Introduction
Metastatic breast cancer (MBC) is a chronic and
incurable disease, with a median survival of approximately 2–3
years. Although advances have been made in the management of MBC,
long-term survivors are rare, with 5-year survival rates varying
from 5 to 10% (1,2).
Conventional anthracyclines are among the most
widely used agents to treat breast cancer in the adjuvant setting,
as well as in metastatic disease (3). In phase II trials the response rate
to anthracyclines in patients naïve to chemotherapy is about 50%,
however, the clinical use of anthracyclines is limited by
dose-related cardiotoxicity, which becomes more relevant with
increasing cumulative dose (4–6). The
mechanism of anthracycline related cardiotoxicity involves the
formation of free radicals that cause lipid perossidation leading
to a dose-dependent cardiomyopathy and to congestive heart failure
(7–9). Cardiotoxicity can appear during
treatment, at the end of therapy, or even years after the end of
anthracycline therapy (10).
Moreover, doxorubicin associated cardiotoxicity is often
unpredictable, because it can happen at lower dose than expected.
It was recently reported that this may be related to a genetic
predisposition in patients carrying selected polymorphism of the
NAD(P)H oxidase and doxorubicin efflux transporters (11).
Non-pegylated liposome-encapsulated doxorubicin
citrate, a liposomal formulation of doxorubicin, was designed to
reduce cardiotoxicity while preserving antitumor efficacy (12–15).
The formulation consists in encapsulation of water-soluble
doxorubicin within a phospholipid membrane to prevent doxorubicin
from exiting the circulation through the capillary junctions in
healthy tissues. Conversely, liposome-encapsulated drug appears to
pass easily through the damaged capillaries of tumor tissues.
Therefore, liposome encapsulated doxorubicin does not easily reach
heart tissue and a higher cumulative dose of liposome-encapsulated
doxorubicin can be delivered to the patient (16,17).
Results from phase III trials demonstrated that
liposome-encapsulated doxorubicin and doxorubicin are equivalent in
terms of antitumor efficacy (18–20).
In particular, Myocet in combination with cyclophosphamide showed
an efficacy equivalent to doxorubicin, with a better profile in
terms of cardiotoxicity and hematological toxicity (20).
Vinorelbine is a semi-synthetic vinca alkaloid, with
leukopenia and constipation as the dose limiting toxicities. Phase
II studies have shown a response rate ranging from 35 to 50% for
vinorelbine as first line chemotherapy for metastatic breast cancer
(21–23).
This multicenter randomized phase III trial was
designed to compare the efficacy and tolerability of the regimen MV
vs. MC. Secondary end-points were ORR, safety and OS.
The principal aim of the study was to assess a
difference in time to progression (TTP) between M/C (Arm A) and M/V
(Arm B). With the use of a one-sided log-rank test with a type I
error of 0.1, we determined that 133 events (disease progression or
death from any cause) would be required for an 80% power to detect
an expected minimum difference of 2.5 months in PFS between the two
arms in favor of vinorelbine containing arm. With a 1:1
randomization of assignment to study groups and considering a total
duration of the study of 60 months (48 months of accrual and 12
months of follow-up), we estimated that we would need to enroll 220
patients to observe 133 events.
Patients and methods
All eligible patients had histological confirmed
locally advanced (LABC) or metastatic breast cancer (MBC) Her-2
negative not previously treated with chemotherapy for advanced
disease. Previous treatment with an anthracyclines-based
chemotherapy regimen in the adjuvant or neo-adjuvant setting was
allowed (doxorubicin cumulative dose <240 mg/m2,
epirubicin cumulative dose <360 mg/m2). Other
inclusion criteria were: age 18–70 years; PS (ECOG) ≤2; at least
one measurable lesion according to RECIST criteria; previous
endocrine therapy allowed; adequate bone marrow, liver and renal
function. Main exclusion criteria were: previous chemotherapy for
metastatic disease, pregnancy or breast-feeding, serious
concomitant systemic disorders, pre-existing sensorial or motor
neuropathy, symptomatic brain metastasis, second primary malignancy
(except in situ carcinoma of the cervix or adequately
treated non-melanoma carcinoma of the skin or other malignancies
treated at least 5 years previously with no evidence of
recurrence). Randomization method 1:1 was performed and any
eligible patient was randomized to receive:
Arm A, non-pegylated liposome encapsulated
doxorubicin citrate (M) 60 mg/m2, Day 1 q3w +
cyclophosphamide (C) 600 mg/m2, Day 1 q3w; Arm B,
non-pegylated liposome encapsulated doxorubicin citrate (M) 50
mg/m2, Day 1 q3w + vinorelbine (V) 25 mg/m2
iv, Day 1 q3w and 60 mg/m2 orally, Day 8 q3w. Treatment
was performed until 6 cycles in case of SD or 8–10 cycles if PR or
CR was reached.
