Introduction
Ovarian cancer is one of the deadliest gynecological
malignancies, and at advanced stages is typically treated with
cytoreductive surgery and platinum-based chemotherapy (1–3).
However, cisplatin resistance can limit the utility of
chemotherapeutic intervention (4–6).
Some cancers have intrinsic resistance to cisplatin, while in
others resistance gradually develops over time (6). The mechanisms underlying cisplatin
resistance are not fully understood, though they are believed to be
affected by multiple molecular factors, including progressive
downregulation of pro-apoptotic pathways and activation of survival
pathways (6). Multiple recent
studies have also reported that chronic oxidative stress could lead
to cisplatin resistance (7–10).
Several studies indicate that cisplatin toxicity
occurs via the increased generation of reactive oxygen species
(ROS) within mitochondria (11,12).
The Nrf2-Keap1 pathway functions as a critical regulator of
cellular defense against oxidative stress by controlling the
expression of many cellular protective proteins (13). Under non-stressed condition, Nrf2
is constitutively degraded through the ubiquitin-proteasome system
by binding to Kelch-like ECH-associated protein 1 (Keap1), an
adaptor of a ubiquitin ligase complex (14). Oxidative stress disrupts the
sequestration of Nrf2 by Keap1, leading to nuclear translocation of
Nrf2. Nuclear Nrf2 then binds to the cis-acting antioxidant
response element (ARE) in the promoter of target genes (14,15).
As a multifunctional protein, p62/SQSTM1
(sequestosome 1) plays important roles in cell differentiation,
proliferation and as an antiapoptotic mediator (16,17).
P62 binds to and targets ubiquitinated proteins for degradation
through autophagy (18).
Additionally, p62 interacts with the Nrf2 binding site on Keap1 via
the Keap1 binding region and functions as an activator of Nrf2
(19,20).
Here we describe the activation of the
Keap1-Nrf2-ARE signaling pathway and subsequent induction of
antioxidant gene expression in SKOV3/DDP cells by p62. We show that
p62 possesses an important role in preventing ROS stress-induced
apoptosis by regulating the Keap1-Nrf2-ARE signaling pathway, which
leads to cisplatin resistance in human ovarian cancer cells
(HOCCs).
Materials and methods
Cell lines
Cisplatin-sensitive ovarian cancer cells SKOV3 and
their cisplatin-resistant clones SKOV3/DDP were obtained from the
Chinese Academy of Medical Sciences and Peking Union Medical
College. Cells were cultured at 37°C with 5% CO2 in
RPMI-1640 culture medium (Gibco, Carlsbad, CA, USA), supplemented
with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA).
Cisplatin-resistant SKOV3/DDP cells were maintained in medium
containing 1 μg/ml cisplatin (Sigma-Aldrich, St. Louis, MO, USA) to
maintain resistance.
Cell viability assays
The MTT assay was used to measure cell viability.
Cells were plated at 1×104 cells per well in 96-well
plates. The following day, indicated concentrations of cisplatin or
H2O2 were added, and incubation proceeded for
the specified times. Each treatment was conducted in triplicate at
a minimum. To each well, 20 μl MTT [3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide; (Sigma-Aldrich)], was added followed
by incubation for 4 h and addition of 150 μl dimethylsulfoxide to
dissolve the formazan crystals. Absorbance was measured with a
Vmax Microplate Reader (Molecular Devices, Sunnyvale,
CA, USA) at a wavelength of 570 nm.
Measurement of intracellular reactive
oxygen species
To determine the intracellular levels of ROS
generated by cisplatin, we used dichlorodihydrofluorescein
diacetate (DCFH-DA), which is cell-permeable and interacts with
intracellular ROS to generate fluorescent dichlorofluorescin as
reported previously (21). Cells
were plated at 1×104 cells per well in 96-well plates,
and cultured with cisplatin or H2O2 for 12 or
24 h. After this preincubation period, the medium was discarded and
the attached cells were rinsed thrice with phosphate-buffered
saline (PBS). Cells were then exposed to 5 μM DCFH-DA solution for
15 min at 37°C. The treated cells were washed three times with PBS.
The cells were subsequently scanned to quantitate the average
fluorescence intensity per cell.
Immunofluorescence staining and confocal
laser microscopy
Apoptotic nuclear changes were assessed with Hoechst
33342 (Sigma-Aldrich). Following treatment with 1 mM
H2O2 for 0 and 24 h, cells were fixed in 4%
paraformaldehyde, stained with Hoechst 33342 (2 μg/ml) for 30 min,
washed with PBS, and examined using an Olympus FV1000 confocal
laser microscope. Cells were cultured on coverslips overnight,
treated with 1 mM H2O2 for different times,
and rinsed with PBS. After incubation, cells were fixed for 20 min
in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 for 5
min, blocked with bovine serum albumin, incubated with primary
antibodies against p62 and Keap1 overnight at 4°C, and then
FITC/Texas-conjugated secondary antibodies (1:400 dilution; all
antibodies were from, Santa Cruz Biotechnology, Santa Cruz, CA,
USA) for 1 h. Cells were examined by confocal laser microscopy.
RNA extraction and reverse-transcriptase
PCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen) according to the manufacturer’s protocol.
First-strand cDNA synthesis was performed by reverse transcription
of RNA samples using the SuperScript preamplification system
(Promega, Madison, WI, USA). Absolute gene transcription was
normalized to glyceraldehyde-3 phosphate dehydrogenase (GAPDH).
Primers for GAPDH: sense strand:
5′-GGG-TGA-TGC-TGG-TGC-TGA-GTA-TGT-3′, antisense strand:
5′-AAG-AAT-GGG-AGT-TGC-TGT-TGA-AGT-3′. Primers for p62: sense
strand: 5′-GAA-CTC-CAG-TCCCTA-CAG-AT-3′, antisense strand:
5′-CGA-TGT-CATAGT-TCT-TGG-TC-3′. PCR products were separated on a
1% agarose gel containing ethidium bromide, visualized using a
Tanon-1600 figure gel image processing system, and analyzed by GIS
1D gel image system software (Tanon, Shanghai, China).
