Introduction
Prostate cancer is second to lung cancer in
incidence world-wide, and it is the third most common cause of
cancer deaths in developed countries (age-standardised rates in
2008) (1). The majority of
patients with advanced prostate cancer will develop bone
metastases, and these metastases represent a significant source of
morbidity and mortality, resulting in a number of clinical
complications of metastatic skeletal lesions that may be present in
up to 80% of cases, including pain, bone loss, fracture,
hypercalcemia and bone marrow replacement (2–4).
Moreover, the 5-year survival rate among prostate cancer patients
with bone metastases continues to be less than 50% (4). Despite the frequency of prostate
carcinogenesis and skeletal metastases, the molecular mechanisms
for their propensity to colonise bone are poorly understood and
treatment options are often unsatisfactory. For nearly 70 years,
endocrine therapy has been the mainstay of treatment, besides
surgery, for patients with early-stage prostate cancer. In general,
40–80% of prostate cancers respond to hormonal therapy, but most of
these tumors will progress to androgen-independent states within
2–3 years (5), and then the tumors
recur in an androgen-independent form that is unresponsive to
additional androgen withdrawal (6). Therefore, there is an urgent need to
elucidate its molecular mechanisms to establish effective therapies
and to allow early detection and treatment strategies.
Ubiquitin-proteasome system is accountable for
regulation of numerous basic cellular processes such as the cell
cycle progression, signal transduction, proliferation, apoptosis,
modulation of surface receptors and regulation of tumor suppression
proteins (7). The
ubiquitin-proteasome pathway is essential to cellular homeostasis
and is considered to be an important regulator of multiple
metabolic processes such as osteoblast differentiation and bone
formation (8).
Ubiquitin-proteasome system dysfunction, with demonstrated
contribution to the development of cancer, renders it a feasible
and rational target for novel therapies (9). Numerous E3 ubiquitin ligases in the
ubiquitin proteasome pathway have been implicated in cell cycle
control and uncontrolled cell proliferation. The neural precursor
cell-expressed developmentally downregulated gene 4 (Nedd4) family
of ubiquitin ligases (E3s) is characterized by a distinct modular
domain architecture, with each member consisting of a C2 domain,
2–4 WW domains, and a HECT-type ligase domain (10). In Nedd4 family, smad ubiquitination
regulatory factors (smurfs) are negative regulators of the
transforming growth factor (TGF) signal-transduction cascade, which
has antineoplastic activity in prostate cancer, indicating a
cancer-fostering role of smurfs (11). The gene for WW domain containing E3
ubiquitin protein ligase 1 (WWP1), another member of Nedd4 family
is located at 8q21, a region frequently amplified in human cancers,
including prostate cancer. Recent studies have shown that WWP1
negatively regulates the TGFβ tumor suppressor pathway by
inactivating its molecular components, including Smad2, Smad4 and
TbR1 (12). However, there has not
been a systemic elaboration of the relationship between
transcription and expression levels of WWP1, Smurf1 and Smurf2
genes and the oncogenesis and bone metastasis of prostate
cancer.
Proteasome in ubiquitin-proteasome system plays a
central role in regulation of the cell cycle, proliferation, cell
death, angiogenesis, metastasis and resistance to chemotherapy and
radiation therapy (13). By
controlling the levels of numerous transcription factors, tumor
suppressors, anti-apoptotic proteins, and other signaling
molecules, proteasome has been shown to be vital for cell cycle
progression as well as programmed cell death (4). Proteasome inhibitors have presented
cytotoxicity against a wide range of tumor cell lines, and these
drugs are thought to impede tumor generation and growth by several
different mechanisms. By limiting the degradation of various cell
cycle regulatory proteins, proteasome inhibitors may induce mitotic
arrest and apoptosis (14,15). As demonstrated in numerous
experiments, proteasome inhibition could be a novel method for
treating androgen-dependent and androgen-independent prostate
cancer (13). Bortezomib (formerly
known as PS-341) is a dipeptidyl boronic acid compound that acts as
an exceedingly potent and selective proteasome inhibitor. By
blocking the ubiquitin-proteasome pathway, bortezomib may inhibit
the degradation of various regulatory proteins and transcription
factors that are involved in cell division, apoptosis, cellular
adhesion, angiogenesis and NF-κB activation, finally leading to the
arrest of tumor growth and metastasis. Bortezomib has also been
demonstrated to arrest cell cycle progression and induce apoptosis
in cultured human prostate cancer cells as well as inhibit the
growth of prostate cancer xenografts in a murine model (14,16).
It was implicated in other clinical studies that bortezomib
promotes osteoblastogenesis and inhibits bone resorption in
patients with multiple myeloma (17–19).
The specific mechanism of bortezomib in treating prostate cancer
and its bone metastasis is still not fully understood, therefore,
the connection with the levels of WWP1, Smurf1 and Smurf2 were
investigated.
In the present study, we detected the transcription
and expression levels of WWP1, Smurf1 and Smurf2 genes in cell
lines and tissues of benign prostate hyperplasia and human prostate
cancer with and without bone metastasis, and came to the conclusion
that increased transcription and expression levels of ubiquitin
ligase E3s WWP1, Smurf1 and Smurf2 genes are involved in the
mechanism of occurrence, development and metastasis of prostate
cancer. Furthermore, by testing the proliferation levels of human
prostate cancer PC3 cell lines treated by bortezomib with different
concentration gradients, we demonstrated that bortezomib can
prevent prostate cancer and its bone metastasis by reducing WWP1,
Smurf1 and Smurf2.
Materials and methods
Ethics statement
This study was conducted according to the principles
expressed in the Declaration of Helsinki and has been approved by
Ethics Committee of Hebei Medical University. The participants
provided their written informed consent to participate in this
study.
Clinical materials and grouping
Forty-five cases of patients diagnosed with prostate
cancer by histopathological examination and hospitalized in the
Fourth Hospital of Hebei Medical University from July, 2011 to
February, 2013 were enrolled. Age range of the patients was from 52
to 88 years and the average was 67.82 years. Whole body bone scan
with Emission Computed Tomography was performed on all the cases,
if bone lesions existed, bone biopsy was further employed to
determine whether bone metastasis had occurred. The cases were
divided into bone metastasis group and non-bone metastasis group.
Twenty-one cases were in the former group, whose age range was from
53 to 88 years with an average of 68.16 years, while the other 24
cases were in the latter group, age range was from 52 to 85 years
with an average of 65.23 years. In addition, 25 patients that were
diagnosed with benign prostate hyperplasia by histopathological
examination after transurethral resection of prostate treatment in
the same hospital were enrolled as control group. The age range of
the control group was from 52 to 86 years with an average of 66.96
years.
Immunohistochemistry
Prostate tissues from three groups were performed
into serial sections with a thickness of 3 μm, dewaxed, placed into
citric acid solution at 27°C to get antigen retrieval, added with
3% hydrogen peroxide to scavenge endogenous peroxidases, and then
washed with PBS. Samples from each group were divided into 3 parts.
Subsequently, rabbit anti-human WWP1 polyclonal antibody, rabbit
anti-human Smurf1 polyclonal antibody and rabbit anti-human Smurf2
polyclonal antibody (all from Santa Cruz Biotechnology Co., Ltd.,
Shanghai, China) were, respectively, added into each sample from
the 3 groups, co-incubated with the samples overnight and then
washed with PBS. Afterwards, goat anti-rabbit IgG (Santa Cruz
Biotechnology) was added to each part, co-incubated with the
complexes at 37°C for 30 min and then washed with PBS. Finally,
color was developed with DAB kit (Biosynthesis Biotechnology Co.,
Ltd., Beijing, China) according to its specifications. The negative
control group was set without rabbit anti-human WWP1, Smurf1 or
Smurf2 polyclonal antibody. After dehydration and sealing sheets,
the samples were observed under a microscope. Image-Pro Plus image
analysis system was applied to analyze the images and measure the
integral optical density (IOD) values of ubiquitin ligase E3s.