Time to progression (TTP) was determined from the
date of randomization to the date of assessment of disease
progression or death from any cause. TTP curves were calculated by
Kaplan-Meier method. Toxicity was graduated according to NCI-CTC v.
4.0. Objective response, according to RECIST criteria, was
evaluated by a tumor assessment performed every 3 cycles. Survival
time was measured from the date of randomization to the date of
death or lost to follow-up. Overall survival (OS) curves were
calculated by Kaplan-Meier method. Cardiotoxicity was defined as
appearance of signs and/or symptoms of CHF and/or a decrease in
LVEF below normal limit (<50%) or a decline ≥15% from baseline
value. To evaluate Left Ventricular Ejection Fraction (LVEF) an
echocardiography was done at baseline and every 3 cycles in all
patients entered into the study.
Results
From July 2006 through December 2011, 233 patients
with metastatic breast cancer, and no prior chemotherapy for
advanced disease, were enrolled at 18 Italian centers: 117 patients
were randomized to receive MC and 116 patients to receive MV. The
results are reported on the basis of data collected on all 233
patients with follow-up through December 2012. The two treatment
groups were equally distributed for baseline characteristics with
respect to age, disease-free interval, ECOG performance status,
estrogen/progesterone receptor status and visceral involvement
(Table I). Prior therapies were
well matched between the two groups: 84% in group A vs. 89% of
group B had received anthracyclines as adjuvant therapy,
