Introduction
Osteosarcoma, the most common primary bone cancer,
occurs predominantly in growing adolescents and young adults and is
characterized by frequent distant metastasis, particularly to the
lung (1). Although the overall
survival rate for osteosarcoma has increased to ~70%, <30% of
patients presenting with metastases survive 5 years after the
initial diagnosis (2). Since
pulmonary metastasis is the major cause of death in osteosarcoma,
identifying molecular alterations that lead to metastasis is
essential for developing novel therapies.
The first step in metastasis is the migration from
the primary tumour site and invasion through the basement membrane.
The degradation of the extracellular matrix contributes to the
ability of osteosarcoma cells to metastasize. In osteosarcoma the
gelatinases matrix metalloproteinase (MMP)-2 and -9 promote
invasion and metastasis (3–5),
intracellular protease m-calpain modulates cell adhesion and
motility (6), and urokinase
plasminogen activator (uPA) and its receptor uPAR promote cell
adhesion, migration and invasion through the activation of
plasminogen and pro-MMPs (7).
Integrin-β4 (8) and the
Wnt/β-catenin (9,10) and Notch (11,12)
signalling pathways, both of which activate metalloproteinases
(13,14), have also been shown to promote
osteosarcoma metastasis. Finally, secreted factors, including the
cytokine interleukin-6 (IL-6) (15), parathyroid hormone (PTH), PTH
peptides and the PTH receptor (PTHR) (16,17),
autocrine motility factor (AMF) (18,19)
and matricellular protein Cyr61 (20) are known to promote metastasis.
The expression of both soluble and membrane bound
APN is strongly correlated with the invasive capacity of numerous
tumour cell types (21–23) and APN is widely believed to
influence the invasion mechanism. A previous study showed that APN
activity is correlated with IL-6-mediated osteosarcoma invasiveness
(24). APN is a zinc-dependent
membrane-bound aminopeptidase with a short N-terminal cytoplasmic
domain, a single transmembrane part and a large extracellular
domain containing the active site. APN is a multifunctional enzyme,
which can operate as an enzyme for peptide cleavage, a receptor in
endocytosis and/or a signalling molecule in signal transduction.
Each of these three mechanisms elicits a different biological
effect (23). APN overexpression
or altered enzymatic activity has been reported in skin, ovary,
thyroid, lung, stomach, colon, kidney, bone and prostate neoplasias
(25,26). The mechanism by which APN
participates in cell invasion is linked to its enzymatic activity,
but APN has also been shown to facilitate signal transduction in
endothelial invasion (27).
It has been demonstrated that IL-6 plays an
important role in the progression and invasion of tumours by
stimulating MMP production (28,29).
MMPs have pivotal roles in the degradation of extracellular matrix,
and thereby enhance the invasive and metastatic potential in cancer
(30,31). In human osteosarcoma, MMP-2 has
been implicated in the metastatic process (32) and MMP-9 has been shown to be
associated with poor prognosis (33–35).
In this study we investigate the effect of APN
inhibition and activation on osteosarcoma cell lines using APN
inhibitor bestatin and APN activator IL-6. This study creates a
platform to further explore APN involvement in osteosarcoma
metastasis and identify target signalling networks for novel
therapeutic strategies.
Materials and methods
Cell culture
The human osteosarcoma cell lines MG63 and U-2 OS
were obtained from the Shanghai Institute of Cell Biology
(Shanghai, China). The cells were grown under standard conditions
in RPMI-1640 and supplemented with 10% heat-inactivated FBS and
antibiotics/antimycotics (all from Gibco-BRL, Eggenstein, Germany).
They were incubated at 37°C in a CO2 incubator, released
from the culture surface using trypsin/EDTA (Gibco-BRL) and counted
in a haemocytometer.
Cytokines and APN inhibitors
IL-6 and sIL-6R were purchased from R&D Systems
(Minneapolis, MN, USA). Bestatin was purchased from Sigma (St.
Louis, MO, USA). All treatments with IL-6 were at 1 nM and included
15 nM sIL-6R, which was added to achieve a stable effect in
osteoblastic cells (36–39).
RNA isolation and cDNA synthesis
MG63 or U-2 OS cells (1×106 cells/well)
were placed in 6-well plates and incubated with factors (IL-6,
sIL-6R or bestatin) for the times indicated. Cells were collected
and total RNA was extracted from each treatment using the Qiagen
RNeasy Mini kit (Qiagen Inc., Valencia, CA, USA). RNA samples were
reverse-transcribed at 42°C using the High Capacity cDNA Reverse
Transcription kit for 30 min according to the protocol of the
supplier (Applied Biosystems, Foster City, CA, USA).
Absolute quantitation of APN expression
by competitive RT-PCR
The internal competitive standard RNA was obtained
using the method designed by Kehlen et al and composite
primers were synthesized as shown in Table I (40). For cDNA synthesis, 1,000, 500, 50,
10 or 1 pg APN competitor RNA were added to 5 μg total RNA and
reverse-transcribed at 42°C using the High Capacity cDNA Reverse
Transcription kit for 30 min according to the protocol of the
supplier (Applied Biosystems). Two microliters of cDNA was diluted
in 50 μl of PCR reaction solution containing primers 3 and 4. The
PCR reaction was performed according to the manufacturer’s standard
protocol (Qiagen Inc., Hilden, Germany) in a thermal cycler
(MaxiCycler PTC-100; MJ Research, Inc., Watertown, MA, USA) for 35
cycles of 60 sec at 94°C, 60 sec at 60°C, and 60 sec at 72°C. Each
reaction product (10 μl) was run on a 1.5% agarose gel containing
0.1% μg/ml ethidium bromide in TAE buffer. The relative intensities
of the bands corresponding to the target (573 bp) and internal
standard (434 bp) PCR products were visualized with UV light. The
relative amounts of target and internal standard products were
calculated by densitometric analysis using ImageMaster 1D Prime
software (Amersham Pharmacia Biotech, Freiburg, Germany). The ratio
of standard to target amplification pro ducts was graphed as a
function of the initial amount of internal standard, and lines were
drawn from a linear regression analysis using InStat (GraphPad
Software, Inc., San Diego, CA, USA). The initial amount of target
was calculated from the point where the amount of amplified target
equals the amount of amplified standard (ratio D 1). The analysis
was performed in triplicate.
 | Table IPrimers used in competitive PCR. |
Table I
Primers used in competitive PCR.
| Primers | Sequences |
|---|
| 1 | GTG ATG GCA GTG GAT
GCA CAG CTT CCT GTC CGA GGA CTG TA |
| 2 | GAT TTA GGT GAC ACT
ATA GAA TAC GTG ATG GCA GTG GAT GCA C |
| 3 | GTG ATG GCA GTG GAT
GCA C |
| 4 | CGT CAC ATT GAG GTT
CAG CAG |
Quantitative PCR
The following quantitative PCR conditions were used:
2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 sec at 95°C, 1
min at 60°C using 1 μl of the cDNA reverse-transcribed as described
above, 2X SYBR-Green PCR Master Mix (Applied Biosystems), and 200
nM forward and reverse primers. The primer sequences were APN
forward, GTTCTCCTTCTCCAACCTCATC and reverse, CTGTTTCCTCGTTGTCCTTCT;
MMP-2 forward, CCCCAGACAGGTGATCTTGAC and reverse,
GCTTGCGAGGGAAGAAGTTG; MMP-9 forward, CGCTGGGCTTAGATCATTCC and
reverse, AGGTTGGATACATCACTGCATTAGG; GAPDH forward,
ACACCCACTCCTCCACCTTT and reverse, TAGCCAAATTCGTTGTCATACC. Each
assay was run on an Applied Biosystems 7300 Real-Time PCR System in
triplicate, and expression fold changes were derived using the
comparative threshold cycle (CT) method (41,42).
Enzyme activity
APN activity was assayed using the substrate alanine
p-nitroanilide (Ala-pNA) at a final concentration of 1.5 mM.
Confluent cell monolayers in 48-well plates were rinsed three times
and incubated at 37°C for 20–40 min with pre-warmed substrate.
Supernatant p-nitroanilide was measured at an OD of 405 nm by a
microplate reader (Anthos Labtec Instruments GmbH, Salzburg,
Austria). Assays were run in triplicate, in parallel with cell- and
substrate-free blanks. The cells were detached from the plates and
counted. Catalytic activity was expressed as pkat/106
cells.
