Introduction
Cancer stem cells (CSCs) have been identified in
various cancers and are thought to be involved in metastasis,
recurrence and radio/chemotherapy resistance (1–5). One
of the main properties used to isolate CSCs is their capacity to
form spheres in non-adherent culture conditions (6). Recently CSCs have been identified in
prostate cancer (PCa) (7,8), one of the most diagnosed male
malignancies worldwide (9).
Several PCa CSCs features such as molecular signatures, gene
expression profiles, functional characteristics and metastatic
potential have been published (10,11).
Most data come from CSCs produced from PCa cell lines, mainly from
metastasis, and animal models which are the main bias for the
clinical projection of the results. These studies have identified
several molecular markers for CSCs such as cluster of
differentiation 133, 44, 40 (CD133, CD44, CD40) and α2β1 integrin
(12,13). Furthermore, the capacity to exclude
Hoechst 33342 staining has been used to separate CSCs (side
population) (14,15). ABCG2 transporter is responsible for
the exclusion of this dye and has been found overexpressed in
several CSCs, including PCa (16).
Recently, we have studied several stem markers in biopsy archives
of PCa tumors of different Gleason grades, lymph node and bone
metastases, and also have obtained an enriched CSCs population from
PCa explants (13). These
tumorsphere (prostatosphere) cultures showed a molecular signature
CD133+/CD44+/ABCG2+/CD24−,
and these stem markers were found mainly in medium Gleason samples
of PCa biopsies (13). On the
other hand, PCa is known by its strikingly high intrinsic drug
resistance (17–19). In advanced disease, the gold
standard is androgen-deprivation therapy (20,21)
but in castration-resistant stages, chemotherapy has very limited
impact in patient survival (22).
Previously, we have extensively studied the molecular mechanisms of
multidrug resistance (MDR) in PCa (23,24).
A high expression of ABC transporters seems to be involved in this
MDR phenotype, since the pharmacological blockage and knocking down
of ABC transporters partially sensitize PCa cells to therapeutic
drugs (23). PCa CSCs exhibit a
high expression of ABCG2 (13), a
transporter used by several chemotherapeutic drugs, suggesting that
CSCs may be responsible for the increased MDR phenotype of PCa
tumors. In the present work, a functional characterization,
including drug resistance, of prostatosphere cultures obtained from
PCa explants is reported.
Materials and methods
Prostatic tissue
The prostatic tissue was obtained from patients
undergoing radical prostatectomy for PCa from the Clinical Hospital
of the University of Chile. The tissue was received in sterile
culture medium and transferred subsequently to the laboratory. All
protocols for obtaining these samples were approved by the
Committees of Bioethics of the Faculty of Medicine and the Hospital
of the University of Chile.
Cell cultures
PCa cell cultures were obtained from the tumor
explants according to our methods described (13). After enzymatic digestion, resulting
cell aggregates were washed and seeded in culture plates of 10 cm
diameter using DMEM/F-12 culture medium (Gibco, Invitrogen,
Carlsbad, CA, USA) including fetal bovine serum 7% (FBS), and
supplements as described previously (13), in an atmosphere of 5%
CO2 at 37°C. These cultures in adherent conditions
contained a representative mixed population of tumor cells and were
considered as non-CSC controls.
Tumorsphere (prostatosphere)
cultures
After 3 passages of the cell cultures described
above, cells were detached, washed and cultured in non-adherent
conditions in absence of FBS and with B-27 supplement (Gibco,
Invitrogen), as described previously (13). Resulting tumorspheres were
maintained until at least 2 weeks with medium change every 3 days.
These prostatosphere cultures contained mainly cells with stemness
markers and were considered as CSCs.
Immunocytochemistry
Adherent cells at 60% confluence were washed,
trypsinized, collected and centrifuged at 70 g, whereas, the
prostatospheres were collected on day 10 of culture, washed and
pelleted. Subsequently, both pellets from prostatospheres and
adherent cells, were fixed with paraformaldehyde solution
(paraformaldehyde 16%, PBS 1 M, sucrose 0.2 M) for 12 h.
Afterwards, the pellets were washed and embedded in HistoGel
(Thermo Scientific, Waltham, MA, USA) a matrix that preserves
cellular integrity and facilitates handling during histological
processing. Then, samples were dehydrated in an increasing ethanol
concentration, cleared in butanol and embedded in Paraplast Plus.
Serial sections (3-μm) were obtained and mounted on silanized
slides. Samples from adherent and prostatosphere cultures were
deparaffinized in xylene and hydrated in a decreasing concentration
of ethanol. Subsequently, antigen retrieval was performed and the
preparations were transferred to citrate buffer pH 6.0, exposed for
3 min in a microwave oven and immediately incubated for 30 min in a
steamer. Then, the preparations were incubated in
H2O2 3% to inhibit endogenous peroxidases and
then washed in distilled water. Then, sections were incubated for
30 min in normal goat serum to block non-specific binding. Later,
samples were incubated at 4°C for 12 h with antibodies to
anti-stemness markers, CD44 (Santa Cruz Biotechnology, Inc., Santa
Cruz, USA), CD133 (Bioss, Woburn, MA, USA) and cytokeratin 5 (CK5)
(Thermo Scientific), and anti-differentiation markers, androgen
receptor (AR) (Thermo Scientific), prostatic specific antigen (PSA)
(Santa Cruz Biotechnology, Inc.) and cytokeratin 18 (CK18) (Abcam,
Cambridge, MA, USA). Then, the samples were washed and incubated
for 1 h at room temperature with the corresponding secondary
antibody (goat, anti-mouse, KPL, Inc., Gaithersburg, MD, USA).
After washing, the slides were incubated for 30 min at room
temperature with immuno-peroxidase kit (Vectastain-ABC, Vector
Laboratories, Burlingame, CA, USA), washed and incubated for 10 min
with 3,3′-diaminobenzidine substrate (DAB) (Sigma, St. Louis, MO,
USA) as chromogen, followed by counterstaining with hematoxylin.
Subsequently, sections were dehydrated in increasing ethanol
concentrations, cleared in xylene and mounted with Entellan (Merck,
Germany). For negative control, serial sections were exposed only
to the secondary antibody. For immunostaining intensity
quantification, H-Score method was used (25).
Differential cloning capacity
To evaluate the formation of different types of
clones (holoclones, meroclones and paraclones), prostatospheres
obtained at day 7 of culture and adherent cultures at 60%
confluence, were digested with Accutase (StemCell Technologies,
USA) at 37°C for 10 min and then disrupted mechanically by
pipetting. Resulting cells were cultured at density of 2000
cells/well in plates of 6 cm diameter for 15 days at 37°C in 5% of
CO2. Subsequently, the colonies were stained and fixed
with glutaraldehyde 6% mixed 1:1 with crystal violet 0.5% for 30
min (26). Then, the plates were
washed with water and dried at room temperature. For counting, only
colonies with >50 cells were considered (26). The morphology of the colonies was
evaluated according to a method described previously (27), under a stereoscopic microscope
connected to a digital camera Olympus C-4040 DIG CAM Zoom. The
results were expressed as the percentage of each clone type formed
in both prostatospheres and adherent cell cultures.
Anchorage-independent growth capacity
(soft agar assay)
To assess anchorage-independent colony formation
capacity, prostatospheres obtained at day 7 of culture and adherent
cultures at 60% confluence, were digested with Accutase at 37°C for
10 min and then disrupted mechanically by pipetting. Resulting
cells were resuspended in culture medium mixed 1:1 with agarose
0.3% and plated at density of 1000 cells/well in plates of 6 cm
diameter, coated with agar 1%, for 15 days at 37°C in 5% of
CO2. Subsequently, plates were stained with crystal
violet 0.5% and fixed with methanol for 30 min (26). Then, the plates were washed with
water and dried at room temperature. For counting, only colonies
with diameter >100 μm were considered (28). The results were expressed as the
number of colonies formed from each type of cell culture.
