Introduction
Patients with non-metastatic breast cancer are
treated surgically. Adjuvant treatment is indicated if the risk for
recurrence can be significantly reduced. Risk assessment is, thus,
of utmost importance and is being conducted by means of
TNM-classification and differentiation grade complemented by
estrogen and progesterone receptor status, Her2neu expression and
peri-tumor vascular invasion (1–5).
More recent improvement of the risk assessment is obtained through
the molecular characterization of the tumor (6–11).
These methods identify genetic phenotypes with a higher likelihood
for micrometastasis that can lead to disease recurrence. Detection
of the actual presence of tumor cells beyond the primary tumor is
preferred, but may not be sufficient, as one cannot distinguish
between dormant tumor cells and those giving rise to recurrence of
the disease (12,13). The presence of micrometastases in
bone marrow of breast cancer patients is associated with an
increased risk for disease recurrence and death, but has not been
adapted in standard clinical practice (14–16).
A more attractive approach for the detection of the presence of
tumor cells beyond the primary tumor is the detection of
circulating tumor cells (CTC). For CTC detection a validated method
is available (17) and several
studies have demonstrated that the presence of CTC in patients with
metastatic breast cancer is associated with a significantly shorter
progression free and overall survival (18–21).
In these studies CTC can be found in 7.5 ml of blood in ~70% of
metastatic breast cancer patients. The presence of CTC in the
non-metastatic setting is considerable lower and is similar to that
found in patients with benign breast disease (22). Still a relation between their
presence and an increased risk of disease recurrence has been shown
in several studies (23–27). Whether or not this relation also
exists when blood is investigated for the presence of tumor cells
after surgical removal of the tumor and during follow-up has not
yet been investigated. In a previous study we investigated the
relation between the presence of CTC before breast cancer surgery
and recurrence free survival and breast cancer related survival
(22) and in the present study we
report the relation between CTC in blood of these patients at
several time-points after surgery and extended the follow-up of to
a median of 5.7 years.
Materials and methods
Study design
Four hundred and three patients with stage I–III
breast cancer were enrolled before surgery with curative intend in
this prospective study between September 2003 and January 2009. The
test result was not communicated to the treating physician and thus
could not influence the further therapeutic algorithm. Blood (30
ml) was drawn from all patients into four CellSave Preservative
tubes (Veridex LLC, Raritan, NJ, USA) before surgery (A-Draw), a
median of 1 week after surgery (B-Draw), after completion of
adjuvant chemo- and/or radiotherapy or before start of long-term
hormonal therapy (C-Draw), one (D-Draw), two (E-Draw) and three
(F-Draw) years after surgery to measure CTC. All patients were
treated in accordance with the Dutch national guidelines (28). The ethics board of Medisch Spectrum
Twente (Enschede, The Netherlands) approved the study protocol and
all patients provided informed consent. The primary endpoint of the
present study was an association between the presence of CTC and
breast cancer related recurrence and was reported earlier (22). In the present study, we report the
relation between the presence of CTC and recurrence free and
overall survival before and at several time-points after surgery.
All patient records were reviewed in 2013 and updated for
occurrence of breast related disease recurrence and death resulting
in a follow-up period of 0.5–9.9 years (median 5.7)
CellSearch system
The CellSearch system (Veridex) was used to measure
CTC. Four 7.5-ml aliquots of each patient were analyzed within 72 h
after blood draw. This system immunomagnetically enriches CTC from
7.5 ml of blood targeting the epithelial cell adhesion molecules
(EpCAM). The enriched cells are labeled with the nucleic acid dye
4′,6-diaminodino-2-phenylindole (DAPI) and antibodies specific for
leukocytes (CD45) labeled with allophycocyan (APC) and specific for
epithelial cells (cytokeratin 8, 18 and 19) labeled with
phycoerythrin (PE). Images of CTC candidates were captured by a
semi-automatic magnetic fluorescence microscope and presented to
experienced operators for classification as CTC when the cells were
>4 μm, expressed cytokeratin and lacked CD45 (17). The operators were blinded to the
clinical status of the patient.
Statistical analysis
Two databases were created, one with the results of
the CTC analysis, patient ID and inclusion date and one with the
clinical data from the patient charts both were merged at the time
of the analysis at the hospital. The following clinical data were
included: age, menopausal status, tumor stage on basis of TNM
classification, estrogen/progesterone receptor status, Her2Neu
receptor status, differential grade of the tumor based on the
Bloom-Richardson method, adjuvant treatment, date of recurrence if
occurred, location of recurrence (local vs. distant), date of
breast cancer associated death or non-breast cancer associated
death. ER and PR positivity was defined at 10% or more. Her2Neu
positivity was defined as 3+ or 2+ with confirmation. The time
until recurrence was defined as the time between date of inclusion
and the date on which the recurrence was confirmed with an
appropriate diagnostic test. Follow-up time was defined as the time
between inclusion date and the date of the last check-up. Patients
with no objectified recurrence at the end of follow-up were
considered free of recurrence. Statistical analysis was done using
the SPSS version 20.0 (IBM, Armonk, NY, USA) an R (29). A P-value <0.05 was considered to
indicate a significant difference. All tests were two-sided.