Real-time quantitative PCR
The mRNA levels of WWP1, Smurf1, Smurf2 in prostate
cancer PC3 cells were tested with RT-PCR, as previously described
(20). The expansion effects of
WWP1, Smurf1 and Smurf2 were analyzed by ΔΔCt method, and by
standardization via β-actin the results were expressed by
2−ΔΔCT. RNAiso Reagent [Takara Biotechnology (Dalian)
Co., Ltd., Shanghai, China] was employed according to its
specifications to extract RNAs from prostate cancers and the purity
of the extracting solution was tested with an ultraviolet
spectrophotometer. In the progress of reverse transcription, the
RNase-free DNase and M-MLV kit (both from Promega Corporation, WI,
USA) were applied in accordance with their specifications. The
primers were designed by reference to GeneBank database and
AlleleID4.0 software. β-actin was chosen as internal reference
upstream: 5′-AGCTCTGCTGGTAGGTGCAC-3′ and downstream:
5′-GCTACGACCATCTAGGCACG-3′. The primers of WWP1 were: primer 1
forward, 5′-TGGCATTGGAAAGAAGACG-3′ and primer 2 reverse,
5′-GTTGTGGTCTCTCCCATGT GGT-3′. The primers of Smurf1 were: primer 1
forward, 5′-GTCCAGAAGCTGAAAGTCCTCAGA-3′ and primer 2 reverse,
5′-CACGGAATTTCACCATCAGCC-3′. The primers of Smurf2 were primer 1
forward, 5′-GATCCAAAGTG GAATCAGCA-3′ and primer 2 reverse,
5′-TGGCATTGGAAA GAAGACG-3′; The primers of β-actin were primer 1
forward, 5′-TCTTCCAGCCTTCCTTCCTG-3′ and primer 2 reverse,
5′-TAGAGCCACCAATCCACACA-3′. SYBR-Green I dye method was employed
using real-time quantitative PCR with Ex Tap kit (Takara
Biotechnology) by fluorescence quantitative real-time PCR system
(type ABI 7900, Applied Biosystems, CA, USA), according to the
specifications provided by the manufacturer. The reaction
conditions were as follows: predegeneration for 5 min at 93°C,
degeneration for 30 sec at 93°C, annealing for 1 min at 55°C and
then elongation for 1 min at 71°C, after 30 cycles, another
elongation for 1 min at 71°C. IQ5 software was used to analyze the
readings.
Prostate cancer PC3 cells culture
Under strict aseptic conditions, prostate cancer PC3
cells [American Type Culture Collection (ATCC), Manassas, VA, USA]
were cultured in a 60 ml plastic culture bottle with DMEM medium
(Biohermes Biomedicine and Tecnology Co., Ltd., Boston, MA, USA)
and culture solution was replaced each day. As soon as the cells
formed a dense single wall, they were digested with 0.25%
trypsinase (Gibco Corporation, CA, USA) and subcultured with the
density of 5×105 cells/ml. After 3–5 generations, cells
were used to conduct the experiments.
Brdu ELISA
Prostate cancer PC3 cells were cultured in the
24-well microtiter plates at a concentration of 1×105
cells/ml, bortezomib (Millennium Pharmaceuticals, Inc., MA, USA) at
diverse concentrations (0.1, 1, 10 and 50 μmol/l) were,
respectively, added into different wells. A negative control group
was set without bortezomib. Then 10 μl of 10X Brdu (Hoffmann-La
Roche Inc., Basel, Switzerland) was put into each well,
respectively, at 24, 48 and 72 h. After incubation at 37°C in an
incubator for 72 h, the cells were centrifuged, then supernatant
was discarded. A total of 100 μl of the liquid was put into each
well, and then the cells were incubated at 37°C for 30 min and
washed with PBS several times. Subsequently, 100 μl anti-Brdu-POP
antibody (Hoffmann-La Roche) was added into each well, then the
cells were incubated at 25°C for 60 min and centrifuged, then the
supernatant was discarded. The cells were washed with PBS 3 times ×
5 min, and 100 μl of ECL chromogenic substrate (Thermo Fisher
Scientific Inc., Tokyo, Japan) was added into each well, and then
incubated at 25°C for 10 min. Sulfuric acid (25 μl) with a
concentration of 1 mol/l was put into each well, then optical
density (OD) levels were detected at 450 nm with enzyme-labeling
instrument (ELx808, BioTek Instruments, Inc., Beijing, China) and
cell viability was indicated by OD levels.
Western blot experiment
The western blot experiment were performed in a
routine manner, as previously reported (21). Concentrations of protein were
tested with BCA Protein Assay Reagent (Thermo Fisher Scientific),
and electrophoresis was performed with SDS-PAGE. The voltage of
stacking gel was 80 V while that of separation gel was 100 V.
Half-dry transfer was conducted under a voltage of 20 V. After
washing with TBST and closing, the cells were co-incubated with
first antibody of WWP1, Smurf1, Smurf2, respectively, at 4°C
overnight. After washing 3 times with TBST, the cells were
co-incubated with the second antibody. Color development was
performed by chemiluminescence method, and then optical density
analysis of the bands was made by Gel-Pro analyzer 4.0 software.
The results are expressed by the ratios compared with the optical
density of β-actin.
Statistical analysis
SPSS 18.0 software package was used for statistical
analysis and data are expressed as the average ± standard
deviation. Multiple-group comparisons were made by one-way ANOVA
while 2-group comparisons by SNK t-test.
