Introduction
Prostate cancer (PC) is a major health problem in
men in the Western world. Definitive treatment options for
localized PC routinely include radical retropubic prostatectomy
(RP), external beam radiotherapy (EBRT), and brachytherapy or a
combination of these methods. A recent review of 18,000 studies
compared the outcomes of curative treatment of localized PC in the
post-PSA era (1), and it was
concluded that randomized studies are rare and there is a lack of
evidence to determine whether any of the available treatment
options are superior for prolonging survival. Therefore, it is
necessary for patients to consider the morbidity and side-effects
of the treatment modalities when deciding on treatment for PC. This
underlines the importance of having detailed knowledge of
health-related quality of life (HRQoL) and adverse events
associated with different curative treatments. Early HRQoL research
concerning treatment-related side-effects such as incontinence,
bowel disturbances, and impact on sexual activity showed disparate
profiles for the various treatment modalities (2–4). In
the cited investigations, patients treated with RP reported more
incontinence, which was acute but improved over the first 2 years
after treatment. By comparison, bowel problems were worse in
irradiated patients, continuing for up to 4 years, and urinary
bother was increased in patients treated by brachytherapy with
permanent implants. HRQoL studies concerning sexual problems have
reported fewer initial problems after brachytherapy and EBRT than
after RP, but the results are often hampered by a lack of baseline
data.
During the 1990s, questions were raised about
whether the traditional EBRT treatment dose of 66–70 Gy was
sufficient to cure PC. Since then, several studies have shown
increased disease-free survival after dose escalation (5). Such amplification can be done by
several different methods: three-dimensional conformal
radiotherapy, intensity-modulated radiotherapy and combinations of
these approaches with high-dose-rate brachytherapy (HDRBT),
low-dose-rate brachytherapy or particle beam boosts. Some studies
have compared acute effects of traditional and modern
dose-escalated radiotherapy on HRQoL in PC patients (6,7), and
the results indicated comparable HRQoL outcomes. However, few
investigations have examined long-term effects in this clinical
context.
The aim of the present study was to compare
long-term HRQoL in men who were diagnosed with PC between 1988 and
1997 and had undergone one of the two curative treatment strategies
that, with small adjustments, are still in routine use today. Our
focus was on HRQoL after external beam radiotherapy combined with
high-dose-rate brachytherapy (HDRBT-EBRT) and open retropubic
prostatectomy (RP).
Patients and methods
Patients
The study cohort comprised all men who had received
curative-intent treatment with either RP or HDRBT-EBRT in the
Gothenburg area from 1 January 1988 to 31 December 1997 (n=492).
All members of this cohort who were still alive in October 2000
were asked to participate in the HRQoL study. Two urological
departments and one oncological department were involved. Evaluated
patient records and death certificates covered the period
1999–2001, during which time three standard treatments [RP, EBRT
and EBRT combined with HDRBT Ir-192 (radionuclide, iridium192)] and
one experimental treatment [cryoablation surgery (Cryo)] were
available. Men treated with EBRT alone or Cryo were not included in
the study: the former because they had been treated with an old
technique and inadequate doses according to the present standard;
the latter because only a small selected group of patients under
surveillance received Cryo treatment.
Clinical staging
TNM stage was defined according to the UICC 1992
classification (8). T stage data
were obtained from patient records or from pathology reports.
Patients were divided into low-, intermediate- and high-risk PC
groups. The low- and high-risk groups were, respectively,
classified according to the following criteria: prostate-specific
antigen (PSA) <10 and WHO grade 1 (corresponding to Gleason
score ≤5) and T1; PSA ≥20 and/or WHO grade 3 (corresponding to
Gleason score ≥4+3) and/or T3. The intermediate-risk group
comprised all patients that were not included in either the low-or
the high-risk group. In the high-risk PC group, lymph node
dissection was performed before radiotherapy or during RP, and M
stage was assessed by bone scan.
Radical retropubic prostatectomy (the RP
group)
RP was performed as an open retropubic procedure, if
possible using the nerve-sparing technique. The majority of
patients underwent regional lymph node excision with frozen
section, and only men with N0 were included (9).
Brachytherapy combined with external beam
radiotherapy (the HDRBT-EBRT group)
The radiotherapy technique used has been previously
described in detail (10).
