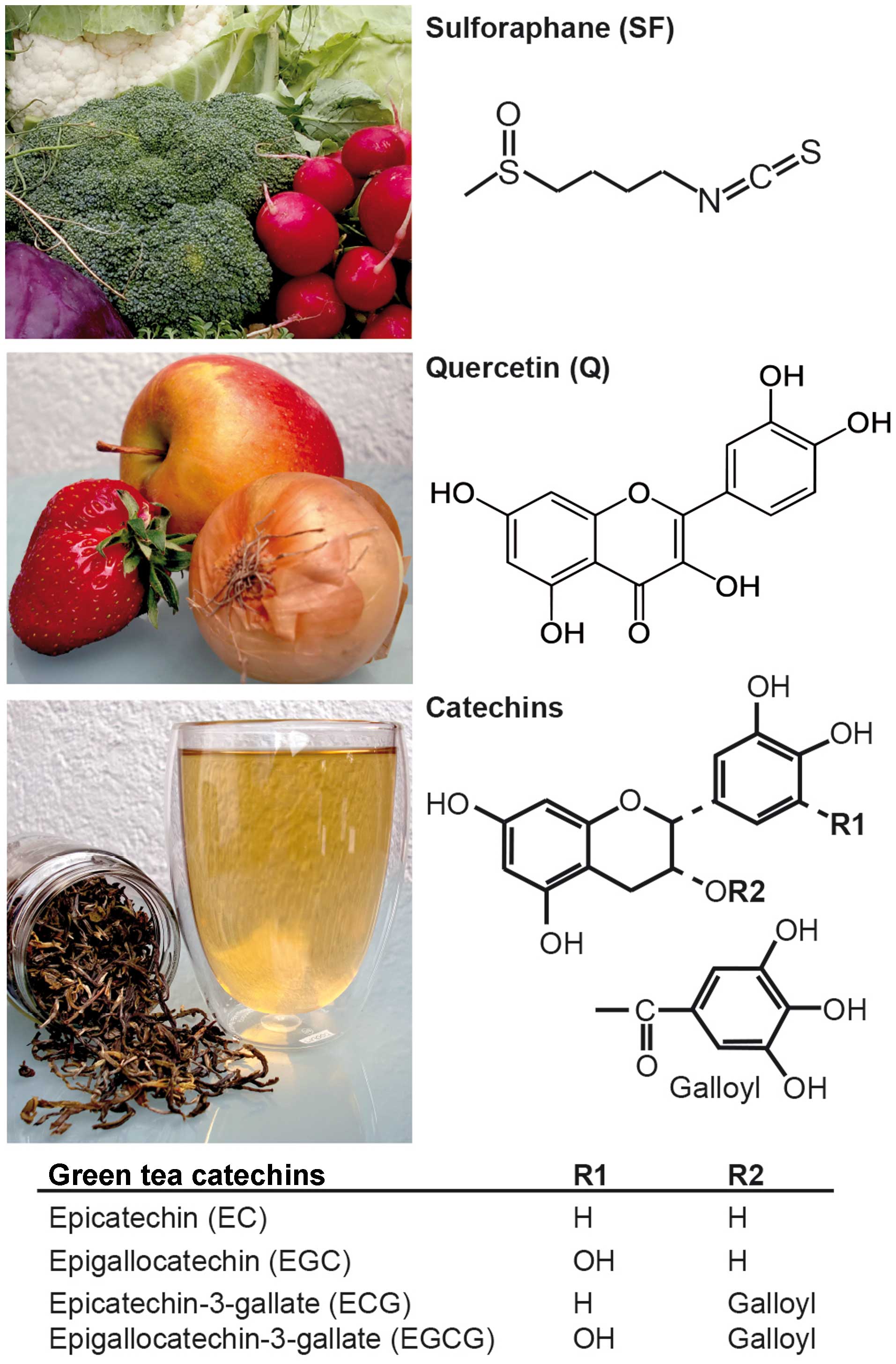
Introduction
Pancreatic ductal adenocarcinoma (PDA) is one of the
most aggressive malignancies usually diagnosed in an advanced
state, with an extensive local invasion, early systemic
dissemination and marked resistance to chemo- and radiotherapy
(1). A frequently dysregulated
gene is K-ras, which is overexpressed in more than 90% of patients
suffering from PDA (2). The
current treatment options for PDA provide a 5-year survival rate of
only 5%. Cancer stem cells (CSCs) are made responsible for the
pronounced therapy resistance and early progression of PDA, because
this small subpopulation within the tumor mass is thought to
survive conventional cytotoxic therapy due to defense and survival
mechanisms (3,4). CSCs are believed to possess
self-renewal potential and the ability to divide asymmetrically,
whereby a mother cell generates a stem cell and a progenitor cell,
which undergoes clonal expansion and then terminally differentiates
(5–7). Because current therapeutics do not
target CSCs, new therapeutic options are urgently needed.
Several epidemiological cancer and nutrition studies
provide new therapeutic perspectives, because they show that the
risk to develop cancer and the risk of cancer progression
correlates with a defined dietary pattern. One well-designed large
population-based case-control study found that frequent consumption
of cruciferous vegetables, three and more servings/week of e.g.,
broccoli, cauliflower, cabbage, radish, horseradish, cress,
arugula, mustard, canola and others, had an about 50% risk
reduction to develop pancreatic cancer (8). Another prospective epidemiological
study found that the high consumption of cruciferous vegetables and
especially of broccoli and cauliflower was associated with a 50 %
reduction of the relative risk of developing metastasis in patients
with prostate cancer (9).
Similarly, a population-based case-control study conducted in urban
Shanghai, China, found that regular green tea drinking was
associated with a 32% reduction of pancreatic cancer risk in women
(10). Well examined bioactive
substances from broccoli, cauliflower and green tea involve the
isothiocyanate sulforaphane (11),
the polyphenol family of catechins (12) and the polyphenol quercetin, which
is one of the most abundant flavonoids found in many fruits and
vegetables, such as broccoli, apples, onions and berries (13).
Our recent in vitro data and mouse
experiments suggest that sulforaphane eliminates pancreatic CSCs by
inhibition of NF-κB activity and self-renewal potential and thereby
sensitizes the cells to apoptosis induction (14–16).
After publication of these results many patients contacted us, and
we recommend a balanced plant-based diet enriched in cruciferous
vegetables. We do not recommend a focus to cruciferous vegetables
or broccoli sprout supplements, because in the meanwhile also other
plant ingredients with anti-CSC activity have been identified
(17). In particular, quercetin
and epigallocatechin-3-gallate (EGCG) have been described to target
pancreatic CSCs (18,19). Other dietary compounds with
experimental proven direct or indirect effects on Wnt/β-catenin,
hedgehog and Notch self-renewal pathways include the turmeric spice
component curcumin, soy isoflavone, resveratrol, lycopene, piperine
and vitamin D3 (17). There may
still be several other plant substances with similar activity.
Therefore, the high intake of a balanced mixture of a plant-based
diet with proven activity toward CSCs may be superior to the intake
of supplements with isolated substances.
Recently, the indole 3,3′-diindolylmethane
(cruciferous vegetables), the polyphenol genistein (soy) and an
analogue of the natural phenol curcumin (turmeric) have been
demonstrated to inhibit the growth of pancreatic and prostate
cancer by upregulation of the micro RNA (miR)-let-7 (20–22).
