Introduction
Malignant pleural mesothelioma (MPM) arises from
exposure to asbestos fibers with a long latency period and has been
a global health problem for the past few decades. Its peak
incidence is yet to be reached. Mortality and morbidity remain
extremely high, and treatment is rarely curative. Surgery alone or
in combination with radiotherapy and chemotherapy (1) remains the standard treatment for
early-stage disease. Combination chemotherapy for advanced disease
has modest survival benefit (2).
Thymidylate synthase (TYMS) converts 2′-deoxyuridine-monophosphate
(dUMP) to deoxythymidine-5′-monophosphate (dTMP) in DNA synthesis
(3). Pemetrexed is an antifolate
that mainly inhibits TYMS to abort DNA synthesis and cell division:
overexpression of TYMS has resulted in pemetrexed resistance
(4,5). Notably, TYMS is a key pharmacological
target in MPM though its role is still controversial (6,7).
Alternative treatment options are very limited, especially for
patients with a poor response to previous chemotherapy.
Most anticancer drugs work through inhibition of
cellular proliferation or reactivation of apoptosis. The mechanisms
of apoptosis in mesothelioma are less well-known, although it is
frequently impeded (8). Thus
development of anticancer drugs with novel targets that can inhibit
cell proliferation and induce apoptosis is urgently needed to
improve the overall outcome for patients with mesothelioma.
Arsenic trioxide (As2O3 or
ATO) is the active component of a traditional Chinese medicine
called Pi Shuang. ATO has demonstrated significant clinical
activity in acute promyelocytic leukemia (APL): an intravenous
formulation was approved by the US Food and Drug Administration
over a decade ago. Our institution has subsequently pioneered the
development of an oral preparation of ATO for clinical use with a
much better safety profile (9).
Interestingly, ATO has recently been shown to decrease TYMS
expression in lung adenocarcinoma (10) and colorectal cancer (11), suggesting a possible role as a TYMS
inhibitor. Nonetheless, the therapeutic role and targets of ATO in
mesothelioma are less well-known and only two publications are
available: ATO induced apoptosis through JNK and ERK (12) and repressed Hedgehog signal
transduction pathway (13).
Specifically the upstream transcription factor of TYMS, E2F1, which
is responsible for controlling proliferation and apoptosis
(14), has not been explored as a
therapeutic target in mesothelioma. We therefore aimed to
investigate the anticancer effect and target of ATO in mesothelioma
in vitro and in vivo, and provide a scientific basis
for the future clinical development of ATO as a treatment for
MPM.
Materials and methods
Cell lines and reagents
A panel of five mesothelioma cell lines [NCI-H28
(sarcomatoid), MSTO-211H (biphasic), NCI-H226 (epithelioid),
NCI-H2052 (sarcomatoid) and NCI-H2452 (epithelioid)] was purchased
(American Type Culture Collection, Manassas, VA, USA). Cells were
incubated in RPMI-1640 medium (Gibco®, Life
Technologies, Carlsbad, CA, USA) enriched with 10% fetal bovine
serum (FBS) (Gibco) in a humidified atmosphere of 5% CO2
at 37°C. ATO (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
sodium hydroxide (1.65 M) with pH adjusted to 7.4 by 6 M
hydrochloric acid.
Cell viability assay
Briefly, cells (5,000/well) were incubated with
different concentrations of ATO. Cells incubated with medium only
served as a negative control. Following incubation for 48 or 72 h,
cells were stained with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
for 2 h, followed by addition of DMSO after removal of all medium.
Absorbance (595 nm) was measured using a microplate reader Fluo
Star Optima (Bmg Labtec GmbH, Ortenberg, Germany (10).
Western blotting study of whole-cell
lysate
Western blotting was performed as previously
described (15). Specific primary
antibodies [mouse monoclonal anti-human β-actin (Sigma-Aldrich),
anti-c-Myc, anti-E2F1, anti-p-c-Jun, anti-pRB1, anti-TYMS,
anti-thymidine kinase 1, anti-ribonucleotide reductase M1,
anti-skp2, anti-Bcl-2, anti-Bcl-xL, anti-Bak and anti-cleaved
caspase-3 (Cell Signaling Technology, Danvers, MA, USA) antibodies]
and corresponding horseradish peroxidase (HRP)-conjugated secondary
antibody (Cell Signaling Technology) were purchased. An enhanced
chemiluminescence (ECL) kit (GE Healthcare) was used to detect
protein expression. β-actin was selected as reference protein.
Quantification of TYMS mRNA
A standard TRIzol/chloroform method was used to
extract total cellular RNA. Reverse transcription and quantitative
polymerase chain reaction (qPCR) were performed using the
PowerSYBR® Green Cells-to-CT™ kit
(Life Technologies) and standard protocol in StepOnePlus Real-Time
PCR System (Applied Biosystems, CA, USA). The sequence of TYMS
forward and reverse primers were TCAAGGGATCCACAAATGCT and
TCTGTCGTCAGGGTTGGTTT, respectively. GAPDH served as internal
control. Relative expression was calculated (10).
TYMS activity using tritium-release
assay
Cells were incubated for 2 h in fresh medium
containing 3 μl [5-3H]-dUMP (American Radiolabeled
Chemical, MO, USA) per well. The medium was collected, and mixed
with charcoal and trichloroacetic acid before centrifugation. One
milliliter scintillation fluid was added to the supernatant and
read using a scintillation counter (16). TYMS activity = total scintillation
count of sample/total scintillation count of control.
TYMS and E2F1 siRNA knockdown
TYMS (sc-44978), E2F1 (sc-29297) or control
(sc-37007) siRNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) was allowed to transfect cells for 6 h using a transfection
reagent (Santa Cruz Biotechnology) in RPMI-1640 medium, followed by
replenishment with new medium containing 1% FBS for 3 days
(10). Cell viability, TYMS and
E2F1 protein expression were measured.
Measurement of phosphatidylserine
externalization
Cells were stained at room temperature for 20 min
with phycoerythrin (PE)-conjugated Annexin V and
7-amino-actinomycin (AAD) (BD Phamingen, CA, USA) in binding buffer
and incubated in darkness. Cells were then analyzed by flow
cytometry using FL-2 (575 nm) and FL-4 channel (675 nm) with
Beckman FC500 (Beckman Coulter, Brea, CA, USA) (15).
Measurement of mitochondrial membrane
potential
In short, cells were incubated for 15 min at 37°C in
500 μl RPMI medium containing
5,5′,6,6′-tetrachloro-1,1′,3,3′tetraethylbenz
imidazolylcarbocyanine iodide (JC-1) (Sigma-Aldrich) in the dark.
