Introduction
Glioblastoma multiforme (GBM) is the most common
primary intracranial tumor in adults, with a combined incidence of
5–8 per 100,000 individuals. It is highly aggressive, with a poor
prognosis (1,2). Although the treatment of GBM has
advanced, the median survival of patients with GBM is generally
only 9–12 months (3). At present,
GBM treatments include surgery, radiotherapy, and chemotherapy.
However, these treatments frequently fail: i) due to its highly
invasive nature, operations cannot remove tumor tissue tho roughly;
and ii) tumors develop resistance to radiotherapy and chemotherapy.
Therefore, it is critical to develop more effective therapies based
on novel molecular targets (4) and
to identify novel therapeutic strategies to improve prognosis for
patients with GBM.
Cox et al (5) first discovered PIWI in a genetic
screen for mutants that affected the asymmetrical division of stem
cells in the Drosophila germline. Early studies revealed
that Drosophila PIWI is an important factor for
gametogenesis, and a key regulator of female germline stem cells.
The PIWI protein family is highly conserved in many species,
including humans. To date, four PIWI proteins have been reported in
humans: PIWIL1/HIWI, PIWIL2/HILI, PIWIL3, and PIWIL4/HIWI2
(6). HIWI (also named PIWIL1), a
human homologue of the PIWI family, plays an important role in the
self-renewal of stem cells (7).
Tumor, germ, and stem cells all share common characteristics, for
example the ability for rapid and infinite self-renewal. Therefore,
it is not surprising that HIWI has been implicated in oncogenesis.
Several studies have demonstrated that HIWI is expressed in tumor
tissues, and is one of the most important elements in the
regulation of cancer cells (8,9).
HIWI was also correlated with patient prognosis in pancreatic,
colon, colorectal, lung, and endometrial cancer (10–14).
Sun et al (15) reported that HIWI was overexpressed
in glioma tissues and cell lines, and associated the prognosis of
patients with glioma. However, it remains unclear whether HIWI
plays an important role in the tumorigenesis and development of
GBM. Therefore, the aim of the present study was to investigate the
effects of HIWI in aspects of GBM including proliferation,
invasion, and migration, and to define the corresponding molecular
mechanisms using RNA interference and flow cytometry analysis.
Materials and methods
Cell culture
The human glioblastoma cell lines A172 and U251 were
purchased from the Peking Union Medical College cell library
(Beijing, China). Cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with penicillin/streptomycin and
10% fetal bovine serum (FBS) in a humidified atmosphere containing
5% CO2 at 37°C.
Transfection
Small interfering RNA against HIWI and a negative
control (scrambled control) were purchased from GenePharma
(Shanghai, China). The following siRNA sequences described
previously (16) were used: HIWI,
5′-GCC GUUCAUACAAGACUAATT-3′; negative control, 5′-UUC
UCCGAACGUGUCACGUTT-3′. Transfection was performed using
Lipofectamine 2000 Reagent (Invitrogen Life Tech nologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions. In
brief, A172 or U251 cells were seeded into 6- or 96-well plates
(Corning Costar, Cambridge, MA, USA). The following day (when the
cells were ~40% confluent), the culture media were aspirated, and
the cell monolayer was washed with pre-warmed sterile
phosphate-buffered saline (PBS). Transfection complexes were
prepared following the manufacturer’s instructions.
Western blotting
U251 and A172 control and treated cells were washed
twice in ice-cold PBS, and lysed in M-PER Mammalian Protein
Extraction Reagent (CW Biotech, Beijing, China) containing protease
and phosphatase inhibitor cocktails. Lysates were centrifuged at
12,000 × g for 15 min at 4°C. The supernatants were then added to
an equal volume of sample buffer (50 mM Tris-HCl, 4% SDS, 0.01%
bromophenol blue, 20% glycerol, and 2% 2-Mercaptoethanol, pH 6.8),
heat-denatured, and placed on ice for 5 min. Protein concentrations
were determined using the BCA Protein Assay kit (CW Biotech). Equal
amounts of protein were loaded onto 12% Tris-glycine SDS-PAGE gels,
and separated at 80–120 V for 1.5–2 h. The proteins were
transferred to nitrocellulose membrane, and blocked with 5% milk in
TBST buffer. Membranes were then incubated with primary antibodies
at 4°C overnight. After washing three times in TBST buffer for 5
min each, membranes were incubated with secondary antibodies
conjugated to horseradish peroxidase (1:10,000) for 1 h at room
temperature, and developed using an Electrochemiluminescence (ECL)
kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Anti-HIWI,
anti-MMP-2, anti-MMP-9, anti-p21, anti-cyclin D1 (Beijing Biosea
Biotechnology Co., Ltd., Beijing, China), anti-Bcl-2, and anti-Bax
antibodies (Signalway Antibody, College Park, MD, USA) were used to
detect the respective proteins. An anti-β-actin antibody (Beijing
Biosea Biotechnology Co., Ltd.) was used as a control for equal
sample loading.
MTT assay
U251 and A172 cells (5×103) were
incubated in 96-well plates, with each well containing 100 μl of
medium. The cells were divided into the following groups: i)
control; ii) scrambled siRNA (cells transfected with control
siRNA); and iii) HIWI siRNA group (cells transfected with HIWI
siRNA). The transfection of siRNAs was performed the day after
plating when cells were ~40% confluent. The rate of cellular
proliferation was measured 48 h after treatment. To each well 15 μl
of 5 mg/ml 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium
bromide (MTT) (Sigma, St. Louis, MO, USA) was added. After 4 h, 150
μl of DMSO were added to the MTT-treated wells, and the absorption
at 490 nm was determined using a spectrometer. Each experimental
condition was performed in triplicate.
Colony formation
Cells in each group described above were
trypsinized, counted, and seeded for colony formation assays in
12-well plates at 100 cells/well. During colony growth, the culture
media were replaced every 3 days. A colony was counted only if it
contained >50 cells, and the number of colonies was counted 14
days after seeding. The colony formation rate was calculated using
the equation: colony formation rate = (number of colonies/number of
seeded cells) × 100%. Each treatment was performed in
triplicate.