The miR-let-7 (from ‘lethal’) is one of the first identified
miRNAs, due to its role in terminally differentiation of C.
elegans seam cells (23). A
function of miR-let-7 in human cancer was detected, and expression
levels of let-7 members are significantly low in human cancers and
CSCs. The major function of let-7 is to promote the terminal
differentiation in development and tumor suppression (24). Let-7 has been demonstrated to be a
direct regulator of K-ras expression in human cells (25). In lung cancer patient samples,
expression of K-ras and let-7 showed reciprocal patterns of low
let-7 and high K-ras in cancerous cells, but high let-7 and low
K-ras in normal cells (25). In
many cancer types, downregulation of miR-let-7 is associated with a
poor outcome for patients (26).
In the present study we asked if green tea-derived
catechins in general may harbor anti-CSC activity, if the
combination with quercetin and sulforaphane may be superior and if
the upregulation of miR-let-7 and the downregulation of K-ras are
involved. We demonstrate that epicatechin-3-gallate (ECG) and
catechin gallate (CG) are as potent as EGCG in inhibition of
colony-formation. Furthermore, sulforaphane, quercetin and a
natural mixture of the complete set of green tea catechins (GTC)
complemented each other in induction of apoptosis and inhibition of
self-renewal potential, migration and expression of the matrix
metalloproteinases MMP-9 and MMP-2. Most importantly, these
bioactive agents led to induction of miR-let-7 and inhibition of
its target gene K-ras with strongest effects after combination.
Materials and methods
Established cell lines and primary
cells
The human established PDA cell lines BxPc-3 and
MIA-PaCa2 and human hTERT-HPNE immortalized pancreatic duct cells
CRL-1097 were obtained from the American Type Culture Collection
(Manassas, VA, USA). The primary human PDA cells PaCaDD-183 were
isolated from a patient PDA tissue as described (27). MIA-PaCa2 and BxPc-3 cells were
cultured in DMEM (PAA, Pasching, Austria) supplemented with 10%
heat-inactivated FCS (Sigma, Deisenhofen, Germany) and 25 mmol/l
HEPES (PAA). Cells were authenticated throughout the culture by the
typical morphology. To maintain authenticity of the cell lines,
frozen stocks were prepared from initial stocks, and every three
months a new frozen stock was used for the experiments. The
authenticity of established cell lines was certified in April 2013.
Mycoplasma-negative cultures were ensured by monthly testing.
Treatment of cells
Epigallocatechin gallate (EGCG), epicatechin gallate
(ECG), and catechin gallate (CG) (Sigma) were diluted in water to a
50-mM stock solution. Green tea extract (GTC) containing 588 mg
polyphenols/600 mg, from which 50% are EGCG (Dr. Loges + Co. GmbH,
Winsen, Germany) were diluted in DMSO to a 300-mM stock solution.
Quercetin (Sigma) was diluted in DMSO to a 200-mM stock solution
and DL-sulforaphane (Sigma) was diluted in EtOH to a 100-mM stock
solution. The final concentration of the solvents in cell culture
assays was 1:1000 or higher.
Viability assay
Viability was measured using 3-(4,5-dimethylthiazol-
2-yl)-2,5-diphenyltetrazolium bromide (MTT) as described previously
(14).
Apoptosis measurement
Cells were stained with fluorescein isothiocyanate
(FITC)-conjugated Annexin V (BD Biosciences, Heidelberg, Germany)
and externalization of phosphatidylserine was identified by flow
cytometry (FACScan, BD Biosciences). DNA fragmentation was detected
by staining of fragmented DNA with propidium iodide buffer
according to Nicoletti et al (28).
Detection of ALDH1 activity
A total of 2.5 μl of Aldefluor substrate (Aldagen,
Durham, USA) was added to 1×106 cells in 500 μl of assay
buffer and incubated for 60 min at room temperature. As a negative
control diethylamino-benzaldehyde (DEAB) was added to the cells.
ALDH1 activity was measured by flow cytometry (FACScan, BD
Biosciences).
Colony-forming assay
Seventy-two hours after treatment cells were seeded
in complete medium in 6-well tissue culture plates (TPP) and
colony-forming assays were performed as described previously
(14).
Spheroid assay
For formation of spheroids, cells were cultured in
NeuroCult NS-A basal serum-free medium (human) (StemCell
Technologies, Vancouver, Canada) supplemented with 2 μg/ml heparin
(StemCell Technologies), 20 ng/ml hEGF (R&D Systems,
Wiesbaden-Nordenstadt, Germany), 10 ng/ml hFGF-b (PeproTech,
Hamburg, Germany) and NeuroCult NS-A Proliferation Supplements
(StemCell Technologies). Cells were seeded at low densities
(2×102 to 1×103 cells/ml) in 12-well
low-adhesion plates (1 ml/well). Five days later, spheroid
formation was visible and spheroids were treated. Forty-eight hours
after treatment spheroids were dissociated by vigorous pipetting up
and down, followed by evaluation of the number of trypan-blue
negative cells, and re-seeding as described above (1st generation).
Upon formation of spheroids, cells were re-treated with natural
substances and the number of viable cells was evaluated by counting
trypan blue-negative cells (2nd generation).
Western blot analysis
Proteins were isolated, and western blot analysis
was performed as described previously (14). The following antibodies were used:
rabbit polyclonal Ab against human ras (Cell Signaling Technology,
Danvers, MA, USA) - this antibody detects endogenous levels of
total K-ras, H-ras and N-ras and it may cross-react with R-ras and
M-ras. Mouse mAb against human β-actin was from Sigma.
Detection of MMP-2, MMP-9 and K-ras mRNA
expression
Total RNA was extracted with the RNeasy Mini Kit
(Qiagen, Hilden, Germany) according to the manufacturer’s
instructions. cDNA was synthesized with the iScript™ cDNA Synthesis
kit (Bio-Rad, Munich, Germany). qRT-PCR was performed by using
reagents and a LightCycler®-PCR machine (Roche
Diagnostics GmbH, Mannheim, Germany) as described recently
(29). Specific primer pairs (MWG
Biotech, Ebersberg, Germany) were: β-actin
5′-TGACGGGGTCACCCACACTGTG CCCATCTA-3′ (sense),
5′-CTAGAAGCATTTGCGGTGGAC GATG-3′ (antisense); MMP-9:
5′-GACCTCAAGTGGCACC ACCA-3′ (sense), 5′-GTGGTACTGCACCAGGGCAA-3′
(antisense); MMP-2 5′-AGTCTGAAGAGCGTGAAG-3′ (sense),
5′-CCAGGTAGGAGTGAGAATG-3′ (antisense), K-ras 5′-AT
TCCTTTTATTGAAACATCAGCA-3′ (sense), 5′-TCGGAT CTCCCTCACCAAT-3′
(antisense). The PCR conditions were for MMP-2 and K-ras: 95′C for
20 sec followed by 58°C for 30 sec; MMP-9: 95°C for 20 sec followed
by 60°C for 30 sec. Fold changes of target gene expression relative
to β-actin were calculated using the 2-ΔΔCt method.