Cells were then analyzed using flow cytometry FL-1 (525 nm) and
FL-2 (575 nm) channel (Beckman FC500).
Tumor growth inhibition in vivo
The H226 xenograft model was created by subcutaneous
injection of 107 cells in phosphate-buffered saline
(PBS) into the upper back of 18 nude mice (female, 4–6-week-old,
10–14 g, BALB/cAnN-nu, Charles River Laboratories, Wilmington, MA,
USA). Mice were randomized into 3 groups after tumor growth was
established. PBS (served as control) or ATO (3.75 and 7.5 mg/kg)
was administered daily intraperitoneally. Tumor dimension (using
standard calipers) and body weight of mice were measured on
alternate days and tumor volume calculated [volume = length × width
× width)/2] (17). For humane
reasons, mice were sacrificed when tumor size reached 17 mm in
diameter. Part of the tumor xenografts were collected and lysed for
western blotting. The remainder was fixed in paraffin block and cut
into sections for immunohistochemical (IHC) staining according to
standard procedures. The study protocol was approved by the
institutional Animal Ethics Committee (approval reference no.
CULATR 2510-11), and standard humane endpoints for animal research
were applied.
Statistical analysis
Experiments were repeated at least 3 times and data
analysed (mean ± standard deviation). The difference between groups
was analyzed using Student’s two-tailed t-test by Prism (GraphPad
Software, La Jolla, CA, USA). A p-value <0.05 defined
statistical significance (*p<0.05,
**p<0.01, ***p<0.001 in the
figures).
Results
In vitro activity of ATO in
mesothelioma
Treatment with ATO induced a dose- and
time-dependent antiproliferative effect in all mesothelioma cell
lines. The IC50 values in different cell lines were
4.7–7.8 μM (H28, 211H, H226, H2052 and H2452 cells: 6.9±1.6,
6.6±1.1, 7.8±1.1, 4.7±2.1 and 5.6±2.6 μM respectively) and 1.7–7 μM
(H28, 211H, H226, H2052 and H2452 cells: 2.1±0.7, 7.0±0.6, 6.6±1.0,
1.7±0.5 and 2.8±0.8 μM respectively) after 48- and 72-h treatment,
respectively.
Effects of ATO on TYMS protein and mRNA
expression, pRB1 and E2F1 protein expression and TYMS activity
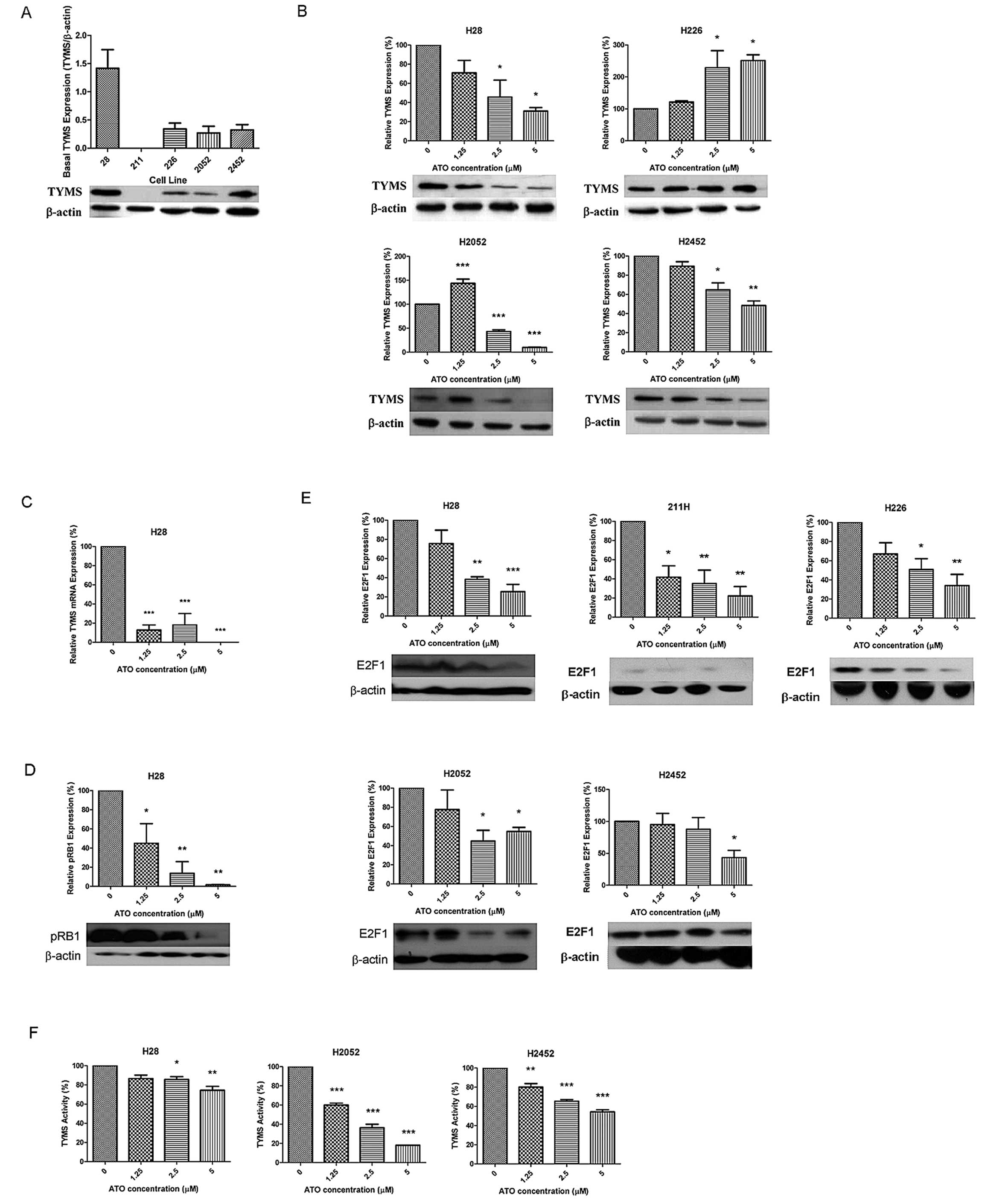
Basal TYMS protein expression is shown in Fig. 1A. H28 cells displayed the highest
TYMS protein expression. The TYMS expression levels in H226, H2052
and H2452 cells were similar, but undetectable in 211H cells.
