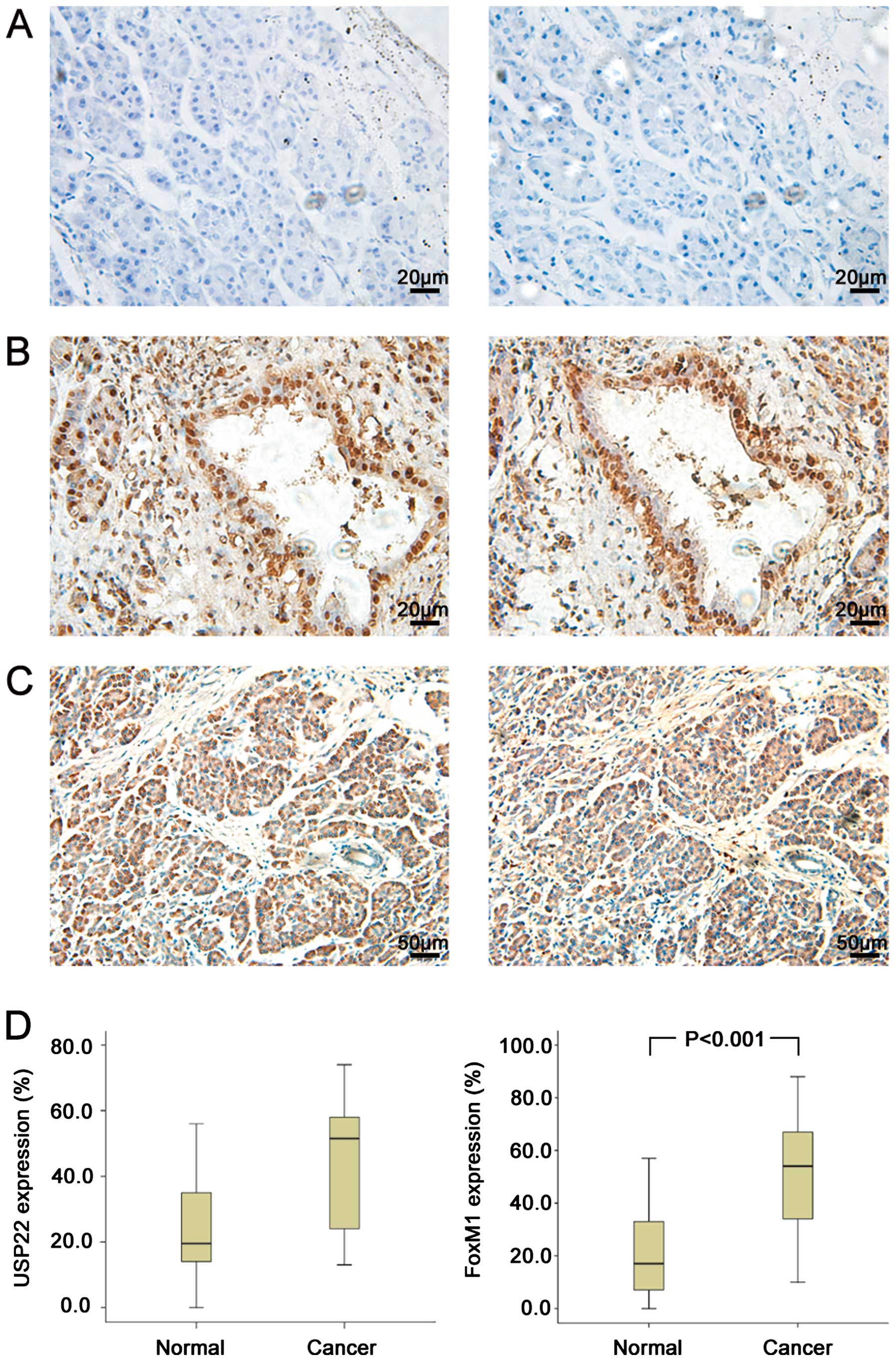
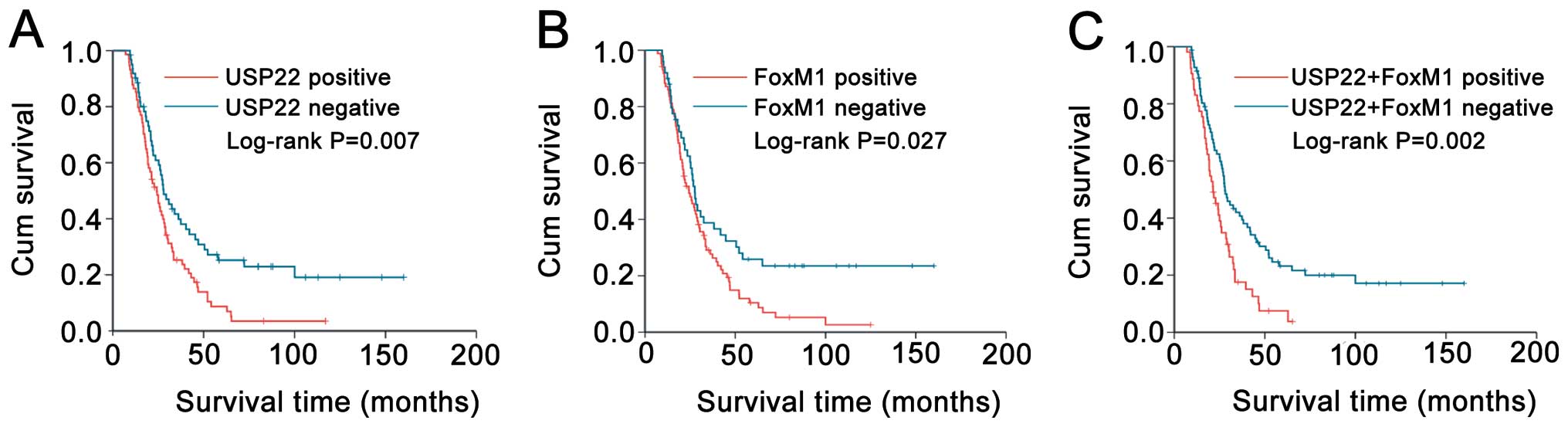
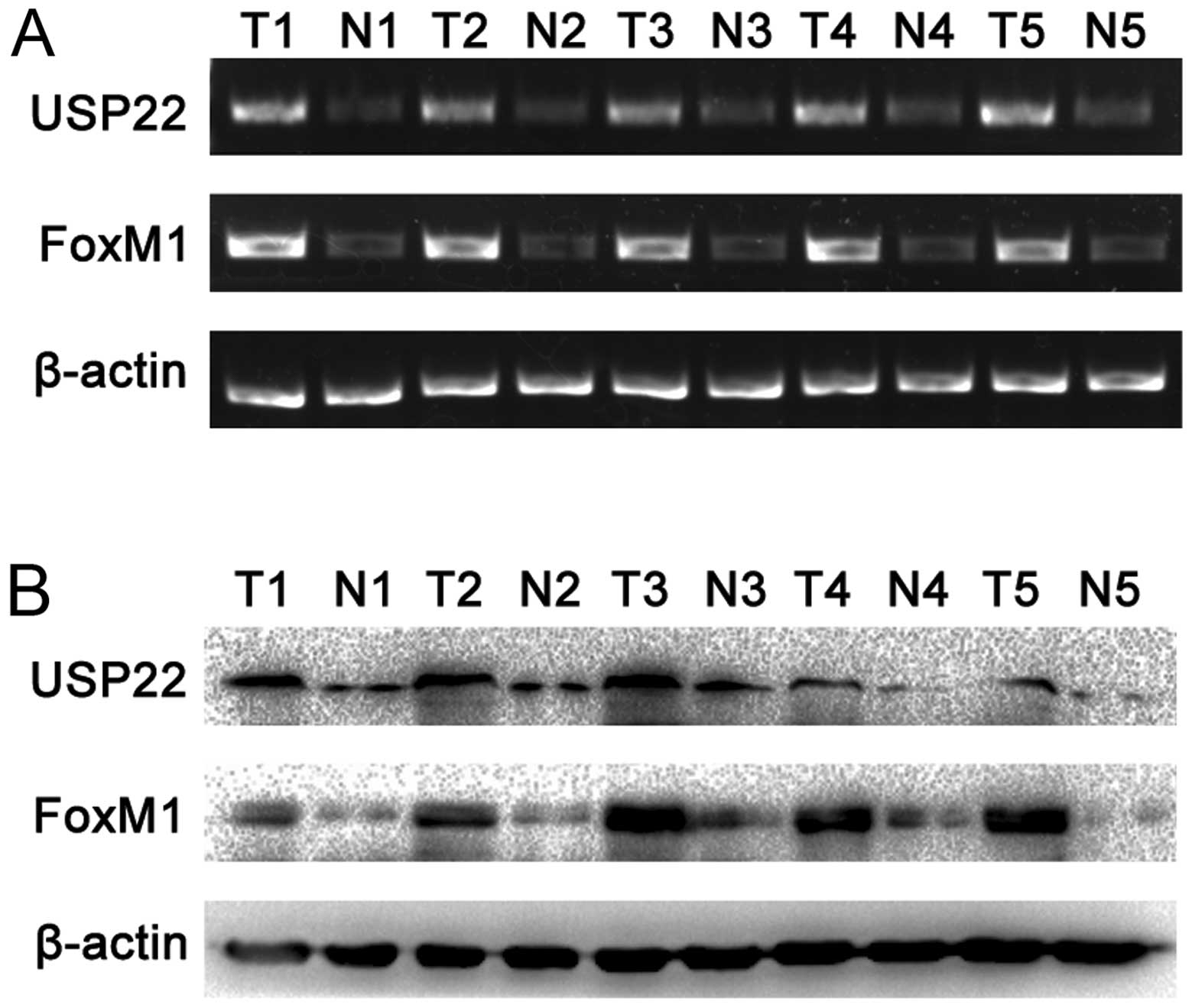