respectively. Up to two lines of previous hormonal treatment were
allowed. A median of 6 cycles (range 2–10) of therapy was performed
in both arms. With regard to response rate, 116/117 patients in Arm
A and 112/116 in Arm B were evaluable per protocol, however,
responses were also assessed on intention to treat basis. In fact,
1 patient and 4 patients for Arms A and B, respectively, were not
evaluable for early drop out (1 refused to continue, 3 lost to
follow-up, 1 drug related death after the first cycle in Arm B).
Overall response rate (Table II),
per protocol, was 53/116 [45.7% (95% CI, 36.5–54.7%)] for Arm A and
51/112 [45.5% (95% CI, 36.3–54.7%)] for Arm B, respectively (P=NS).
On intent to treat basis, responses were 53/117 [45.29% (95% CI,
36.6–54.3%)] and 51/116 [44% (95% CI, 35.3–53.0%)] for Arms A and
B, respectively. The response rate by prognostic factors showed a
high degree of comparability between the two treatment groups
(Table III). The duration of
response was longer for MC treated patients as compared to MV (26
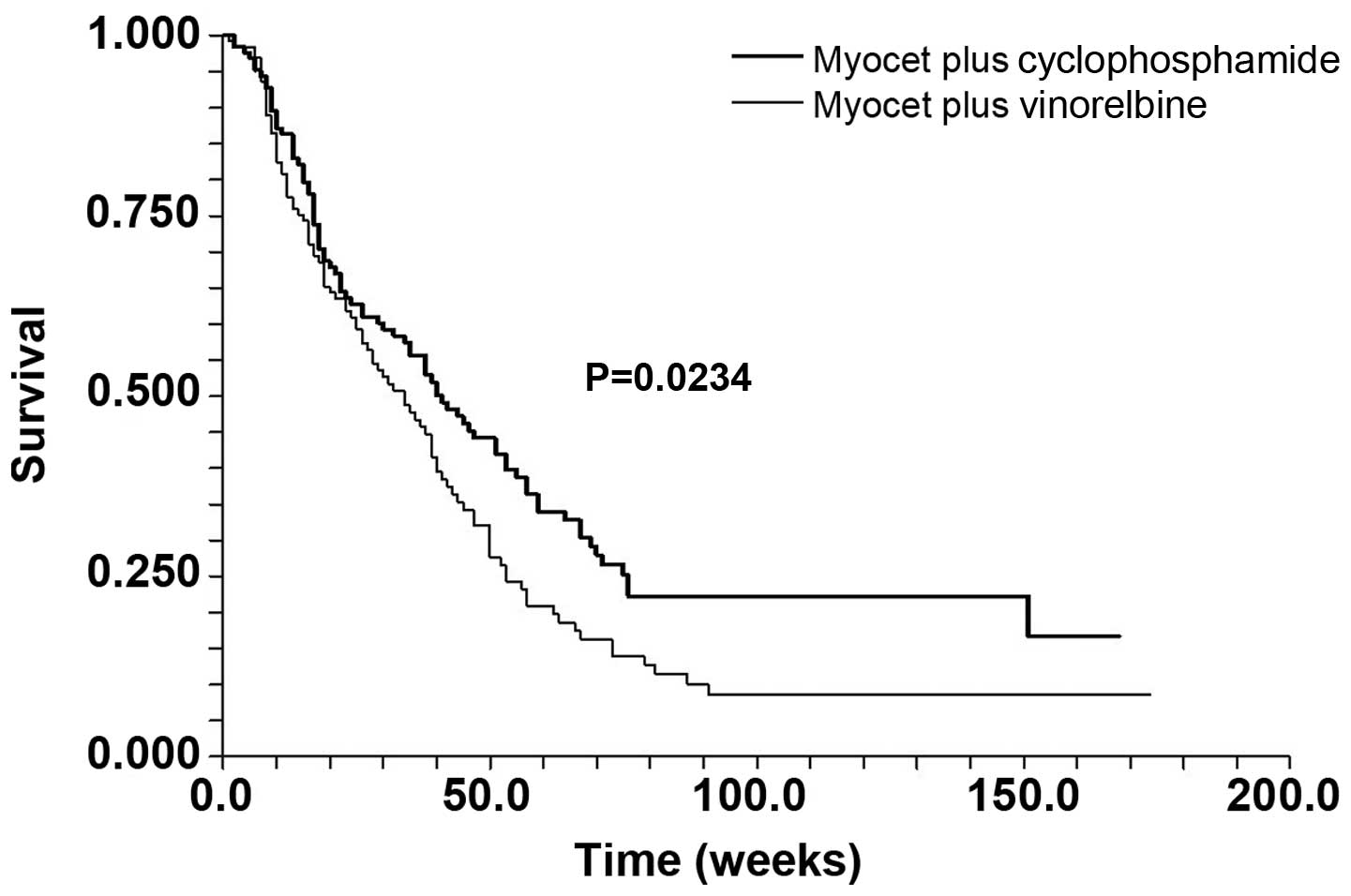
vs. 19 weeks; P=0.327). Median TTP (Fig. 1) was 41 (95% CI, 32–51) and 34 (95%
CI, 26–39) weeks, respectively (P=0.0234). With a median follow-up
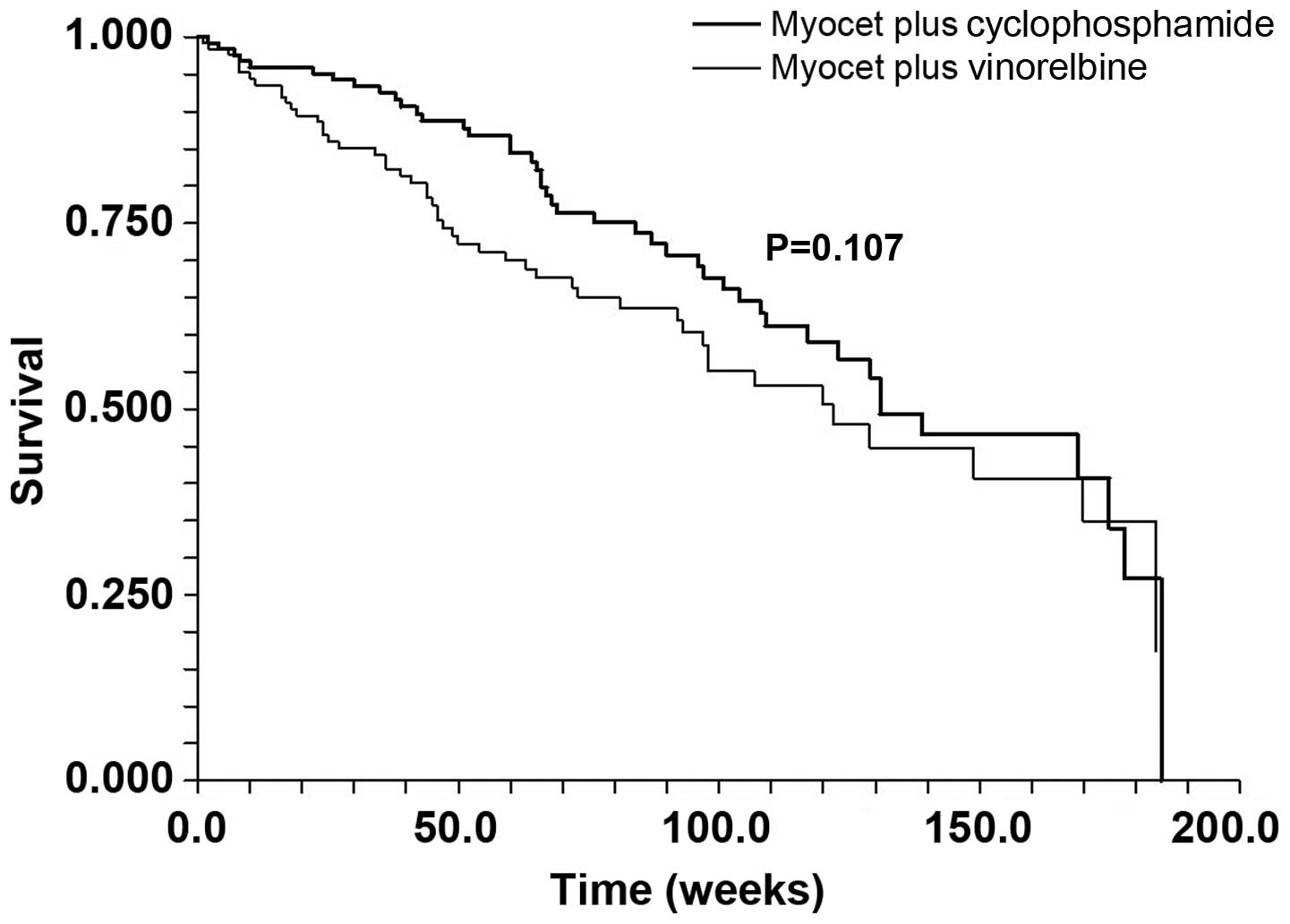
of 36 months, there was little difference in the overall survival
curves. The median survival was 131 weeks in the MC patients vs.
122 weeks in the MV treated patients (P=0.107) (Fig. 2).
 | Table ICharacteristics of the patients. |
Table I
Characteristics of the patients.
| Characteristics | Arm A
no. of patients (%) | Arm B
