Introduction
Epithelial mesenchymal transition (EMT) not only
plays crucial roles in embryonic development or tissue repair, but
is also involved in fibrotic diseases and cancer progression
(1–3). During EMT, cells undergo profound
phenotypic changes including the loss of cell-cell adhesion, the
loss of cell polarity, the reorganization of cytoskeleton, and the
acquisition of migratory and invasive properties (4,5). A
network of transcriptional regulators is involved in the control of
EMT, which is coupled to posttranscriptional and posttranslational
modifications that amplify the initial signals (6). Several transcription factors have
been shown to be involved in this process, such as the Snail family
of zinc-finger transcription factors and the basic helix-loop-helix
factors Twist (7, 8). These transcription factors
downregulate epithelial markers (E-cadherin) and upregulate
mesenchymal markers (fibronectin, vimentin), which induce EMT and
consequently promote the development of metastatic properties
(9,10). Many extracellular matrix components
and cytokines, including Wnt, hepatocyte growth factor, epidermal
growth factor (EGF) and transforming growth factor-β (TGF-β), can
elicit EMT. Among them, TGF-β is a powerful inducer of EMT
(11). Depending on the specific
cellular context, different signaling pathways during TGF-β-induced
EMT can be activated and contribute to establish an organizing
center that in turn controls morphogenetic movement and
specification (12).
Idiopathic pulmonary fibrosis (IPF) is a specific
form of chronic progressive fibrosing interstitial pneumonia of
unknown cause that is limited to the lungs (13). A major factor in IPF pathogenesis
is thought to be an aberrant activation of alveolar epithelial
cells (AECs). AECs can transformed into (myo) fibroblasts, which
secrete an excessive amount of collagen to form fibers, impairing
organ function (14). There is
increasing evidence that EMT is involved in these processes during
pulmonary fibrogenesis and TGF-β is a major inducer of EMT in the
lungs (2). Previous studies
indicated the alveolar epithelial cells (AEC) underwent EMT during
the pulmonary fibrosis induced by TGF-β, and TGF-β1 induced A549
AEC to undergo EMT via Smad2 activation (15,16).
These results suggest that AEC serve as a source of fibroblasts in
lung fibrosis and highlight the potentially critical role of EMT in
the induction of fibrosis in the lung. Core of this process is
cytoskeleton reorganization (5).
After induction by proinflammatory cytokines, actin filament
architecture changes from cortical actin to stress fibers. However,
the precise mechanism underlying these structural rearrangements
remains unknown.
Ezrin, radixin and moesin, known as the ERM
proteins, are a group of membrane-cytoskeleton linkers, which are
closely associated with actin cytoskeleton remodeling (17,18).
Two groups have shown the role of ERM proteins in the actin
filaments rearrangement during EMT (19,20).
ERM proteins may be involved in regulating alveolar structure and
lung homeostasis. Moreover, dysregulation of the ERM-RAGE complex
might be an important step in rearrangement of the actin
cytoskeleton during proinflammatory cytokine-induced EMT of human
alveolar epithelial cells. Recent study showed that increased
moesin expression promotes EMT by regulating actin filament
remodeling, but ezrin expression decreased in NMuMG cells or
remained unchanged in A549 cells during TGF-β-induced EMT,
suggesting that the regulating mechanism of EMT by ezrin may be
different from that by moesin if it does play a role in this
process (21).
Podocalyxin (PODXL), as a member of the CD34 family,
is a type I transmembrane glycoprotein, playing an important role
in regulating cell adhesion and cell morphology (22). It has a number of interacting
partners, including the actin binding protein ezrin, the adhesion
molecule L-selectin and Na+/H+ exchanger
regulatory factor (NHERF) (23,24).
PODXL could increase migration and invasion, MMP expression, and
activation of MAPK and PI3K activity in MCF7 and PC3 cells through
interaction with ezrin (25).
Moreover, PODXL is markedly increased and required for TGF-β
induced EMT of A549 cells (26).
During this process, PODXL interacts with collagen type I, which
may control cell migration by regulating the dynamics of cell
protrusion formation.
In our study, we examine the role of ezrin in actin
filament reorganization and cell metastasis during TGF-β1-induced
alveolar EMT. Our finding also revealed an association between
ezrin and PODXL during EMT, suggesting ezrinpodocalyxin complex
might be important in actin filament remodeling during
TGF-β1-induced alveolar EMT.
Materials and methods
Antibodies
Monoclonal ezrin antibody, monoclonal or polyclonal
podocalyxin antibodies and monoclonal vimentin antibody were
obtained from Santa Cruz Biotechnology. Monoclonal E-cadherin
antibody was purchased from BD (Becton-Dickinson Co.). Polyclonal
GAPDH antibody was from Cell Signaling Technologies (Beverly, MA,
USA). Monoclonal β-actin antibody was from Proteintech. Secondary
antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 594 was
from Beyotime Institute of Biotechnology (China). Secondary
antibodies conjugated to peroxidase were obtained from Jackson
ImmunoResearch Laboratories Inc. (West Grove, PA, USA).
Cell culture and treatment
Human bronchoalveolar carcinoma cell H358, human
lung adenocarcinoma cell A549 and human lung adenocarcinoma cell
H1299 were purchased from the American Type Culture Collection
(ATCC, USA). All cell lines were grown in high-glucose Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) and maintained at 37°C with 5% CO2. A549
cells were treated with 5 ng/ml recombinant human TGF-β1 (Pepro
Tech) for 24 or 48 h to induce EMT.
RNA interference
Sequences of ezrin siRNA were designed and
synthesized by Thermo Scientific Dharmacon. Ezrin siRNA contains 4
individual siRNAs, Ezrin-siRNA-1: 5′-GCU CAA AGA UAA UGC UAU GTT-3′
(sense) and 5′-CAU AGC AUU AUC UUU GAG CTT-3′ (antisense).
Ezrin-siRNA-2: 5′-GGA AUC AAC UAU UUC GAG ATT-3′ (sense) and 5′-UCU
CGA AAU AGU UGA UUC CTT-3′ (antisense). Ezrin-siRNA-3: 5′-GCG CAA
GGA GGA UGA AGU UTT-3′ (sense) and 5′-AAC UUC AUC CUC CUU GCG
CTT-3′ (antisense). Ezrin-siRNA-4: 5′-GCG CGG AGC UGU CUA GUG
ATT-3′ (sense) and 5′-UCA CUA GAC AGC UCC GCG CTT-3′ (antisense).
The negative control siRNA was also purchased from Thermo
Scientific Dharmacon. The cells were transfected with ezrin siRNA
or negative control siRNA using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s
instructions.