Detection of microRNA expression
MicroRNA was extracted with the mirVana miRNA
isolation kit (Applied Biosystems/Life technologies, Darmstadt,
Germany) according to the manufacturer’s instructions. Total RNA
containing miRNA was synthesized to cDNA with miScript II RT kit
(Qiagen). Quantification of miRNA was performed by using the
miScript SYBR-Green PCR kit (Qiagen) and a LightCycler-PCR (Roche
Diagnostics GmbH). Transcripts obtained with U6 primers were used
for normalization. The sense sequence of miR-let-7a was
5′-TGAGGTAGTAGGTTGTATAGTTGG-3′ (MWG Biotech); A universal antisense
primer and the U6 control primers were provided in the miScript
SYBR-Green PCR kit. The PCR conditions were 95°C for 15 sec, 60°C
for 20 sec. Fold changes of miR-let-7a gene expression relative to
U6 were calculated using the 2-ΔΔCt method.
Transwell migration assay
To analyze the cell invasive potential we used a
standard transwell assay. Transwell polycarbonate filters of 8-μm
pore size (Corning, Inc., Lowell, MA) were used. FCS was used as a
chemo-attractant with DMEM in the lower compartment. Sulforaphane,
quercetin and catechin pre-treated (18 h) or untreated cells were
seeded at a concentration of 105 cells/cm2 in
24-well plates. The cells in boyden chambers were incubated at 37°C
and 5% CO2. After 24 h the number of transmigrated cells
was counted. The percentage of transmigrated cells was normalized
to the percentage of cell vitality evaluated by the MTT assay at
the end-point of the experiment.
Statistical analysis
The quantitative data are presented as the mean ±
SD. The groups were compared using the nonparametric Mann-Whitney
test. We report point-wise P-values not adjusted for multiple
comparisons since this project is exploratory in nature. The
control was compared to the single treatment groups and the single
treatment groups were compared to the double treatment groups.
*P<0.05 and **P<0.01 were deemed to be
statistically significant.
Results
The combination of dietary agents is
superior in reducing the self-renewal potential compared to single
agents
Recently, sulforaphane, quercetin and EGCG (Fig. 1) were described to target
pancreatic CSC features (14,18,29,30).
We asked if other green tea catechins may have similar activity and
if the combination of bioactive agents may enhance the efficacy.
The human established PDA cell lines MIA-PaCa2 with high CSC
features and BxPc-3 with low CSC features served as model cell
lines (compare Table I).
 | Table ICharacteristics of the established
pancreatic cancer cell lines. |
Table I
Characteristics of the established
pancreatic cancer cell lines.
| CSC-properties |
CSClow
BxPc-3 |
CSChigh
MIA-PaCa2 | Refs. |
|---|
| Source | Primary tumor | Primary tumor | ATCC |
| Tumor grade | G2 | G3 | (31) |
| p53 status | MT | MT | (31) |
| K-ras status | WT | MT | (31) |
| In vitro
morphology | Densely
attached | Loosely
attached | (15) |
| Self renewal
capacity |
|
Colony-formation | + | +++ | (14,32) |
| Spheroid
formation | − | +++ | (14,32) |
| ALDH1
activity | + | +++ | (14,32) |
| Gemcitabine
resistance | + | +++ | (14) |
| E-cadherin | +++ | − | (18) |
| Vimentin | + | +++ | (18) |
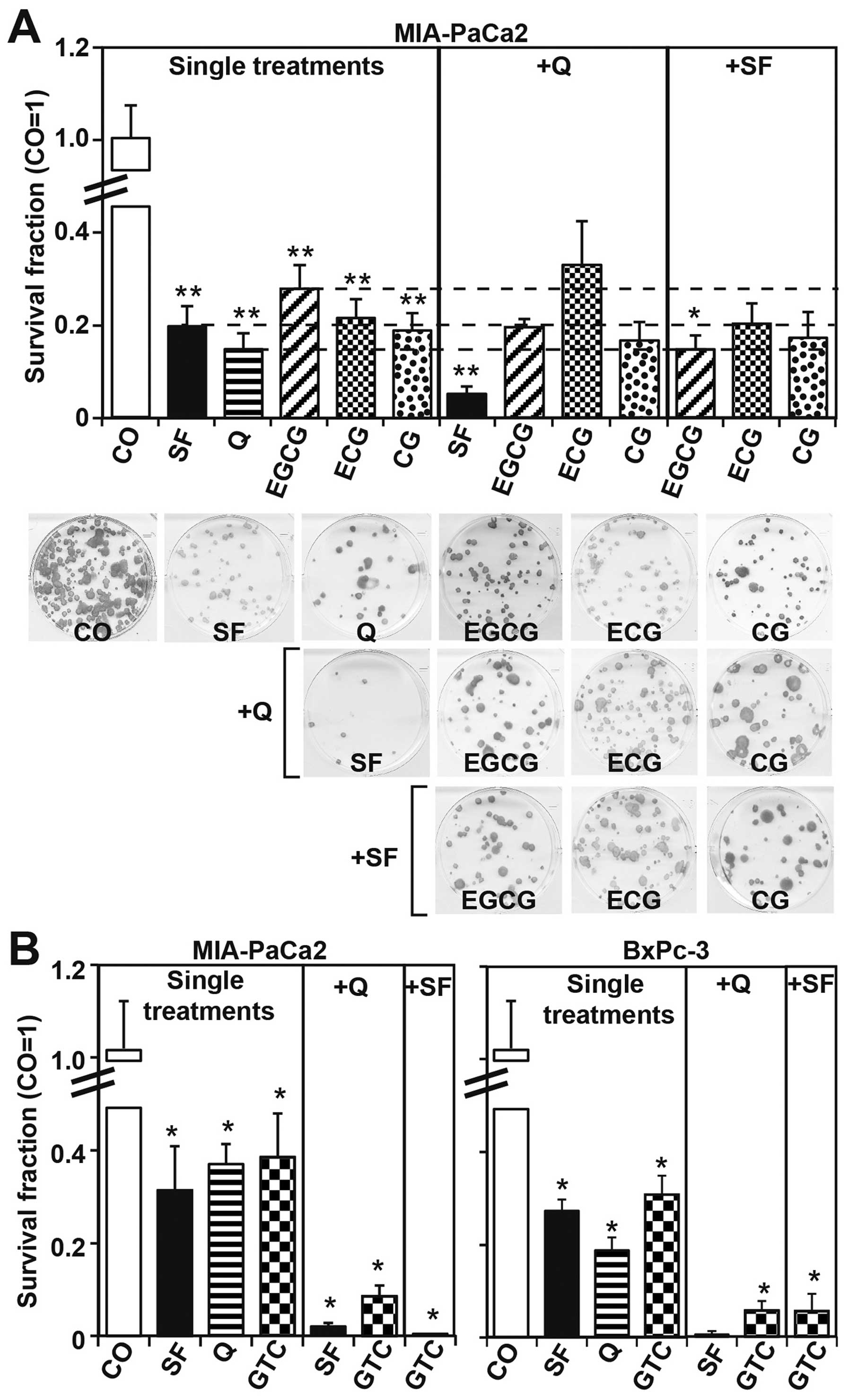
To evaluate the influence of single or combined
bioactive agents to the self-renewal potential, MIA-PaCa2 cells
were treated with sulforaphane, quercetin, EGCG, ECG and CG alone
or in combination. Seventy-two hours later the cells were seeded in
clonal density, and after additional 2 weeks the formation of
colonies was analyzed (Fig. 2A).