Following a 48-h incubation with ATO, TYMS protein expression was
decreased in H28 and H2452 cells, and increased in H226 cells in a
dose-dependent manner. The TYMS protein expression was first
increased (1.25 μM ATO), then decreased at higher concentrations of
ATO in H2052 cells (Fig. 1B). TYMS
mRNA expression was significantly decreased in H28 cells (Fig. 1C), while basal TYMS mRNA expression
was undetectable in other cells. Basal pRB1 was highly expressed
and downregulated in H28 cells in a dose-dependent manner after ATO
treatment (Fig. 1D), but
undetectable in other cells. Upon ATO treatment, expression of E2F1
protein was decreased significantly in all cell lines (Fig. 1E). ATO inhibited TYMS activity in
H28, H2052 and H2452 cells, but increased TYMS activity in H226
cells (Fig. 1F).
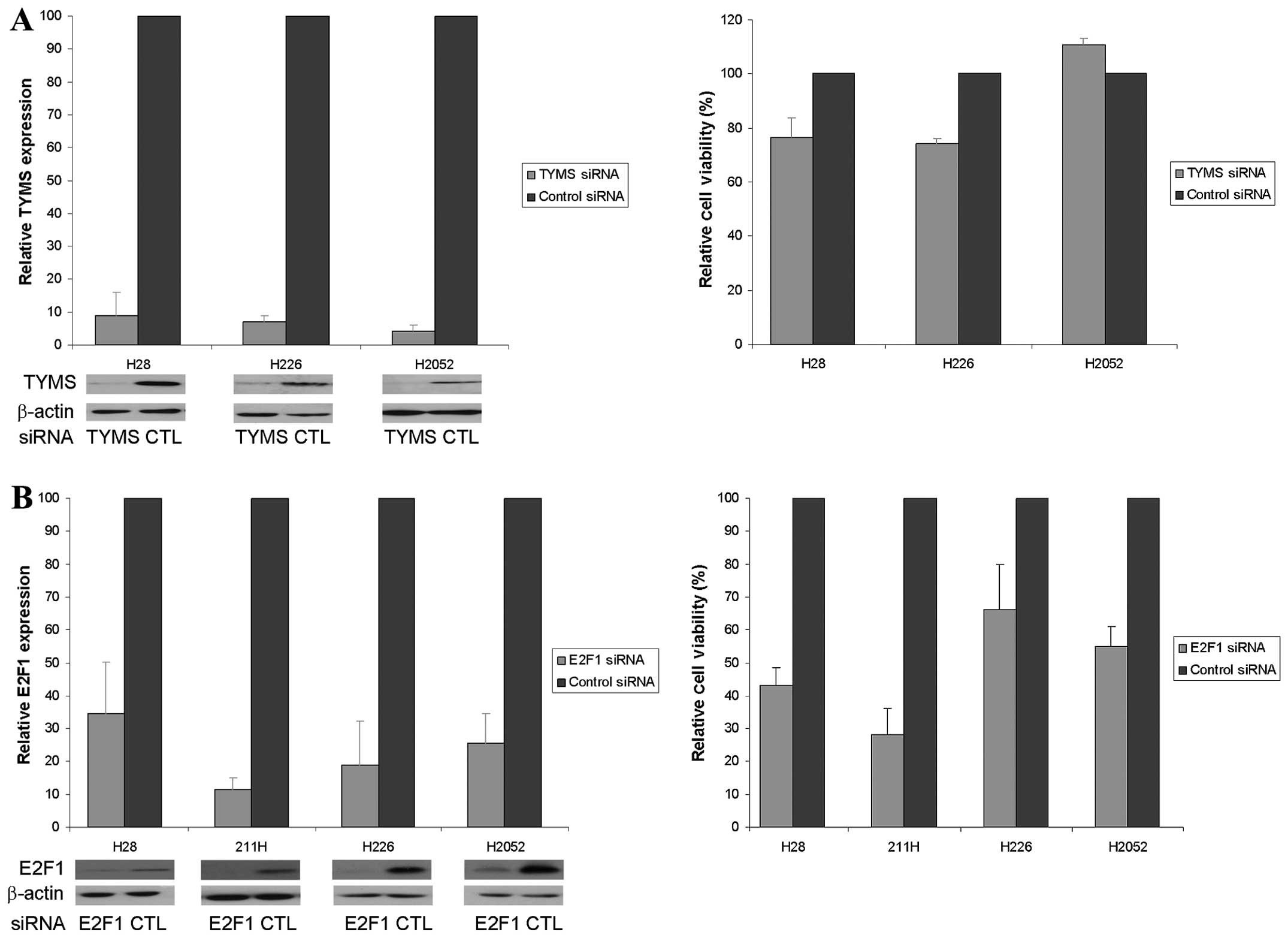
Decreased cell viability after knockdown
of TYMS and E2F1
H28, H226, H2052 and H2452 cells were used in TYMS
silencing experiment, however, H2452 cells could not survive in 1%
FBS condition. TYMS siRNA reduced the relative TYMS protein
expression to <10% that of the control siRNA and was associated
with a 25% reduction in cell viability in H28 and H226 cells but
unaltered viability in H2052 cells (Fig. 2A). E2F1 siRNA decreased the
relative E2F1 protein expression to ~35, 12, 19 and 26% that of the
control siRNA and was associated with 57, 72, 37 and 45% reduction
in cell viability in H28, 211H, H226 and H2052 cells, respectively
(Fig. 2B).
