Introduction
Glioblastomas are highly radio- and
chemotherapy-resistant cancers, because they contain cellular
hierarchies with cancer stem cells (CSCs) that are radio- and
chemoresistant (1–4). CSCs are maintained within the niche
for stem cells, such as the hypoxic microenvironment (5), as previously shown in normal stem
cells (6). Hypoxia contributes to
the self-renewal, differentiation, and quiescence of CSCs (7). Hypoxia-inducible factors (HIFs)
become stable under the hypoxic condition and transcriptionally
upregulate genes that promote cell survival, motility, and tumor
angiogenesis, leading to the promotion of cancer cells to a more
malignant state. However, the role of HIFs in the maintenance of
CSCs is not fully understood. Recent studies have shown that HIFs
maintain glioma stem cells via overexpression of ZNF217 (8), promote expansion of glioma stem cells
(9), and upregulate the expression
of CD133 protein in glioblastoma-derived neurospheres (10). Furthermore, HIFs may mediate the
expression of chemoresistance markers in CSCs (11). It has been shown that in
glioblastoma-derived neurospheres, hypoxia induces increases in the
expression of not only HIFs and CD133 but also
chemoresistance-related markers (MGMT, TIMP-1, Lamp-1, MRP1 and
MDR-1). Similarly, in cancer cell lines as well as in CSCs, HIFs
contribute to the regulation of stemness-related cellular responses
such as neurosphere formation (7)
and the upregulation of CD133 (12) and the stem cell factors OCT4,
NANOG, SOX2, KLF4 and c-MYC (7,13).
These findings indicate that the hypoxic condition play an
important role in the stemness state of cancer cells as well as
CSCs.
CD133 has recently attracted much attention as a
marker for identifying CSCs in glioblastoma, hepatic cancer,
melanoma, osteosarcoma and colon cancer (14–19).
However, the physiological function of the transmembrane protein
CD133 remains unknown. On the basis of the assessment of graft
survival rate in a xenograft model and ability of sphere formation
under the routine culture condition, it was demonstrated that
CD133-positive cells exhibit properties of CSCs. In contrast,
controversial results indicating that CD133-negative cells also
exhibit properties of CSCs have been reported (20–23).
A possible explanation for this discrepancy is the plasticity of
CD133 expression, which is dependent on the cellular
microenvironment. In glioblastoma neurosphere cells, the level of
CD133 expression was reported to vary with oxygen concentration
(24). In addition,
hypoxia-induced CD133 expression has been reported in human lung
cancer cell lines (12). It has
been reported that in SW620 colorectal cancer cells, CD133
expression changes depending on the type of the microenvironment
(25). Under the low-nutrient
culture condition, CD133-negative SW620 colorectal cancer cells
re-expressed CD133. These results indicate that CD133 expression is
dynamic and modulated by the cellular microenvironment. It seems
that CD133 is not a critical marker of glioblastoma CSCs. The
plasticity of CD133 expression in the non-stem and stem-like
glioblastoma populations provides careful consideration about
effects of the hypoxic microenvironment on both cell
populations.
Spheroids, larger cell masses compared with spheres,
can be used as an in vitro tumor model, which exhibits
marked similarity to the three-dimensional growth and morphological
characteristics of in vivo tumors (26). Cells in the outer region of
spheroids are exposed to a normoxic, nutrient-rich, and neutral pH
microenvironment, but cells in the central area of spheroids are
exposed to a hypoxic, low-nutrient, and low pH microenvironment.
Cellular oxygen consumption of V79 and EMT6 spheroid cells
decreases ~3-fold with the increase in the size of spheroids
(27,28). Such three-dimensional
characteristics of spheroids are expected to provide useful
information for the behavior of stemness-related markers under a
hypoxic microenvironment. In this study, we examined the expression
of CD133 using cryostat sections of glioblastoma spheroids formed
using the T98G cell line.
Materials and methods
Cell culture
A human glioblastoma cell line (T98G) was maintained
in α-minimum essential medium (α-MEM) supplemented with 20 mM
4-(2-hydroxyethyl) piperazine ethane sulfonic acid (HEPES), 8 mM
NaHCO3, 50 μg/ml streptomycin, 50 U/ml penicillin and
10% fetal calf serum. T98G cells were cultured in a humidified
incubator at 37°C with a mixture of 98% air and 2%
CO2.