Gelatin zymography
MMP-2 and -9 activity was determined by gelatin
zymography after exposure to IL-6 or bestatin. Cells
(1×106 cells/well) were plated in 12-well tissue culture
plates and incubated in serum-free McCoy’s 5A medium in the
presence of 1 nM IL-6 or 100 μM bestatin for 24 and 48 h. The
conditioned medium was then collected and separated by
electrophoresis on 10% SDS-PAGE containing 0.2% gelatin (Sigma). At
the end of electrophoresis, the gels were soaked twice in 2.5%
Triton X-100 in dH2O at 25°C for a total of 60 min, then
incubated in substrate buffer (50 mM Tris HCl, 5 mM
CaCl2, 0.02% NaN3 and 1% Triton X-100, pH
8.0) at 37°C for 18 h. Bands corresponding to MMP-2 and -9 activity
were visualized by negative staining using 0.2% Coomassie Blue in
50% methanol and 10% acetic acid as described elsewhere (43,44).
Bands were quantified using NIH ImageJ software (NIH, Bethesda, MD,
USA) as previously described (45,46).
In vitro migration and invasion
assays
Cell mobility was determined in migration and
invasion assays through 24-well Transwell inserts (8 mm pore size)
coated with 30 μg type 1 collagen (both from Millipore, Billerica,
MA, USA) (migration assay) or Matrigel (BD Biosciences, Bedford,
MA, USA) (invasion assay) (41,44).
U-2 OS and MG63 cells were maintained in serum-free medium for 24
h, after which they were trypsinized, resuspended in serum-free
McCoy’s 5A medium and placed in the upper chamber of the Transwell
insert (5×104 cells/well). Cells were then treated for
24 or 48 h with 1 nM IL-6 or 100 μM bestatin. McCoy’s 5A medium
containing 10% FBS was then placed in the lower chamber.
Non-migrating cells in the upper chamber were removed by wiping
with a cotton swab. Cells that had penetrated the filter and were
located on the lower chamber side of the filter were fixed with 4%
formaldehyde in PBS, stained with 2% crystal violet in 2% ethanol
and counted under a light microscope at ×200 magnification.
NF-κB immunofluorescence
Cells placed on 6-well chamber slides were treated
with 1 nM IL-6 or 100 μM bestatin for 24 h,
immunofluorescence-labeled using a Cellular NF-κB Translocation kit
(Beyotime Institute of Biotechnology, Jiangsu, China) according to
the manufacturer’s protocol. Briefly, after fixing, the cells were
stained using anti-NF-κB p65 (1:100) overnight and then stained
with Cy3 fluorescein-conjugated secondary antibody followed by
nuclear counterstain propidium iodide. Photomicrographs were
obtained using a Leica TCS SP2 confocal spectral microscope.
Western blotting
U-2 OS cells (1×106 cells/well) were
placed in 6-well plates and each well was incubated for 24 or 48 h
in 1 nM IL-6 or 100 μM bestatin. Cells were harvested and lysed
with ice-cold 50 mM potassium phosphate buffer (pH 7.4) containing
2 mM EDTA and 0.1% Triton X-100, sonicated and then centrifuged at
13,000 g for 10 min at 4°C. The supernatant was collected and total
protein was determined using a Bio-Rad Protein Assay kit (Bio-Rad,
Hercules, CA, USA) with bovine serum albumin (BSA) as the standard.
At the end of electrophoresis, proteins were electrotransferred to
nitrocellulose membranes, blotted with primary antibody against
GRB2, PI3K, AKT, PKC, p38, NF-κB p65, p38, ERK1/2, JNK, MMP-2,
MMP-9, p-p38, p-ERK1/2 or p-JNK (Cell Signaling Technology, Inc.,
Danvers, MA, USA) then washed and stained with secondary antibody.
Bands were visualized with an enhanced chemiluminescence reagent
(ECL™; Amersham Biosciences Corp., Piscataway, NJ, USA) and
quantified using an NIH Image analyzer (NIH).
Statistical analysis
Data are presented as the means ± standard deviation
(SD) from at least three independent experiments. Differences
between groups were determined using Student’s t-test. P<0.05
was considered to indicate a statistically significant result.
Results
APN mRNA expression and hydrolysing
activity is affected by IL-6 and bestatin
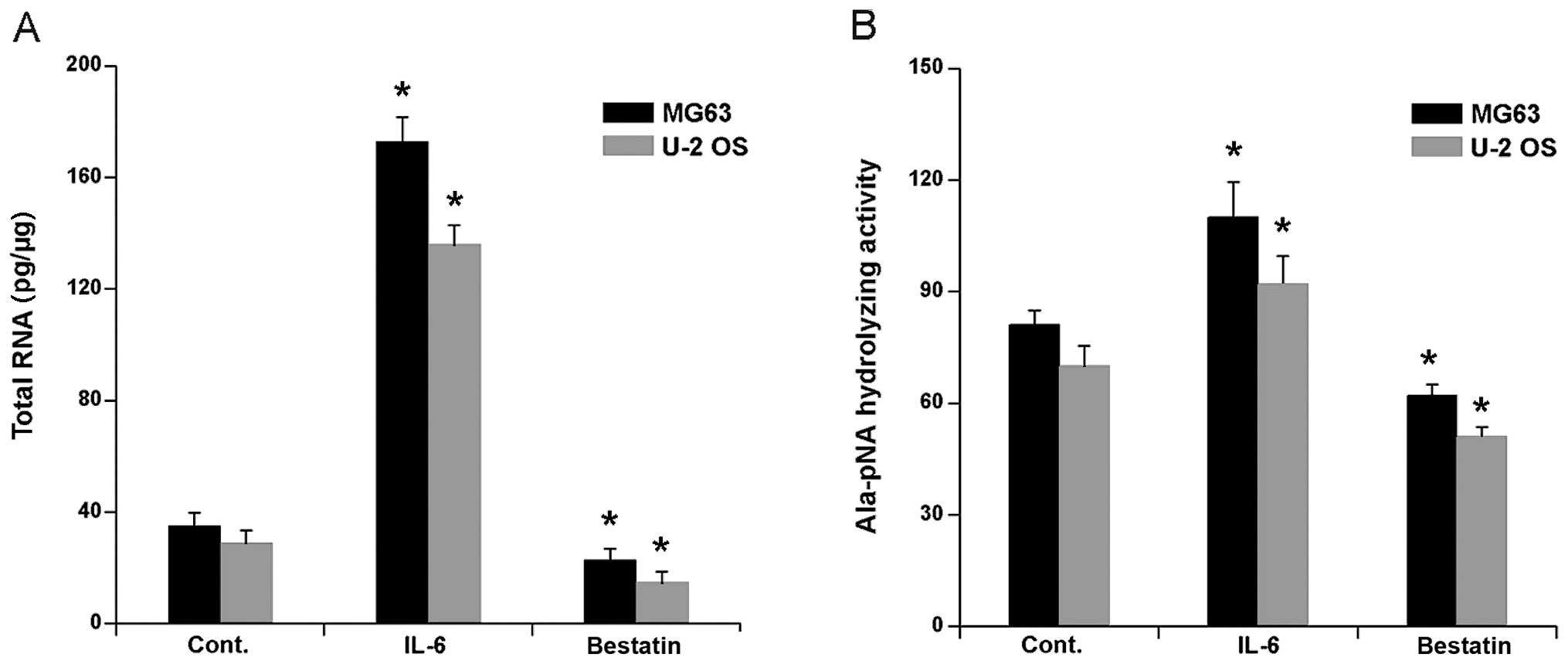
The treatment of human osteosarcoma cell lines HOS
and MG63 with cytokine IL-6 has been shown to upregulate APN mRNA
(24). To confirm and extend these
observations we first determined APN mRNA expression in human
osteosarcoma cells lines U-2 OS and MG63 after exposure to IL-6 (1
nM) for 24 h. Competitive PCR showed that APN mRNA expression in
both cell lines increased significantly (Fig. 1A). We next investigated whether
bestatin, a widely used and potent inhibitor of APN, inhibited APN
mRNA expression. A 24 h exposure of U-2 OS and MG63 to 100 μM
bestatin decreased the mRNA levels slightly, but nevertheless
significantly (Fig. 1A).