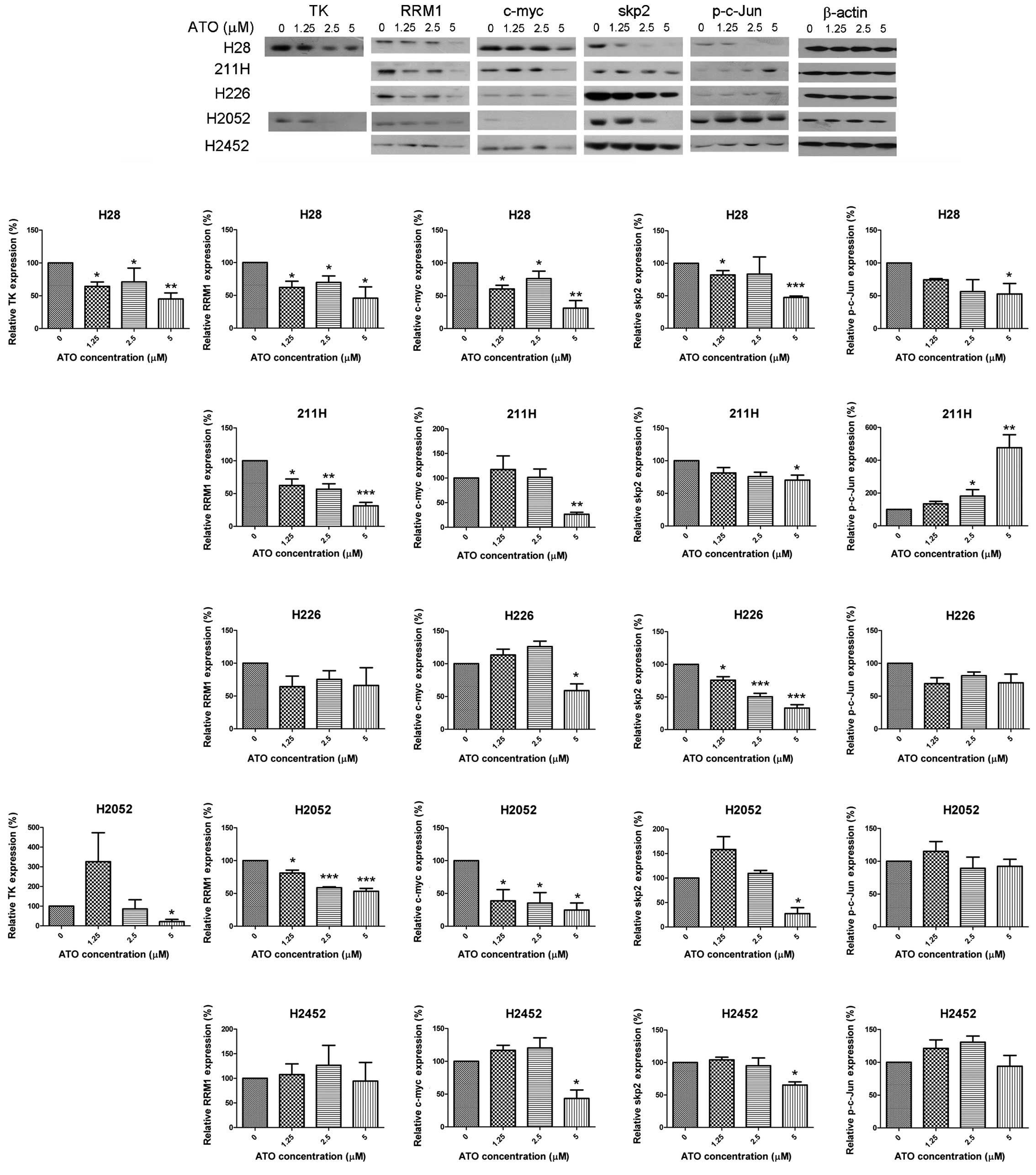
Alteration of E2F1 downstream targets by
ATO
ATO decreased expression of thymidine kinase 1 (TK)
in H28 cells, but upregulated TK expression initially at 1.25 μM
and then downregulated with higher concentrations in H2052 cells.
The expression of ribonucleotide reductase M1 (RRM1) was suppressed
in H28, 211H and H2052 cells. ATO downregulated the expression of
c-myc and skp2 in all cell lines. The expression of p-c-Jun was
decreased in H28 cells while upregulated in 211H cells (Fig. 3).
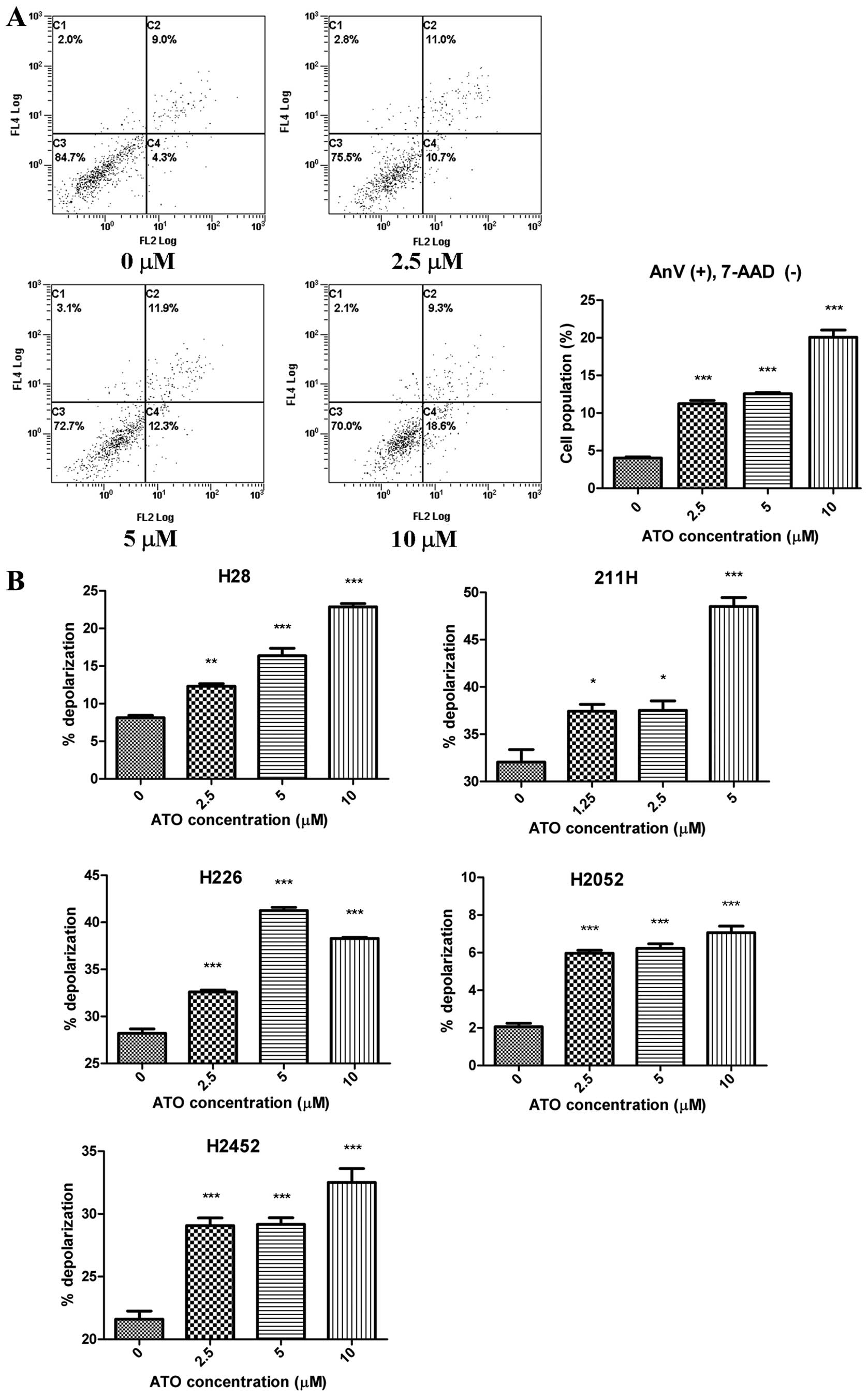
Phosphatidylserine (PS) externalization
and mitochondrial membrane depolarization induced by ATO
PS externalization induced by ATO was observed in
H2452 cells (Fig. 4A) although ATO
enhanced mitochondrial membrane depolarization in all cell lines in
a dose-dependent manner (Fig.