Patients were divided into two groups: those without any CTC, and
those with at least one CTC. Between-group differences in
categorical variables were tested by the Pearson’s Chi-squared test
and in case of small numbers the Fisher’s exact test. For between
group differences in continues variables the Mann-Whitney U was
performed. All significant univariate prognostic factors where
included in a multivariate Cox proportional regression model.
Interdependent covariates where removed from the multivariate model
using stepwise elimination, using P>0.10 as criteria.
Kaplan-Meier curves for disease free survival, cancer related death
and overall survival were generated and compared using the log-rank
test.
Results
Patient characteristics and relation to
recurrence and survival
Between September 2003 and January 2009, 403
patients with stage I–III breast cancer were included into the
present study. Their age ranged from 29–90 years (mean and median
of 59). Recurrence of disease was observed in 57 of 403 (14.4%)
patients, 43 (10.7%) patients died of causes related to breast
cancer and 57 (14.1%) patients died of breast cancer or other
causes. Patient characteristics and whether or not a significant
relation was present between the presence of CTC, RFS and OS is
shown in Table I.
 | Table ICharacteristics of the 403 patients
and their relation to recurrence free survival (RFS) and breast
cancer related death (BCRD). |
Table I
Characteristics of the 403 patients
and their relation to recurrence free survival (RFS) and breast
cancer related death (BCRD).
| N | % | % CTC ≥1 | RFS P-value | OS P-value |
|---|
| Stage | | | | 0.001 | <0.001 |
| I | 178 | 44 | 16 | | |
| IIA | 122 | 30 | 18 | | |
| IIB | 52 | 12 | 17 | | |
| IIIAa | 40 | 10 | 31 | | |
| IIIBa | 9 | 2 | | | |
| IIICa | 2 | 1 | | | |
| T stage | | | | 0.026 | <0.001 |
| T1 | 229 | 57 | 15 | | |
| T2 | 154 | 38 | 22 | | |
| T3a | 12 | 3 | 30 | | |
| T4a | 8 | 2 | | | |
| N stage | | | | <0.001 | <0.001 |
| N0 | 261 | 65 | 18b | | |
| N1 | 98 | 25 | 12b | | |
| N2a | 42 | 10 | 34b | | |
| N3a | 2 | 1 | | | |
| Histology | | | | 0.603 | 0.971 |
| Lobular | 44 | 11 | 23 | | |
| Ductal | 341 | 84 | 19 | | |
| Other | 18 | 5 | 11 | | |
|
Differentiation | | | | <0.001 | <0.001 |
| I | 98 | 24 | 11b | | |
| II | 184 | 46 | 18b | | |
| III | 121 | 30 | 25b | | |
| ER | | | | 0.017 | 0.002 |
| Positive | 341 | 85 | 18 | | |
| Negative | 62 | 15 | 24 | | |
| PR | | | | 0.068 | 0.028 |
| Positive | 289 | 72 | 14b | | |
| Negative | 114 | 28 | 30b | | |
| Her2/neu | | | | <0.001 | 0.107 |
| Positive | 81 | 20 | 24 | | |
| Negative | 322 | 80 | 17 | | |
| Adjuvant
chemotherapy | | | | 0.597 | 0.959 |
| Yes | 150 | 37 | 22 | | |
| No | 253 | 63 | 16 | | |
| Adjuvant
radiotherapy | | | | 0.879 | 0.94 |
| Yes | 295 | 73 | 17 | | |
| No | 108 | 27 | 22 | | |
| Adjuvant hormonal
therapy | | | | 0.193 | 0.282 |
| Yes | 192 | 48 | 13b | | |
| No | 211 | 53 | 24b | | |
| Age |
| Continues |
| Menopausal
status | | | | 0.441 | 0.183 |
| Pre | 119 | 30 | 22 | | |
| Post | 284 | 70 | 17 | | |
Prevalence of CTC
The prevalence of CTC in the patients before and at
several time-points after surgery is shown in Table II. In 75 out of the 403 patients
(19%) CTC were detected before surgery. At the time-points after
surgery this frequency slowly decreased to 18, 15, 12, 11 and 13%,
respectively.
 | Table IIPrevalence of circulating tumor cells
before surgery and at several time points after surgery. |
Table II
Prevalence of circulating tumor cells
before surgery and at several time points after surgery.