Briefly, the prescribed target dose was 50 Gy, which was given in
2-Gy fractions using high-energy photons delivered by a standard
four-field box technique to the prostate and seminal vesicles. The
HDRBT target dose was 20 Gy in two 10-Gy fractions, which were
delivered 2 weeks apart to the prostate gland and the base of the
vesicles with a 3-mm margin.
Neoadjuvant hormonal therapy (NHT)
In addition to the treatment modalities described
above, NHT using a GNRH analogue was given 3–6 months before start
of treatment to patients with high-risk features. For irradiated
patients, NHT was administered throughout the course of
radiotherapy. NHT was also given to 51% of the patients in the RP
group who were participating in a clinical study (11).
Follow-up
A majority of the patients underwent annual
follow-up at the Department of Oncology or Urology at Sahlgrenska
University Hospital, including clinical examination and measurement
of PSA. A bone scan was performed if PSA relapse occurred or was
suspected on clinical grounds. Most patients with relapse of
disease were treated with early hormone therapy or were given the
best supportive care, as recommended by the treating physician.
Data collection
After a mean follow-up time of 7 years (range, 4–16
years) in October 2000 to April 2001, all men who were still alive
were asked by mail to participate in the HRQoL assessment by
completing a HRQoL questionnaire and returning it in a prepaid
envelope. The Regional Cancer Registry in Gothenburg handled all
questionnaires and entered data in the study database. One reminder
was sent.
Instruments
The European Organization of Research and Treatment
of Cancer (EORTC) developed the Quality of Life Questionnaire C30
(EORTC QLQ-C30) to measure HRQoL in cancer patients participating
in clinical trials (12). This
instrument includes 30 items comprising five functional scales
(physical, role, emotional, social and cognitive), three symptom
scales (fatigue, pain, nausea and vomiting), a global health
status/QoL scale, and six single items (dyspnea, loss of appetite,
insomnia, constipation, diarrhea and financial impact of disease).
In addition to the EORTC QLQ-C30, we used the EORTC QLQ-PR25
(13), which is a disease-specific
questionnaire assessing problems related to treatment of PC by use
of 25 questions on areas such as sexual function and bladder and
bowel problems. During the period covered by the present study, the
EORTC QLQ-PR25 had not yet been validated.
Statistical methods
The items of the EORTC QLQ-C30 and QLQ-PR25
instruments were scaled according to the scoring manual (14). Raw scores were transformed into a
scale ranging from 0 to 100. Higher scores indicate better
functioning on the functional subscales and the global quality of
life scale, and more symptoms on the symptom scales. The expected
mean value for each of the EORTC QLQ-C30 subscales was calculated
using the age distribution in all groups combined with age-specific
mean reference scores from the Swedish population (15). Differences in categorical variables
were tested using Fisher’s exact test. Continuous variables were
modeled using linear regression. Group differences were assessed by
Wald tests. Results from the regression models are presented as
mean differences together with 99% confidence intervals. In the
interpretation of the QLQ-C30 scores, a difference of ≥5 points on
the 0–100 scale was considered clinically significant. Differences
of 5–9 points were considered small, 10–20 as moderate and >20
as large (16). Due to multiple
testing, the level of significance was set at 0.01. All statistical
analyses were performed using the Stata statistical software
version 11.
Results
The initial cohort comprised 492 patients, but 71
(14.4%) of those individuals died before onset of the study. Thus,
421 patients were asked to participate in the HRQoL evaluation; 347
(82%) completed the questionnaires, and 42 declined to take part or
did not respond. The initial number of patients in each treatment
group, the number of respondents, and the number of deaths before
administration of the questionnaires are listed in Table I.
 | Table INumber of deaths before
questionnaires, and number and proportions of respondents per
treatment group. |
Table I
Number of deaths before
questionnaires, and number and proportions of respondents per
treatment group.
| Treatment group | Initial cohort n | Deceased before
questionnaires, n (%) | Questionnaires sent,
n (%) | Respondents, n
(%) |
|---|
| RP | 379 | 48 (13) | 331 (79) | 261 (79) |
| HDRBT/EBRT | 113 | 23 (20) | 90 (21) | 86 (96) |
| Total | 492 | 71 (14) | 421 | 347 (82) |
Clinical and demographic patient characteristics for
each treatment group are presented in Table II. There were no statistically
significant differences in clinical parameters between the groups,
except regarding T stage and PSA recurrence at the time of the
questionnaires. The proportion of patients with locally advanced
disease (T3–T4 tumors) was larger in the HDRBT-EBRT group than in
the RP group (13 and 6%, respectively; P=0.01). PSA relapse at the
time of the questionnaires was noted in 44% of the men in the RP
group compared to 9% in the HDRBT-EBRT group (P<0.0001). All
demographic parameters except education were equally distributed
between the treatment groups.