Flow cytometric analysis of
apoptosis
U251 and A172 cells at 40% confluence were
transfected with negative control or HIWI siRNA. At 48 h
post-transfection, cells were harvested by trypsinization. Cells
(1×105) were washed with PBS, and resuspended in 250 μl
of binding buffer containing 5 μl Annexin V-FITC and 5 μl propidium
iodide (PI) (BD Pharmingen, San Diego, CA, USA). After incubation
for 20 min at room temperature in the dark, another 400 μl of
binding buffer was added, and the samples were analyzed immediately
using FACSCalibur. Flow cytometry data were analyzed using FlowJo
(BD Pharmingen).
Flow cytometric analysis of the cell
cycle
Control U251 and A172 cells, and those treated with
HIWI or control siRNA (50 nM) for 48 h were harvested, washed twice
with cold PBS, and fixed with 5 ml of ice-cold 70% ethanol
overnight. The cells were then incubated with 1 mg/ml RNase A, 1%
Triton X-100 (both from Sigma), and 50 μg/ml PI at 4°C overnight,
and the DNA content was determined using flow cytometry (BD
Pharmingen). The data were then analyzed using MLT software
(Becton-Dickinson, San Jose, CA, USA).
Transwell invasion and migration
assays
Cell invasion and migration assays were performed
using a Transwell system that incorporated a polycarbonate filter
membrane with a diameter of 6.5 mm and pore size of 8 μm (Corning
Costar), according to the manufacturer’s protocol. To assess
invasion, filters were pre-coated with 50 μl of mixed Matrigel
(Matrigel:DMEM = 1:5; BD Biosciences, Franklin Lakes, NJ, USA).
Cell suspensions (1×105) in serum-free culture media
were added into the inserts, and each insert was placed in the well
of a plate filled with culture media containing 10 % FBS as a
chemoattractant. After 24 h incubation at 37°C, the non-invasive
cells were removed from the upper chamber by wiping with
cotton-tipped swabs. Filters were fixed using methanol and glacial
acetic acid (1:3) for 30 min, and then stained with 3% crystal
violet solution for 30 min at room temperature. Five fields of
adherent cells in each well were photographed randomly under a
Nikon-TE2000-U inverted fluorescence microscope, and counted using
ImageJ software.
The same experimental design was used for migration
experiments, except that filters were not precoated with Matrigel,
and cells were counted after 8 h incubation. All experiments were
performed in triplicate.
Nude mouse tumor xenograft model
The animal experimental protocols (total mice
number, 28) were approved by the Animal Care and Use Committee of
Tianjin Medical University. Female, SPF grade BALB/C-A nude mice
aged four weeks, were purchased from the Laboratory Animal Center
of the PLA Military Academy of Medical Sciences. The U251 glioma
subcutaneous model was established as described previously
(17). The mice were randomly
divided into three groups with six mice in each treatment group:
HIWI siRNA group, scramble group and control group. A mixture of 20
μl Lipofectamine 2000 and HIWI siRNA, nonsense siRNA (20 nmol/l ×
20 μl) or PBS mixture was injected into the xenograft model in a
multisite injection manner. Treatment was conducted every 2 days,
and the tumor volume was measured with a caliper every 2 days,
using a formula (volume = long diameter × short
diameter2/2) (18).
After being observed for 28 days, the mice bearing xenograft tumors
were sacrificed and the tumor tissues were removed for formalin
fixation and the preparation of paraffin embedded sections for
immunohistochemical analysis. The mice were maintained at an animal
facility under pathogen-free conditions. The handling of mice and
experimental procedures were conducted in accordance with
experimental animal guidelines.
Immunohistochemistry assay
The immunohistoche mical staining was detected using
streptavidin-biotin-horseradish peroxidase complex (SABC) compound
(SABC kits; Wuhan Boster Biological Technology, Ltd., Hubei, China)
according to the manufacturer’s protocol. Briefly, after incubation
with endogenous peroxidase by 3% H2O2, the
slides were blocked with normal goat serum for 30 min in a
humidified chamber at room temperature and then incubated at 4°C
overnight with appropriate primary antibody (dilution 1:100). Next,
these slides were treated with biotinylated secondary antibody for
60 min, and finally incubated with SABC-AP conjugate for 30 min.
Immunologic reaction was developed using 3,3′-diaminobenzidine (DAB
substate kit; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China). Positive cells were mounted by Image-Pro
Plus5.0 software. Negative controls were performed by substituting
the primary antibody with Tris-buffered saline.
Statistical analysis
Results were analyzed using SPSS software 17.0, and
compared using one-way analysis of variance (ANOVA). Data are
presented as mean ± standard deviation (SD) of three or six
independent experiments, as appropriate. P<0.05 was considered
to be statistically significant.