It has previously been shown that IL-6 treatment of
human osteosarcoma cell lines MG63 and HOS significantly increased
Ala-pNA hydrolysing activity (24). We confirmed and extended these
observations by investigating APN Ala-pNA hydrolysing activity in
the cells lines U-2 OS and MG63 after a 24 h treatment with IL-6 (1
nM) or bestatin (100 μM). As expected, IL-6 treatment significantly
increased Ala-pNA hydrolysing activity in both cell lines (Fig. 1B). In contrast, the bestatin
treatment significantly decreased Ala-pNA hydrolysing activity
(Fig. 1B). Together these data
confirm that IL-6 treatment activates, whereas bestatin treatment
inhibits, APN hydrolysing activity and APN mRNA expression in human
osteoblast cell lines.
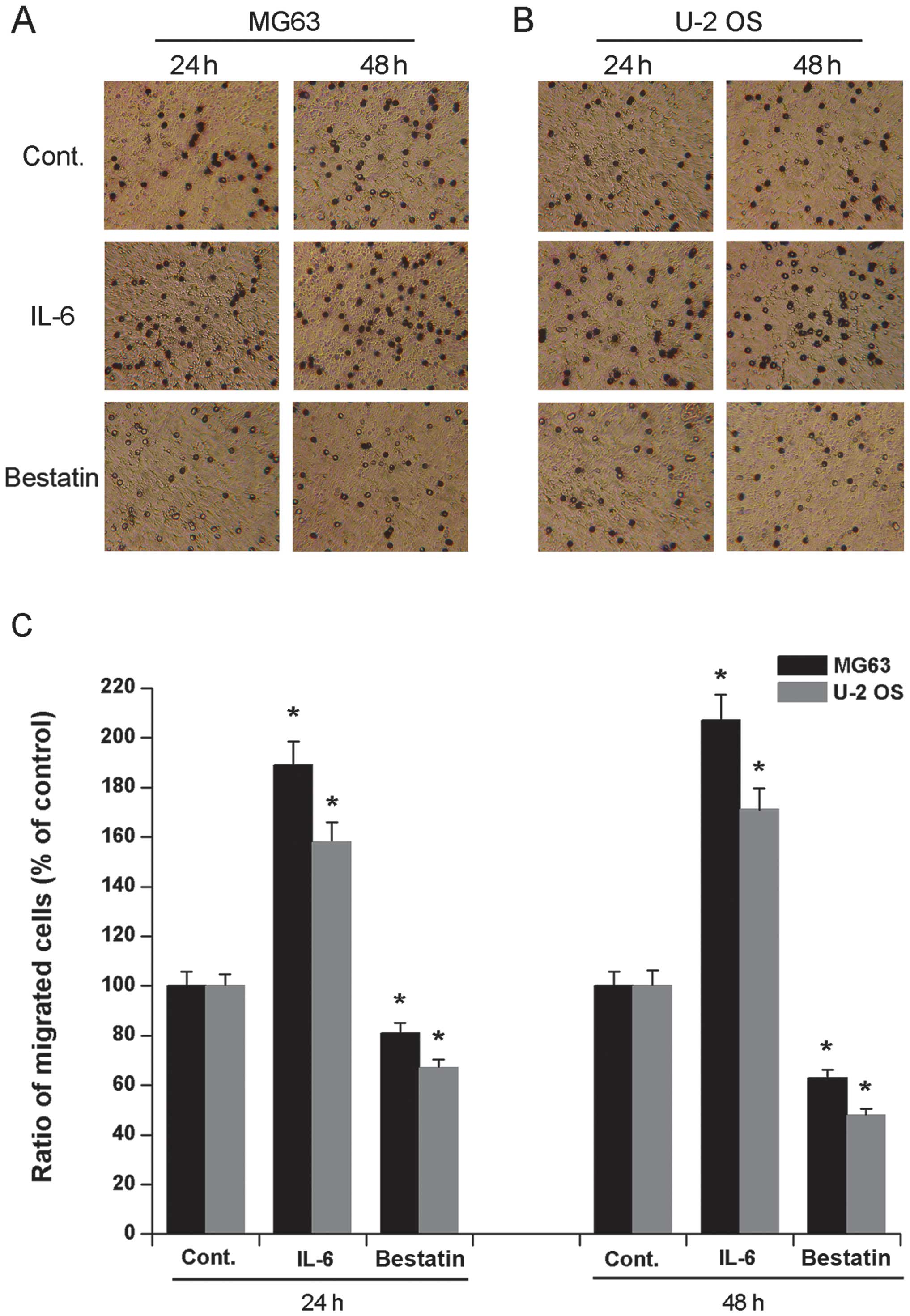
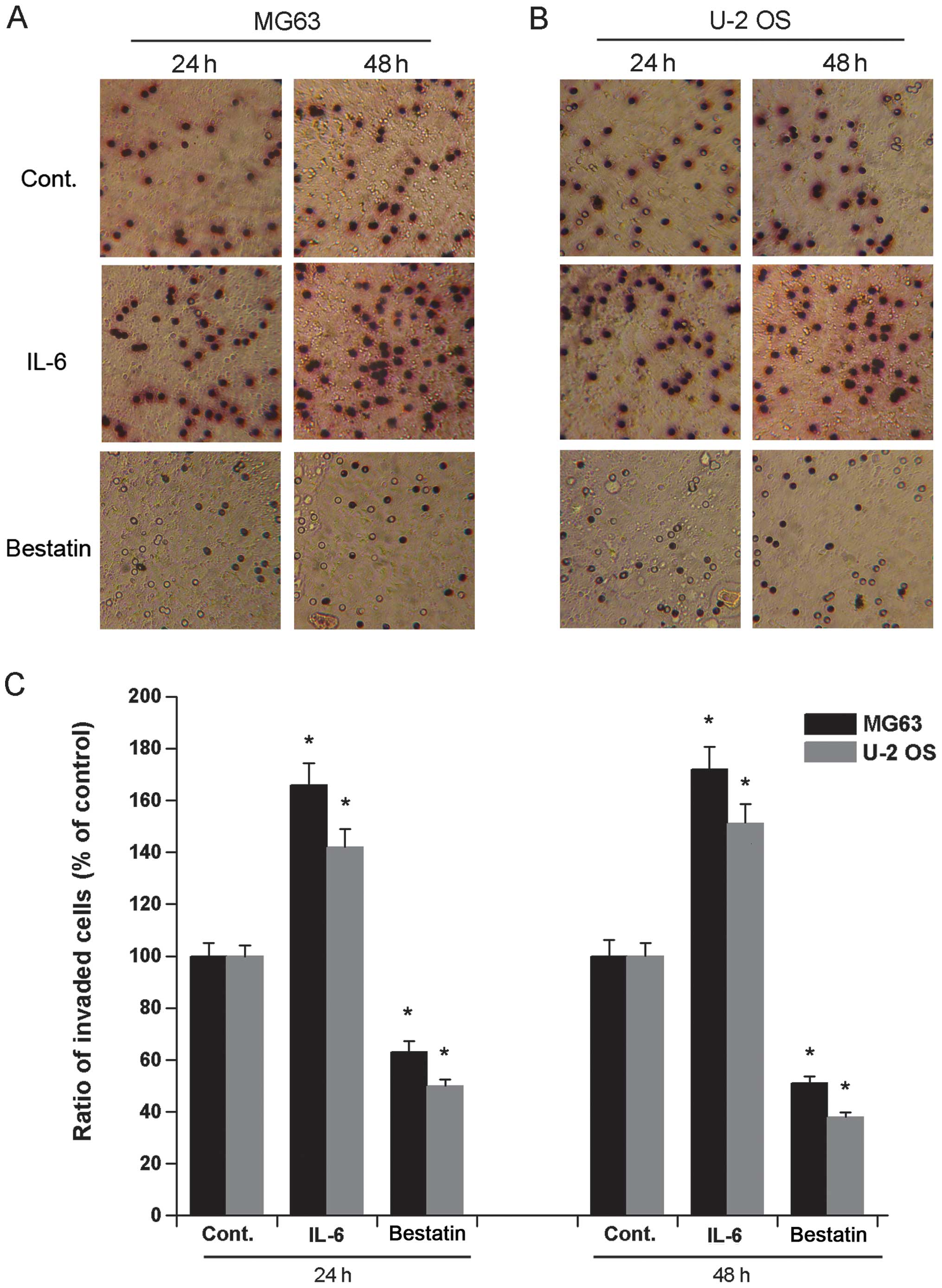
Osteosarcoma cell migration and invasion
after IL-6 and bestatin treatment
It has previously been demonstrated that a 24 h
treatment of human osteosarcoma cell lines HOS and MG63 with IL-6
(1 nM) or bestatin (100 μM) enhances or represses their invasive
potential, respectively (24). To
confirm and extend these observations we assessed the invasive
potential of cell lines U-2 OS and MG63 exposed to IL-6 (1 nM) or
bestatin (100 μM) for 24 or 48 h. The invasiveness of both MG63 and
U-2 OS cells significantly increased upon exposure to IL-6 and
significantly decreased upon exposure to bestatin after both 24 and
48 h (Fig. 2). We also assessed
the migration potential of MG63 and U-2 OS when exposed to IL-6 (1
nM) or bestatin (100 μM) for 24 or 48 h. The migration of both U-2
OS and MG63 cells significantly increased upon exposure to IL-6 and
significantly decreased upon exposure to bestatin after both 24 and
48 h (Fig. 3). The data shown here
confirm that APN stimulator IL-6 and APN inhibitor bestatin enhance
and reduce cell invasiveness and migration, respectively. Since the
invasive potential of cell lines HOS and MG63 correlates with the
relative enzymatic activity of APN (24), it is possible that the increased
invasive potential and migration of U-2 OS and MG63 cells observed
here is also due to increased APN activity.