Western blot analysis
Cells were harvested following the various
treatments described above, washed with cold PBS, and incubated in
ice-cold radio-immunoprecipitation buffer. Cell lysates were
sonicated for 30 sec on ice and then incubated at 4°C for 60 min.
Lysates were then centrifuged for 30 min at 12,000 g to remove
debris. Protein concentration was determined using the Protein
Assay kit (Bio-Rad, Hercules, CA, USA). For western blot analysis,
protein lysates (30–50 μg) were separated on a 12% w/v
SDS-polyacrylamide gel by electrophoresis and transferred onto
nitrocellulose membranes (Millipore, Bedford, MA, USA). Membranes
were blocked with 5% non-fat dry milk in buffer [10 mM Tris-HCl (pH
7.6), 100 mM NaCl, and 0.1% Tween-20] for 1 h at room temperature,
incubated with the desired primary antibody overnight at 4°C, and
then incubated with horseradish peroxidase-conjugated secondary
antibody (Thermo, Waltham, MA, USA) at a 1:2,000 dilution for 1 h
at room temperature. Immunoreactive bands were visualized using the
diaminobenzene (Sigma) coloration method.
p62 knockdown by small interfering
RNA
Small interfering RNA (siRNA) sequences targeting
human p62/SQSTM1 (GenBank accession NM_003900) and a scrambled
non-targeting control sequence were constructed by Genechem
(Shanghai, China). Described previously, the p62 siRNA (si-p62)
sequence was GAC-ATC-TTC-CGA-ATC-TAC-A, and that of the control
siRNA (Scramble) was TTC-TCC-GAA-CGT-GTC-ACG-T (18). Transfection with siRNA was
performed using Lipofectamine 2000 (Invitrogen) according to the
manufacturer’s protocol. Briefly, cisplatin-resistant SKOV3/DDP
cells were seeded into 6-well plates, and transfected the next day
with si-p62 or si-Scramble. Cells were harvested 2 days
post-transfection, and whole cell lysates were prepared for western
blots. For the MTT assay, transfected cells were treated with
different concentrations of H2O2 for 24 h and
subjected to the MTT assay to determine cell viability.
Flow cytometry
Following exposure to different experimental
conditions, cells were trypsinized and incubated with propidium
iodide (PI, 1 μg/ml) and Annexin V-FITC (1 μg/ml; Invitrogen) for
15 min at 37°C. Samples were then analyzed for apoptosis using a
FACScan flow cytometer (Becton-Dickinson, Franklin Lakes, NJ,
USA).
Analysis of luciferase reporter gene
activity
The Dual-Luciferase Reporter Assay System (Promega)
was used to examine reporter gene activity. Cells were plated at
1×105 per well in 24-well plates. The next day, the
cells were transiently transfected with various ARE-luciferase