Spheroid culture
T98G cells were seeded onto non-adherent U-shape
bottom 96-well plates (PrimeSurface 96U, MS-9096U, Sumitomo
Bakelite Co., Ltd., Tokyo, Japan) in α-MEM at a density of 5,000 or
10,000 cells/well and cultured for 3 days at 37°C with a mixture of
98% air and 2% CO2. Three days later, spheroids were
transferred to non-adherent 30-mm dishes (PrimeSurface 35Φ,
MS-9035X, Sumitomo Bakelite Co., Ltd.) at a density of 6–10
spheroids/dish to decrease the frequency of medium change during
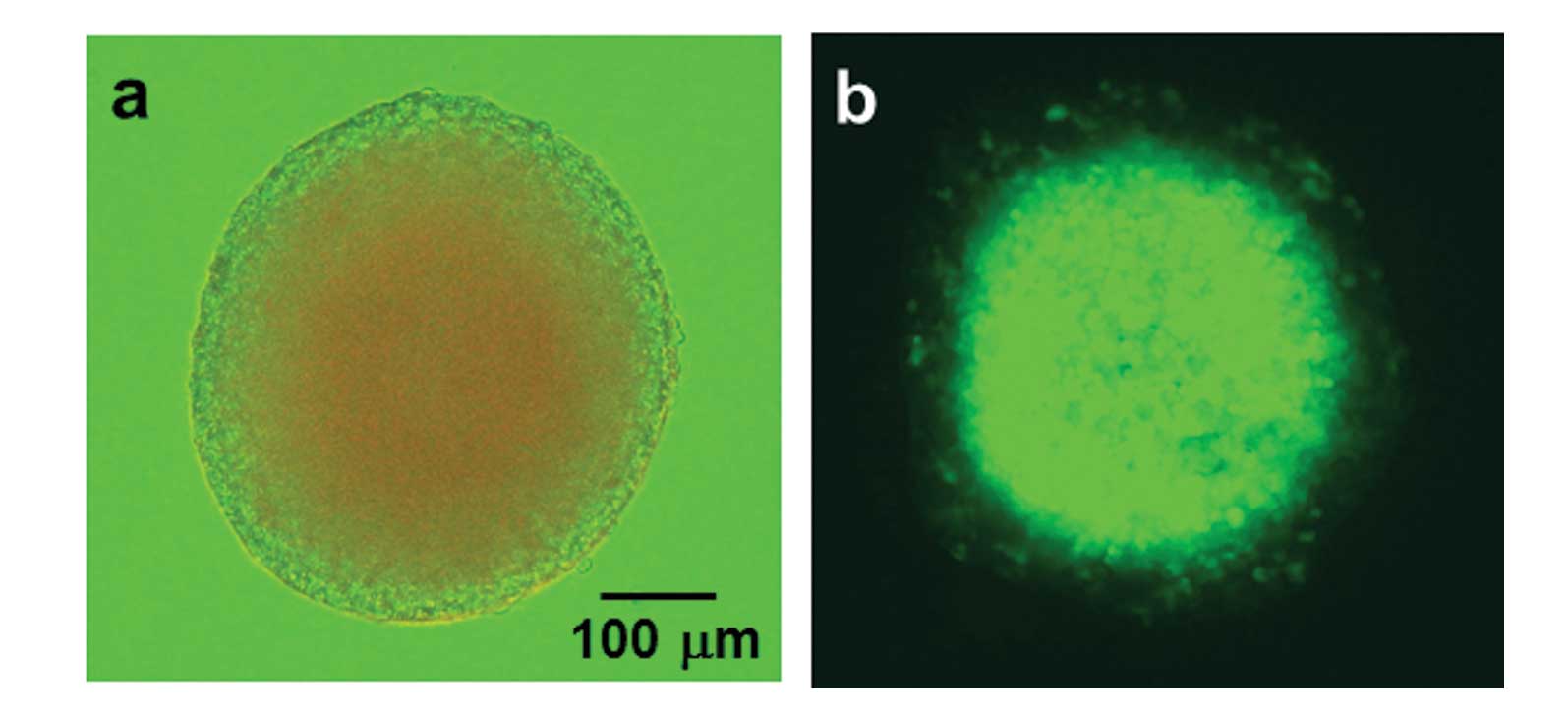
culturing for 7–10 days. The spheroids grew to a diameter of ~300
μm (Fig. 1a).
Preparation of frozen cryostat
sections
Spheroids were rinsed with PBS and fixed with 10%
formalin containing 10% sucrose for ≥1 h. After rinsing with PBS,
spheroids were embedded in Tissue-Tek O.C.T. Compound (Sakura
Finetechnical Co., Ltd., Tokyo, Japan) and cut into 10–20-μm-thick
frozen sections using a cryostat.
Stable transfection
To monitor tumor hypoxia, a stable
reporter-transfectant, T98G/5HRE-EGFP, which expressed GFP in
response to hypoxic stress, was isolated. A plasmid, p5HRE-EGFP,
into which was inserted a GFP reporter gene under the regulation of
an artificial HIF-1α-dependent promoter, 5HRE, was transfected into
T98G cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s protocol. Cells were trypsinized 24
h after the transfection and replated onto 100-mm dishes in α-MEM
containing G418 (200 μg/ml, Sigma Chemical Co., St. Louis, MO, USA)
and then incubated at 37°C for 7–10 days to enable colony
formation. Colonies were isolated using cloning cylinders. The
hypoxic area was monitored by GFP fluorescence in spheroids formed
using T98G/5HRE-EGFP transfectants, as shown in Fig. 1b.
Immunofluorescence staining
Cryostat sections were rinsed twice with PBS and
incubated with anti-CD133/1 (AC133) monoclonal antibody (Miltenyi
Biotechnology, Auburn, CA, USA), anti-HIF-1α polyclonal antibody
(Novus Biologicals, Littleton, CO, USA), or anti-nestin polyclonal
antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at
37°C. After the incubation, cells were washed with PBS three times
and then incubated with Alexa Fluor 488 anti-rabbit and/or 546
anti-mouse secondary antibodies (Nacalai Tesque, Kyoto, Japan) for
1 h at 37°C. The sections were then washed with PBS, treated with
SlowFade Gold antifade regent with DAPI (Invitrogen), and covered
with a glass cover slip.
