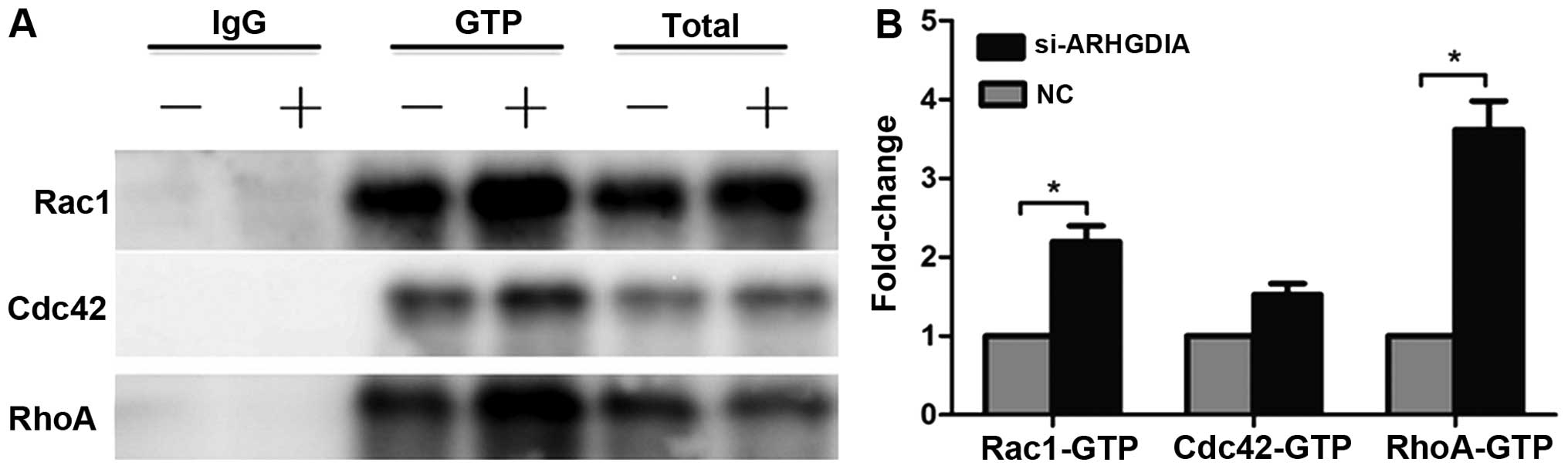
assays to determine the status of Rho GTPases in HCC cells. The
mouse monoclonal antibody directed against the active form of
Cdc42, RhoA and Rac1 were used in the immunoprecipitation assays.
The results indicated that loss of ARHGDIA significantly induced
RhoA and Rac1 activation in SMMC-7721 cells (Fig. 7A), particularly the activity of
RHOA increased nearly 4-fold compared to the control. The Cdc42
activity was also slightly increased, but did not reach statistical
significance (Fig. 7B). Numerous
studies have confirmed that activation of signaling of Rho GTPases
plays an important role in cancer progression and metastasis
(9). Therefore, the activation of
Rho GTPase proteins induced by silencing ARHGDIA might contribute
to tumor invasion and metastasis of HCC.
Discussion
Changes in ARHGDIA expression levels have been
associated with many cancers (10). Previous studies indicated that the
changes vary depending on the tumor type. For instance, ARHGDIA
expression is upregulated in colorectal and ovarian cancers, and
high expression levels correlate with increased invasion and
resistance to chemotherapy (11–13).
By contrast, ARHGDIA expression is reduced in brain cancers, and
inversely correlate with the degree of malignancy (14). In breast cancers, Jiang et
al found a significant reduction of ARHGDIA expression in tumor
versus normal breast (15).