reporter plasmids using Lipofectamine 2000 reagent. Following
transfection, cells were incubated for 0, 4, and 8 h in medium
containing H2O2 and then harvested.
The luciferase activity present in lysates was
measured using a luminometer following addition of Luciferase Assay
Reagent II. The relative luciferase activity was calculated by
normalizing firefly luciferase activity to that of Renilla
luciferase.
Statistical analyses
Experiments were performed on at least three
occasions, and are presented as the means ± SD. Comparisons were
made between treatments using paired Student’s t-test, or one-way
ANOVA for multiple group comparisons to single controls;
differences between treatment means were examined with Dunnett’s
test. We used SPSS version 16.0 (SPSS/IBM, Chicago, IL, USA).
*p<0.05 was considered statistically significant.
Results
Cisplatin induces ROS generation and
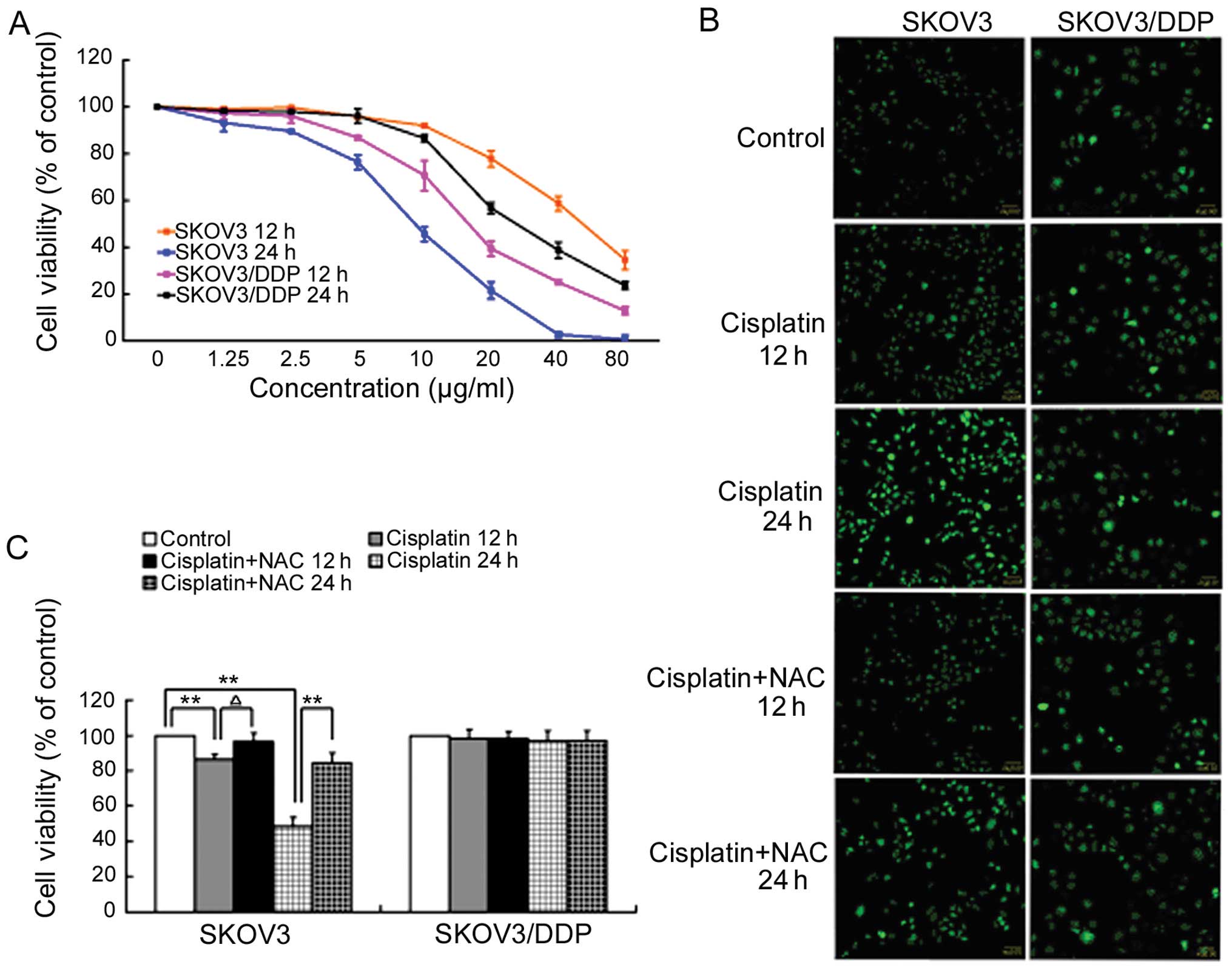
inhibits growth in ovarian cancer cells
Cisplatin-sensitive SKOV3 cells and
cisplatin-resistant SKOV3/DDP cells were treated with increasing
doses of cisplatin for 12 h and 24 h and examined for growth
inhibition using MTT assays. While cisplatin inhibited the growth
of both cell lines, SKOV3 cells were more sensitive to cisplatin
than SKOV3/DDP cells (Fig.
1A).
Based on these MTT results and previous studies
(18), we next treated both cell
lines with 6 μg/ml cisplatin for 12 and 24 h and then used DCFH-DA
to measure hydrogen peroxide levels by fluorescent confocal
microscopy. ROS levels increased significantly in SKOV3 cells
treated with 6 μg/ml cisplatin after 12 and 24 h. When cisplatin
treatment was combined with the antioxidant N-acetylcysteine (NAC;
80 μM), the intracellular ROS level did not increase and was
similar to the control group. ROS levels underwent no significant
change in SKOV3/DDP cells treated with 6 μg/ml cisplatin after 12
or 24 h (Fig. 1B).
Additionally, the MTT assay showed that the cell
survival rate was significantly improved when cisplatin treatment
was combined with NAC relative to cisplatin treatment alone. There
was no similar effect in SKOV3/DDP cells treated with cisplatin and
NAC (Fig. 1C).
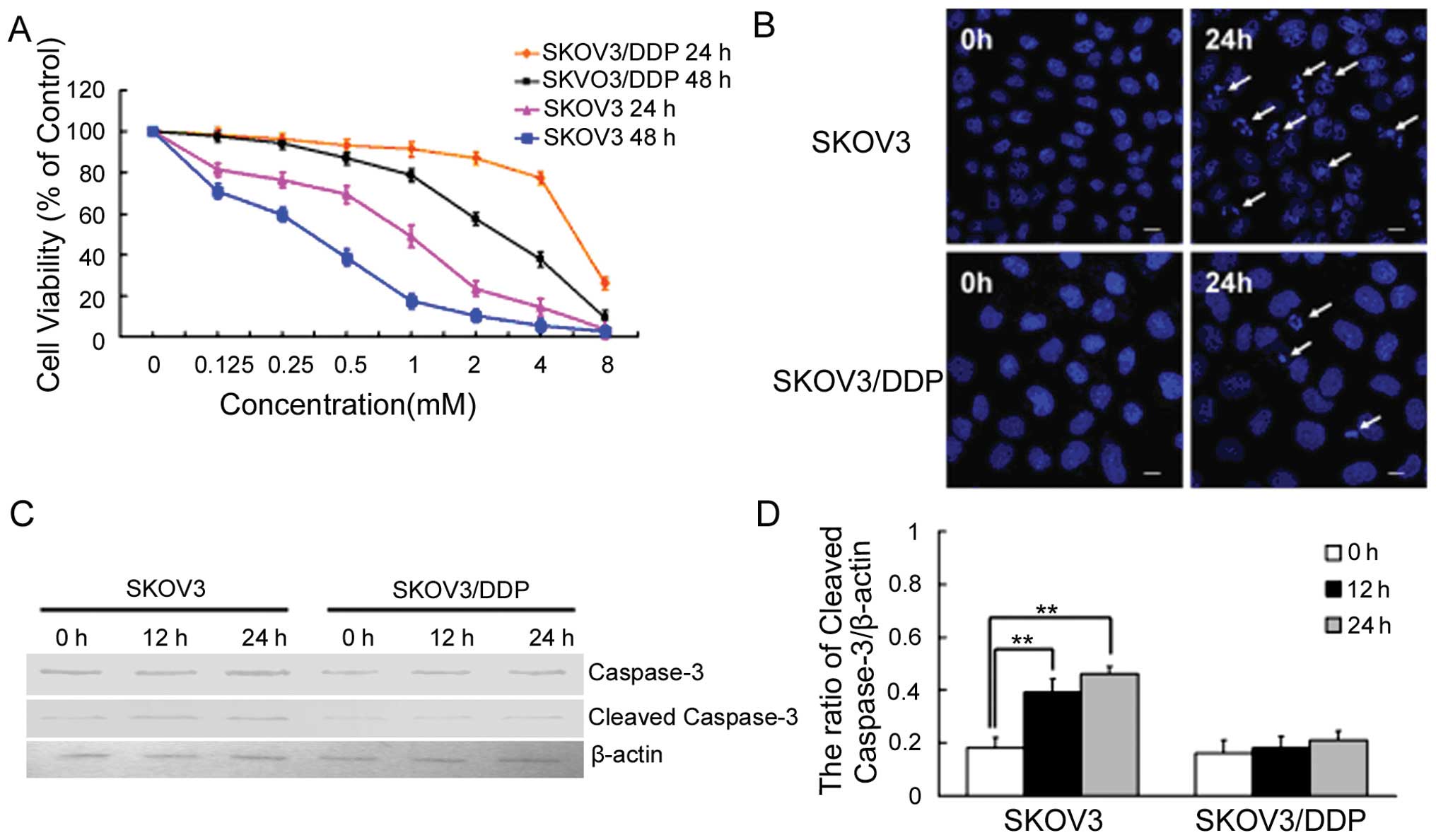
H2O2 inhibits
growth and induces apoptosis in ovarian cancer cells
We then treated SKOV3 and SKOV3/DDP cells with
increasing doses of H2O2 for 24 and 48 h and
measured growth inhibition using the MTT assay. While
H2O2 inhibited the growth of both cell lines,
SKOV3 cells were more sensitive to H2O2 than
SKOV3/DDP cells (Fig. 2A).