4B).
Alteration of apoptosis-related factors
by ATO
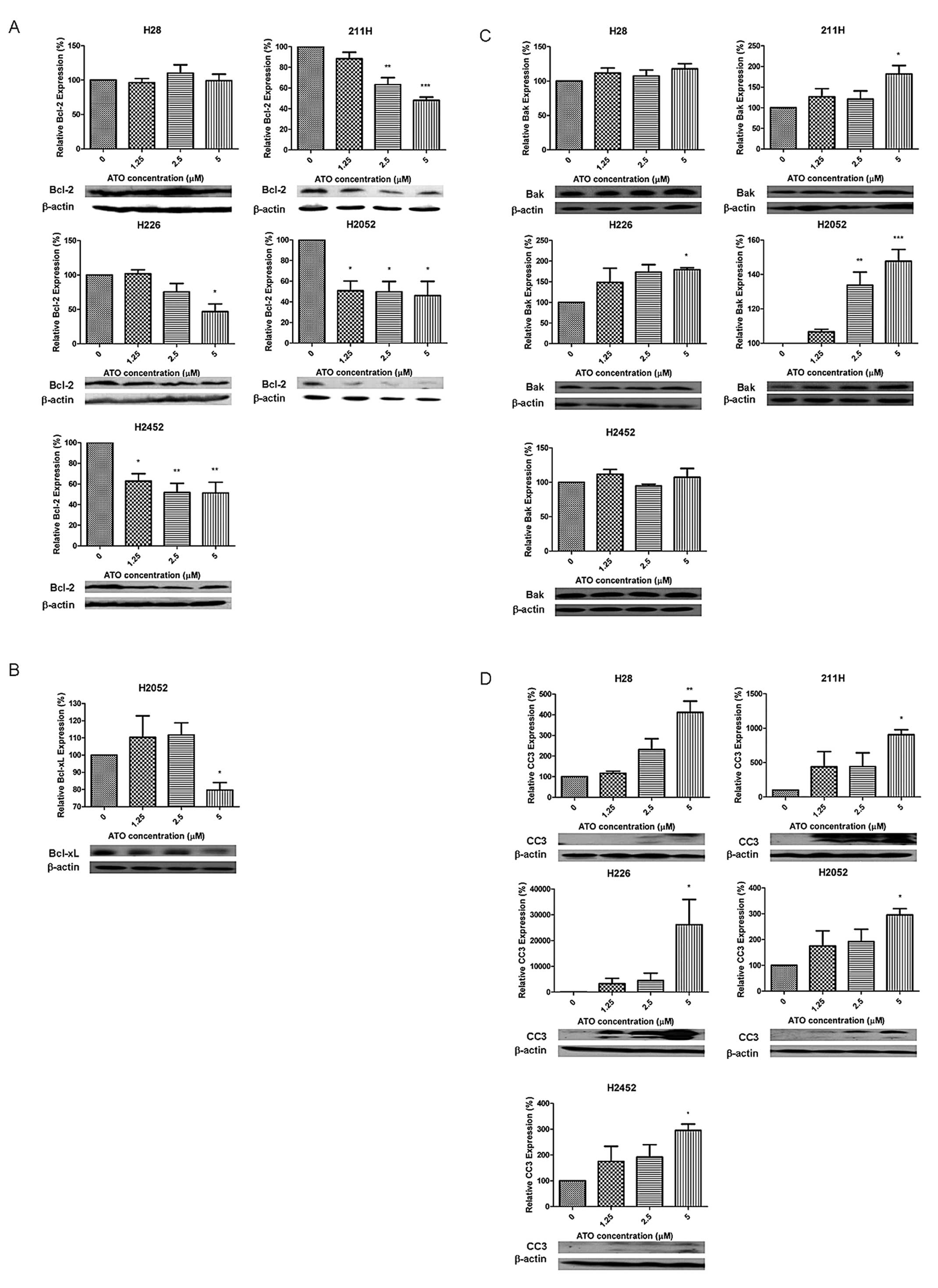
The expression of anti-apoptotic factor Bcl-2 was
downregulated in a dose-dependent manner with ATO treatment in all
cell lines except H28 cells (Fig.
5A). The anti-apoptotic factor Bcl-xL was suppressed in H2052
cells (Fig. 5B). The expression of
the downstream Bak in 211H, H226 and H2052 cells was upregulated
when the concentration of ATO increased (Fig. 5C). Expression of cleaved caspase-3
(CC3) was increased by ATO in all cell lines (Fig. 5D).
