Introduction
Cellular proliferation, differentiation, senescence
and apoptosis are cell cycle-dependent processes (1). Regulation of the cell cycle is
closely linked to tumor development and progression, as it is
impaired in almost all tumors (2,3).
Moreover, many proto-oncogenes and tumor suppressor genes function
as major factors or are directly involved in the regulation of cell
cycle (4). Carcinogenic factors
can induce mutation, deletion, translocation or amplification of
these genes, resulting in de-regulation of the cell cycle, abnormal
cell proliferation and tumor development. A new strategy for cancer
therapy involves regulating cell cycle, proliferation and apoptosis
of tumor cells, and select inhibition of tumor tissue activity
(5).
B cell translocation gene 1 (BTG1) is a member of
the TOB/BTG family of proteins known to inhibit cell proliferation
and negatively regulate the cell cycle that was first identified in
B lymphoblastic leukemia (6–9).
TOB/BTG family members regulate these processes by acting on cell
cycle genes in response to various internal and external stimuli.
For example, high PC3 expression in NIH/3T3 cells arrests the cell
cycle in the G1 phase and inhibits cell growth (10). Enhanced BTG1 expression, which
peaks in the G0/G1 phase, promotes the differentiation of neural
stem and germ cells and plays an important role in angiogenesis.
BTG1 also facilitates the formation of the CCR4/NOT transcriptional
complex, which regulates the deadenylation and turnover of
cytoplasmic mRNAs. BTG1 is structurally characterized by the
presence of a specific BTG domain in the N-terminus along with a
large anti-proliferative homologous region (11). Although BTG1 displays some
characteristics of tumor suppressor genes (12,13),
it is not known if BTG1 is a thyroid cancer suppressor gene.
Therefore, this study aimed to determine the roles BTG1 plays in
the growth, proliferation, invasion, metastasis and apoptosis of
thyroid cancer cells.
Materials and methods
Reagents
The rabbit anti-human BTG1 monoclonal, rabbit
anti-human cyclin D1 polyclonal, rabbit anti-human Bcl-2 polyclonal
and rabbit anti-human MMP-9 monoclonal antibodies were purchased
from Abcam (Cambridge, UK). Goat anti-rabbit fluorescent secondary
antibody (IRDye800) was obtained from LI-COR Bioscience, Inc.
(Lincoln, NE, USA). The β-actin primary antibody, polyoxymethylene
and crystal violet were purchased from Sigma (St. Louis, MO, USA).
The Plenti6/V5-DEST Vector, lentiviral packaging mix, Opti-MEM,
Lipofectamine 2000 and TRIzol were obtained from Invitrogen (Thermo
Fisher Scientific, Waltham, MA, USA). An immunohistochemistry kit
and the Annexin V-FITC/PI apoptosis detection kit were purchased
from 4A Biotech Co., Ltd. (Beijing, China). Fetal bovine serum
(FBS), cell-culture media and supplementary materials were obtained
from Gibco (Thermo Fisher Scientific, Waltham, MA, USA). Reverse
transcriptase reagents and SYBR Premix ExTaq (Perfect Real-Time)
were purchased from Takara (Otsu, Shiga, Japan). Invasion chambers
and Matrigel for invasion and migration assays were purchased from
BD Biosciences (Franklin Lakes, NJ, USA).
Clinical data
All patients enrolled in this study provided
informed consent in advance. There were 23 males and 60 females,
aged from 26 to 73 years old, with a median age of 44 years. The
studies contained 46 cases of papillary cancer, 22 cases of
follicular cancer, and 11 cases of medullary cancer, and 4 cases of
undifferentiated cancer. Of the 83 cases of thyroid cancer, 31 had
lesions ≤4 cm and 52 had esions >4 cm. Fifty-five of the
patients demonstrated lymph node metastasis. Twenty-seven patients
had grade I (well differentiated) tumors, and 56 patients had grade
II or III (moderately to poorly differentiated) tumors. Clinical
staging showed 25 cases of stage I–II, and 58 cases of stage
III–IV. Two endoscopic biopsy samples were collected from each
patient and either immediately stored in liquid nitrogen for
western blot analysis, or fixed in a 4% formaldehyde solution and
paraffin-embedded for immunohistochemistry.
Cell culture and gene transfection
The human thyroid cancer cell line FTC-133 was
maintained in DMEM medium supplemented with 10% FBS, which was
changed every 2–3 days. Upon reaching confluence, cells were
subcultured with 0.25% trypsin and 1% ethylenediaminetetraacetic
acid. BTG1 cDNA sequences were cloned into the BamHI and
AscI sites of the plenti6/V5-DEST vector, and cells were
transfected with pLenti6-BTG1 or plenti6/V5-DEST vector using
Lipofectamine 2000. Transfected cells were maintained in
blastidicin (5 μg/ml)-containing RPMI-1640 medium for selection of
stable vector-containing sublines.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (14). Briefly, 4-μm
sections were prepared from paraffin-embedded biopsy samples and
dehydrated. Sections were incubated in 3% hydrogen peroxide for 10
min to block endogenous peroxidase, followed by 20 min in 0.05%
trypsin. Sections were incubated for 20 min at room temperature in
a blocking solution containing 10% goat serum followed by BTG1
antibody (1:100) at 4°C overnight. For a negative control, the
primary antibody was replaced with phosphate-buffered saline (PBS).
Sections were subsequently incubated for 20 min each in secondary
and tertiary antibodies at room temperature, visualized by DAB
staining and countered with a hematoxylin stain. Two pathologists
blind to patient condition examined and quantified the sections.
Five randomly selected fields from three slides for each specimen
were examined under a microscope and counted. BTG1 expression was
determined based on the percentage of positive cells (0 points,
≤5%; 1 point, 5–25%; 2 points, 25–50%; and 3 points, >50%
positive cells) and the staining intensity [0 points, no staining;
1 point, weak staining (light yellow); 2 points, moderate staining
(yellowish-brown); and 3 points, strong staining (brown)]. The
final score of BTG1 expression was the product of the BTG1
expression rate (percentage score) and intensity: − for 0 points, +
to +++ for positive (+ for 1–3 points, ++ for 4–6 points and +++
for 7–9 points).
Quantitative real-time RT-PCR
Total RNA was extracted from FTC-133 cells using
TRIzol reagent according to the manufacturer’s protocol. Total RNA
(500 ng) was reverse transcribed using reverse transcriptase, and
quantitative real-time RT-PCR (qRT-PCR) was performed on an ABI
PRISM 7300 Real-Time PCR system (Applied Biosystems, Inc., by Life
Technologies/Thermo Fisher Scientific, Waltham, MA, USA) according
to the standard manufacturer’s protocol for SYBR Premix ExTaq. Gene
specific primers used include: BTG1, sense
5′-GGAATTCATGCATCCCTTCTACACCCGG and antisense
5′-CGACGCGTTTAACCTGATACAGTCATCAT; and β-actin for normalization,
sense 5′-ATCGTCCACC GCAAATGCTTCTA and antisense 5′-AGCCATGCCAA
TCTCATCTTGTT. Thermal cycling conditions were 95°C for 1 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
expression level relative to β-actin was calculated using the
2−ΔΔCt method in SDS 1.3 software.