Each single substance significantly decreased the colony-forming
capacity and ECG or CG had a stronger effect than EGCG. The colony
formation was further reduced by the combinations
quercetin/sulforaphane and sulforaphane/EGCG, whereas the
combinations quercetin/ECG, quercetin/CG, SF/ECG and SF/CG had no
significant effect compared to single treatments. Because each of
the evaluated catechins strongly reduced the colony-forming
capacity, we evaluated the total green tea catechins in their
natural composition and used a purified and caffeine-free green tea
extract. These green tea catechins (GTC) consisted of 588 mg
catechin polyphenols/600 mg extract, from which 50% were EGCG (HPLC
data are available from the manufacturer: Dr. Loges + Co. GmbH).
MIA-PaCa2 and BxPc-3 cells were treated with sulforaphane,
quercetin or GTC alone, or in combination. Thereafter MIA-PaCa2
cells and BxPc-3 cells were seeded for colony formation. Two weeks
later, the single treatments significantly reduced colony
formation, whereas the combination treatments were more effective
and nearly totally abolished colony-formation (Fig. 2B). Similarly, the measurement of
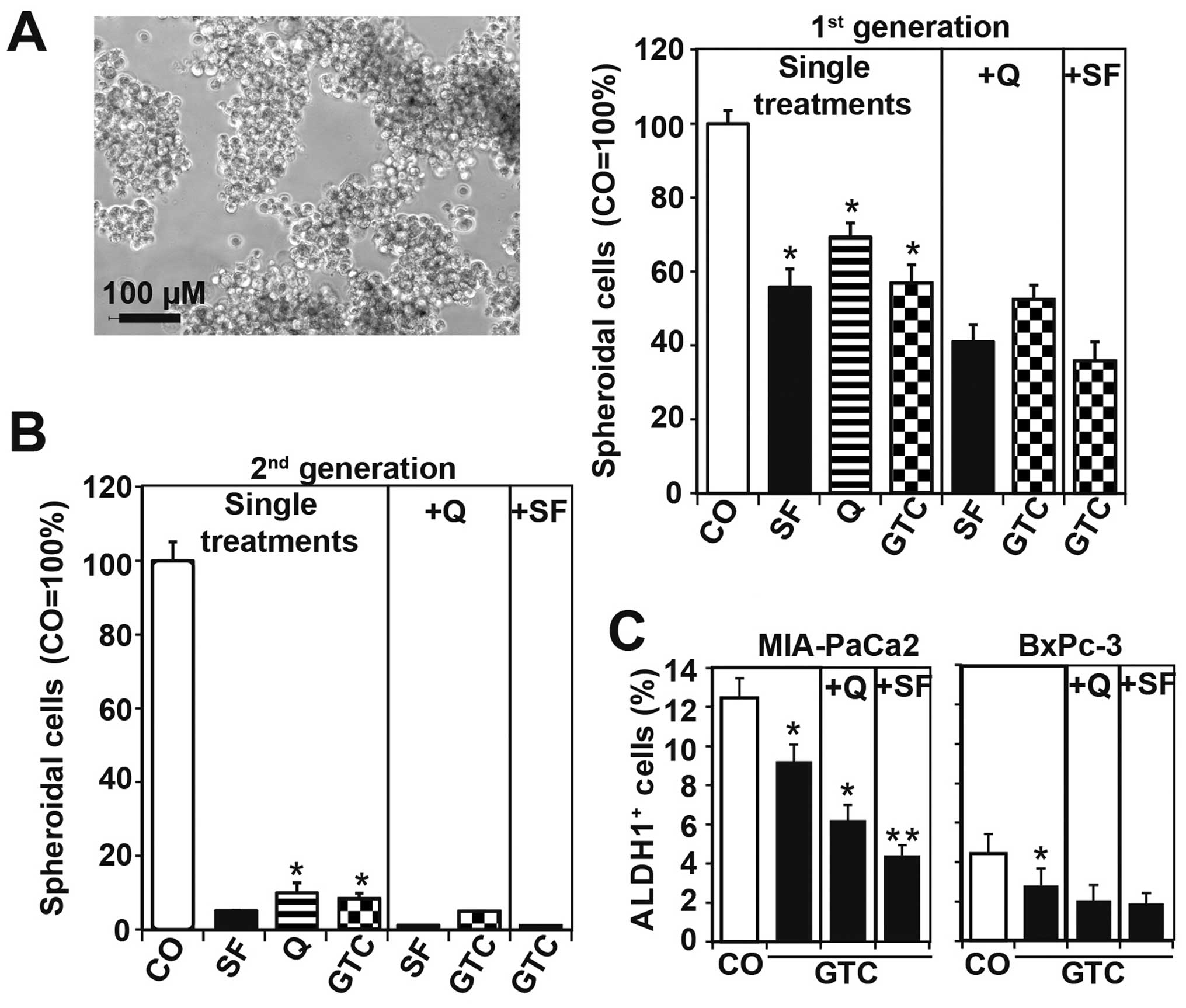
the self-renewal potential by the treatment of spheroidal-growing
MIA-PaCa2 cells revealed a strongly reduced number of
spheroidal-growing cells 48 h after single treatment, which was
further reduced by combination treatment (Fig. 3A). BxPc-3 cells were not used for
evaluation of spheroid formation, because this cell line is less
aggressive and does not form spheroids (Table I). To evaluate if the surviving
MIA-PaCa2 cells from the first treatment may be completely
eliminated by a second round of treatment, we re-seeded equal
amounts of surviving cells. After spheroid formation, the cells
were treated again. This resulted in a nearly complete elimination
of spheroidal-growing cells after single treatments and a nearly
complete elimination by combination treatments (Fig. 3B). The observed effects on the
self-renewal potential were confirmed by the measurement of ALDH1
activity, which is another indicator of the self-renewal capacity.
After treatment of MIA-PaCa2 and BxPc-3 cells, the activity of
ALDH1 was measured by the ALDEFLUOR substrate assay and FACS
analysis. GTC alone reduced ALDH1 activity significantly, but the
combinations had more pronounced effects (Fig. 3C). These data suggest that the
self-renewal potential is strongly inhibited by treatment with
sulforaphane, quercetin or GTC alone, but the combinations
GTC/sulforaphane, GTC/quercetin or sulforaphane/quercetin are
superior. Interestingly, the combination of the isothiocyanate with
either polyphenol (GTC or quercetin) was stronger than the
combination of two different polyphenolic agents.