Results
Expression levels of WWP1, Smurf1 and
Smurf2
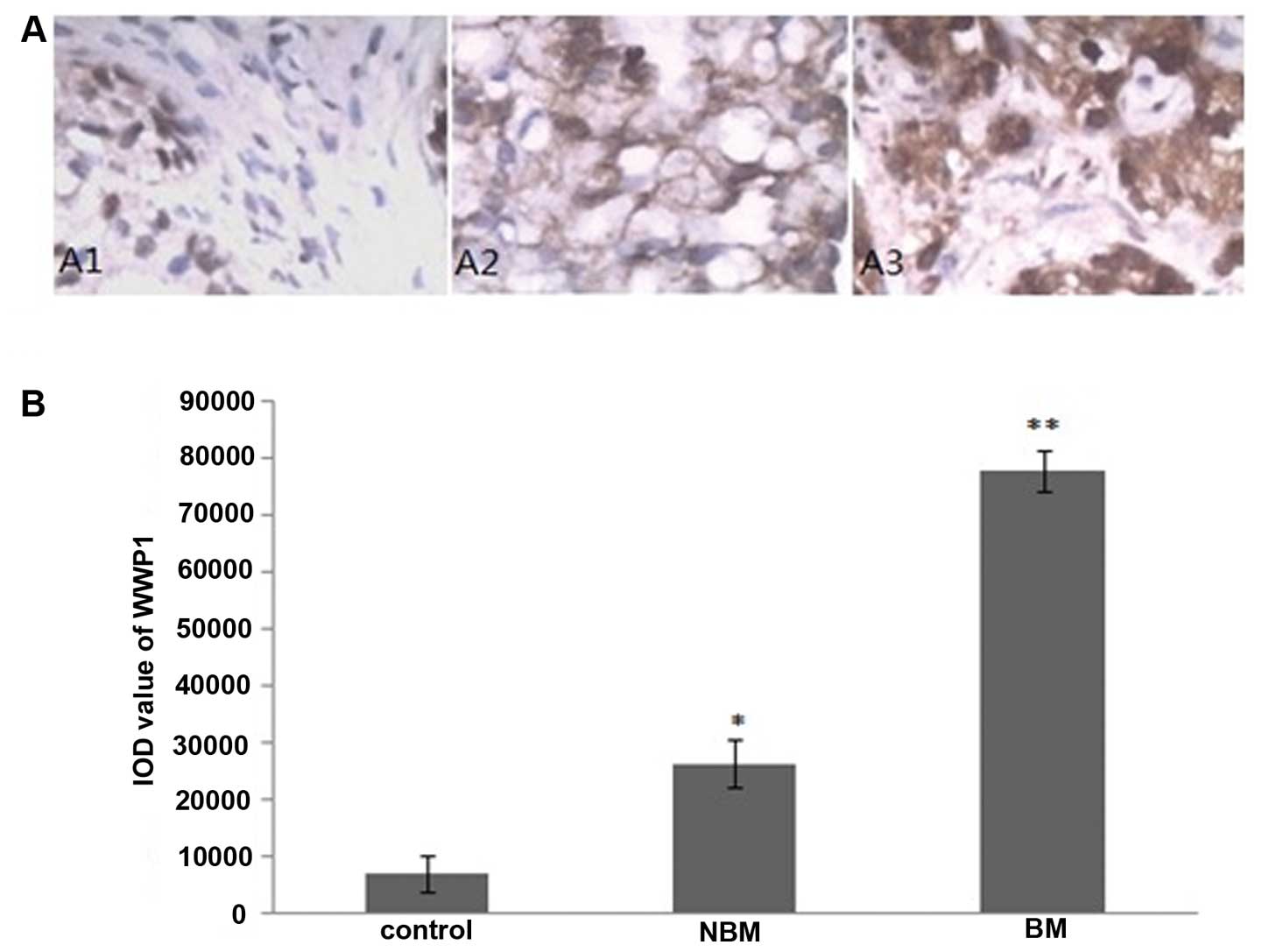
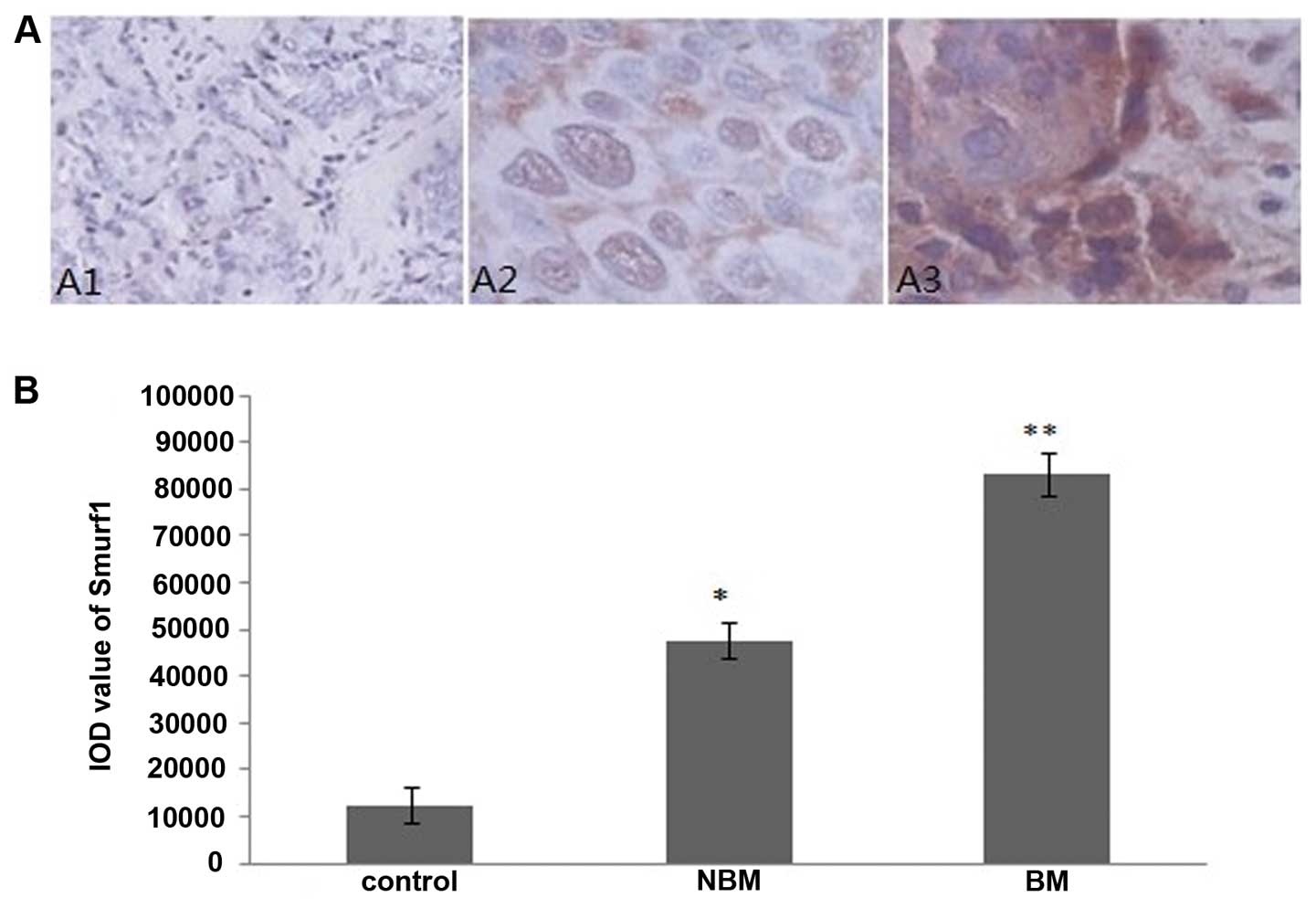
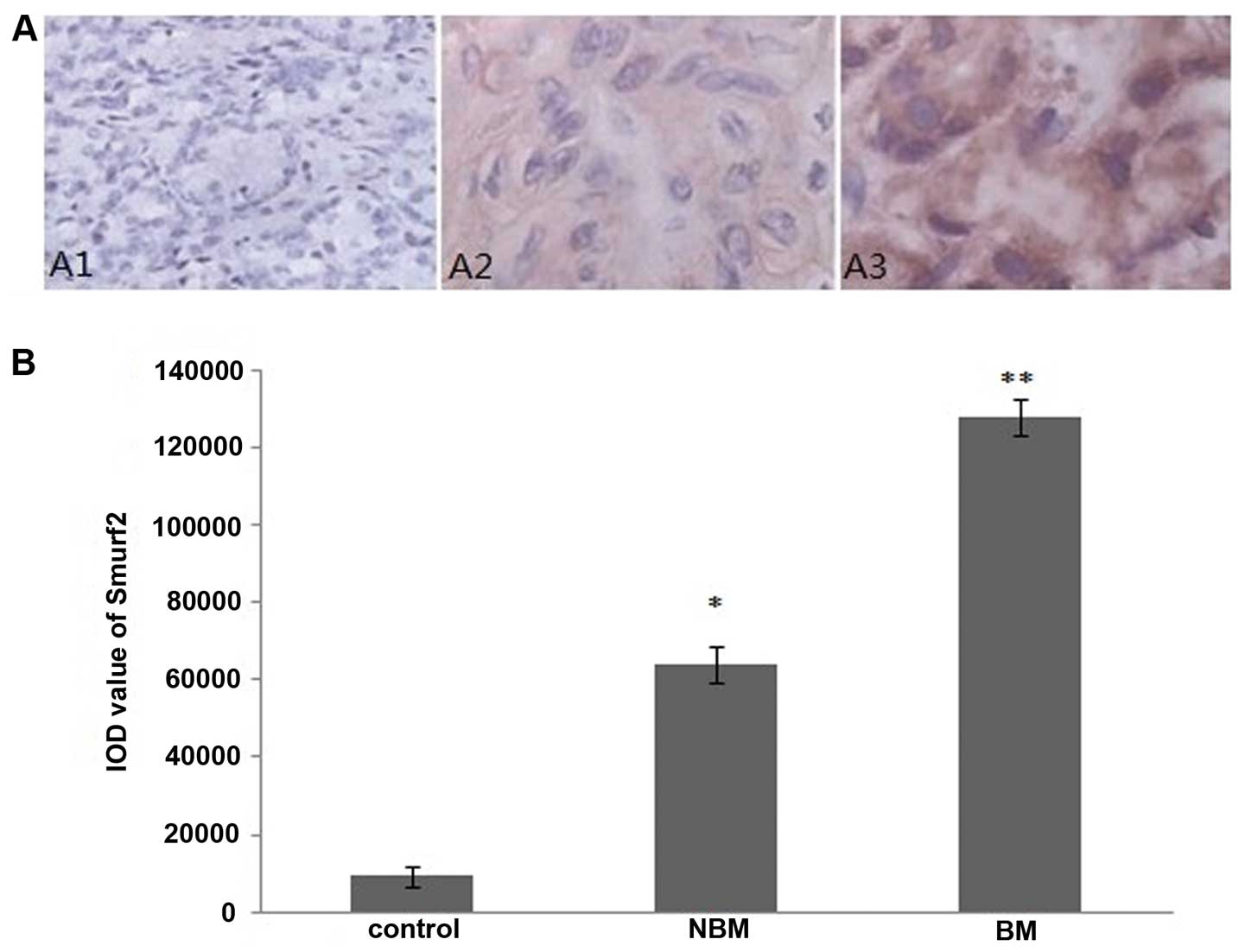
The immunohistochemistry staining sections were
observed under a microscope, the ubiquitin ligase E3 WWP1, Smurf1
and Smurf2 positive staining cells had brownish yellow particles in
the cytoplasm. We found that positive staining cells were rare in
prostate tissues from the control group, furthermore, prostate
tissues of patients with prostate cancer from non-bone metastasis
group presented more positive-staining cells compared with the
control group, whereas less than those of the bone metastasis group
(Figs. 1A, 2A and 3A). Integrated optical density (IOD)
analysis showed that, compared with the control group, the IOD
values of WWP1, Smurf1 and Smurf2 staining in non-bone metastasis