Based on these MTT results, we next treated both
cell lines with 1 mM H2O2, and examined
apoptotic chromatin condensation with Hoechst 33342 staining by
fluorescence microscopy (Fig. 2B).
Compared with controls at 24 h, H2O2-induced
apoptotic chromatin condensation was obvious in cisplatin-sensitive
SKOV3 cells, but rare in cisplatin-resistant SKOV3/DDP cells. The
effect on apoptosis was assessed through measurement of caspase-3
activation by western blotting with an antibody that specifically
recognizes cleaved caspase-3. H2O2 enhanced
the expression of cleaved caspase-3 in SKOV3 cells at 12 and 24 h
(Fig. 2C and D). These results
indicate that H2O2 can efficiently induce
apoptosis in SKOV3 cells but not in SKOV3/DDP cells.
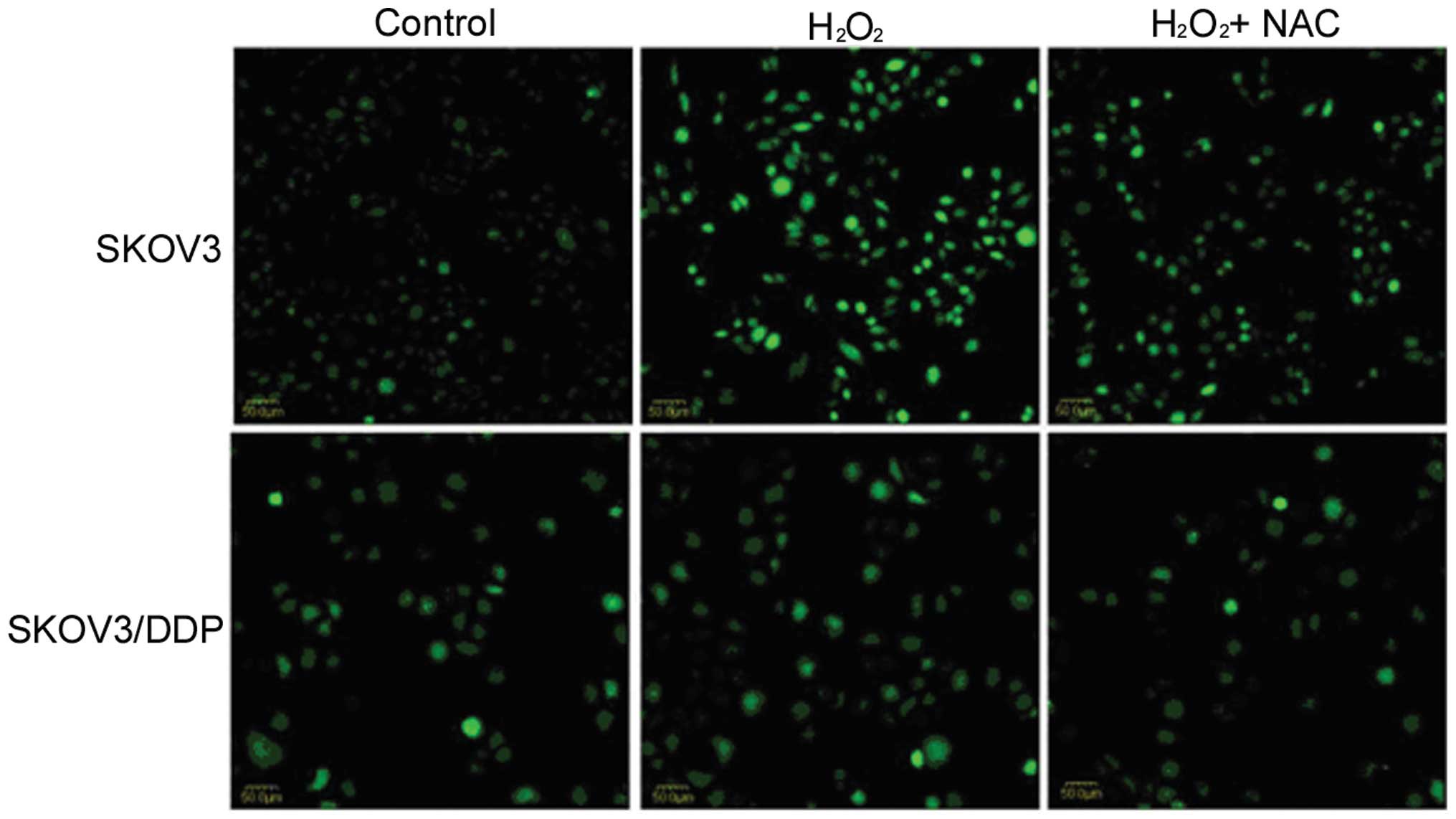
H2O2 induces ROS in
ovarian cancer cells
To evaluate the ROS level in HOCCs treated with
H2O2, DCFH-DA was employed. After 4-h
treatment, H2O2 was shown to induce ROS in
SKOV3 cells, but not in SKOV3/DDP cells. Furthermore, in
combination with NAC, the ROS level was decreased in SKOV3 cells
(Fig. 3).