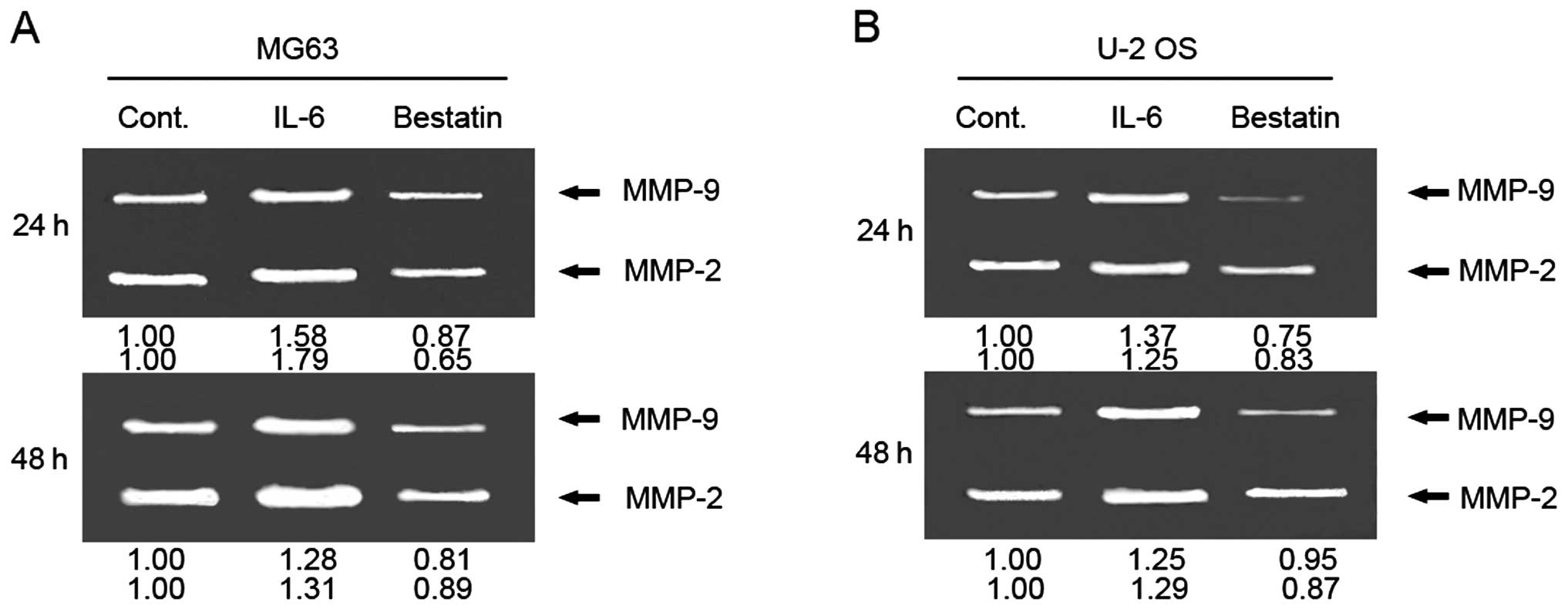
Effect of IL-6 and bestatin on MMP-2 and
-9 activity
To investigate whether APN regulates MMP-2 and -9
activity in osteosarcoma cell lines, we measured MMP-2 and -9
enzyme activity by gelatin zymography after inhibiting APN in MG63
and U-2 OS cells with bestatin (100 μM) for 24 and 48 h. We found
that both 24- and 48-h bestatin treatments decreased MMP-2 and -9
activity (Fig. 4A and B). These
results suggest that the decreased invasiveness of bestatin-treated
cells could be due APN’s reduced activation of MMP-2 and -9. By
comparison, a 24 and 48 h incubation of MG63 and U-2 OS cells with
IL-6 (1 nM) increases MMP-2 and -9 activity (Fig. 4A and B). Together these results
raise the possibility that the increased invasiveness of MG63 and
U-2 OS by IL-6 could be due to IL-6’s activation of MMP-2 and -9
via APN activation.
IL-6 and bestatin alter levels and
phosphorylation states of signalling proteins
Cell invasion and migration are orchestrated by
multiple signalling cascades, including the mitogen-activated
protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)
pathways (47,48). We determined the effect of APN
inhibition on protein levels and phosphorylation states of MAPK and
PI3K signalling pathway members by western blotting. The treatment
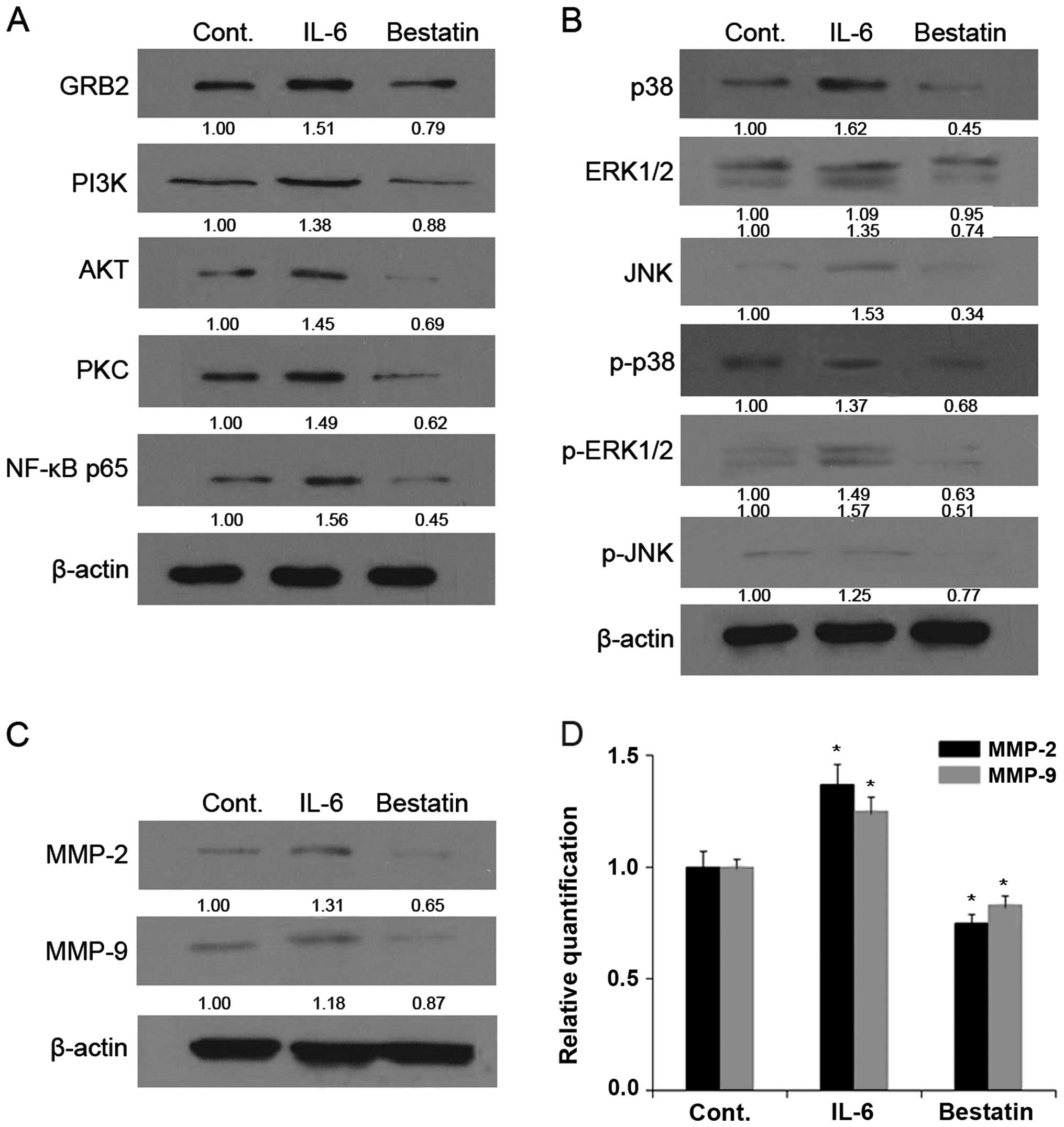
of MG63 (Fig. 5) and U-2 OS
(Fig. 6) cell lines with bestatin
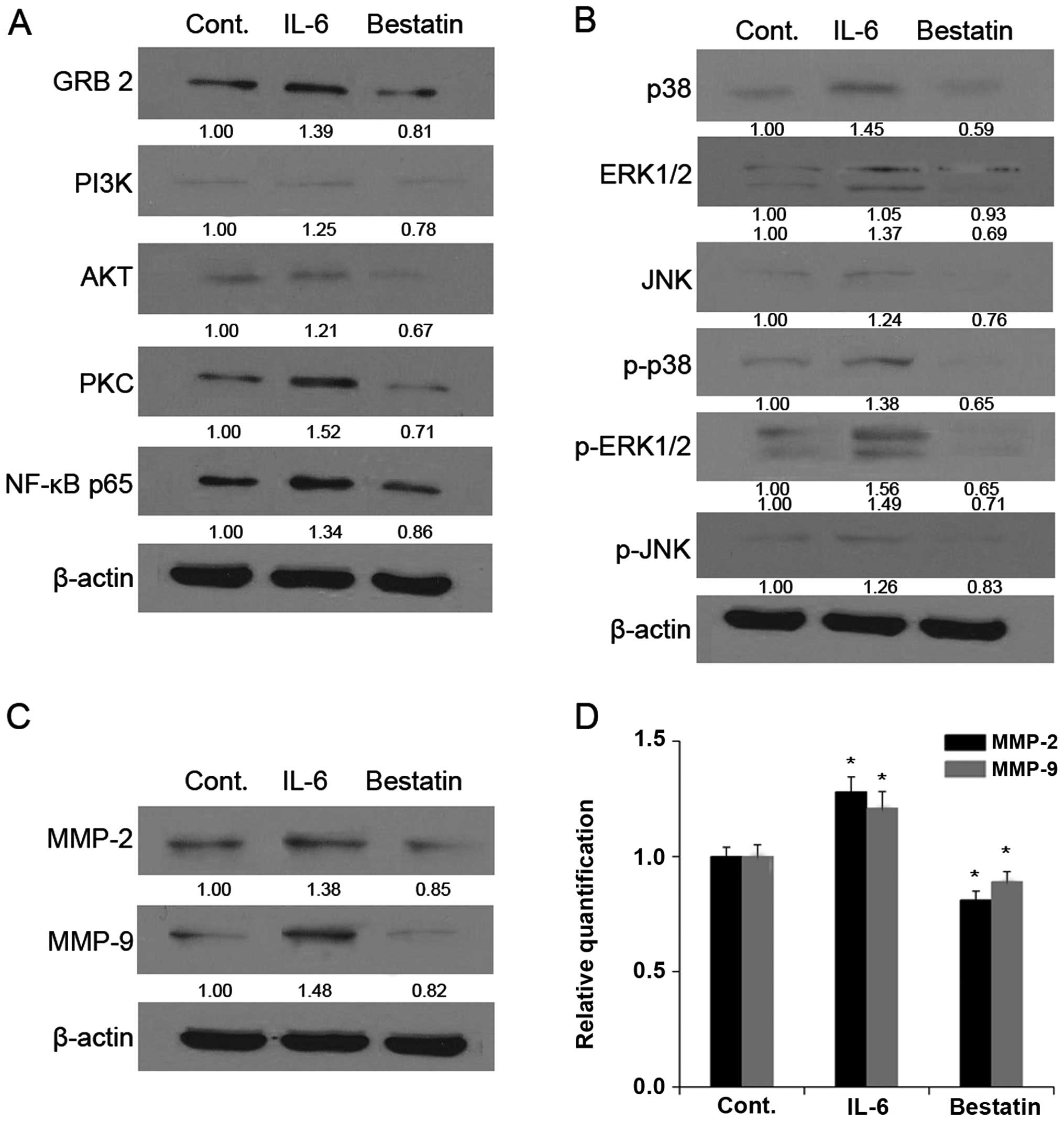
(100 μM) for 24 h resulted in decreased levels of GRB2, PI3K, AKT,
PKC, NF-κB p65 (Figs. 5A and
6A), p38, ERK1/2, JNK, p-p38,
p-ERK1/2 and p-JNK (Figs. 5B and
6B). By comparison, the treatment
of MG63 and U-2 OS cell lines with APN stimulator IL-6 for 24 h
resulted in increased levels of GRB2, PI3K, AKT, PKC, NF-κB p65
(Figs. 5A and 6A), p38, ERK1/2, JNK, p-p38, p-ERK1/2 and
p-JNK (Figs. 5B and 6B). Together these results show that a
reduction of APN activity in osteosarcoma cells dampens MAPK and