Immunofluorescent analysis
Cells were fixed with 4% paraformaldehyde for 30
min, permeated with 0.1% Triton X-100 for 10 min at room tempreture
(RT), followed by blocking in 10% normal goat serum for 1 h, and
then incubated with the primary antibody at 4°C overnight. After
washing, the slides were incubated with Alexa flour 488-conjugated
secondary antibody, followed by nuclear counterstaining with DAPI
for 10 min. F-actin was stained using rhodamine
conjugated-phalloidin (Invitrogen). Cells were imaged using a 40X
EC Plan Neofluar/1.30 oil immersion objective on an inverted
laser-scanning confocal microscope (LSM510 META, Carl Zeiss) and
images were captured using Zeiss software.
Coimmunoprecipitation
Cells were rinsed with PBS twice and lysed in lysis
buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100) with 1X
proteinase inhibitor cocktail (Roche), 1 mM sodium fluoride, and 1
mM sodium orthovanadate (Amersco) for 30 min at 4°C. The lysates
were clarified by centrifugation at 13200 rpm for 20 min. Protein
concentrations were detected using the BCA protein assay kit. After
pre-clear, 1.5 mg total protein was immunoprecipitated with the
indicated primary antibody overnight, and incubated with 20 μl
Protein A/G Plus-Agarose beads for 4 h at 4°C. The
immunoprecipitates and the lysates were subjected to western
blotting using the antibody indicated.
Wetstern blotting
Cells were grown to confluence on culture dishes,
and lysed in ice-cold cell lysis buffer. Lysates were centrifuged