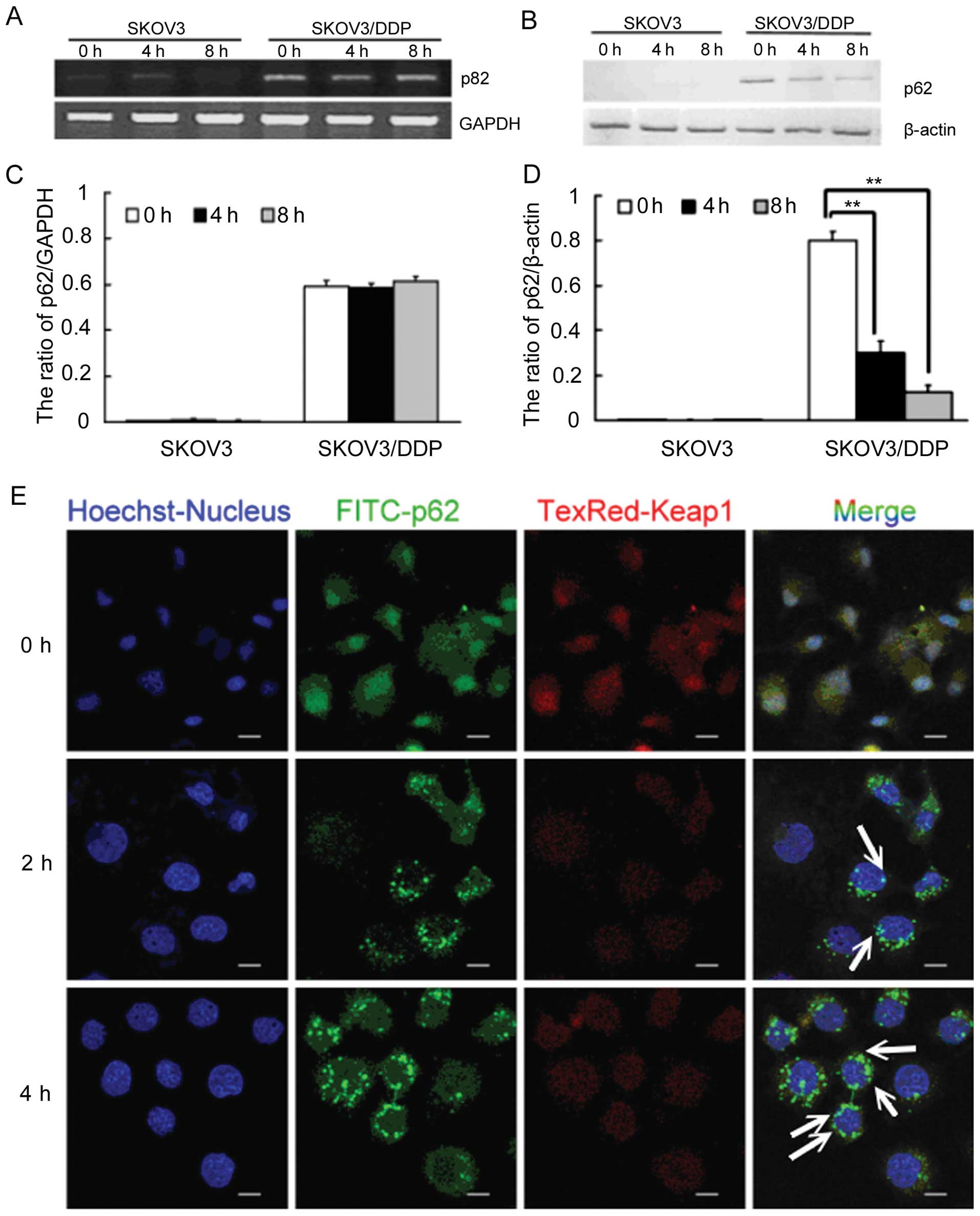
Cisplatin-resistant SKOV3/DDP cells
express the highest level of p62
Based on our previous studies, cisplatin-resistant
SKOV3/DDP cells express much higher levels of p62 than do
cisplatin-sensitive SKOV3 cells. As a multifunctional protein, p62
has been reported to recruit ubiquitinated proteins to
autophagosomes for degradation, and also to regulate the
Keap1-Nrf2-ARE system in cancer cells. We next determined the
expression levels of p62 protein and mRNA after
H2O2-mediated ROS induction in HOCCs.
Following a 4-h treatment with 1 mM H2O2, p62
protein levels in SKOV3/DDP cells decreased gradually while p62
mRNA transcripts remained constant. This indicates that
H2O2 decreases p62 at the protein level
(Fig. 4A–D).