In vivo effect of ATO on tumor
xenografts
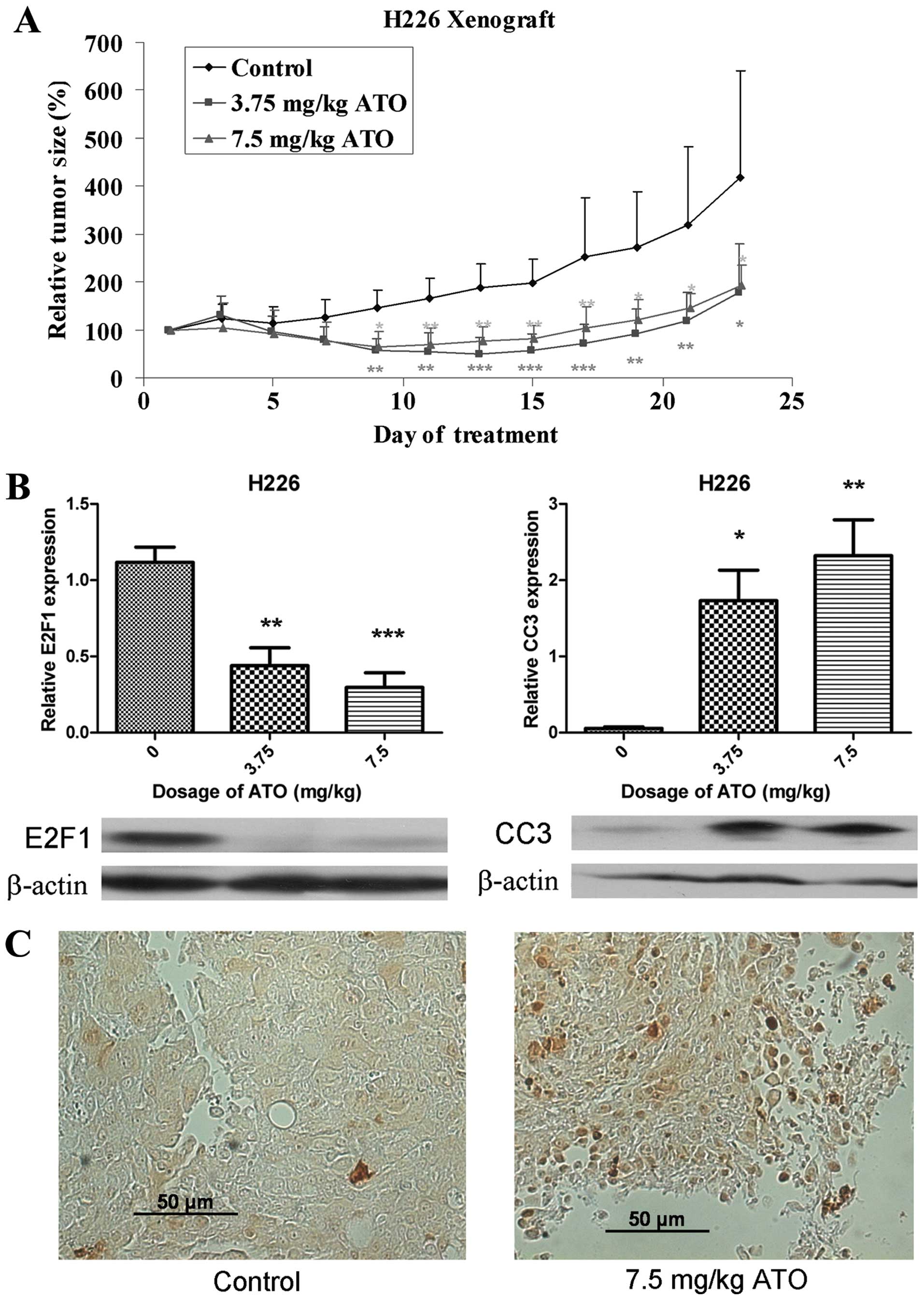
After inoculation of H226 cells for 7 days, obvious
tumors were established. There was no significant difference in
baseline tumor size amongst different groups of mice. The relative
tumor size in the control and ATO treatment groups during treatment
was determined (Fig. 6A). After 23
days of ATO treatment, the relative tumor volume in the 3.75 and
7.5 mg/kg treatment groups were 42.9 and 46.5% that of the control
group respectively (p<0.05). There was no difference in body
weight between different groups (p>0.05) and no pathological
changes (H&E staining) were observed in the liver of mice in
the ATO treatment arms. E2F1 was suppressed and CC3 was upregulated
in the ATO treatment arm (Fig.
6B), with IHC showing localization of CC3 to the nucleus
(Fig. 6C).
Discussion
ATO showed antiproliferative effects as demonstrated
by MTT cell viability assay with clinically achievable
concentrations (18) in our cell
line model accompanied by downregulation of TYMS (except H226
cells) and E2F1. The significance of TYMS in cell viability was
demonstrated in vitro with TYMS knockdown, nonetheless with
rather modest effect. In contrast, knockdown of the upstream target
E2F1 resulted in more significant inhibition of cell viability. Our
findings of downregulation of E2F1 and its various downstream
signals would support E2F1 as a significant target of ATO leading
to its antiproliferative effect. Upon treatment with ATO, apoptosis
was also observed. In the H226 xenograft model, tumor growth was
suppressed, E2F1 was downregulated while cleaved caspase-3 was
upregulated and translocated to the nucleus in ATO treated
mice.
It is evident that arsenic contamination in drinking
water and food is related to liver, skin, kidney, bladder and lung
cancers (19,20). Contrary to this though, ATO is
being used for treatment of APL, mediated through SUMOylation of
retinoic acid receptor α oncoprotein (21). ATO is also known to exert its
anti-leukemic action through reactive oxygen species induction,
mitochondrial membrane destruction, cytochrome c release,
caspase activation and finally apoptosis (22–27).
The US Food and Drug Administration has approved APL as an
indication for treatment with ATO. Since TYMS is a key target in
mesothelioma and ATO has recently been shown to suppress TYMS
expression in lung adenocarcinoma (10) and colorectal cancer (11), we postulated that ATO would exert a
TYMS inhibitory effect in mesothelioma cell lines.
In order to understand the significance of reduced
protein expression of TYMS and TYMS activity by ATO in the 3
mesothelioma cell lines, the pivotal role of TYMS in cellular
proliferation was studied using a TYMS knockdown technique.
However, the impact of TYMS knockdown on cell viability was
unexpectedly low. The role of TYMS in mesothelioma is controversial
which has been shown to be non-essential (6,7).
Notably, the expression of E2F1 has demonstrated a strong
correlation with cancer cell proliferation (28,29),
leading to our further exploration of a new actionable target the
E2F1.
E2F1 is a well-known transcription factor which is
involved in proliferation, apoptosis, cell cycle, tumor growth and
senescence. It is released and activated upon phosphorylation of
retinoblastoma tumor suppressor protein (RB) (14). However, the role of E2F1 in
mesothelioma has never been elucidated. Thus, E2F1-targeted siRNA
experiment was performed to investigate the functional role of
E2F1. Decrease in cell viability upon E2F1 knockdown was more
profound when compared with TYMS, in support of a more important
role of E2F1 than TYMS on cancer cell proliferation. Moreover,
cleaved caspase-3 was elevated only in 211H cells (data not shown)
after E2F1 knockdown showing that the relationship between E2F1 and
apoptosis is cell line-specific. As such, the downstream targets of
E2F1 related to cell proliferation and apoptosis were also
investigated.