Single colony formation assay
To evaluate the single colony formation capacity,
prostatospheres obtained at day 7 of culture and adherent cultures
at 60% confluence, were digested with Accutase at 37°C for 10 min
and then disrupted mechanically by pipetting. Resulting cells were
seeded at a density of 1 cell/well in 96-well culture plates coated
with agarose 1%, for 15 days at 37°C in 5% of CO2.
Subsequently, the spheres formed were counted using a phase
contrast microscope (29).
Evaluation of cell proliferation
Cell proliferation rates of prostatospheres and
adherent cultures were evaluated by immunocytochemistry, following
the protocol and conditions described above, using antibodies
against the proliferation markers Ki67 (Dako/Agilent Technologies,
Santa Clara, CA, USA), PCNA (Novocastra, UK) and BrdU (Zymed, Life
Technologies USA).
Evaluation of apoptosis
Apoptosis was evaluated using the TUNEL assay (In
situ cell death detection kit Rhodamine; Roche Diagnostics
GmbH, Mannheim, Germany). Both prostatospheres and adherent cells
were processed as for immunocytochemistry. The preparations
obtained were subjected to TUNEL-Rhodamine technique following the
manufacturer’s instructions. Slides were blocked with BSA 3%, and
exposed to TUNEL assay at 37°C for 1 h in a moist chamber in
darkness. For negative control, serial sections were exposed only
to the staining solution. As a positive control, cells exposed to
daunorubicin 8 μM (Sigma) were used. The preparations obtained were
analyzed in a confocal laser scanning microscope, Nikon C1 Plus
model.
Drug resistance assay
Both adherent cells and prostatospheres were