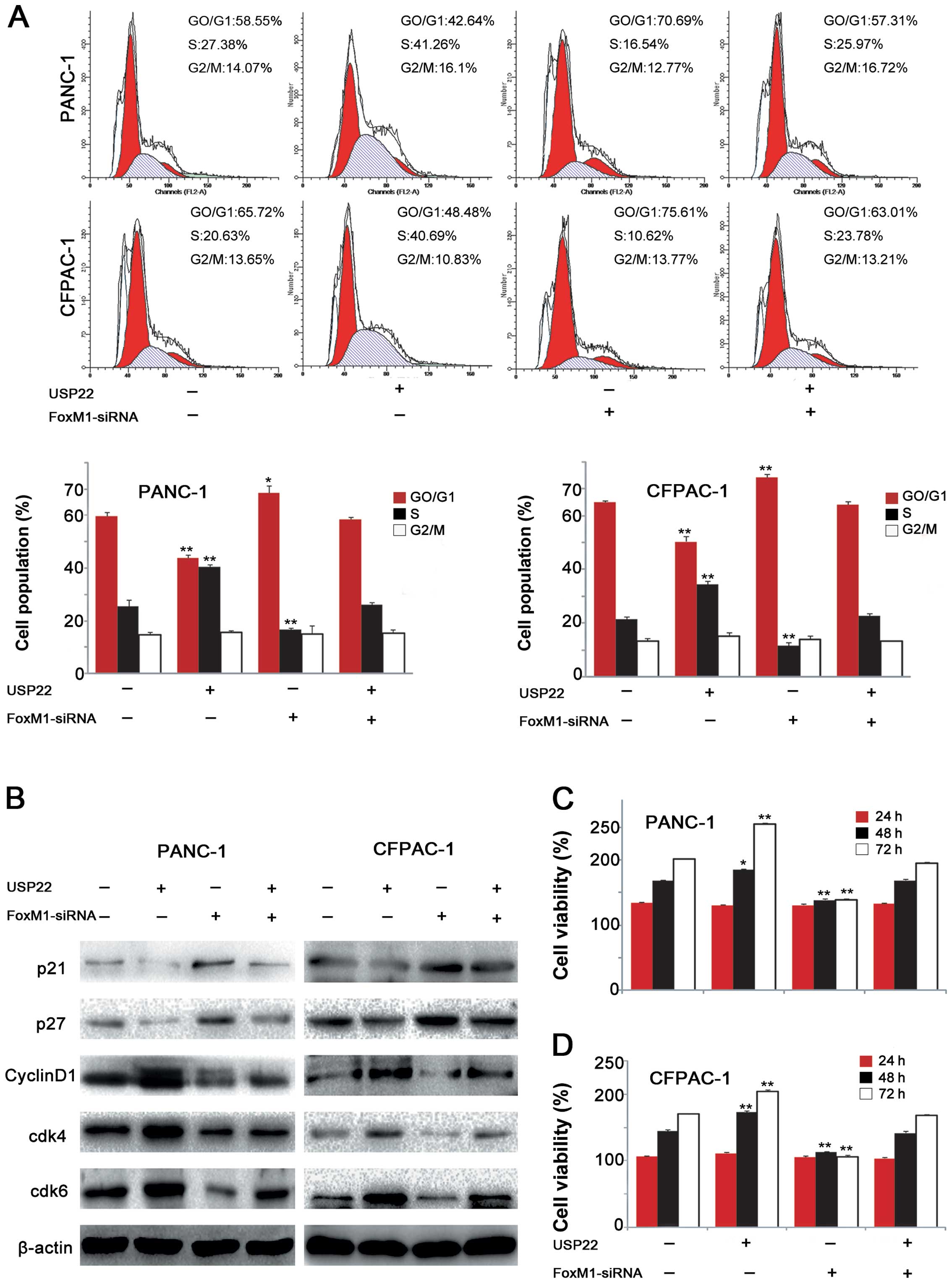
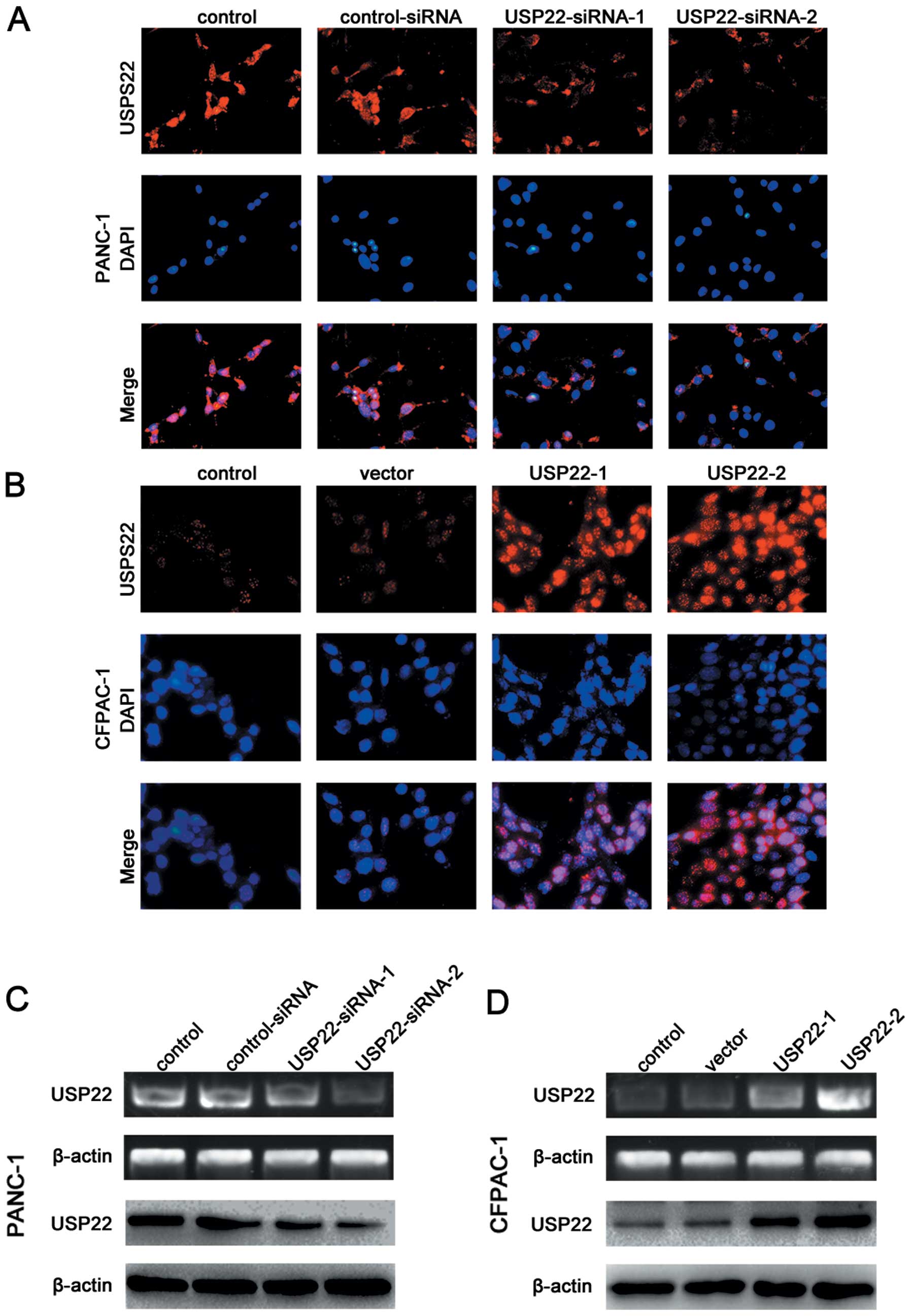
