Introduction
Cell migration pathways play significant roles in a
variety of physiological processes that can be ‘hijacked’ by cancer
cells and thus, increase the metastatic potential of the disease
and increase the incidence of cancer-related deaths (1). Regardless of when it occurs, the
dissemination of tumor cells from the primary tumor is the
principle reason for the mortality and morbidity of cancer
patients. This appears to be particularly important for ovarian
surface epithelial (OSE) cancer, which is often diagnosed only
after cells have been released into the peritoneal cavity.
Therefore, investigation into the molecular mechanisms of tumor
cell motility has been intensified. Therapies that specifically
target the motility of tumor cells could significantly improve
cancer treatment by removing the threat of systemic disease and
decreasing the sole dependence on cytotoxic therapeutics with
detrimental side-effects (2).
The dynamic interaction between a cell and
extracellular matrix (ECM) proteins influences cell migration and
the invasive behavior of cancer cells. These proteins influence
both the motility and migratory capacity of normal cells, and the
metastasis of tumor cells. Although the extracellular matrix was
initially perceived as a barrier to tumor cell dissemination, it is
becoming evident that the architecture and the matrix composition
of the microenvironment can promote tumor cell dissemination.
Specifically ECM proteins have been shown to influence movement in
ovarian cancer cells through interaction with integrins (3).
As groups, integrins mediate adhesion and
mechano-transduction to extracellular ligands via α2β1 integrin
predominantly binding to fibrillar collagen; αvβ3, αvβ1 and α5β1
interacting with fibronectin; and α3β1 and α6β1 engaging with
laminin (4). After associating
with ligands, the cytoplasmic tails of integrins interact with
cytoskeletal adaptor proteins (5,6).
Adaptor and mechanosensing modulator proteins engage with the actin
cytoskeleton and trigger signaling to protein kinases, including
focal adhesion kinase (FAK) and Src (5,7,4).
Downstream integrin effectors further include the small GTPases Rac
and Rho, which reinforce cell protrusion and rear contraction
(8). The Rho-family GTPases have
been directly linked to motility and protrusion formation through
their ability to activate the signaling targets that regulate actin
cytoskeleton modification (9).
Cell migration can be subdivided into random
motility and directed migration, the latter indicating whether a
cell is able to maintain a single direction of migration for
prolonged periods of time (10).
Directional motility requires polarization, and maintaining
polarity determines persistence with which a cell moves
directionally. Morphological polarization is a consequence of an
internal asymmetry in distribution of signaling molecules and
cellular structures, primarily cytoskeletal (11). External stimuli are not necessarily
required to trigger and maintain polarity, although they can
contribute and shift equilibria in favor of polarized states.
Chemotactic and haptotactic gradients serve as external guides by
keeping up cell polarity and reducing the probability of changing
direction. Polarized motility is governed by organization of a
leading edge in the direction of cell movement. The leading edge is
stabilized by the formation of new focal adhesions or cell-ECM
contact sites (12). While
directional cell migration facilitates the coordinated movement of
cells during the development and tissue repair, the pathways
involved with regulating the interplay between the extracellular
environment, the actin cytoskeleton and the plasma membrane that
result in directional movement remain inadequately understood.
It is believed that intracellular signaling, often
mediated at the leading edge by the Rho family of small GTPases,
operates at each step of the cell motility cycle to promote
directional migration by regulating leading edge formation. Among
the downstream targets of the Rho family of small GTPases, PAK
(p21-activaed kinases) serine/threonine protein kinases have been
implicated as effectors of cell motility. These kinases are
subdivided into two groups, PAK1-3 (group I) and PAK4-6 (group II).
The group I isoforms contain an autoinhibitory domain (PID) which
is absent in group II PAK proteins (13). The group I PAKS share a p21-binding
domain (PBD), a serine/threonine kinase domain, an acidic region
and multiple proline-rich regions that serve as binding sites for
SH3 domain-containing proteins. Pak kinases were first identified
in screens for Rac and Cdc42 effectors (14).
Several targets of Paks are directly implicated in
regulating cytoskeletal dynamics, including LIM domain kinase 1
(15), which phosphorylates and
inactivates cofilin, an F-actin-severing and -depolymerizing
protein, or myosin light chain (16) and MLC kinase (17), which control myosin contractility.
Paks are also involved in the reorganization of the focal adhesions
(18,19). So it appears logical that PAK(s)
could play important role(s) in modulating the ability of cancer
cells to move and metastasize. A number of human breast cancer
lines exhibit constitutively elevated PAK1 and PAK2 activity, in
some cases associated with the presence of an activated Rac GTPase
(20) and PAK activity has been
linked to tumor invasiveness and motility of a variety of human
cancer cell lines (21).
A better understanding of motility and its role in
cancer metastasis is crucial to reducing the number of annual
deaths due to metastatic disease. Therefore, the experiments
reported herein were designed to test the hypothesis that ECM
proteins (specifically collagen I and fibronectin abundant in the
peritoneal mesenteries) are important in ovarian cancer metastasis,
facilitating cellular motility. We identified Pak2 as a possibly
important mediator of ovarian cancer cell migration on ECM.
Materials and methods
Materials
Fetal bovine serum (FBS) was obtained from
Innovative Research (Novi, MI). RPMI-1640 media with L-glutamine
and without sodium bicarbonate was purchased from HyClone
Laboratories, Inc. (Logan, UT). CellTiter 96® AQueousOne
Solution Cell Proliferation Assay (MTS) was purchased from Promega
(Madison, WI). Antibiotic antimycotic solution (100X) stabilized,
with 10,000 units penicillin, 10 mg streptomycin and 25 μg
amphotericin B per ml (PSA) was obtained from Mediatech, Inc.
(Manassas, VA). Human plasma fibronectin and bovine collagen type I
was ordered from BD Biosciences (Franklin Lakes, NJ). Sodium
bicarbonate, HEPES and trypsin solution from porcine pancreas were
purchased from Sigma-Aldrich (St. Louis, MO). T-25 cm2
cell culture flasks were produced by Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA).
Cell lines and culture conditions
The cisplatin-sensitive cell line, OV2008, was
derived from ovarian serous cystadeno-carcinoma from a patient
without prior chemotherapy (22);
and its resistant variant, C13, originated from in vitro
cisplatin challenges of OV2008 cells (23). These cells were acquired as a gift
from Dr Barbara Vanderhyden (University of Ottawa, Canada). The
OV2008 and C13 cell lines were maintained in RPMI-1640 (1X) medium,
supplemented with 10% fetal bovine serum (FBS) and 1% PSA, sodium
bicarbonate (24 mM) and HEPES (10 mM). All cell cultures were
maintained at 37°C in a humidified atmosphere with 5%
CO2.
All wound healing assays were performed in modified
35-mm cell culture dishes. These dishes were created by punching a
hole in the bottom of the dish followed by adherence of a
22-mm2 glass cover slip (Corning) to the bottom of the
dish. These dishes were baked at 60°C for 2 days before being
soaked overnight in a CytoClean solution. The dishes were then
rinsed, dried and sterilized via exposure to UV light for 2.5
h.
Culture of ovarian cancer cell lines on
collagen I and fibronectin
The substrata that were used in the current
investigation were selected to represent some of the different
types of ECM that OSE cells may contact, in vivo, when
disseminated into the peritoneal cavity. Glass cover slips were
coated with extracellular matrix proteins by suspending the ECM
proteins in a solvent (fibronectin in PBS, collagen I in 0.1 N
HCl). Collagen was used as a thin coating at 10 μg/cm2
and fibronectin was applied at a 5-μg/cm2 as per the
manufacturer’s recommendations. The ECM solution was then added to
the cover slips and allowed to incubate for 1 h at room
temperature. The cover slips were then quickly rinsed with PBS
before plating the cells onto the coated cover slips.
Wound healing assay
Cell migration was measured using an in vitro
wound healing assay. OV2008 and C13 cells were allowed to form a
confluent monolayer in modified 35-mm tissue culture dishes until
confluent. The wound was created by scraping monolayer cells with a
sterile pipette tip to scratch a ‘wound’ into the confluent
monolayer. The media was changed to remove debris and cells. The
dish was placed into a stage top incubation LiveCell device
(Pathology Devices, Exton, PA). The LiveCell device maintained the
temperature at 37°C, the CO2 at 5%, and the relative
humidity at 75% within the stage top chamber. Slidebook software
was used to take a picture at time point zero and every 10 min for
a total of 10 h using an Olympus IX70 inverted microscope (Center
Valley, PA). TScratch software (developed by Tobias Gebäck and
Martin Schulz, ETH Zürich) was used to analyze the images,
measuring the differences in migration. Values are presented as
percentage (%) of open area (‘wound’) remaining at 10 h compared to
0 h. The time lapse stacks of images were also analyzed using
ImageJ and the two following plug-ins: i) Manual Tracking
(developed by Fabrice Cordeli, Institute Curie, Orsay, France) and
ii) Chemotaxis and Migration Tool (Ibidi, Martinsried, Germany).
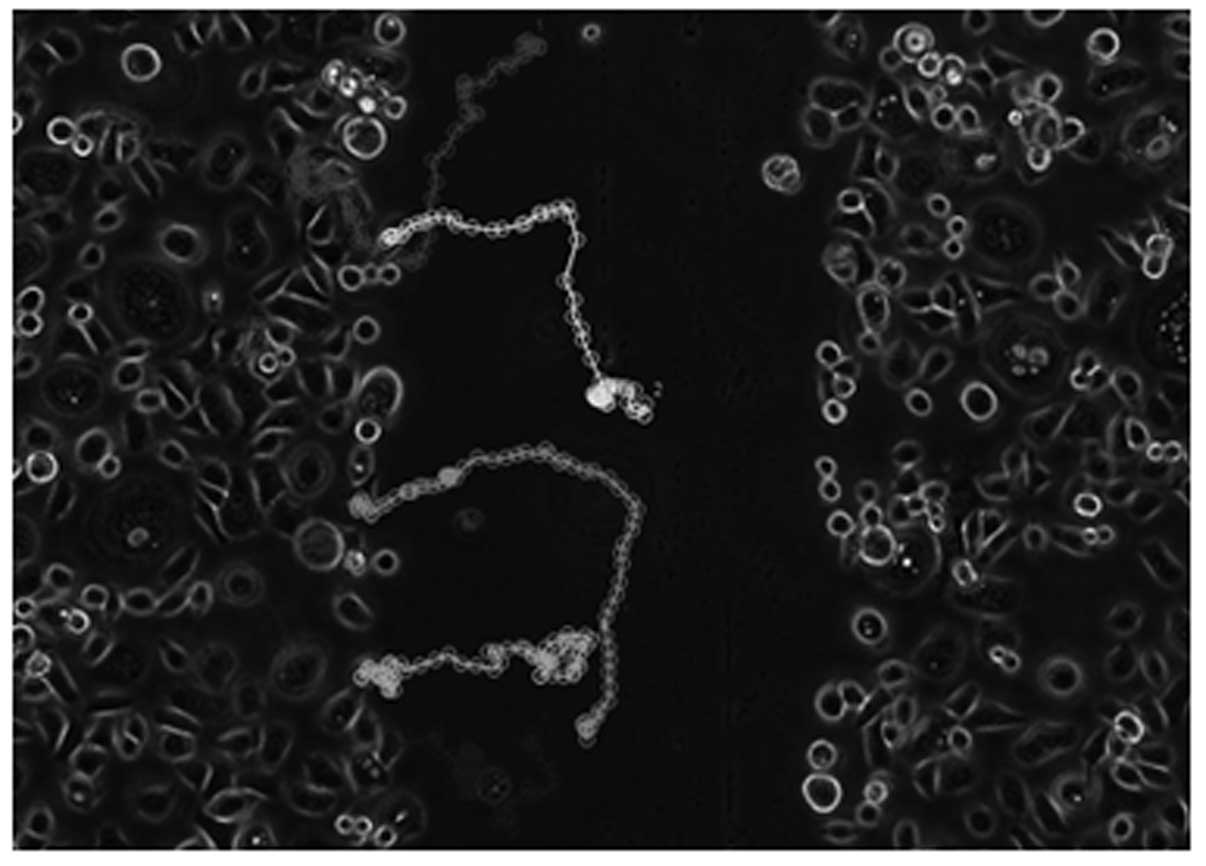
Individual cells were randomly selected and tracked throughout the
10-h time period, as demonstrated in Fig. 1.