Results
Endogenous expression and knockdown of
HIWI in glioma cells
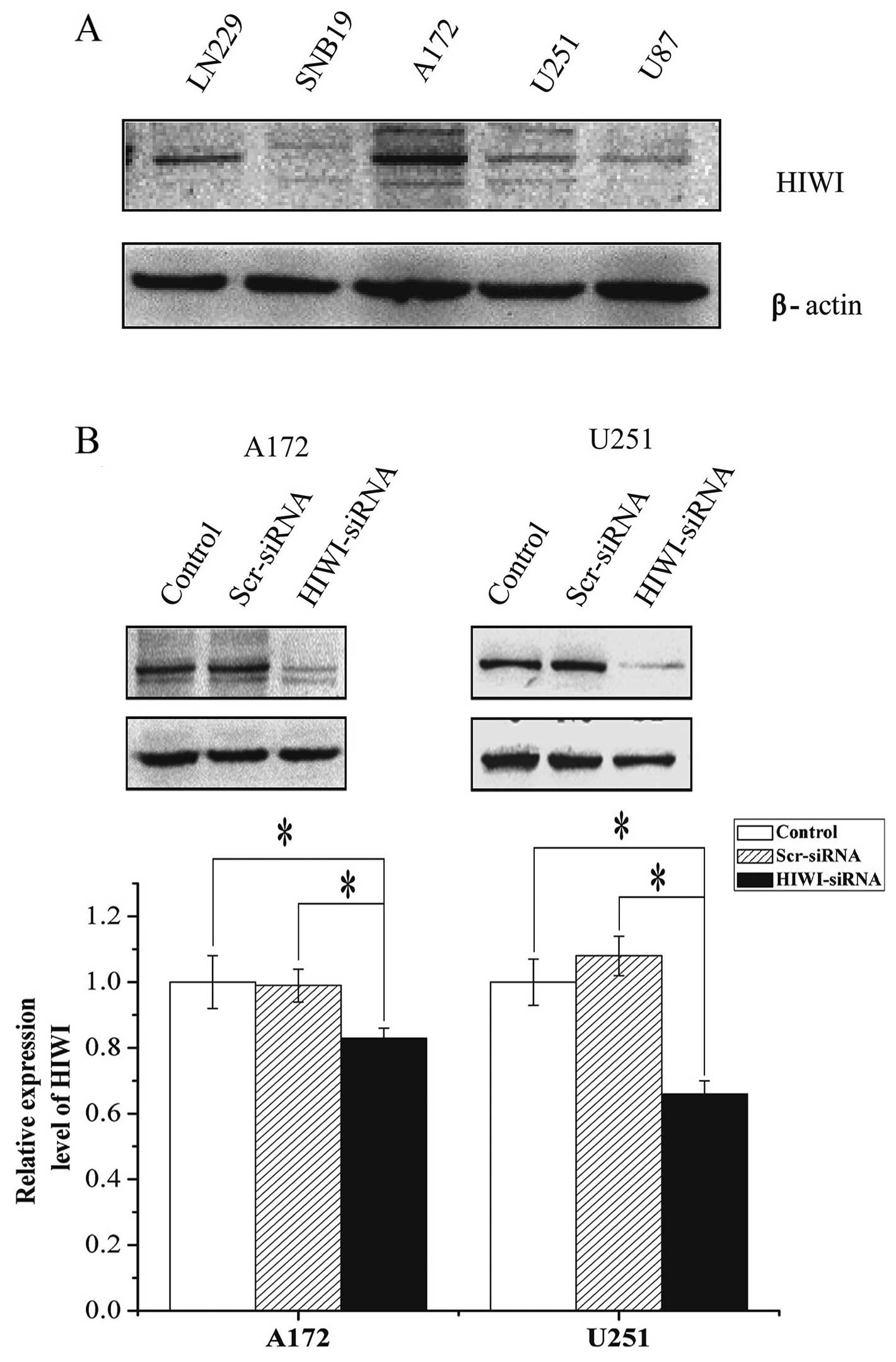
HIWI expression was assessed in five glioma cell
lines (LN229, SNB19, A172, U251, and U87) by western blotting. HIWI
was expressed to a variable extent in the different cell lines
(Fig. 1A). HIWI or scrambled siRNA
was then transiently transfected into U251 and A172 cells. After 48
h, the HIWI protein levels were quantified with western blotting.
Although the β-actin internal control revealed equal loading
between the three groups, the expression of HIWI in U251 or A172
cells treated with HIWI siRNA was reduced by ~80 or 65%,
respectively, compared with both untreated and negative
siRNA-transfected cells.
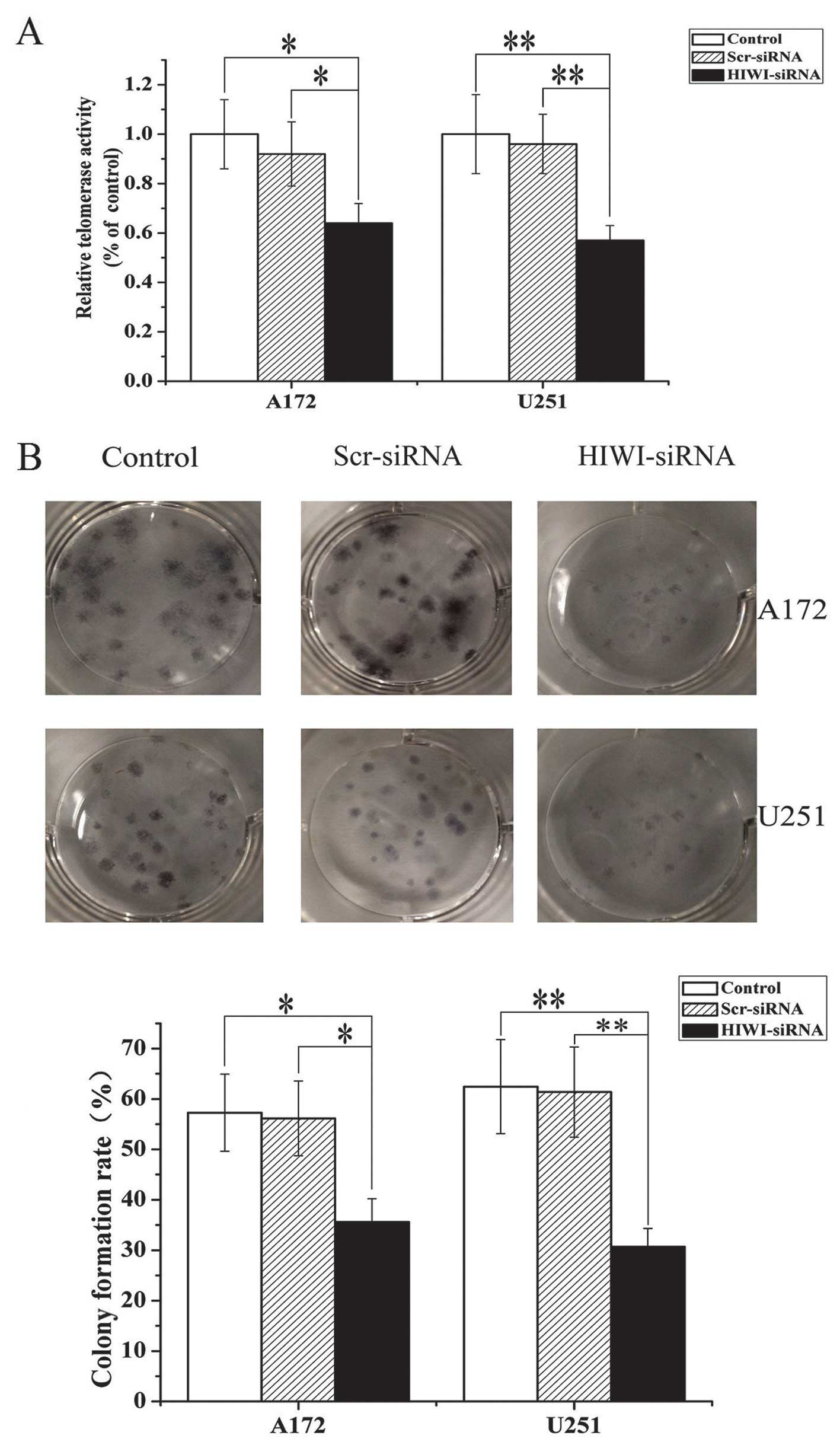
Silencing HIWI inhibited cell viability
in vitro
To determine whether HIWI functions as an oncogene,
we examined the effects of HIWI knockdown on glioma cell
proliferation. Cells were transfected with HIWI siRNA, and the
number of viable cells was determined by MTT assay 48 h after
transfection. As shown in Fig. 2A,
HIWI siRNA significantly decreased the percentage of viable cells
in both U251 and A172 cell lines. These results confirmed that
expression level of HIWI modulated cell growth, and that knockdown
of HIWI exerted anti-proliferative effects.