The combination of dietary agents is
superior in reducing viability, migratory potential along with
induction of apoptosis compared to single agents
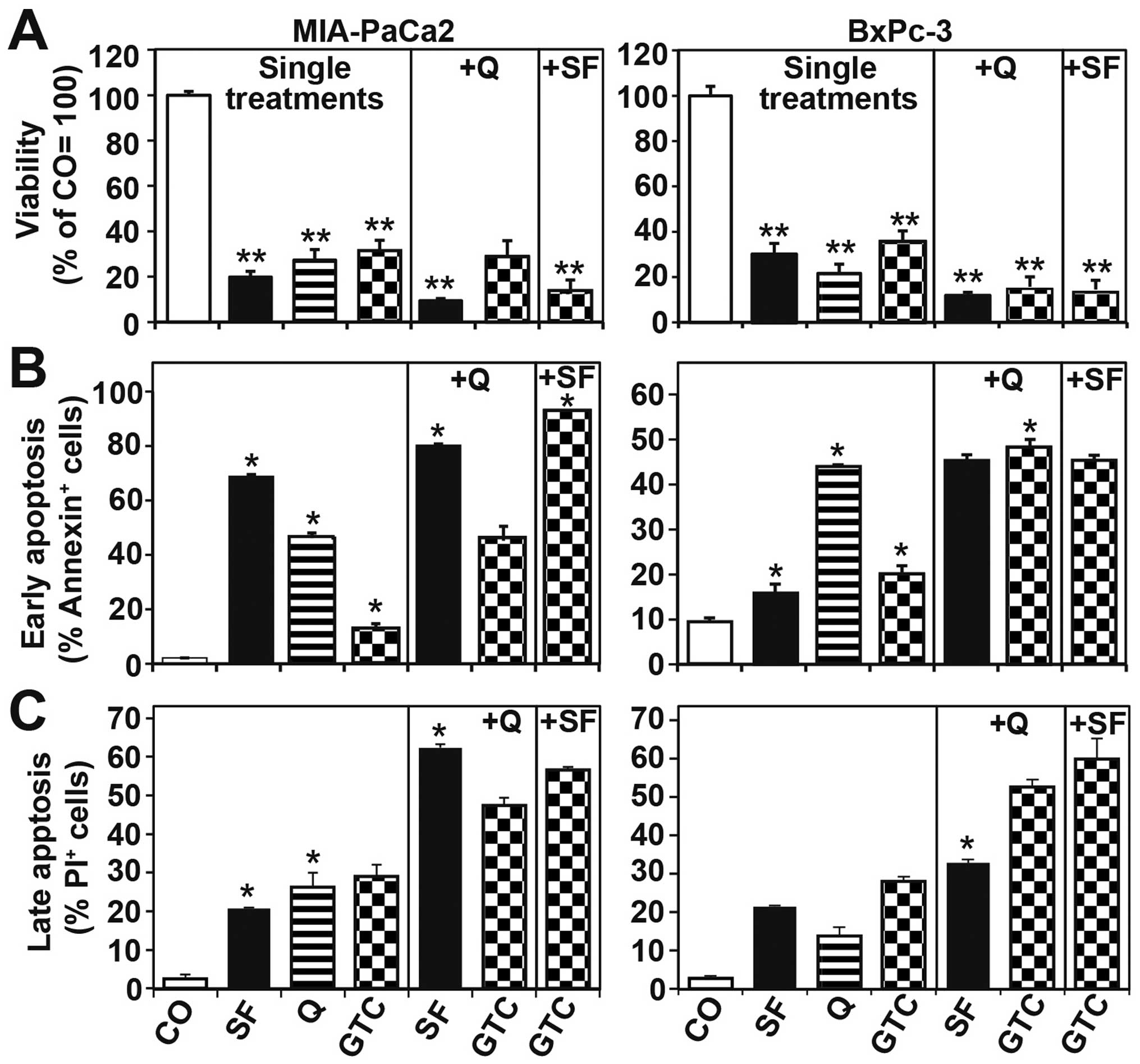
To investigate whether GTC, sulforaphane and
quercetin, alone or combined, may affect viability, apoptosis and
migration potential, MIA-PaCa2 and BxPc-3 cells were treated as
described above. Ninty-six hours later the NAD(P)H content was
detected by the MTT assay, which reflects viability (33). Whereas all single agents strongly
inhibited the viability, the combinations were stronger (Fig. 4A). Likewise, early apoptosis was
induced in MIA-PaCa2 cells by single agents, but the combinations
were more effective as measured by annexin staining and
FACS-analysis (Fig. 4B).
Comparable results were obtained by the measurement of late
apoptosis by staining of the cells with propidium iodide and FACS
analysis. The combination of a isothiocyanate with a polyphenol had
more pronounced effects than the combination of two polyphenols,
whereas all combinations were significantly stronger than the
single treatments (Fig. 4C). To
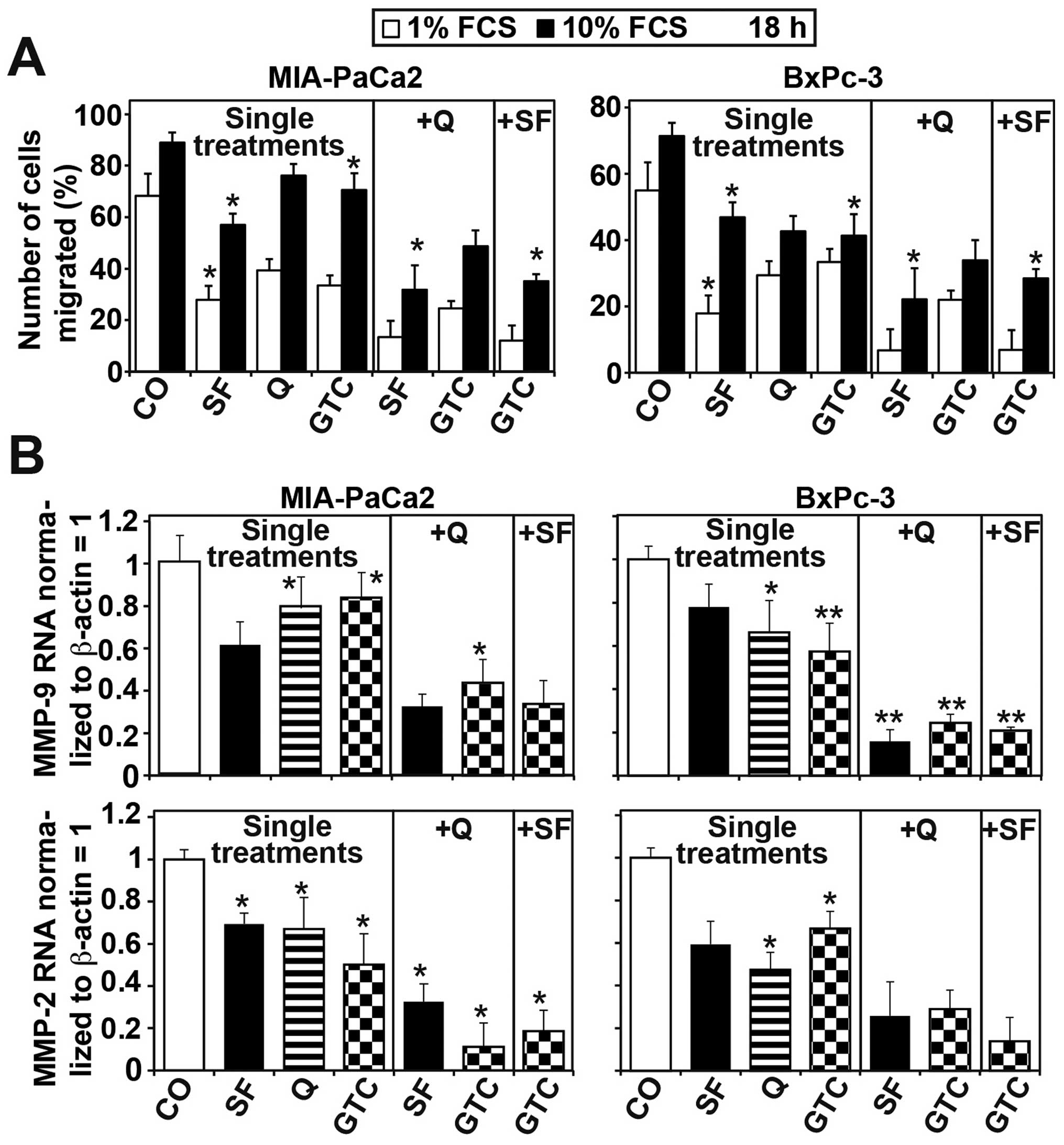
gain knowledge on whether the dietary agents also inhibit the
migratory potential, we performed a boyden chamber assay. MIA-PaCa2
and BxPc-3 cells were treated with single agents or combinations
thereof and 24 h later living cells in 10% FCS were put to the
upper chamber and 1 or 10% FCS were added to the lower chamber. The
transmigration of the cells was analyzed 18 h later and the number
of control cells seeded to the upper chamber was set to 100%. Each
single component significantly inhibited migration whereas the
total effects were stronger with a gradient toward 1% FCS compared
to a gradient toward 10% FCS. However, the combinations were most
effective in both cell lines and with both gradients (Fig. 5A). Accordingly, the RNA expression
of matrix metalloproteinase MMP-2 and MMP-9 was inhibited with
strongest effects upon combination treatments (Fig. 5B). These data suggest that the
combinations of quercetin, sulforaphane and GTC sensitize
pancreatic cancer cells to apoptosis and inhibit their invasion
potential stronger than each single agent.