at 13,200 rpm for 20 min at 4°C to obtain the proteins, and then
protein concentration was determined by the BCA protein assay. The
protein lysates were separated by SDS-PAGE and then transferred to
PVDF membrane (Millipore). The membranes were blocked with 5%
non-fat milk solution for 1 h at room temperature and incubated in
primary antibody dissolved in block solution at 4°C overnight. The
proteins were probed by antibody against ezrin, E-cadherin,
vimentin or β-actin. After washing, the membrane was incubated with
horseradish peroxidase-conjugated secondary antibody corresponding
to the primary antibody for 1 h at room temperature, and visualized
using ECL.
Would-healing assay
Adhered cell monolayers were scratched with a 200-μl
pipette tip and grown in DMEM medium with 10% FBS at 37°C with 5%
CO2. Wound healing capacity was monitored by microscopy
at 0, 12 and 24 h.
Cell invasion assay
After siRNA transfection, TGF-β1 induction was
started 48 h before the assay. The inserts were precoated with 40
μl BD Matrigel and then cells (5×104) were seeded in the
upper chamber and incubated for 12 h. The cells were fixed and
stained with crystal violet. Migrated cells in 5 randomly chosen
fields of each well were counted.
Results
Suppressing ezrin expression limits
morphological changes and actin filament remodeling during EMT
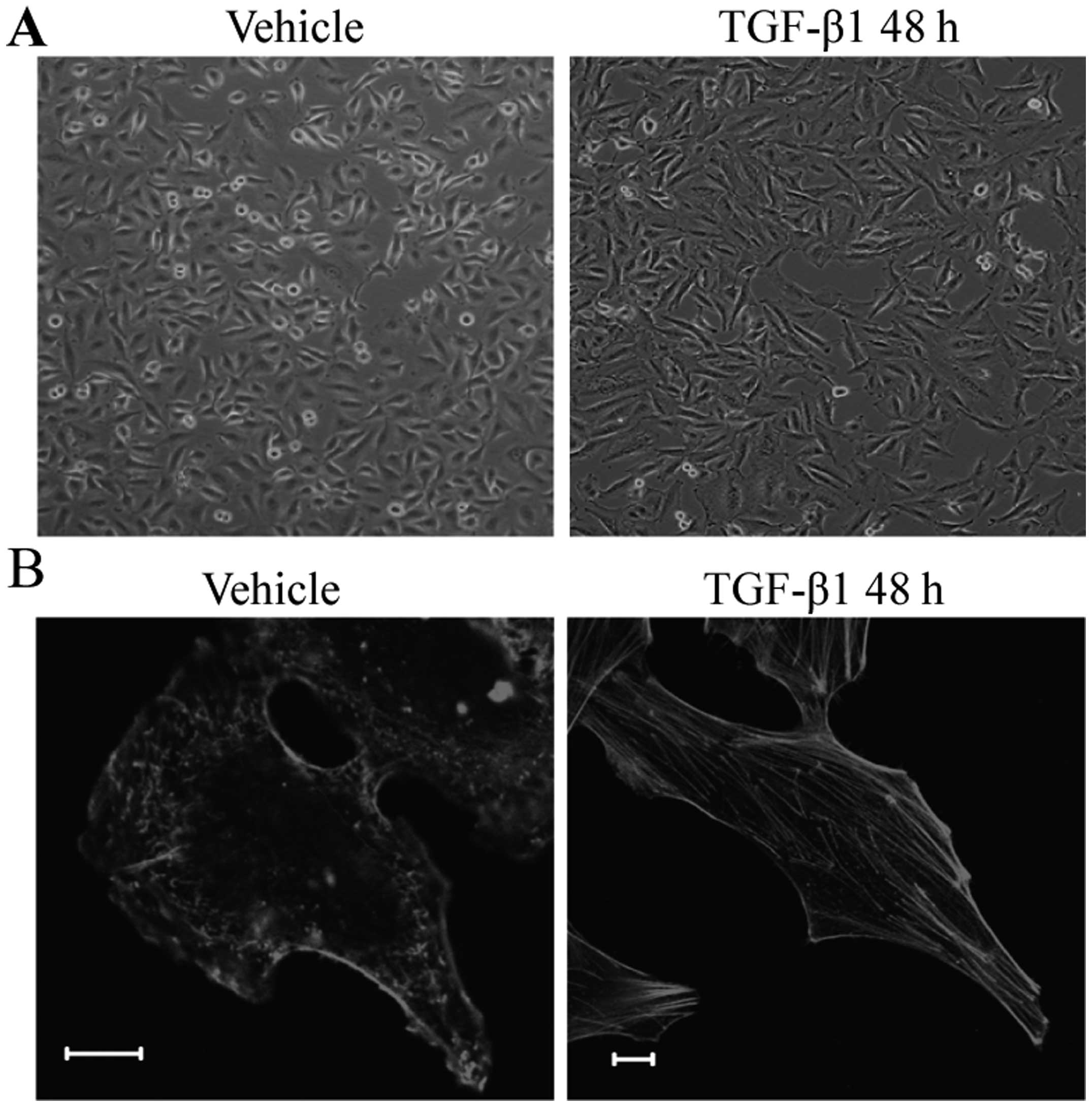
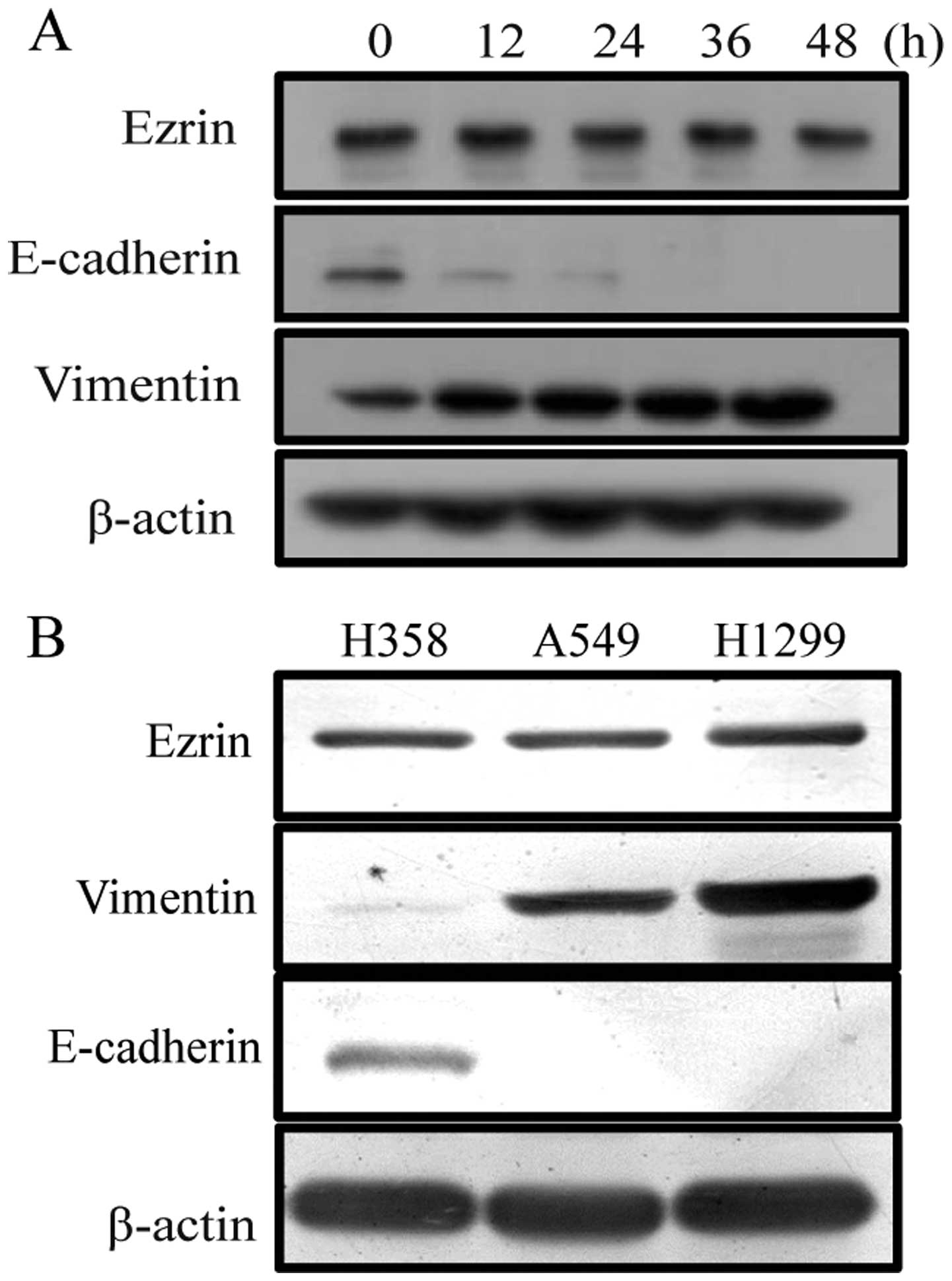
The morphological and biochemical features of A549