To further assess the effects of HIWI on the growth
of glioma cells, a colony formation assay was performed. As is
shown in Fig. 2B, the number of
colonies formed from U251 and A172 cells transfected with HIWI
siRNA was significantly lower than untreated cells or those
transfected with control siRNA. This suggests that knockdown of
HIWI suppressed colony formation in U251 and A172 cells. These
results were consistent with data obtained from the MTT assay, and
further suggested that HIWI might be an oncogene.
Suppression of HIWI induced cell cycle
arrest in G0/G1 phase, and altered the expression of cell
cycle-related proteins in U251 and A172 cells
To further elucidate the growth suppressive effects
of silencing HIWI in U251 and A172 cells, we performed cell cycle
distribution analysis by flow cytometry 48 h after transfection
with HIWI siRNA. As shown in Fig. 3A
and B, the number of cells in G1 phase increased, but the
number of cells in S phase decreased sharply after transfection
with HIWI siRNA. To determine the molecular mechanism behind the
inhibition of cell cycle after the downregulation of HIWI, we
assessed the expression of important cell cycle regulatory proteins
by western blotting. The expression of cyclin D1 decreased, whereas
the expression of p21 increased in U251 and A172 cells that had
been transfected with HIWI siRNA (Fig.
3D).
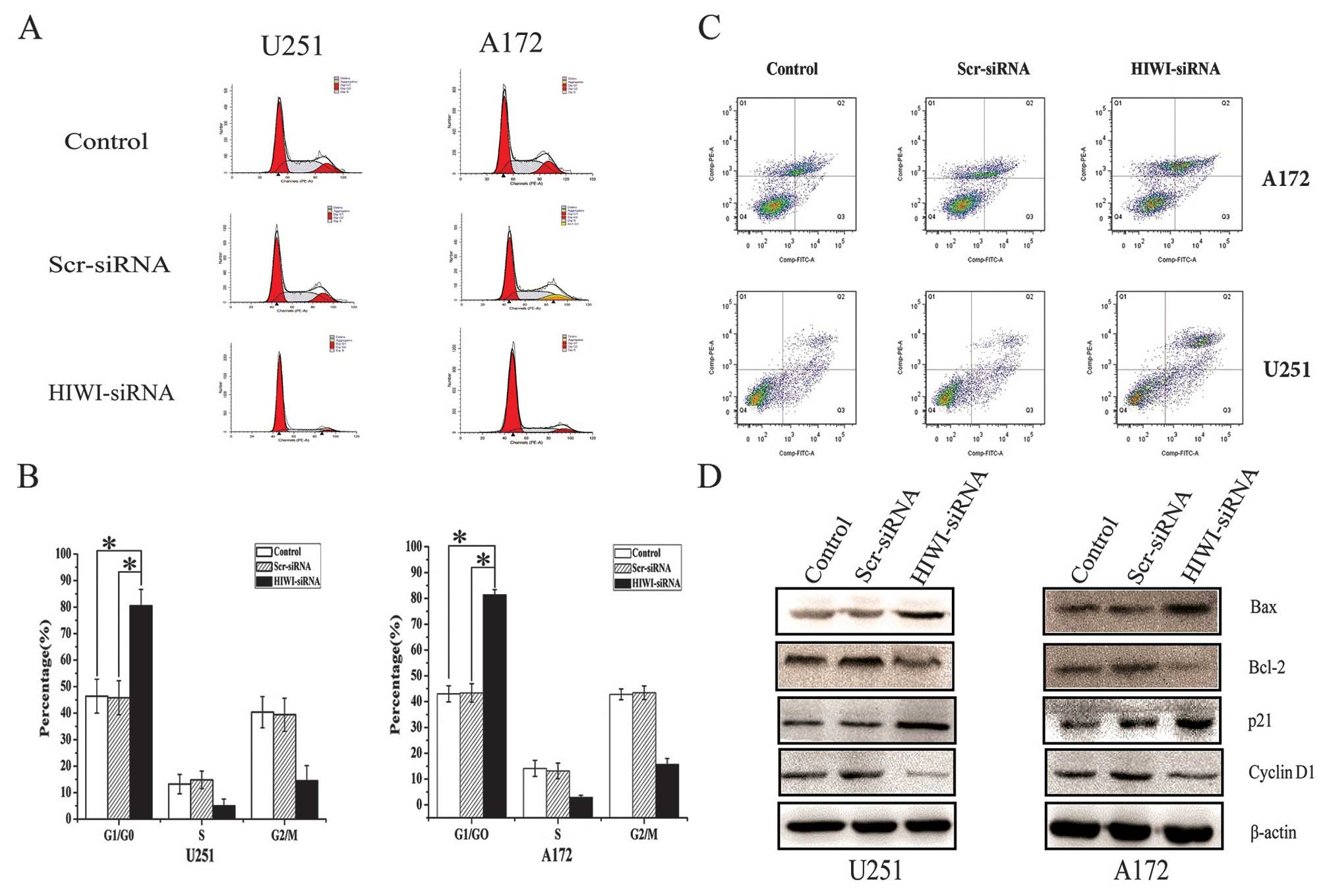 | Figure 3HIWI knockdown induced cell cycle
arrest in G0/G1, induced apoptosis, and altered the expression of
related proteins in glioma cell lines. (A) Silencing of HIWI by
siRNA transfection affected cell cycle distribution in U251 and
A172 glioma cancer cells. At 48 h post-transfection, DNA content
was measured using propidium iodide (PI) staining by flow
cytometry. (B) Knockdown of HIWI by RNAi in U251 and A172 cells
induced cell cycle arrest in G0/G1 phase 48 h after transfection.