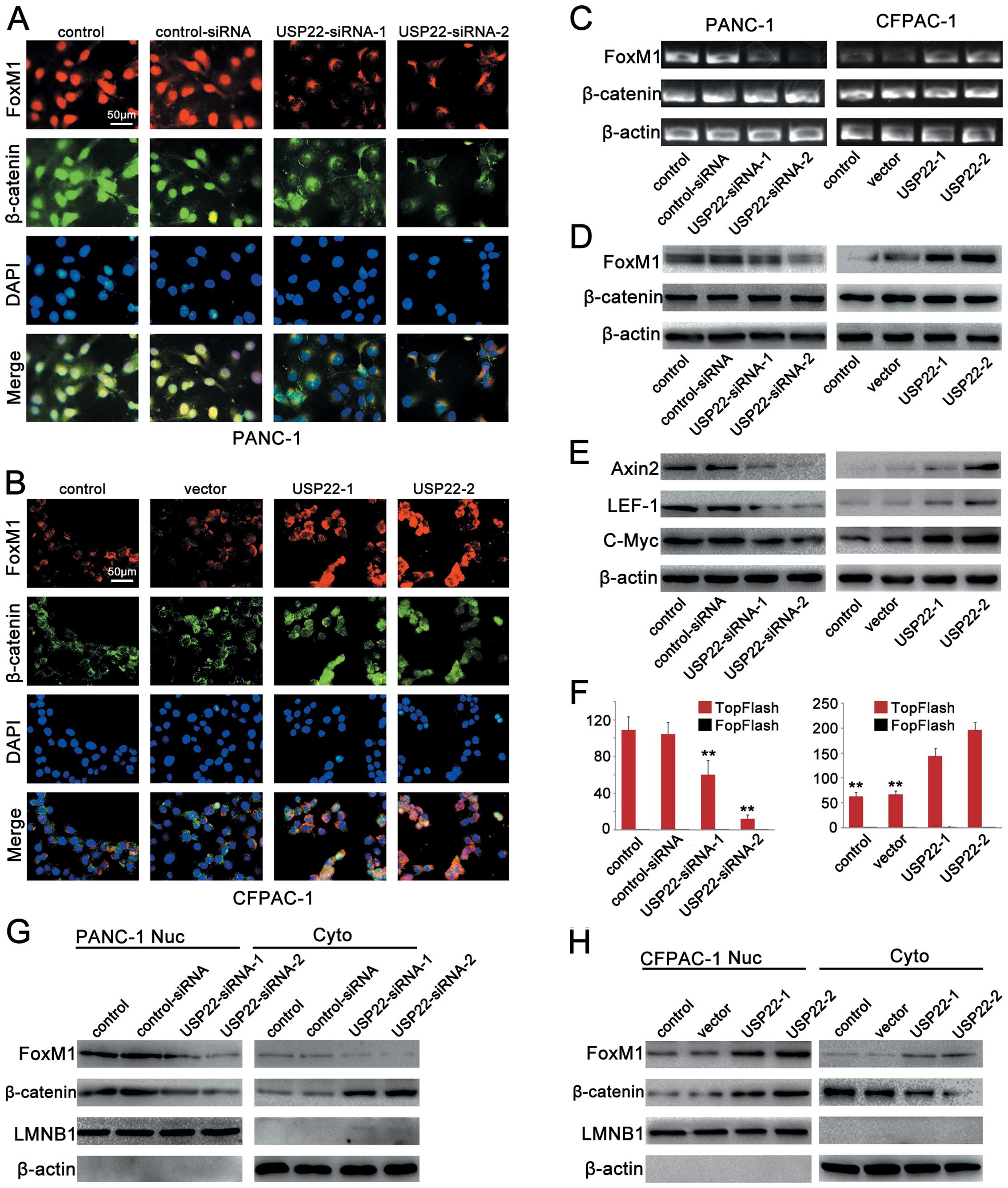
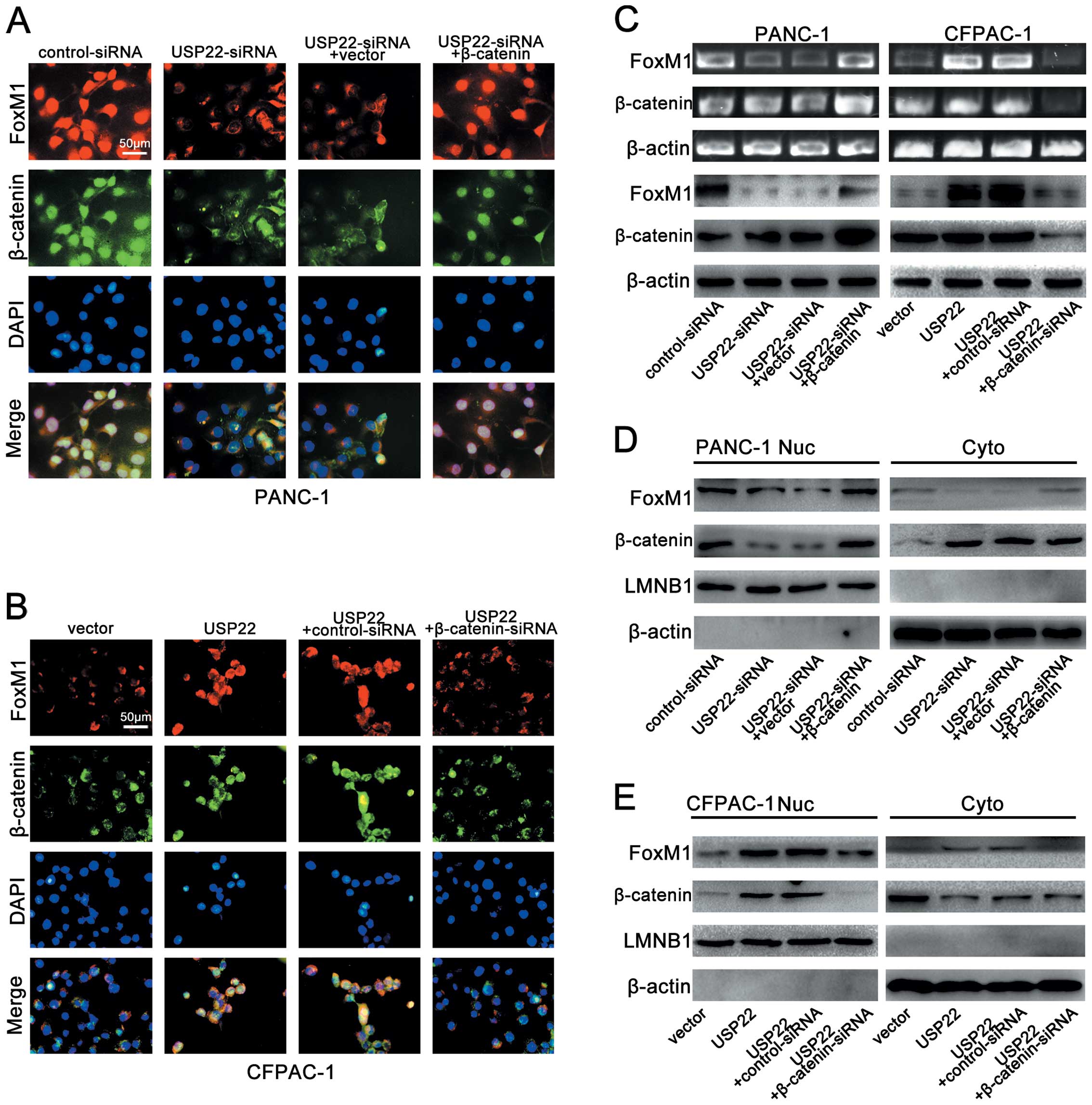
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
3
|
Singh P, Srinivasan R and Wig JD: Major
molecular markers in pancreatic ductal adenocarcinoma and their
roles in screening, diagnosis, prognosis, and treatment. Pancreas.