group and bone metastasis group were significantly increased
(P<0.05), moreover, compared with the non-bone metastasis group,
the IOD values of WWP1, Smurf1, Smurf2 staining in the metastasis
group were significantly elevated in the bone metastasis group
(P<0.05) (Figs. 1B, 2B and 3B).
Transcription levels of WWP1, Smurf1 and
Smurf2 genes
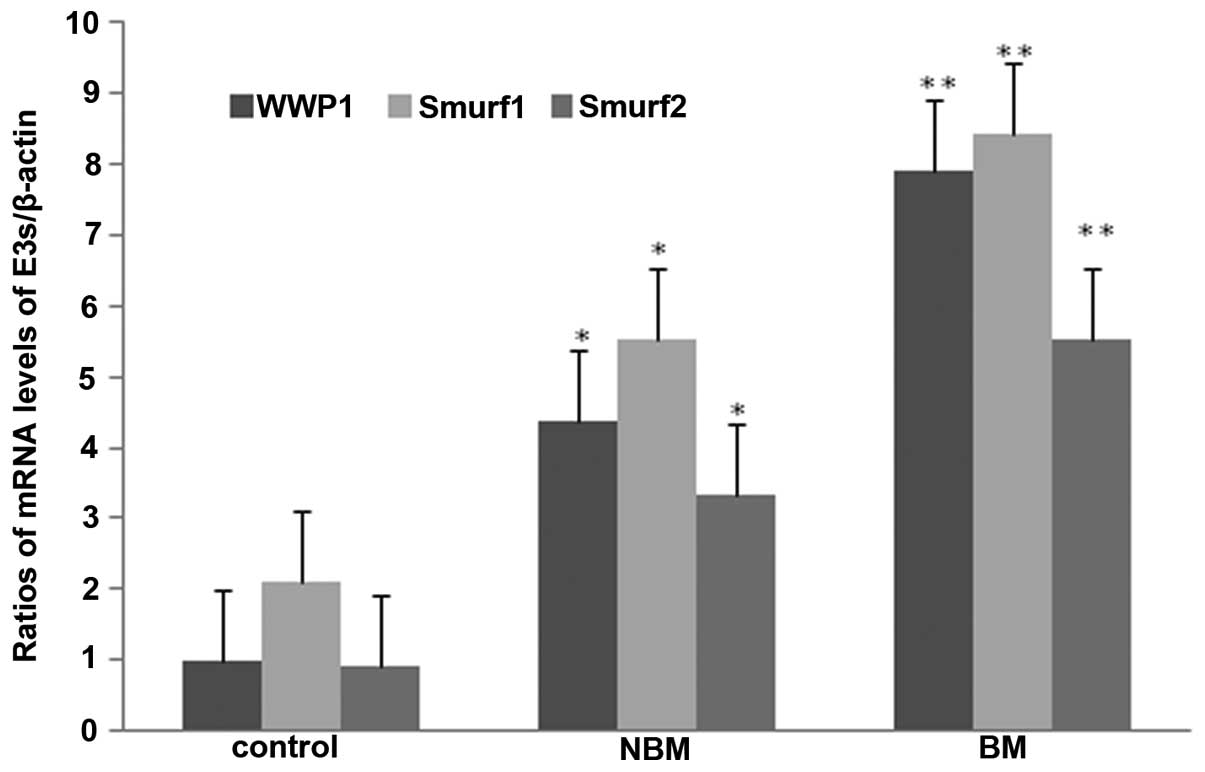
To test the transcription and expression levels of
WWP1, Smurf1 and Smurf2 genes, real-time PCR was performed. The
results showed that compared with the control group, the levels of
WWP1, Smurf1 and Smurf2 mRNA in non-bone metastasis group and bone
metastasis group were significantly increased (P<0.05),
moreover, compared with non-bone metastasis group, the levels of
WWP1, Smurf1 and Smurf2 mRNA were significantly elevated in bone
metastasis group (P<0.05) (Fig.
4).