*P<0.05 in comparison with control group and
scrambled siRNA group. (C) For flow cytometry, control cells or
those transfected with HIWI or scrambled siRNA were harvested 48 h
after transfection, and stained with Annexin V-FITC and PI. Annexin
V-FITC and PI double staining flow cytometry analysis showed that
the cells transfected with HIWI siRNA exhibited significantly
increased apoptosis (42.71±3.14%, 31.46±2.53%) compared with normal
cells (23.43±1.68%, 19.82±1.45%) and cells treated with scrambled
siRNA (25.17±1.42%, 20.06±1.36%). Data represent the mean ±
standard deviation (SD) of three independent experiments.
**P<0.01 in comparison with control group and
scrambled siRNA group. (D) Total cellular proteins were treated as
described above, and harvested after 72 h. Western blotting was
then performed using antibodies against p21, cyclin D1, Bcl-2, and
Bax; β-actin was used as a loading control. |
Knockdown of HIWI expression increased
apoptosis in glioma cells by inducing Bcl-2 and Bax expression
We next determined whether the decrease in cell
viability caused by HIWI knockdown was associated with increased
apoptosis. The number of apoptotic untreated cells or those
transfected with negative control or HIWI siRNA was assessed using
Annexin V-FITC and PI labeling followed by flow cytometry. As shown
in Fig. 3C, the number of late
apoptotic cells was increased significantly 48 h after transfection
with HIWI siRNA. In addition, the expression of Bcl-2 decreased,
and Bax increased (Fig. 3D).
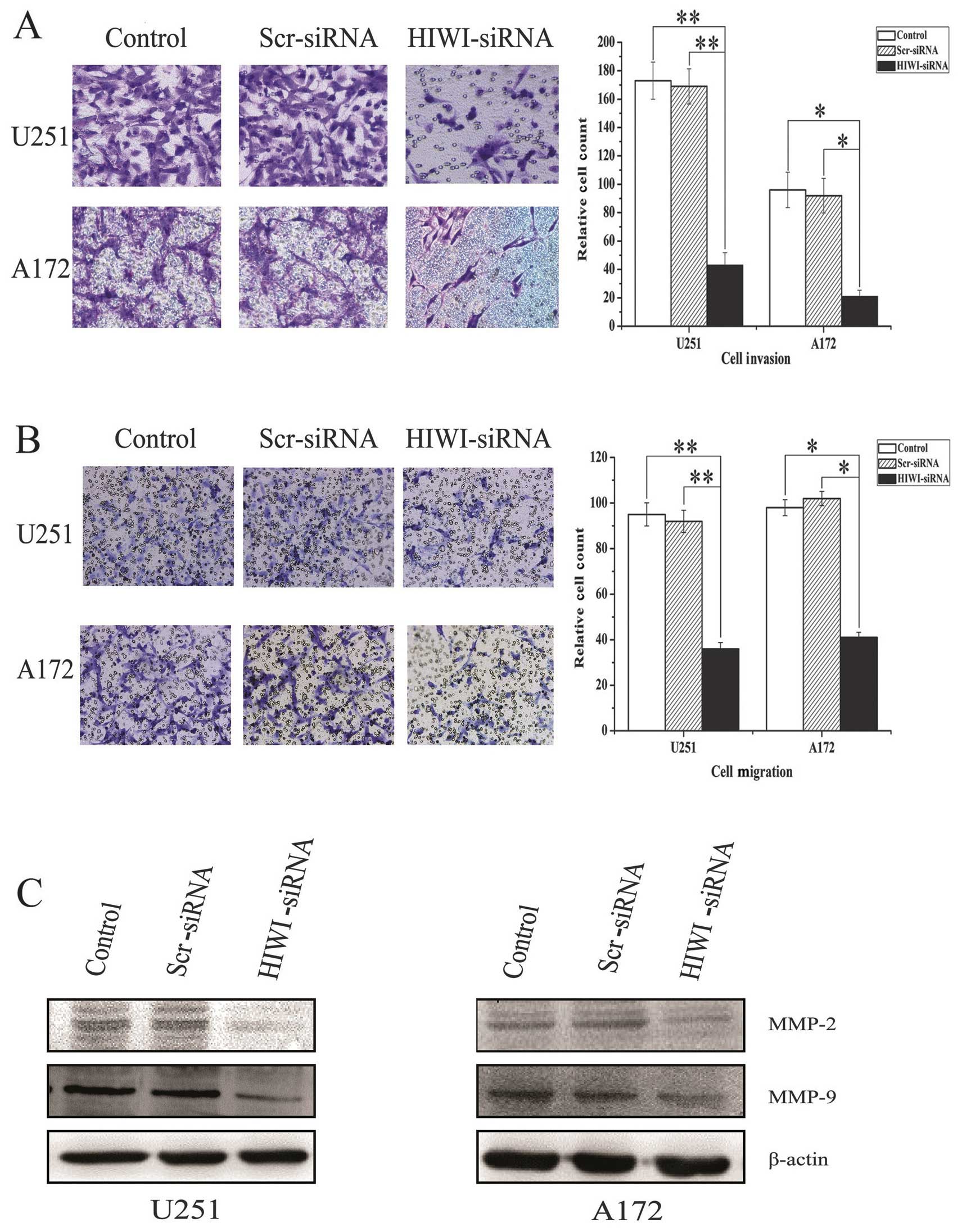
Downregulation of HIWI expression
inhibited the migration and invasion of glioma cells in vitro
Transwell assays were performed to determine whether
HIWI expression affected the migration and invasion of malignant
glioma cells. Because the cells in tumor tissues exist in a
three-dimensional environment, migration and invasion behavior is
more complicated than in cell culture plates. Therefore, we used
Transwell chambers to mimic the three-dimensional environment of
tumor cells to assess the migration and invasion behavior of cells.