cultured for 7 days, in their specific conditions, in 48-well
plates at 37°C in 5% of CO2. Following this, the media
were changed to include topotecan (Sigma) or daunorubicin (Sigma),
drugs that are substrates for ABCG2 transporter, at different
concentrations for 48 h. Afterwards, culture media containing the
drugs were removed and replaced with 100 μl of MTT
(dimethyl-thiazol-diphenyl tetrazolium) solution (Sigma). The
incubation was performed for 2 h at 37°C in darkness. After the
incubation, the MTT solution was removed and replaced by DMSO and
plates incubated with stirring at room temperature. Then, each
plate was analyzed in a micro-plate reader at 570 nm (BioTek
Instruments, Inc., Winooski, VT, USA). The results were expressed
as the percentage of survival respect to the control cells
incubated without drugs, which were considered as 100% survival.
Furthermore, in parallel experiments, the ABCG2 inhibitor
fumitremorgin C (Sigma) was used alone or in combination with both
drugs. For topotecan and daunorubicin, dose-response curves for
each culture type (adherent and prostatospheres) were carried out.
The corresponding half maximal effective concentrations (EC50) for
both drugs were determined by analyzing the resulting dose-response
curve using GraphPad Prism 6.0 software.
Statistical analysis
The statistical evaluation of the results was
performed using unpaired two-tailed Student’s t-test or ANOVA
followed by Bonferroni post test. Statistic significance was
considered for p<0.05. Results were expressed as means ± SE.
Results
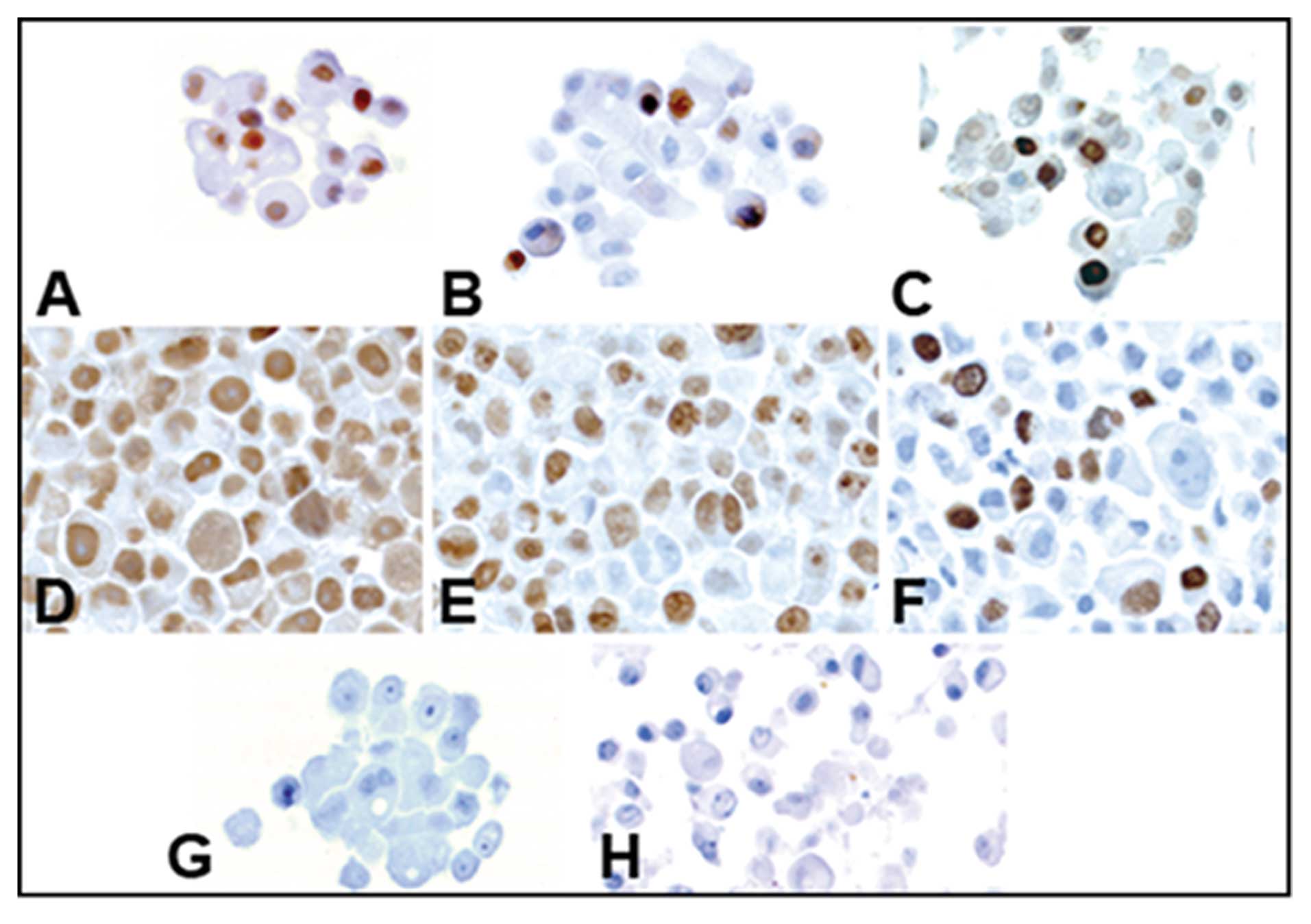
Expression of stemness and