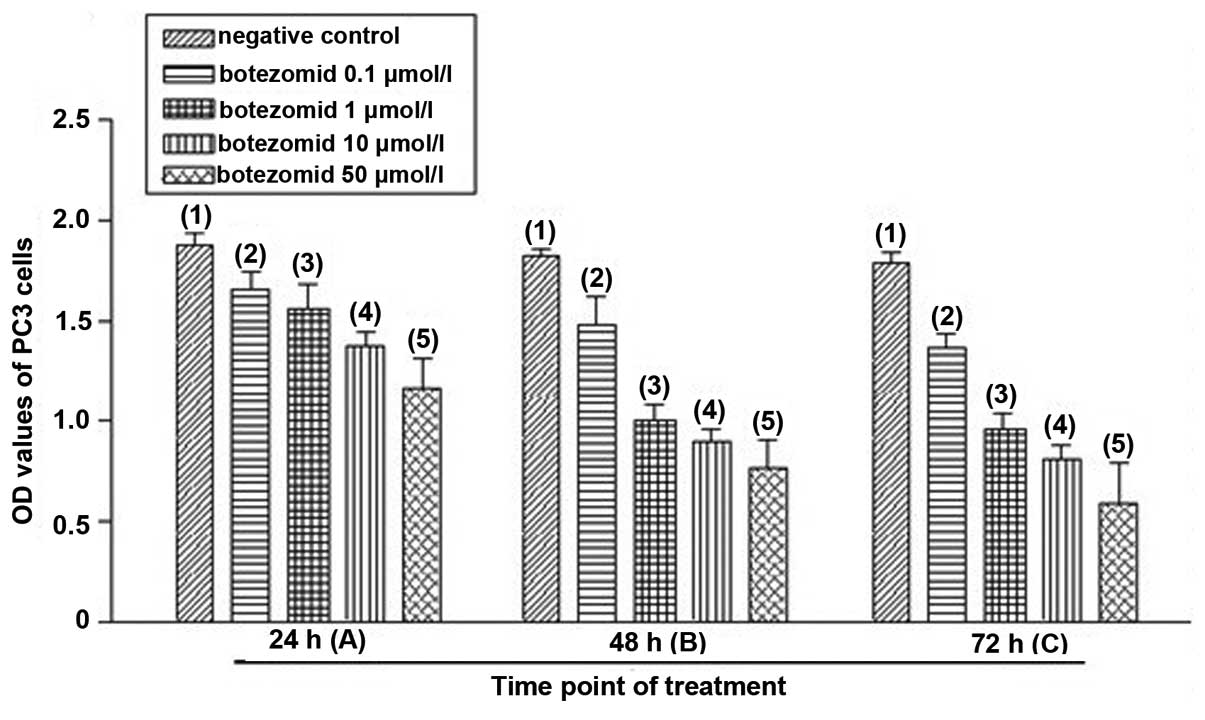
Effects of bortezomib treatment on PC3
cells
PC3 cell proliferation activities were evaluated by
OD values. Compared with the negative control group, bortezomib
treatment reduced PC3 cell proliferation activity in a
dose-dependent manner with the most obvious effect at 50 μmol/l 24,
48 and 72 h after treatment (P<0.01) (Fig. 3, Table
I). Except for 0.1 μmol/l, bortezomib of other concentrations
reduced PC3 cell proliferation activity in a time-dependent manner
(P<0.05) (Fig. 3, Table II). In addition, bortezomib
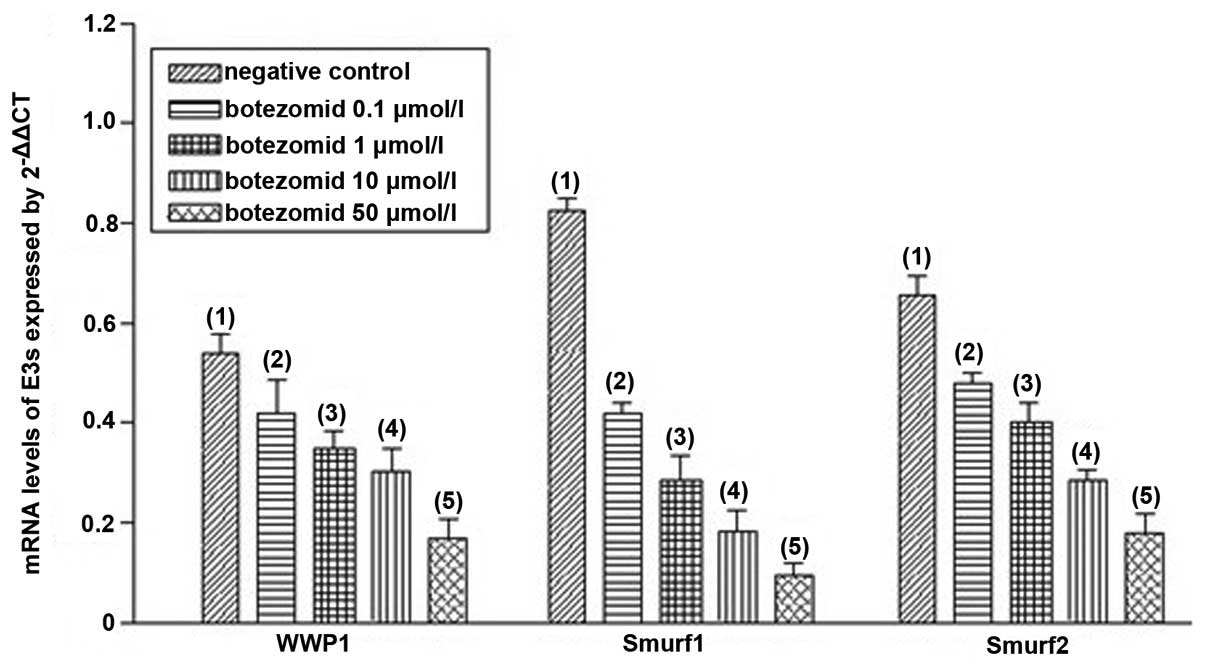
reduced WWP1, Smurf1 and Smurf2 mRNA levels in a dose-dependent
manner with most obvious effect at 50 μmol/l on 72 h after
treatment (P<0.01) (Fig. 4,
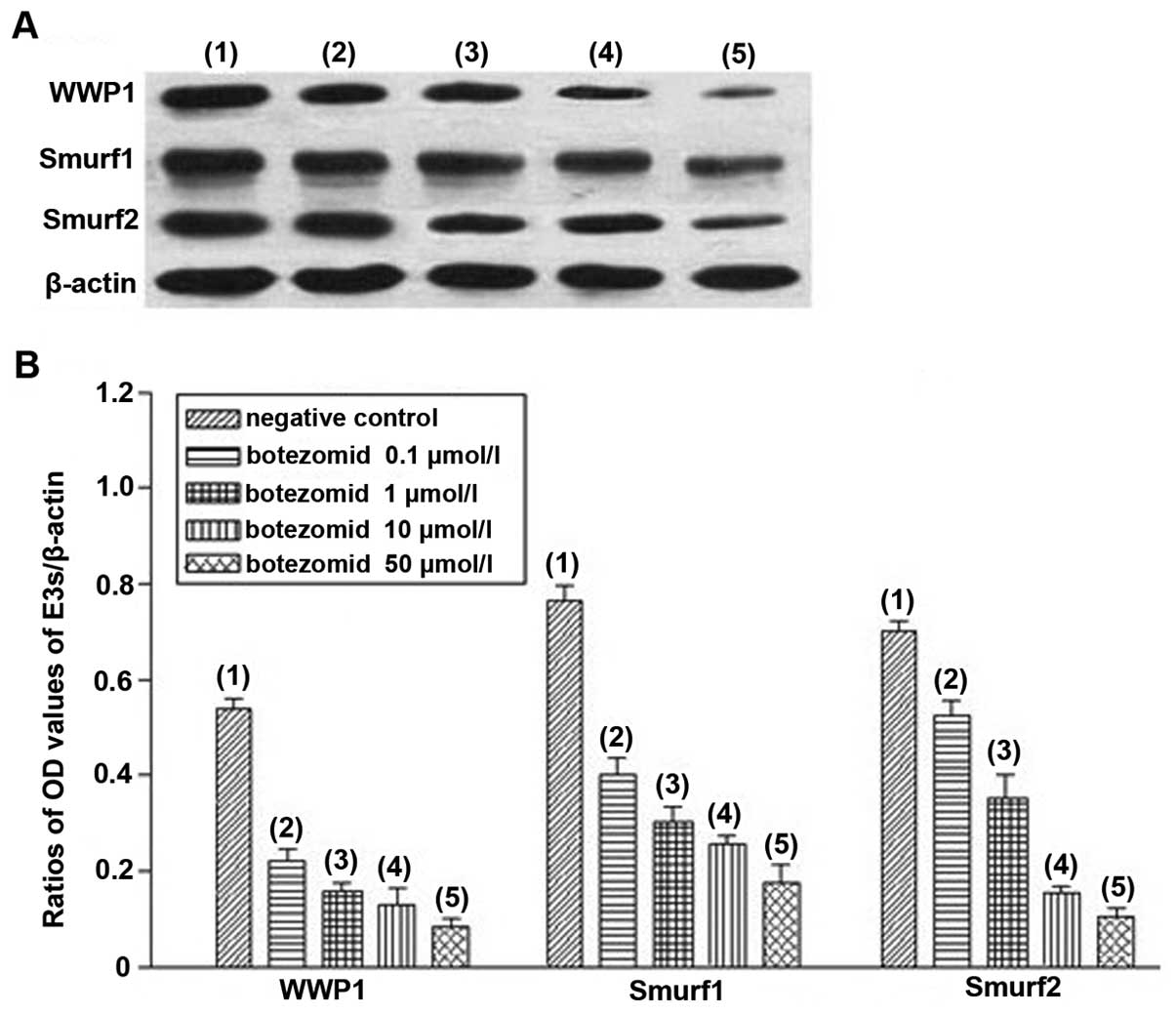
Tables II and III). After treatment for 72 h, protein
expression levels of WWP1, Smurf1 and Smurf2 became consistent with
their mRNA levels (data not shown). Moreover, bortezomib treatment
decreased protein expression levels of WWP1, Smurf1 and Smurf2 in a
dose-dependent manner with a maximum effect at 50 μmol/l
(P<0.01) (Fig. 5, Table IV).