40:644–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HJ, Kim MS, Shin JM, Park TJ, Chung HM
and Baek KH: The expression patterns of deubiquitinating enzymes,
USP22 and Usp22. Gene Expr Patterns. 6:277–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XY, Varthi M, Sykes SM, et al: The
putative cancer stem cell marker USP22 is a subunit of the human
SAGA complex required for activated transcription and cell-cycle
progression. Mol Cell. 29:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Z, Yang H, Kong Q, et al: USP22
antagonizes p53 transcriptional activation by deubiquitinating
Sirt1 to suppress cell apoptosis and is required for mouse
embryonic development. Mol Cell. 46:484–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Atanassov BS, Evrard YA, Multani AS, et
al: Gcn5 and SAGA regulate shelterin protein turnover and telomere
maintenance. Mol Cell. 35:352–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Liu YL, Yang YM and Dong XS:
Knock-down of ubiquitin-specific protease 22 by micro-RNA
interference inhibits colorectal cancer growth. Int J Colorectal
Dis. 27:21–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv L, Xiao XY, Gu ZH, Zeng FQ, Huang LQ
and Jiang GS: Silencing USP22 by asymmetric structure of
interfering RNA inhibits proliferation and induces cell cycle
arrest in bladder cancer cells. Mol Cell Biochem. 346:11–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling SB, Sun DG, Tang B, et al: Knock-down
of USP22 by small interfering RNA interference inhibits HepG2 cell
proliferation and induces cell cycle arrest. Cell Mol Biol
(Noisy-le-grand). 58:L1803–L1808. 2012.PubMed/NCBI
|
|
11
|
Glinsky GV: Genomic models of metastatic
cancer: functional analysis of death-from-cancer signature genes
reveals aneuploid, anoikis-resistant, metastasis-enabling phenotype
with altered cell cycle control and activated Polycomb Group (PcG)
protein chromatin silencing pathway. Cell Cycle. 5:1208–1216.
2006.
|
|
12
|
Glinsky GV, Berezovska O and Glinskii AB:
Microarray analysis identifies a death-from-cancer signature
predicting therapy failure in patients with multiple types of
cancer. J Clin Invest. 115:1503–1521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jackson BC, Carpenter C, Nebert DW and
Vasiliou V: Update of human and mouse forkhead box (FOX) gene
families. Hum Genomics. 4:345–352. 2010.PubMed/NCBI
|
|
14
|
Laoukili J, Kooistra MR, Bras A, et al:
FoxM1 is required for execution of the mitotic programme and
chromosome stability. Nat Cell Biol. 7:126–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wierstra I and Alves J: FOXM1, a typical
proliferation-associated transcription factor. Biol Chem.
388:1257–1274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luscher-Firzlaff JM, Lilischkis R and
Luscher B: Regulation of the transcription factor FOXM1c by Cyclin
E/CDK2. FEBS Lett. 580:1716–1722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Major ML, Lepe R and Costa RH: Forkhead
box M1B transcriptional activity requires binding of Cdk-cyclin
complexes for phosphorylation-dependent recruitment of p300/CBP
coactivators. Mol Cell Biol. 24:2649–2661. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Hung NJ and Costa RH: Earlier
expression of the transcription factor HFH-11B diminishes induction
of p21(CIP1/WAF1) levels and accelerates mouse hepatocyte entry
into S-phase following carbon tetrachloride liver injury.
Hepatology. 33:1404–1414. 2001. View Article : Google Scholar
|
|
19
|
Wang X, Krupczak-Hollis K, Tan Y,
Dennewitz MB, Adami GR and Costa RH: Increased hepatic Forkhead Box
M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration
through diminished p27Kip1 protein levels and increased Cdc25B
expression. J Biol Chem. 277:44310–44316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laoukili J, Stahl M and Medema RH: FoxM1:
at the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.PubMed/NCBI
|
|
21
|
Wang Z, Ahmad A, Banerjee S, et al: FoxM1
is a novel target of a natural agent in pancreatic cancer. Pharm
Res. 27:1159–1168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Banerjee S, Kong D, Li Y and
Sarkar FH: Down-regulation of Forkhead Box M1 transcription factor
leads to the inhibition of invasion and angiogenesis of pancreatic
cancer cells. Cancer Res. 67:8293–8300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao B, Wang Z, Ali S, et al:
Over-expression of FoxM1 leads to epithelial-mesenchymal transition
and cancer stem cell phenotype in pancreatic cancer cells. J Cell
Biochem. 112:2296–2306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Qiu W, Liu B, et al: Forkhead box
transcription factor 1 expression in gastric cancer: FOXM1 is a
poor prognostic factor and mediates resistance to docetaxel. J
Transl Med. 11:2042013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Wen L, Zhao SH, Ai ZH, Guo JZ and
Liu WC: FoxM1 expression is significantly associated with
cisplatin-based chemotherapy resistance and poor prognosis in
advanced non-small cell lung cancer patients. Lung Cancer.