differentiation markers in prostatospheres
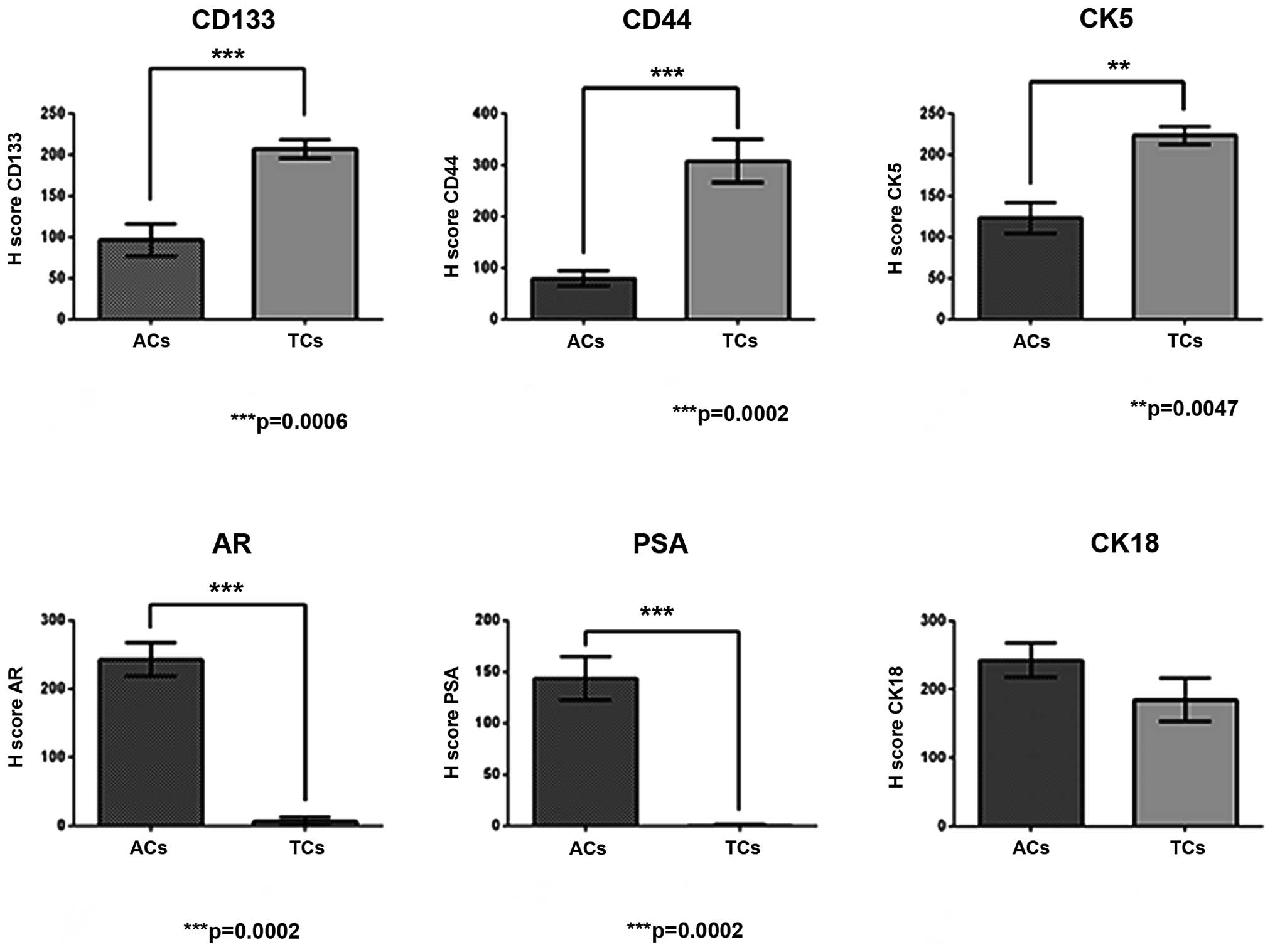
Stemness markers CD133, CD44 and CK5, and
differentiation markers AR, PSA and CK18 were analyzed in
prostatospheres and adherent control cell cultures, by
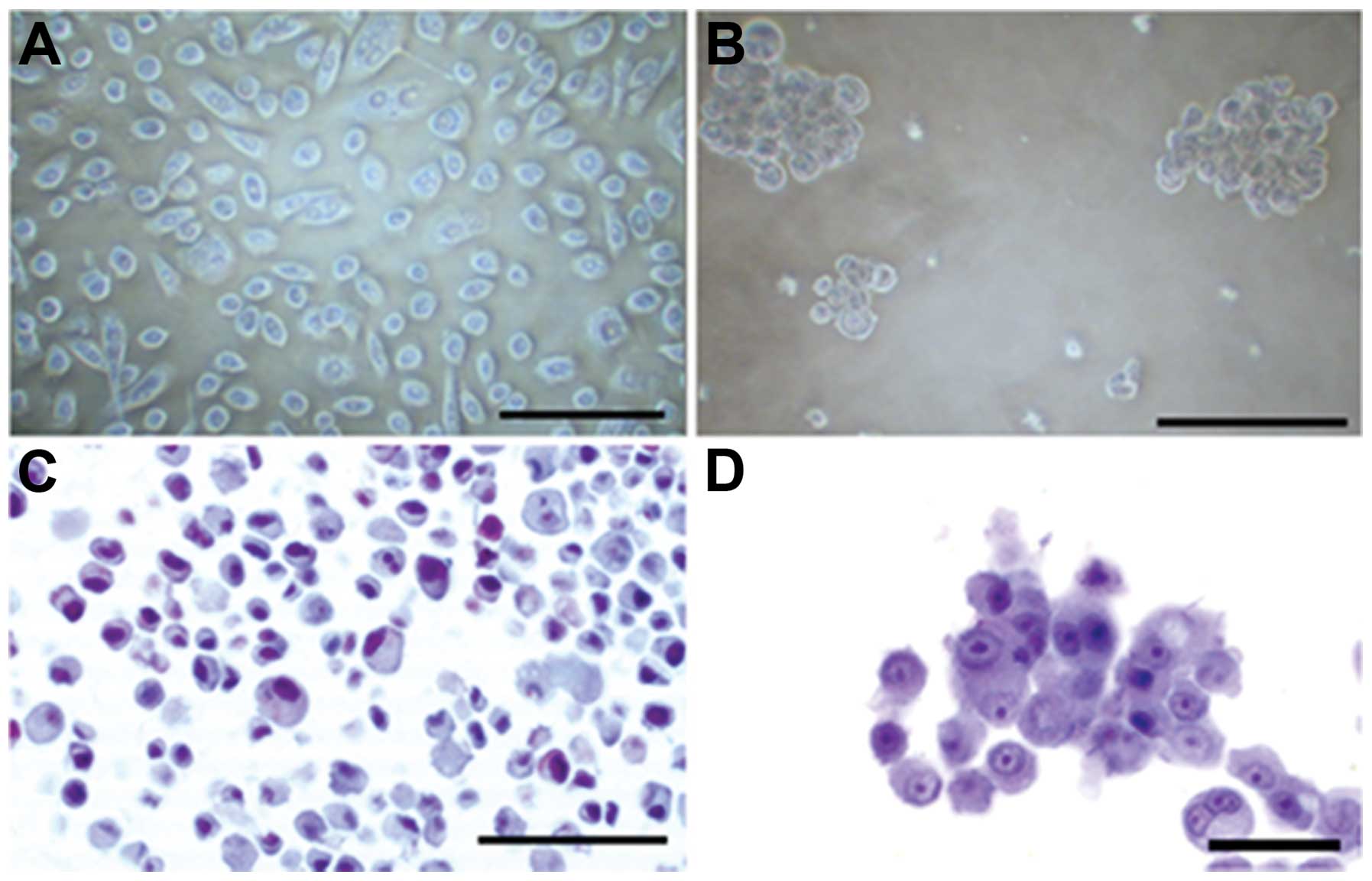
immunocytochemistry. Morphology of both types of cultures is shown
in Fig. 1. Prostatospheres are
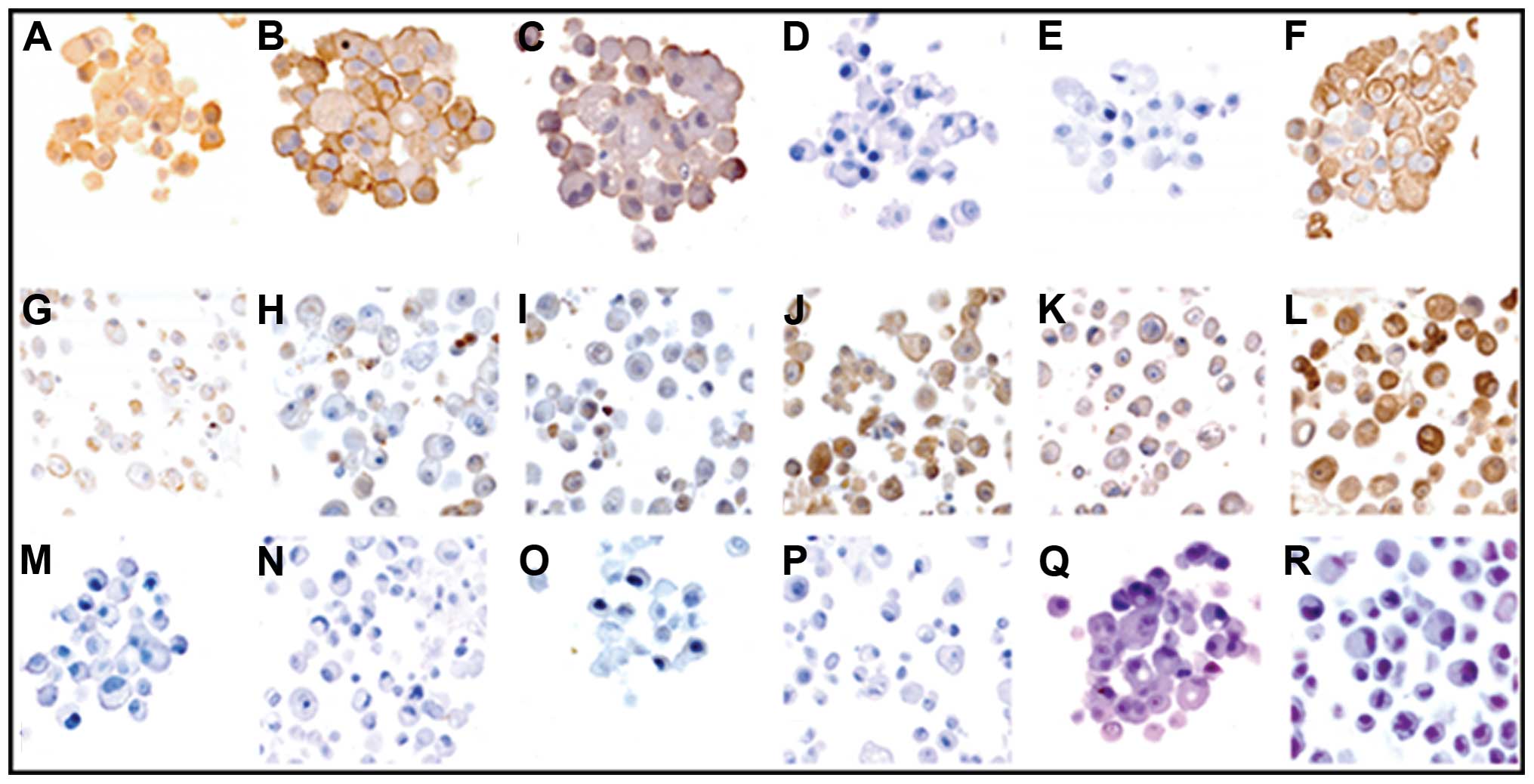
highly positive for CD133, CD44, CK5 and CK18 (Fig. 2A–C and F, respectively). However,
spheres were almost negative for AR and PSA (Fig. 2D and E, respectively). Adherent
control cell cultures exhibited very weak staining for stemness
markers (Fig. 2G–I), and strongly
positive staining for differentiation markers (Fig. 2J–L). Haematoxylin and eosin
controls are also shown (Fig.
2M–R). Immunostaining quantification of each marker for
prostatospheres and control cultures are shown in Fig. 3.
Differential cloning capacity
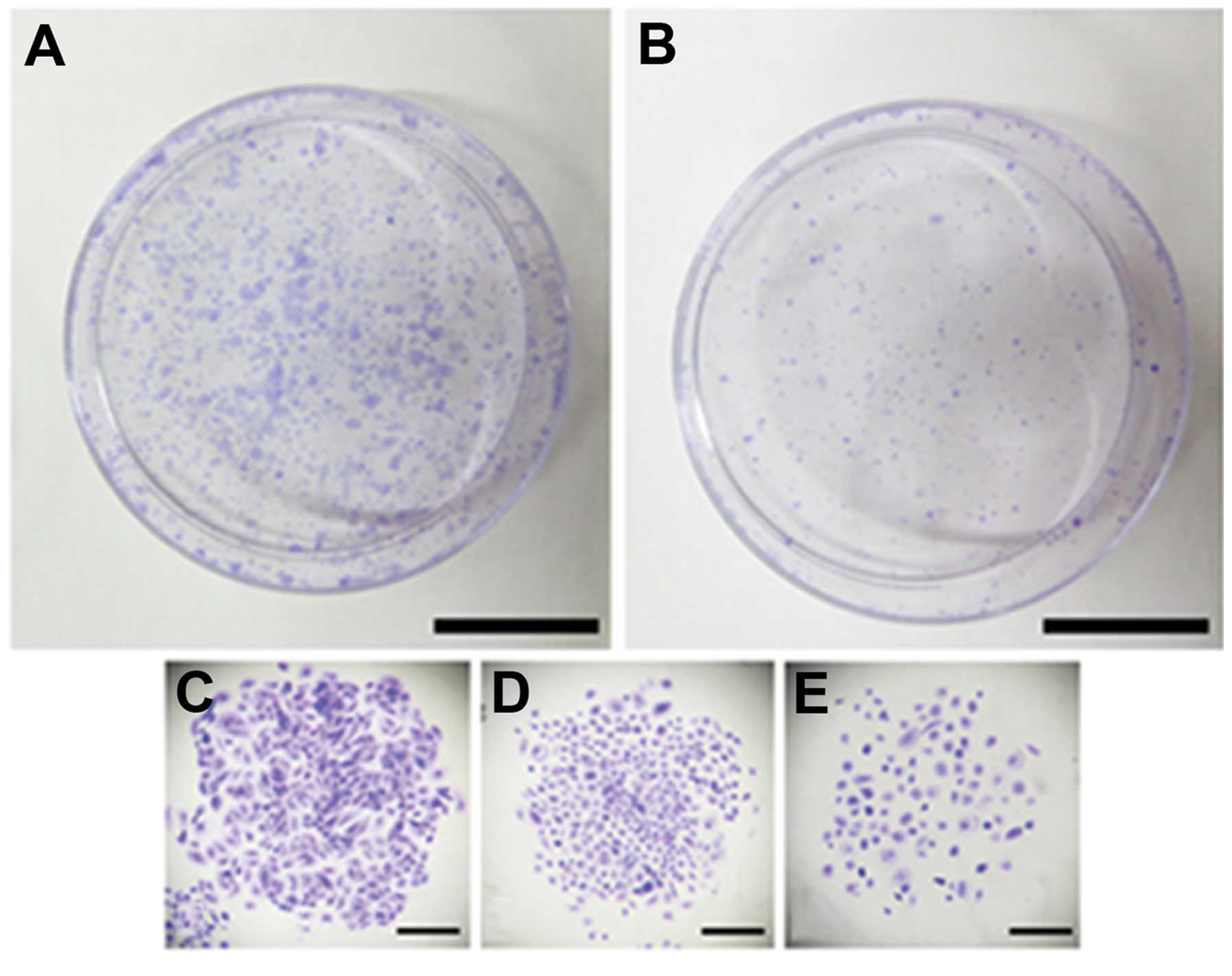
The ability to form holoclones (compact colonies),
meroclones (loose colonies) and paraclones (dispersed colonies) was
evaluated in prostatospheres and adherent cultures (Fig. 4A and B). Only clones formed by at
least 50 cells were considered. The formation of the three types of
clones was observed in both cultures (Fig. 4C–E). PCa tumorspheres formed a
larger percentage of holoclones than control adherent cultures
(Fig. 4F), whereas, the cells from
adherent cultures formed a high percentage of paraclones compared
to cells from spheres cultures (Fig.
4F). Meroclones were observed in both types of cultures without
significant difference (Fig.
4F).