 | Table IStatistics analysis for the effect of
diverse bortezomib concentrations on PC3 cell proliferation. |
Table I
Statistics analysis for the effect of
diverse bortezomib concentrations on PC3 cell proliferation.
| 24 h | 48 h | 72 h |
|---|
| F | 22.567a | 42.564a | 89.281a |
| P(1):(2) | 0.031 | 0.025 | 0.012 |
| (1):(3) | 0.023 | 0.005 | 0.002 |
| (1):(4) | 0.013 | 0.002 | <0.001 |
| (1):(5) | <0.001 | <0.001 | <0.001 |
| (2):(3) | 0.014 | 0.018 | 0.009 |
| (2):(4) | 0.036 | 0.025 | 0.003 |
| (2):(5) | <0.001 | <0.001 | <0.001 |
| (3):(4) | 0.017 | 0.025 | 0.006 |
| (3):(5) | 0.009 | 0.005 | <0.001 |
| (4):(5) | 0.011 | 0.008 | <0.001 |
 | Table IIStatistics analysis for the effect of
bortezomib at different time point on PC3 cell proliferation. |
Table II
Statistics analysis for the effect of
bortezomib at different time point on PC3 cell proliferation.
| 0.1 μmol/l | 1 μmol/l | 10 μmol/l | 50 μmol/l |
|---|
| F | 7.383 | 12.274a | 21.236b | 32.115b |
| P(A):(B) | 0.654 | <0.001 | 0.012 | <0.001 |
| (A):(C) | 0.032 | 0.032 | 0.032 | <0.001 |
| (B):(C) | 0.548 | 0.041 | 0.028 | 0.008 |
 | Table IIIStatistics analysis for the effect of
diverse bortezomib concentrates on E3 transcription levels. |
Table III
Statistics analysis for the effect of
diverse bortezomib concentrates on E3 transcription levels.
| WWP1 | Smurf1 | Smurf2 |
|---|
| F | 42.784a | 86.321a | 74.583a |
| P(1):(2) | <0.001 | <0.001 | <0.001 |
| (1):(3) | <0.001 | <0.001 | <0.001 |
| (1):(4) | <0.001 | <0.001 | <0.001 |
| (1):(5) | <0.001 | <0.001 | <0.001 |
| (2):(3) | 0.038 | <0.001 | <0.001 |
| (2):(4) | 0.024 | <0.001 | <0.001 |
| (2):(5) | <0.001 | <0.001 | <0.001 |
| (3):(4) | 0.012 | 0.013 | <0.001 |
| (3):(5) | 0.005 | 0.007 | <0.001 |
| (4):(5) | 0.022 | 0.016 | 0.013 |
 | Table IVStatistics analysis for the effect of
diverse bortezomib concentrates on E3s protein levels. |
Table IV
Statistics analysis for the effect of
diverse bortezomib concentrates on E3s protein levels.
| WWP1 | Smurf1 | Smurf2 |
|---|
| F | 22.567a | 42.564a | 89.281a |
| P(1):(2) | <0.001 | <0.001 | <0.001 |
| (1):(3) | <0.001 | <0.001 | <0.001 |
| (1):(4) | <0.001 | <0.001 | <0.001 |
| (1):(5) | <0.001 | <0.001 | <0.001 |
| (2):(3) | 0.024 | 0.013 | <0.001 |
| (2):(4) | 0.013 | <0.001 | <0.001 |
| (2):(5) | <0.001 | <0.001 | <0.001 |
| (3):(4) | 0.027 | 0.015 | <0.001 |
| (3):(5) | 0.005 | <0.001 | <0.001 |
| (4):(5) | 0.018 | 0.022 | 0.008 |
Discussion
In the present study, by evaluating the
transcription and expression levels of WWP1, Smurf1 and Smurf2
genes in cell lines and tissues of benign prostate hyperplasia and
human prostate cancer with and without bone metastasis, and those
of human prostate cancer PC3 cell lines treated by bortezomib with
different concentrations (0.1, 1, 10, 50 μmol/l) at different time
points, as well as the proliferation levels of PC3 cells, we
demonstrated that increased transcription and expression levels of
ubiquitin ligase E3s WWP1, Smurf1 and Smurf2 genes may be parts of
the mechanisms of occurrence, development and metastasis of
prostate cancer. Moreover, bortezomib inhibits prostate cancer and
its bone metastasis by downregulating WWP1, Smurf1 and Smurf2.
Prostate cancer is a commonly diagnosed cancer and a
major cause of cancer death in elderly men. Metastatic prostate
cancers are lethal because they are heterogeneously composed of
both androgen-dependent and non-androgen-dependent prostate cancer
cells and androgen ablation does not induce apoptotic death of the
non-androgen-dependent cells because they activate survival
pathways that do not require androgenic stimulation, therefore, it
is the continuing survival and proliferation of the
non-androgen-dependent prostate cancer cells that eventually kill,
no matter how complete the androgen ablation is within the prostate
cancer patient (22). Therefore,
additional targets and therapies that can eliminate the
non-androgen-dependent cancer cells are urgently needed in
conjunction with androgen ablation.
Protein ubiquitination is a post-translational
modification that can direct proteins for degradation by the 26S
proteasome or plasma membrane proteins for endocytosis, sorting and
destruction in the lysosome (23).
Besides, ubiquitination is involved in many other biological
processes, including regulation of protein stability, cell cycle
progression, gene transcription, receptor transport and immune
responses (24). Ubiquitination is
the covalent attachment of the 76-amino acid-comprising protein
ubiquitin via its C terminus to an amino group on a target protein.