no. of patients (%) |
|---|
|
Assessable/entered | 116/117 | 112/116 |
| Median age
(range) | 59 (37–71) | 58 (25–70) |
| ECOG PS | | |
| 0–1 | 96 (77) | 89 (76) |
| 2 | 21 (23) | 27 (23) |
| Receptor status | | |
| Positive | 96 (77) | 93 (74) |
| Negative | 22 (17) | 26 (20) |
| Unknown | 9 (6) | 7 (6) |
| Her-2 status | | |
| Positive | 8 (6) | 8 (6) |
| Negative | 101 (80) | 105 (84) |
| Unknown | 18 (14) | 13 (10) |
| Prior surgery | 92 (74) | 104 (83) |
| Prior adjuvant
radiotherapy | 46 (37) | 42 (33) |
| Prior hormonal
therapy | 52 (42) | 67 (53) |
| Prior adjuvant
therapy with anthracyclines | 98 (84) | 103 (89) |
| Dominant site of
disease | | |
| Soft tissue | 25 (19) | 17 (13) |
| Bone | 12 (10) | 17 (13) |
| Viscera | 90 (71) | 92 (74) |
| No. of metastatic
sites | | |
| 1 | 44 (35) | 39 (31) |
| 2 | 43 (34) | 51 (40) |
| ≥3 | 40 (31) | 36 (29) |
 | Table IIResponse rate in per-protocol
evaluable patients according to dominant site of disease. |
Table II
Response rate in per-protocol
evaluable patients according to dominant site of disease.
| Dominant site of
disease | No. of
patients | CR | PR | SD | PD |
|---|
| Arm A |
| Viscera | 81 | 3 | 31 | 28 | 19 |
| Bone | 11 | 0 | 4 | 4 | 3 |
| Soft tissue | 24 | 5 | 10 | 8 | 1 |
| Total (%) | 116 | 8 (7) | 45 (39) | 40 (34) | 23 (20) |
| Arm B |
| Viscera | 82 | 2 | 37 | 23 | 20 |
| Bone | 15 | 0 | 5 | 8 | 2 |
| Soft tissue | 15 | 1 | 6 | 6 | 2 |
| Total (%) | 112 | 3 (3) | 48 (43) | 37 (33) | 24 (21) |
 | Table IIIResults with regard to prognostic
factors. |
Table III
Results with regard to prognostic
factors.