cells after TGF-β1 treatment were examined to confirm that EMT
properties were induced under our culture conditions. After TGF-β1
treatment, A549 cells underwent phenotypic changes including the
acquisition of spindle shape, the reorganization of actin filaments
from cortical thin bundles to thick parallel bundles or actin
stress fibers. In addition, the loss of expression of the
epithelial marker, E-cadherin, and an increase of expression of the
mesenchymal marker, vimentin further confirmed that EMT properties
were induced under our culture conditions (Fig. 1). To examine the functional
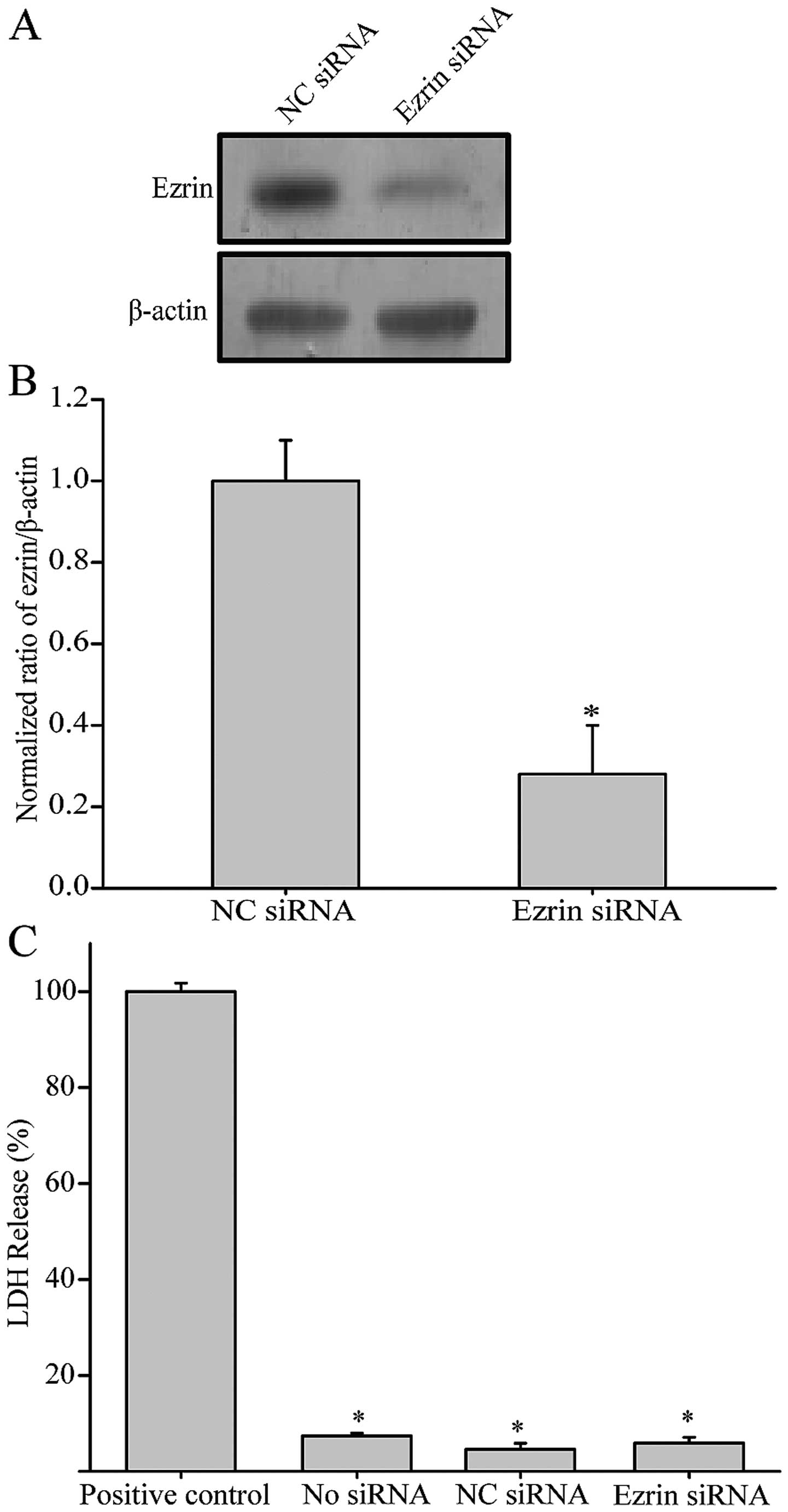
significance of ezrin during EMT, we chose to knock down ezrin
expression by using siRNA. Two days following the transfection with
ezrin siRNA, the level of ezrin expression was reduced by ~70% as
compared with the cells transfected with control siRNA (Fig. 2A and B). Additionally, there was no
significant difference in LDH release in ezrin siRNA transfected
cells as compared with the control, suggesting ezrin siRNA has no
cytotoxicity on the cells tested (Fig.
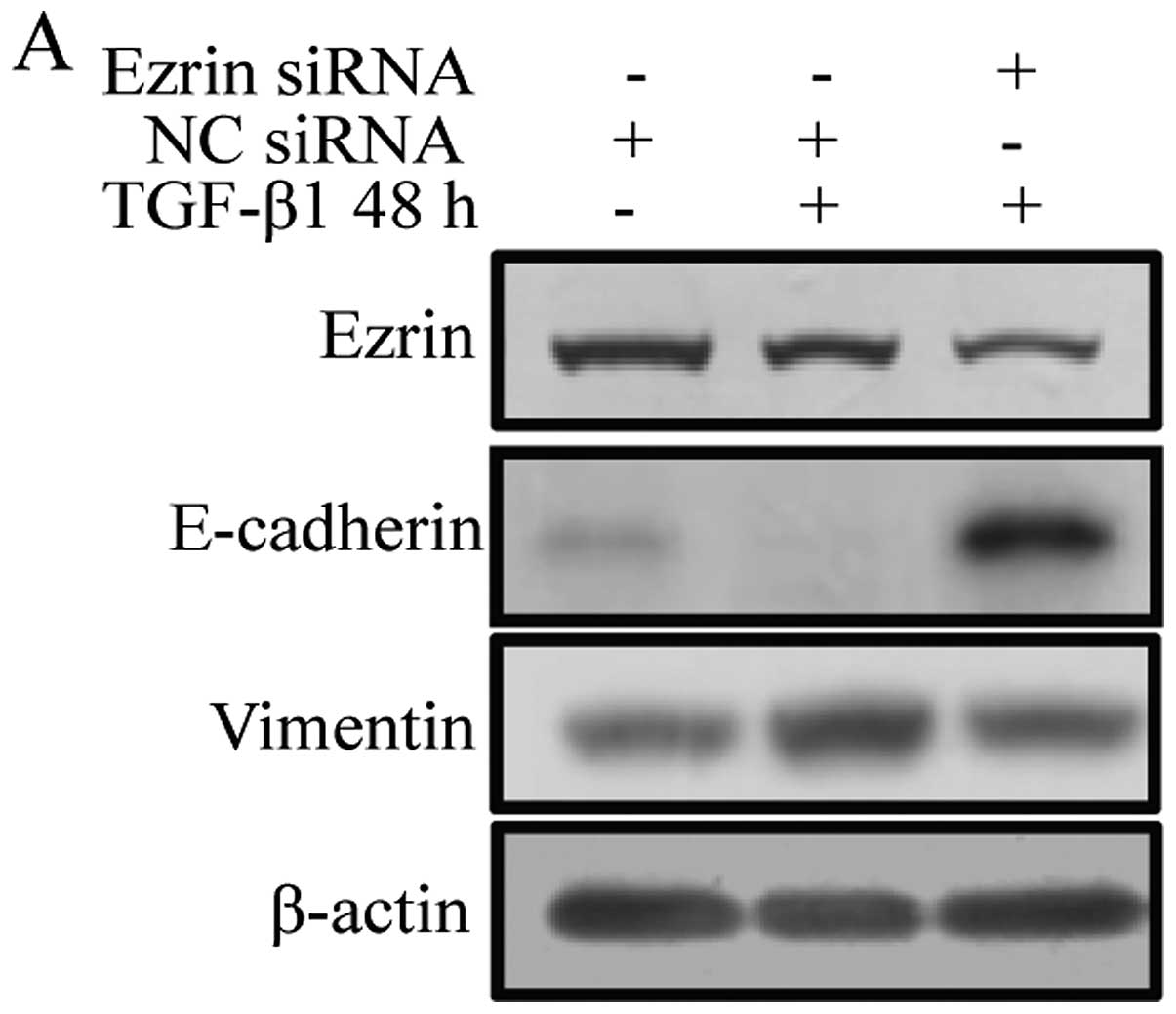
2C). After TGF-β1 treatment, control cells expressing negative
control siRNA showed decreased expression of E-cadherin and
increased expression of vimentin during EMT. Cells transiently
transfected with ezrin siRNA exhibited increased expression of
E-cadherin and decreased expression of vimentin in contrast to
negative control siRNA cells (Fig.