The transfer of the activated ubiquitin to substrates occurs
through a series of enzymes. These enzymes include a ubiquitin
activating enzyme (E1), multiple ubiquitin conjugating enzymes
(E2), and hundreds of ubiquitin-protein ligases (E3) (10). The three-step catalytic cascade is
initiated by E1-mediated ATP-dependent activation of ubiquitin,
which is subsequently conjugated to a cysteine residue within the
E2, before finally being attached by an E3 to a lysine residue on a
target protein, and the E3 ubiquitin ligases (E3s) play a critical
role in the ubiquitin conjugation cascade by recruiting
ubiquitin-loaded E2s, recognizing specific substrates, and
facilitating or directly catalyzing ubiquitin transfer to either
the Lys residues (in most cases) or the N terminus of their
molecular targets (25). Based on
the sequence homology of their E2-binding domains, E3s can be
generally classified into three subfamilies: the homologous to
E6-AP carboxyl-terminus (HECT) domain containing E3s, the really
interesting new gene (RING) finger domain-containing E3s, and the U
box E3s, additionally, given their substrate specificity, the E3s
represent attractive targets for cancer therapy (25). For HECT E3s, ubiquitin is initially
transferred to a catalytically active cysteine residue of the E3
and subsequently to a specific target; by contrast, RING and U-Box
E3s do not possess enzymatic activity and rather serve as adaptors,
bridging the catalytically active E2 and the E3-selected target
protein, facilitating the direct transfer of ubiquitin from E2 to
substrate (24). There are nine
members of the Nedd4-like E3 family, all of which share a similar
structure, including a C2 domain at the N-terminus, two to four WW
domains in the middle of the protein, and a homologous to E6-AP
COOH terminus domain at the C terminus (23).
It is well known that TGFβ is a potent tumor
suppressor in the development of cancer, but it becomes a promoter
of metastasis during cancer progression. The TGFβ signaling pathway
involves a series of molecules, among them are the TGFβ receptor 1
(TbR1), Smad2 and Smad4, the protein levels of which are negatively
regulated by WWP1 while increased when WWP1 expression was knocked
down by siRNA in PC-3 cells (12).
In this study, it has been shown that both the mRNA and protein
levels of WWP1 in prostate cancer tissues are significantly higher
than those in benign prostate hyperplasia (BPH) (Figs. 1 and 4), which suggests that elevated WWP1
level may be one of the mechanisms for oncogenesis of prostate
cancer. It has been shown that abrogation of smurfs potentiated
TGF-mediated antineoplastic activity and simultaneously suppressed
genomic instability by activating DNA repair proteins, suppressing
FLIP (a protein that negatively regulates apoptosis) and elevating
TRAIL (a positive regulator of apoptosis), which indicated a
cancer-fostering role of smurfs (11). In the present study, we have
demonstrated that both the mRNA and protein levels of Smurf1 and
Smurf2 in prostate cancer tissues are obviously increased compared
to those in benign prostate hyperplasia (Figs. 2, 3 and 4),
indicating essential roles of smurfs in oncogenesis of prostate
cancer. Bone morphogenetic proteins, members of the TGFβ
superfamily, were originally identified as osteoinductive proteins
in bone that induce ectopic bone and cartilage formation in
vivo (26). Smurf1 binds to
receptor-regulated Smads for bone morphogenetic proteins Smad1/5
and promotes their degradation. In addition, Smurf1 associates with
TGFβ type I receptor through the inhibitory Smad (I-Smad) Smad7 and
induces their degradation (26).
Smurf2, which is structurally similar to Smurf1, also targets Smad1
for degradation, moreover, Smurf2 was shown to associate with
activated TGFβ-specific RSmad Smad2 and to induce its
ubiquitin-dependent degradation (26). In addition, Smurf1 and Smurf2
interact with nuclear Smad7 and induce nuclear export of Smad7. The
Smurfs-Smad7 complexes then associate with type I receptor for TGFβ
and enhance its turnover (26).
The E3 ubiquitin ligase Smurf1 mediates the ubiquitination and
degradation of the main osteoblast transcription factor Runx2, as
well as the signaling proteins JunB, MEKK2 and BMP2-activated Smad1
and Smad4 (27). Runx2 degradation
is also mediated by WWP1, resulting in decreased osteoblast
differentiation and bone formation, consistently, WWP1 deletion in
mice leads to increased Runx2 and bone mass (28). WWP1 also promotes the
ubiquitination and degradation of JunB, an AP-1 transcription
factor that positively regulates osteoblast differentiation, which
was demonstrated in WWP1 knockout mice that do not exhibit the
TNF-α-induced JunB ubiquitination and subsequent inhibition of
osteoblast differentiation observed in wild-type mice (29,30).
Consistently, our study found that the mRNA and protein levels of
WWP1, Smurf1 and Smurf2 in protein tissues from bone-metastasis
group are significantly elevated compared to those from the
non-bone metastasis group (Figs.
1, 2, 3 and 4),
determined by inducing the effects of WWP1, Smurf1 and Smurf2 on
prostate cancer metastatic to the bone. Our research suggested
potential roles of WWP1, Smurf1 and Smurf2 as novel targets in
cancer therapy.
NF-κB is a transcription factor that stimulates the
production of inflammatory mediators, adhesion molecules, and
anti-apoptotic proteins that promote tumor proliferation,
metastasis and chemoresistance (4). By stabilizing the inhibitory protein
of NF-κB, proteasome inhibitors may block NF-κB-mediated
transcription in tumor cells and enhance their susceptibility to
antineoplastic drugs and radiation therapy (4,31).
In March, 2005, the FDA granted regular approval for bortezomib
therapy in progressive multiple myeloma for one prior treatment
(32). Experiments with bortezomib
show that this agent induces apoptosis in androgen-dependent and
androgen-independent prostate cancer cell lines (14). In this study, we have shown that
bortezomib treatment decreased PC3 cell proliferation activity in a
dose-dependent manner with the most obvious effect at 50 μmol/l 24,
48 and 72 h after treatment (Fig.
5, Table I), additionally,
except for 0.1 μmol/l, bortezomib of other concentrations reduced
PC3 cell proliferation activity in a time-dependent manner
(Fig. 5, Table II), which confirmed the
anti-oncogenesis function of bortezomib on prostate cancer. In one
study, nude mice implanted with prostate cancer PC3 cells
experienced a 60% decrease in tumor burden following weekly
intravenous bortezomib therapy, furthermore, when directly injected
into PC3 xenografts, bortezomib generated an even larger decrease
in tumor volume (4). By augmenting
the radiosensitivity of prostate cancer cells, circumventing
multicellular drug resistance in slow-growing prostate cancer
tumors, exerting anti-angiogenic and direct cytotoxic effects and
blocking androgen-dependent prostate cancer growth, bortezomib
prevents the occurrence and development of prostate cancer
(4). In osteoblasts, β-catenin (a
negative regulator of chondrogenesis) accumulation induced by
proteasome inhibition leads to increased osteoblastic cell
proliferation, differentiation and survival (33). In addition to β-catenin, the
bortezomib-induced bone formation is mediated by reduced
degradation of Dickkopf1 (Dkk1), an extracellular Wnt/β-catenin
antagonist (18). Additionally,
proteasome inhibition decreases the degradation of the zinc-finger
transcription factor Gli2 that mediates bone morphogenetic
protein-2 expression in response to Hedgehog signaling, resulting
in increased bone formation (27).
Thus, several proteins can be targeted by proteasome inhibitors in
osteoblasts, leading to increased osteoblastogenesis and bone
formation. In addition to its effect on osteoblasts, the proteasome
inhibitors exert essential impact on bone resorption. Notably,
proteasome inhibitors suppress osteoclastogenesis and decrease bone
resorption mainly by acting on the NF-κB signaling pathway, causing
a reduction in the expression of receptor activator of NF-κB ligand
(RANKL), which is essential for osteoclastogenesis (27). In the present study, we verified
that bortezomib reduced both mRNA and protein levels of WWP1,
Smurf1 and Smurf2 from prostate cancer PC3 cells in a
dose-dependent manner with most obvious effect at 50 μmol/l on 72 h
after treatment (Figs. 6 and
7), which may be part of the
mechanisms for its inhibiting effect on prostate cancer. However,
it has also been shown that after treatment by bortezomib for 72 h,
the mRNA level of WWP1 were not consistent with but higher than its
protein level (Figs. 6 and
7), which suggests that by
contrast to Smurfs, bortezomib additionally promotes degradation of
WWP1, in other words, time difference exists in regulating effects
of bortezomib on Smurfs and WWP1, however, the specific mechanism
is still not clear.