| (A) Before
surgery
n=403 | (B) After
surgery
n=367 | (C) After adjuvant
therapya
n=263 | (D) One year after
surgery
n=235 | (E) Two years after
surgery
n=162 | (F) Three years
after surgery
n=83 |
|---|
|
|
|
|
|
|
|
|---|
| CTC | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
|---|
| 0 | 328 (81) | 301 (82) | 223 (85) | 215 (88) | 144 (89) | 72 (87) |
| ≥1 | 75 (19) | 66 (18) | 40 (15) | 30 (12) | 18 (11) | 11 (13) |
| 1 | 48 (12) | 33 (9) | 31 (12) | 21 (9) | 13 (8) | 9 (11) |
| 2 | 4 (1) | 12 (3) | 6 (2) | 4 (2) | 2 (1) | 0 (0) |
| 3 | 6 (2) | 8 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| 4 | 5 (1) | 4 (1) | 0 (0) | 1 (0) | 0 (0) | 0 (0) |
| >4 | 12 (3) | 9 (3) | 3 (1) | 4 (2) | 3 (3) | 1 (1) |
Association between presence of CTC
before surgery, disease recurrence and survival
A total of 57 (14.4%) patients developed disease
recurrence and 43 (10.7%) patients died during follow-up. Of the 75
patients with CTC before surgery, 17 (22.7%) developed a recurrence
compared to 40 of 328 (12.2%) patients without CTC (P=0.019). Of
the 75 patients with CTC before surgery, 19 (25.3%) died compared
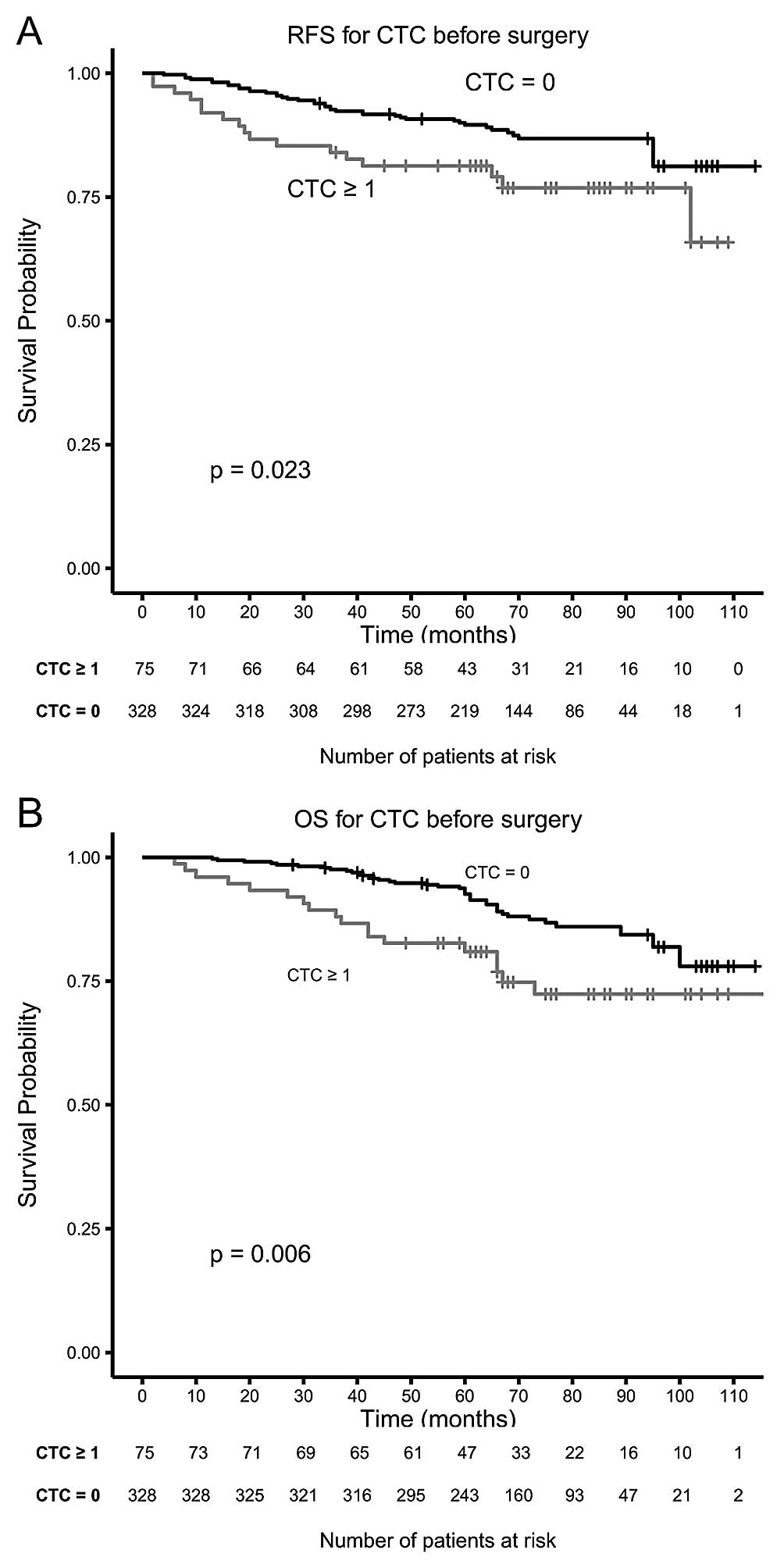
to 39 of 328 (11.9%) patients without CTC (P=0.003). Kaplan-Meier
plots for RFS and OS for patients with and without CTC before
surgery in Fig. 1 show a
significant difference in RFS (P=0.022) and OS (P=0.006) indicating
that the presence of CTC is a negative prognostic indicator.