PI3K signalling, as shown by the reduced levels of phosphorylated
p38, ERK1/2 and JNK. This raises the possibility that APN might
have a signalling function in osteosarcoma invasiveness by
activating the transcription factor NF-κB through the MAPK and PI3K
pathways. NF-κB would then activate downstream target genes such as
MMP-2 and -9.
 | Figure 5Mitogen-activated protein kinase
(MAPK) and phosphatidylinositol 3-kinase (PI3K) pathway proteins in
interleukin-6 (IL-6)- and bestatin-treated MG63 cells. Cells were
treated with IL-6 (1 nM) or bestatin (100 μM) for 24 h. The levels
of (A) GRB2, PI3K, AKT, PKC, NF-κB p65; (B) p38, ERK1/2, JNK,
p-p38, p-ERK1/2 and p-JNKp; (C) matrix metalloproteinase (MMP)-2
and -9 are depicted by SDS-PAGE and western blotting. Results shown
here are representative of at least three independent experiments.
(D) Relative quantification of MMP-2 and -9 by real-time PCR. The
ratios between MMP-2, -9 and GAPDH mRNA are displayed and data
represent the mean ± standard deviation (SD) in duplicate of at
least three independent experiments. *P<0.05 was
considered significant. β-actin, control. |
We next investigated whether the enzymatic activity
changes in MMP-2 and -9 upon bestatin or IL-6 treatment could be
due to reduced MMP-2 and -9 protein levels. Indeed, we showed by
western blotting that the treatment of MG63 (Fig. 5) and U-2 OS (Fig. 6) cell lines with bestatin (100 μM)
for 24 h resulted in decreased levels of MMP-2 and -9 (Figs. 5C and 6C). By comparison, the treatment of with
APN stimulator IL-6 resulted in increased levels of MMP-2 and -9
(Figs. 5C and 6C). We next determined MMP-2 and -9 mRNA
levels by quantitative PCR and found that these were reduced upon
bestatin treatment and increased upon IL-6 treatment. The changes
in MMP-2 and -9 protein and mRNA levels (Figs. 5D and 6D) are therefore in accordance with the
changes in MMP-2 and -9 activity (Fig.
4).