Migration assay
OV2008 and C13 cells were grown in 35-mm tissue
culture dishes until confluent. Cells were then trypsinized and
migration assays were performed using ThinCerts migration inserts
with 8 μm pore size (Bioexpress, Kaysville, UT). Briefly,
2×105 cells suspended in 200 μl of serum-free RPMI were
added to the upper compartment of the insert, which rests in the
well of a 24-well plate. RPMI (650 μl) containing 10% FBS was added
to the bottom compartment with serum providing the chemoattractant
signal. The cells were cultured at 37°C and 5% CO2 and
allowed to migrate for 24 h. The inserts were removed and the
remaining non-migrating cells on the upper surface of the membrane
were removed with a cotton swab. The cells that migrated to the
lower surface of the membrane were fixed with 4% formaldehyde for 5
min at room temperature, washed twice with PBS and stained with
Harris Hematoxylin Solution (Sigma-Aldrich) for 45 min at room
temperature. Inserts were washed several times with tap water until
the membrane was clean. The membrane was peeled off the plastic
inserts, placed on glass microscope slides and mounted using
HistoChoice mounting media (Amresco, Solon, OH).
Migrating cells were examined by microscopy at ×200
magnification with Olympus IX70 microscope. Pictures were taken of
5 randomly chosen different fields and migrating cells were counted
manually. The average of number of migratory cells for each
condition was calculated and the differences in the number of cells
that migrated through the membrane were calculated.
Western blot analysis
Cells were grown until approximately 90% confluent
and washed twice in cold PBS. Cells were lysed on ice, using RIPA
buffer (1% NP40, 0.5% Na deoxycholate, 0.1% SDS, 150 mM NaCl)
containing Halt Protease Inhibitor Single-Use Cocktail (100X)
(Pierce, Rockford, IL). The protein concentration in these whole
cell lysates was measured using the bicinchoninic acid (BCA)
Protein Assay (Pierce) and calculated based on the BSA standard
curve. Protein (50–80 μg) was prepared for SDS-PAGE by denaturing
the lysate in 6X loading buffer containing β-mercaptoethanol and
DTT followed by boiling for 5 min. Protein preparations were loaded
into 10% SDS-PAGE gels and run for 90 min at 150 V. Proteins on the
gel were transferred onto nitrocellulose membrane (Bio-Rad,
Hercules, CA) for 1 h at 110 V, followed by washing 3 times in TBS
with 0.1% Tween-20. Membranes were blocked in TTBS with 5% non-fat
dry milk for 45 min and then incubated overnight at 4°C with
polyoclonal antibodies in primary antibody diluent solution from
the SuperSignal Western Blot Enhancer kit (Thermo Scientific)
containing either anti-Pak2 or anti-β-actin (Cell Signaling
Technologies, Danvers, MA). Membranes were washed 3 times in TTBS
and incubated for 1 h at room temperature with the appropriate
HRP-conjugated secondary antibody (anti-mouse for β-actin, Santa
Cruz Biotechnology, Inc.; anti-rabbit for Pak2, Santa Cruz
Biotechnology, Inc.). Membranes were washed three times in TTBS and
developed by ECL western blotting detection system from Santa Cruz
Biotechnology, Inc. Finally, chemiluminescence was detected via
exposure to HyBlot CL autoradiography film (Denville Scientific,
Metuchen, NJ). Digital images of the membranes were scanned and
bands intensities were quantified using ImageJ analysis.
Small interfering RNA transfections
For the wound healing assays, OV2008 and C13 cells
were grown in modified 35-mm tissue culture dishes until 50–60%
confluent. Cells were transfected with 10 μl of TransIT-TKO
transfection reagent (Mirus Bio LLC, Madison, WI) and either 50 nM
non-targeting control siRNA or 50 nM SignalSilence Pak2 siRNA (Cell
Signaling Technology). The siRNA sequences are proprietary. The
transfection was performed in antibiotic-free media and the cells
were incubated for 24 h before changing the media. When the cells
reached confluence (usually one additional day) the wound was
created with a 10-μl pipette and time point zero was photographed
with the IX70 microscope. Cells were then incubated for 20 h at
37°C and 5% CO2 until time point 20 h was then captured.
Mock transfection with TransIT-TKO reagent alone revealed that the
transfection process decreased cell movement in general. Therefore
longer time periods were used to assess wound healing and migration
assays post-transfection.
For the migration assays, cells were first plated in
35-mm dishes and allowed to attach overnight. The next day, cells
were transfected with either control siRNA or Pak2 siRNA. The media
was changed after 24 h of incubation. Forty-eight hours
post-transfection, the cells were trypsinized, counted and seeded
into the inserts as described above. Cells were allowed to migrate
for 48 h before the inserts were stained, washed and mounted on
slides as described above.
Microarray
Cells were plated and cultured on uncoated,
fibronectin-coated or collagen I-coated glass cover slips for 24 h.
Total RNA of the cells was then isolated using the RNeasy Mini kit
(Qiagen). RNA yield was determined using the ND-1000
spectrophotometer (NanoDrop). The Ambion WT Expression kit (Ambion)
was used to synthesize first-strand cDNA from the isolated RNA.
Next, a template for transcription was created by synthesizing
second-strand cDNA. Antisense cRNA was synthesized and amplified by
in vitro transcription in a thermal cycler (Eppendorf
Mastercycler Gradient). The resulting cRNA was purified using
nucleic acid binding beads. Next, second cycle cDNA was synthesized
by the reverse transcription of cRNA using random primers before
hydrolyzing the cRNA template with RNase H. Second-cycle cDNA was
purified using nucleic acid binding beads before the yield was
determined using the ND-1000.
The second-cycle cDNA was fragmented and labeled
using the GeneChip WT Terminal Labeling kit (Affymetrix). The
fragmented and labeled DNA samples were hybridized to a GeneChip
Human Gene 1.0 ST Array cartridge for 17 h at 45°C at 60 rpm in a
GeneChip Hybridization Oven 640 (Affymetrix). The chips were then
stained and washed using the GeneChip Hybridization, Wash and Stain
kit (Affymetrix) and a GeneChip Fluidics Station 450 (Affymetrix).
Finally, the chips were scanned using the Affymetrix GeneChip
Scanner 3000 using Affymetrix GeneChip Command Console software.
The resulting cell files were analyzed using BRB-ArrayTools
developed by Dr Richard Simon and BRB-ArrayTools Development Team.
JMP Genomics Suite 6.0 was also used to analyze and graph the
results.
Statistical analysis
Means derived from multiple treatments in the two
ovarian cancer cell lines were analyzed by one way analysis of
variance (ANOVA) using SPSS, version 20. Tukey and LSD were the two
post-hoc tests used. In all cases, p<0.05 was considered
significant.
Results
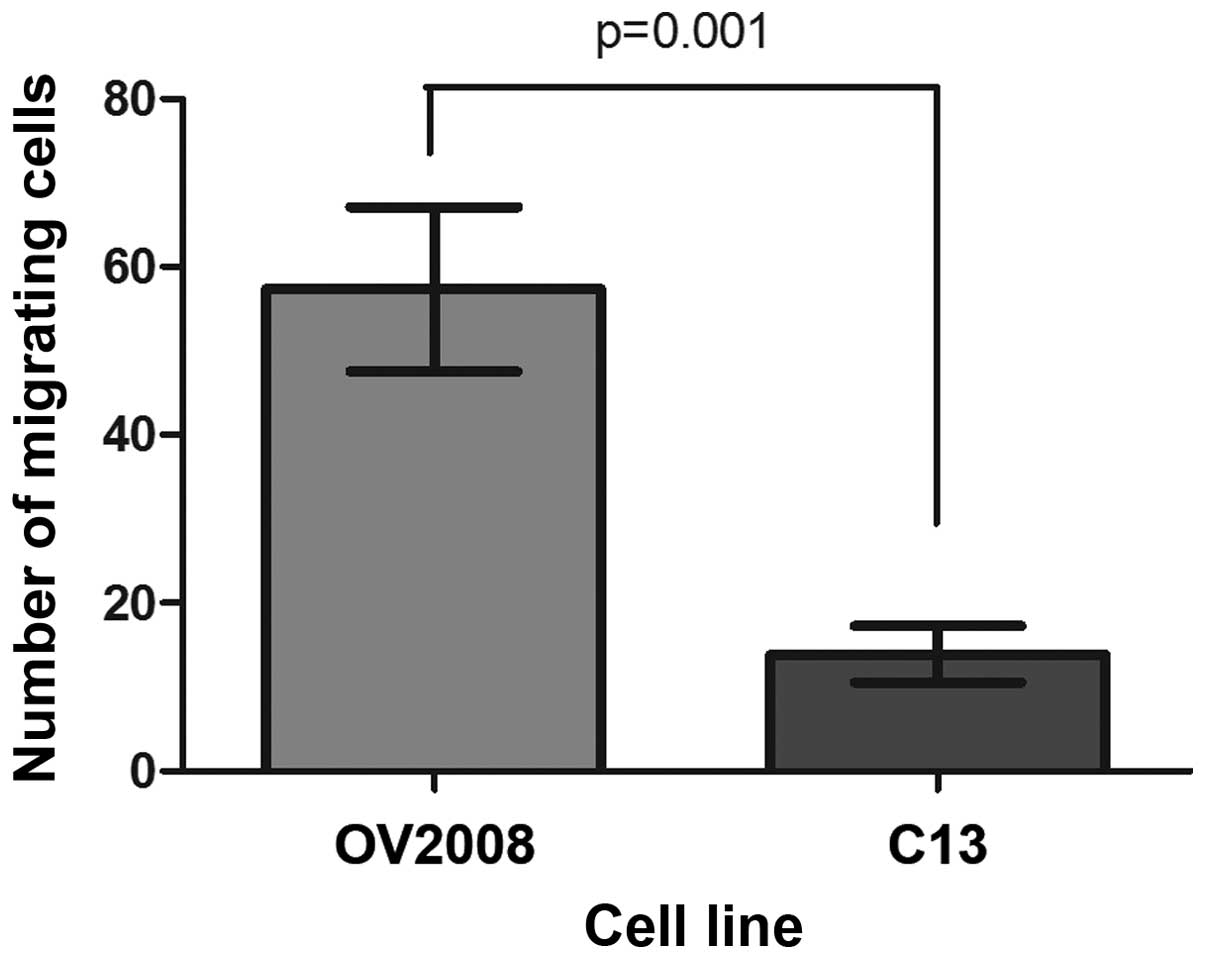
Migration of OV2008 and C13 cells
Migration through the porous membrane of transwell
inserts was used to measure 3-D motility of the selected cell
lines. More than 3 times as many OV2008 cells migrated through the
cell culture insert membranes than C13 cells over a 24-h period and
the difference was significant (Fig.