3A).
Compared with negative control siRNA cells, ezrin
siRNA cells treated with TGF-β had different morphology and actin
filament remodeling. After TGF-β1 treatment, actin filaments in
negative control siRNA cells changed from a cortical actin network
to thick bundles, or actin stress fibers and the cells elongated
and became spindle shape. However, cells transfected with ezrin
siRNA had fewer and thinner actin filaments, accompanied by
incomplete morphological transition as compared with control siRNA
cells (Fig. 3B). These results
indicate that ezrin is associated with morphological changes and
actin filament remodeling during EMT.
Suppressing ezrin expression during EMT
decreases cell migration and invasion
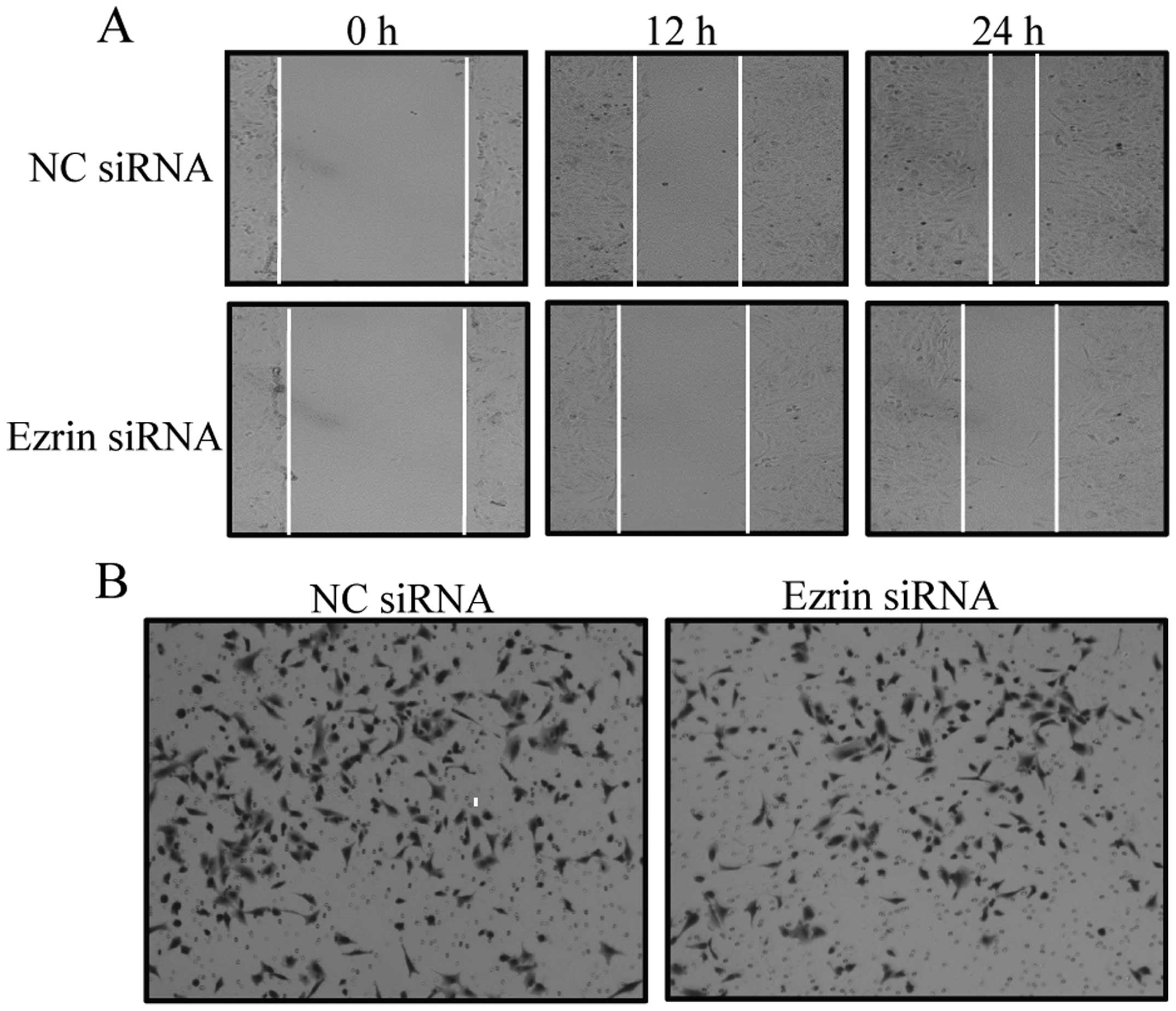
The acquisition of migratory and invasive properties
is one of the phenotypic changes during EMT. To determine the
relationship between ezrin and cell migratory and invasive
abilities during EMT, we used ezrin siRNA to suppress ezrin
expression and observed cell migration and invasion after TGF-β1
treatment. In a wound-healing assay, cells transfected with ezrin
siRNA showed decreased wound healing at the indicated time as
compared with control siRNA cells (Fig. 4A). Moreover, the invasive potential
of cells transfected with ezrin siRNA decreased as compared with
control siRNA cells (Fig. 4B and
C). These results demonstrated that ezrin is involved in
regulating cell migration and invasion during EMT, further
confirming ezrin is required for TGF-β1-induced EMT in A549
cells.
Differential ezrin localization is
associated with EMT characteristics in lung cancer cells
To further assess the involvement of ezrin
expression in TGF-β1-induced EMT, we observed the change of ezrin
expression level in A549 cells treated with TGF-β. However, the
result showed that the expression level of ezrin has no change in
A549 cells following TGF-β treatment (Fig. 5A), which is consistent with the
report from Haynes et al (21). Subsequently, we observed the change
of ezrin expression level in different lung cancer cell lines
(H358, A549 and H1299). Though these cell lines underwent EMT to
different extent, there was no obvious difference in the levels of
ezrin protein (Fig. 5B). However,
we found that ezrin was differently distributed in these lung cell
lines (Fig. 5C). In H358 cells,
ezrin was localized at microvilli throughout the entire cell. In
contrast, A549 cells showed that ezrin was distributed in the
cytoplasm. In H1299 cells, ezrin was found in the cytoplasm, and a
proportion of it was colocalized with F-actin. The localization of
ezrin and its colocalization with F-actin were in accordance with
the invasion ability of these cell lines, which is one of the EMT
properties. Taken together, the results demonstrated that
differential ezrin localization, rather than total ezrin protein
levels, is associated with EMT characteristics in lung cell lines,
suggesting ezrin may be involved in the mechanism of TGF-β1-induced
EMT through the change of its distribution.