Association between presence of CTC at
several time intervals after surgery, disease recurrence and
survival
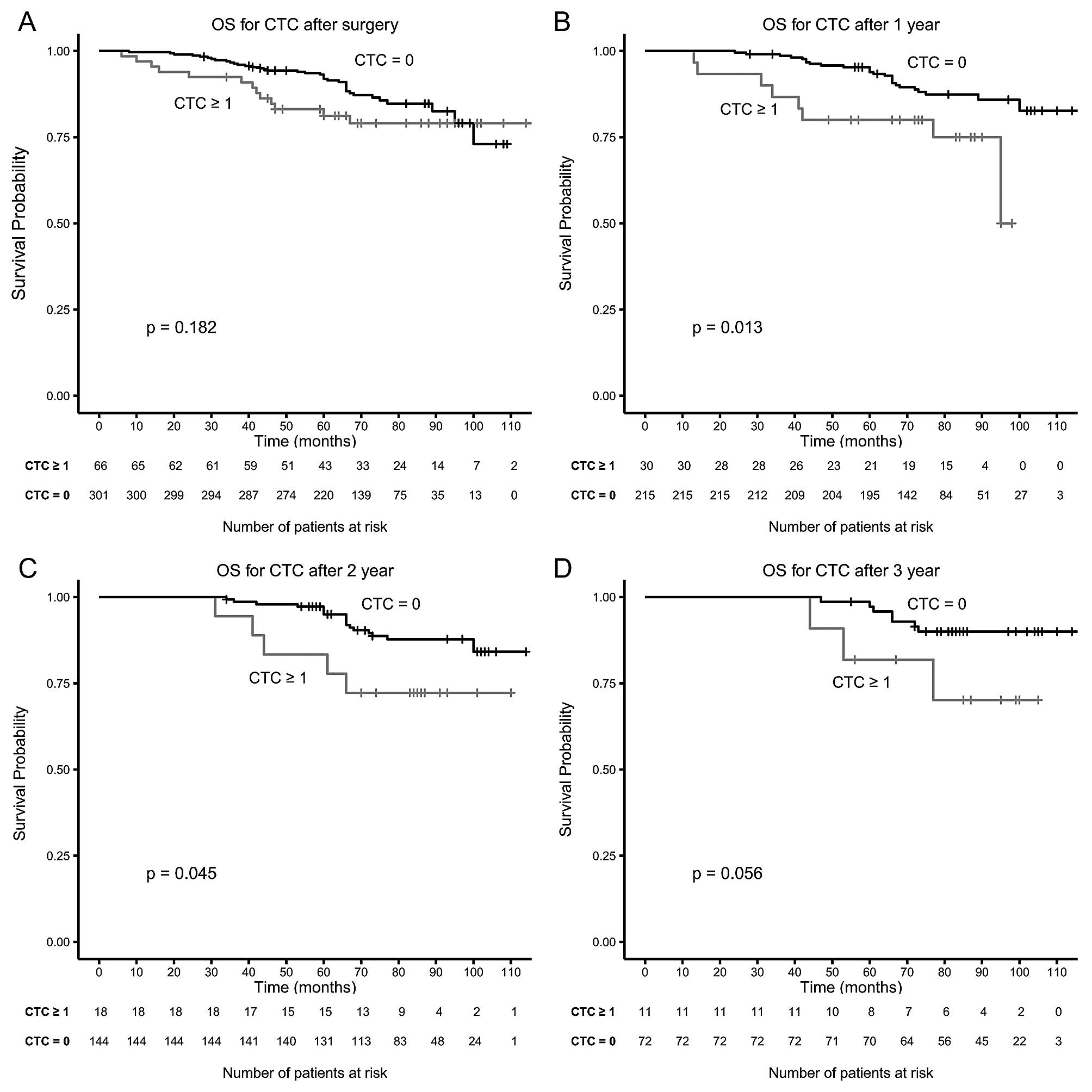
Blood was drawn in 367 of the 403 (93%) of patients
a median of 1 week after surgery. Although the proportion of
patients with CTC was similar (19 vs. 18%) no significant
associated was found between the presence of CTC and RFS and OS
(Table III). To investigate
whether this was due to the difference in the number of patients
with blood drawn after surgery the same analysis was done for the
blood drawn of these 367 patients taken before surgery and is shown
in italics in Table III. This
clearly shows that the presence of CTC in the same group of
patients is highly significant in the blood drawn before, but not
after surgery. After completion of adjuvant therapy blood was drawn
in 263 of the 348 patients that received adjuvant therapy. Presence
of CTC was significantly associated with RFS and OS (Table III). Presence of CTC in blood
drawn 1 year (n=245) and 2 years (n=162) after surgery was also
significantly associated with RFS and OS (Table III). This significant difference
was not reached in the same group of patients when considering the
CTC in the blood drawn pre-surgery. The presence of CTC in 83
patients with blood drawn 3 years after surgery was not
significantly associated with RFS and OS (Table III). Fig. 2 shows the Kaplan-Meier plots for OS
for patients with and without CTC after surgery (Fig. 2A), 1 year after surgery (Fig. 2B), 2 years after surgery and 3
years after surgery (Fig. 2D).