2).
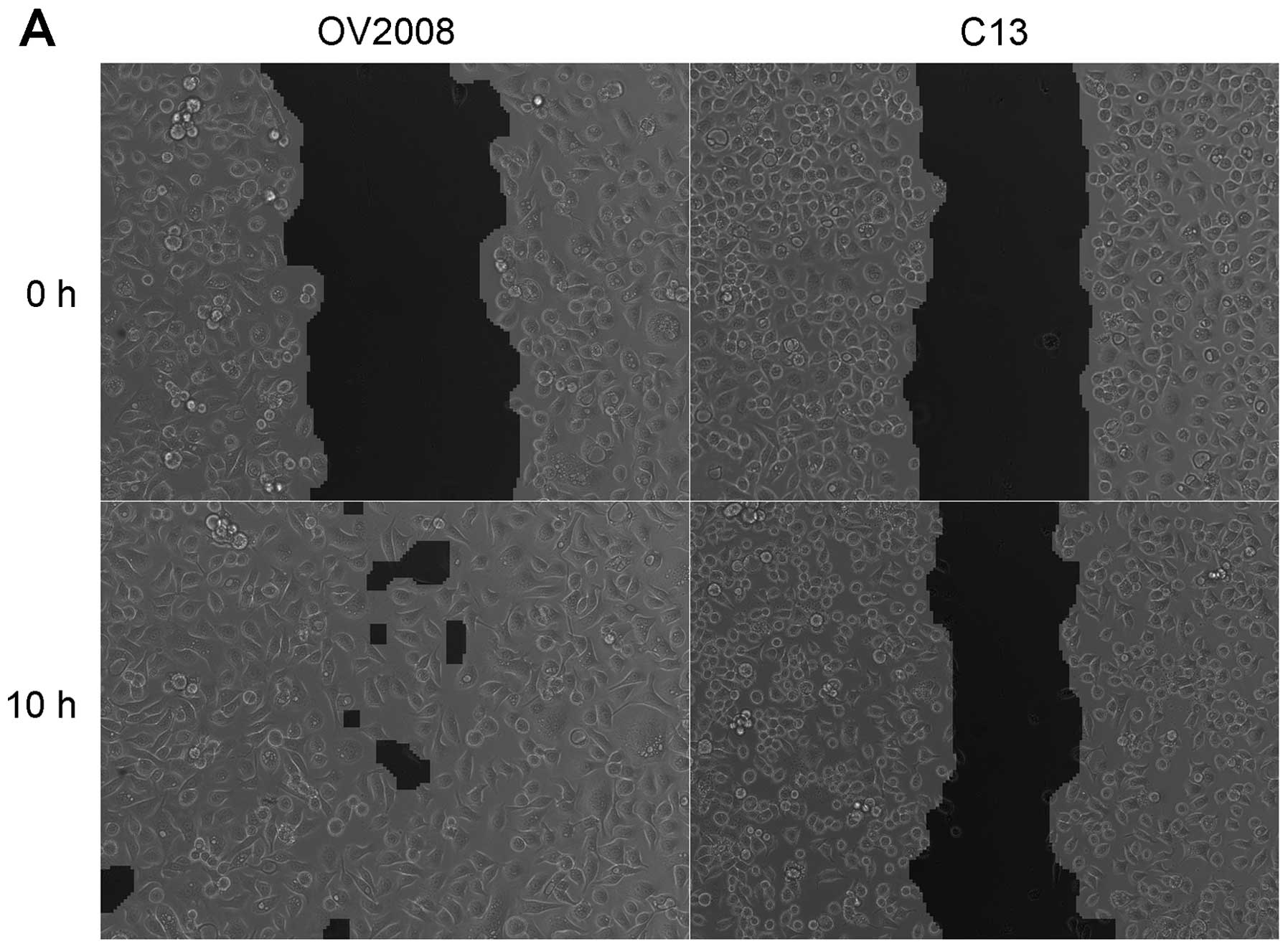
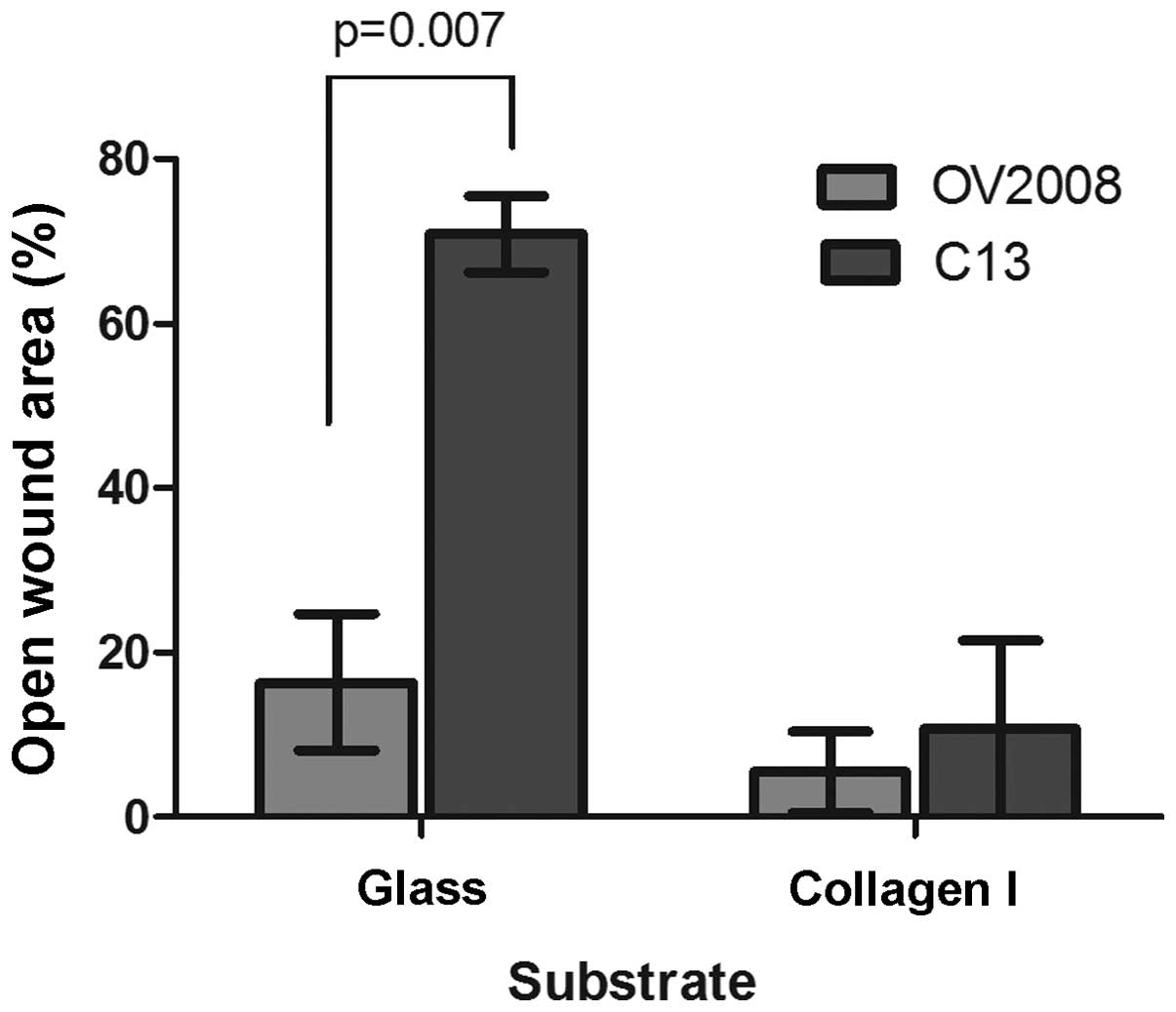
Removing cells from a confluent culture creates a
scratch wound (Fig. 3A). Although
the overall area of the wound is slightly greater for OV2008 cells,
the wound is almost completely closed 10 h post-wounding, whereas
the wound for the C13 cells remained substantially open during the
same time period. Thus, the OV2008 cells had a much greater
capacity to heal a scratch wound than the C13 cells when cultured
on untreated glass cover slips (Fig.
3B). When the amount of wound remaining open 10 h post-wounding
was compared as a percentage of the area of the original wound (0
h), there was over 3-fold more open wound area remaining in C13
than OV2008 cells (Fig. 3A).