Furthermore, the reduction of ARHGDIA had a significant, poor
prognostic correlation when tumors were stratified by node status
or by recurrence and disease-specific death. Therefore, the effects
of the ARHGDIA on cancers are complex and context-dependent. In the
present study, we first clarified the role of ARHGDIA in HCC to
ensure that ARHGDIA is indeed a tumor suppressor gene involved in
HCC invasion and metastasis. We found the following evidence: a)
ARHGDIA was frequently downregulated in HCC compared with
non-cancer liver tissues. Within the same IHC slide, ARHGDIA
expression remarkably decreased at invasive cancer in situ
such as tumor embolus. b) ARHGDIA expression level was
significantly associated with vascular invasion. HCC with vascular
invasion had a lower ARHGDIA expression than those without vascular
invasion. c) The level of ARHGDIA in the high-metastatic HCC cell
lines was lower than that in the low-metastatic cell lines. d) In
the Kaplan-Meier analyses, the expression level of ARHGDIA was
significantly associated with OS and TTR. The patients with low
level of ARHGDIA exhibited a decreased postoperative OS and a
shorter TTR. e) The functional assay indicated that in vitro
and in vivo phenotypes of ARHGDIA correlated well with the
patterns of its expression and prognosis in HCC, as well as
clinical profiles. Loss of ARHGDIA could promote HCC cell migration
and invasion in vitro and increase lung metastasis in
vivo. Therefore, the evidence above proves that ARHGDIA is a
tumor suppressor and plays an important role in HCC progression
especially in invasion and metastasis.
The changes in ARHGDIA expression are manifested
through their actions on multiple RHO GTPases, and the levels and
activity vary significantly in the different cell types and
cancers. A single Rho family member can have opposite effects in
different tumor types (16,17),
possibly leading to the biological diversity of ARHGDIA. Many
experiments have reported that loss of ARHGDIA might reduce
inhibition on endogenous Rho family GTPases exerting a negative
regulator of Rho-family GTPase activity. Turner et al found
that the amount of RhoGTP increased significantly in the HEL cells
transfected with ARHGDIA siRNA (18). In ARHGDIA-knockout mice, renal
abnormality is associated with increased Rac1 (but not RhoA)
(19), while the abnormal basal
permeability of the pulmonary vascular endothelium correlates with
the increasing activity of RhoA (20). On the contrary, overexpression of
ARHGDIA significantly inhibits the activities of RhoA, Rac1, Cdc42
and reduces the positioning of these active proteins in membranes
of myocardial cells (21). In HCC,
we confirmed that loss of ARHGDIA significantly induced Rac1, RhoA
activation in SMMC-7721 cells. Numerous studies indicate that
deregulated signaling of Rho GTPases plays an important role in HCC
progression and metastasis (9).
RhoA pathway associates with venous invasion, cell differentiation
and poor prognosis (22),
correlating with tumor progression and metastasis (23–25).
Rac1 GTPase is crucial for actin cytoskeleton reorganization at the
cell cortex and is involved in processes of HCC migration and
invasion (26–29). Therefore, activating Rho GTPase
initiated by silencing ARHGDIA in HCC cells may at least in part
mediate the effect of tumor invasion and metastasis.
In conclusion, the present study identified ARHGDIA
as a suppressor of HCC invasion and metastasis by the RhoGTP
pathway. The above findings may contribute to better understanding
of the processes of hepatic tumorigenesis, especially invasion and
metastasis thus providing a potential therapeutic target in
HCC.
References
|
1
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: worldwide incidence and trends.
Gastroenterology. 127(Suppl 1): S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Portolani N, Coniglio A, Ghidoni S, et al:
Early and late recurrence after liver resection for hepatocellular
carcinoma: prognostic and therapeutic implications. Ann Surg.
243:229–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Boix L, Sala M and Llovet JM:
Focus on hepatocellular carcinoma. Cancer Cell. 5:215–219. 2004.
View Article : Google Scholar
|
|
4
|
Takai Y, Sasaki T and Matozaki T: Small
GTP-binding proteins. Physiol Rev. 81:153–208. 2001.PubMed/NCBI
|
|
5
|
Fukumoto Y, Kaibuchi K, Hori Y, et al:
Molecular cloning and characterization of a novel type of
regulatory protein (GDI) for the rho proteins, ras p21-like small
GTP-binding proteins. Oncogene. 5:1321–1328. 1990.PubMed/NCBI
|
|
6
|
Leonard D, Hart MJ, Platko JV, et al: The
identification and characterization of a GDP-dissociation inhibitor
(GDI) for the CDC42Hs protein. J Biol Chem. 267:22860–22868.
1992.PubMed/NCBI
|
|
7
|
Sun HC, Zhang W, Qin LX, et al: Positive
serum hepatitis B e antigen is associated with higher risk of early
recurrence and poorer survival in patients after curative resection
of hepatitis B-related hepatocellular carcinoma. J Hepatol.
47:684–690. 2007. View Article : Google Scholar
|
|
8
|
Gao Q, Qiu SJ, Fan J, et al: Intratumoral
balance of regulatory and cytotoxic T cells is associated with
prognosis of hepatocellular carcinoma after resection. J Clin
Oncol. 25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ellenbroek SI and Collard JG: Rho GTPases:
functions and association with cancer. Clin Exp Metastasis.