Effect of IL-6 and bestatin on NF-κB p65
signalling
Western blotting revealed that IL-6 and bestatin
treatments increased and decreased, respectively, the levels of the
transcription factor NF-κB, which has been shown to activate MMP-1,
-3 and -9 transcription (49). We
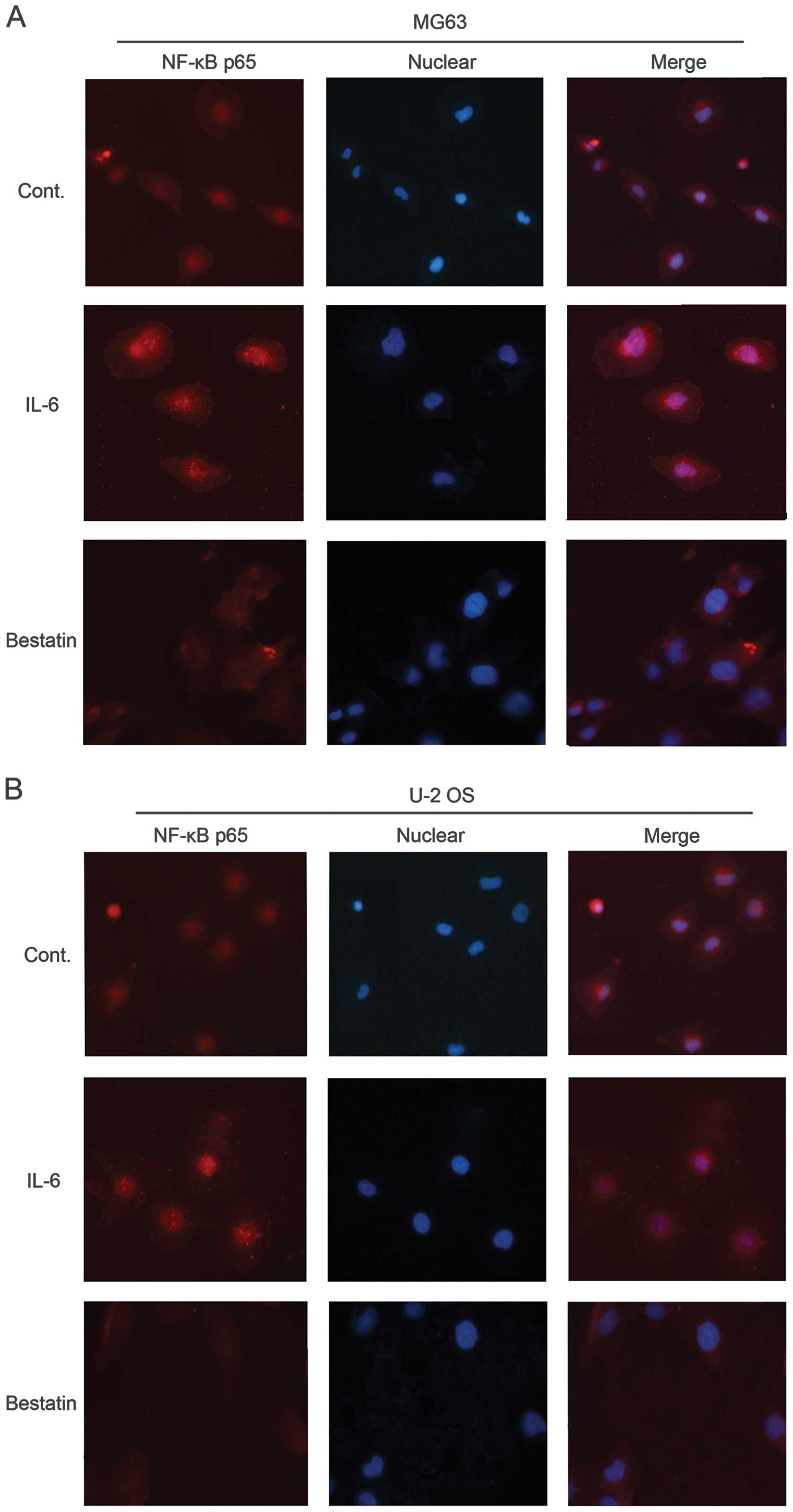
therefore investigated the expression and localization of NF-κB in
IL-6 or bestatin-treated MG63 and U-2 OS cells by
immunocytochemisty with an antibody against the p65 subunit. In
untreated cells, NF-κB was visible in both the cytosol and nucleus
(control panel in Fig. 7A and B).
The cytoplasmic form is inactive due to its association with
inhibitory protein IκB. IL-6 treatment concentrated the NF-κB p65
in the nucleus (Fig. 7A and B),
while bestatin reduced overall NF-κB p65 levels, consistent with
our western blotting data (Fig.
5). These results support our model of MMP-2 and -9 activation
by NF-κB, which has been activated by APN signalling via the MAPK
or PI3K pathway.
Discussion
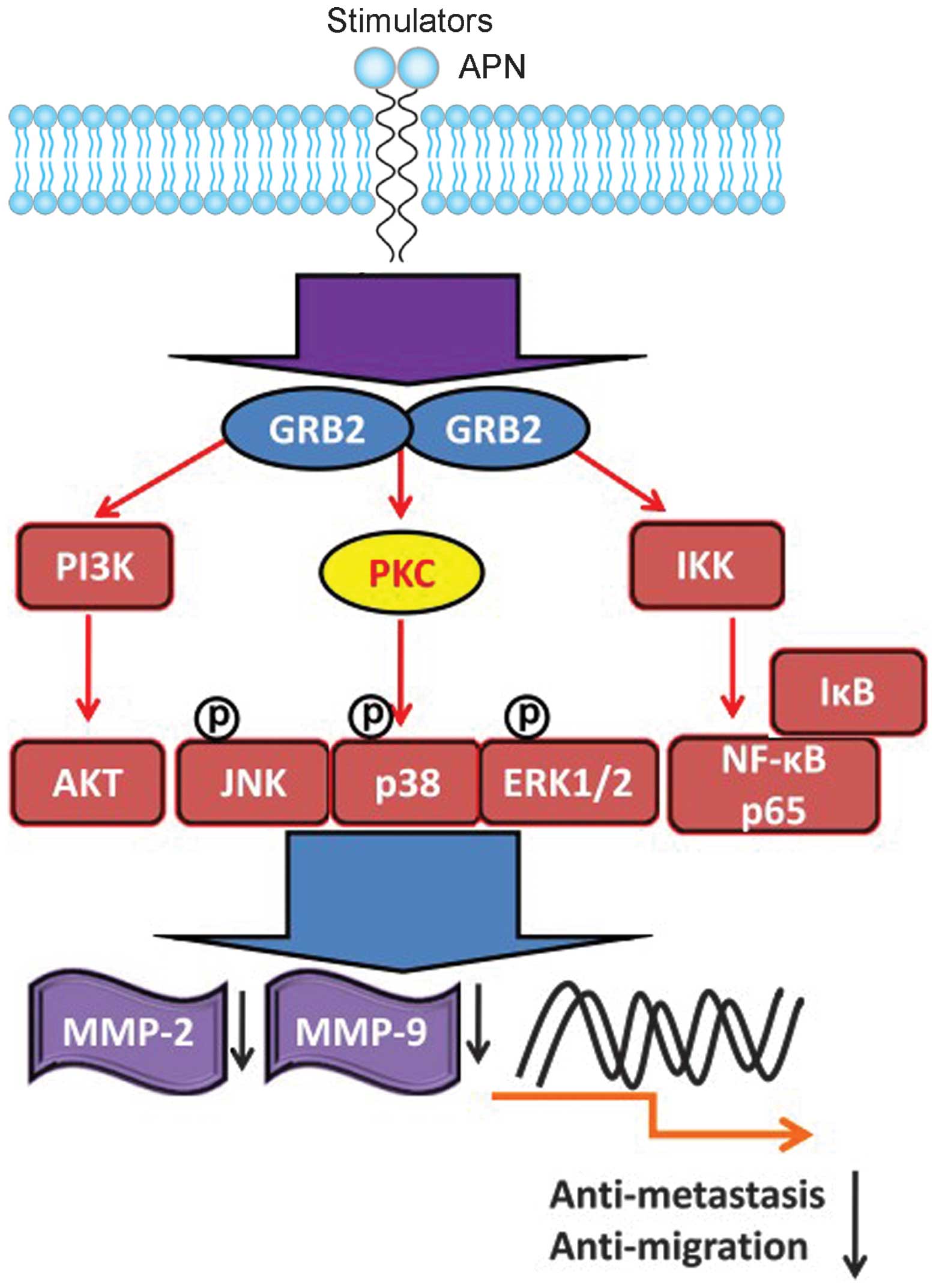
Our study supports the involvement of enzymatic and
signalling functions of APN in osteosarcoma invasion. Foremost, the
inhibition by bestatin of APN in osteosarcoma cell lines reduces
cell invasiveness and migration potential concomitant with a
downregulation of APN mRNA and hydrolysing activity. Reduced cell
motility is accompanied by reduced MAPK and PI3K signalling and
reduced levels of transcription factor NF-κB, and its targets MMP-2
and -9. Together these data support the notion that the reduced
invasiveness is caused by reduced APN enzymatic and signalling
activity. That is, APN enzymatic activity not only degrades the ECM
to facilitate migration but also activates the MAPK and PI3K
pathway, leading to activation of MMP-2 and -9. These data place
APN in a potentially important position for regulating key
signalling pathways which activate MMP-2 and -9 via MAPK and NF-κB
to promote invasiveness (Fig. 8).