 | Table IIClinical and demographic
characteristics of the patients in the two treatment groups. |
Table II
Clinical and demographic
characteristics of the patients in the two treatment groups.
| Prostatectomy | HDRBT-EBRT | P-valuee | Total |
|---|
| No. of patients
(%) | 261 (75) | 86 (25) | | 347 (100) |
| Age (years) |
| Mean (SD) | 70 (6.0) | 70 (6.2) | NSf | 70 (6.1) |
| Median (range) | 70 (51–83) | 70 (56–83) | | 70 (51–83) |
| WHO, n (%) |
| 1 | 108 (41) | 33 (38) | | 141 (41) |
| 2 | 113 (43) | 35 (41) | | 148 (43) |
| 3 | 32 (12) | 4 (5) | NS | 36 (10) |
| Missing | 8 (3) | 14 (16) | | 22 (6) |
| Clinical T stage, n
(%) |
| 1 | 111 (42) | 25 (29) | | 136 (39) |
| 2 | 132 (51) | 49 (57) | | 181 (52) |
| 3 | 16 (6) | 11 (13) | | 27 (8) |
| 4 | 0 (0) | 1 (1) | 0.01 | 1 (0) |
| Missing | 2 (1) | 0 | | 2 (1) |
| PSA (ng/ml) |
| Mean | 16 (29.9) | 12 (9.1) | NSf | 15.3 (26.4) |
| Median (range) | 9.2 (0.9–410) | 9.6 (0.5–36) | | 9.4 (0.5–410) |
| Risk group,a n (%) |
| Low | 37 (14) | 5 (6) | | 42 (12) |
| Intermediate | 123 (47) | 39 (45) | | 162 (47) |
| High | 92 (35) | 30 (35) | NS | 122 (35) |
| Missing | 9 (3) | 12 (14) | | 21 (6) |
| Neoadjuvant hormonal
therapy, n (%) | 133 (51) | 49 (57) | NS | 182 (52) |
| Relapse at time of
questionnaires, n (%) | 114 (44) | 8 (9) | <0.000 | 122 (35) |
| Civil status, n
(%) |
| Married | 215 (82) | 73 (85) | | 288 (83) |
| Single | 20 (8) | 8 (9) | | 28 (8) |
| Single with
partner | 14 (5) | 4 (5) | | 18 (5) |
| Widower | 11 (4) | 1 (1) | NS | 12 (3) |
| Missing | 1 (0) | | | 1 (0) |
| Employment, n
(%) |
| Gainfully
employed | 29 (11) | 13 (15) | | 42 (12) |
| Retired (age
>65) | 217 (83) | 66 (77) | | 283 (82) |
| On sick
leaveb | 11 (4) | 6 (7) | | 17 (5) |
| Other | 3c (1) | 1d (1) | | 4 (1) |
| Missing | 1 (0) | 1 (1) | NS | 2 (1) |
| Education, n
(%) |
| Comprehensive
school | 162 (62) | 42 (49) | | 204 (59) |
| Higher school
degree | 23 (9) | 7 (8) | | 30 (9) |
| University
degree | 74 (28) | 37 (43) | 0.007 | 111 (32) |
| Missing | 2 (1) | | | 2 (1) |
| Nationality, n
(%) |
| Swedish | 232 (89) | 77 (90) | | 309 (89) |
| Scandinavian | 10 (4) | 4 (5) | | 14 (4) |
| European (other
than above) | 15 (6) | 4 (5) | | 19 (5) |
| Non-European | 3 (1) | 1 (1) | NS | 4 (1) |
| Missing | 1 (0) | | | 1 (0) |
HRQoL results for the normative population and the
treatment groups are shown in Fig.
1. In general, the patients’ mean HRQoL scores were high and
similar to the mean scores for the normative sample, with the
exception of physical and role function, for which the patients’
scores were higher. Considering physical symptoms, compared to the
normal population, the patients reported less pain but more
pronounced problems with sleep disturbances.