 | Table IIIRelation between the presence of CTC
and recurrence free survival (RFS) or overall survival (OS). |
Table III
Relation between the presence of CTC
and recurrence free survival (RFS) or overall survival (OS).
| N | Unfavorable
(%) | RFS P-value | OS P-value |
|---|
| Before surgery | 403 | 19 | 0.022 | 0.006 |
| After surgery | 367 | 18 | 0.852 | 0.182 |
| (before surgery
draw) | | 19 | 0.006 | 0.005 |
| After adjuvant
TX | 263 | 15 | <0.001 | 0.018 |
| (before surgery
draw) | | 17 | 0.026 | 0.013 |
| After 1 year | 245 | 12 | 0.006 | 0.013 |
| (before surgery
draw) | | 16 | 0.672 | 0.320 |
| After 2 years | 162 | 11 | <0.001 | 0.045 |
| (before surgery
draw) | | 23 | 0.720 | 0.275 |
| After 3 years | 83 | 13 | 0.439 | 0.056 |
| (before surgery
draw) | | 27 | 0.591 | 0.787 |
Multivariate analysis for disease
recurrence and survival
Multivariate logistic regression analysis was
performed to identify the parameters most significant for RFS and
OS of breast cancer patients. For this the univariate significant
parameters for RFS and OS were used. Table IV shows this analysis in which
conditionally stepwise elimination of least significant parameters
was applied for recurrence RFS and OS.
 | Table IVMultivariate Cox proportional hazard
regression analysis of univariate significant parameters with a
conditional stepwise elimination of the least significant for
recurrence free survival (RFS) and overall survival (OS). |
Table IV
Multivariate Cox proportional hazard
regression analysis of univariate significant parameters with a
conditional stepwise elimination of the least significant for
recurrence free survival (RFS) and overall survival (OS).
| Variables | Categories | P-value | Hazard ratio | 95% CI |
|---|
| RFS | CTC | Negative vs.
positive | 0.057 | 1.96 | 0.98–3.17 |
| Her2Neu | Negative vs.
positive | 0.039 | 1.82 | 1.03–3.20 |
|
Differentiation | I vs. II | 0.157 | 2.02 | 0.76–5.38 |
|
Differentiation | I vs. III | 0.017 | 3.38 | 1.25–9.15 |
| N-stage | N0 vs. N1 | 0.023 | 2.04 | 1.10–3.76 |
| N-stage | N0 vs. N2 and
N3 | 0.028 | 2.17 | 1.09–4.34 |
| OS | Age | | <0.001 | 1.05 | 1.03–1.08 |
| CTC | Negative vs.
positive | 0.002 | 2.60 | 1.43–4.72 |
| ER | Negative vs.
positive | 0.003 | 0.36 | 0.19–0.71 |
|
Differentiation | I vs. II | 0.423 | 1.46 | 0.58–3.68 |
|
Differentiation | I vs. III | 0.021 | 3.06 | 1.18–7.90 |
| N-stage | N0 vs. N1 | <0.001 | 3.14 | 1.69–5.84 |
| N-stage | N0 vs. N2 and
N3 | 0.050 | 2.20 | 1.00–4.85 |
| T-stage | T1 vs. T2 | 0.595 | 1.17 | 0.66–2.08 |
| T-stage | T1 vs. T3 and
T4 | 0.016 | 3.00 | 1.23–7.33 |
Discussion
Several studies have shown that the presence of
tumor cells in blood of breast cancer patients before surgery
(22,24,25,27)
and with metastatic disease (18–20)
is associated with poor outcome. To simplify the interpretation of
the CTC results patients with metastatic disease are subdivided
into those with favorable CTC (<5/7.5 ml of blood) and those
with unfavorable CTC (>5/7.5 ml of blood) (18). In actuality, the peripheral blood
circulating tumor cell load reflects the prognosis of the patient
and the larger the CTC load the lower the survival time (30,31).