Quantitative PCR analysis
Total RNA was extracted with a RNAiso Plus kit
(Takara, Siga, Japan). RNA extracts (1 μg) were treated with DNase
to degrade genomic DNA and then reverse transcribed according to
the protocol of PrimeScript RT regent kit (Takara). Quantitative
PCR was performed with SYBR Premix Ex Taq™ II (Takara) in 25-μl
reactions using 1/25 of the cDNA. The following conditions were
used for PCR by Thermal Cycler Dice Real Time System II (Takara):
one cycle at 95°C for 30 sec and 95°C for 5 sec, 40 cycles at 60°C
for 30 sec, and one cycle at 95°C for 30 sec, 60°C for 30 sec, 95°C
for 15 sec for dissociation. The sequence-specific primers of CD133
and β-actin were as follows: CD133: 5′-GGA CCCATTGGCATTCTC-3′
(sense) and 5′-CAGGACACA GCATAGAATAATC-3′ (antisense); β-actin:
5′-GGCACCC AGCACAATGAAG-3′ (sense) and 5′-TCATAGTCCGCCTA GAAGCA-3′
(antisense).
For each sample, nonspecific PCR products were
checked using dissociation curves. The threshold cycle (Ct) values
for gene expression in each sample were normalized by the relative
expression of β-actin.
Results
Observation of CD133AC133- and