Anchorage-independent growth
capacity
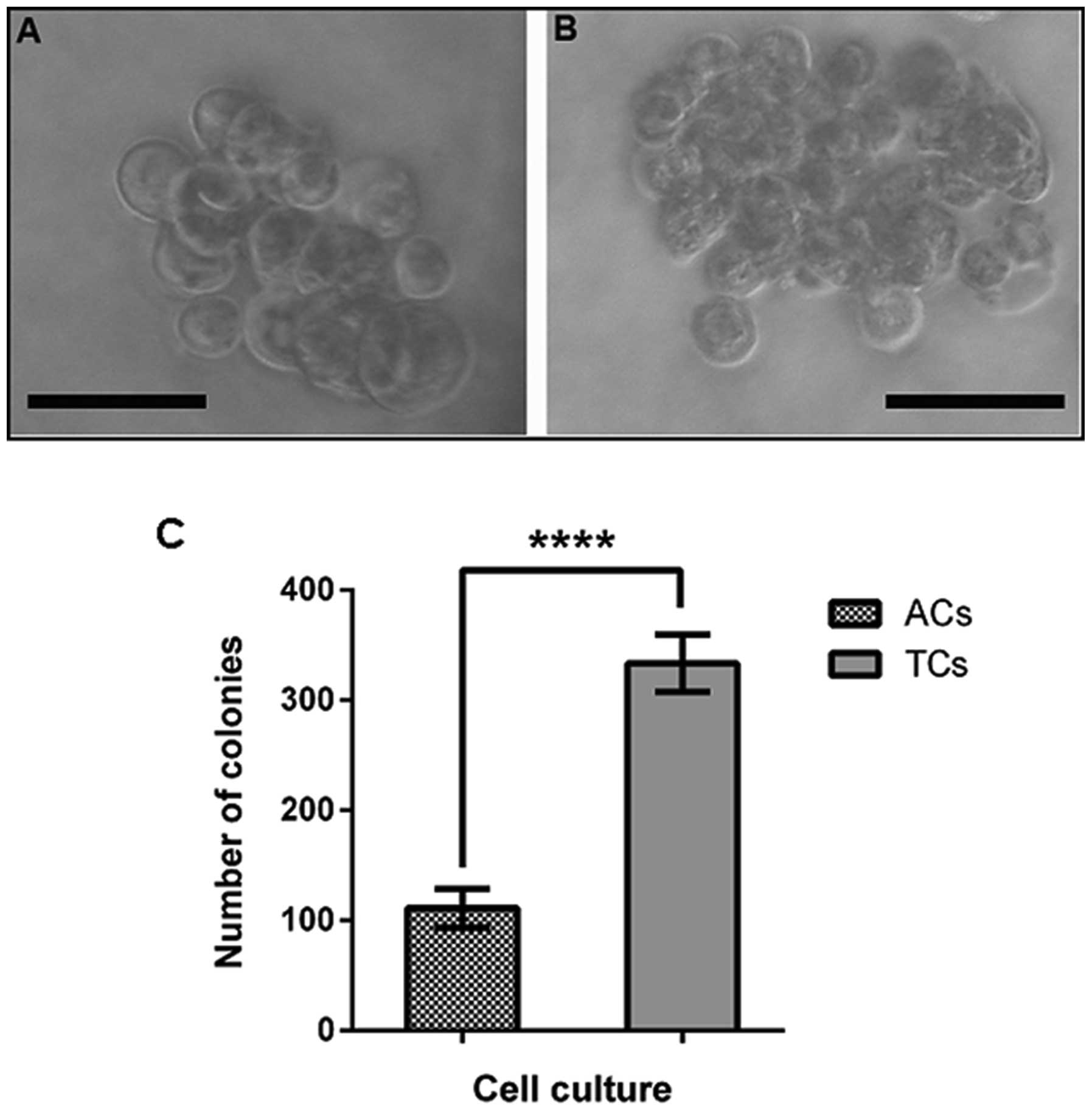
The ability to form colonies in soft agar, in an
anchorage-independent manner, was assessed in prostatospheres and
adherent control cells. Only colonies with diameter >100 μm were
considered. Spheroid-derived colonies reached a diameter larger
than the colonies formed from adherent cultures (Fig. 5A and B). In addition, it was
observed that cells derived from tumorspheres formed a larger
number of colonies than cells from adherent cell cultures (Fig. 5C).
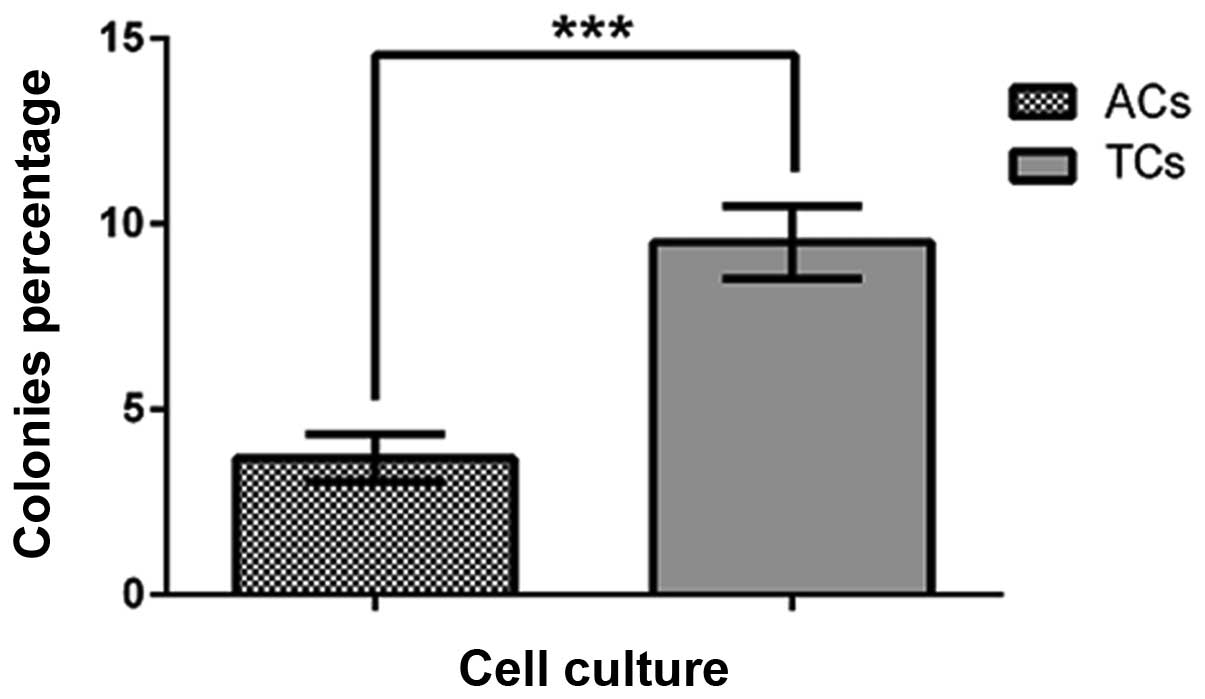
Single colony formation ability
The ability to form colonies from a single cell, an
assay often used to assess self-renewal potential, was evaluated in
prostatospheres and adherent control cultures. Single cells from
PCa tumorspheres showed a significantly higher percentage of
colonies compared with cell from control adherent cultures
(Fig. 6).
Cell proliferation activity
Cell proliferation in prostatospheres and adherent
cell cultures was assessed using immunocytochemistry for Ki67, PCNA
and BrdU (Fig. 7A–F). Haematoxylin
and eosin controls are also shown (Fig. 7G and H). Tumorspheres cultures
showed a smaller number of positive cells than adherent cultures
for the three proliferation markers (Fig. 7I). Furthermore, the Ki67 marker
showed the largest difference between the culture types (Fig. 7I).