The association of ezrin and podocalyxin
increases during EMT
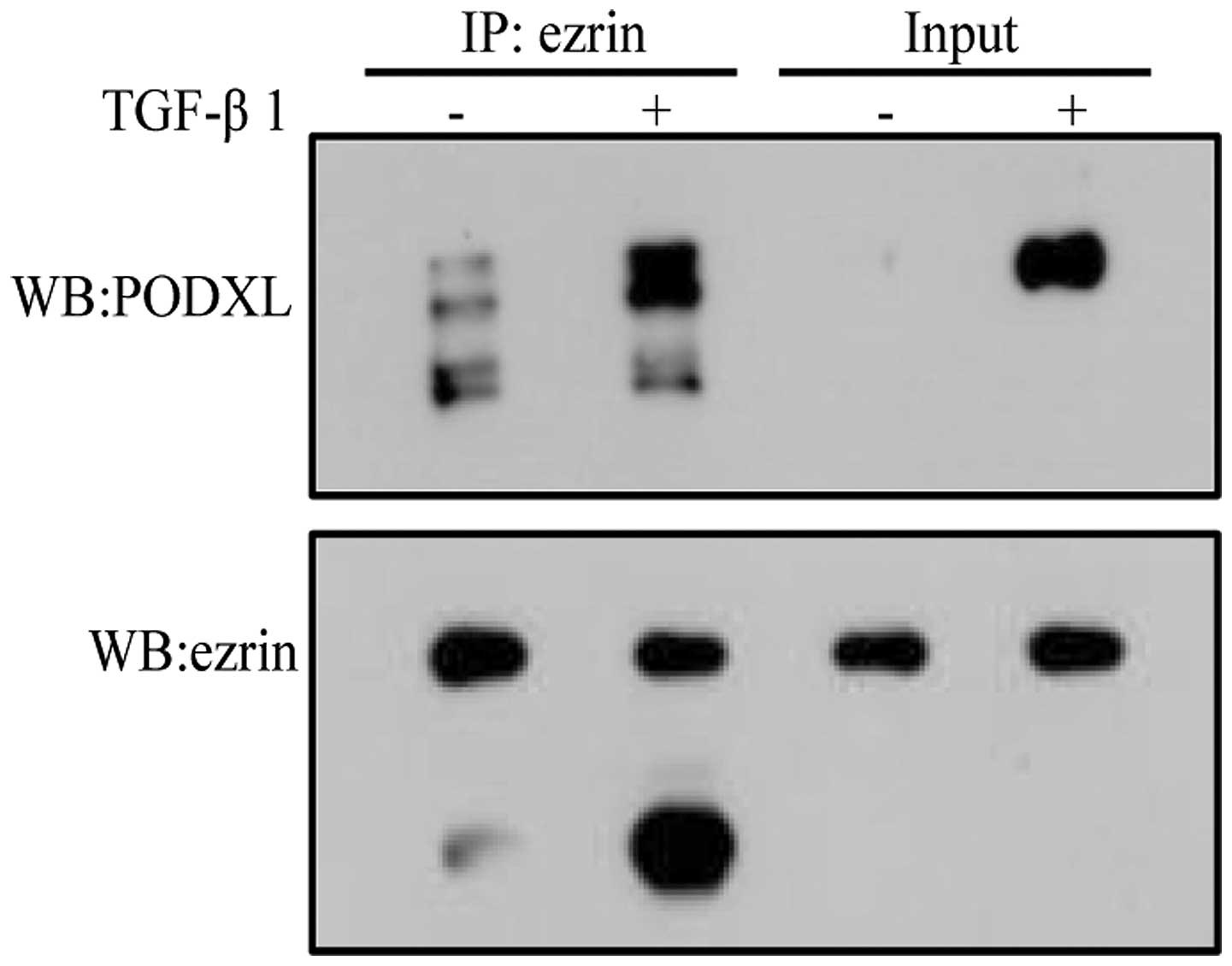
A recent study found that PODXL is involved in the
EMT process through regulating the loss of epithelial features and
acquisition of a motile phenotype. PODXL expression was induced
upon TGF-β1 treatment (26). We
asked whether PODXL is associated with ezrin during TGF-β1-induced
EMT, and then used coimmunoprecipitation to detect the interaction
between PODXL and ezrin. Our result showed that PODXL interacted
with ezrin during TGF-β1-induced EMT (Fig. 6). These results suggest that ezrin
may be involved in regulating TGF-β1-induced EMT through
interaction with PODXL.
Discussion
A number of investigations into the molecular events
of TGF-β1-induced EMT have paved the way for the design of improved
specific therapies. In the present study, we provide information
regarding the role of ezrin in TGF-β1-induced EMT, which may be
associated with its localization and target proteins. These
observations broaden knowledge of the precise molecular mechanism
mediating TGF-β1-induced EMT, which is important for developing
strategies to inhibit or reverse EMT.
Ezrin, as a membrane-cytoskeleton linker, plays a
privotal role in tumor invasion and metastasis (27–29).
Ezrin can regulate the assembly of cytoskeleton elements to promote
cytoskeletal reorganization and phenotypic alternation in cells,
and facilitate cell migration and invasion (30,31).
Overexpression of ezrin has been shown to enhance metastatic
potential in various types of tumors, while downregulation of ezrin
reduced the expression of β-catenin but enhanced the expression of
E-cadherin (32,33). It has been found that ERM proteins
are involved in regulating cytokine-induced EMT of human alveolar
epithelial cells (19,20). The expression of moesin increased,
which promotes EMT by regulating actin filament remodeling.
However, the expression of ezrin has no change during
TGF-β1-induced EMT (21). The role
of ezrin in TGF-β1-induced EMT remains unknown. In the present
study, we demonstrated that ezrin is associated with morphological
changes and actin filament remodeling, and regulates cell migration
and invasion in TGF-β1-induced EMT, suggesting ezrin plays an
important role in TGF-β1-induced EMT of human alveolar epithelial
cells and its involvement is independent on the expression level
(Figs. 3 and 4).