By fitting the distribution of the CTC frequency in patients with
metastatic disease in whom CTC were detected it was calculated that
a 10-fold increase in CTC corresponds to a decrease in survival of
6.6 month and suggested that this trend continues at CTC
concentrations that cannot be detected with current technology
(31). Efforts are ongoing to
determine whether CTC indeed can be identified in all patients with
metastatic carcinomas by probing larger blood volumes for the
presence of CTC (32,33). To detect a breast cancer tumor
before the disease is disseminated using circulating tumor cells
will be even more challenging (34). One of the largest difficulties is
the discrimination between those tumor cells that can cause harm
and those that may remain dormant. This is exemplified by the
presence of micro-metastasis in the bone marrow in ~30% of patients
at the time of diagnosis, but although their presence is associated
with an increased risk for disease recurrence not all of the
patients with micrometastasis will develop a disease recurrence
(15,16). Likewise, not all patients in which
CTC are detected before surgery will develop a disease recurrence
(22,25,35).
Aneusomie of CTC has been demonstrated in patients suspect for
dormant breast cancer suggesting that at least some of these cells
are indeed tumor cells but in a dormant state (12). Furthermore, cells classified as CTC
can also be detected at low frequency in patients with benign
disease suggesting that they may not be tumor cells which can
explain part of the absence of recurrence in CTC positive patients
with cancer (22).
In the present study, we investigated the presence
of CTC in 30 ml of blood before and during follow-up after surgery
to determine whether CTC could be detected and whether or not their
presence after surgery was still related to poor outcome. Results
of the pre-surgery blood were presented before (22), but here we extended the follow-up
and investigated the role of CTC in blood drawn during 3 years of
follow-up. The longer follow-up increased the significance of the
presence of CTC and CTC are highly significant in multivariate
analysis for OS. The proportion of patients with CTC detected
before surgery (19%) was not different in the weeks after surgery
(18%). Surprisingly, CTC presence was highly significant for RFS
(P=0.022) and OS (P=0.006) in blood draw pre-surgery, but not in
the post-surgery blood for RFS (P=0.852) and OS (P=0.182). Possible
explanations are that the cells detected in the post-surgery blood
are epithelial cells or tumor cells with no metastatic potential
shed during surgery. Alternatively, these cells are similar to
those observed in patients with benign disease (22). Remarkably, the same phenomenon is
observed in patients undergoing surgery for colorectal cancer
(36).
Presence of CTC in blood drawn after adjuvant
therapy, but before starting long-term hormonal therapy was highly
significant for both RFS (P<0.001) and OS (P=0.018). This may
not be surprising as this patient group already has a higher risk
for disease recurrence as compared to those not receiving adjuvant.
This significance was, however, also observed in the patient group
comprising patients that did not receive adjuvant therapy as the
presence of CTC was significant for RFS and OS in blood drawn one
and two years after surgery. Remarkably, CTC in blood of the same
patients drawn before surgery was not significant. These results
indeed indicate a potential important role for the monitoring of
patients treated for breast cancer, but also highlight that
improvement of CTC technology is needed before it can play a role
in clinical practice. Relative simple improvement in CTC technology
can be obtained by automation of the assignment of objects as CTC
and optimization of the definition by which the human error and
subjectivity can be eliminated (37). Further needed is confirmation at
the genetic level that the cells identified as CTC are indeed tumor
cells. Technology to detect genetic abnormalities associated with
cancer in CTC is indeed feasible (12,33,38–43)
but will need to be implemented in this setting. Still remaining is
the challenge to discriminate between those tumor cells signifying
impeding relapse and those that do not (44). Availability of larger numbers of
CTC will help to address these questions and be obtained by probing
larger blood volumes for the presence of CTC (30,33,34)
and alternative phenotypes (45,46)
or physical characteristics (47–49).
In conclusion, CTC found in the blood at several
time-points after surgery for breast cancer extends our knowledge
to what already has been shown for CTC detection before surgery, it
is also associated with a shorter RFS and OS. However, improvement
in the determination and characterization of CTC is necessary in
order to design randomized trials guiding the effect of the
adjuvant therapy or starting pre-emptive therapy in those patients
with presence of CTC after surgery.