Western blot analysis
Western blotting was performed as previously
described (15). Briefly, 50 μg of
protein (determined using a BCA Protein Assay kit (Tiangen Biotech
Co., Ltd., Beijing, China) per samples were subjected to sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
to a nitrocellulose membrane. Membranes were incubated for 2 h in
5% non-fat dry milk followed by an overnight incubation at 4°C in
primary antibody (BTG1, 1:1000; β-actin, 1:5000). After washing,
the membranes were incubated with goat anti-rabbit fluorescent
secondary antibody (IRDye800, 1:20,000 dilution) in the dark, for 1
h, at room temperature. The blots were then scanned and analyzed
using the Odyssey Infrared Imaging System (LI-COR Bioscience,
Inc.). Western blot data were quantified by normalizing the BTG1
signal intensity of each sample to that of β-actin.
MTT assay
Cell viability was determined using the tetrazolium
salt MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide) assay, as previously described (16). Briefly, FTC-133 cells were plated
into 96-well culture plates at an optimal density of
5×103 cells/ml with 200 μl/well. After 24–96 h, 20 μl of
5 mg/ml MTT was added to each well and incubated at 37°C for 4 h.
The medium was then gently aspirated and 150 μl of dimethyl
sulfoxide was added to each well to solubilize the formazan
crystals. The optical density of each sample was immediately
measured at 570 nm using a microplate reader (Bio-Rad, Hercules,
CA, USA).
Flow cytometry
An Annexin V-FITC flow cytometry assay was used as
previously described (17) to
detect the apoptosis rate. Cells were plated into 60-mm dishes for
48 h and grown to 70–75% confluency. Cells were then collected,
washed with ice-cold PBS, and resuspended at a density of
1×106 cells/ml in a binding buffer and incubated for 15
min in the dark at 25°C with 5 μl of Annexin V-FITC and 10 μl of
propidium iodide (20 μg/ml). Ten thousand cells were analyzed with
a FACScan flow cytometer with CellQuest software (BD Biosciences)
for apoptosis rate determination. For cell cycle distribution,
1×106 cells were fixed in 70% ethanol and resuspended in
1 ml of a solution containing 3.8 mM sodium citrate, 50 μg/ml
propidium iodide, and 0.5 μg of RNase A, and analyzed with the flow
cytometer using the ModFit software program (Verity Software House,
Topsham, ME, USA).
Invasion and migration assays
Invasion and migration assays were performed as
previously described (18).
Briefly, 10×105 cells were plated into Invasion Chambers
with Costar Transwell 8 μm inserts coated with 50 μg reduced serum
Matrigel according to the manufacturer’s instructions. Medium
supplemented with 10% FBS was used in the lower chamber. Migration
assays were performed in the same manner excluding the Matrigel.
After 12 h, non-invading cells and media were removed with a cotton
swab. Cells on the lower surface of the membrane were fixed with
polyoxymethylene and stained with 0.1% crystal violet for 30 min.
Stained cells were counted under a microscope in four randomly
selected fields, and the average was used to indicate cell
migration and invasion.