| Percentage of
responding patients |
|---|
|
|
|---|
| MC (N=116) | MV (N=112) |
|---|
| Age (years) |
| <50 | 52 | 54 |
| 50–59 | 45 | 43 |
| >60 | 35 | 32 |
| Disease-free
interval |
| <2 years | 44 | 45 |
| >2 years | 42 | 43 |
| ECOG PS |
| 0 | 48 | 47 |
| 1 | 47 | 45 |
| 2 | 25 | 24 |
| Receptor
status |
| Positive | 44 | 43 |
|
Negative/unknown | 46 | 46 |
| Prior
radiotherapy | 43 | 42 |
| Prior hormonal
therapy | 44 | 45 |
| Prior adjuvant
therapy with anthracyclines | 43 | 40 |
| Dominant site of
disease |
| Soft tissue | 75 | 80 |
| Bone | 35 | 38 |
| Viscera | 45 | 44 |
| No. of metastatic
sites |
| 1 | 45 | 45 |
| 2 | 45 | 45 |
| ≥3 | 38 | 40 |
Safety
Therapy was generally well tolerated in both groups
(Table IV). Myelosuppression was
the most frequent and severe adverse side effect on both arms, and
was the most common cause of dose reduction and delay. Still, dose
reductions and delays were not common: median duration of cycles
was 21 days on both arms, and patients achieved 90% of the planned
dose intensity. Grade 3 or 4 thrombocytopenia was observed in 2% of
the MC vs. 3% of MV patients. Grade 3–4 neutropenia, however, was
significantly lower in the MC arm: 16% of MC patients as compared
to 32% of MV patients (P=0.008). G-CSF was administered in 35% of
MV cycles vs. 19% of MC cycles. Eight patients (7%) on the MC arm
compared with 18 patients (16%) on the MV arm developed neutropenic
fever requiring IV antibiotics and/or hospitalization.
Additionally, fewer RBC transfusions were required with MC (5
transfusions to 3 patients) relative to MV (10 transfusions to 7
patients). The incidence of all grade mucositis/stomatitis was
comparable in both arms although a slight increase in percent of
grade 3–4 mucositis was observed in MV arm (8 vs. 3%). With regard
to gastrointestinal toxicity, incidence of grade 3–4 diarrhea was
negligible in both arms (1%). However, grade 1–2 diarrhea was more
frequent on MV as compared to MC (11 vs. 3%; P=0.042). Conversely,
constipation was significantly higher on MV arm (16 vs. 4%;
P=0.003), with 7% of grade 3–4.
 | Table IVToxicity. |
Table IV
Toxicity.
| Arm A (N=117) | Arm B (N=116) |
|---|
|
|
|
|---|
| Toxicity | Grade I–II (%) | Grade III–IV
(%) | Grade I–II (%) | Grade III–IV
(%) |
|---|
| Leucopenia | 25 (21) | 19 (16) | 23 (20) | 37 (32) |
| Febrile
neutropenia | - | 8 (7) | - | 18 (16) |
| Anemia | 40 (34) | 3 (3) | 39 (34) | 7 (6) |
|
Thrombocytopenia | 10 (9) | 2 (2) | 9 (8) | 4 (3) |
|
Nausea/vomiting | 49 (42) | 2 (2) | 30 (26) | 3 (3) |
| Mucositis | 20 (17) | 3 (3) | 19 (16) | 9 (8) |
| Diarrhoea | 4 (3) | 1 (1) | 13 (11) | 1 (1) |
| Asthenia | 17 (15) | 2 (2) | 15 (13) | 1 (1) |
| Constipation | 3 (3) | 1 (1) | 10 (9) | 8 (7) |
| Hair loss | 43 (37) | 73 (62) | 42 (36) | 70 (60) |
| Neuropathy | 3 (3) | - | 4 (3) | 1 (1) |
| Cardiac
toxicity | 8 (7) | - | 9 (8) | - |
With regard to cardiotoxicity, overall 17/233 (7%)
patients after 6–8 cycles of either arm, developed a decrease of
LVEF below 50%, or showed >15% decline of LVEF below basal
examination, without any clinical sign of or symptom. Most of these
patients (75%) were previously treated with anthracyclines. All
patients recovered after appropriate treatment. The median dose of
non-pegylated liposomal doxorubicin administered was 420 mg (range
120–660). No patient developed palmar-plantar erythrodisesthesia
which is a common side effect with pegylated liposomal doxorubicin.
Moreover, we observed only one treatment related death in Arm B,
because of sepsis.
Discussion
Doxorubicin is a mainstay in the treatment of breast
cancer. In fact, this drug is generally included in the treatment
of early as well as of advanced/metastatic disease. However, the
most important drawback in its use is the development of
cardiotoxicity, which is fatal in more than 5% of the patients when
cumulative dose of 450 mg2 is reached (4–6).