HIF-1α-positive cells in adherent monolayer cells and
spheroids
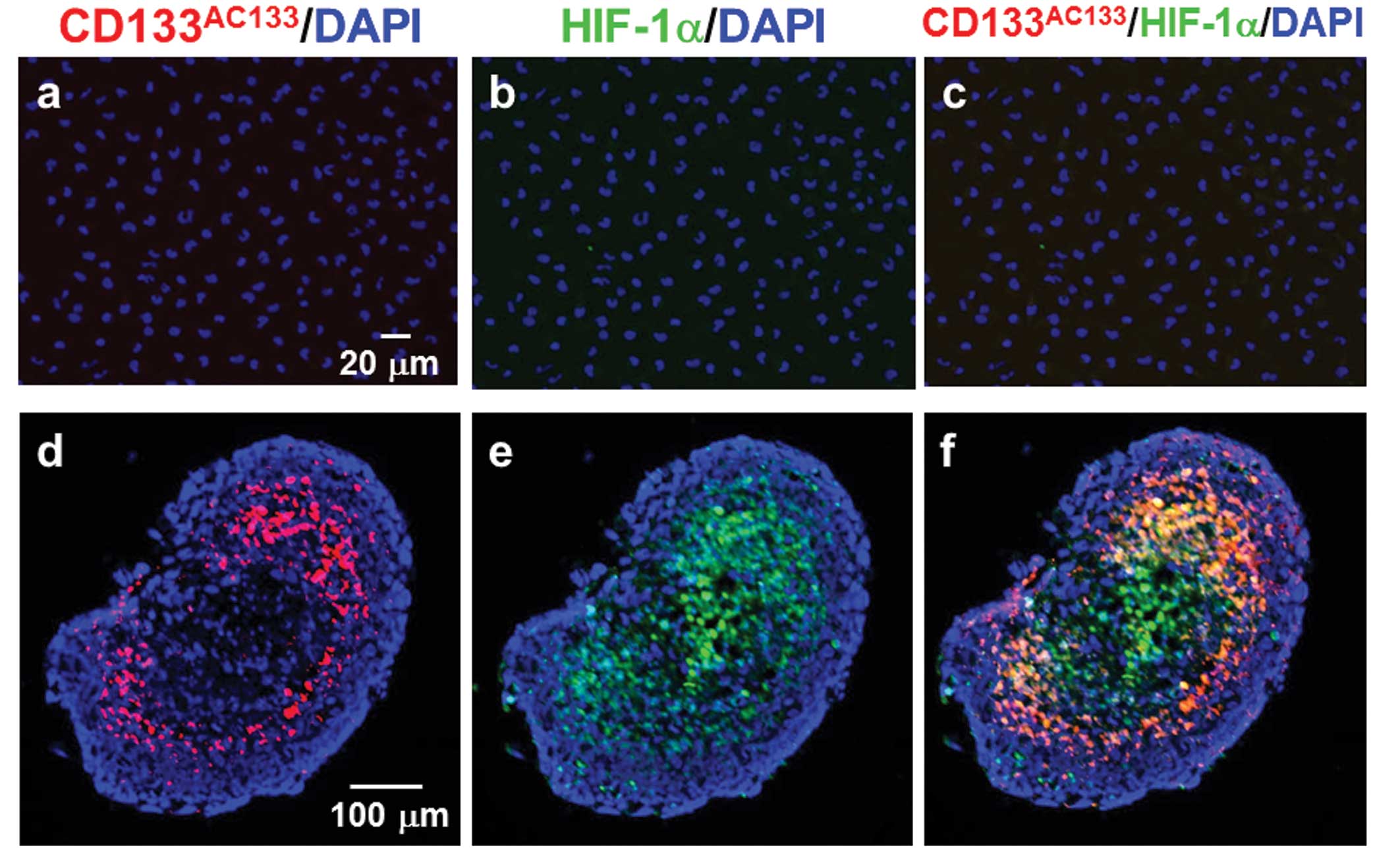
Double immunofluorescence staining with
CD133AC133 and HIF-1α antibodies showed that T98G cells
under the adherent monolayer culture condition were not positive
for either CD133AC133 or HIF-1α (Fig. 2a–c). However, immunofluorescence
staining of cryostat sections showed that 10 days after spheroid
formation, many CD133AC133- and HIF-1α-positive cells
were located mainly in the marginal region of the central area of
T98G spheroids positive for HIF-1α (Fig. 2d–f). In addition, a few
CD133AC133- and HIF-1α-positive cells were observed in
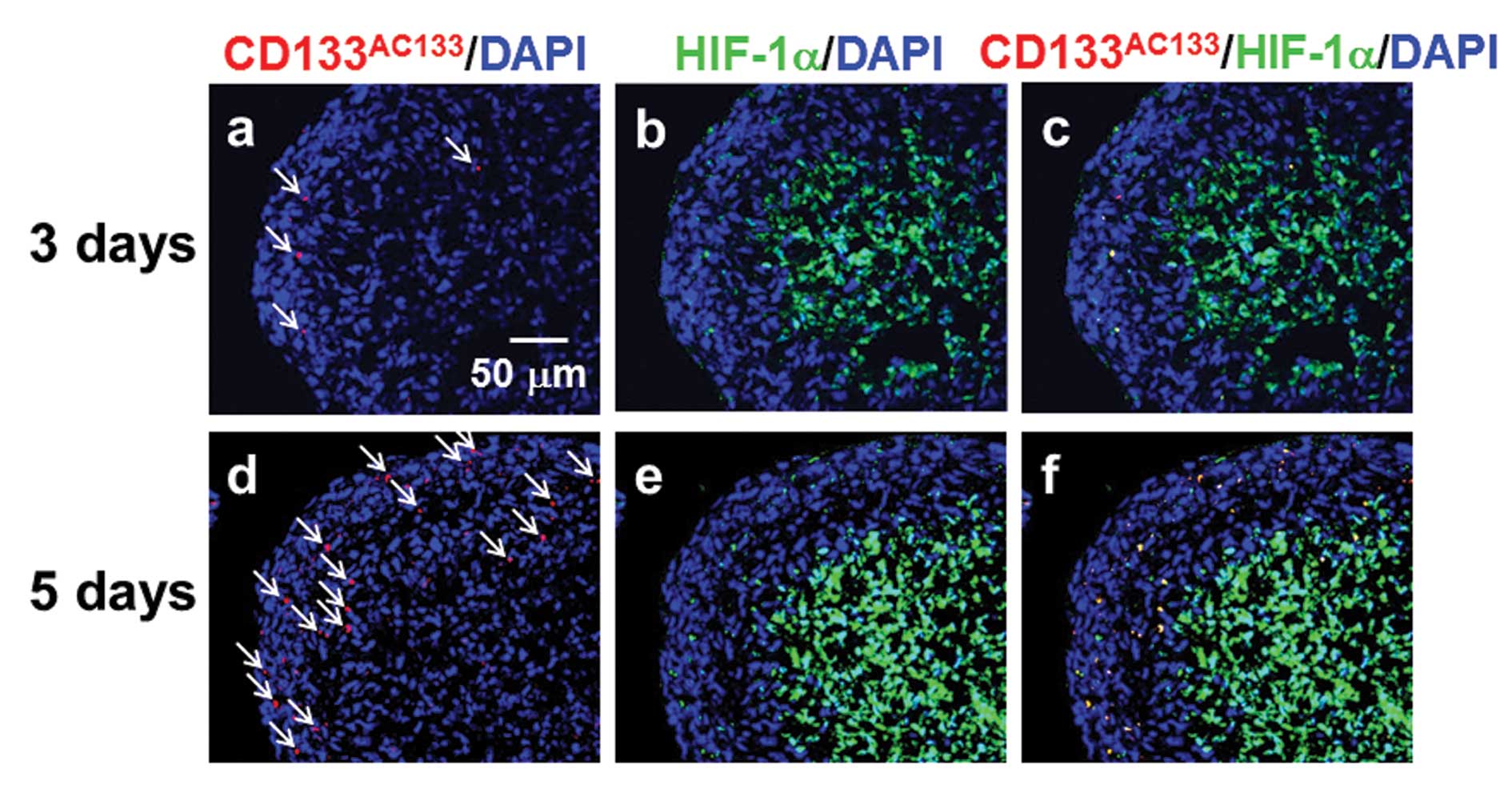
the outer region near the surface of spheroids. Three days after
spheroid formation, CD133AC133- and HIF-1α-positive
cells were sparsely distributed in the outer region of spheroids
(Fig. 3a–c) and were more abundant
5 days after spheroid formation (Fig.
3d–f). At this early stage after spheroid formation, a few
CD133AC133- and HIF-1α-positive cells were observed in
the central areas of spheroids positive for HIF-1α.