Following both 2- and 4-h treatment of SKOV3/DDP
cells with H2O2, p62 levels increased while
Keap1 levels decreased. We then used confocal microscopy to
identify the locations of p62 and Keap1 in these cells, and
observed colocalization of p62 and Keap1 in the cytosol. This
suggests that p62 may interact with Keap1 in SKOV3/DDP cells
(Fig. 4E).
p62 induces the Keap1-Nrf2-ARE system to
reduce the ROS in resistant cells
The importance of the Keap1-Nrf2-ARE system in the
cellular response to oxidative stress has been well established
(22). The next step was therefore
to determine whether the antioxidant gene Nrf2 was activated in
cisplatinresistant SKOV3/DDP cells treated with
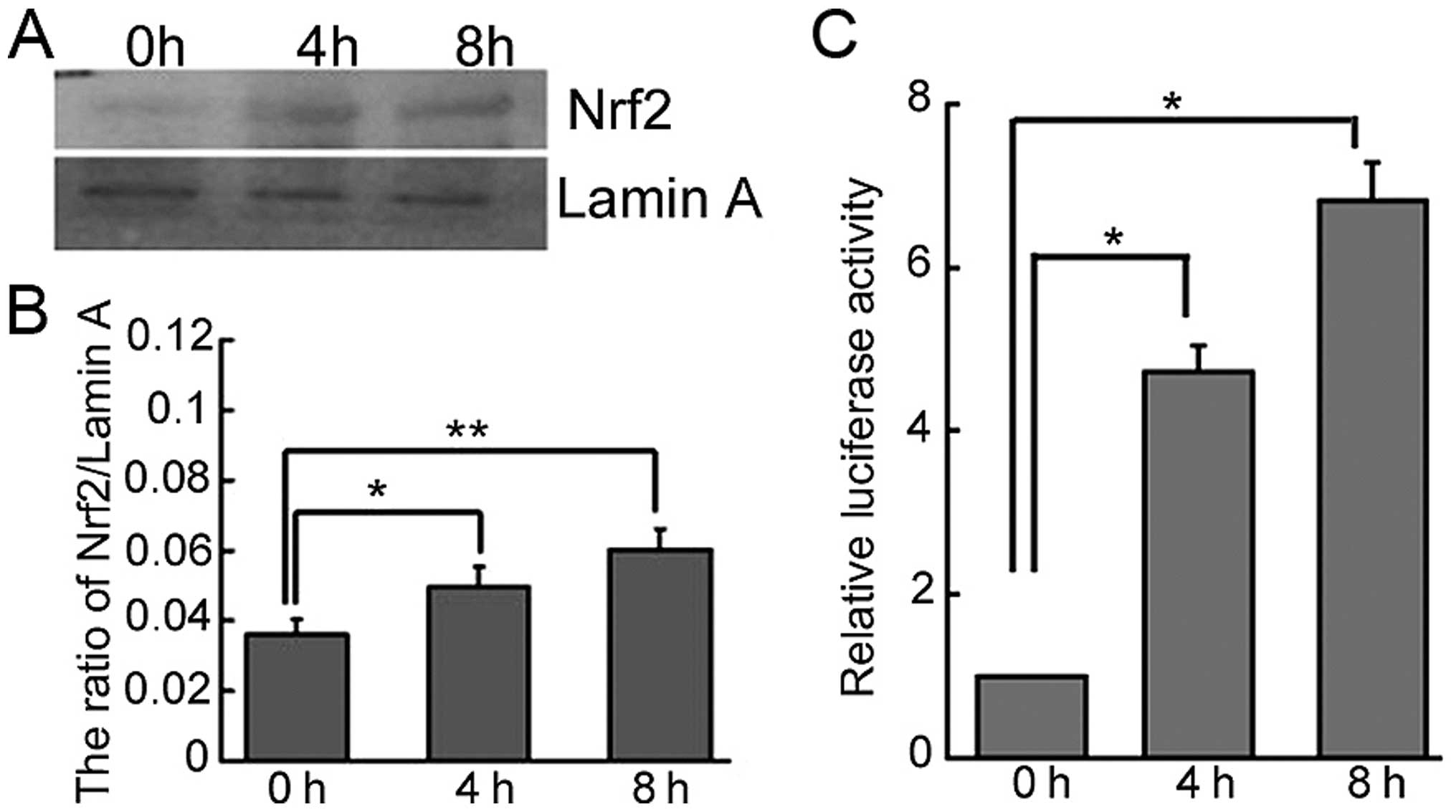
H2O2. When treated with 1 mM
H2O2 over a time course, the level of Nrf2
protein gradually increased in the nucleus of SKOV3/DDP cells
(Fig. 5A and B). Additionally, an
ARE-luciferase reporter construct was employed to determine the
response of ARE-containing genes to ROS in SKOV3/DDP cells. Here,
H2O2 significantly increased luciferase
activity (Fig. 5C).