Statistical analyses
All statistical analyses were performed using SPSS
16.0 software (IBM, Armonk, NY, USA), according to published
guidelines (19). Survival
distributions were estimated with the Kaplan-Meier method and
compared with the log-rank test. Student’s t-test, χ2
and Fisher’s exact tests were used to analyze the differences
between groups. Data are presented as the mean ± the standard
error, and a P<0.05 was considered to be statistically
significant.
Results
BTG1 protein expression in normal tissue
and thyroid cancer
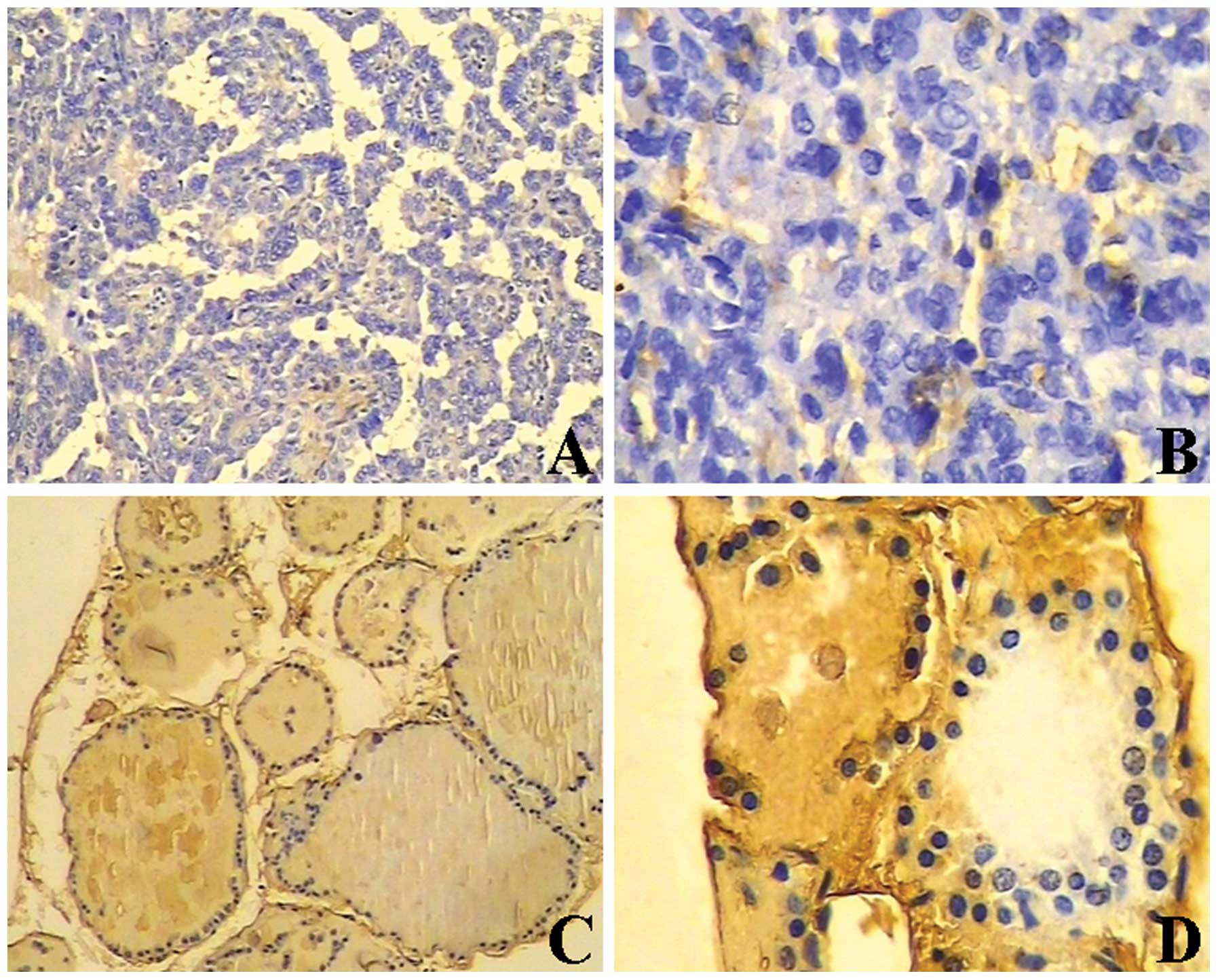
Immunohistochemistry for BTG1 revealed light yellow
to brown staining in 80.0% (28/35) of normal thyroid tissues, and
negative or weak staining in 36.1% (30/83) thyroid cancer tissues
(P<0.05) (Table I, Fig. 1). Furthermore, BTG1 protein
expression was significantly lower in cancer lesion samples
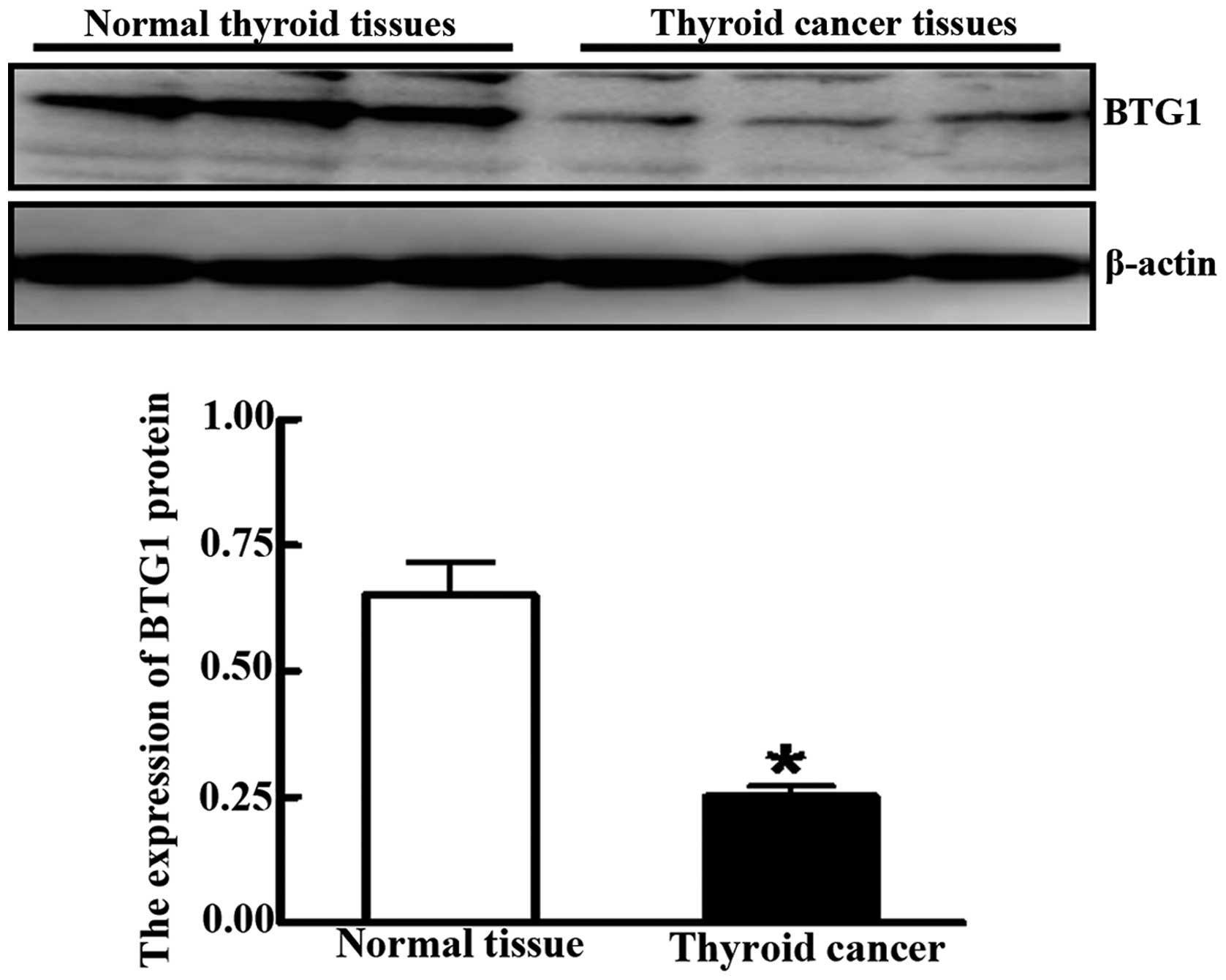
compared to adjacent normal tissue, as determined by western blot
analysis (0.251±0.021 vs. 0.651±0.065; P<0.05) (Fig. 2). BTG1 expression levels correlated
with lymph node metastasis, clinical stage and pathological
differentiation (P<0.05), regardless of age, gender, tumor size
and pathological types (P>0.05) (Table II).
 | Table IExpressions of BTG1 in normal and
cancerous thyroid tissue. |
Table I
Expressions of BTG1 in normal and
cancerous thyroid tissue.
| | Expression of BTG1
protein |
|---|
| |
|
|---|
| Group | Case | − | + | ++ | +++ | χ2 | P-value |
|---|
| Normal tissue | 35 | 7 | 6 | 13 | 9 | 22.721 | 0.000 |
| Cancer tissue | 83 | 53 | 13 | 10 | 7 | | |
 | Table IIThe relationship between BTG1
expression and thyroid cancer characteristics. |
Table II
The relationship between BTG1
expression and thyroid cancer characteristics.
| | Expression of BTG1
protein |
|---|
| |
|
|---|
| Group | Case | − | + to +++ | χ2 | P-value |
|---|
| Gender |
| Male | 23 | 15 | 8 | 0.026 | 0.873 |
| Female | 60 | 38 | 22 | | |
| Age (years) |
| ≤40 | 63 | 40 | 23 | 0.015 | 0.903 |
| >40 | 20 | 13 | 7 | | |
| Pathological
types |
| Papillary
cancer | 46 | 29 | 17 | 0.845 | 0.839 |
| Follicular
cancer | 22 | 13 | 9 | | |
| Medullary
cancer | 11 | 8 | 3 | | |
| Undifferentiated
cancer | 4 | 3 | 1 | | |
| Tumor length
(cm) |
| ≤4 | 31 | 18 | 13 | 0.719 | 0.396 |
| >4 | 52 | 35 | 17 | | |
| Lymph node
metastasis |
| N0 | 28 | 12 | 16 | 8.072 | 0.004 |
| N+ | 55 | 41 | 14 | | |
| Clinical
stages |
| I–II | 25 | 10 | 15 | 8.821 | 0.003 |
| III–IV | 58 | 43 | 15 | | |
| Histological
grade |
| I | 27 | 11 | 16 | 9.264 | 0.002 |
| II–III | 56 | 42 | 14 | | |
BTG1 expression and prognosis
Follow-up examinations were performed on cancer
patients for up to 120 months, with 36 patients remaining at the
conclusion of the study. Overall survival (OS) rates were
determined between patients positive for BTG1 expression and those
negative for expression. Sixteen of the 53 individuals showing no
BTG1 expression remained at the conclusion of the study, with an OS
rate of 30.2%. Patients positive for BTG1 expression had a
significantly higher OS rate of 66.7% (20/30) (P<0.05) (Fig. 3).