As is shown in Fig. 4A,
downregulation of HIWI reduced the number of migratory cells
significantly compared with the scrambled siRNA and untreated
control groups (Fig. 4B).
Consistent with this, decreased invasion of U251 and A172 cells was
also measured in the Matrigel Transwell assay after HIWI knockdown
(Fig. 4C). Taken together, these
results suggest that HIWI plays a vital role in the migration and
invasion of glioma cells. To further explore the molecular
mechanism by which HIWI promotes cellular migration and invasion,
we measured the levels of matrix metalloproteinases (MMPs), which
regulate these processes. Western blotting showed that the levels
of MMP-2 and MMP-9 were reduced in U251 and A172 cells after HIWI
knockdown (Fig. 4D).
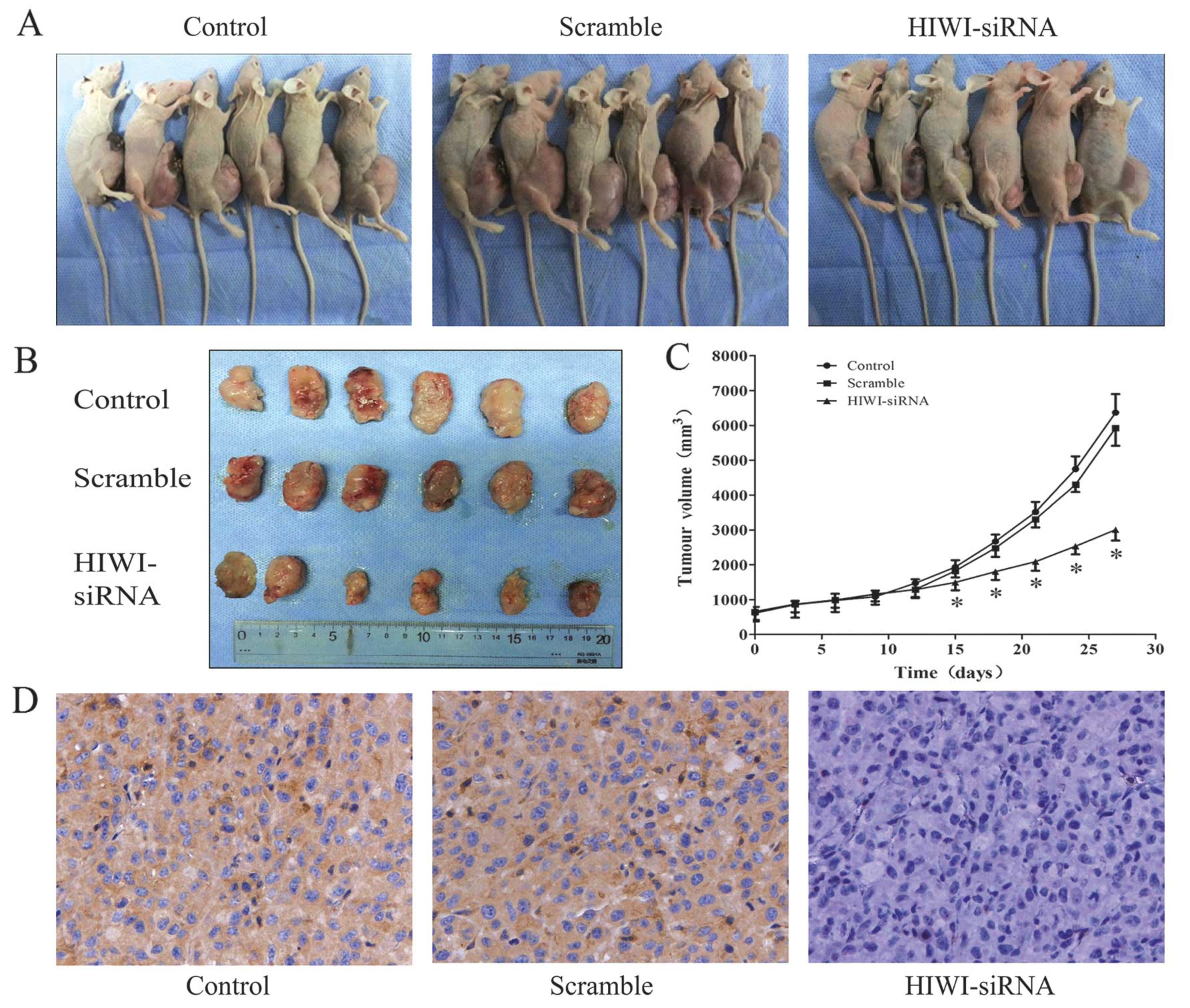
Reduction of HIWI inhibits glioma growth
in vivo
To explore the effect of HIWI activity on tumor
growth in vivo, we employed a xenograft mouse model using
U251 glioma cells treated with HIWI siRNA. On day 15, the tumor
size of the HIWI siRNA group started to reach statistical
significance compared with control groups (p<0.05). At the
termination of the study, there was a marked reduction in tumor
mass between the HIWI siRNA group and the control groups (Fig. 5A–C). After observation for 28 days,
tumor samples were dissected from mice, and paraffin-embedded
sections were prepared for immunohistochemical assay. Similar to
the results obtained from the in vitro study, the expression
of HIWI in tumor specimens of the HIWI siRNA group was highly
downregulated (Fig. 5D).