Observation of CD133AC133- and
nestin-positive cells in adherent monolayer cells and
spheroids
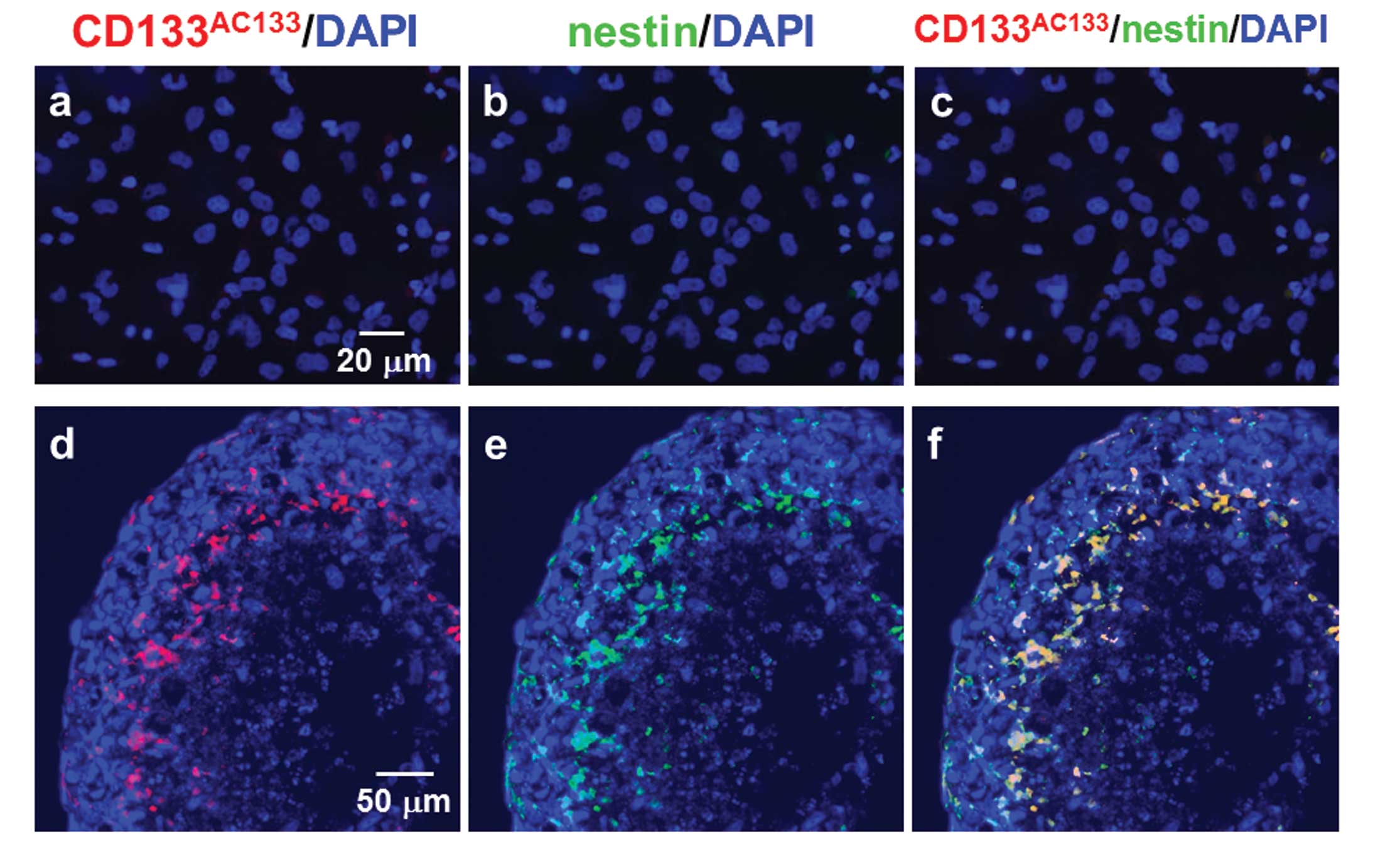
T98G cells under the adherent monolayer culture
condition were not positive for CD133AC133 or nestin
after double immunofluorescence staining with CD133AC133
and nestin antibodies (Fig. 4a–c).
However, immunofluorescence staining of cryostat sections showed
that CD133AC133- and nestin-positive cells were located
mainly in the marginal region of the central area of spheroids
positive for HIF-1α 10 days after spheroid formation (Fig. 4d–f). A few CD133AC133-
and nestin-positive cells were also observed in the outer region of
spheroids.