As further understanding leads to the development of
potential anticancer therapeutics that target the activities of
Nedd4-like E3s, a deeper exploration of the mechanisms underlying
the association of Nedd4L with its molecular interplay in prostate
cancer progression could potentially lead to more effective
clinical management, as well as provide new target pathways for
prostate cancer detection, early diagnosis and therapy.
Additionally, due to its cytotoxic activity against cancer cells,
bortezomib may represent a novel adjunctive therapy for prostate
cancer and its bone metastasis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation (81202037).
References
|
1
|
Obertova Z, Brown C, Holmes M and
Lawrenson R: Prostate cancer incidence and mortality in rural men -
a systematic review of the literature. Rural Remote Health.
12:20392012.PubMed/NCBI
|
|
2
|
Armstrong AP, Miller RE, Jones JC, Zhang
J, Keller ET and Dougall WC: RANKL acts directly on RANK-expressing
prostate tumor cells and mediates migration and expression of tumor
metastasis genes. Prostate. 68:92–104. 2008. View Article : Google Scholar
|
|
3
|
Buijs JT, Henriquez NV, van Overveld PG,
van der Horst G, ten Dijke P and van der Pluijm G: TGF-beta and
BMP7 interactions in tumour progression and bone metastasis. Clin
Exp Metastasis. 24:609–617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whang PG, Gamradt SC, Gates JJ and
Lieberman JR: Effects of the proteasome inhibitor bortezomib on
osteolytic human prostate cancer cell metastases. Prostate Cancer
Prostatic Dis. 8:327–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satoh F, Mimata H, Nomura T, et al:
Autocrine expression of neurotrophins and their receptors in
prostate cancer. Int J Urol. 8:S28–S34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Festuccia C, Gravina GL, Muzi P, et al: In
vitro and in vivo effects of bicalutamide on the expression of TrkA
and P75 neurotrophin receptors in prostate carcinoma. Prostate.
67:1255–1264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hellwinkel OJ, Asong LE, Rogmann JP, et
al: Transcription alterations of members of the
ubiquitin-proteasome network in prostate carcinoma. Prostate Cancer
Prostatic Dis. 14:38–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garrett IR, Chen D, Gutierrez G, et al:
Selective inhibitors of the osteoblast proteasome stimulate bone
formation in vivo and in vitro. J Clin Invest. 111:1771–1782. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Micel LN, Tentler JJ, Smith PG and
Eckhardt GS: Role of ubiquitin ligases and the proteasome in
oncogenesis: novel targets for anticancer therapies. J Clin Oncol.
31:1231–1238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang B and Kumar S: Nedd4 and Nedd4-2:
closely related ubiquitin-protein ligases with distinct
physiological functions. Cell Death Differ. 17:68–77. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farooqi AA, Waseem MS, Riaz AM and Bhatti
S: SMURF and NEDD4: sharp shooters monitor the gate keepers and ion
traffic controllers of lead astray cell. J Membr Biol. 244:1–8.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Sun X, Guo P, et al: Ubiquitin E3
ligase WWP1 as an oncogenic factor in human prostate cancer.
Oncogene. 26:2386–2394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papandreou CN and Logothetis CJ:
Bortezomib as a potential treatment for prostate cancer. Cancer
Res. 64:5036–5043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams J, Palombella VJ, Sausville EA, et
al: Proteasome inhibitors: a novel class of potent and effective
antitumor agents. Cancer Res. 59:2615–2622. 1999.PubMed/NCBI
|
|
15
|
Sunwoo JB, Chen Z, Dong G, et al: Novel
proteasome inhibitor PS-341 inhibits activation of nuclear
factor-kappa B, cell survival, tumor growth, and angiogenesis in
squamous cell carcinoma. Clin Cancer Res. 7:1419–1428.
2001.PubMed/NCBI
|
|
16
|
Williams S, Pettaway C, Song R, Papandreou
C, Logothetis C and McConkey DJ: Differential effects of the
proteasome inhibitor bortezomib on apoptosis and angiogenesis in
human prostate tumor xenografts. Mol Cancer Ther. 2:835–843.
2003.PubMed/NCBI
|
|
17
|
Uy GL, Trivedi R, Peles S, et al:
Bortezomib inhibits osteoclast activity in patients with multiple
myeloma. Clin Lymphoma Myeloma. 7:587–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oyajobi BO, Garrett IR, Gupta A, et al:
Stimulation of new bone formation by the proteasome inhibitor,
bortezomib: implications for myeloma bone disease. Br J Haematol.
139:434–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giuliani N, Morandi F, Tagliaferri S, et
al: The proteasome inhibitor bortezomib affects osteoblast
differentiation in vitro and in vivo in multiple myeloma patients.
Blood. 110:334–338. 2007. View Article : Google Scholar
|
|
20
|
Alaaeddine N, De Montigny C and Sadouk M:
Real-time reverse transcription-polymerase chain reaction
quantification of tumor necrosis factor alpha messenger in human
leukocytes. Clin Lab. 57:799–802. 2011.
|
|
21
|
Zhu Y, Li T, Song J, et al: The
TIR/BB-loop mimetic AS-1 prevents cardiac hypertrophy by inhibiting
IL-1R-mediated MyD88- dependent signaling. Basic Res Cardiol.
106:787–799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weeraratna AT, Dalrymple SL, Lamb JC, et
al: Pan-trk inhibition decreases metastasis and enhances host
survival in experimental models as a result of its selective
induction of apoptosis of prostate cancer cells. Clin Cancer Res.
7:2237–2245. 2001.
|
|
23
|
Chen C and Matesic LE: The Nedd4-like
family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev.
26:587–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rieser E, Cordier SM and Walczak H: Linear
ubiquitination: a newly discovered regulator of cell signalling.
Trends Biochem Sci. 38:94–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bernassola F, Karin M, Ciechanover A and
Melino G: The HECT family of E3 ubiquitin ligases: multiple players
in cancer development. Cancer Cell. 14:10–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murakami G, Watabe T, Takaoka K, Miyazono
K and Imamura T: Cooperative inhibition of bone morphogenetic
protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell.
14:2809–2817. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Severe N, Dieudonne FX and Marie PJ: E3
ubiquitin ligase-mediated regulation of bone formation and
tumorigenesis. Cell Death Dis. 4:e4632013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jones DC, Wein MN, Oukka M, Hofstaetter
JG, Glimcher MJ and Glimcher LH: Regulation of adult bone mass by
the zinc finger adapter protein Schnurri-3. Science. 312:1223–1227.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao L, Huang J, Zhang H, et al: Tumor
necrosis factor inhibits mesenchymal stem cell differentiation into
osteoblasts via the ubiquitin E3 ligase Wwp1. Stem Cells.
29:1601–1610. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sterz J, von Metzler I, Hahne JC, et al:
The potential of proteasome inhibitors in cancer therapy. Expert
Opin Investig Drugs. 17:879–895. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma MH, Yang HH, Parker K, et al: The
proteasome inhibitor PS-341 markedly enhances sensitivity of
multiple myeloma tumor cells to chemotherapeutic agents. Clin
Cancer Res. 9:1136–1144. 2003.PubMed/NCBI
|
|
32
|
Kane RC, Farrell AT, Sridhara R and Pazdur
R: United States Food and Drug Administration approval summary:
bortezomib for the treatment of progressive multiple myeloma after
one prior therapy. Clin Cancer Res. 12:2955–2960. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiang YW, Hu B, Chen Y, et al: Bortezomib
induces osteoblast differentiation via Wnt-independent activation
of beta-catenin/TCF signaling. Blood. 113:4319–4330. 2009.
View Article : Google Scholar : PubMed/NCBI
|