Discussion
HIWI, a human homolog of the PIWI family that is
important for stem cell self-renewal, is expressed highly in a
variety of human cancers. Some studies have demonstrated that HIWI
plays a key role in the development of tumors in cervical (19), colon (20), and liver cancer (21). Sun et al (15) also found that HIWI was expressed
specifically in glioma tissues and cell lines, but its role and
molecular mechanisms related to the development of glioma remain
unclear. In this study, we first confirmed the expression of HIWI
in glioma cell lines. To explore whether HIWI plays a significant
role in the tumorigenesis and development of glioma, we next
knocked down its expression in U251 and A172 glioma cells using
RNAi. Inhibiting HIWI expression significantly decreased cell
proliferation, increased apoptosis, and induced cell cycle arrest
at G0/G1. We determined that decreased HIWI expression was
accompanied by decreased migration and invasion of U251 and A172
cells, as measured by Transwell assays. Finally, we found that
reduction of HIWI inhibited glioma growth in vivo.
A main feature of cancer is uncontrolled cell growth
and proliferation. Previous studies revealed that cell
proliferation is inhibited following the silencing of HIWI in human
gastric cancer and lung cancer tumor stem cells (13,22).
This was due to inducing cell cycle arrest at G2/M phase and
inducing apoptosis, respectively, after the suppression of HIWI
expression. However, a different study found that cell
proliferation was reduced dramatically and apoptosis was induced in
KG1 cells, a human leukemia cell line that lacks HIWI expression,
when HIWI was expressed transiently (23). Our study revealed that cell
proliferation was decreased significantly after knockdown of HIWI
in glioma cells, which was confirmed by MTT and colony formation
assays. The decline in cell proliferation was explained by both
increased apoptosis and cell cycle arrest, which is partly
consistent with previous studies. We speculated that HIWI could
exert varying effects in a cell- and tissue-specific manner.
Therefore, further studies will be needed to clarify these
mechanisms.
Inhibiting cell cycle progression in cancer cells is
considered to be one of the most effective strategies for
regulating tumor growth. The shift from a dormant quiescent stage
(G0) to an actively growing state is a prerequisite for entry into
the cell cycle in most cells, and a crucial step for cancer cells
(24). The cyclins and
cyclin-dependent kinase (CDK) inhibitors strictly regulate cell
cycle progression. The first of these proteins to be identified and
cloned was p21. p21 is a universal cell cycle inhibitor that binds
to cyclin-CDK complexes and proliferating cell nuclear antigen,
thereby inducing cell arrest at G1 and blocking cell entry into S
phase (25). Therefore, we
assessed the relationships between G1 arrest and cell cycle related
regulatory proteins, including p21 and cyclin D1, after HIWI
knockdown. Data revealed that HIWI-mediated G1 arrest in glioma
cancer cells was linked with p21. The upregulation of p21 enhances
the formation of complexes with the G1/S CDKs and cyclins,
inhibiting their activities and promoting apoptosis. Future studies
should examine the underlying mechanisms by which HIWI upregulates
p21 expression and modulates the downstream pathways.
Oncogenesis is also associated with abnormal
apoptosis. As such, tumorigenesis can be defined by the imbalance
between cell proliferation and apoptosis. Many studies have
suggested that cell cycle arrest and apoptosis may be linked
(26,27). In addition, it was reported that
the overexpression of p21 resulted in increased Bax expression and
the induction of apoptosis. Liang et al demonstrated that
silencing HIWI induced apoptosis in lung cancer stem cells
(13). In this study, data showed
that silencing HIWI also stimulated U251 and A172 apoptosis,
increased Bax expression, and decreased Bcl-2 expression.
In addition to the function of HIWI as a potent
growth regulator, another study revealed that low levels of
expression of HIWI led to a significant decrease in the migration
and invasion of hepatocellular carcinoma cells (16). The invasion of glioma cells into
brain tissue is a pathologic hallmark of World Health Organization
(WHO) grade II–IV gliomas, which contributes significantly to the
failure of current treatments (28). Glioma cell invasion involves the
attachment of tumor cells to the extracellular matrix (ECM), the
degradation of ECM components, and the subsequent penetration into
adjacent brain structures (29).
MMPs are a family of extracellular zinc-dependent endopeptidases
that selectively cleave the protein components of the ECM (30). Previous reports suggested that the
expression of gelatinase-A (MMP-2) and gelatinase-B (MMP-9) play an
important role in the invasion or metastasis of neoplastic tissue,
since these are critical factors in basement membrane degradation
(31). Numerous studies have
demonstrated that malignant glioma cells secrete MMPs to facilitate
their migration and invasion (32,33).
Therefore, MMPs may be involved in glioma cell invasion. A better
understanding of the mechanisms of glioma invasion will facilitate
the development of therapeutic strategies to decrease glioma
invasion. Our data suggest that the silencing of HIWI inhibited
glioma cell invasion and migration by suppressing MMP-2 and MMP-9
production. The activation of some signaling pathways can promote
the expression of MMP-2 and MMP-9; however, it is unclear whether
HIWI plays a role in these signaling pathways. The potential
relationship between HIWI and MMPs will be assessed in future
studies.
In summary, these findings suggest that HIWI plays
an important role in the development of human glioma. Silencing
HIWI expression could inhibit glioma cell proliferation, attenuate
migration and invasion, promote apoptosis, and cause cell cycle
arrest. This suggests that targeting HIWI could be a promising
treatment modality for glioma in the future.
Acknowledgements
The study was supported by the Scientific and
Technological Project of Tianjin Bureau of Public Health (11KG115),
the National Key disciplines Fund of the Ministry of Health of the
People’s Republic of China, the Foundation of Tianjin Science and
Technology Committee (12ZCDZSY17700, 14JCZDJC35600).
References
|
1
|
Maher EA, Furnari FB, Bachoo RM, et al:
Malignant glioma: genetics and biology of a grave matter. Genes
Dev. 15:1311–1333. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Furnari FB, Fenton T, Bachoo RM, et al:
Malignant astrocytic glioma: genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R and Roila F; ESMO Guidelines
Working Group. Malignant glioma: ESMO clinical recommendations for
diagnosis, treatment and follow-up. Ann Oncol. 20(Suppl 4):
S126–S128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Argyriou AA and Kalofonos HP: Molecularly
targeted therapies for malignant gliomas. Mol Med. 15:115–122.
2009.PubMed/NCBI
|
|
5
|
Cox DN, Chao A, Baker J, Chang L, Qiao D
and Lin H: A novel class of evolutionarily conserved genes defined
by piwi are essential for stem cell self-renewal. Gen Dev.