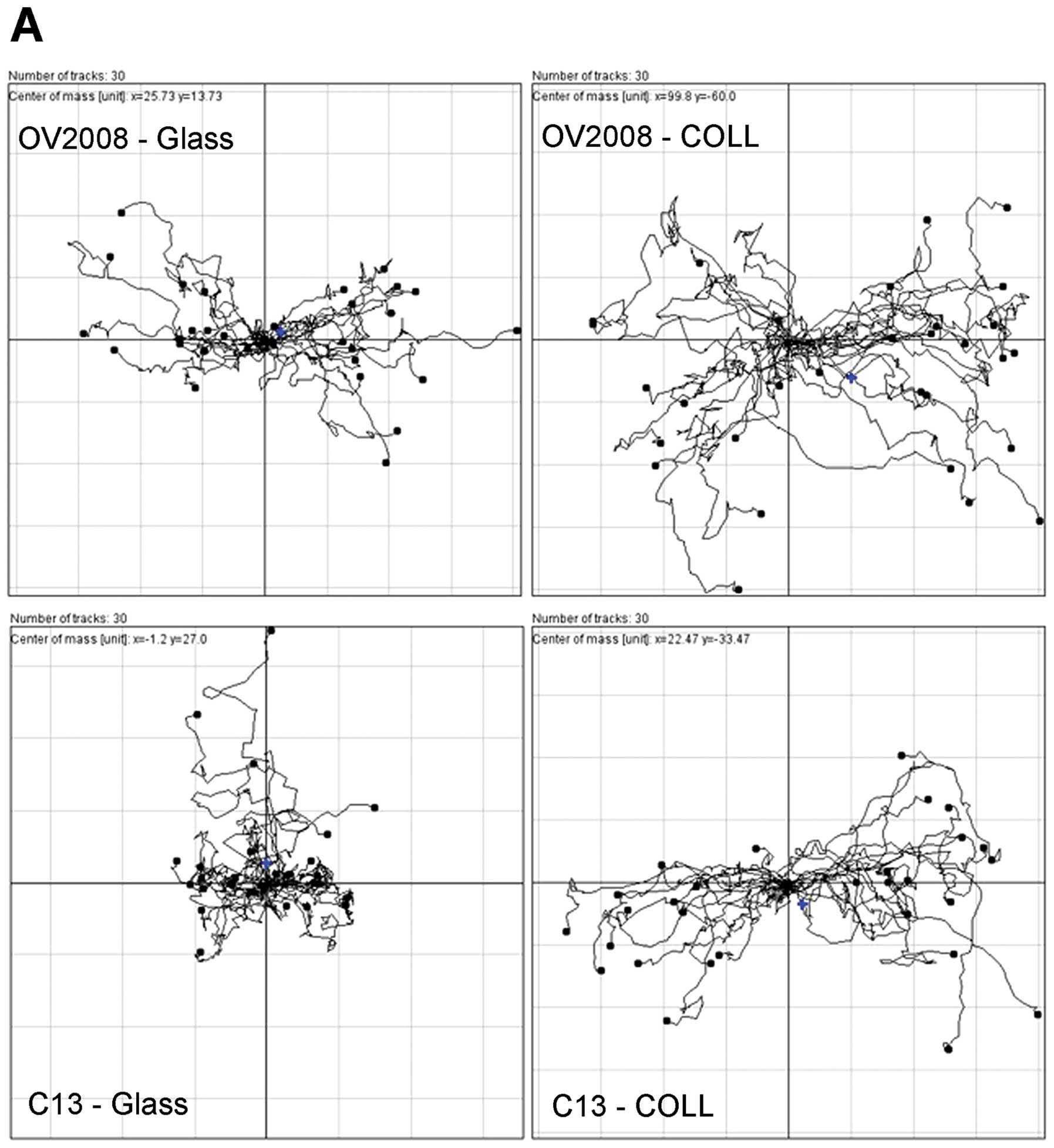
In order to determine if the wound was being closed
due to cell movement into the wound, individual cells randomly
selected along the border of the wounds were tracked in each frame
of the time-lapse recordings. Inspection of the time lapse
recording reveals that the OV2008 cells do migrate into the open
area to heal the wound whereas the C13 cells do not generally move
into the wound. Somewhat surprisingly, both cell lines have highly
motile cells. The OV2008 cells primarily moved perpendicular to the
edges of the wound into the open area to effectively close the
wound. In contrast to the OV2008 cells, the C13 cells did not move
towards the open area, rather C13 cells tended to move parallel to
the wound edges and to often change direction. Although the C13
cells were motile, the cell movement did not result in wound
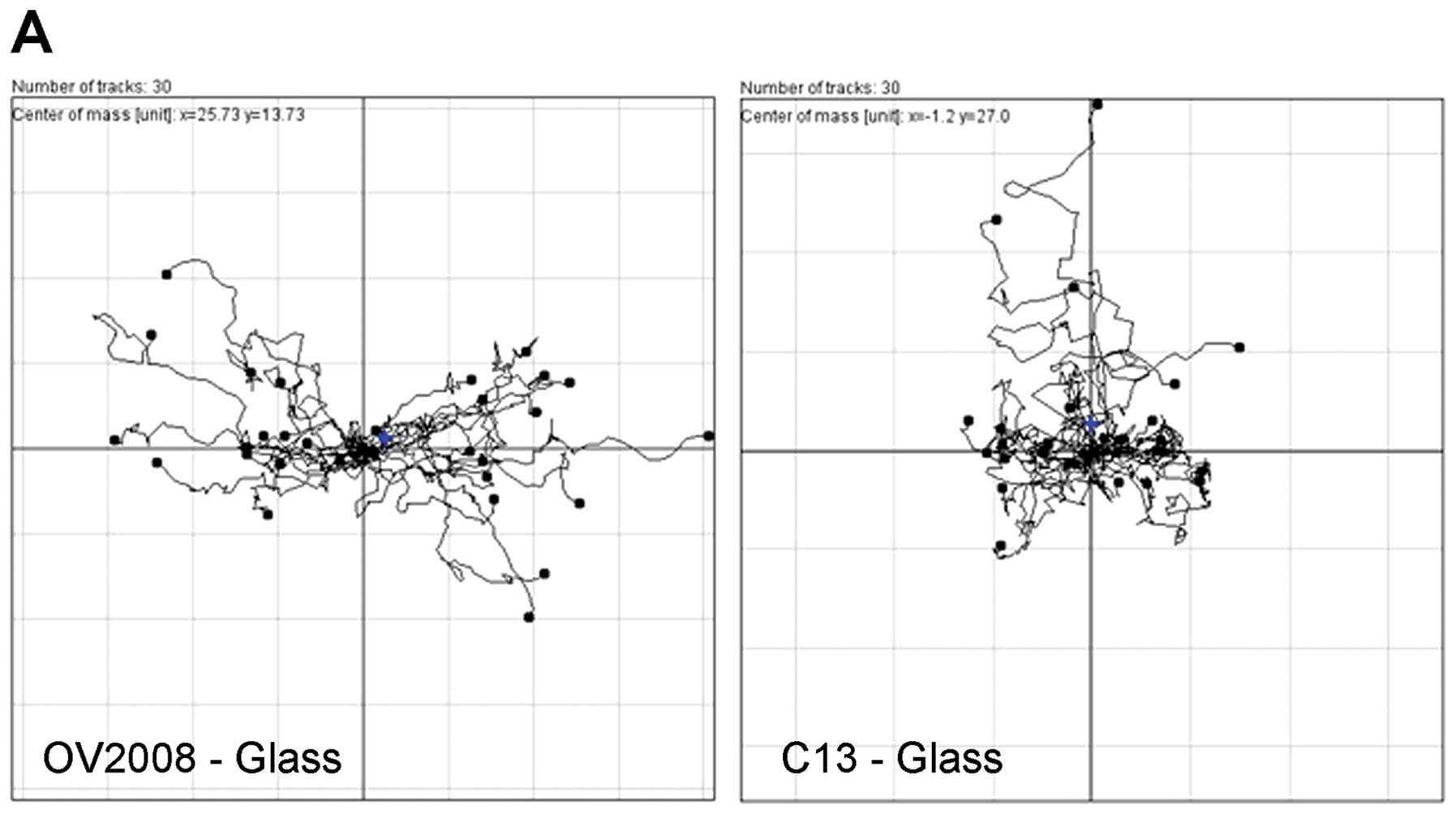
closure. When the trajectory of cells was plotted on a polar
coordinate grid (Fig. 4), a
directionality index (Euclidean distance/total distance moved) can
be developed where an index of 1.0 represents completely
directional movement in a straight line perpendicular to the wound
edge and an index of 0.0 represents totally random cell movement.
There is a 1.3-fold greater tendency of the OV2008 cells to move in
a directional fashion than for C13 cells. Thus, as measured by two
different motility assays, OV2008 cells appear to have a greater
capacity for directional movement as compared to the C13 cell
line.
Collagen type I enhances the migration of
ovarian cancer cells
The effect of collagen I on the migratory behavior
of the OV2008 and C13 cells plated on collagen I-coated cover slips
is illustrated in Fig. 5. Although
there was a slight decrease in the amount of wound remaining open
10 h post-wounding when compared as a percentage of the area of the
original wound (0 h) for cells plated on collagen I compared to
cells plated on glass for the OV2008 cells, the difference was not
significant. In contrast, collagen I significantly increased total
wound healing motility in C13 cells such that only about 10% of the
wound area remained open. Furthermore, there was no longer a wound
healing difference between the two cell lines and thus collagen I
makes the two cell lines phenotypically similar in regard to
migratory capacity.
Analysis of time lapse recordings for directional
cell movement demonstrates that as with wound healing, collagen I
did not alter the directionality of OV2008 cells during wound
healing (Fig. 6). In contrast, the
directionality of individual cells during wound healing was
increased in C13 cells by collagen I as compared to glass (Fig. 6A) as was the directionality index
(Fig. 6B). Thus collagen I allows
C13 cells to express a migratory behavior similar to that of OV2008
cells.
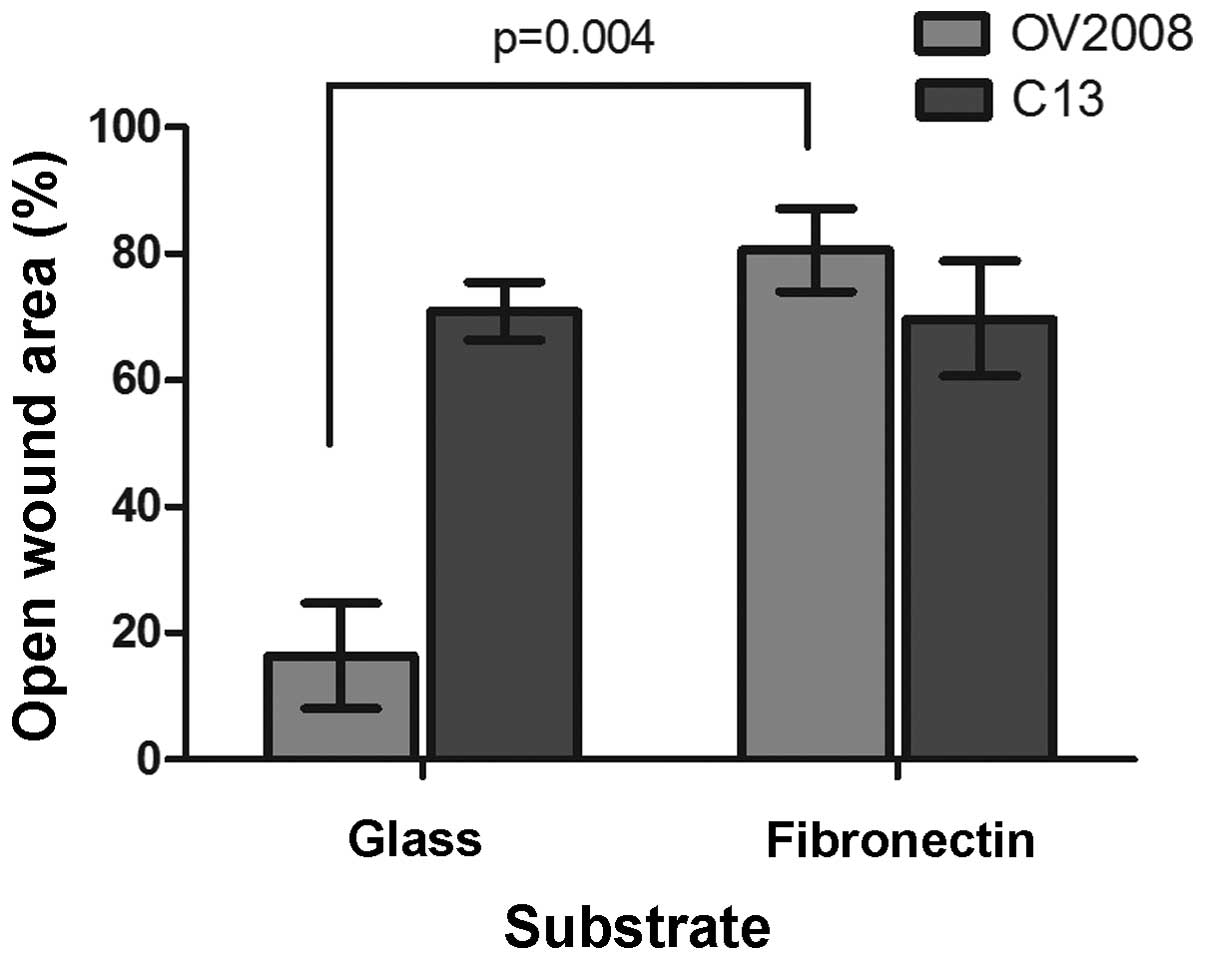
Fibronectin reduces the migratory
capacity of OV2008 cells
OV2008 and C13 cells cultured on fibronectin-coated
cover slips move differently than on those coated with collagen I.