Knockdown of p62 resensitizes
cisplatin-resistant SKOV3/DDP cells to
H2O2
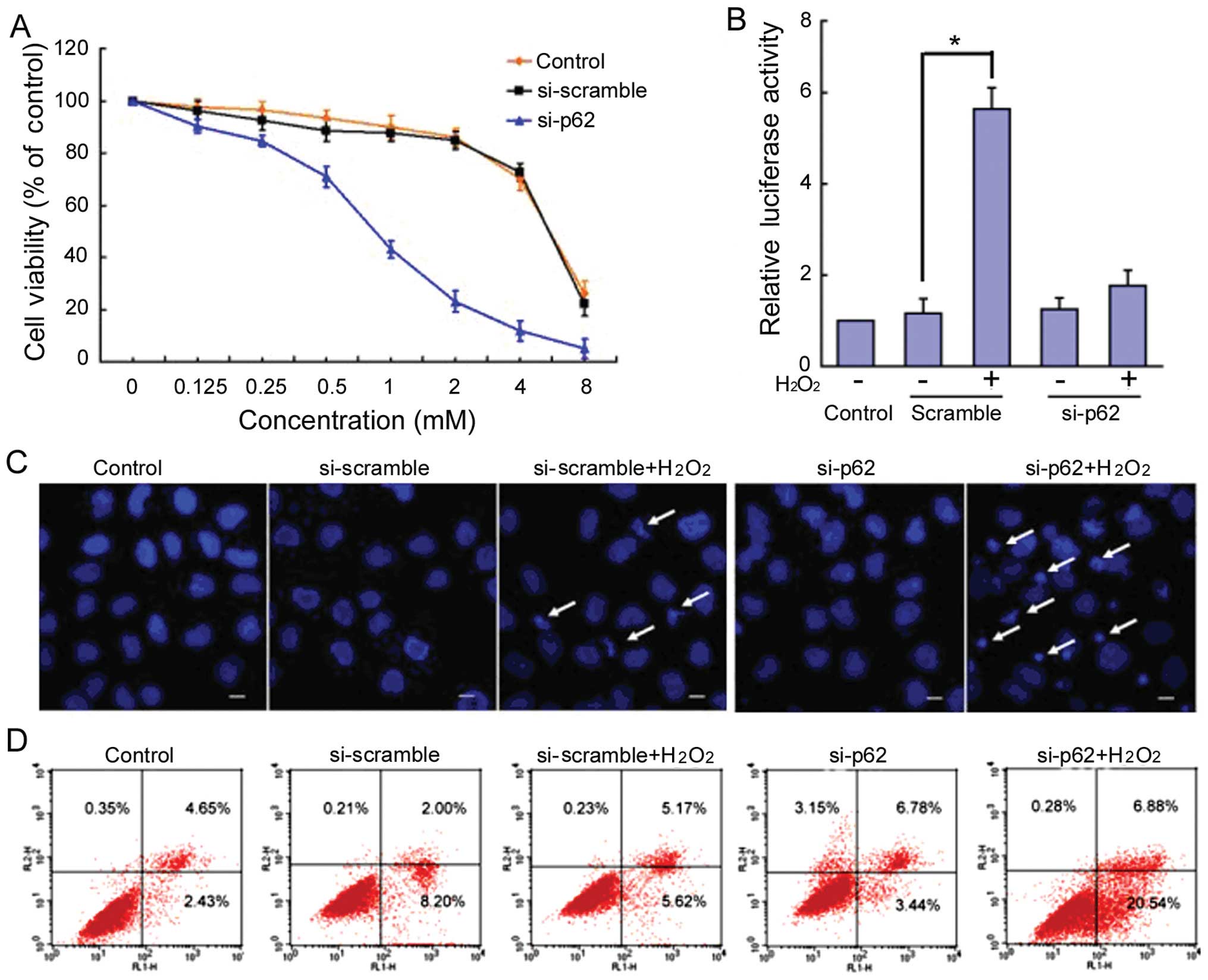
To investigate the direct role of p62 in cisplatin
resistance, we used siRNA to knock down p62 expression. We
transfected cisplatin-resistant SKOV3/DDP cells with siRNA against
p62 or with a non-targeting scrambled sequence. Compared with the
cells transfected with scrambled siRNA, p62 knockdown increased
H2O2-induced growth inhibition in
cisplatin-resistant SKOV3/DDP cells (Fig. 6A). The ARE-luciferase reporter
system revealed that the reporter could not be activated by 1 mM
H2O2 (Fig.
6B). Hoechst 33342 staining and confocal microscopy was used to
detect apoptosis in the si-p62-SKOV3/DDP cells. Compared with
controls at 24 h, H2O2-induced apoptotic
chromatin condensation was clearly observed in si-p62 SKOV3/DDP
cells, but rarely in si-Scramble SKOV3/DDP cells (Fig. 6C).
Additionally, si-p62-SKOV3/DDP cells were subjected
to PI and Annexin V-FITC staining followed by flow cytometry
analysis to quantify apoptotic cell populations. After 24-h
incubation following treatment with 1 mM
H2O2, 10.79% of si-Scramble SKOV3/DDP cells
and 27.42% of sip62-SKOV3/DDP cells underwent apoptosis. This
difference was significant (p<0.05; Fig. 6D).
Discussion
Cisplatin is one of the major chemotherapeutic drugs
used in the treatment of various human cancers. Still, the
mechanisms by which it induces apoptosis are not fully understood
(23,24). Nevertheless, acquired resistance to
cisplatin limits its use. While approximately 70% of ovarian cancer
patients respond to cisplatin initially, most relapse as resistance
to cisplatin develops (25,26).
An improved understanding of the detailed mechanisms underlying
cisplatin resistance is therefore critical.
The cell possesses various defenses to protect
against oxidant insult from ROS and xenobiotic toxicants (27). Nrf2 is a key transcriptional
regulator implicated in protecting cells against oxidative and
xenobiotic stresses (21). Nrf2
has emerged as the master regulator of a cellular defense mechanism
that elicits an adaptive response and promotes cell survival under
stress through transcriptional activation of an array of
ARE-bearing genes, including detoxifying genes, drug transporters,
and cellular redox regulators. High constitutive expression of Nrf2
is observed in many cancer cells that demonstrate resistance to
anticancer drugs (28). Nrf2
promotes cancer cell survival under therapeutic regimens and thus
contributes to chemoresistance. In this study, we have also
implicated Nrf2 in cisplatin resistance in HOCCs.
Our early studies showed that p62 binds and targets
ubiquitinated proteins for degradation through autophagy, thus
maintaining cellular homeostasis and contributing to cisplatin
resistance in HOCCs. Herein, we demonstrated that oxidative stress
tolerance may also contribute to cisplatin resistance in ovarian
cancer. We found that cisplatin-resistant SKOV3/DDP cells express
much higher p62 levels than do cisplatin-sensitive SKOV3 cells.
Following treatment with H2O2, the level of
p62 decreased gradually in SKOV3/DDP cells. Furthermore, confocal
microscopy revealed that p62 increased while Keap1 decreased in
SKOV3/DDP cells. Thus, the multifunctional protein p62 exerts its
important role in drug resistance through multiple avenues,
including recruitment of ubiquitinated proteins to autophagosomes
for degradation, and the regulation of the Keap1-Nrf2-ARE
system.