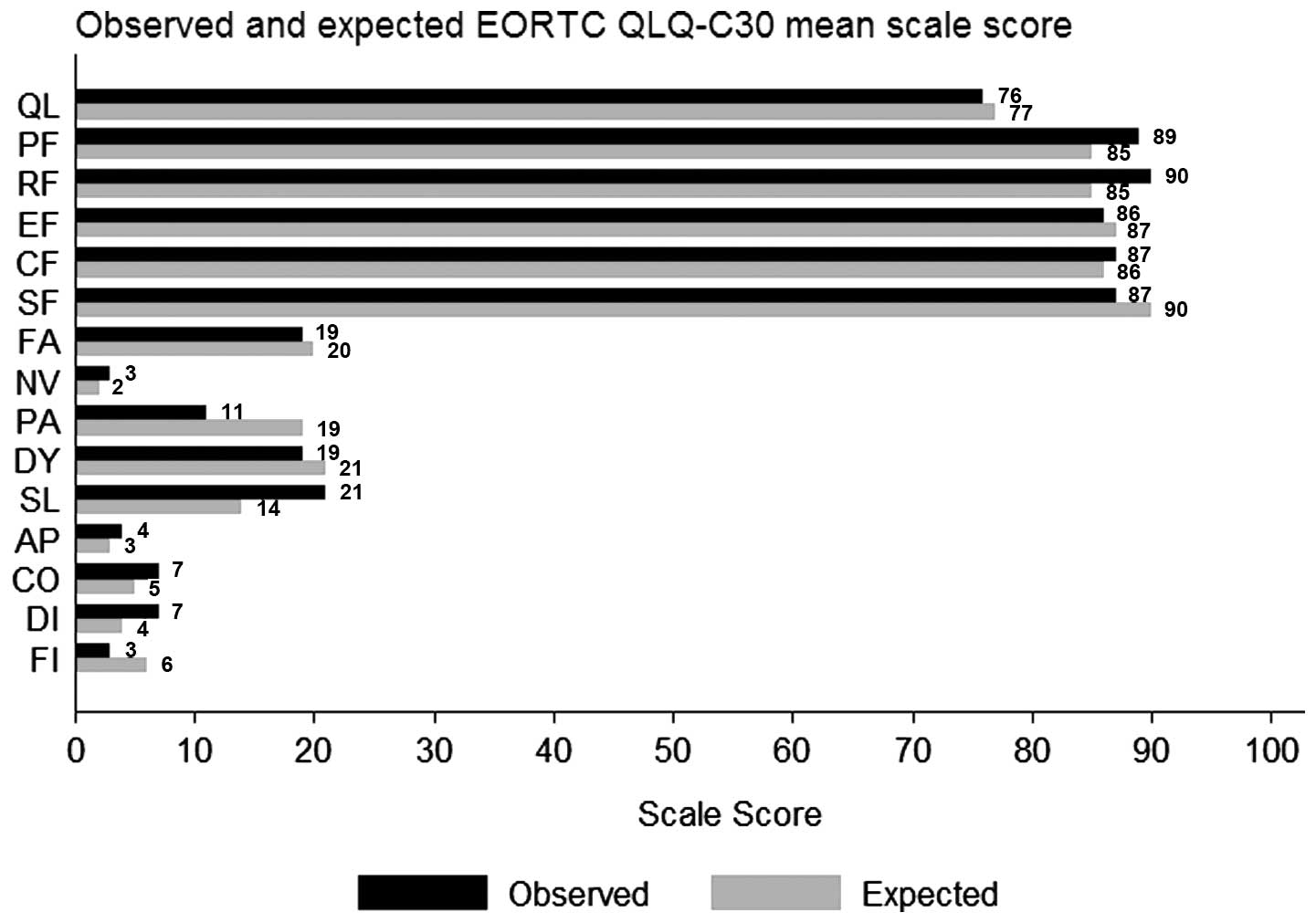 | Figure 1Comparison between HRQoL, mean scores
in the study population (observed) and an age-adjusted normative
sample (expected). Functional scales (high scores=high levels of
functioning): QL, global quality of life; PF, physical function;
RF, role function; EF, emotional function; CF, cognitive function;
SF, social function. Symptom scales (high scores=high levels of
sypmtoms); FA, fatigue; NV, nausea; PA, pain; DY, dyspnea; SL,
sleeping disturbances; AP, appetite loss; CO, constipation; DI,
diarrhea; FI, financial difficulties. |
The results of the univariate and multivariate
analyses comparing the two treatment groups are outlined in
Table III. Taking into account
age, PSA recurrence, and neo-adjuvant hormonal treatment, there
were no statistically significant differences between the groups in
either the univariate or the multivariate regression analysis of
the functional domains in the EORTC QLQ C-30 questionnaire. Adding
‘risk group at diagnosis’ or ‘time from diagnosis to time for
questionnaire’ as confounding factors did not change the results
(data not shown). Concerning the symptom scale, a higher level of
problem with diarrhea was found in the HDRBT-EBRT group, and this
difference was both statistically and clinically significant. Small
clinically significant differences favoring the RP group were found
for global quality of life, role functioning, fatigue, dyspnea,
insomnia and diarrhea, but none of these differences were
statistically significant.