TYMS, TK and RRM1 are three key enzymes that take
part in DNA synthesis and thus important in cell proliferation. TK
activity in sarcomatoid type was reported to be higher than
epithelioid type mesothelioma (30), which is in line with our observed
relatively higher TK expression in sarcomatoid cells (H28 and H2052
cells). RRM1 polymorphisms and haplotypes were related to efficacy
of gemcitabine treatment in mesothelioma (31). Interestingly, RRM1 downregulation
by ATO was only observed in sarcomatoid mesothelioma cell lines in
this study, suggesting a differential effect of ATO in different
histological types of mesothelioma. Degradation of tumor suppressor
proteins, e.g., p21 and p27, are induced by skp2 so as to
accelerate cell cycle (32).
Nonetheless, the role of skp2 in mesothelioma has so far not been
described. Our findings have provided the first evidence of TK,
RRM1 and skp2 downregulation by ATO in mesothelioma.
c-myc is a transcription factor which governs cell
proliferation and metastasis. Suppression of c-myc has been shown
to have antiproliferative (33)
and apoptotic (34) effect in
mesothelioma. In addition, growth inhibition in APL by ATO was
partially mediated through suppression of c-myc (35).
JNK-c-JUN pathway regulates cell proliferation and
upregulation of p-c-Jun mediates cell apoptosis (36). Apoptosis induced by ATO was
reported only in one mesothelioma cell line (H2052) (12). Hence, we further studied the
apoptotic effects of ATO in our 5 mesothelioma cell lines.
Phosphatidylserine (PS) externalization (37) and mitochondrial membrane
depolarization (38) are
well-known hallmarks of apoptosis. In addition, our findings of
upregulated pro-apoptotic (Bak and cleaved caspase-3) and
downregulated anti-apoptotic (Bcl-2 and Bcl-xL) proteins have
provided supportive evidence that apoptosis was induced by ATO in
mesothelioma cell lines.
The expression of cleaved caspase-3 in all
mesothelioma cell lines increased in a dose-dependent manner with
ATO, substantiated in our H226 xenograft model. However, H28, 211H,
H2052 and H2452 xenograft models could not be generated despite
multiple attempts. Based on IHC in H226 tumor xenografts,
cytoplasmic cleaved caspase-3 was translocated to the nucleus,
leading to subsequent chromatin condensation, DNA fragmentation
and/or nuclear disruption with eventual apoptosis (39). In addition, downregulation of E2F1
expression in H226 xenograft model with ATO treatment was observed.
Our findings provide strong evidence for the in vitro and
in vivo antiproliferative and pro-apoptotic effect of ATO in
mesothelioma which is similar in lung adenocarcinoma recently
reported (40).
In conclusion, ATO has demonstrated an
antiproliferative effect at least partially mediated through
downregulation of E2F1, as well as a cytotoxic effect through
apoptosis in both in vitro and in vivo mesothelioma
models. Moreover, we propose the E2F1 as a new actionable target in
MPM. Our findings provide the scientific basis for future
exploration of the clinical application of ATO in treatment of MPM.
This is particularly feasible with the recent development of an
oral-ATO preparation by our institution for clinical use.
Acknowledgements
This study was supported by the Hong Kong
Pneumoconiosis Compensation Fund Board.
References
|
1
|
Borasio P, Berruti A, Bille A, et al:
Malignant pleural mesothelioma: clinicopathologic and survival
characteristics in a consecutive series of 394 patients. Eur J
Cardiothorac Surg. 33:307–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abakay A, Abakay O, Tanrikulu AC, et al:
Effects of treatment regimens on survival in patients with
malignant pleural mesothelioma. Eur Rev Med Pharmacol Sci.
17:19–24. 2013.PubMed/NCBI
|
|
3
|
Rahman L, Voeller D, Rahman M, et al:
Thymidylate synthase as an oncogene: a novel role for an essential
DNA synthesis enzyme. Cancer Cell. 5:341–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bunn PA Jr: Incorporation of pemetrexed
(Alimta) into the treatment of non-small cell lung cancer (thoracic
tumors). Semin Oncol. 29:17–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sigmond J, Backus HH, Wouters D, Temmink
OH, Jansen G and Peters GJ: Induction of resistance to the
multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon
cancer cells is associated with thymidylate synthase
overexpression. Biochem Pharmacol. 66:431–438. 2003. View Article : Google Scholar
|
|
6
|
Lustgarten DE, Deshpande C, Aggarwal C, et
al: Thymidylate synthase and folyl-polyglutamate synthase are not
clinically useful markers of response to pemetrexed in patients
with malignant pleural mesothelioma. J Thorac Oncol. 8:469–477.
2013. View Article : Google Scholar
|
|
7
|
Mairinger F, Vollbrecht C, Halbwedl I, et
al: Reduced folate carrier and folylpolyglutamate synthetase, but
not thymidylate synthase predict survival in pemetrexed-treated
patients suffering from malignant pleural mesothelioma. J Thorac
Oncol. 8:644–653. 2013. View Article : Google Scholar
|
|
8
|
Fennell DA and Rudd RM: Defective
core-apoptosis signalling in diffuse malignant pleural
mesothelioma: opportunities for effective drug development. Lancet
Oncol. 5:354–362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumana CR, Au WY, Lee NS, et al: Systemic
availability of arsenic from oral arsenic-trioxide used to treat
patients with hematological malignancies. Eur J Clin Pharmacol.
58:521–526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lam SK, Mak JC, Zheng CY, Li YY, Kwong YL
and Ho JC: Downregulation of thymidylate synthase with arsenic
trioxide in lung adenocarcinoma. Int J Oncol. 44:2093–2102.
2014.PubMed/NCBI
|
|
11
|
Subbarayan PR, Lee K and Ardalan B:
Arsenic trioxide suppresses thymidylate synthase in 5-FU-resistant
colorectal cancer cell line HT29 in vitro re-sensitizing cells to
5-FU. Anticancer Res. 30:1157–1162. 2010.PubMed/NCBI
|
|
12
|
Eguchi R, Fujimori Y, Takeda H, et al:
Arsenic trioxide induces apoptosis through JNK and ERK in human
mesothelioma cells. J Cell Physiol. 226:762–768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You M, Varona-Santos J, Singh S, Robbins
DJ, Savaraj N and Nguyen DM: Targeting of the Hedgehog signal
transduction pathway suppresses survival of malignant pleural
mesothelioma cells in vitro. J Thorac Cardiovasc Surg. 147:508–516.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slee EA and Lu X: Requirement for
phosphorylation of P53 at Ser312 in suppression of chemical
carcinogenesis. Sci Rep. 3:31052013.PubMed/NCBI
|
|
15
|
Li YY, Lam SK, Mak JC, Zheng CY and Ho JC:
Erlotinib-induced autophagy in epidermal growth factor receptor
mutated non-small cell lung cancer. Lung Cancer. 81:354–361. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pressacco J, Mitrovski B, Erlichman C and
Hedley DW: Effects of thymidylate synthase inhibition on thymidine
kinase activity and nucleoside transporter expression. Cancer Res.