79:173–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia JT, Wang H, Liang LJ, et al:
Overexpression of FOXM1 is associated with poor prognosis and
clinicopathologic stage of pancreatic ductal adenocarcinoma.
Pancreas. 41:629–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang H and He X: Wnt/beta-catenin
signaling: new (and old) players and new insights. Curr Opin Cell
Biol. 20:119–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karayiannakis AJ, Syrigos KN,
Polychronidis A and Simopoulos C: Expression patterns of alpha-,
beta- and gamma-catenin in pancreatic cancer: correlation with
E-cadherin expression, pathological features and prognosis.
Anticancer Res. 21:4127–4134. 2001.PubMed/NCBI
|
|
29
|
Lowy AM, Fenoglio-Preiser C, Kim OJ, et
al: Dysregulation of beta-catenin expression correlates with tumor
differentiation in pancreatic duct adenocarcinoma. Ann Surg Oncol.
10:284–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pasca DMM, Biankin AV, Heiser PW, et al:
Common activation of canonical Wnt signaling in pancreatic
adenocarcinoma. PLoS One. 2:e11552007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang N, Wei P, Gong A, et al: FoxM1
promotes beta-catenin nuclear localization and controls Wnt
target-gene expression and glioma tumorigenesis. Cancer Cell.
20:427–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deer EL, Gonzalez-Hernandez J, Coursen JD,
et al: Phenotype and genotype of pancreatic cancer cell lines.
Pancreas. 39:425–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang DD, Cui BB, Sun LY, et al: The
co-expression of USP22 and BMI-1 may promote cancer progression and
predict therapy failure in gastric carcinoma. Cell Biochem Biophys.
61:703–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Li YP, Chen JH, et al: Prognostic
significance of USP22 as an oncogene in papillary thyroid
carcinoma. Tumour Biol. 34:1635–1639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Yao L, Zhang X, et al: Elevated
expression of USP22 in correlation with poor prognosis in patients
with invasive breast cancer. J Cancer Res Clin Oncol.
137:1245–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu J, Liu YL, Piao SL, Yang DD, Yang YM
and Cai L: Expression patterns of USP22 and potential targets
BMI-1, PTEN, p-AKT in non-small-cell lung cancer. Lung Cancer.
77:593–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Wang Z and Li Y: USP22 nuclear
expression is significantly associated with progression and
unfavorable clinical outcome in human esophageal squamous cell
carcinoma. J Cancer Res Clin Oncol. 138:1291–1297. 2012. View Article : Google Scholar
|
|
38
|
Liu Y, Yang Y, Xu H and Dong X:
Implication of USP22 in the regulation of BMI-1, c-Myc, p16INK4a,
p14ARF, and cyclin D2 expression in primary colorectal carcinomas.
Diagn Mol Pathol. 19:194–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagy Z, Riss A, Romier C, et al: The human
SPT20-containing SAGA complex plays a direct role in the regulation
of endoplasmic reticulum stress-induced genes. Mol Cell Biol.
29:1649–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nagy Z and Tora L: Distinct
GCN5/PCAF-containing complexes function as co-activators and are
involved in transcription factor and global histone acetylation.
Oncogene. 26:5341–5357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang XY, Pfeiffer HK, Thorne AW and
McMahon SB: USP22, an hSAGA subunit and potential cancer stem cell
marker, reverses the polycomb-catalyzed ubiquitylation of histone
H2A. Cell Cycle. 7:1522–1524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Atanassov BS and Dent SY: USP22 regulates
cell proliferation by deubiquitinating the transcriptional
regulator FBP1. EMBO Rep. 12:924–930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu Z, Malureanu L, Huang J, et al:
Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional
programme required for mitotic progression. Nat Cell Biol.
10:1076–1082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Anders L, Ke N, Hydbring P, et al: A
systematic screen for CDK4/6 substrates links FOXM1 phosphorylation
to senescence suppression in cancer cells. Cancer Cell. 20:620–634.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X, Bhattacharyya D, Dennewitz MB, et
al: Rapid hepatocyte nuclear translocation of the Forkhead Box M1B
(FoxM1B) transcription factor caused a transient increase in size
of regenerating transgenic hepatocytes. Gene Expr. 11:149–162.