 | Table IIIUnivariate and multivariate analyses
of HRQoL subscales and single items, taking into account age,
recurrence of PC at time of questionnaires, and neoadjuvant
hormonal treatment. |
Table III
Univariate and multivariate analyses
of HRQoL subscales and single items, taking into account age,
recurrence of PC at time of questionnaires, and neoadjuvant
hormonal treatment.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Subscale | Mean (SD) | Mean
difference | 99% CI | P-value | Mean
difference | 99% CI | P-value |
|---|
| Global quality of
lifea |
| HDRBT-EBRT | 74 (20) | −3 | (−10 to 4) | | −6c | (−14 to 2) | |
| RP | 77 (22) | ref | | 0.245 | ref | | 0.053 |
| Physical
functiona |
| HDRBT-EBRT | 88 (16) | −1 | (−6 to 4) | | −3 | (−8 to 2) | |
| RP | 89 (16) | ref | | 0.61 | ref | | 0.219 |
| Role
functiona |
| HDRBT-EBRT | 89 (22) | −2 | (−9 to 5) | | −5c | (−12 to 3) | |
| RP | 91 (21) | ref | | 0.448 | ref | | 0.094 |
| Emotional
functiona |
| HDRBT-EBRT | 86 (19) | −0.3 | (−6 to 6) | | −2 | (−8 to 5) | |
| RP | 86 (19) | ref | | 0.893 | ref | | 0.459 |
| Cognitive
functiona |
| HDRBT-EBRT | 85 (15) | −3 | (−8 to 2) | | −4 | (−9 to 1) | |
| RP | 87 (16) | ref | | 0.168 | ref | | 0.06 |
| Social
functiona |
| HDRBT-EBRT | 85 (20) | −2 | (−9 to 5) | | −4 | (−11 to 4) | |
| RP | 87 (22) | ref | | 0.454 | ref | | 0.224 |
| Fatigueb |
| HDRBT-EBRT | 20 (19) | 2 | (−5 to 9) | | 5c | (−3 to 12) | |
| RP | 18 (22) | ref | | 0.446 | ref | | 0.112 |
|
Nausea/vomitingb |
| HDRBT-EBRT | 3 (7) | −0.4 | −3 to 3 | | 0.6 | −3 to 4 | |
| RP | 3 (10) | | | 0.729 | | | 0.615 |
| Painb |
| HDRBT-EBRT | 12 (21) | 2 | (−6 to 9) | | 3 | (−5 to 11) | |
| RP | 10 (23) | ref | | 0.567 | ref | | 0.357 |
| Dyspneab |
| HDRBT-EBRT | 20 (24) | 0.7 | (−8 to 9) | | 6c | (−3 to 15) | |
| RP | 19 (27) | ref | | 0.832 | ref | | 0.108 |
| Insomniab |
| HDRBT-EBRT | 23 (26) | 3 | (−6 to 12) | | 63 | (−4 to 15) | |
| RP | 20 (28) | ref | | 0.413 | ref | | 0.121 |
| Appetite
lossb |
| HDRBT-EBRT | 4 (10) | −0.3 | (−5 to 4) | | 1 | (−4 to 6) | |
| RP | 4 (14) | ref | | 0.870 | ref | | 0.556 |
|
Constipationb |
| HDRBT-EBRT | 7 (18) | 0.02 | (−6 to 6) | | 2 | (−5 to 9) | |
| RP | 7 (19) | ref | | 0.032 | ref | | 0.443 |
| Diarrheab |
| HDRBT-EBRT | 10 (19) | 5 | (−0.05 to 10) | | 7c | (1 to 12) | |
| RP | 5 (15) | ref | | 0.011 | ref | | <0.002 |
Results concerning the disease-specific and
treatment-related symptoms are presented in Table IV. Levels of bowel-related
symptoms and urinary problems were higher in the HDRBT-EBRT
patients than in the RP patients, and these results were
statistically significant but the clinical significance were small.