55:1505–1508. 1995.PubMed/NCBI
|
|
17
|
Kousparou CA, Yiacoumi E, Deonarain MP and
Epenetos AA: Generation of a selectively cytotoxic fusion protein
against p53 mutated cancers. BMC Cancer. 12:3382012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen ZX, Chen GQ, Ni JH, et al: Use of
arsenic trioxide (As2O3) in the treatment of
acute promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360.
1997.PubMed/NCBI
|
|
19
|
Aballay LR, del Diaz MP, Francisca FM and
Munoz SE: Cancer incidence and pattern of arsenic concentration in
drinking water wells in Cordoba, Argentina. Int J Environ Health
Res. 22:220–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naujokas MF, Anderson B, Ahsan H, et al:
The broad scope of health effects from chronic arsenic exposure:
update on a worldwide public health problem. Environ Health
Perspect. 121:295–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de The H, Le Bras M and
Lallemand-Breitenbach V: The cell biology of disease: Acute
promyelocytic leukemia, arsenic, and PML bodies. J Cell Biol.
198:11–21. 2012.PubMed/NCBI
|
|
22
|
Cai X, Shen YL, Zhu Q, et al: Arsenic
trioxide-induced apoptosis and differentiation are associated
respectively with mitochondrial transmembrane potential collapse
and retinoic acid signaling pathways in acute promyelocytic
leukemia. Leukemia. 14:262–270. 2000. View Article : Google Scholar
|
|
23
|
Jing Y, Dai J, Chalmers-Redman RM, Tatton
WG and Waxman S: Arsenic trioxide selectively induces acute
promyelocytic leukemia cell apoptosis via a hydrogen
peroxide-dependent pathway. Blood. 94:2102–2111. 1999.PubMed/NCBI
|
|
24
|
Mahieux R, Pise-Masison C, Gessain A, et
al: Arsenic trioxide induces apoptosis in human T-cell leukemia
virus type 1- and type 2-infected cells by a caspase-3-dependent
mechanism involving Bcl-2 cleavage. Blood. 98:3762–3769. 2001.
View Article : Google Scholar
|
|
25
|
Park WH, Seol JG, Kim ES, et al: Arsenic
trioxide-mediated growth inhibition in MC/CAR myeloma cells via
cell cycle arrest in association with induction of cyclin-dependent
kinase inhibitor, p21, and apoptosis. Cancer Res. 60:3065–3071.
2000.
|
|
26
|
Shim MJ, Kim HJ, Yang SJ, Lee IS, Choi HI
and Kim T: Arsenic trioxide induces apoptosis in chronic
myelogenous leukemia K562 cells: possible involvement of p38 MAP
kinase. J Biochem Mol Biol. 35:377–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang ZG, Rivi R, Delva L, et al: Arsenic
trioxide and melarsoprol induce programmed cell death in myeloid
leukemia cell lines and function in a PML and PML-RARalpha
independent manner. Blood. 92:1497–1504. 1998.PubMed/NCBI
|
|
28
|
Gorgoulis VG, Zacharatos P, Mariatos G, et
al: Transcription factor E2F-1 acts as a growth-promoting factor
and is associated with adverse prognosis in non-small cell lung
carcinomas. J Pathol. 198:142–156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zacharatos P, Kotsinas A, Evangelou K, et
al: Distinct expression patterns of the transcription factor E2F-1
in relation to tumour growth parameters in common human carcinomas.
J Pathol. 203:744–753. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsuji AB, Sogawa C, Sugyo A, et al:
Comparison of conventional and novel PET tracers for imaging
mesothelioma in nude mice with subcutaneous and intrapleural
xenografts. Nucl Med Biol. 36:379–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Erculj N, Kovac V, Hmeljak J, Franko A,
Dodic-Fikfak M and Dolzan V: The influence of gemcitabine pathway
polymorphisms on treatment outcome in patients with malignant
mesothelioma. Pharmacogenet Genomics. 22:58–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kitagawa K, Kotake Y and Kitagawa M:
Ubiquitin-mediated control of oncogene and tumor suppressor gene
products. Cancer Sci. 100:1374–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nuvoli B, Santoro R, Catalani S, et al:
CELLFOOD induces apoptosis in human mesothelioma and colorectal
cancer cells by modulating p53, c-myc and pAkt signaling pathways.
J Exp Clin Cancer Res. 33:242014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kitamura A, Matsushita K, Takiguchi Y, et
al: Synergistic effect of non-transmissible Sendai virus vector
encoding the c-myc suppressor FUSE-binding protein-interacting
repressor plus cisplatin in the treatment of malignant pleural
mesothelioma. Cancer Sci. 102:1366–1373. 2011. View Article : Google Scholar
|
|
35
|
Ghaffari SH, Momeny M, Bashash D, Mirzaei
R, Ghavamzadeh A and Alimoghaddam K: Cytotoxic effect of arsenic
trioxide on acute promyelocytic leukemia cells through suppression
of NFkbeta-dependent induction of hTERT due to down-regulation of
Pin1 transcription. Hematology. 17:198–206. 2012. View Article : Google Scholar
|
|
36
|
Song W, Ma Y, Wang J, Brantley-Sieders D
and Chen J: JNK signaling mediates EPHA2-dependent tumor cell
proliferation, motility, and cancer stem cell-like properties in
non-small cell lung cancer. Cancer Res. 74:2444–2454. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yedjou C, Tchounwou P, Jenkins J and
McMurray R: Basic mechanisms of arsenic trioxide (ATO)-induced
apoptosis in human leukemia (HL-60) cells. J Hematol Oncol.
3:282010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin HO, Yoon SI, Seo SK, et al:
Synergistic induction of apoptosis by sulindac and arsenic trioxide
in human lung cancer A549 cells via reactive oxygen
species-dependent down-regulation of survivin. Biochem Pharmacol.
72:1228–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo M, Lu Z, Sun H, et al: Nuclear entry
of active caspase-3 is facilitated by its p3-recognition-based
specific cleavage activity. Cell Res. 20:211–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lam SK, Li YY, Zheng CY, Leung LL and Ho
JC: E2F1 downregulation by arsenic trioxide in lung adenocarcinoma.
Int J Oncol. 45:2033–2043. 2014.PubMed/NCBI
|