Since bestatin is an inhibitor of various leucine and arginine
aminopeptidases and an efficient inhibitor of LTA4 hydrolase and
lacks selectivity toward exopeptidases (50), further experiments are required to
determine whether the decreases in APN, MMP-2 and -9 activity were
caused exclusive by bestatin inhibition of APN or its inhibition of
other molecules. An exclusive role of APN in activating signalling
pathways and MMP-2 and -9 activity was demonstrated with gene
silencing of APN by small interfering RNA.
We also show that IL-6 treatment of osteosarcoma
cell lines increases cell invasiveness and migration potential
concomitant with an upregulation of APN mRNA and hydrolysing
activity. Increased cell motility is accompanied by activation of
the MAPK and the PI3K signalling pathways. IL-6 treatment also
increased levels of transcription factor NF-κB, and its targets
MMP-2 and -9. This supports the possibility that APN activation
promotes cell invasiveness. It has previously been shown that
inflammatory cytokines such as IL-6 increase the invasive capacity
of malignant cells (15,51–53).
An involvement of APN in IL-6-induced osteosarcoma invasiveness has
already been suggested because invasiveness correlates with the
increased relative enzymatic activity of APN and APN inhibitor
bestatin reduces IL-6-induced invasiveness (24). These data do not exclude the
possibility that APN activates MMP-2 and -9 via MAPK activation of
NF-κB. However, further experiments are required to exclude the
involvement of other factors known to be induced by IL-6. For
example IL-6 activation of intercellular adhesion molecule-1
(ICAM-1) via the integrin-linked kinase (ILK)/AKT/AP-1 pathway
promotes osteosarcoma cell motility (15). IL-6 also promotes invasion and
migration of human osteosarcoma cell lines through the signal
transducer and activator of transcription 3 (STAT3) signalling
pathway (54). Thus to exclude the
possibility that our observed effects are due to APN activation and
not other IL-6 downstream effectors, a specific activation of APN
would be required.
Inflammatory cytokine IL-6 treatment resulted in an
activation of all three arms of the MAPK signalling pathway, which
regulates diverse processes including gene expression and cell
morphology (48). Specifically, we
observed increased levels of phosphorylated p38, ERK1/2 and JNK. In
addition, IL-6 treatment increased the protein levels of these
three MAPKs. Furthermore, levels of NF-κB p65, a downstream
effector of IL-6 signalling and a MAPK substrate, were increased.
Our analysis of signalling pathway proteins also revealed that IL-6
altered expression levels of PI3K, AKT and PKC, members of the PI3K
signalling pathway, whose activation in tumours contributes to
metastatic competence (47). In
contrast, bestatin had the opposite effect. Together these data
establish a signalling footprint of IL-6-stimulated osteosarcoma
cells and represent a platform on which to explore crosstalk with
signalling pathways activated by APN.
APN performs multiple functions by numerous
mechanisms, including the enzymatic cleavage of peptides,
endocytosis and signal transduction (55). The strong correlation of APN
expression and enzymatic activity with the invasive capacity of
numerous cell types makes it an attractive target molecule for
therapy. Our study raises the possibility that APN is involved in
osteosarcoma metastasis and that its functions do not always depend
on its enzymatic activity. APN performs multiple functions by
numerous mechanisms, including the enzymatic cleavage of peptides,
endocytosis and signal transduction (56). Our study suggests that these
mechanisms also occur in osteosarcoma, and lays the foundation for
future studies.
Acknowledgements
The study was supported by the Natural Science
Foundation of Zhejiang province (LY13H060005), Public Technology
Applied Research Projects of Zhejiang province (2014C33254),
General Foundation of Zhejiang province (2013KYA201), General
Research Plan B of Zhejiang province (2012KYB213), and Shaoxing
Science Project (2013B70081).
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
2
|
Sung L, Anderson JR, Donaldson SS, Spunt
SL, Crist WM and Pappo AS; Soft Tissue Sarcoma Committee of the
Children’s Oncology Group. Late events occurring five years or more
after successful therapy for childhood rhabdomyosarcoma: a report
from the Soft Tissue Sarcoma Committee of the Children’s Oncology
Group. Eur J Cancer. 40:1878–1885. 2004.
|
|
3
|
Cheng YY, Huang L, Lee KM, Li K and Kumta
SM: Alendronate regulates cell invasion and MMP-2 secretion in
human osteosarcoma cell lines. Pediatr Blood Cancer. 42:410–415.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho HJ, Lee TS, Park JB, et al: Disulfiram
suppresses invasive ability of osteosarcoma cells via the
inhibition of MMP-2 and MMP-9 expression. J Biochem Mol Biol.
40:1069–1076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xin ZF, Kim YK and Jung ST: Risedronate
inhibits human osteosarcoma cell invasion. J Exp Clin Cancer Res.
28:1052009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan DG, Dai JY, Tang J, et al: Silencing
of calpain expression reduces the metastatic potential of human
osteosarcoma cells. Cell Biol Int. 33:1263–1267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dass CR, Nadesapillai AP, Robin D, et al:
Downregulation of uPAR confirms link in growth and metastasis of
osteosarcoma. Clin Exp Metastasis. 22:643–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan X, Kim SY, Guenther LM, et al: Beta4
integrin promotes osteosarcoma metastasis and interacts with ezrin.
Oncogene. 28:3401–3411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haydon RC, Deyrup A, Ishikawa A, et al:
Cytoplasmic and/or nuclear accumulation of the beta-catenin protein
is a frequent event in human osteosarcoma. Int J Cancer.
102:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwaya K, Ogawa H, Kuroda M, Izumi M,
Ishida T and Mukai K: Cytoplasmic and/or nuclear staining of
beta-catenin is associated with lung metastasis. Clin Exp
Metastasis. 20:525–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Engin F, Bertin T, Ma O, et al: Notch
signaling contributes to the pathogenesis of human osteosarcomas.
Hum Mol Genet. 18:1464–1470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hughes DP: How the NOTCH pathway
contributes to the ability of osteosarcoma cells to metastasize.
Cancer Treat Res. 152:479–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leow PC, Tian Q, Ong ZY, Yang Z and Ee PL:
Antitumor activity of natural compounds, curcumin and PKF118-310,
as Wnt/β-catenin antagonists against human osteosarcoma cells.
Invest New Drugs. 28:766–782. 2010.PubMed/NCBI
|
|
14
|
Li Y, Zhang J, Ma D, et al: Curcumin
inhibits proliferation and invasion of osteosarcoma cells through
inactivation of Notch-1 signaling. FEBS J. 279:2247–2259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin YM, Chang ZL, Liao YY, Chou MC and
Tang CH: IL-6 promotes ICAM-1 expression and cell motility in human
osteosarcoma. Cancer Lett. 328:135–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berdiaki A, Datsis GA, Nikitovic D, et al:
Parathyroid hormone (PTH) peptides through the regulation of
hyaluronan metabolism affect osteosarcoma cell migration. IUBMB
Life. 62:377–386. 2010.PubMed/NCBI
|
|
17
|
Yang R, Hoang BH, Kubo T, et al:
Over-expression of parathyroid hormone Type 1 receptor confers an
aggressive phenotype in osteosarcoma. Int J Cancer. 121:943–954.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dobashi Y, Watanabe H, Matsubara M, et al:
Autocrine motility factor/glucose-6-phosphate isomerase is a
possible predictor of metastasis in bone and soft tissue tumours. J
Pathol. 208:44–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niinaka Y, Harada K, Fujimuro M, et al:
Silencing of autocrine motility factor induces
mesenchymal-to-epithelial transition and suppression of
osteosarcoma pulmonary metastasis. Cancer Res. 70:9483–9493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fromigue O, Hamidouche Z, Vaudin P, et al:
CYR61 downregulation reduces osteosarcoma cell invasion, migration,
and metastasis. J Bone Miner Res. 26:1533–1542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bauvois B: Transmembrane proteases in cell
growth and invasion: new contributors to angiogenesis? Oncogene.