The combination of dietary agents
enhances the expression of miR-let-7a associated with inhibition of
K-ras stronger than the single agents
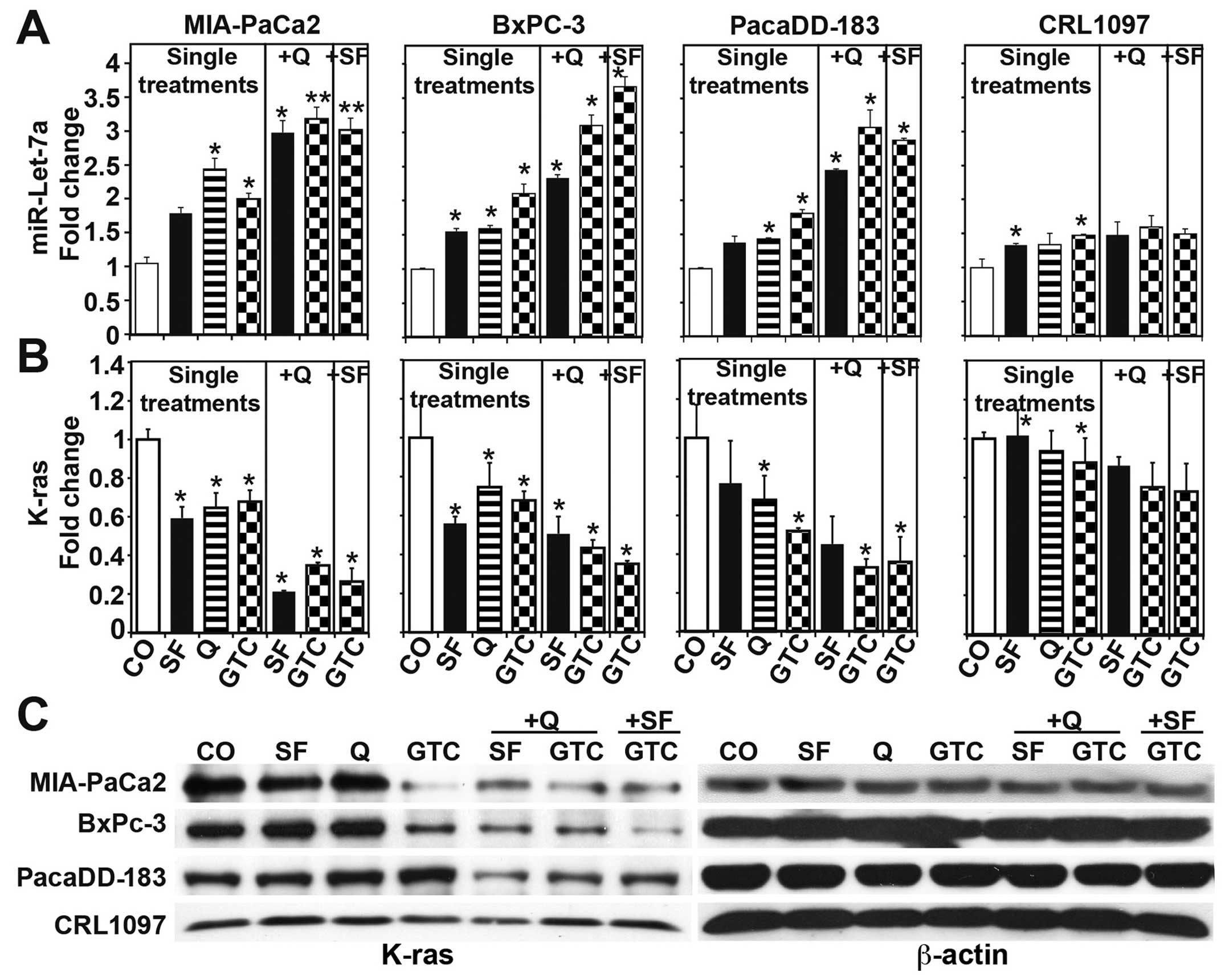
MIA-PaCa2, BxPC-3, primary PDA cells PacaDD-183 and
CRL 1097 non-malignant pancreatic ductal cells were treated with
dietary agents alone or in combination and 72 h later the
expression of the miR-let-7a and its target gene K-ras was examined
by qRT-PCR. The single treatments significantly enhanced the
expression of miR-let-7a but inhibited the expression of K-ras in
the 3 malignant cells with strongest effects after combination
treatment (Fig. 6A and B). In
contrast, the induction of miR-let-7a expression along with the
inhibition of K-ras expression were minimal in the non-malignant
CRL 1097 cells, as expected. Likewise, the basal expression of
miR-let-7a was lower in malignant cells compared to non-malignant
cells (data not shown). The strong inhibition of K-ras in malignant
cells only was confirmed by western blot analysis (Fig. 6C). These data suggest that
sulforaphane, quercetin and GTC mediate the induction of miR-let-7a
expression, which in turn inhibits K-ras expression and CSC
features in pancreatic cancer.
Discussion
Considering the still devastating prognosis of
patients with pancreatic cancer, we evaluated in the present study
an experimental combination strategy of bioactive dietary agents
for elimination of CSCs. We tested the efficacy of quercetin,
sulforaphane and GTC to inhibit the self-renewal potential,
apoptosis resistance and migratory potential and found that the
combination of bioactive agents is more effective than the each
single agents. Further, we addressed whether the induction of
miR-let-7a along with inhibition of its target gene K-ras is
involved. We show that sulforaphane, quercetin and EGCG strongly
inhibit colony formation and demonstrate that the catechins ECG and
CG are even more effective than the well examined EGCG. To mimic
the natural composition of catechins in green tea, all the
subsequent experiments were performed with purified green tea
extract (GTC). GTC effectively inhibited the self-renewal
potential, as exemplified by colony and spheroid formation as well
as ALDH1 activity, but the combination with sulforaphane or
quercetin increased the effect. Similar results were obtained for
apoptosis resistance, migratory potential, and expression of matrix
metalloproteinases. Most importantly, our data provide evidence
that the combination of sulforaphane or quercetin with GTC
activates the expression of miR-let-7a along with inhibition of
K-ras expression much stronger than the single agents. Because
upregulation of miR-let-7a and downregulation of K-ras was specific
for cancer cells and occurred only minimally in nonmalignant
pancreatic ductal cells, these data suggest a cancer cell-specific
action of the evaluated dietary agents.