Stable transfection of BTG1 in thyroid
cancer cells
BTG1 overexpressing FTC-133 cells (named LeBTG1)
were obtained by a stable transfection of BTG1 cDNA, and compared
with FTC-133 cells overexpressing an empty vector (named LeEmpty)
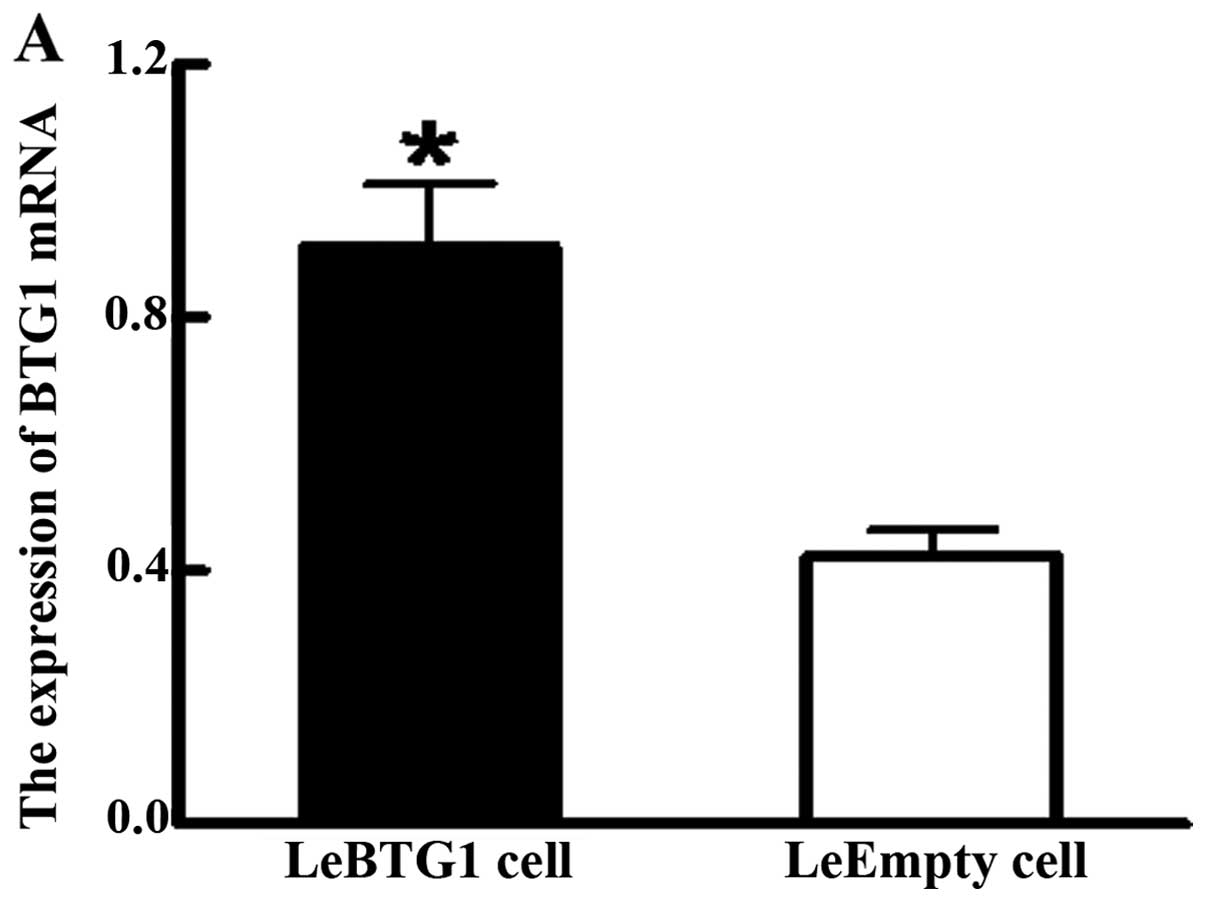
as a control. Analysis of qRT-PCR data showed LeBTG1 cells had a
significantly higher expression of BTG1 mRNA compared to LeEmpty
cells (0.912±0.097 vs. 0.423±0.042; P<0.05) (Fig. 4A). Furthermore, western blot
analysis showed that LeBTG1 cells had a significantly higher
expression of BTG1 protein compared to LeEmpty cells (0.873±0.086
vs. 0.395±0.042; P<0.05) (Fig.
4B).
Cellular effects of BTG1
overexpression
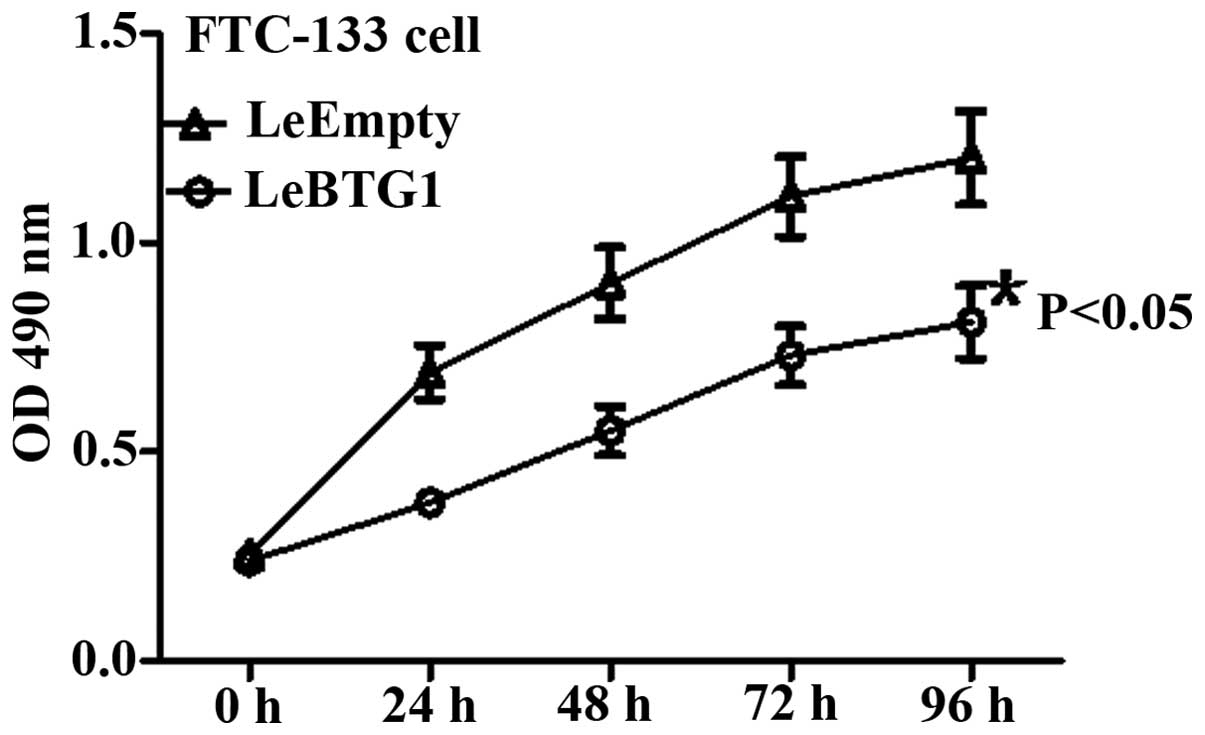
LeBTG1 cells had a significantly lower viability at
24, 48, 72 and 96 h compared to LeEmpty cells as assessed by the
MTT assay (P<0.05) (Fig. 5).