Acknowledgements
The present study was supported by the Immunicon
Corporation responsible for the development of the CellSearch
system.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Boyages J, Chua B, Taylor R, Bilous M,
Salisbury E, Wilcken N and Ung O: Use of the St Gallen
classification for patients with node-negative breast cancer may
lead to overuse of adjuvant chemotherapy. Br J Surg. 89:789–796.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colomer R, Viñas G, Beltran M, et al:
Validation of the 2001 St Gallen risk categories for node-negative
breast cancer using a database from the Spanish Breast Cancer
Research Group (GEICAM). J Clin Oncol. 22:961–962. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lundin J, Lehtimäki T, Lundin M, et al:
Generalisability of survival estimates for patients with breast
cancer--a comparison across two population-based series. Eur J
Cancer. 42:3228–3235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olivotto IA, Bajdik CD, Ravdin PM, et al:
Population-based validation of the prognostic model ADJUVANT! for
early breast cancer. J Clin Oncol. 23:2716–2725. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cronin M, Pho M, Dutta D, et al:
Measurement of gene expression in archival paraffin-embedded
tissues: development and performance of a 92-gene reverse
transcriptase-polymerase chain reaction assay. Am J Pathol.
164:35–42. 2004. View Article : Google Scholar
|
|
7
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001.PubMed/NCBI
|
|
8
|
Perou CM, Sørlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Klijn JGM, Zhang Y, et al:
Gene-expression profiles to predict distant metastasis of
lymph-node-negative primary breast cancer. Lancet. 365:671–679.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
West M, Blanchette C, Dressman H, et al:
Predicting the clinical status of human breast cancer by using gene
expression profiles. Proc Natl Acad Sci USA. 98:11462–11467. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van ‘t Veer LJ, Dai H, van de Vijver MJ,
et al: Gene expression profiling predicts clinical outcome of
breast cancer. Nature. 415:530–536. 2002.PubMed/NCBI
|
|
12
|
Meng S, Tripathy D, Frenkel EP, et al:
Circulating tumor cells in patients with breast cancer dormancy.
Clin Cancer Res. 10:8152–8162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vessella RL, Pantel K and Mohla S: Tumor
cell dormancy: an NCI workshop report. Cancer Biol Ther.
6:1496–1504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fehm T, Braun S, Muller V, et al: A
concept for the standardized detection of disseminated tumor cells
in bone marrow from patients with primary breast cancer and its
clinical implementation. Cancer. 107:885–892. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Braun S and Marth C: Circulating tumor
cells in metastatic breast cancer - toward individualized
treatment? N Engl J Med. 351:824–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Braun S, Vogl F and Naume B: A pooled
analysis of bone marrow micrometastasis in breast cancer. N Engl J
Med. 353:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allard WJ, Matera J, Miller MC, et al:
Tumor cells circulate in the peripheral blood of all major
carcinomas but not in healthy subjects or patients with
nonmalignant diseases. Clin Cancer Res. 10:6897–6904. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cristofanilli M and Budd G: Circulating
tumor cells, disease progression, and survival in metastatic breast
cancer. Engl J Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu MC, Shields PG, Warren RD, et al:
Circulating tumor cells: a useful predictor of treatment efficacy
in metastatic breast cancer. J Clin Oncol. 27:5153–5159. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pierga JY, Hajage D, Bachelot T, et al:
High independent prognostic and predictive value of circulating
tumor cells compared with serum tumor markers in a large
prospective trial in first-line chemotherapy for metastatic breast
cancer patients. Ann Oncol. 23:618–624. 2012. View Article : Google Scholar
|
|
21
|
Bidard F, Peeters D and Fehm T: Clinical
validity of circulating tumour cells in patients with metastatic
breast cancer: a pooled analysis of individual patient data. Lancet
Oncol. 15:406–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Franken B, de Groot MR, Mastboom WJB,
Vermes I, van der Palen J, Tibbe AGJ and Terstappen LWMM:
Circulating tumor cells, disease recurrence and survival in newly
diagnosed breast cancer. Breast Cancer Res. 14:R1332012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mathiot C, Brain E, Delaloge S, Pierga J,
Marty M, Giachetti S and de Cremoux P: Circulating tumor cell
detection predicts early metastatic relapse after neoadjuvant
chemotherapy in large operable and locally advanced breast cancer
in a phase II randomized trial. Clin Cancer Res. 14:7004–7010.
2008. View Article : Google Scholar
|
|
24
|
Jueckstock JK, Rack BK, Zwingers T, Hepp
PGM, Schneeweiss A, Beckmann MW, Lichtenegger W, Sommer HL, Pantel
K, Tesch H, Forstbauer H, Lorenz R, Rezai M, Neugebauer JK,
Andergassen U, Friese K, et al: Prognostic relevance of circulating
tumor cells (CTC) before adjuvant chemotherapy in patients with
breast cancer: results of the German SUCCESS trial. J Clin Oncol.