In contrast to collagen I, fibronectin significantly reduced OV2008
total wound healing motility as illustrated in Fig. 7 and there was essentially no effect
of fibronectin on C13 total wound healing motility. So, OV2008
cells cultured on fibronectin- coated glass appeared to express a
phenotype similar to C13 cells migrating on uncoated glass or
fibronectin.
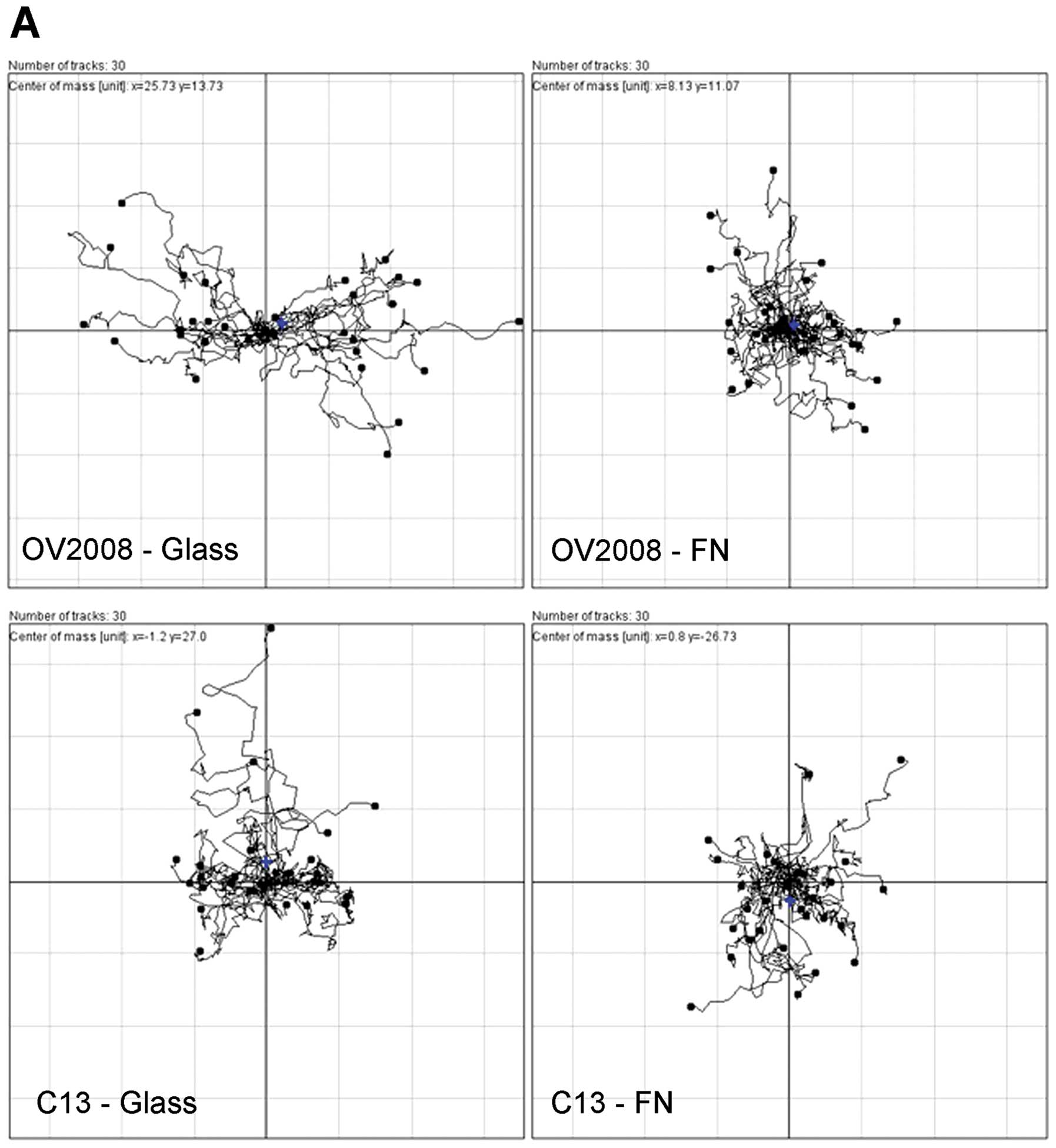
In terms of directionality as determined from time
lapse microscopy, the effect of fibronectin was opposite of that
for collagen I, such that the directionality of C13 cells was not
changed when the cells were cultured on fibronectin and the
directionality of individual cells during wound healing was
decreased for OV2008 cells by fibronectin as compared to glass
(Fig. 8).
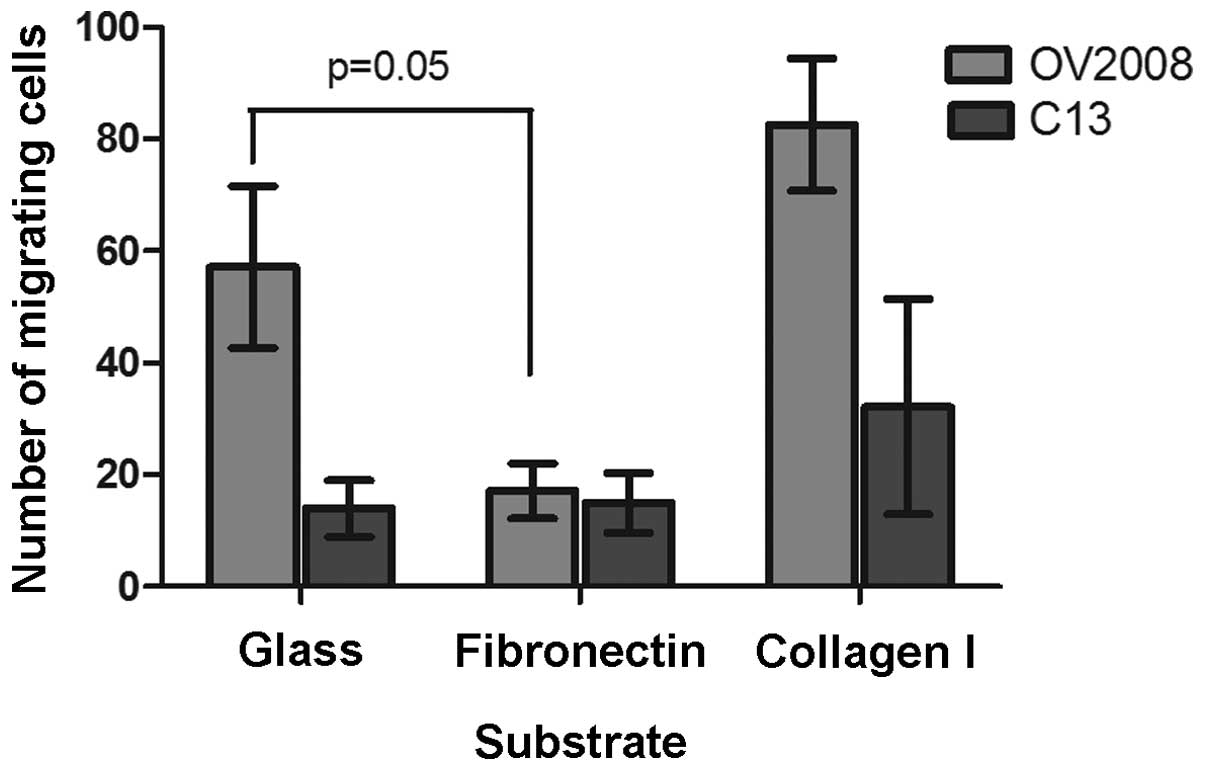
In addition to the effect of collagen I and
fibronectin on wound healing and directionality, migration across a
membrane was effected. There was a significant 65% reduction in the
number of OV2008 cells that migrated through the fibronectin-coated
membrane than through the uncoated membranes as illustrated in
Fig. 9. There was no effect of the
fibronectin coating on migration of C13 cells through the insert
membranes. In contrast and consistent with the effect observed in
the wound healing assays, collagen type I increased the migration
of the cells through the culture insert membranes such that there
was a 2.3-fold increase in the number of C13 cells that migrated
through the collagen I coated membranes than through the uncoated
membranes as illustrated in Fig.
9. Although not statistically significant, there also was a
1.7-fold increase in the number of OV2008 cells migrating through
the collagen I coated membranes. Thus, two different motility
assays showed that fibronectin decreases and collagen I increases
the migratory ability of the OV2008 and C13 cells.