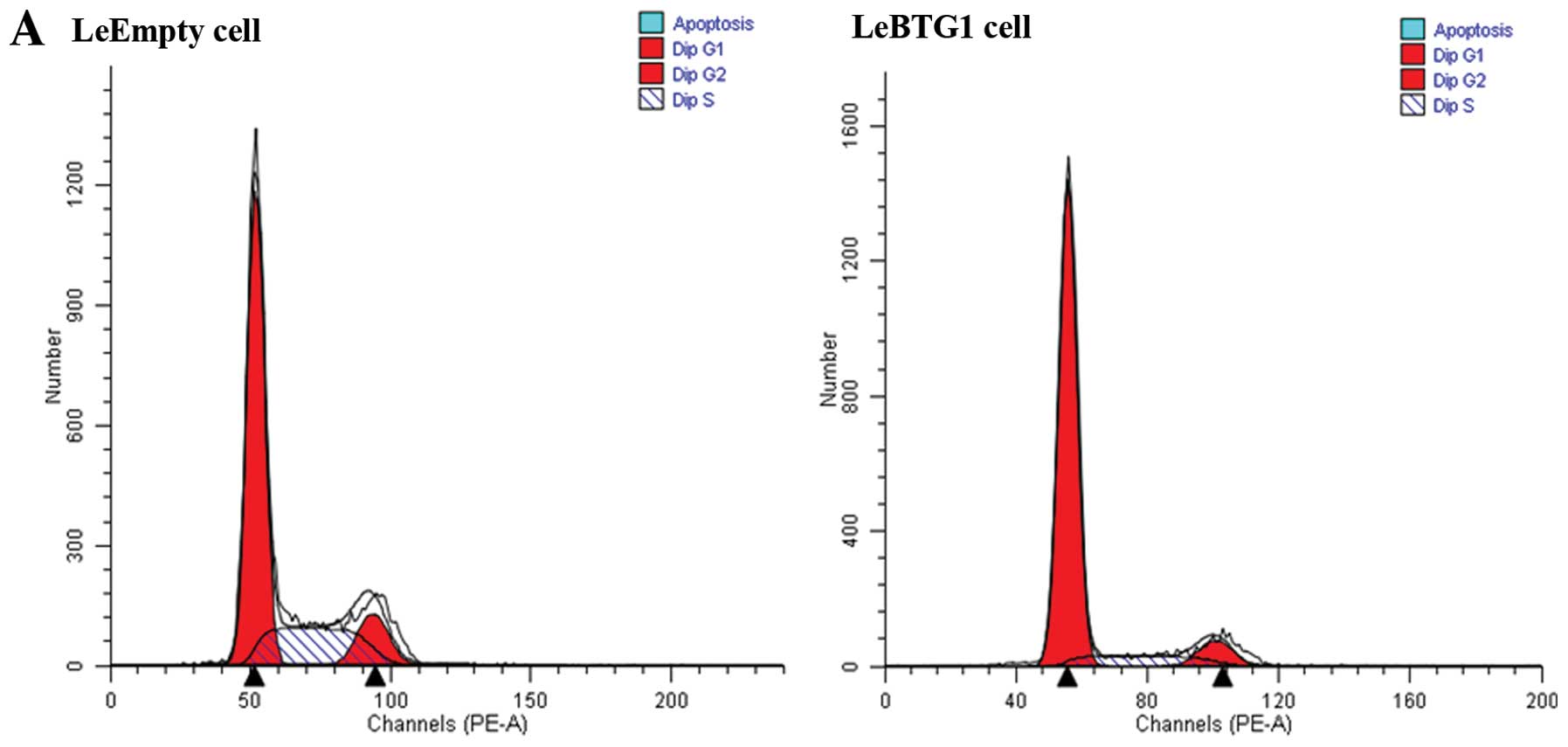
Cell cycle analysis using flow cytometry showed that the proportion
of LeBTG1 cells in G0/G1 and S phases of the cell cycle were
significantly different compared to the control LeEmpty cells
(81.8±6.3 and 10.2±1.0%, vs. 62.4±4.9 and 25.5±2.6%, respectively;
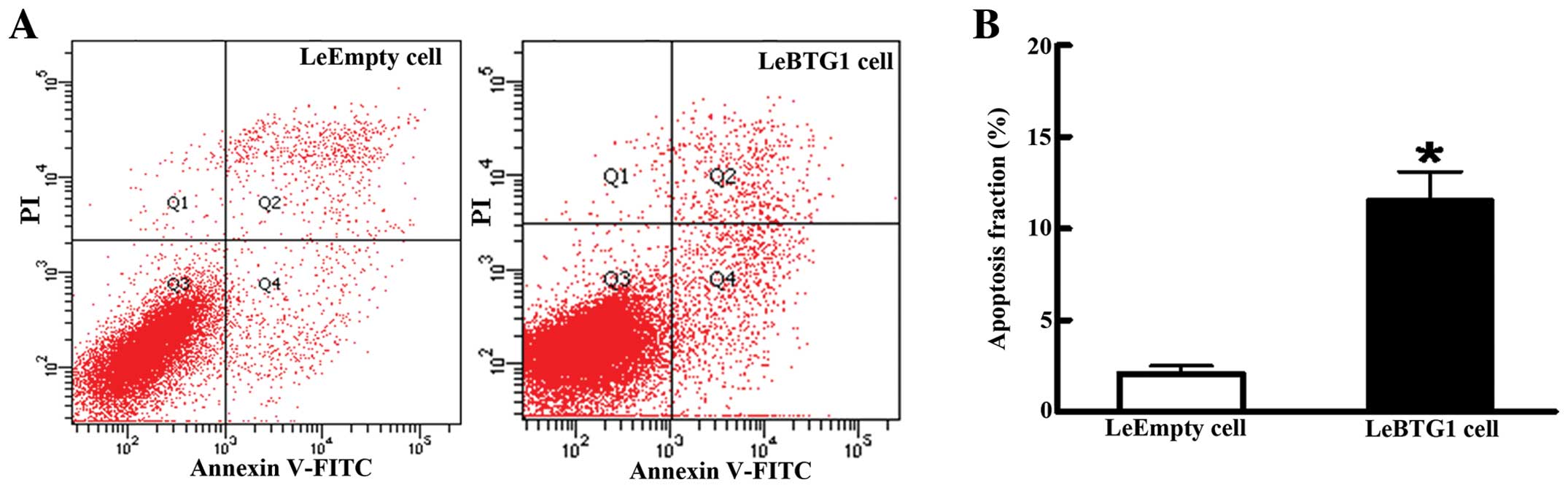
P<0.05) (Fig. 6). In addition,