12:3715–3727. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaki T, Shiohama A, Minoshima S and
Shimizu N: Identification of eight members of the Argonaute family
in the human genome. Genomics. 82:323–330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hutvagner G and Simard MJ: Argonaute
proteins: key players in RNA silencing. Nat Rev Mol Cell Biol.
9:22–32. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siddiqi S, Terry M and Matushansky I: Hiwi
mediated tumorigenesis is associated with DNA hypermethylation.
PLoS One. 7:e337112012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Liu Y, Shen X, et al: The PIWI
protein acts as a predictive marker for human gastric cancer. Int J
Clin Exp Pathol. 5:315–325. 2012.PubMed/NCBI
|
|
10
|
Grochola LF, Greither T, Taubert H, et al:
The stem cell-associated Hiwi gene in human adenocarcinoma of the
pancreas: expression and risk of tumour-related death. Br J Cancer.
99:1083–1088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li S, Meng L, Zhu C, et al: The universal
overexpression of a cancer testis antigen hiwi is associated with
cancer angiogenesis. Oncol Rep. 23:1063–1068. 2010.PubMed/NCBI
|
|
12
|
Zeng Y, Qu LK, Meng L, et al: HIWI
expression profile in cancer cells and its prognostic value for
patients with colorectal cancer. Chin Med J (Engl). 124:2144–2149.
2011.PubMed/NCBI
|
|
13
|
Liang D, Fang Z, Dong M, et al: Effect of
RNA interference-related HiWi gene expression on the proliferation
and apoptosis of lung cancer stem cells. Oncol Lett. 4:146–150.
2012.PubMed/NCBI
|
|
14
|
Liu C, Qu L, Dong B, et al: Combined
phenotype of 4 markers improves prognostic value of patients with
colon cancer. Am J Med Sci. 343:295–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun G, Wang Y, Sun L, et al: Clinical
significance of Hiwi gene expression in gliomas. Brain Res.
1373:183–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao YM, Zhou JM, Wang LR, et al: HIWI is
associated with prognosis in patients with hepatocellular carcinoma
after curative resection. Cancer. 118:2708–2717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou X, Ren Y, Moore L, et al:
Downregulation of miR-21 inhibits EGFR pathway and suppresses the
growth of human glioblastoma cells independent of PTEN status. Lab
Invest. 90:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hahnfeldt P, Panigrahy D, Folkman J and
Hlatky L: Tumor development under angiogenic signaling: a dynamical
theory of tumor growth, treatment response, and postvascular
dormancy. Cancer Res. 59:4770–4775. 1999.PubMed/NCBI
|
|
19
|
Liu WK, Jiang XY and Zhang ZX: Expression
of PSCA, PIWIL1 and TBX2 and its correlation with HPV16 infection
in formalin-fixed, paraffin-embedded cervical squamous cell
carcinoma specimens. Arch Virol. 155:657–663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L, Yu C, Gao H and Li Y: Argonaute
proteins: potential biomarkers for human colon cancer. BMC Cancer.
10:382010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang J, Zhang H, Tang Q, Hao B and Shi R:
Expression of HIWI in human hepatocellular carcinoma. Cell Biochem
Biophys. 61:53–58. 2011. View Article : Google Scholar
|
|
22
|
Liu X, Sun Y, Guo J, et al: Expression of
hiwi gene in human gastric cancer was associated with proliferation
of cancer cells. Int J Cancer. 118:1922–1929. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma AK, Nelson MC, Brandt JE, et al:
Human CD34(+) stem cells express the hiwi gene, a human homologue
of the Drosophila gene piwi. Blood. 97:426–434. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Janssen A and Medema RH: Mitosis as an
anti-cancer target. Oncogene. 30:2799–2809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cornils H, Kohler RS, Hergovich A and
Hemmings BA: Human NDR kinases control G(1)/S cell cycle transition
by directly regulating p21 stability. Mol Cell Biol. 31:1382–1395.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghate NB, Chaudhuri D, Sarkar R, et al: An
antioxidant extract of tropical lichen, Parmotrema
reticulatum, induces cell cycle arrest and apoptosis in breast
carcinoma cell line MCF-7. PLoS One. 8:e822932013.PubMed/NCBI
|
|
27
|
Nakada M, Nakada S, Demuth T, Tran NL,
Hoelzinger DB and Berens ME: Molecular targets of glioma invasion.
Cell Mol Life Sci. 64:458–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Itoh Y, Palmisano R, Anilkumar N, Nagase
H, Miyawaki A and Seiki M: Dimerization of MT1-MMP during cellular
invasion detected by fluorescence resonance energy transfer.
Biochem J. 440:319–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kargiotis O, Chetty C, Gondi CS, et al:
Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in
impaired invasion and tumor-induced angiogenesis, induces apoptosis
in vitro and inhibits tumor growth in vivo in glioblastoma.
Oncogene. 27:4830–4840. 2008. View Article : Google Scholar
|
|
30
|
Zhou X, Ma L, Li J, Gu J, Shi Q and Yu R:
Effects of SEMA3G on migration and invasion of glioma cells. Oncol
Rep. 28:269–275. 2012.PubMed/NCBI
|
|
31
|
Munaut C, Noël A, Hougrand O, Foidart JM,
Boniver J and Deprez M: Vascular endothelial growth factor
expression correlates with matrix metalloproteinases MT1-MMP, MMP-2
and MMP-9 in human glioblastomas. Int J Cancer. 106:848–855. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshida D, Watanabe K, Noha M, Takahashi
H, Teramoto A and Sugisaki Y: Anti-invasive effect of an
anti-matrix metalloproteinase agent in a murine brain slice model
using the serial monitoring of green fluorescent protein-labeled
glioma cells. Neurosurgery. 52:187–197. 2003.
|
|
33
|
Bellail AC, Hunter SB, Brat DJ, Tan C and
Van Meir EG: Microregional extracellular matrix heterogeneity in
brain modulates glioma cell invasion. Int J Biochem Cell Biol.
36:1046–1069. 2004. View Article : Google Scholar : PubMed/NCBI
|