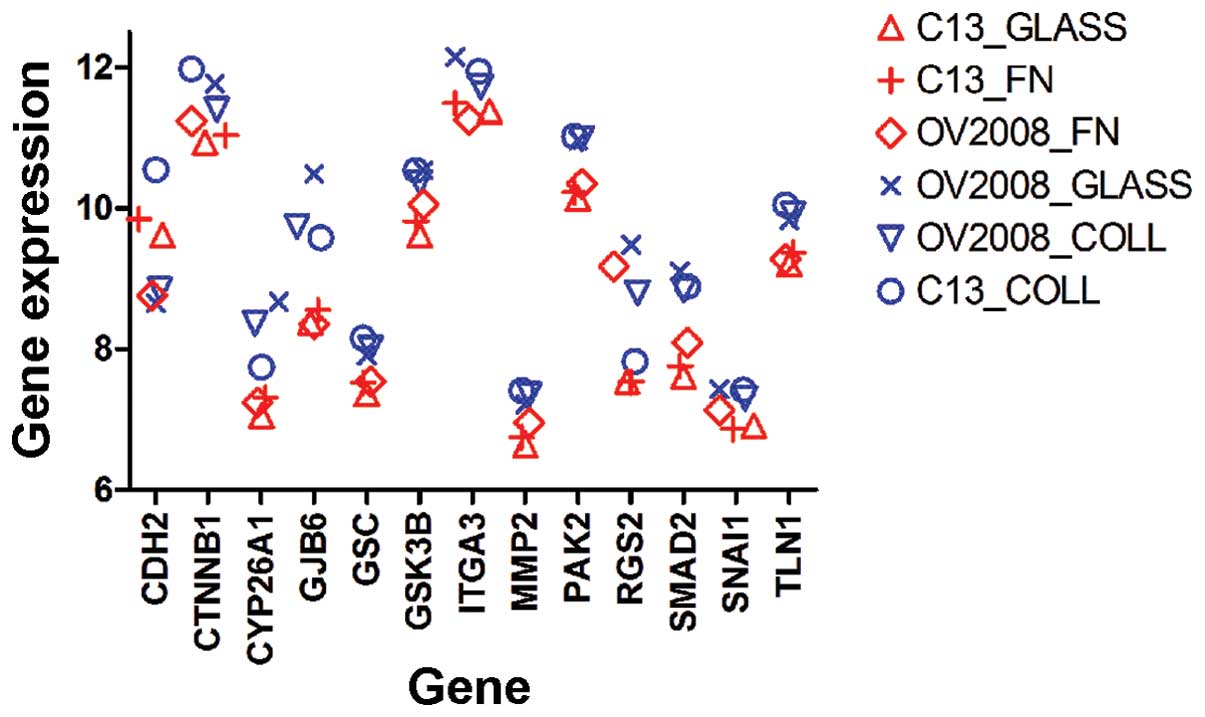
Identification of relevant genes
Exploratory analyses of gene expression in cells
expressing the different cell movement phenotypes observed when the
cells were cultured on glass, collagen I or fibronectin, were
performed for each of the cell lines for each of the above
conditions. Treatments were divided by phenotype into two groups:
motile/directional cells (OV2008-Glass, OV2008-Collagen,
C13-Collagen) and the less motile/less directional cells
(C13-Glass, C13-Fibronectin, OV2008-Fibronectin) and it was
apparent that there was differential expression of several genes
known to play a role in cell motility. Treatments were divided by
phenotype into two groups (Fig.
10): motile cells (OV2008-Glass, OV2008- Collagen,
C13-Collagen) and the less motile cells (C13-Glass,
C13-Fibronectin, OV2008-Fibronectin).
Pak2 inhibition and knockdown reduces the
ability of ovarian cancer cells to migrate
Among the candidate shown in Fig. 10, p21-activated kinase 2 (PAK2)
provided tight grouping and distinct separation of the motility
phenotypes and has been reported to be either upregulated or
hyperactivated in a variety of human cancers. In order to determine
the role of PAK in the migratory changes that were observed when
the cells were grown on collagen type I, both the OV2008 and C13
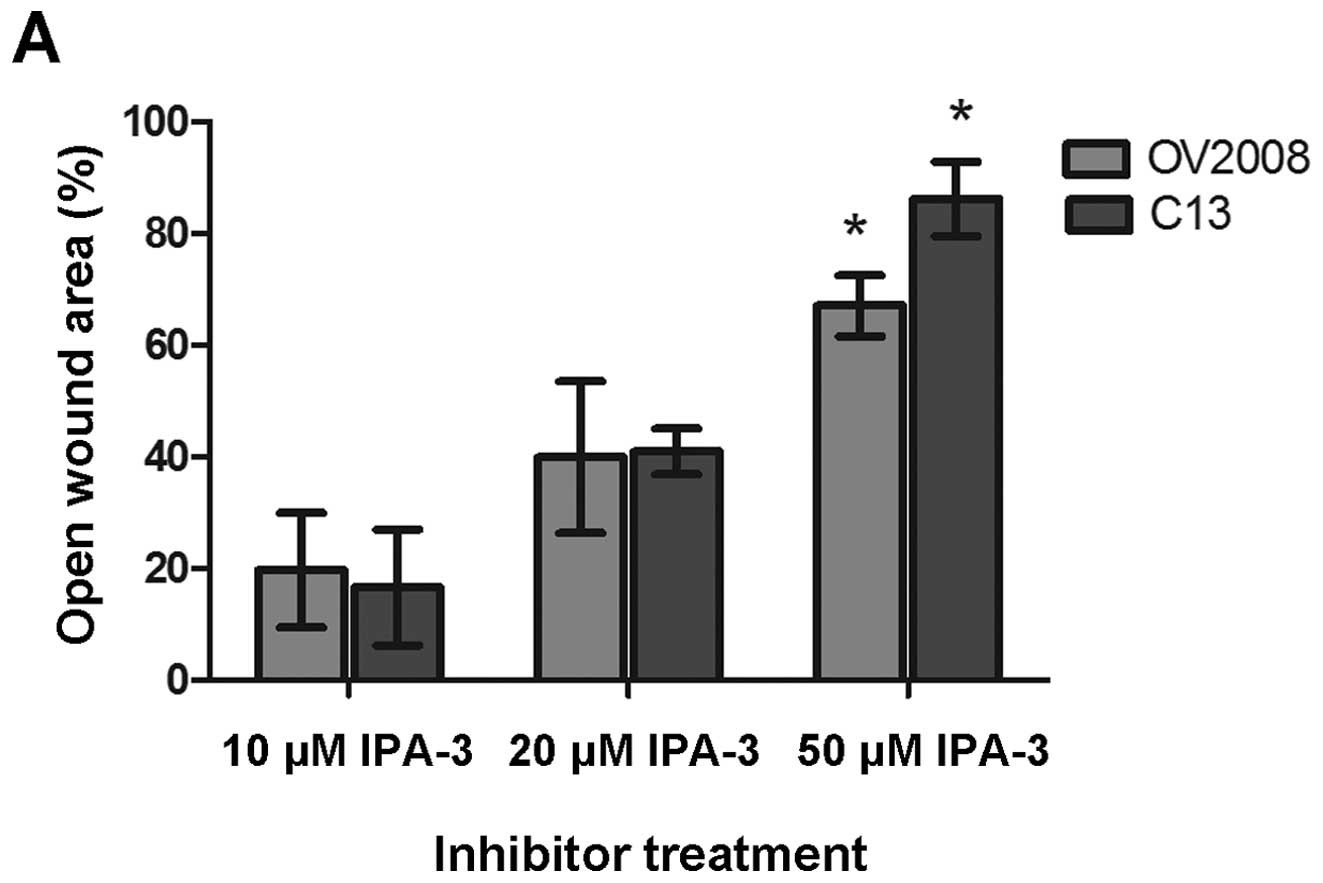
cells were pretreated with the PAK inhibitor, IPA-3, that targets
the auto-regulatory mechanism of group I PAKs. When C13 cells were
cultured on collagen I and treated with increasing concentrations
of the PAK inhibitor (IPA-3) for 2 h prior to the wound healing
assay, there was a dose-dependent response such that there was
increase in the percent of open wound area with an increasing
inhibitor concentration (Fig.
11A). There was a similar effect on wound healing in OV2008
cells cultured on collagen I and treated with increasing
concentrations of IPA-3.
As shown in Fig.
11B, when C13 cells were cultured on collagen I and treated
with increasing concentrations of IPA-3 for 2 h prior to the wound
healing assay, there was also a dose-dependent response in terms of
individual cell directionality. Directionality of OV2008 cells was
similarly affected. Thus, inhibition of PAK prevented the
motility/directionality promoting effect of collagen I on
cells.
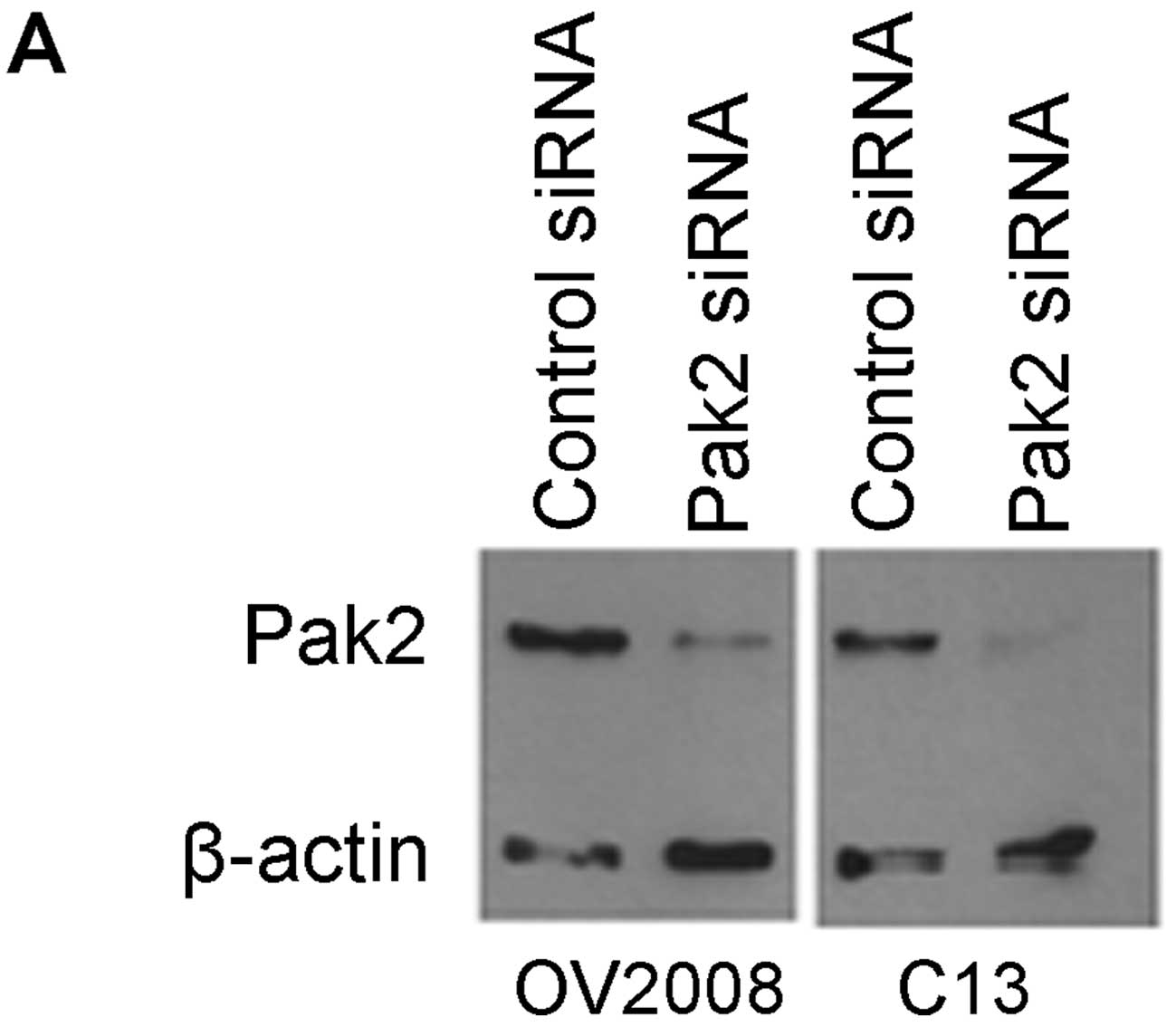
Since there appeared to be an effect of IPA-3
inhibition on both OV2008 and C13 cell migration, siRNA
transfection was used to specifically inhibit the Pak2 isoform of
PAK prior to wound healing assays on collagen I-coated coverslips.