Observation of CD133AC133- and
nestin-positive cells in adherent monolayer cells dissociated from
spheroids
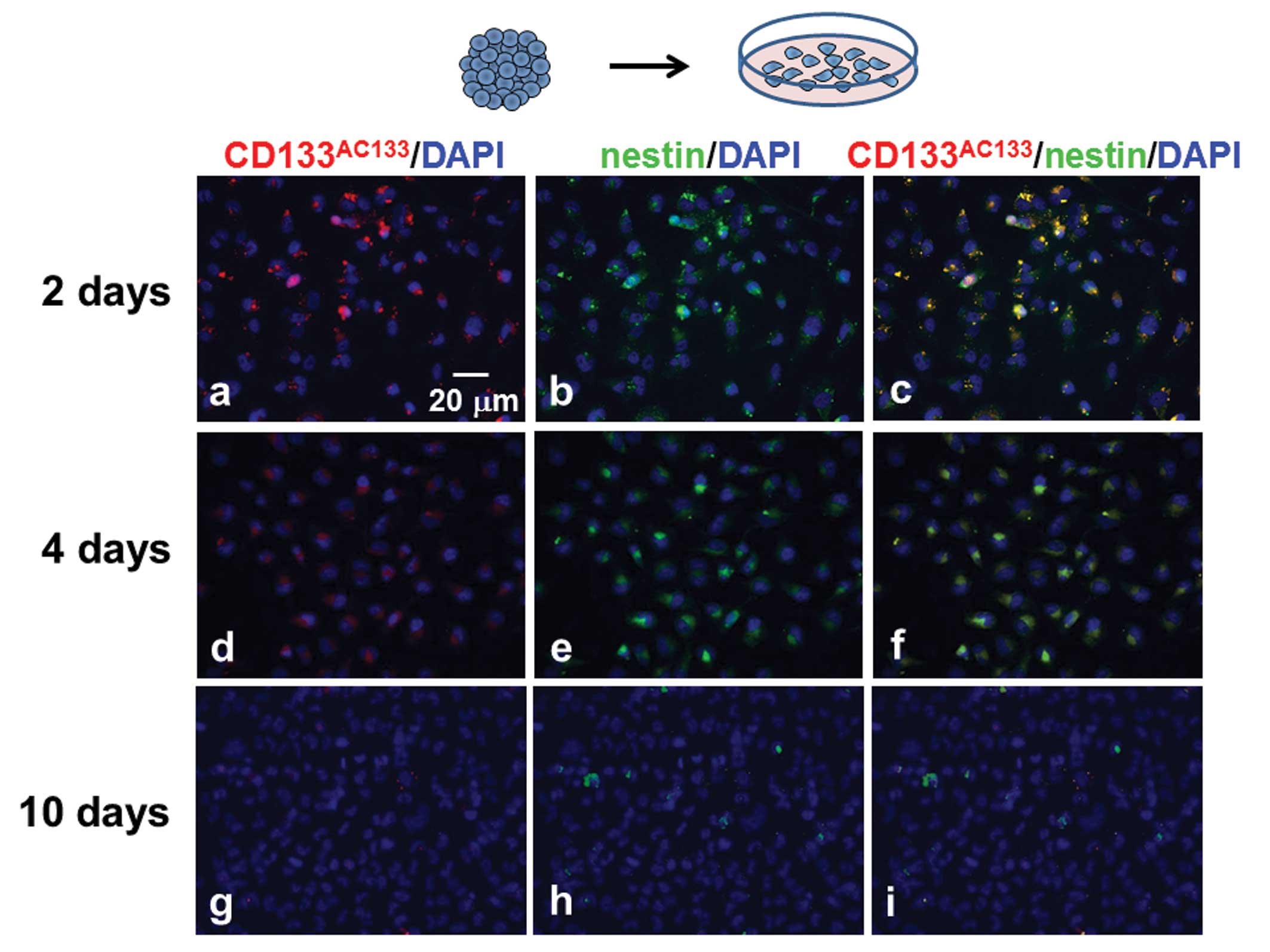
T98G spheroids were trypsinized 10 days after
spheroid formation, and the dissociated cells were then cultured
under the adherent monolayer condition for scheduled periods (2, 4
and 10 days). The monolayer-cultured cells were immunofluorescently
stained with CD133AC133 and nestin antibodies. Many
CD133AC133- and nestin-positive cells were observed 2
days after culturing under the adherent monolayer culture condition
(Fig. 5a–c), but these cells were
slightly decreased after 4 days (Fig.
5d–f). After further culture for 6 days, hardly any
CD133AC133- or nestin-positive cells were observed in
the adherent monolayer cells (Fig.
5g–i).