there was a large increase in the early apoptosis rate in LeBTG1
cells compared to control LeEmpty cells (11.6±2.1 vs. 2.1±0.4%;
P<0.05) (Fig. 7). Furthermore,
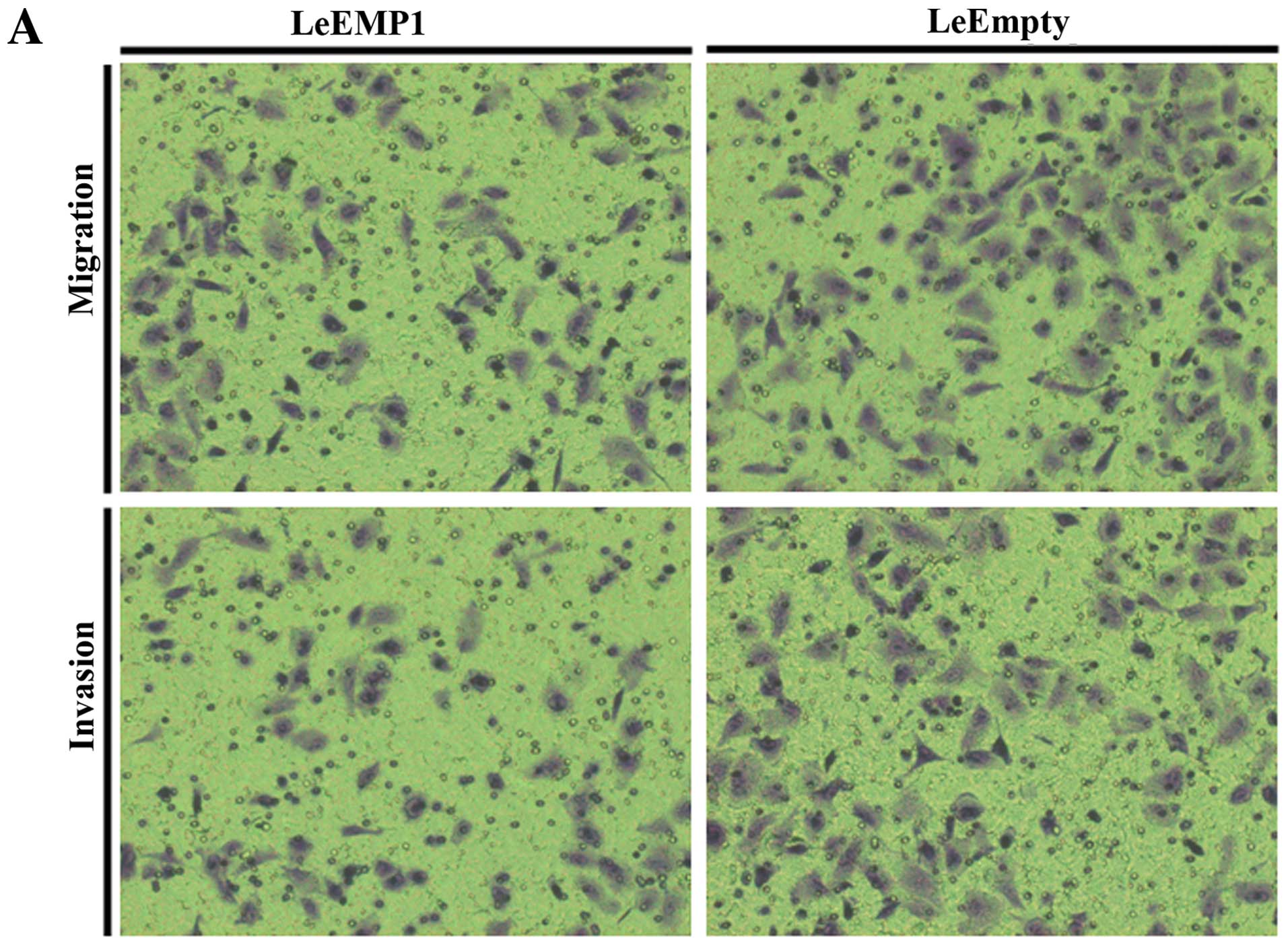
LeBTG1 cells had a reduced capability for invasion and migration
through Transwell inserts (72.0±8.0 and 55.0±7.0, respectively)
compared to control LeEmpty cells (113.0±16.0 and 89.0±9.0,
respectively; P<0.05) (Fig.
8).