This risk is particularly high when patients with advanced disease
have been already treated with anthracyclines as a component of
their adjuvant treatment of early disease. Thus, doxorubicin
cardiac toxicity can limit patient ability to receive further
potentially active therapy, in particular when the tumor is
responding to the current treatment including anthracyclines.
With this regard, considerable research has been
undertaken to reduce the potential cardiotoxicity of this drug.
Drugs having potential protective effect as dexrazoxane (24,25)
or the administration of doxorubicin as continuous infusion
(26), have been proposed as tools
able to reduce risk of hearth damage, but none has been considered
really effective. Non-pegylated liposomal doxorubicin
(Myocet©) is a unique formulation of doxorubicin, able
to alter the tissue distribution of this doxorubicin.
Pharmacokinetic studies have demonstrated that doxorubicin in
Myocet is bioavailable, metabolized and excreted in a manner
similar to that of conventional doxorubicin, but in a slower rate
sparing myocardial cells. Moreover, a number of clinical studies
(18–20) have demonstrated comparable activity
and lesser toxicity of Myocet vs. conventional doxorubicin. In
addition, data from a phase III randomized controlled clinical
trial, comparing Myocet plus cyclophosphamide (MC) vs. adriamycin
plus cyclophosphamide (AC) in advanced breast cancer, demonstrated
that Myocet significantly reduced the cumulative cardiac toxicity,
while providing comparable antitumor efficacy. In fact, in this
study which enrolled 296 patients, an objective response of 43% was
observed in both arms, with a significant reduction of
cardiotoxicity (6 vs. 21%; P=0.0002) in favor of MC patients
(20). A further phase III study
(19) randomized 160 patients to
receive cyclophosphamide 600 mg/m2 plus either
epirubicin 75 mg/m2 or liposomal doxorubicin 75
mg/m2. No significant differences were observed in the
rate of asymptomatic reduction in LVEF (11 vs. 10%). In this study,
no patient developed clinical heart failure. In 2010, the Cochrane
Library reported a systematic review of the different anthracycline
compounds and their cardiotoxicity (27). Studies by Harris et al
(18) and Batist et al
(20) were analyzed together, and
authors concluded that non-pegylated liposomal anthracyclines did
reduce the overall risk of cardiotoxicity (RR=0.38, P<0.0001)
and the risk of clinical heart failure (RR=0.20, P=0.02).
The aim of the present study was to compare the
efficacy of the regimen Myocet plus vinorelbine (MV) vs. the
standard MC in a population mostly pretreated with anthracyclines
as adjuvant treatment. Secondary end-points of the study were ORR,
safety and OS.
The rationale of the choice of the association of
Myocet and vinorelbine as comparator of the standard
Myocet-cyclophosphamide was the synergism observed in breast cancer
cell lines between doxorubicin and vinorelbine (28). In cell lines, an increased p38
activity was demonstrated following vinorelbine, with unchanged
mitogen-activated protein kinase (MAPK) activity and p53
expression. On the other hand, doxorubicin treatment resulted in
increased p53 expression, without changes in MAPK or p38 activity.
This different mechanism of action may result in increased cell
apoptosis in vitro as well as in vivo. Nevertheless,
the first randomized trial of the combination of doxorubicin plus
vinorelbine vs. doxorubicin alone, reported disappointing results
(29) with no difference in
response rate as well as in overall survival between doxorubicin
alone and the combination with vinorelbine. However, following this
study, Pawlicki et al (30)
reported an objective response rate of nearly 80%, in 38
chemotherapy naïve metastatic breast cancer treated with
doxorubicin plus vinorelbine, and Lorvidhaya et al showed an
overall response of 67% in 27 patients (31) with this combination. In addition,
few years later, a Scandinavian group reported the results of a
phase III study of 387 patients who were randomly assigned to
receive IV epirubicin 90 mg/m2 on Day 1 plus vinorelbine
25 mg/m2 on Days 1 and 8 or epirubicin alone at dosage
of 90 mg/m2 IV on Day 1 (32). Both regimens were given every 3
weeks for a maximum of 1 year but discontinued prematurely in the
event of progressive disease or severe toxicity. In addition,
epirubicin was discontinued at a cumulative dose of 1000
mg/m2 (950 mg/m2 from June 1999). It is
noteworthy that prior anthracycline-based adjuvant chemotherapy and
prior chemotherapy for metastatic breast cancer was not allowed.