There was no statistically significant difference in sexual
activity between the two treatment groups, although only 37% of the
patients reported that they were sexually active and sexual
function was low (mean score 57, SD 25).
 | Table IVProstate cancer specific problems
QLQ-PR25: univariate and multivariate analyses of HRQoL subscales
EORTC QLQ-PR25, taking into account age, recurrence of PC at time
of questionnaires and neoadjuvant hormonal treatment. |
Table IV
Prostate cancer specific problems
QLQ-PR25: univariate and multivariate analyses of HRQoL subscales
EORTC QLQ-PR25, taking into account age, recurrence of PC at time
of questionnaires and neoadjuvant hormonal treatment.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Subscale | Mean (SD) | Mean
difference | 99% CI | P-value | Mean
difference | 99% CI | P-value |
|---|
| Urinary
functiona | | | | | | | |
| HDRBT-EBRT | 19 (17) | 4 | (−1 to 9) | | 6b | (0.2 to 11) | |
| RP | 15 (16) | ref | | <0.046 | ref | | 0.008 |
| Bowel
functiona | | | | | | | |
| HDRBT-EBRT | 9 (12) | 5 | (0.7 to 9) | | 5b | (1 to 10) | |
| RP | 4 (9) | ref | | 0.003 | ref | | 0.001 |
| Sexual
activitya | | | | | | | |
| HDRBT-EBRT | 30 (27) | −3 | (−13 to 7) | | −3 | (−13 to 7) | |
| RP | 33 (30) | ref | | 0.408 | ref | | 0.431 |
| Sexual
function | | | | | | | |
| HDRBT-EBRT | 60 (21) | 4 | (−11 to 18) | | −4 | (−12 to 20) | |
| RP | 56 (27) | ref | | 0.528 | ref | | 0.546 |
| Hormone
treatment-related symptomsa | | | | | | | |
| HDRBT-EBRT | 12 (10) | −0.9 | (−5 to 3) | | 1 | (−4 to 5) | |
| RP | 13 (13) | ref | | 0.557 | ref | | 0.616 |
| Incontinence
aida | | | | | | | |
| HDRBT-EBRT | 27 (38) | 11 | (−13 to 35) | | 7b | (−19 to 33) | |
| RP | 16 (25) | ref | | 0.256 | ref | | 0.491 |
|
| EORTC QLQ-PR25 item
20 | | | | | | | |
|
| Sexually
active | HDRBT-EBRT | RP | | P-value |
|
| Yes, n (%) | 32 (37) | 96 (37) | | |
| No, n (%) | 54 (63) | 165 (63) | | 0.521 |
Discussion
Clinically localized PC can be treated effectively
by use of conceptually different treatment approaches with
contrasting patterns of acute and late side-effects. Hence overall
and disease-specific HRQoL following treatment has become an
important aspect in patients with such disease. In general, the
studies published to date concern HRQoL during the first 3–5 years
after treatment. The number of longer follow-ups is limited, and,
in particular, few investigations have compared late effects of
surgery and modern dose-escalated radiotherapy.
The present cross-sectional cohort study explored
long-term HRQoL in PC patients 7 years after curative treatment by
two different methods, RP and HDRBT-EBRT. The results were compared
with age-matched HRQoL data on the normal male population in
Sweden. We found that the levels of overall quality of life were
high, irrespective of curative treatment modality. In short, the
scores were high on functional scales and low on symptom scales in
all domains, and they concurred with age-adjusted normative data.
The data from the disease-specific questionnaire revealed a small
but statistically significant difference in bowel and urinary
problems between the treatment groups, in favor of the RP
group.
The differences we observed between the study groups
agree with the findings of other investigators concerning general
HRQoL in PC patients following curative treatment (17–20).
In the disease-specific EORTC QLQ-PR25 instrument, more bowel and
urinary tract symptoms were reported by patients in the HDRBT-EBRT
group than by those in the RP group. Similar results concerning
bowel symptoms have been reported by other researchers (3,17–20).