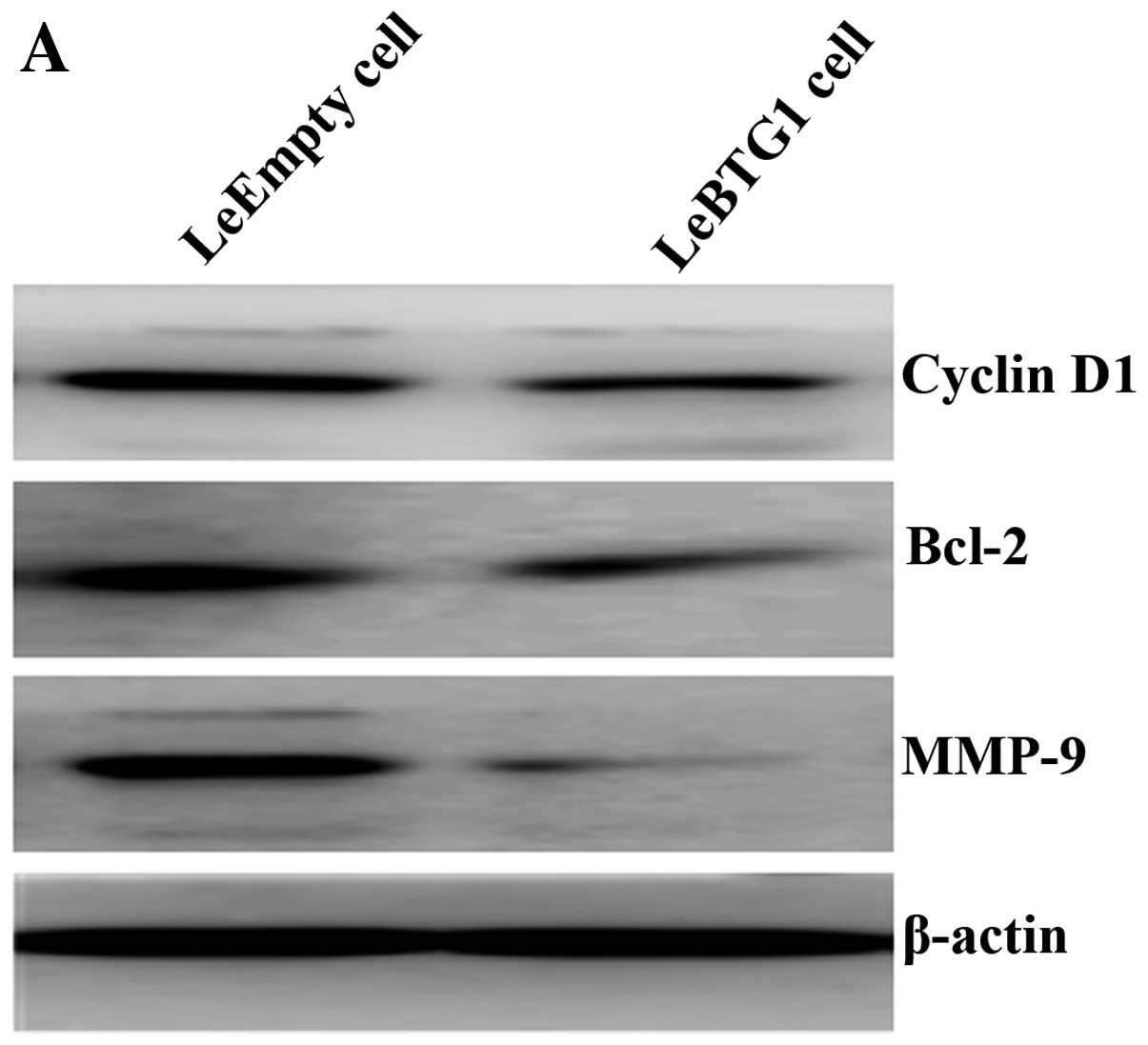
To further identify the mechanisms by which BTG1
overexpression regulated these cellular changes in cancer cells,
expression levels of proteins critical for the regulation of cell
cycle, apoptosis and migration, were examined. Western blot
analysis revealed that LeBTG1 cells had significantly reduced
levels of cyclin D1, Bcl-2 and MMP-9 (0.234±0.018, 0.209±0.021 and
0.155±0.017, respectively) compared to control LeEmpty cells
(0.551±0.065, 0.452±0.043 and 0.609±0.072, respectively; P<0.05)
(Fig. 9).
Discussion
Tumor development and progression are associated
with uncontrolled proliferation and reduced apoptosis of tumor
cells. BTG1 has been show to act as a tumor suppressor gene in
breast cancer, by inhibiting proliferation, regulating cell cycle
and inducing apoptosis (20). This
study examined BTG1 protein expression in thyroid cancer tissue and
showed that levels were significantly lower and were correlated
with lymph node metastasis, clinical stage and cancer
differentiation. Current studies suggest that tumor stage is the
preferred prognostic indicator (21), though prognoses can still vary
considerably among patients in the same stage. Therefore, it is of
particular importance to identify reliable molecular markers for
use in clinical practice. The results of this study suggest that
BTG1 deletion is a major contributor to the development and
progression of thyroid cancer. As expression-positive patients had
a significantly higher 10-year overall survival rate, BTG1 may be a
useful prognostic indicator for patients with thyroid cancer. The
combination of the tumor-node-metastasis classification system and
BTG1 expression scores may provide some valuable information for
clinicians in choosing treatment options, and for predicting
disease severity and prognosis.
The development of thyroid cancer is driven by the
abnormal proliferation of cells that normally undergo apoptosis
(22). Recent studies (20) have indicated that overexpression of
BTG1 can affect the cell cycle and suppress tumor growth. This
study utilized in vitro tests to confirm that thyroid cancer
cells with high BTG1 expression had significantly weakened
viability and proliferation potential. In addition, BTG1
overexpression resulted in decreased protein levels of cyclin D1,
which is considered to be a proto-oncogene product that is highly
expressed or mutated in a variety of human tumors (23). The increased BTG1 expression in
thyroid cancer FTC-133 cells resulted in a higher proportion of
cells in the G0/G1 phase, suggesting the occurrence of G0/G1 arrest
and inhibition of growth. Taken together, the data implicate BTG1
in cell cycle regulation and cyclin D1 expression.
In this study, increased BTG1 expression in thyroid
cancer cells reduced the amount of the anti-apoptotic protein Bcl-2
and induced apoptosis. Apoptosis is a programmed death process that
involves a series of changes in relevant genes, including Bcl-2 and
caspase family genes, oncogenes such as C-myc, and the tumor
suppressor gene p53 (24), and is
regulated by numerous internal and external factors (25). These results are in agreement with
previous work linking BTG1 expression with apopotosis. Corjay et
al demonstrated a high level of BTG1 expression in apoptotic
cells within macrophage-rich tissues in patients with hereditary
hyperlipidemia (26), and Lee
et al showed that BTG1 could induce apoptosis in glioma
cells (27). Moreover, a study by
Nahta et al found that apoptosis in breast cancer MCF7 cells
induced by knockdown of Bcl-2 was regulated by BTG1 expression
(28). Therefore, the evidence
suggests that BTG1 can inhibit the growth of thyroid cancer cells
by reducing Bcl-2 expression.
Tumor invasion and metastasis are major causes for
treatment failures, thus the ultimate research goals are to
identify the molecular mechanisms underlying metastasis and target
key pathways that inhibit this process. Tumor invasion and
metastasis share common molecular mechanisms and involve a number
of changes in tumor cells and the microenvironment, including
altered tumor cell adhesion properties, enhanced proliferation,
survival, chemotaxis and migration of tumor cells,
lymphangiogenesis, evasion of immune attack, and hydrolysis of
surrounding matrix proteins (29).
A key step in tumor invasion and clonal growth is the remodeling of
the extracellular matrix and basement membranes through proteolytic
degradation by MMPs, which are highly expressed by tumor cells with
malignant, invasive and metastatic phenotypes. Moreover, the degree
of malignancy and patient prognosis are associated with excessive
expression of MMP-2 and MMP-9 (30,31).
Tumor cells can also regulate the expression of MMPs produced by
stromal cells by secreting chemokines, cytokines and extracellular
MMP inducer, a cell surface glycoprotein. This study showed that
BTG1-overexpressing thyroid cancer cells had decreased MMP-9
protein levels and reduced invasion and migration in vitro,
suggesting that BTG1 could modulate tumor cell metastasis by
downregulating MMP-9 expression.