Overall response rates to vinorelbine and epirubicin, and
epirubicin alone, were 50 and 42%, respectively (P=0.15). The
complete response rate was significantly superior in the
combination arm (17 vs. 10%; P=0.048) as was median duration of
progression-free survival (10.1 vs. 8.2 months; P=0.019). Median
survival was similar in the two arms (19.1 vs. 18.0 months;
P=0.50). Leukopenia related complications, stomatitis and
peripheral neuropathy were more common in the combination arm. The
incidences of cardiotoxicity and constipation were similar in both
arms. The Authors concluded that addition of vinorelbine to
epirubicin conferred a significant advantage in terms of complete
response rate and progression-free survival, but not in terms of
survival.
The results of the present study demonstrate that
the combination of Myocet plus cyclophosphamide had a significantly
longer TTP compared to Myocet plus vinorelbine while the two
combinations showed similar response rate and overall survival.
Also safety of the two combination was comparable, except for a
higher incidence of leukopenia and neurotoxicity in MV arm.
In conclusion, the combination of Myocet plus
cyclophosphamide showed a good profile of efficacy and safety in a
population with previous exposure to anthracyclines in the early
phase of the disease and remains an unbeaten standard in the
treatment of advanced/metastatic breast cancer.
References
|
1
|
Clark GM, Sledge GW Jr, Osborne CK and
McGuire WL: Survival from first recurrence: relative importance of
prognostic factors in 1,015 breast cancer patients. J Clin Oncol.
5:55–61. 1987.PubMed/NCBI
|
|
2
|
Greenberg PA, Hortobagyi GN, Smith TL,
Ziegler LD, Frye DK and Budzar AU: Long-term follow-up of patients
with complete remission following combination chemotherapy for
metastatic breast cancer. J Clin Oncol. 14:2197–2205.
1996.PubMed/NCBI
|
|
3
|
Launchbury AP and Habbubi N: Epirubicin
and doxorubicin: a comparison of their characteristics, therapeutic
activity and toxicity. Cancer Treat Rev. 3:197–228. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith LA, Cornelius VR, Plummer CJ, Levitt
G, Verrill M, Canney P and Jones A: Cardio-toxicity of
anthracycline agents for the treatment of cancer: systematic review
and meta-analysis of randomized controlled trials. BMC Cancer.
10:3372010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Von Hoff DD, Layard MW, Basa P, et al:
Risk factors for doxorubicin-induced congestive heart failure. Ann
Intern Med. 91:710–717. 1979.PubMed/NCBI
|
|
6
|
Bristow MR: Anthracycline cardio-toxicity.
Drug-Induced Heart Disease. Elsevier; New York: pp. 191–215.
1980
|
|
7
|
Jackson JA, Reeves JP, Muntz KH, et al:
Evaluation of free radical effects and catecholamine alterations in
adriamycin cardio-toxicity. Am J Pathol. 117:140–153.
1984.PubMed/NCBI
|
|
8
|
Rajagopalan S, Politi PM, Sinha BK, et al:
Adriamycin induced free radical formation in the perfused rat
heart: implications for cardio-toxicity. Cancer Res. 48:4766–4769.
1988.PubMed/NCBI
|
|
9
|
Myers CE, McGuire WP, Liss RH, et al:
Adriamycin: the role of lipid peroxidation in cardiac toxicity and
tumor response. Science. 197:165–167. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steinherz LJ, Steinherz PG, Tan CT, Heller
G and Murphy ML: Cardiac toxicity 4 to 20 years after completing
anthracycline therapy. JAMA. 266:1672–1677. 1991.
|
|
11
|
Wojnowski L, Kulle B, Schirmer M, et al:
NAD(P)H oxidase and multidrug resistance protein genetic
polymorphisms are associated with doxorubicin induced
cardio-toxicity. Circulation. 112:3754–3762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tardi PG, Boman NL and Cullis PR:
Liposomal doxorubicin. J Drug Target. 4:129–140. 1996. View Article : Google Scholar
|
|
13
|
Kanter PM, Bullard GA, Ginsberg RA, et al:
Comparison of the cardiotoxic effects of liposomal doxorubicin (TLC
D-99) versus free doxorubicin in beagle dogs. In Vivo. 7:17–26.
1993.PubMed/NCBI
|
|
14
|
Kanter PM, Klaich G, Bullard GA, et al:
Preclinical toxicology study of liposome encapsulated doxorubicin
(TLC D-99) given intraperitoneally to dogs. In Vivo. 8:975–982.