This study provides data to support the speculation,
that miR-let7a-mediated inhibition of K-ras expression directly
mediates the observed inhibition of CSC features. In agreement with
data derived from mouse models of lung and colon cancer, oncogenic
K-ras accelerated tumor progression by imposing an immature
stem-like state in which the differentiation is inhibited (reviewed
in ref. 34). Compelling evidence
also exists for K-ras-induced reprogramming of pancreatic acinar
cells into ductal intraepithelial neoplasia, a histologically
well-defined precursor to PDA (34). A recent study shows the direct
reprogramming of primary mouse cells by conditional expression of
K-ras (34) and it was concluded
that non-CSCs have the potential to dedifferentiate and acquire
stem cell properties as a direct consequence of K-ras-induced
plasticity. This allows the formation of CSCs with high metastatic
capacity at any time during cancer progression, or vice versa, the
reversal of the stem cell phenotype of existing CSCs at any time
during cancer development and progression.
We found that the green tea catechins ECG and CG are
more effective than EGCG in inhibition of colony formation of the
highly aggressive and CSC-enriched PDA cell line MIA-PaCa2. A
previous study (19) has already
demonstrated that ECG and CG exert much stronger anti-proliferative
and anti-inflammatory activities on the established PDA cell lines
PancTu-I, Panc1, Panc89 and BxPc-3 than the most widely studied
catechin EGCG. Further, our in vitro data are confirmed by a
recent in vivo examination, in which EGCG inhibited the
growth of orthotopically implanted human pancreatic Panc1
xenografts in BALB/c nude mice (35). Most importantly, in the latter
in vivo study, no obvious side effects of EGCG in mice were
observed. These data match our observation of a cancer
cell-specific induction of miR-let- 7a and inhibition of K-ras. Our
study provides data to support the hypothesis that the antitumor
effect in vivo will be much more pronounced upon the use of
the total green tea catechins combined with sulforaphane or
quercetin.
We observed that the combination of the
isothiocyanate sulforaphane with green tea polyphenols was similar
or slightly higher in inhibition of self-renewal potential than the
combination of green tea polyphenols with the polyphenol quercetin.
It is tempting to speculate that this might be due to targeting of
identical pathways by bioactive agents with similar chemical
structures. Consequently, the combination of bioactive agent with
different chemical structures may be more effective than, e.g., the
combination of two different polyphenols, because a broader
spectrum of anticancer pathways may be induced. The recent findings
that the polyphenols resveratrol, genistein and curcumin possess
anti-CSC activity (17) together
with our data emphasizes that bioactive polyphenols should be
combined with chemically different plant substances with anti-CSC
activity to activate a wide spectrum of stem cell signaling
pathways. In addition to sulforaphane and other isothiocyanates,
the diterpenoid triepoxide triptolide may be a promising
combination partner. Triptolide has a long history in Traditional
Chinese medicine for the treatment of rheumatoid arthritis and
cancer and it is derived from the vine-like herb Tripterygium
wilfordii Hook f (36). In our
prior study we demonstrated that triptolide effectively inhibits
NF-κB activity, epithelial-mesenchymal transition and stem-like
features in PDA cells (32).
Regarding the upregulation of miR-let-7a after
treatment with sulforaphane, quercetine and green tea catechins,
these data are similar to the recent notion that curcumin-induces
miR-let-7a expression (20).
Moreover, upregulation of miRNA-210 may contribute, which was
observed after EGCG treatment and this suppressed the growth of
lung cancer cells (37). As well,
miRNA-30b may be involved, because it was downregulated by the
treatment of HepG2 liver cancer cells with EGCG (38). Besides, the watercress-derived
phenethyl-isothiocyanate downregulated miRNA-141, which repressed
the expression of the androgen receptor and the PSA level in
prostate cancer cell lines (39).
In another study, Shan et al demonstrated the induction of
miRNA-200c by sulforaphane in bladder cancer cells and this
inhibited Cox-2, epithelial-mesenchymal transition and MMP-2 and -9
expression (40). In this respect,
previous studies including our own add important information to the
growing body of evidence that dietary agents may prevent cancer by
epigenetic signaling. Future studies will have to address the
effect of a special ‘epigenetic diet’ created for the presence of
selected bioactive agents for prevention and treatment of cancer
(41). Indeed, previous studies
have shown that chemopreventive nutritional polyphenols and
isothiocyanates may neutralize genetic defects by epigenetic
regulation, which is the potential reason why these substances
attenuated the processes of tumorigenesis, progression and
metastasis and sensitized for drug treatment (42,43).
Such epigenetic regulation does not only include the modification
of miRNA expression, but may as well involve changes in histone
acetylation and promoter methylation.
Together, the translational conclusion from our
study is that a defined diet consisting of fruits and vegetables
with experimentally proven anti-CSC activity may be a highly
effective prevention of cancer growth and progression.
Acknowledgements
This manuscript is dedicated to the memory of
Professor Werner Hunstein. We thank F. Rückert for providing human
primary PaCaDD-138 PDA cells and Yiyao Zhang, Li Liu, Frank
Schönsiegel and Vanessa Rausch for technical support. This study
was supported by grants from the German Cancer Aid (Deutsche
Krebshilfe 109362), German Research Community (DFG HE 3186/11-1),
German-Israeli Foundation for Scientific Research and Development
(GIF 1058-7.11/2008), Heidelberger Stiftung Chirurgie, Stiftung für
Krebs und Scharlachforschung Dietmar Hopp-Stiftung and the Hanns A.
Pielenz Stiftung.
Abbreviations:
|
CSC
|
cancer stem cell
|
|
CG
|
catechin gallate
|
|
ECG
|
epicatechin gallate
|
|
EGCG
|
epigallocatechin gallate
|
|
GTC
|
green tea catechins
|
|
PDA
|
pancreatic ductal adenocarcinoma
|
References
|
1
|
Gukovskaya AS and Pandol SJ: Cell death
pathways in pancreatitis and pancreatic cancer. Pancreatology.
4:567–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuveson DA and Neoptolemos JP:
Understanding metastasis in pancreatic cancer: a call for new
clinical approaches. Cell. 148:21–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rasheed ZA, Kowalski J, Smith BD and
Matsui W: Concise review: emerging concepts in clinical targeting
of cancer stem cells. Stem Cells. 29:883–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simeone DM: Pancreatic cancer stem cells:
implications for the treatment of pancreatic cancer. Clin Cancer
Res. 14:5646–5648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silverman DT, Swanson CA, Gridley G,
Wacholder S, Greenberg RS, Brown LM, Hayes RB, Swanson GM,
Schoenberg JB, Pottern LM, Schwartz AG, Fraumeni JF Jr and Hoover
RN: Dietary and nutritional factors and pancreatic cancer: a
case-control study based on direct interviews. J Natl Cancer Inst.
90:1710–1719. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kirsh VA, Peters U, Mayne ST, Subar AF,
Chatterjee N, Johnson CC and Hayes RB: Prospective study of fruit
and vegetable intake and risk of prostate cancer. J Natl Cancer
Inst. 99:1200–1209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Zhang W, Sun L, Yu H, Ni QX, Risch
HA and Gao YT: Green tea drinking and risk of pancreatic cancer: a
large-scale, population-based case-control study in urban Shanghai.