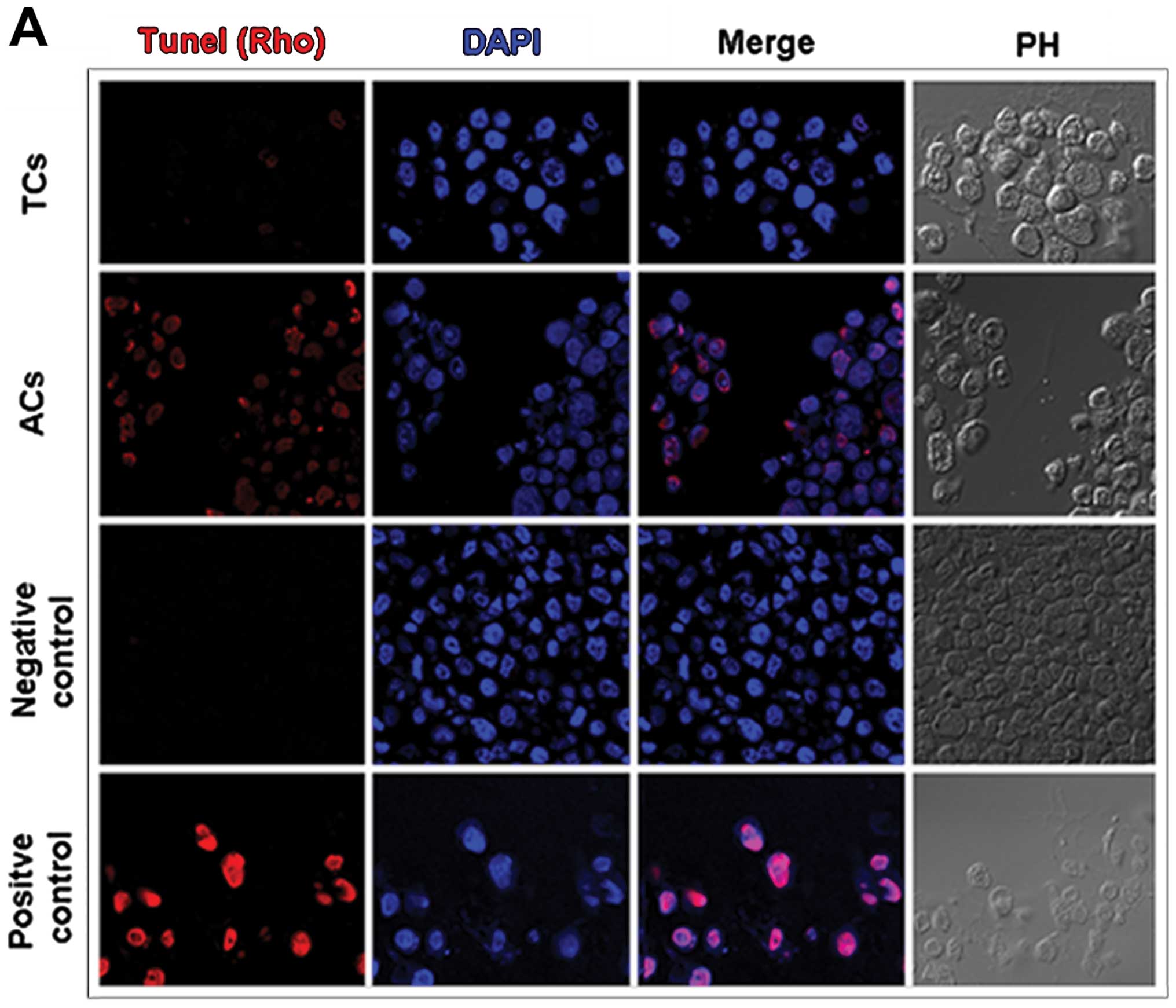
Apoptosis
Programmed cell death was assessed, by the TUNEL
method, both in prostatospheres and adherent control cultures
(Fig. 8A). Adherent cells exposed
to daunorubicin 8 μM were used as positive control. Tumorspheres
showed significantly smaller number of apoptotic cells than
adherent cultures (Fig. 8B).
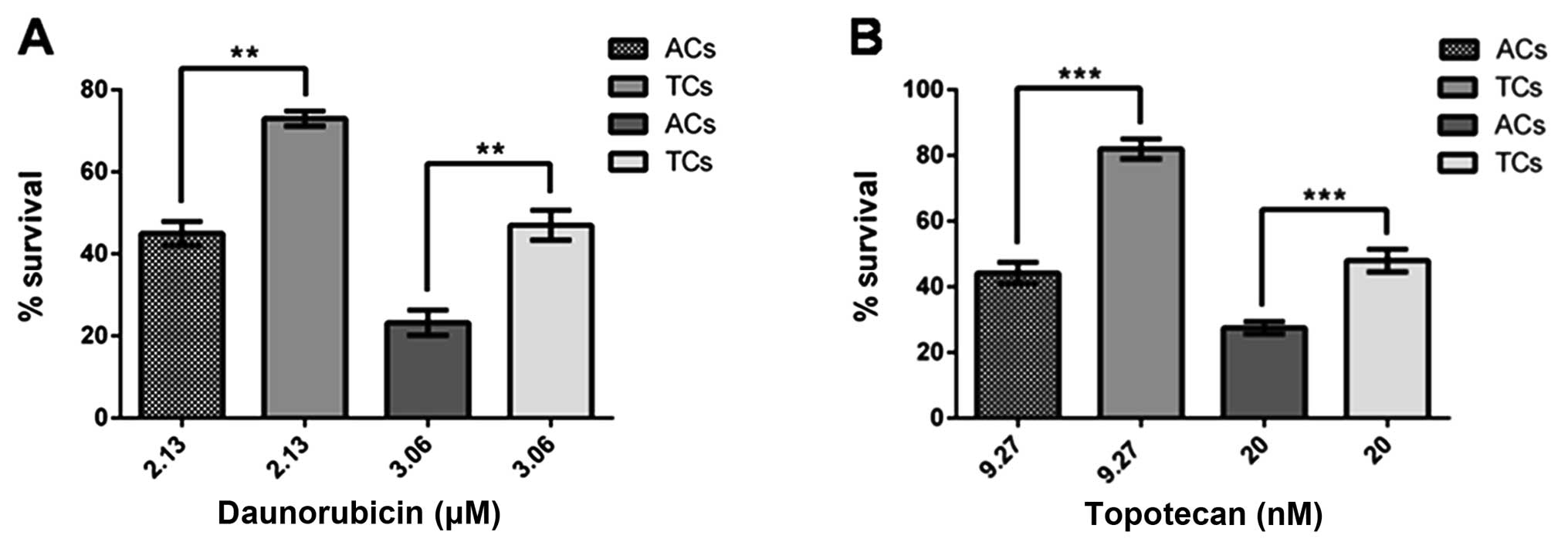
Drug resistance and ABCG2 transporter in
prostatospheres
To assess the cell sensitivity to chemotherapeutic
drugs, both prostatospheres and control cultures were treated with
topotecan or daunorubicin at their corresponding EC50 previously
determined for each drug. Daunorubicin EC50 was 2.13 and 3.06 μM
for adherent cells and prostatospheres, respectively. Topotecan
EC50 was 9.27 and 20 nM for adherent cells and prostatospheres,
respectively. Also, the pharmacological inhibitor of ABCG2
transporter, fumitremorgin C, was used to evaluate the influence of
this transporter in the drug resistance phenotype. Cell survival
was evaluated using MTT assay. Prostatospheres showed higher cell
survival (more resistance) than adherent cells to both drugs used
(Fig. 9). In addition,
fumitremorgin C 5 μM, a concentration that does not affect cell
survival (data not shown), re-sensitized cells, from both types of
cultures, to daunorubicin and topotecan when treated with their
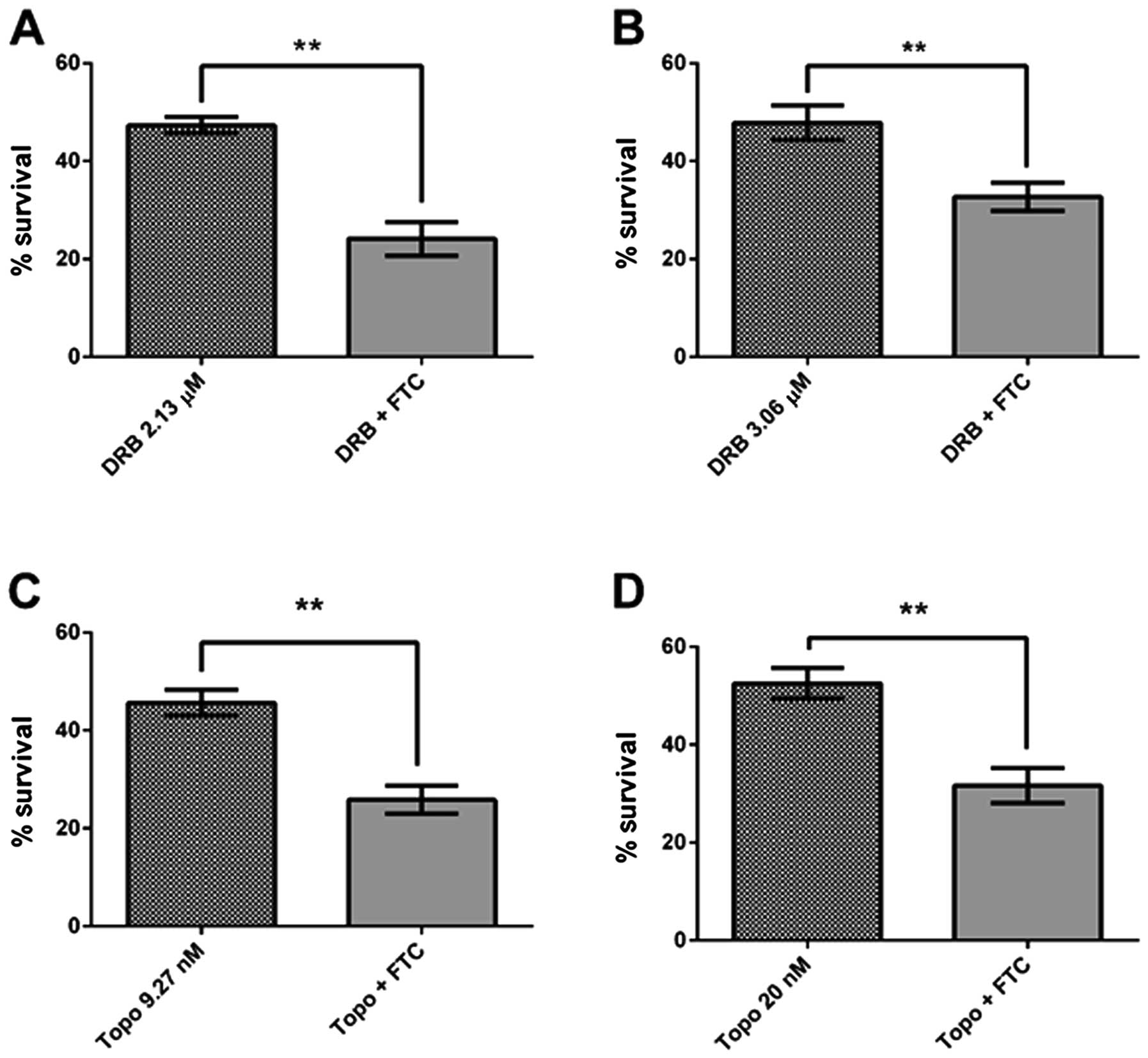
corresponding EC50 (Fig. 10).
 | Figure 9Effect of chemotherapeutic drugs,
substrates for ABCG2 transporter, on survival of tumorspheres and
adherent cells. (A) Daunorubicin at EC50 for tumorspheres (TCs),
3.06 μM and adherent cells (ACs), 2.13 μM, for 48 h; (B) Topotecan
at EC50 for tumorspheres (TCs), 20.0 nM and adherent cells (ACs),
9.27 nM, for 48 h. Cell cultures without treatment were considered
as 100% survival. Data are expressed as means ± SE.
**p<0.05, ***p<0.01, n=6. |
Discussion
CSCs represent a small cell population within
malignant tumors. They have been identified in several cancer
types, including PCa, and are thought to be involved in metastasis,
relapse and therapy resistance (11,30–32).
PCa is a frequent malignancy in men in most countries (9). In advanced castration-resistant
stages, chemotherapy is the only therapeutic option. Unfortunately,
PCa exhibits a high intrinsic drug resistance so the treatment has
little impact on patient survival (18,19).
Most studies on PCa CSCs come from cell lines and animal models
(8,33,34)
limiting the clinical conclusions. Only a few reports obtaining
tumorspheres from PCa explants have been published (13,35).
The main feature of CSCs is their ability to form spheres when
growing in non-adherent conditions. Many tumorspheres have been
obtained and characterized from different cancer samples. Most of
them exhibit high expression of stemness markers (6,33).
Recently, we obtained tumorspheres from PCa explants (13). These prostatospheres show a
molecular signature compatible with CSCs. Also, we have found that
the same stemness markers observed in CSCs from explants are most
expressed in medium Gleason grade biopsies (13). On the other hand, we have
extensively studied the multidrug resistance phenomenon affecting
PCa (23,24). The involvement of ABC transporter
has been clearly demonstrated (23). In our PCa tumorspheres, as in other
models, ABCG2 transporter is highly expressed. Indeed, this