2003. View Article : Google Scholar
|
|
46
|
Wang IC, Chen YJ, Hughes D, et al:
Forkhead box M1 regulates the transcriptional network of genes
essential for mitotic progression and genes encoding the SCF
(Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 25:10875–10894. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Allende-Vega N and Saville MK: Targeting
the ubiquitin-proteasome system to activate wild-type p53 for
cancer therapy. Semin Cancer Biol. 20:29–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clurman BE, Sheaff RJ, Thress K, Groudine
M and Roberts JM: Turnover of cyclin E by the ubiquitin-proteasome
pathway is regulated by cdk2 binding and cyclin phosphorylation.
Genes Dev. 10:1979–1990. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bae Y, Choi D, Rhim H and Kang S: Hip2
interacts with cyclin B1 and promotes its degradation through the
ubiquitin proteasome pathway. FEBS Lett. 584:4505–4510. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pagano M, Tam SW, Theodoras AM, et al:
Role of the ubiquitin-proteasome pathway in regulating abundance of
the cyclin-dependent kinase inhibitor p27. Science. 269:682–685.
1995. View Article : Google Scholar
|
|
51
|
Ding GX, Liu J, Feng CC, Jiang HW, Xu JF
and Ding Q: Slug regulates Cyclin D1 expression by
ubiquitin-proteasome pathway in prostate cancer cells. Panminerva
Med. 54:219–223. 2012.PubMed/NCBI
|
|
52
|
Mandal S, Freije WA, Guptan P and Banerjee
U: Metabolic control of G1-S transition: cyclin E degradation by
p53-induced activation of the ubiquitin-proteasome system. J Cell
Biol. 188:473–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang IC, Chen YJ, Hughes DE, et al: FoxM1
regulates transcription of JNK1 to promote the G1/S transition and
tumor cell invasiveness. J Biol Chem. 283:20770–20778. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nakamura S, Hirano I, Okinaka K, et al:
The FOXM1 transcriptional factor promotes the proliferation of
leukemia cells through modulation of cell cycle progression in
acute myeloid leukemia. Carcinogenesis. 31:2012–2021. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Priller M, Poschl J, Abrao L, et al:
Expression of FoxM1 is required for the proliferation of
medulloblastoma cells and indicates worse survival of patients.
Clin Cancer Res. 17:6791–6801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
He SY, Shen HW, Xu L, et al: FOXM1
promotes tumor cell invasion and correlates with poor prognosis in
early-stage cervical cancer. Gynecol Oncol. 127:601–610. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wierstra I and Alves J: FOXM1c
transactivates the human c-myc promoter directly via the two TATA
boxes P1 and P2. FEBS J. 273:4645–4667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
De Boer VC, de Goffau MC, Arts IC, Hollman
PC and Keijer J: SIRT1 stimulation by polyphenols is affected by
their stability and metabolism. Mech Ageing Dev. 127:618–627.
2006.PubMed/NCBI
|
|
59
|
Zhu GY, Shi BZ and Li Y: FoxM1 regulates
Sirt1 expression in glioma cells. Eur Rev Med Pharmacol Sci.
18:205–211. 2014.PubMed/NCBI
|
|
60
|
Jiang L, Li J and Song L: Bmi-1, stem
cells and cancer. Acta Biochim Biophys Sin (Shanghai). 41:527–534.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu YL, Jiang SX, Yang YM, Xu H, Liu JL
and Wang XS: USP22 acts as an oncogene by the activation of
BMI-1-mediated INK4a/ARF pathway and Akt pathway. Cell Biochem
Biophys. 62:229–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li SK, Smith DK, Leung WY, et al: FoxM1c
counteracts oxidative stress-induced senescence and stimulates
Bmi-1 expression. J Biol Chem. 283:16545–16553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Heiser PW, Lau J, Taketo MM, Herrera PL
and Hebrok M: Stabilization of beta-catenin impacts pancreas
growth. Development. 133:2023–2032. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rulifson IC, Karnik SK, Heiser PW, et al:
Wnt signaling regulates pancreatic beta cell proliferation. Proc
Natl Acad Sci USA. 104:6247–6252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Heiser PW, Cano DA, Landsman L, et al:
Stabilization of beta-catenin induces pancreas tumor formation.
Gastroenterology. 135:1288–1300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang Y, Morris JT, Yan W, et al:
Canonical wnt signaling is required for pancreatic carcinogenesis.
Cancer Res. 73:4909–4922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ripka S, Konig A, Buchholz M, et al: WNT5A
- target of CUTL1 and potent modulator of tumor cell migration and
invasion in pancreatic cancer. Carcinogenesis. 28:1178–1187. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Griesmann H, Ripka S, Pralle M, et al:
WNT5A-NFAT signaling mediates resistance to apoptosis in pancreatic
cancer. Neoplasia. 15:11–22. 2013.PubMed/NCBI
|
|
70
|
Bowman A and Nusse R: Location, location,
location: FoxM1 mediates beta-catenin nuclear translocation and
promotes glioma tumorigenesis. Cancer Cell. 20:415–416. 2011.
View Article : Google Scholar : PubMed/NCBI
|