Our study reveals that p62 knockdown significantly
enhances the sensitivity of cisplatin-resistant SKOV3/DDP cells to
H2O2. Knockdown of p62 reduced the activity
of the ARE in SKOV3/DDP cells, resulting in apoptosis. This
indicates that p62 plays an important role in cisplatin-resistant
HOCCs.
In conclusion, we have identified much higher p62
levels in cisplatin-resistant SKOV3/DDP cells compared with
cisplatin-sensitive SKOV3 cells. Furthermore, p62 underwent gradual
degradation in SKOV3/DDP cells treated with
H2O2, and p62 knockdown resensitizes
cisplatin-resistant SKOV3/DDP cells to H2O2.
It is possible that p62 could interact with Keap1 to regulate Nrf2
entry to the nucleus, as well as act downstream of ARE. This
evidence indicates that cisplatinresistant cells effectively avoid
oxidative stress-induced apoptosis as a result of p62 efficiently
regulating the Keap1- Nrf2-ARE system to mediate cisplatin
resistance in HOCCs. Therefore, p62 represents a candidate
therapeutic target for the improvement of cisplatin efficacy.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (nos. 81201268, 81272876 and 81372793),
the Postdoctoral Science Foundation of China (no. 2013M540256) and
the Fundamental Research Project of Jilin University (no.
450060481216).
References
|
1
|
Goff BA, Mandel LS, Drescher CW, et al:
Development of an ovarian cancer symptom index: possibilities for
earlier detection. Cancer. 109:221–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shank JJ, Yang K, Ghannam J, et al:
Metformin targets ovarian cancer stem cells in vitro and in vivo.
Gynecol Oncol. 127:390–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pils D, Bachmayr-Heyda A, Auer K, et al:
Cyclin E1 (CCNE1) as independent positive prognostic factor in
advanced stage serous ovarian cancer patients - a study of the
OVCAD consortium. Eur J Cancer. 50:99–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Florea AM and Busselberg D: Cisplatin as
an anti-tumor drug: cellular mechanisms of activity, drug
resistance and induced side effects. Cancers. 3:1351–1371. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galluzzi L, Senovilla L, Vitale I, et al:
Molecular mechanisms of cisplatin resistance. Oncogene.
31:1869–1883. 2012. View Article : Google Scholar
|
|
7
|
Cho JM, Manandhar S, Lee HR, et al: Role
of the Nrf2- antioxidant system in cytotoxicity mediated by
anticancer cisplatin: implication to cancer cell resistance. Cancer
Lett. 260:96–108. 2008. View Article : Google Scholar
|
|
8
|
Hall MD, Handley MD and Gottesman MM: Is
resistance useless? Multidrug resistance and collateral
sensitivity. Trends Pharmacol Sci. 30:546–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruiz S, Pergola PE, Zager RA, et al:
Targeting the transcription factor Nrf2 to ameliorate oxidative
stress and inflammation in chronic kidney disease. Kidney Int.
83:1029–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke HJ, Chambers JE, Liniker E, et al:
Endoplasmic reticulum stress in malignancy. Cancer Cell.
25:563–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HJ, Lee JH, Kim SJ, et al: Roles of
NADPH oxidases in cisplatin-induced reactive oxygen species
generation and ototoxicity. J Neurosci. 30:3933–3946. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong YY, Chen HP, Tan BZ, et al:
Triptolide avoids cisplatin resistance and induces apoptosis via
the reactive oxygen species/nuclear factor-kappaB pathway in SKOV3
platinum-resistant human ovarian cancer cells. Oncol Lett.
6:1084–1092. 2013.
|
|
13
|
Taguchi K, Motohashi H and Yamamoto M:
Molecular mechanisms of the Keap1-Nrf2 pathway in stress response
and cancer evolution. Genes Cells. 16:123–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang DD: The Nrf2-Keap1-ARE signaling
pathway: the regulation and dual function of Nrf2 in cancer.
Antioxid Redox Signal. 13:1623–1626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pi J, Zhang Q, Fu J, et al: ROS signaling,
oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol
Appl Pharmacol. 244:77–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moscat J, Diaz-Meco MT and Wooten MW:
Signal integration and diversification through the p62 scaffold
protein. Trends Biochem Sci. 32:95–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mazure NM and Pouyssegur J:
Hypoxia-induced autophagy: cell death or cell survival? Curr Opin
Cell Biol. 22:177–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu H, Su J, Xu Y, et al: p62/SQSTM1
involved in cisplatin resistance in human ovarian cancer cells by
clearing ubiquitinated proteins. Eur J Cancer. 47:1585–1594. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lau A, Zheng Y, Tao S, et al: Arsenic
inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a
p62-dependent manner. Mol Cell Biol. 33:2436–2446. 2013. View Article : Google Scholar
|
|
20
|
Lau A, Wang XJ, Zhao F, et al: A
noncanonical mechanism of Nrf2 activation by autophagy deficiency:
direct interaction between Keap1 and p62. Mol Cell Biol.
30:3275–3285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y1, Parsons KK, Chi L, et al:
Glutathione S-transferase-microl regulates vascular smooth muscle
cell proliferation, migration, and oxidative stress. Hypertension.
54:1360–1368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarkaria JN, Schwingler P, Schild SE, et
al: Phase I trial of sirolimus combined with radiation and
cisplatin in non-small cell lung cancer. J Thorac Oncol. 2:751–757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tummala MK and McGuire WP: Recurrent
ovarian cancer. Clin Adv Hematol Oncol. 3:723–736. 2005.
|
|
27
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jaramillo MC and Zhang DD: The emerging
role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev.
27:2179–2191. 2013. View Article : Google Scholar : PubMed/NCBI
|