Western blotting was used to verify that Pak2 protein was
effectively knocked down following transfection with Pak2 siRNA
(Fig. 12A). When the mean amount
of wound remaining open 20 h post-wounding was compared as a
percentage of the area of the original wound (0 h), there was a
significant 4-fold greater open wound area remaining in OV2008
cells receiving Pak2 siRNA than the cells receiving control siRNA
(Fig. 12B). Although there was a
similar 4-fold greater open wound area remaining in C13 cells
receiving Pak2 siRNA than the cells receiving control siRNA, the
difference did not achieve statistical significance (Fig. 12B). Additionally, transfection
with Pak2 siRNA significantly decreased the ability of OV2008 cells
to move in a directional manner on collagen I (Fig. 12C). However, the directionality of
C13 cells on collagen I was not affected by Pak2 knockdown.
Discussion
The current studies investigated the motility
characteristics of two related ovarian cancer cell lines (OV2008
and C13) and found that OV2008 cells had a much greater capacity to
migrate through a porous membrane of a transwell insert and heal a
scratch wound when cultured on glass than the C13 cells.
Furthermore, the studies indicate that wound healing is due to
characteristics associated with cell movement rather than cell
proliferation and specifically attributable to a difference in
directionality as opposed to differences in the total distance that
the cells moved. These differences were unexpected because the C13
cells were selected to have reduced chemo-sensitivity via a series
of in vitro cisplatin challenges of the parental OV2008 cell
(23) with no attention given to
changes in motility. In subsequent experiments, these differences
in cell motility could be negated or exaggerated by culturing the
cells on one ECM substrate versus another.
Culturing cells on collagen I coated cover slips or
transwell membranes increased total wound healing and individual
cell directionality, as well as migration through a porous membrane
for both cell lines with the effect being the most dramatic on the
migration of C13 cells. While the two cell lines migrated
differentially on uncoated glass, they become phenotypically
similar when plated on collagen I coatings. These findings are
consistent with previous reports in the literature which used
collagen I as a chemo- and haptotactic attractant for ovarian
cancer cells. Recently, Shen et al (24) observed that ovarian cancer cells
demonstrate enhanced migration through a collagen type I-coated
transwell invasion assay. Similarly, Ahmed et al (25) showed that collagen I enhanced the
migration of ovarian cancer cell lines through a Boyden chamber
when collagen I was used as a chemo-attractant in the lower part of
the chamber as well as when it coated the upper part of the
chamber. The current studies confirm these findings and further
reveal that the effect of collagen type I on the migration of
ovarian cancer cells is at least in part due to an increase in
directionality. Although the complex process of cellular motility
is well studied and it is known that cells must polarize, protrude,
adhere and reorganize the cytoskeleton in order to move, it is not
clearly understood how the basic motility machinery is coupled to a
steering mechanism that integrates environmental cues such as ECM
(26). By showing in the present
investigation that collagen I provides ‘steering’ cues to migrating
ovarian cancer cells, we can better understand how collagen type I
facilitates cancer cell movement.
Understanding the effect of collagen type I is
important because ovarian tumor cells have been shown to attach
preferentially at locations where the mesothelium is disrupted and
the underlying collagen I-rich stromal matrix is exposed (27). Collagen type I is abundant in the
interstitial matrix and therefore is a substrate that ovarian
cancer cells disseminated to the peritoneal cavity will inevitably
encounter upon attachment and exposure to the submesothelial ECM
during invasion (28). Moser et
al (29) demonstrated that
ovarian carcinoma cells adhere preferentially to type I collagen
and Burleson et al (28)
reported that ovarian cancer spheroids can completely disaggregate
on type I collagen coatings. Both of these studies examined
disaggregation of cells from spheroids on collagen type I, so the
effect of collagen I on cell motility was only indirectly inferred,
whereas the current studies tracked individual cells and provide
direct measures of distance and direction for cell movement in
response to being cultured on collagen I.
In contrast to the effects of collagen I on cell
movement, in the current investigation fibronectin (FN) reduced the
migration of OV2008 cells as measured by two different migration
assays. While the two cell lines migrated differentially on
uncoated glass, they become phenotypically similar when plated on
fibronectin coatings, adopting the movement characteristic of C13
on glass. That is, the cells migrate less directionally on FN than
on collagen type I or uncoated glass. Fibronectin is another
important constituent of the ECM at the metastatic site of ovarian
cancer. Mesothelial cells express fibronectin on the apical surface
and soluble FN has been detected in the ascities fluid from ovarian
cancer patients. However, the impact of fibronectin on ovarian
cancer cells is less clear than that of collagen I with evidence
for both increased and decreased movement in association with FN.
It has been reported that FN released by peritoneal mesothelial
cells stimulates ovarian cancer cell motility (30). In a study by Ahmed et al
(25), fibronectin increased the
migration of ovarian cancer cells through a Boyden chamber when
used as a chemoattractant in the lower part of the chamber.
Fibronectin has been reported to increase the migration of
pancreatic (31) and breast cancer
(32) cells as well. On the other
hand, there is evidence indicating that metastatic potential is
inversely correlated with FN expression (33,34).
Also, a correlation between the loss of FN and acquisition of the
malignant phenotype in vitro and of tumorigenic and
metastatic phenotypes in vivo has been reported (35,36).
It is possible that migration on fibronectin is
mediated by other proteins that are differentially expressed in
OV2008 and C13 cells. Jiao et al (37) recently showed that human melanoma
cell migration on fibronectin was greatly decreased when matrix
metallopeptidase-2 (MM P-2) activity was inhibited. The authors
(37) suggested that active MMP-2
is recruited to the leading edge of invasive tumor cells and
cleaves fibronectin into shorter fibronectin products. The
fibronectin fragments promote αvβ3 integrin recruitment to the area
of cleaved fibronectin products to facilitate tumor cell adhesion
and migration. Interestingly, MMP-2 was identified in the current
investigation via microarray as being expressed higher in the more
motile cells than in the less motile cells and the expression was
decreased by FN in both cell lines. An elegant study by Kenny and
Lengyel (38) used several
different models to examine the expression of MM P-2 in ovarian
cancer cells. In one model, a novel organotypic 3D coculture model
mimicking human omentum was created using collagen I, human primary
mesothelial cells (HPMCs), and human primary fibroblasts (HPFs)
that were extracted from human omentum. The relative expression of
MM P-2 mRNA was 20-fold higher in ovarian cancer cells attached to
the 3D coculture than in ovarian cancer cells adhering to plastic.
Thus it is possible that in the current study that the differential
expression of MMP-2 influenced the ability of cells to interact
with the FN and their ability to migrate on fibronectin-coated
surfaces.
The results of the migration assays in the present
investigation clearly demonstrated that collagen I and fibronectin
modify the migratory process of ovarian cancer cells and
microarrays were used to study gene expression patterns and narrow
down the list of possible mediators. When comparing the gene
expression profiles of OV2008 and C13 cells grown on glass,
collagen I and fibronectin, a relatively short list of candidate
genes was established. Among the candidates, p21-activated kinase
has been reported to be either upregulated or hyperactivated in a
variety of human cancers such as breast, ovary, colorectal, thyroid
and pancreatic (21). In addition,
it is activated by the Rho family GTPases Cdc42, Rac and Rho, which
are activated in response to ECM adhesion (39).
Initial screening for the role of PAKs in mediating
directional movement of OV2008 and C13 cells was accomplished using
the group I PAK (PAK1, PAK2, PAK3) chemical inhibitor, IPA-3 which
was first identified as a highly selective inhibitor of group I PAK
kinases by testing its effect on over 200 different kinases
(40). It was reported that this
selectivity was achieved by targeting the distinct autoregulatory
mechanism conserved in group I PAKs (40). In the present study, inhibition of
group I PAKs with IPA-3 resulted in decreased total wound healing
and directionality of OV2008 and C13 cells grown on collagen I and
confirmed a role for one or more of the group I PAKs. While a
majority of the literature is focused on the Pak1 isoform, both
Pak1 and Pak2 have been implicated in cell motility. Coniglio et
al (41) reported that Pak2
was needed to generate new focal adhesions and to limit the sizes
of focal adhesions in breast cancer cells. In prostate cancer
cells, Bright et al (42)
observed that both Pak1 and Pak2 affected migration speed.
Knockdown of Pak1 and Pak2 in ovarian cancer cell lines reduced
cell migration and invasion (43).
Pak2 has also been implicated in the migration of hepatocellular
carcinoma cells (44) and
monocytes (45). In order to
specifically assess the role of the Pak2 isoform, which was
identified as a candidate in the microarray experiments, knockdown
of Pak2 with an isoform specific siRNA sequence was performed and
resulted in decreased overall cell migration as well as decreased
directionality of individually migrating cells.
Pak2 appears to play a role in generating new focal
adhesions as well as limiting the sizes of focal adhesions.
Pak2-depleted breast cancer cells contain significantly larger
focal adhesions and are unable to generate new focal adhesions
(41). During cell migration, the
assembly, maturation, translocation and disassembly of focal
adhesions mediate, respectively, cell attachment, contraction,
protrusion of leading edges and retraction of trailing edges
(46). Therefore, by controlling
the generation and size of focal adhesions, Pak2 may play an
important role in the regulation of cell migration. Thus the loss
of general movement and directionality in cells that were
transfected with Pak2 siRNA could be due to unrestricted, large
focal adhesions anchoring the cells to the substrate which would
prevent any movement of the cells. Another of our candidate genes,
talin-1 (TLN1), would be worthy of investigation in the future.
TLN1 has been shown to play important roles in adhesion,
cytoskeletal organization and regulation of integrin signals during
cell migration (47).