1994.PubMed/NCBI
|
|
15
|
Bally MB, Nayar R, Masin D, Hope MJ,
Cullis PR and Mayer LD: Liposomes with entrapped doxorubicin
exhibit extended blood residence times. Biochim Biophys Acta.
1023:133–139. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Yang L, Chen Z and Shin DM:
Application of nanotechnology in cancer therapy and imaging. CA
Cancer J Clin. 58:97–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Symon Z, Peyser A, Tzemach D, et al:
Selective delivery of doxorubicin to patients with breast carcinoma
metastases by stealth liposomes. Cancer. 86:72–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harris L, Batist G, Belt R, et al:
Liposome-encapsulated doxorubicin compared with conventional
doxorubicin in a randomized multicenter trial as first-line therapy
of metastatic breast carcinoma. Cancer. 94:25–36. 2002. View Article : Google Scholar
|
|
19
|
Chan S, Davidson N, Juozaityte E, et al:
Phase III trial of liposomal doxorubicin and cyclophosphamide
compared with epirubicin and cyclophosphamide as first-line therapy
for metastatic breast cancer. Ann Oncol. 15:1527–1534. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batist G, Ramakrishnan G, Rao CS, et al:
Reduced cardiotoxicity and preserved antitumor efficacy of
liposome-encapsulated doxorubicin and cyclophosphamide compared
with conventional doxorubicin and cyclophosphamide in a randomized,
multicenter trial of metastatic breast cancer. J Clin Oncol.
19:1444–1454. 2001.
|
|
21
|
Romero A, Rabinovich MG, Vallejo CT, et
al: Vinorelbine as first line chemotherapy for metastatic breast
carcinoma. J Clin Oncol. 12:336–341. 1994.PubMed/NCBI
|
|
22
|
Smith IE: Navelbine in combination
chemotherapy for advanced breast cancer. J Cancer Res Clin Oncol.
116:10521990.
|
|
23
|
Abeloff MD: Vinorelbine (Navelbine) in the
treatment of breast cancer: a summary. Semin Oncol. 22:1–4.
1995.PubMed/NCBI
|
|
24
|
Swain SM, Whaley FS, Gerber MC, et al:
Cardioprotection with dexrazoxane for doxorubicin containing
therapy in advanced breast cancer. J Clin Oncol. 15:1318–1332.
1997.PubMed/NCBI
|
|
25
|
Swain SM, Whaley FS, Gerber MC, et al:
Delayed administration of dexrazoxane provides cardioprotection for
patients with advanced breast cancer treated with
doxorubicin-containing therapy. J Clin Oncol. 15:1333–1340.
1997.
|
|
26
|
Legha SS, Benjamin RS, Mackay B, et al:
Reduction of doxorubicin cardiotoxicity by prolonged continuous
intravenous infusion. Ann Intern Med. 19:133–139. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Dalen EC, Michiels EM, Caron HN and
Kremer LC: Different anthracycline derivatives for reducing
cardiotoxicity in cancer patients. Cochrane Database Syst Rev.
5:CD0050062010.
|
|
28
|
Liemm AA, Appleyard MV, O’Neill MA, Hupp
TR, Chamberlain MP and Thompson AM: Doxorubicin and vinorelbine act
independently via p53 expression and p38 activation respectively in
breast cancer cell lines. Br J Cancer. 88:1281–1284. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Norris BI, Pritchard KI, James K, et al:
Phase III comparative study of vinorelbine combined with
doxorubicin versus doxorubicin alone in disseminated
metastatic/recurrent breast cancer. National Cancer Institute of
Canada Clinical Trials Group Study MA8. J Clin Oncol. 18:2385–2394.
2000.
|
|
30
|
Pawlicki MI, Rolski J, Zaluski J,
Siedlecki P, Ramlau C and Tomzak P: A phase II study of intravenous
navelbine and doxorubicin combination in previously untreated
advanced breast carcinoma. Oncologist. 7:205–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lorvidhaya VI, Kamnerdsupaphon P,
Chitapanarux I, et al: Combination of vinorelbine + doxorubicin in
advanced breast cancer. Gan To Kagaku Ryoho. 30:1131–1138.
2003.PubMed/NCBI
|
|
32
|
Ejlertsen B1, Mouridsen HT, Langkjer ST,
Andersen J, Sjöström J and Kjaer M: Scandinavian Breast Group Trial
(SBG9403): Phase III study of intravenous vinorelbine in
combination with epirubicin versus epirubicin alone in patients
with advanced breast cancer: a Scandinavian Breast Group Trial
(SBG9403). J Clin Oncol. 22:2313–2320. 2004. View Article : Google Scholar
|