transporter has been used to isolate CSCs from many tumors (side
population). In the present work we confirmed the high expression
of stemness markers CD133 and DC44 (13). Also, CK5 and CK18 expression were
observed in tumorspheres. This is an interesting finding since CK5
is expressed mainly in prostate basal cells (33) and has been extensively associated
with invasiveness and metastasis in breast cancer (36,37).
Furthermore, this cytokeratin has also been found in PCa spheres
(35), suggesting a contribution
of basal cells in CSCs population. The presence of epithelial
marker CK18 support the idea that PCa CSCs would come from a
divergence of the epithelial mesenchymal transition (EMT) process
rather than a malignant transformation of normal prostate stem cell
(38). The absence of AR
expression suggests that PCa CSCs are the most androgen-resistant
cell type within the tumor. Low expression of PSA in
prostatospheres may be consequence of EMT. Our results on
differential clonogenic capacity (mainly holoclones),
anchorage-independent growth and self-renewal properties are
absolute congruent with the main features of stem cells published
in the literature (33,39,40).
Also, low proliferation and apoptotic rate are common
characteristic of CSCs. Ki67 protein showed the largest difference
between prostatospheres and control cultures. This may be due to
the protein being present during all cell cycle phases (41), while PCNA is mainly synthetized in
G1 and S phases (42) and BrdU
evidence only DNA synthesis (42,43).
ABCG2 is one of the most highly expressed ABC transporters in CSCs,
including our prostatosphere preparation. Therefore, we have tested
the sensitivity of PCa tumorspheres to daunorubicin and topotecan,
both ABCG2 substrates and commonly used chemotherapeutic drugs. The
corresponding dose-response curves resulted in a higher EC50 for
prostatospheres than for control adherent cultures with both drugs
(3.06 vs. 2.13 μM for daunorubicin and 20 vs. 9.27 nM for
topotecan), showing that CSCs are significant more resistant than
other cancer cells to ABCG2 transported drugs. Interestingly,
fumitremorgin C, a selective pharmacologic inhibitor of the ABCG2
transporter, re-sensitizes, at least partially, the prostatospheres
and adherent cells to both drugs, when used at their corresponding
EC50. This result suggests strongly that ABCG2 transporter is
involved in drug resistance and may be a suitable therapeutic
target for CSCs-selective therapy in PCa, especially in
castration-resistant advanced stages.
Acknowledgements
This study was supported by FONDECYT Grants No.
1100183 (E.A.C.) and 1110269 (H.R.C.). We acknowledge the
collaboration of the Laboratory of Reproductive Biology and
Laboratory of Advanced Fluorescence Microscopy from University of
Valparaiso and Biotechnology Center ‘Dr Daniel Alkalay Lowitt’ from
University Federico Santa Maria. We also thank Ms. Graciela Caroca
for her excellent technical assistance and Professor Donald Brown,
from University of Valparaiso, for his helpful advice in H-Score
calculations.
References
|
1
|
Nagler C, Zanker KS and Dittmar T: Cell
fusion, drug resistance and recurrence CSCs. Adv Exp Med Biol.
714:173–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnhart BC and Simon MC: Metastasis and
stem cell pathways. Cancer Metastasis Rev. 26:261–271. 2007.