In the traditional analysis of wound healing assays,
the width of a scratch is compared at time point 0 to that at the
end of the experiment. When the wound has not healed, the cells are
assumed to not have migrated (48). However, time lapse microscopy
revealed that even when the cells were not healing the wound, they
were indeed moving. By tracking individually migrating cells, we
were able to measure the distance and direction of the cells moved
and show that collagen I has the ability to direct cell movement
into the wound and thus promote healing. In contrast, fibronectin
was able to decrease directionality in OV2008. Directed cellular
migration is important in facilitating the coordinated movement of
cells throughout development and in wound repair (49). Directional movement may also affect
the ability of metastasizing ovarian cancer cells to colonize the
peritoneal microenvironment. The ability of fibronectin to inhibit
metastasis in some studies may be in part to the effect on
directionality. Finally, our results also suggest that the use of
PAK inhibitors should be explored for possible use in conjunction
with tumor debulking to limit the spread of ovarian metastases
throughout the peritoneal cavity.
References
|
1
|
Siegel R, Ma J, Zhou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
Palmer TD, Ashby WJ, Lewis JD and Zijlstra
A: Targeting tumor cell motility to prevent metastasis. Adv Drug
Deliv Rev. 63:568–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fishman DA, Kearns A, Chilukuri K, et al:
Metastatic dissemination of human ovarian epithelial carcinoma is
promoted by α2β1-integrin-mediated interaction with type I
collagen. Invasion Metastasis. 18:15–26. 1998.
|
|
4
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geiger B, Spatz JP and Bershadsky AD:
Environmental sensing through focal adhesions. Nat Rev Mol Cell
Biol. 10:21–33. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grashoff C, Hoffman BD, Brenner MD, et al:
Measuring mechanical tension across vinculin reveals regulation of
focal adhesion dynamics. Nature. 466:263–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hodivala-Dilke KM, McHugh KP, Tsakiris DA,
et al: β3-integr-indeficient mice are a model for Glanzmann
thrombasthenia showing placental defects and reduced survival. J
Clin Invest. 103:229–238. 1999.
|
|
8
|
Ridley AJ: Rho GTPases and actin dynamics
in membrane protrusions and vesicle trafficking. Trends Cell Biol.
16:522–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spiering D and Hodgson L: Dynamics of the
Rho-family small GTPases in actin regulation and motility. Cell Adh
Migr. 5:170–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Horssen R, Galjart N, Rens JA,
Eggermont AM and ten Hagen TL: Differential effects of matrix and
growth factors on endothelial and fibroblast motility: application
of a modified cell migration assay. J Cell Biochem. 99:1536–1552.
2006.PubMed/NCBI
|
|
11
|
Vorotnikov AV: Chemotaxis: movement,
direction, control. Biochemistry (Mosc). 76:1528–1555. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vicente-Manzanares M, Webb DJ and Horwitz
AR: Cell migration at a glance. J Cell Sci. 118:4917–4919. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arias-Romero LE and Chernoff J: A tale of
two Paks. Biol Cell. 100:97–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manser E, Leung T, Salihuddin H, Zhao ZS
and Lim L: A brain serine/threonine protein kinase activated by
Cdc42 and Rac1. Nature. 367:40–46. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edwards DC, Sanders LC, Bokoch GM and Gill
GN: Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase
signalling to actin cytoskeletal dynamics. Nat Cell Biol.
1:253–259. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chew TL, Masaracchia RA, Goeckeler ZM and
Wysolmerski RB: Phosphorylation of non-muscle myosin II regulatory
light chain by p21-activated kinase (γ-PAK). J Muscle Res Cell
Motil. 19:839–854. 1998.
|
|
17
|
Sanders LC, Matsumura F, Bokoch GM and de
Lanerolle P: Inhibition of myosin light chain kinase by
p21-activated kinase. Science. 283:2083–2085. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manser E, Huang HY, Loo TH, Chen XQ, Dong
JM, Leung T and Lim L: Expression of constitutively active α-PAK
reveals effects of the kinase on actin and focal complexes. Mol
Cell Biol. 17:1129–1143. 1997.
|
|
19
|
Nayal A, Webb DJ, Brown CM, Schaefer EM,
Vicente-Manzanares M and Horwitz AR: Paxillin phosphorylation at
Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and
protrusion dynamics. J Cell Biol. 173:587–589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bokoch GM: Biology of the p21-activated
kinases. Ann Rev Biochem. 72:743–781. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar R, Gururaj AE and Barnes CJ:
p21-activated kinases in cancer. Nat Rev Cancer. 6:459–471. 2006.
View Article : Google Scholar
|
|
22
|
Di Saida PJ, Sinkovics JG, Rutledge FN and
Smith JP: Cell mediated immunity to human malignant cells. Am J
Obstet Gynecol. 114:979–989. 1972.
|
|
23
|
Andrews PA, Muphy MP and Howell SB:
Differential potentiation of alkylating and platinating agent
cytotoxicity in human ovarian carcinoma cells by glutathione
depletion. Cancer Res. 45:6250–6253. 1985.
|
|
24
|
Shen Y, Shen R, Ge L, Zhu Q and Li F:
Fibrillar type I collagen matrices enhance metastasis/invasion of
ovarian epithelial cancer via beta1 integrin and PTEN signals. Int
J Gynecol Cancer. 22:1316–1324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahmed N, Riley C, Rice G and Quinn M: Role
of integrin receptors for fibronectin, collagen and laminin in the
regulation of ovarian carcinoma functions in response to a matrix
microenvironment. Clin Exp Metastasis. 22:391–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petrie RJ, Doyle AD and Yamada KM: Random
versus directionally persistent cell migration. Nat Rev Mol Cell
Biol. 10:538–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mochizuki Y, Nakanishi H, Kodera Y, et al:
TNF-alpha promotes progression of peritoneal metastasis as
demonstrated using a green fluorescence protein (GFP)-tagged human
gastric cancer cell line. Clin Exp Metastasis. 21:39–47. 2004.
View Article : Google Scholar
|
|
28
|
Burleson KM, Hansen LK and Skubitz AP:
Ovarian carcinoma spheroids disaggregate on type I collagen and
invade live human mesothelial cell monolayers. Clin Exp Metastasis.
21:685–697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moser TL, Pizzo SV, Bafetti LM, Fishman DA
and Stack MS: Evidence for prefer-ential adhesion of ovarian
epithelial carcinoma cells to type I collagen mediated by the α2β1
integrin. Int J Cancer. 67:695–701. 1996.PubMed/NCBI
|
|
30
|
Rieppi M, Vergani V, Gatto C, Zanetta G,
Allavena P, Taraboletti G and Giavazzi R: Mesothelial cells induce
the motility of human ovarian carcinoma cells. Int J Cancer.
80:303–307. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryschich E, Khamidjanov A, Kerkadze V,
Büchler MW, Zöller M and Schmidt J: Promotion of tumor cell
migration by extracellular matrix proteins in human pancreatic
cancer. Pancreas. 38:804–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maity G, Choudhury PR, Sen T, Ganguly KK,
Sil H and Chatterjee A: Culture of human breast cancer cell line
(MDA-MB -231) on fibronectin-coated surface induces pro-matrix
metalloproteinase-9 expression and activity. Tumour Biol.
32:129–138. 2011. View Article : Google Scholar
|
|
33
|
Urtreger AJ, Werbajh SE, Verrecchia F, et
al: Fibronectin is distinctly downregulated in murine mammary
adenocarcinoma cells with high metastatic potential. Oncol Rep.
16:1403–1410. 2006.PubMed/NCBI
|
|
34
|
Zhao H, Jhanwar-Uniyal M and Datta PK:
Expression profile of genes associated with anti-metastatic gene:
nm23-mediated metastasis inhibition in breast carcinoma cells. Int
J Cancer. 109:65–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akamatsu H, Ichihara-Tanaka K, Ozono K,
Kamiike W, Matsuda H and Sekiguchi K: Suppression of transformed
phenotypes of human fibrosarcoma cells by overexpression of
recombinant fibronectin. Cancer Res. 56:4541–4546. 1996.PubMed/NCBI
|
|
36
|
Der CJ and Stanbridge EJ: Alterations in
the extracellular matrix organization associated with the
reexpression of tumorigenicity in human cell hybrids. Int J Cancer.
26:451–459. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiao Y, Feng X, Zhan Y, Wang R, Zheng S,
Liu W and Zeng X: Matrix metalloproteinase-2 promotes αVβ3
integrin-mediated adhesion and migration of human melanoma cells by
cleaving fibronectin. PloS One. 7:1–12. 2012.
|
|
38
|
Kenny HA and Lengyel E: MMP-2 functions as
an early response protein in ovarian cancer metastasis. Cell Cycle.
8:683–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cox EA, Sastry SK and Huttenlocher A:
Integrin-mediated adhesion regulates cell polarity and membrane
protrusion through the Rho family of GTPases. Mol Biol Cell.
12:265–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deacon SW, Beeser A, Fukui JA, Rennefahrt
UE, Myers C, Chernoff J and Peterson JR: An isoform-selective,
small-molecule inhibitor targets the autoregulatory mechanism of
p21-activated kinase. Chem Biol. 15:322–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Coniglio SJ, Zavarella S and Symons MH:
Pak1 and Pak2 mediate tumor cell invasion through distinct
signaling mechanisms. Mol Cell Biol. 28:4162–4172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bright MD, Garner AP and Ridley AJ: PAK1
and PAK2 have different roles in HGF-induced morphological
responses. Cell Signal. 21:1738–1747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siu MK, Wong ES, Chan HY, et al:
Differential expression and phosphorylation of Pak1 and Pak2 in
ovarian cancer: effects on prognosis and cell invasion. Int J
Cancer. 127:21–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sato M, Matsuda Y, Wakai T, et al:
P21-activated kinase-2 is a critical mediator of transforming
growth factor-β-induced hepatoma cell migration. J Gastroenterol
Hepatol. 28:1047–1055. 2013.PubMed/NCBI
|
|
45
|
Gadepalli R, Kotla S, Heckle MR, Verma SK,
Singh NK and Rao GN: Novel role for p21-activated kinase 2 in
thrombin-induced monocyte migration. J Biol Chem. 288:30815–30831.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huttenlocher A and Horwitz AR: Integrins
in cell migration. Cold Spring Harb Perspect Biol. 3:a0050742011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cram EJ, Clark SG and Schwarzbauer JE:
Talin loss-of-function uncovers roles in cell contractility and
migration in C. elegans. J Cell Sci. 116:3871–3878. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ashby WJ and Zijlstra A: Established and
novel methods of interrogating two-dimensional cell migration.
Integr Biol (Camb). 4:1338–1350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lauffenburger DA and Horwitz AF: Cell
migration: a physically integrated molecular process. Cell.
84:359–369. 1996. View Article : Google Scholar : PubMed/NCBI
|