29(Suppl): 10332011.
|
|
25
|
Lucci A, Hall CS, Lodhi AK, et al:
Circulating tumour cells in non-metastatic breast cancer: a
prospective study. Lancet Oncol. 13:688–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Janni WJ, Rack BK, Terstappen LWMM, et al:
A pooled analysis of the prognostic relevance of circulating tumor
cells in early breast cancer. Cancer Res. 73(Suppl 24): PD6–6.
2013. View Article : Google Scholar
|
|
27
|
Bidard FC, Mathiot C, Delaloge S, et al:
Single circulating tumor cell detection and overall survival in
nonmetastatic breast cancer. Ann Oncol. 21:729–733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
National guidelines breast cancer
(richlijn mamacarcinoom 2008). http://www.cbo.nl.
|
|
29
|
R Core Team. R: A Language and Environment
for Statistical Computing. Version 3.0.1 (2013-05-16).
|
|
30
|
Coumans FAW, Ligthart ST, Uhr JW and
Terstappen LWMM: Challenges in the enumeration and phenotyping of
CTC. Clin Cancer Res. 18:5711–5718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coumans FAW, Ligthart ST and Terstappen
LWMM: Interpretation of changes in circulating tumor cell counts.
Transl Oncol. 5:486–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barradas AM and Terstappen LWMM: Towards
the biological understanding of CTC: capture technologies,
definitions and potential to create metastasis. Cancers (Basel).
5:1619–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fischer JC, Niederacher D, Topp SA, et al:
Diagnostic leuka-pheresis enables reliable detection of circulating
tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci
USA. 110:16580–16585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coumans FAW, Siesling S and Terstappen
LWMM: Detection of cancer before distant metastasis. BMC Cancer.
13:2832013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pierga JY, Bidard FC, Mathiot C, et al:
Circulating tumor cell detection predicts early metastatic relapse
after neoadjuvant chemotherapy in large operable and locally
advanced breast cancer in a phase II randomized trial. Clin Cancer
Res. 14:70042008. View Article : Google Scholar
|
|
36
|
Van Dalum G, de Groot M, Stam GJ, et al:
Importance of circulating tumor cells in newly diagnosed colorectal
cancer. In: Proc 105th Annual Meeting Amer Assoc for Cancer Res;
San Diego, CA. abst 3064. 2014, PubMed/NCBI
|
|
37
|
Ligthart ST, Coumans FAW, Attard G,
Cassidy AM, de Bono JS and Terstappen LWMM: Unbiased and automated
identification of a circulating tumour cell definition that
associates with overall survival. PLoS One. 6:e274192011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Swennenhuis JF, Tibbe AGJ, Levink R,
Sipkema RCJ and Terstappen LWMM: Characterization of circulating
tumor cells by fluorescence in situ hybridization. Cytometry A.
75:520–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Swennenhuis JF, Reumers J, Thys K,
Aerssens J and Terstappen LWMM: Efficiency of whole genome
amplification of single circulating tumor cells enriched by
CellSearch and sorted by FACS. Genome Med. 5:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klein CA, Blankenstein TJF,
Schmidt-Kittler O, Petronio M, Polzer B and Stoecklein NH:
Mechanisms of disease Genetic heterogeneity of single disseminated
tumour cells in minimal residual cancer. Lancet. 360:683–689. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meng S, Tripathy D, Shete S, et al: uPAR
and HER-2 gene status in individual breast cancer cells from blood
and tissues. Proc Natl Acad Sci USA. 103:17361–17365. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fehm T, Sagalowsky A, Clifford E, et al:
Cytogenetic evidence that circulating epithelial cells in patients
with carcinoma are malignant. Clin Cancer Res. 8:2073–2084.
2002.
|
|
43
|
Meng S, Tripathy D, Shete S, et al: HER-2
gene amplification can be acquired as breast cancer progresses.
Proc Natl Acad Sci USA. 101:9393–9398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Uhr JW and Pantel K: Controversies in
clinical cancer dormancy. Proc Natl Acad Sci USA. 108:12396–12400.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sieuwerts AM, Kraan J, Bolt J, et al:
Anti-epithelial cell adhesion molecule antibodies and the detection
of circulating normal-like breast tumor cells. J Natl Cancer Inst.
101:61–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mostert B, Kraan J, Sieuwerts AM, et al:
CD49f-based selection of circulating tumor cells (CTCs) improves
detection across breast cancer subtypes. Cancer Lett. 319:49–55.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coumans FAW, van Dalum G, Beck M and
Terstappen LWMM: Filter characteristics influencing circulating
tumor cell enrichment from whole blood. PLoS One. 8:e617702013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hou HW, Warkiani ME, Khoo BL, et al:
Isolation and retrieval of circulating tumor cells using
centrifugal forces. Sci Rep. 3:12592013.PubMed/NCBI
|
|
49
|
Vona G, Sabile A, Louha M, et al:
Isolation by size of epithelial tumor cells: a new method for the
immunomorphological and molecular characterization of
circulatingtumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|