23:317–329. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carl-McGrath S, Lendeckel U, Ebert M and
Röcken C: Ectopeptidases in tumour biology: a review. Histol
Histopathol. 21:1339–1353. 2006.
|
|
23
|
Hitzerd SM, Verbrugge SE, Ossenkoppele G,
Jansen G and Peters GJ: Positioning of aminopeptidase inhibitors in
next generation cancer therapy. Amino Acids. 46:793–808. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kido A, Krueger S, Haeckel C and Roessner
A: Possible contribution of aminopeptidase N (APN/CD13) to invasive
potential enhanced by interleukin-6 and soluble interleukin-6
receptor in human osteosarcoma cell lines. Clin Exp Metastasis.
17:857–863. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujii H, Nakajima M, Saiki I, Yoneda J,
Azuma I and Tsuruo T: Human melanoma invasion and metastasis
enhancement by high expression of aminopeptidase N/CD13. Clin Exp
Metastasis. 13:337–344. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kehlen A, Lendeckel U, Dralle H, Langner J
and Hoang-Vu C: Biological significance of aminopeptidase N/CD13 in
thyroid carcinomas. Cancer Res. 63:8500–8506. 2003.PubMed/NCBI
|
|
27
|
Petrovic N, Schacke W, Gahagan JR, et al:
CD13/APN regulates endothelial invasion and filopodia formation.
Blood. 110:142–150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong DY, Lee BJ, Lee JC, Choi JS, Wang SG
and Ro JH: Expression of VEGF, HGF, IL-6, IL-8, MMP-9, telomerase
in peripheral blood of patients with head and neck squamous cell
carcinoma. Clin Exp Otorhinolaryngol. 2:186–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loo WT, Sasano H and Chow LW:
Pro-inflammatory cytokine, matrix metalloproteinases and TIMP-1 are
involved in wound healing after mastectomy in invasive breast
cancer patients. Biomed Pharmacother. 61:548–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Güllü IH, Kurdoğlu M and Akalin I: The
relation of gelatinase (MMP-2 and -9) expression with distant site
metastasis and tumour aggressiveness in colorectal cancer. Br J
Cancer. 82:2492000.PubMed/NCBI
|
|
31
|
Mizutani K, Kofuji K and Shirouzu K: The
significance of MMP-1 and MMP-2 in peritoneal disseminated
metastasis of gastric cancer. Surg Today. 30:614–621. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Korpi JT, Hagström J, Lehtonen N, et al:
Expression of matrix metalloproteinases-2, −8, −13, −26, and tissue
inhibitors of metalloproteinase-1 in human osteosarcoma. Surg
Oncol. 20:e18–e22. 2011.
|
|
33
|
Ferrari C, Benassi S, Ponticelli F, et al:
Role of MMP-9 and its tissue inhibitor TIMP-1 in human
osteosarcoma: findings in 42 patients followed for 1–16 years. Acta
Orthop Scand. 75:487–491. 2004.PubMed/NCBI
|
|
34
|
Foukas AF, Deshmukh NS, Grimer RJ, Mangham
DC, Mangos EG and Taylor S: Stage-IIB osteosarcomas around the
knee. A study of MMP-9 in surviving tumour cells. J Bone Joint Surg
Br. 84:706–711. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Himelstein BP, Asada N, Carlton MR and
Collins MH: Matrix metalloproteinase-9 (MMP-9) expression in
childhood osseous osteosarcoma. Med Pediatr Oncol. 31:471–474.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bellido T, O’Brien CA, Roberson PK and
Manolagas SC: Transcriptional activation of the p21(WAF1,CIP1,SDI1)
gene by interleukin-6 type cytokines. A prerequisite for their
pro-differentiating and anti-apoptotic effects on human
osteoblastic cells. J Biol Chem. 273:21137–21144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Franchimont N, Gangji V, Durant D and
Canalis E: Interleukin-6 with its soluble receptor enhances the
expression of insulin-like growth factor-I in osteoblasts.
Endocrinology. 138:5248–5255. 1997.PubMed/NCBI
|
|
38
|
Jilka RL, Weinstein RS, Bellido T, Parfitt
AM and Manolagas SC: Osteoblast programmed cell death (apoptosis):
modulation by growth factors and cytokines. J Bone Miner Res.
13:793–802. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nishimura R, Moriyama K, Yasukawa K, Mundy
GR and Yoneda T: Combination of interleukin-6 and soluble
interleukin-6 receptors induces differentiation and activation of
JAK-STAT and MAP kinase pathways in MG-63 human osteoblastic cells.
J Bone Miner Res. 13:777–785. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kehlen A, Göhring B, Langner J and Riemann
D: Regulation of the expression of aminopeptidase A, aminopeptidase
N/CD13 and dipeptidylpeptidase IV/CD26 in renal carcinoma cells and
renal tubular epithelial cells by cytokines and cAMP-increasing
mediators. Clin Exp Immunol. 111:435–441. 1998. View Article : Google Scholar
|
|
41
|
Chen YY, Chiang SY, Lin JG, et al: Emodin,
aloe-emodin and rhein inhibit migration and invasion in human
tongue cancer SCC-4 cells through the inhibition of gene expression
of matrix metalloproteinase-9. Int J Oncol. 36:1113–1120.
2010.PubMed/NCBI
|
|
42
|
Lin CC, Chen JT, Yang JS, et al: Danthron
inhibits the migration and invasion of human brain glioblastoma
multiforme cells through the inhibition of mRNA expression of focal
adhesion kinase, Rho kinases-1 and metalloproteinase-9. Oncol Rep.
22:1033–1037. 2009.
|
|
43
|
Lai KC, Huang AC, Hsu SC, et al: Benzyl
isothiocyanate (BITC) inhibits migration and invasion of human
colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9
and urokinase plasminogen (uPA) through PKC and MAPK signaling
pathway. J Agric Food Chem. 58:2935–2942. 2010. View Article : Google Scholar
|
|
44
|
Liu KC, Huang AC, Wu PP, et al: Gallic
acid suppresses the migration and invasion of PC-3 human prostate
cancer cells via inhibition of matrix metalloproteinase-2 and -9
signaling pathways. Oncol Rep. 26:177–184. 2011.PubMed/NCBI
|
|
45
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
46
|
Wen YF, Yang JS, Kuo SC, et al:
Investigation of anti-leukemia molecular mechanism of ITR-284, a
carboxamide analog, in leukemia cells and its effects in WEHI-3
leukemia mice. Biochem Pharmacol. 79:389–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qi M and Elion EA: MAP kinase pathways. J
Cell Sci. 118:3569–3572. 2005. View Article : Google Scholar
|
|
49
|
Bond M, Chase AJ, Baker AH and Newby AC:
Inhibition of transcription factor NF-kappaB reduces matrix
metalloproteinase-1, -3 and -9 production by vascular smooth muscle
cells. Cardiovasc Res. 50:556–565. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bauvois B and Dauzonne D:
Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: chemistry,
biological evaluations, and therapeutic prospects. Med Res Rev.
26:88–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Balkwill F, Charles KA and Mantovani A:
Smoldering and polarized inflammation in the initiation and
promotion of malignant disease. Cancer Cell. 7:211–217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lo CW, Chen MW, Hsiao M, et al: IL-6
trans-signaling in formation and progression of malignant ascites
in ovarian cancer. Cancer Res. 71:424–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tang CH, Chen CF, Chen WM and Fong YC:
IL-6 increases MMP-13 expression and motility in human
chondrosarcoma cells. J Biol Chem. 286:11056–11066. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tu B, Du L, Fan QM, Tang Z and Tang TT:
STAT3 activation by IL-6 from mesenchymal stem cells promotes the
proliferation and metastasis of osteosarcoma. Cancer Lett.
325:80–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mina-Osorio P: The moonlighting enzyme
CD13: old and new functions to target. Trends Mol Med. 14:361–371.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liao CL, Lai KC, Huang AC, et al: Gallic
acid inhibits migration and invasion in human osteosarcoma U-2 OS
cells through suppressing the matrix metalloproteinase-2/-9,
protein kinase B (PKB) and PKC signaling pathways. Food Chem
Toxicol. 50:1734–1740. 2012. View Article : Google Scholar : PubMed/NCBI
|