These studies also revealed a pattern of increased urinary problems
and incontinence in RP patients, but more extensive urinary bother
(e.g., urgency and frequency) among irradiated patients, especially
those treated with permanent seeds. HRQoL data on the combined
HDRBT-EBRT treatment are limited. However, similar to our findings,
a study of HRQoL 5 years after treatment in a cohort of PC patients
given HDR brachytherapy and EBRT indicated that urinary symptoms
were more common than bowel symptoms (21). The authors suggested that the
standard strategy applied at the time of their investigation (i.e.,
assuming that a central position of the urethra represents a good
estimate of the location of urethra, when defining the treatment
dose) might have led to administration of higher doses to the
urethra than intended and hence affected the incidence of
treatment-induced chronic urethritis. A similar technique was used
in Gothenburg in the 1990s, which might explain the difference
observed in the present study. Moreover, other investigators
(19) have reported that urinary
and bowel symptoms, as well as sexual problems, are increased after
radiotherapy combined with NHT.
Only about one third of the patients in our study
were sexually active, and no differences in sexual function were
found between the treatment groups. The interpretation of the
results in this domain was limited by lack of individual baseline
data and data on the normal population. Our analyses were also
restricted by the small sample size with respect to this variable.
In a comparison of men treated with EBRT, permanent seed implants,
and RP, Litwin et al (18)
noted that the EBRT group reported superior sexual function after
24 months compared to the other groups. However, severe sexual
problems were experienced to the same extent in all groups,
regardless of treatment. Other studies of HRQoL have demonstrated a
general decrease in sexual function after 3–5 years of follow-up in
patients treated with RP and EBRT (2019).
The differences we observed between the patient
cohort and the normal population agree with the results of a 5-year
follow-up investigation of HRQoL in Swedish PC patients (20), which showed that the patients had
better physical and role function and lower levels of pain compared
to men in the normal population. An explanation for this might be
that the PC patients based their questionnaire responses on
different standards compared to the non-PC subjects, a phenomenon
that has been referred to as ‘response shift’ (22).
Limitations of the present study include the lack of
baseline HRQoL assessment and the lack of randomization to
treatment, thus, it is possible that confounding factors affected
the results. Although, the RP and the HDRBT-EBRT groups were
equivalent regarding the majority of clinical parameters previously
shown to be relevant, there was a striking difference between the
two groups concerning the PSA recurrence rate at the time of the
questionnaires (44 and 9%, respectively). A plausible explanation
for this discrepancy is that different definitions of PSA
recurrence were applied in our study depending on the treatment
modality used: for RP, PSA ≥0.2 ng/ml; for HDR-EBRT, PSA ≥2 ng/ml
above nadir. The present study was performed in the early 2000s, at
which time RP and dose-escalated radiotherapy were the standard
treatment options for localized PC. Technically, both the open RP
and the HDRBT-EBRT are still performed in manners similar to those
that were employed during the study period. Therefore, our results
can provide physicians and PC patients with valuable information
concerning long-term effects on HRQoL after treatment of PC, which
is particularly important considering that few investigations have
compared long-term HRQoL after RP and dose-escalated
radiotherapy.
The present study also had several strengths. First
of all, it was large, population based, and had a high overall
response rate (82%). Furthermore, the public health system in
Sweden offers a unique opportunity to follow patients over long
periods, and thus, potential inter-individual differences in
staging, grading, treatment procedures, and follow-up during the
study period were at a minimum in our investigation. Another
advantage is that two validated questionnaires were used in our
evaluation, one of which is disease specific, and age-adjusted
normative data were available.
In conclusion, we found that long-term HRQoL after
curative treatment of localized PC by RP or HDRBT-EBRT was high and
agreed with age-adjusted normative data. Statistically significant
differences in bowel and urinary symptoms were observed in favor of
the RP group, but the clinical significance concerning these
disparities was small.
Acknowledgements
The present study was funded by the Swedish Cancer
Society, Radiumhemmets Research Foundation, and Af Jocknick
Research Foundation. Grants for data collection were provided by
the Jubilee Clinical Research Foundation against Cancer.
References
|
1
|
Grimm P, Billiet I, Bostwick D, et al:
Comparative analysis of prostate-specific antigen free survival
outcomes for patients with low, intermediate and high risk prostate
cancer treatment by radical therapy. Results from the Prostate
Cancer Results Study Group. BJU Int. 109(Suppl 1): 22–29. 2012.
View Article : Google Scholar
|
|
2
|
Frank SJ, Pisters LL, Davis J, et al: An
assessment of quality of life following radical prostatectomy, high
dose external beam radiation therapy and brachytherapy iodine
implantation as monotherapies for localized prostate cancer. J
Urol. 178:2151–2156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei JT, Dunn RL, Sandler HM, et al:
Comprehensive comparison of healthrelated quality of life after
contemporary therapies for localized prostate cancer. J Clin Oncol.