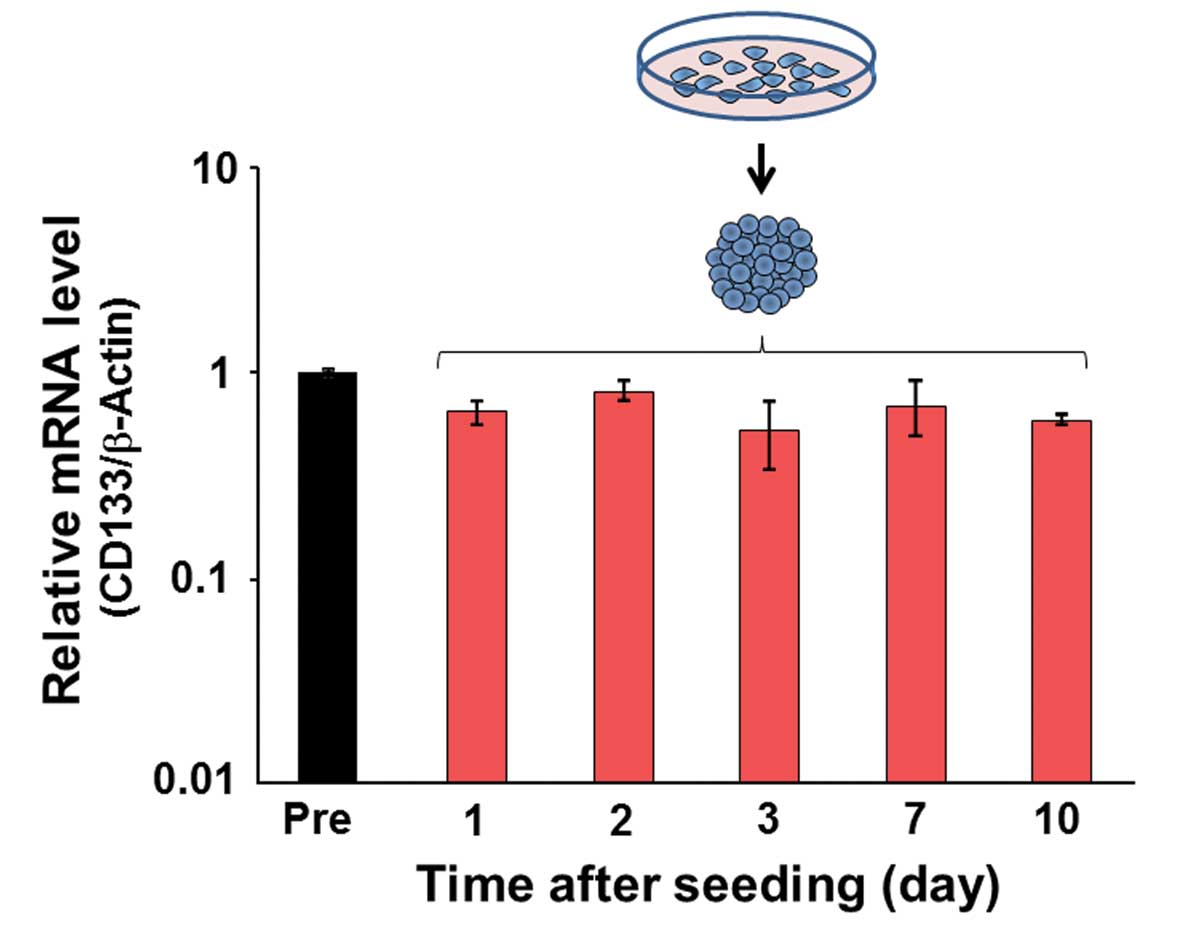
CD133 expression in spheroids
Real-time quantitative PCR analysis was performed to
evaluate CD133 expression in spheroids. No significant differences
were observed in the relative mRNA level from 1 to 10 days after
spheroid formation (Fig. 6).
Discussion
CD133AC133-positive cells were observed
in the outer region of spheroids 3 days after spheroid formation.
The oxygenic condition of this region was considered to be
normoxic, given that the region was HIF-1α-negative. At this early
stage of spheroid formation, CD133AC133-positive cells
appear to be oxygen-independently induced. At this stage, most
CD133AC133-positive cells were HIF-1α-positive.
Oxygen-independent stabilization of HIF-1α is likely to mediate the
induction of CD133AC133-positive cells. In contrast to
this stage, at a late stage ~10 days after spheroid formation, most
CD133AC133-positive cells were observed in the marginal
region of the central area of spheroids positive for HIF-1α
indicating a hypoxic microenvironment. These results suggest that
the different microenvironments within spheroids (the normoxic
microenvironment in the outer region and the hypoxic
microenvironment in the central region) contribute to the induction
and maintenance of CD133AC133-positive cells. However,
it is unknown whether CD133AC133-positive cells
translocate from the normoxic to the hypoxic microenvironment in
the spheroid.
The number of CD133AC133-positive cells
dramatically varied in accordance with the change in the culture
condition between spheroid and monolayer culture. This finding
suggests that the positivity to CD133AC133 may be
plastic according to the cellular microenvironments such as low
oxygen, low nutrients, and low pH. Given that CD133
expression was not affected by the cell culture condition (Fig. 6), the positivity to
CD133AC133 may result from a post-translational
modification that enables the CD133AC133 antibody to
bind to the AC133 epitope. CD133 is probably differentially folded
in CD133AC133-positive and
CD133AC133-negative cells as a result of differential
glycosylation to mask specific epitopes. Such a post-translational
modification of the AC133 epitope has been reported elsewhere in
association with the differentiation of
CD133AC133-positive cells (29). However, it should be kept in mind
that the change in CD133 expression might not be detected by
the present quantitative PCR analysis because of low number of
CD133AC133-positive cells.
CD133AC133-positive cells in spheroids
were also nestin-positive. Because nestin is known to be a marker
for undifferentiated neural cells, it has been suggested that the
CD133AC133-positive cells may be undifferentiated neural
cell-like cells. This finding proposes the possibility that CD133
is not only restricted to CSCs but is also expressed on cancer
cells that began to express an undifferentiated phenotype. Tumor
cells are exposed to a wide range of microenvironments (low oxygen,
low nutrients, and low pH), and these microenvironments may
strongly affect the phenotypic expression of their malignant
properties.
The present study demonstrated that
CD133AC133-positive cells are plastically induced under
the different culture conditions, spheroid and monolayer, in
relation to stability of HIF-1α. Spheroids as an in vitro
tumor model are useful to study the dynamic changes in the tumor
cell phenotype in the different cell microenvironments.