Cancer Epidemiol. 36:e354–e358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herr I, Lozanovski V, Houben P, Schemmer P
and Buchler MW: Sulforaphane and related mustard oils in focus of
cancer prevention and therapy. Wien Med Wochenschr. 163:80–88.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang CS and Wang H: Cancer therapy
combination: green tea and a phosphodiesterase 5 inhibitor? J Clin
Invest. 123:556–558. 2013.PubMed/NCBI
|
|
13
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: from antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kallifatidis G, Rausch V, Baumann B, Apel
A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G,
Altevogt P, Wirth T, Werner J, Schemmer P, Büchler MW, Salnikov A
and Herr I: Sulforaphane targets pancreatic tumour-initiating cells
by NF-kappaB-induced antiapoptotic signalling. Gut. 58:949–963.
2009. View Article : Google Scholar
|
|
15
|
Rausch V, Liu L, Kallifatidis G, Baumann
B, Mattern J, Gladkich J, Wirth T, Schemmer P, Büchler MW, Zöller
M, Salnikov A and Herr I: Synergistic activity of sorafenib and
sulforaphane abolishes pancreatic cancer stem cell characteristics.
Cancer Res. 70:5004–5013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kallifatidis G, Labsch S, Rausch V,
Mattern J, Gladkich J, Moldenhauer G, Büchler MW, Salnikov A and
Herr I: Sulforaphane increases drug-mediated cytotoxicity towards
cancer stem-like cells of pancreas and prostate. Mol Ther.
19:188–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Wicha MS, Schwartz SJ and Sun D:
Implications of cancer stem cell theory for cancer chemoprevention
by natural dietary compounds. J Nutr Biochem. 22:799–806. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou W, Kallifatidis G, Baumann B, Rausch
V, Mattern J, Gladkich J, Giese N, Moldenhauer G, Wirth T, Buchler
MW, Salnikov AV and Herr I: Dietary polyphenol quercetin targets
pancreatic cancer stem cells. Int J Oncol. 37:551–561.
2010.PubMed/NCBI
|
|
19
|
Kurbitz C, Heise D, Redmer T, Goumas F,
Arlt A, Lemke J, Rimbach G, Kalthoff H and Trauzold A: Epicatechin
gallate and catechin gallate are superior to epigallocatechin
gallate in growth suppression and anti-inflammatory activities in
pancreatic tumor cells. Cancer Sci. 102:728–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bao B, Ali S, Banerjee S, Wang Z, Logna F,
Azmi AS, Kong D, Ahmad A, Li Y, Padhye S and Sarkar FH: Curcumin
analogue CDF inhibits pancreatic tumor growth by switching on
suppressor microRNAs and attenuating EZH2 expression. Cancer Res.
72:335–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong D, Heath E, Chen W, Cher ML, Powell
I, Heilbrun L, Li Y, Ali S, Sethi S, Hassan O, Hwang C, Gupta N,
Chitale D, Sakr WA, Menon M and Sarkar FH: Loss of let-7
up-regulates EZH2 in prostate cancer consistent with the
acquisition of cancer stem cell signatures that are attenuated by
BR-DIM. PLoS One. 7:e337292012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, VandenBoom TG II, Kong D, Wang Z,
Ali S, Philip PA and Sarkar FH: Up-regulation of miR-200 and let-7
by natural agents leads to the reversal of
epithelial-to-mesenchymal transition in gemcitabine-resistant
pancreatic cancer cells. Cancer Res. 69:6704–6712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
25
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nair VS, Maeda LS and Ioannidis JP:
Clinical outcome prediction by microRNAs in human cancer: a
systematic review. J Natl Cancer Inst. 104:528–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruckert F, Aust D, Bohme I, Werner K,
Brandt A, Diamandis EP, Krautz C, Hering S, Saeger HD, Grutzmann R
and Pilarsky C: Five primary human pancreatic adenocarcinoma cell
lines established by the outgrowth method. J Surg Res. 172:29–39.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rausch V, Liu L, Apel A, Rettig T,
Gladkich J, Labsch S, Kallifatidis G, Kaczorowski A, Groth A, Gross
W, Gebhard MM, Schemmer P, Werner J, Salnikov AV, Zentgraf H,
Buchler MW and Herr I: Autophagy mediates survival of pancreatic
tumour-initiating cells in a hypoxic microenvironment. J Pathol.
227:325–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang SN, Fu J, Nall D, Rodova M, Shankar S
and Srivastava RK: Inhibition of sonic hedgehog pathway and
pluripotency maintaining factors regulate human pancreatic cancer
stem cell characteristics. Int J Cancer. 131:30–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sipos B, Moser S, Kalthoff H, Torok V,
Lohr M and Kloppel G: A comprehensive characterization of
pancreatic ductal carcinoma cell lines: towards the establishment
of an in vitro research platform. Virchows Arch. 442:444–452.
2003.PubMed/NCBI
|
|
32
|
Liu L, Salnikov AV, Bauer N,
Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J,
Schemmer P, Werner J and Herr I: Triptolide reverses
hypoxia-induced EMT and stem-like features in pancreatic cancer by
NF-kappa B downregulation. Int J Cancer. 134:2489–2503. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vistica DT, Skehan P, Scudiero D, Monks A,
Pittman A and Boyd MR: Tetrazolium-based assays for cellular
viability: a critical examination of selected parameters affecting
formazan production. Cancer Res. 51:2515–2520. 1991.PubMed/NCBI
|
|
34
|
Ischenko I, Zhi J, Moll UM, Nemajerova A
and Petrenko O: Direct reprogramming by oncogenic Ras and Myc. Proc
Natl Acad Sci USA. 110:3937–3942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shankar S, Marsh L and Srivastava RK: EGCG
inhibits growth of human pancreatic tumors orthotopically implanted
in Balb C nude mice through modulation of FKHRL1/FOXO3a and
neuropilin. Mol Cell Biochem. 372:83–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brinker AM, Ma J, Lipsky PE and Raskin I:
Medicinal chemistry and pharmacology of genus
Tripterygium(Celastraceae). Phytochemistry. 68:732–766.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang H, Bian S and Yang CS: Green tea
polyphenol EGCG suppresses lung cancer cell growth through
upregulating miR-210 expression caused by stabilizing HIF-1alpha.
Carcinogenesis. 32:1881–1889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arola-Arnal A and Blade C:
Proanthocyanidins modulate microRNA expression in human HepG2
cells. PLoS One. 6:e259822011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiao J, Gong AY, Eischeid AN, Chen D, Deng
C, Young CY and Chen XM: miR-141 modulates androgen receptor
transcriptional activity in human prostate cancer cells through
targeting the small heterodimer partner protein. Prostate.
72:1514–1522. 2012. View Article : Google Scholar
|
|
40
|
Shan Y, Zhang L, Bao Y, Li B, He C, Gao M,
Feng X, Xu W, Zhang X and Wang S: Epithelial-mesenchymal
transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail,
ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J
Nutr Biochem. 24:1062–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hardy TM and Tollefsbol TO: Epigenetic
diet: impact on the epigenome and cancer. Epigenomics. 3:503–518.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vanden Berghe W: Epigenetic impact of
dietary polyphenols in cancer chemoprevention: lifelong remodeling
of our epigenomes. Pharmacol Res. 65:565–576. 2012.PubMed/NCBI
|
|
43
|
Gerhauser C: Epigenetic impact of dietary
isothiocyanates in cancer chemoprevention. Curr Opin Clin Nutr
Metab Care. 16:405–410. 2013. View Article : Google Scholar : PubMed/NCBI
|