Several studies indicated that the localization of
ezrin, rather than its expression level, is correlated with its
function (34–36). In breast cancer lines, the total
ezrin protein level has no difference, but its localization is
associated with dedifferentiation and adverse features in invasive
breast tumors and cancer cell lines. In salivary acinar cells from
Sjögren’s syndrome, the structure and organization of microvilli
are linked to the localization changes of ezrin. In the present
study, we also found that differential ezrin localization, rather
than total ezrin protein levels, is associated with EMT
characteristics in lung cell lines (Fig. 5). Increasing number of studies have
shown ezrin is concentrated in the microvilli of the cell surface
playing a normal role under normal physiological condition or
before cytokine stimulation, while it could translocate to the
cytoplasm after cytokine stimulation (37). In the cytosol, ezrin exists in a
monomeric form and is thought to be ‘inactive’, provoking
dysfunction in ezrin-mediated cellular processes (38–40).
Following the stimulation, ezrin is phosphorylated, binds to actin
and membrane proteins, colocalizes with F-actin, and redistributes
to membrane, which is associated with enhanced migration and
invasion (41–44). Our observations were very close to
these studies (Fig. 3B). Before
TGF-β1 induction, ezrin mainly distributes in the microvilli and
the nucleus. After TGF-β1 treatment, ezrin translocates to the
cytosol, and with the increase of stimulation time, it co-localizes
with F-actin and redistributes to cell membrane. These results
indicated that ezrin may be involved in the mechanism of
TGF-β1-induced EMT through the change of its distribution.
Ezrin is known to link membrane proteins and actin
cytoskeleton. Membrane proteins include hyaluronate receptor
(CD44), sodium-hydrogen exchanger (NHE), and cell adhesion
molecules (ICAM-1, -2 and -3) (45–47).
After activated with cytokine, membrane proteins could transduce
extracellular signals into intracellular signals through the
interaction between their extracellular domain and ligands. Ezrin
also unmasks membrane protein and F-actin binding sites. The
N-terminal FERM domain of ezrin can bind to the cytoplasmic tail of
membrane proteins and its C-terminal domain interacts with F-actin,
resulting in actin filament remodeling and provoking migration and
invasion (17). We hypothesized
that membrane proteins interacting with ezrin may be different with
the distribution change of ezrin, initiating a regulatory mechanism
of TGF-β1-induced EMT. PODXL has been found to participate in
cytoskeleton rearrangement and tumor metastasis through interaction
with ezrin. PODXL could activate RhoA and induces actin
reorganization through NHERF1 and ezrin in MDCK cells (48). It also increases the aggressive
phenotype of breast and prostate cancer cells in vitro
through its interaction with ezrin (25). In our study, we indicated that
PODXL significantly interacts with ezrin with the increased
expression of PODXL after TGF-β1 treatment (Fig. 6). The result suggests PODXL may be
involved in regulating TGF-β1-induced EMT through interaction with
ezrin, and ezrin may act as a linker between PODXL and actin
cytoskeleton during TGF-β1-induced EMT.
In conclusion, we demonstrated that ezrin is
associated with actin filament reorganization and cell metastasis
during TGF-β1-induced alveolar EMT, indicating that ezrin is
required for EMT induced by TGF-β1. The interaction of ezrin and
PODXL may be important in regulating TGF-β1-induced EMT.
Acknowledgements
This study was supported by National Program on Key
Basic Research Project (973 Program) (grant no. 2011CB910700),
High-Level Talents Project of the Universities of Guangdong (no.
[2011]431), National Natural Science Foundation of China (Grant no.
31000628), Fundamental Research Funds for the Central Universities
(grant nos. 21611430, 21610101 and 21609317), and Natural Science
Foundation of Guangdong Province (grant no. S2013030013315).
References
|
1
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Willis BC and Borok Z: TGF-beta-induced
EMT: mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:525–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21(Suppl 7): vii89–vii92.
View Article : Google Scholar : 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Wang H, Wang F, Gu Q and Xu X: Snail
involves in the transforming growth factor β1-mediated
epithelial-mesenchymal transition of retinal pigment epithelial
cells. PLoS One. 6:e233222011.
|
|
8
|
Margetts PJ: Twist: a new player in the
epithelial-mesenchymal transition of the peritoneal mesothelial
cells. Nephrol Dial Transplant. 27:3978–3981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo W, Fang W, Li S and Yao K: Aberrant
expression of nuclear vimentin and related epithelial-mesenchymal
transition markers in nasopharyngeal carcinoma. Int J Cancer.
131:1863–1873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pagan R, Sánchez A, Martin I, Llobera M,
Fabregat I and Vilaró S: Effects of growth and differentiation
factors on the epithelial-mesenchymal transition in cultured
neonatal rat hepatocytes. J Hepatol. 31:895–904. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saitoh M and Miyazawa K: Transcirptional
and post-transcriptional regulation in TGF-β-mediated
epithelial-mesenchymal transition. J Biochem. 151:563–571.
2012.
|
|
13
|
American Thoracic Society, European
Respiration Society. American Thoracic Society/European Respiration
Society international multidisciplinary consensus classification of
the idiopathic interstitial pneumonias. Am J Respir Crit Care Med.
165:277–304. 2002.
|
|
14
|
Selman M and Pardo A: Alveolar epithelial
cell disintegrity and subsequent activation: a key process in
pulmonary fibrosis. Am J Respir Crit Care Med. 186:119–121. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marmai C, Sutheriand RE, Kim KK, et al:
Alveolar epithelial cells express mesenchymal proteins in patients
with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol
Physiol. 301:L71–L78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kolosova I, Nethery D and Kern JA: Role of
Smad2/3 and p38 MAP kinase in TGF-β1-induced epithelial-mesenchymal
transition of pulmonary epithelial cells. J Cell Physiol.
226:1248–1254. 2011.
|
|
17
|
Tsukita S and Yonemura S: Cortical actin
organization: lessons from ERM (ezrin/radixin/moesin) proteins. J
Biol Chem. 274:34507–34510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsukita S, Oishi K, Sato N, Sagara J,
Kawai A and Tsukita S: ERM family members as molecular linkers
between the cell surface glycoprotein CD44 and actin-based
cytoskeletons. J Cell Biol. 126:391–401. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buckley ST, Medina C, Kasper M and
Ehrhardt C: Interplay between RAGE, CD44, and focal adhesion
molecules in epithelial-mesenchymal transition of alveolar
epithelial cells. Am J Physiol Lung Cell Mol Physiol.