View Article : Google Scholar
|
|
3
|
Kuhn NZ and Tuan RS: Regulation of
stemness and stem cell niche of mesenchymal stem cells:
implications in tumorigenesis and metastasis. J Cell Physiol.
222:268–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayashida T, Jinno H, Kitagawa Y, et al:
Cooperation of cancer stem cell properties and
epithelial-mesenchymal transition in the establishment of breast
cancer metastasis. J Oncol. 2011:5914272011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sampieri K and Fodde R: Cancer stem cells
and metastasis. Semin Cancer Biol. 22:187–193. 2012. View Article : Google Scholar
|
|
6
|
Chen SF, Chang YC, Nieh S, et al:
Nonadhesive culture system as a model of rapid sphere formation
with cancer stem cell properties. PLoS One. 7:e318642012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tu SM and Lin SH: Prostate cancer stem
cells. Clin Genitourin Cancer. 10:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H and Tang DG: Prostate cancer stem
cells and their potential roles in metastasis. J Surg Oncol.
103:558–562. 2011. View Article : Google Scholar
|
|
9
|
Siegel R, Ma J, Zou Z, et al: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
10
|
Tirino V, Desiderio V, Paino F, et al:
Cancer stem cells in solid tumors: an overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugihara E and Saya H: Complexity of
cancer stem cells. Int J Cancer. 132:1249–1259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Filho MS and Nor JE: The biology
of head and neck cancer stem cells. Oral Oncol. 48:1–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castellon EA, Valenzuela R, Lillo J, et
al: Molecular signature of cancer stem cells isolated from prostate
carcinoma and expression of stem markers in different Gleason
grades and metastasis. Biol Res. 45:297–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukaya R, Ohta S, Yamaguchi M, et al:
Isolation of cancer stem-like cells from a side population of a
human glioblastoma cell line, SK-MG-1. Cancer Lett. 291:150–157.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hiraga T, Ito S and Nakamura H: Side
population in MDA-MB-231 human breast cancer cells exhibits cancer
stem cell-like properties without higher bone-metastatic potential.
Oncol Rep. 25:289–296. 2011.
|
|
16
|
Bleau AM, Huse JT and Holland EC: The
ABCG2 resistance network of glioblastoma. Cell Cycle. 8:2936–2944.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Corcoran C, Rani S, O’Brien K, et al:
Docetaxel-resistance in prostate cancer: evaluating associated
phenotypic changes and potential for resistance transfer via
exosomes. PLoS One. 7:e509992012. View Article : Google Scholar
|
|
18
|
Singh S, Chitkara D, Mehrazin R, et al:
Chemoresistance in prostate cancer cells is regulated by miRNAs and
Hedgehog pathway. PLoS One. 7:e400212012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semenas J, Allegrucci C, Boorjian SA, et
al: Overcoming drug resistance and treating advanced prostate
cancer. Curr Drug Targets. 13:1308–1323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooperberg MR, Small EJ, D’Amico A, et al:
The evolving role of androgen deprivation therapy in the management
of prostate cancer. Minerva Urol Nefrol. 55:219–238.
2003.PubMed/NCBI
|
|
21
|
Buyyounouski MK: Androgen deprivation
therapy in high-risk prostate cancer. Oncology (Williston Park).
24:806–809. 2010.PubMed/NCBI
|
|
22
|
Petrylak DP: Current state of
castration-resistant prostate cancer. Am J Manag Care. 19(Suppl
18): S358–S365. 2013.PubMed/NCBI
|
|
23
|
Sanchez C, Mercado A, Contreras HR, et al:
Chemotherapy sensitivity recovery of prostate cancer cells by
functional inhibition and knock down of multidrug resistance
proteins. Prostate. 71:1810–1817. 2011. View Article : Google Scholar
|
|
24
|
Sanchez C, Mendoza P, Contreras HR, et al:
Expression of multidrug resistance proteins in prostate cancer is
related with cell sensitivity to chemotherapeutic drugs. Prostate.
69:1448–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kerfoot C, Huang W and Rotenberg SA:
Immunohistochemical analysis of advanced human breast carcinomas
reveals downregulation of protein kinase C alpha. J Histochem
Cytochem. 52:419–422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Franken NA, Rodermond HM, Stap J, et al:
Clonogenic assay of cells in vitro. Nat Protoc. 1:2315–2319. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Chen X, Calhoun-Davis T, et al: PC3
human prostate carcinoma cell holoclones contain self-renewing
tumor-initiating cells. Cancer Res. 68:1820–1825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Jiao M, Li L, et al: Tumorspheres
derived from prostate cancer cells possess chemoresistant and
cancer stem cell properties. J Cancer Res Clin Oncol. 138:675–686.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bisson I and Prowse DM: WNT signaling
regulates self-renewal and differentiation of prostate cancer cells
with stem cell characteristics. Cell Res. 19:683–697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Freitas DP, Teixeira CA, Santos-Silva F,
et al: Therapy-induced enrichment of putative lung cancer stem-like
cells. Int J Cancer. 134:1270–1278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ratajczak M, Tarnowski M, Staniszewska M,
et al: Mechanisms of cancer metastasis: involvement of cancer stem
cells? Minerva Med. 101:179–191. 2010.PubMed/NCBI
|
|
32
|
Chen X, Rycaj K, Liu X, et al: New
insights into prostate cancer stem cells. Cell Cycle. 12:579–586.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miki J: Investigations of prostate
epithelial stem cells and prostate cancer stem cells. Int J Urol.
17:139–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Huang X, Zheng X, et al:
Enrichment of prostate cancer stem-like cells from human prostate
cancer cell lines by culture in serum-free medium and
chemoradiotherapy. Int J Biol Sci. 9:472–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garraway IP, Sun W, Tran CP, et al: Human
prostate sphere-forming cells represent a subset of basal
epithelial cells capable of glandular regeneration in vivo.
Prostate. 70:491–501. 2010.
|
|
36
|
Sutton LM, Han JS, Molberg KH, et al:
Intratumoral expression level of epidermal growth factor receptor
and cytokeratin 5/6 is significantly associated with nodal and
distant metastases in patients with basal-like triple-negative
breast carcinoma. Am J Clin Pathol. 134:782–787. 2010. View Article : Google Scholar
|
|
37
|
de Silva Rudland S, Platt-Higgins A,
Winstanley JH, et al: Statistical association of basal cell
keratins with metastasis-inducing proteins in a prognostically
unfavorable group of sporadic breast cancers. Am J Pathol.
179:1061–1072. 2011.PubMed/NCBI
|
|
38
|
Celia-Terrassa T, Meca-Cortes O, Mateo F,
et al: Epithelial-mesenchymal transition can suppress major
attributes of human epithelial tumor-initiating cells. J Clin
Invest. 122:1849–1868. 2012. View
Article : Google Scholar
|
|
39
|
Patrawala L, Calhoun-Davis T,
Schneider-Broussard R, et al: Hierarchical organization of prostate
cancer cells in xenograft tumors: the
CD44+alpha2beta1+cell population is enriched
in tumor-initiating cells. Cancer Res. 67:6796–6805. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Patrawala L, Calhoun T,
Schneider-Broussard R, et al: Highly purified
CD44+prostate cancer cells from xenograft human tumors
are enriched in tumorigenic and metastatic progenitor cells.
Oncogene. 25:1696–1708. 2006.
|
|
41
|
Kee N, Sivalingam S, Boonstra R, et al:
The utility of Ki-67 and BrdU as proliferative markers of adult
neurogenesis. J Neurosci Methods. 115:97–105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Coltrera MD and Gown AM: PCNA/cyclin
expression and BrdU uptake define different subpopulations in
different cell lines. J Histochem Cytochem. 39:23–30. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Staszkiewicz J, Gimble J, Cain C, et al:
Flow cytometric and immunohistochemical detection of in vivo
BrdU-labeled cells in mouse fat depots. Biochem Biophys Res Commun.
378:539–544. 2009. View Article : Google Scholar : PubMed/NCBI
|