In conclusion, this study provides clinical and
in vitro evidence implicating BTG1 in the development and
progression of thyroid cancer. The results show that BTG1 protein
levels were significantly reduced in thyroid cancer biopsy
specimens and were associated with disease progression and
prognosis. Furthermore, the effects of BTG1 expression on the
regulation of cancer cell proliferation, apoptosis, invasion and
metastasis suggest that BTG1 expression may serve as a prognostic
marker for thyroid cancer patients.
References
|
1
|
Agathocleous M and Harris WA: Metabolism
in physiological cell proliferation and differentiation. Trends
Cell Biol. 23:484–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hofmockel G: Molecular genetic principles
of tumor development and progression. Urologe A. 39:212–213.
2000.(In German).
|
|
3
|
Shibata D and Aaltonen LA: Genetic
predisposition and somatic diversification in tumor development and
progression. Adv Cancer Res. 80:83–114. 2001. View Article : Google Scholar
|
|
4
|
Lee EY and Muller WJ: Oncogenes and tumor
suppressor genes. Cold Spring Harb Perspect Biol.
2:a0032362010.PubMed/NCBI
|
|
5
|
Okuyama T, Maehara Y, Kabashima A,
Takahashi I, Kakeji Y and Sugimachi K: Combined evaluation of
expressions of p53 and p21 proteins as prognostic factors for
patients with gastric carcinoma. Oncology. 63:353–361. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vadgama JV, Scuric Z, Chakrabarti R, Marzo
E, Shen D and Wu Y: Insulin-like growth factor I differentially
regulates the expression of HIRF1/hCAF1 and BTG1 genes in human
MCF-7 breast cancer cells. Int J Mol Med. 18:129–139.
2006.PubMed/NCBI
|
|
7
|
Cortes U, Moyret-Lalle C, Falette N,
Duriez C, Ghissassi FE, Barnas C, Morel AP, Hainaut P, Magaud JP
and Puisieux A: BTG gene expression in the p53-dependent and
-independent cellular response to DNA damage. Mol Carcinog.
27:57–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winkler GS: The mammalian
anti-proliferative BTG/Tob protein family. J Cell Physiol.
222:66–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rouault JP, Rimokh R, Tessa C, Paranhos G,
Ffrench M, Duret L, Garoccio M, Germain D, Samarut J and Magaud JP:
BTG1, a member of a new family of antiproliferative genes. EMBO J.
11:1663–1670. 1992.PubMed/NCBI
|
|
10
|
Rouault JP, Falette N, Guéhenneux F,
Guillot C, Rimokh R, Wang Q, Berthet C, Moyret-Lalle C, Savatier P,
Pain B, Shaw P, Berger R, Samarut J, Magaud JP, Ozturk M, Samarut C
and Puisieux A: Identification of BTG2, an antiproliferative
p53-dependent component of the DNA damage cellular response
pathway. Nat Genet. 14:482–486. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuda S, Rouault J, Magaud J and Berthet
C: In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS
Lett. 497:67–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bozec A, Peyrade F and Milano G: Molecular
targeted therapies in the management of head and neck squamous cell
carcinoma: recent developments and perspectives. Anticancer Agents
Med Chem. 13:389–402. 2013.PubMed/NCBI
|
|
13
|
Suzuki K, Nakamura K, Kato K, Hamada H and
Tsukamoto T: Exploration of target molecules for prostate cancer
gene therapy. Prostate. 67:1163–1173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turashvili G, Bouchal J, Ehrmann J,
Fridman E, Skarda J and Kolar Z: Novel immunohistochemical markers
for the differentiation of lobular and ductal invasive breast
carcinomas. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
151:59–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ranganathan V and De PK: Western blot of
proteins from Coomassie-stained polyacrylamide gels. Anal Biochem.
234:102–104. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Meerloo J, Kaspers GJ and Cloos J:
Cell sensitivity assays: the MTT assay. Methods Mol Biol.
731:237–245. 2011.PubMed/NCBI
|
|
17
|
Rasola A and Geuna M: A flow cytometry
assay simultaneously detects independent apoptotic parameters.
Cytometry. 45:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kramer N, Walzl A, Unger C, Rosner M,
Krupitza G, Hengstschläger M and Dolznig H: In vitro cell
migration and invasion assays. Mutat Res. 752:10–24. 2013.
View Article : Google Scholar
|
|
19
|
Richards RJ: Responsibility for
statistical analyses. Endocr Pract. 9:3292003.PubMed/NCBI
|
|
20
|
Zhu R, Zou ST, Wan JM, Li W, Li XL and Zhu
W: BTG1 inhibits breast cancer cell growth through induction of
cell cycle arrest and apoptosis. Oncol Rep. 30:2137–2144.
2013.PubMed/NCBI
|
|
21
|
Manjili MH, Najarian K and Wang XY:
Signatures of tumor-immune interactions as biomarkers for breast
cancer prognosis. Future Oncol. 8:703–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martinez-Outschoorn UE, Pavlides S, Sotgia
F and Lisanti MP: Mitochondrial biogenesis drives tumor cell
proliferation. Am J Pathol. 178:1949–1952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koff A, Cross F, Fisher A, Schumacher J,
Leguellec K, Philippe M and Roberts JM: Human cyclin E, a new
cyclin that interacts with two members of the CDC2 gene family.
Cell. 66:1217–1228. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tirone F: The gene PC3(TIS21/BTG2),
prototype member of the PC3/BTG/TOB family: regulator in control of
cell growth, differentiation, and DNA repair? J Cell Physiol.
187:155–165. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicholson DW and Thornberry NA: Apoptosis.
Life and death decisions. Science. 299:214–215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Corjay MH, Kearney MA, Munzer DA, Diamond
SM and Stoltenborg JK: Antiproliferative gene BTG1 is highly
expressed in apoptotic cells in macrophage-rich areas of advanced
lesions in Watanabe heritable hyperlipidemic rabbit and human. Lab
Invest. 78:847–858. 1998.
|
|
27
|
Lee H, Cha S, Lee MS, Cho GJ, Choi WS and
Suk K: Role of antiproliferative B cell translocation gene-1 as an
apoptotic sensitizer in activation-induced cell death of brain
microglia. J Immunol. 171:5802–5811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nahta R, Yuan LX, Fiterman DJ, Zhang L,
Symmans WF, Ueno NT and Esteva FJ: B cell translocation gene 1
contributes to antisense Bcl-2-mediated apoptosis in breast cancer
cells. Mol Cancer Ther. 5:1593–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wiseman BS and Werb Z: Stromal effects on
mammary gland development and breast cancer. Science.
296:1046–1049. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alok C and Bharat B: Nuclear factor-kappa
Band cancer: its role in prevention and therapy. Biochem Phamacol.
64:883–888. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Virós D, Camacho M, Zarraonandia I, García
J, Quer M, Vila L and León X: Prognostic role of MMP-9 expression
in head and neck carcinoma patients treated with radiotherapy
orchemoradiotherapy. Oral Oncol. 49:322–325. 2013.PubMed/NCBI
|