Acknowledgements
This study was supported by Grants-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
References
|
1
|
Bao S, Wu Q, McLendon RE, Hao Y, et al:
Glioma stem cells promote radioresistance by preferential
activation of the DNA damage response. Nature. 444:756–760. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mannino M and Chalmers AJ: Radioresistance
of glioma stem cells: intrinsic characteristic or property of the
‘microenvironment-stem cell unit’? Mol Oncol. 5:374–386. 2011.
|
|
3
|
Liu G, Yuan X, Zeng Z, et al: Analysis of
gene expression and chemoresistance of CD133+ cancer
stem cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang MK and Kang SK: Tumorigenesis of
chemotherapeutic drug-resistant cancer stem-like cells in brain
glioma. Stem Cells Dev. 16:837–847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohyeldin A, Garzón-Muvdi T and
Quiñones-Hinojosa A: Oxygen in stem cell biology: a critical
component of the stem cell niche. Cell Stem Cell. 7:150–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parmar K, Mauch P, Vergilio JA, et al:
Distribution of hematopoietic stem cells in the bone marrow
according to regional hypoxia. Proc Natl Acad Sci USA.
104:5431–5436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heddleston JM, Li Z, McLendon RE, et al:
The hypoxic microenvironment maintains glioblastoma stem cells and
promotes reprogramming towards a cancer stem cell phenotype. Cell
Cycle. 8:3274–3284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao XG, Yan M, Xue XY, et al:
Overexpression of ZNF217 in glioblastoma contributes to the
maintenance of glioma stem cells regulated by hypoxia-inducible
factors. Lab Invest. 91:1068–1078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soeda A, Park M, Lee D, et al: Hypoxia
promotes expansion of the CD133-positive glioma stem cells through
activation of HIF-1alpha. Oncogene. 28:3949–3959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bar EE, Lin A, Mahairaki V, et al: Hypoxia
increases the expression of stem-cell markers and promotes
clonogenicity in glioblastoma neurospheres. Am J Pathol.
177:1491–1502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kolenda J, Jensen SS, Aaberg-Jessen C, et
al: Effects of hypoxia on expression of a panel of stem cell and
chemoresistance markers in glioblastoma-derived spheroids. J
Neurooncol. 103:43–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iida H, Suzuki M, Goitsuka R, et al:
Hypoxia induces CD133 expression in human lung cancer cells by
up-regulation of OCT3/4 and SOX2. Int J Oncol. 40:71–79.
2012.PubMed/NCBI
|
|
13
|
Mathieu J, Zhang Z, Zhou W, et al: HIF
induces human embryonic stem cell markers in cancer cells. Cancer
Res. 71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monzani E, Facchetti F, Galmozzi E, et al:
Melanoma contains CD133 and ABCG2 positive cells with enhanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O’Brien CA, Pollett A, Gallinger S, et al:
A human colon cancer cell capable of initiating tumour growth in
immunodeficient mice. Nature. 445:106–110. 2007.PubMed/NCBI
|
|
16
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma S, Chan KW, Hu L, et al: Identification
and characterization of tumorigenic liver cancer stem/progenitor
cells. Gastroenterology. 132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tirino V, Desiderio V, Paino F, et al:
Human primary bone sarcomas contain CD133+ cancer stem
cells displaying high tumorigenicity in vivo. FASEB J.
25:2022–2030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beier D, Hau P, Proescholdt M, et al:
CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show
differential growth characteristics and molecular profiles. Cancer
Res. 67:4010–4015. 2007.
|
|
21
|
Joo KM, Kim SY, Jin X, et al: Clinical and
biological implications of CD133-positive and CD133-negative cells
in glioblastomas. Lab Invest. 88:808–815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogden AT, Waziri AE, Lochhead RA, et al:
Identification of A2B5+CD133−
tumor-initiating cells in adult human gliomas. Neurosurgery.
62:505–514. 2008.
|
|
23
|
Wang J, Sakariassen P, Tsinkalovsky O, et
al: CD133 negative glioma cells form tumors in nude rats and give
rise to CD133 positive cells. Int J Cancer. 122:761–768. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Platet N, Liu SY, Atifi ME, et al:
Influence of oxygen tension on CD133 phenotype in human glioma cell
cultures. Cancer Lett. 258:286–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Z, Wang Z, Fan Y, et al: Expression
of CD133 in SW620 colorectal cancer cells is modulated by the
microenvironment. Oncol Lett. 4:75–79. 2012.PubMed/NCBI
|
|
26
|
Sutherland RM: Cell and environment
interactions in tumor microregions: the multicell spheroid model.
Science. 240:177–184. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Freyer JP, Tustanoff E, Franko AJ, et al:
In situ oxygen consumption rates of cells in V-79 multicellular
spheroids during growth. J Cell Physiol. 118:53–61. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freyer JP and Sutherland RM: A reduction
in the in situ rates of oxygen and glucose consumption of cells in
EMT6/Ro spheroids during growth. J Cell Physiol. 124:516–524. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kemper K, Sprick MR, de Bree M, et al: The
AC133 epitope, but not the CD133 protein, is lost upon cancer stem
cell differentiation. Cancer Res. 70:719–729. 2010. View Article : Google Scholar : PubMed/NCBI
|