24:657–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harding MA and Theodorescu D: RhoGDI
signaling provides targets for cancer therapy. Eur J Cancer.
46:1252–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones MB, Krutzsch H, Shu H, et al:
Proteomic analysis and identification of new biomarkers and
therapeutic targets for invasive ovarian cancer. Proteomics.
2:76–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao L, Wang H, Li J, Liu Y and Ding Y:
Overexpression of Rho GDP-dissociation inhibitor α is associated
with tumor progression and poor prognosis of colorectal cancer. J
Proteome Res. 7:3994–4003. 2008.
|
|
13
|
Zhao L, Wang H, Sun X and Ding Y:
Comparative proteomic analysis identifies proteins associated with
the development and progression of colorectal carcinoma. FEBS J.
277:4195–4204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Forget MA, Desrosiers RR, Del M, et al:
The expression of rho proteins decreases with human brain tumor
progression: potential tumor markers. Clin Exp Metastasis. 19:9–15.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang WG, Watkins G, Lane J, Cunnick GH,
Douglas-Jones A, Mokbel K and Mansel RE: Prognostic value of rho
GTPases and rhoguanine nucleotide dissociation inhibitors in human
breast cancers. Clin Cancer Res. 9:6432–6440. 2003.PubMed/NCBI
|
|
16
|
Habets GG, Scholtes EH, Zuydgeest D, et
al: Identification of an invasion-inducing gene, Tiam-1, that
encodes a protein with homology to GDP-GTP exchangers for Rho-like
proteins. Cell. 77:537–549. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hordijk PL, ten Klooster JP, van der
Kammen RA, et al: Inhibition of invasion of epithelial cells by
Tiam1-Rac signaling. Science. 278:1464–1466. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Turner SJ, Zhuang S, Zhang T, et al:
Effects of lovastatin on Rho isoform expression, activity, and
association with guanine nucleotide dissociation inhibitors.
Biochem Pharmacol. 75:405–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata S, Nagase M, Yoshida S, et al:
Modification of mineralocorticoid receptor function by Rac1 GTPase:
implication in proteinuric kidney disease. Nat Med. 14:1370–1376.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gorovoy M, Neamu R, Niu J, et al: RhoGDI-1
modulation of the activity of monomeric RhoGTPase RhoA regulates
endothelial barrier function in mouse lungs. Circ Res. 101:50–58.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei L, Imanaka-Yoshida K, Wang L, et al:
Inhibition of Rho family GTPases by Rho GDP dissociation inhibitor
disrupts cardiac morphogenesis and inhibits cardiomyocyte
proliferation. Development. 129:1705–1714. 2002.PubMed/NCBI
|
|
22
|
Li XR, Ji F, Ouyang J, et al:
Overexpression of RhoA is associated with poor prognosis in
hepatocellular carcinoma. Eur J Surg Oncol. 32:1130–1134. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang D, Dou K, Xiang H, et al: Involvement
of RhoA in progression of human hepatocellular carcinoma. J
Gastroenterol Hepatol. 22:1916–1920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fuku K, Tamura S, Wada A, et al:
Expression and prognostic role of RhoA GTPases in hepatocellular
carcinoma. J Cancer Res Clin Oncol. 132:627–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu X, Chen H, Gao Q, et al: Downregulation
of JWA promotes tumor invasion and predicts poor prognosis in human
hepatocellular carcinoma. Mol Carcinog. 53:325–336. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takenawa T and Suetsugu S: The WASP-WAVE
protein network: connecting the membrane to the cytoskeleton. Nat
Rev Mol Cell Biol. 8:37–48. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee TK, Man K, Ho JW, et al: Significance
of the Rac signaling pathway in HCC cell motility: implications for
a new therapeutic target. Carcinogenesis. 26:681–687.
2005.PubMed/NCBI
|
|
28
|
Liu S, Yu M, He Y, et al: Melittin
prevents liver cancer cell metastasis through inhibition of the
Rac1-dependent pathway. Hepatology. 47:1964–1973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Chan TH, Yuan YF, et al: CHD1L
promotes hepatocellular carcinoma progression and metastasis in
mice and is associated with these processes in human patients. J
Clin Invest. 120:1178–1191. 2010. View
Article : Google Scholar : PubMed/NCBI
|