300:L548–L559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ehrhardt C, Medina C and Buckley ST:
Interaction of RAGE with focal adhesion molecules during alveolar
epithelial-mesenchymal transition. FASEB J. 25:8652011.
|
|
21
|
Haynes J, Srivastava J, Madson N, Wittmann
T and Barber DL: Dynamic actin remodeling during
epithelial-mesenchymal transition depends on increased moesin
expression. Mol Biol Cell. 22:4750–4764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nielsen JS and McNagny KM: The role of
podocalyxin in health and disease. J Am Soc Nephrol. 20:1669–1676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orlando RA, Takeda T, Zak B, et al: The
glomerular epithelial cell anti-adhesin podocalyxin associates with
the actin cytoskeleton through interaction with ezrin. J Am Soc
Nephrol. 12:1589–1598. 2001.PubMed/NCBI
|
|
24
|
Li Y, Li J, Straight SW and Kershaw DB:
PDZ domain-mediated interaction of rabbit podocalyxin and
Na(+)/H(+) exchange regulatory factor-2. Am J Physiol Renal
Physiol. 282:1129–1139. 2002.PubMed/NCBI
|
|
25
|
Sizemore S, Cicek M, Sizemore N, Ng KP and
Casey G: Podocalyxin increases the aggressive phenotype of breast
and prostate cancer cells in vitro through its interaction with
ezrin. Cancer Res. 67:6183–6191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng X, Ezzati P and Wilkins JA:
Requirement of podocalyxin in TGF-beta induced epithelial
mesenchymal transition. PLoS One. 6:e187152011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohtani K, Sakamoto H, Rutherford T, Chen
Z, Satoh K and Naftolin F: Ezrin, a membrane-cytoskeletal linking
protein, is involved in the process of invasion of endometrial
cancer cells. Cancer Lett. 147:31–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elliott BE, Meens JA, SenGupta SK, Louvard
D and Arpin M: The membrane cytoskeletal crosslinker ezrin is
required for metastasis of breast carcinoma cells. Breast Cancer
Res. 7:R365–R373. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Osawa H, Smith CA, Ra YS, Kongkham P and
Rutka JT: The role of the membrane cytoskeleton cross-linker ezrin
in medulloblastoma cells. Neuro Oncol. 11:381–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cant SH and Pitcher JA: G protein-coupled
receptor kinase 2-mediated phosphorylation of ezrin is required for
G protein-coupled receptor-dependent reorganization of the actin
cytoskeleton. Mol Biol Cell. 16:3088–3099. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Crepaldi T, Gautreau A, Comoglio PM,
Louvard D and Arpin M: Ezrin is an effector of hepatocyte growth
factor-mediated migration and morphogenesis in epithelial cells. J
Cell Biol. 138:423–434. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang HY, Li CF, Fang FM, Tsai JW, Li SH,
Lee YT and Wei HM: Prognostic implication of ezrin overexpression
in myxofibrosarcomas. Ann Surg Oncol. 17:3212–3219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Gao H, Xu H, et al: Expression of
ezrin correlates with malignant phenotype of lung cancer, and in
vitro knockdown of ezrin reverses the aggressive biological
behavior of lung cancer cells. Tumour Biol. 33:1493–1504. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sarrió D, Rodríguez-Pinilla SM, Dotor A,
Calero F, Hardisson D and Palacios J: Abnormal ezrin localization
is associated with clinicopathological features in invasive breast
carcinomas. Breast Cancer Res Treat. 98:71–79. 2006.PubMed/NCBI
|
|
35
|
Pérez P, Aguilera S, Olea N, et al:
Aberrant localization of ezrin correlates with salivary acini
disorganization in Sjögren’s Syndrome. Rheumatology. 49:915–923.
2010.PubMed/NCBI
|
|
36
|
Arslan AA, Silvera D, Arju R, Giashuddin
S, Belitskaya-Levy I, Formenti SC and Schneider RJ: Atypical ezrin
localization as a marker of locally advanced breast cancer. Breast
Cancer Res Treat. 134:981–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berryman M, Franck Z and Bretscher A:
Ezrin is concentrated in the apical microvilli of a wide variety of
epithelial cells whereas moesin is found primarily in endothelial
cells. J Cell Sci. 105:1025–1043. 1993.
|
|
38
|
Bretscher A, Edwards K and Fehon RG: ERM
proteins and merlin: integrators at the cell cortex. Nat Rev Mol
Cell Biol. 3:586–599. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moilanen J, Lassus H, Leminen A, Vaheri A,
Bützow R and Carpén O: Ezrin immunoreactivity in relation to
survival in serous ovarian carcinoma patients. Gynecol Oncol.
90:273–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tokunou M, Niki T, Saitoh Y, Imamura H,
Sakamoto M and Hirohashi S: Altered expression of the ERM proteins
in lung adenocarcinoma. Lab Invest. 80:1643–1650. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gautreau A, Louvard D and Arpin M:
Morphogenic effects of ezrin require a phosphorylation-induced
transition from oligomers to monomers at the plasma membrane. J
Cell Biol. 150:193–203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bretscher A: Rapid phosphorylation and
reorganization of ezrin and spectrin accompany morphological
changes induced in A-431 cells by epidermal growth factor. J Cell
Biol. 108:921–930. 1989. View Article : Google Scholar
|
|
43
|
Jiang WG, Hiscox S, Singhrao SK, Puntis
MC, Nakamura T, Mansel RE and Hallett MB: Induction of tyrosine
phosphorylation and translocation of ezrin by hepatocyte growth
factor/scatter factor. Biochem Biophys Res Commun. 217:1062–1069.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Z, Fadiel A, Feng Y, Ohtani K,
Rutherford T and Naftolin F: Ovarian epithelial carcinoma tyrosine
phosphorylation, cell proliferation, and ezrin translocation are
stimulated by interleukin 1alpha and epidermal growth factor.
Cancer. 92:3068–3075. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Martin TA, Harrison G, Mansel RE and Jiang
WG: The role of the CD44/ezrin complex in cancer metastasis. Crit
Rev Oncol Hematol. 46:165–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Weinman EJ, Steplock D, Donowitz M and
Shenolikar S: NHERF associations with sodium-hydrogen exchanger
isoform 3 (NHE3) and ezrin are essential for cAMP-mediated
phosphorylation and inhibition of NHE3. Biochemistry. 39:6123–6129.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Heiska L, Alfthan K, Grönholm M, Vilja P,
Vaheri A and Carpén O: Association of ezrin with intercellular
adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by
phosphatidylinositol 4, 5-bisphosphate. J Biol Chem.
273:21893–21900. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schmieder S, Nagai M, Oriando RA, Takeda T
and Farquhar MG: Podocalyxin activates RhoA and induces actin
reorganization through NHERF1 and ezrin in MDCK cells. J Am Soc
Nephrol. 15:2289–2298. 2004. View Article : Google Scholar : PubMed/NCBI
|