20:557–566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Namiki S and Arai Y: Health-related
quality of life in men with localized prostate cancer (Rewiev). Int
J of Urol. 17:125–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viani GA, da Silva LG and Stefano EJ:
High-dose conformal radiotherapy reduces prostate cancer-specific
mortality: results of a meta-analysis. Int J Radiat Oncol Biol
Phys. 83:e619–e625. 2012. View Article : Google Scholar
|
|
6
|
Vordermark D, Wulf J, Markert K, et al:
3-D conformal treatment of prostatecancer to 74 Gy vs.
high-dose-rate brachytherapy boost: a cross-sectional
quality-of-life survey. Acta Oncol. 45:708–716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joseph KJ, Alvi R, Skarsgard D, et al:
Analysis of health related quality of life of patients with
clinically localized prostate cancer, one year after treatment with
external beam radiotherapy (EBRT) alone versus EBRT and high dose
rate brachytherapy. Radiat Oncol. 3:202008.PubMed/NCBI
|
|
8
|
American Joint Committee on Cancer. Manual
for Staging of Cancer. 4th edition. Lippincott Raven; Philadelphia:
1992
|
|
9
|
Grenabo L, Grundtman S and Hedelin H:
Laparoscopic obturator lymph node dissection in patients with
prostatic cancer. Scand J Urol Nephrol. 29:51–55. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lennernäs B, Rikner G, Letocha H, et al:
External beam radiotherapy of localized prostatic adenocarcinoma.
Acta Oncol. 34:953–958. 1995.PubMed/NCBI
|
|
11
|
Hugosson J, Abrahamsson PA, Ahlgren G, et
al: The risk of malignancy in the surgical margin at radical
prostatectomy reduced almost three-fold in patients given
neo-adjuvant hormone treatment. Eur Urol. 29:413–419. 1996.
|
|
12
|
Aaronson NK, Ahmedzai S, Bergman B, et al:
The European Organization for Research and Treatment of Cancer
QLQ-C30: a quality-of-life instrument for use in international
clinical trials in oncology. J Natl Cancer Inst. 85:365–376. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Andel G, Bottomley A, Fosså SD, et al:
An international field study of the EORTC QLQ-PR25: a questionnaire
for assessing the health-related quality of life of patients with
prostate cancer. Eur J Cancer. 44:2418–2424. 2008.
|
|
14
|
Fayers P, Aaronson N, Bjordahl K, et al:
The EORTC QLQ-C30 scoring Manual. 3rd edition. European
Organization for Cancer Research and Treatment of Cancer; Brussels:
2001
|
|
15
|
Michelson H, Bolund C, Nilsson B, et al:
Health-related quality of life measured by the EORTC QLQ-C30 -
reference values from a large sample of Swedish population. Acta
Oncol. 39:477–484. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osoba D, Rodrigues G, Myles J, et al:
Interpreting the significance of changes in health related
quality-of-life scores. J Clin Oncol. 16:139–144. 1998.PubMed/NCBI
|
|
17
|
Hoffman RM, Gilliland FD, Penson DF, et
al: Cross-sectional and longitudinal comparisons of health-related
quality of life between patients with prostate carcinoma and
matched controls. Cancer. 101:2011–2019. 2004. View Article : Google Scholar
|
|
18
|
Litwin MS, Gore JL, Kwan L, et al: Quality
of life after surgery, external beam irradiation, or brachytherapy
for early-stage prostate cancer. Cancer. 109:2239–2247. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanda MG, Dunn RL, Michalski J, et al:
Quality of life and satisfaction with outcome among prostate-cancer
survivors. N Engl J Med. 358:1250–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Potosky AL, Davis WW, Hoffman RM, et al:
Five-year outcomes after prostatectomy or radiotherapy for prostate
cancer: the prostate cancer outcomes study. J Natl Cancer Inst.
96:1358–1367. 2004.PubMed/NCBI
|
|
21
|
Wahlgren T, Nilsson S, Lennernäs B and
Brandberg Y: Promising long-term health related quality of life
after high-dose-rate brachytherapy boost for localized prostate
cancer. Int J Radiat Oncol Biol Phys. 69:662–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sprangers MAG and Schwartz CE: Integrating
response shift into health-related quality of life research: a
theoretical model. Soc Sci Med. 48:1507–1515. 1999. View Article : Google Scholar : PubMed/NCBI
|















