Introduction
As immunomodulatory cytokines, type 1 interferons
(IFNs), have a long history of efficacy in treating hematological
malignancies, especially in chronic myeloid leukemia (CML)
(1). Before imatinib, IFN-α based
regimens were the most important treatments for early CML patients.
Recently, IFN-α was renewed to be a vital candidate for CML
treatment. Many studies exist reporting that the combination of
IFN-α and imatinib significantly increased the rates of molecular
responses, comparing to single imatinib treatment (2–4).
Molecular mechanisms underlying this phenomenon may be the
inhibition of CML progenitors or the recruitment of quiescent CML
stem cells into cell cycle by IFN-α (2,4).
However, those mechanisms have not been clearly demonstrated. Bone
marrow mesenchymal stromal cells, which may also be defined as
mesenchymal stem cells, are important in regulating cell cycle
arrest and survival of dormant CML cells (5,6). To
ascertain the effect of IFN-α on hMSCs will provide insights into
the mechanism of action of IFN-α therapy.
Patient tolerability is the main obstacles for
long-term IFN-α treatment (2). As
one of the most serious side-effects caused by IFN-α therapy,
myelosuppression (including anemia, lymphopenia and
thrombocytopenia) is IFN-α dose-dependent (7), and the growth inhibition on bone
marrow cells induced by IFN-α is suggested to be one of the most
possible mechanisms (8,9). As a negative regulator of cell
apoptosis and cell cycle (9–11),
IFN-α was described as a potential inhibitor of hMSCs in several
studies (12). However, the exact
mechanism involved in IFN-α and hMSCs remains unclear.
Promyelocytic leukemia (PML) gene is known as a
tumor suppressor, which locates downstream of IFN-α pathway
(13,14). Multiple isoforms of PML have been
identified, and all of them are characterized by the presence of
RBCC, or the recently termed, TRIM motif. The RBCC domain mediates
the subnuclear localization and protein-protein interaction of PML
(15,16). Thus, PML protein localizes in
discrete, speckled subnuclear structures named PML-NBs, where it
co-localizes with many other proteins. Through the interaction with
other proteins, PML regulates numerous fundamental processes, such
as transcription, cell apoptosis, cell cycle regulation, senescence
and others (15,17). To our knowledge, although PML has
been extensively studied in tumor cells, little is known about PML
gene regulation in hMSCs. In our previous research, we proved that
PML stably expressed in hMSCs, which was important in maintaining
the normal proliferation capacity of hMSCs (18). However, the biological function and
regulatory mechanism of PML induced by IFN-α in hMSCs remains
undefined. In this study, we investigated the effects of IFN-α on
hMSCs and defined the role of PML involved in this process.
Materials and methods
Cell culture
The present study was approved by the Ethics
Committee of the First Affiliated Hospital, Medical School of
Zhejiang University (2012-101). Additionally, an informed consent
was obtained from each healthy donor prior to obtaining bone marrow
samples. hMSCs were isolated by density gradient centrifugation and
cultured in low-glucose Dulbecco’s modified Eagle’s medium (L-DMEM;
Corning, Danville, VA, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco, Life Technologies, Australia), which was changed
every 3 days. Passage 3–6 hMSCs were used in the following
experiments. Every experiment was repeated using 3 different hMSCs
strains isolated from 3 different randomly selected donors.
Colony formation assay
For colony formation assay in vitro, hMSCs
were plated at a density of 1,000 cells per well into a 6-well
plate. Cells were cultured in the presence of different
concentrations of IFN-α (Peprotech, Rocky Hill, NJ, USA) or PBS as
vehicle control. After 14 days, the cultures were fixed and stained
with 0.1% crystal violet for 30 min, and the number of colonies
(≥50 cells) was counted using a microscope (Nikon, Tokyo,
Japan).
Senescence associated β-galactosidase
(SA-β-gal) staining
SA-β-gal staining was performed by senescence
β-galactosidase staining kit according to the manufacturer’s
instructions (Cell Signaling Technology, Inc. Danvers, MA, USA).
Briefly, cells in different treatments were grown on 6-well culture
plates, washed, and fixed with 4% paraformaldehyde for 15 min at
room temperature. Then, cells were washed with PBS twice and
incubated with β-galactosidase staining solution for 16 h at 37°C
(pH 6.0). Image files were collected under a light microscope and
the percentage of blue cells per 400 cells was calculated using
Imagepro-Plus software (IPP, Media Cybernetics, Inc. Rockville, MD,
USA).
Reverse transcription and real-time PCR
analysis
PCR analysis was performed as described in our
previous studies. Briefly, total cellular RNA was extracted using
TRIzol reagent (Invitrogen, Life Technologies, MD, USA) and 1 μg
RNA was reverse-transcribed to cDNA using PrimeScript™ 1st Strand
cDNA Synthesis kit (Takara Bio, Dalian, China). cDNA was amplified
in a final reaction volume of 20 μl using a Takara Taq™ kit or
SYBR® Premix Ex Taq™ kit. The forward and reverse
primers for amplification of PML and β-actin are as follows: PML
forward, 5′-TGTACCGGCAGATTGTGGAT-3′; and reverse,
5′-AGATGTTGTTGGTCTTGCGG-3′. β-actin forward,
5′-AGCGAGCATCCCCCAAAGTT-3′; and reverse,
5′-GGGCACGAAGGCTCATCATT-3′.
Western blot analysis
hMSCs proteins were lysed with
radioimmunoprecipitation assay (RIPA) buffer and proteinase
inhibitors (both from Beyotime, Jiangsu, China). Equal amounts of
cell proteins were subjected to 10% SDS-polyacrylamide gel
electrophoresis and electrotransferred onto polyvinylidene
difluoride (PVDF) membranes, which were blocked with 5%
phosphatase-free powdered milk in Tris-buffered saline with 0.1%
Tween (TBST) for 2 h. Then, the membranes were incubated overnight
at 4°C with primary antibodies consisting of PML, P21, P53 (all
from Abcam, Hong Kong, China) and β-actin (Cell Signaling
Technology, Inc.). The membrane was then rinsed with TBST and
incubated with an IRDye secondary antibody (LI-COR Biosciences,
Lincoln, NE, USA) for 1.5 h at room temperature. The membranes were
visualized using Odyssey infrared imaging system (LI-COR
Biosciences).
Immunofluorescence
Cells were cultured in 12-well-plates and treated at
different conditions. After the treatment, cells were fixed with 4%
paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100
for 10 min, and then blocked for 1 h in 5% BSA at room temperature.
Primary antibodies diluted 1:500 were incubated overnight at 4°C.
After incubation with the secondary antibodies (1:500) for 1 h at
room temperature, cells were counterstained with DAPI to visualize
cell nuclei. The primary antibodies used include rabitt monoclonal
anti-PML and mouse or rabbit monoclonal anti-P53 (Abcam). The
secondary antibody was Alexa fluor-488 conjugated goat anti-mouse
and Alexa fluor-555 conjugated goat anti-rabbit (Invitrogen, Life
Technologies). Cells were examined under flurescence microscope and
images were collected (Nikon).
Flow cytometry analysis of cell
apoptosis
After the treatments, the adherent and supernatant
cells were harvested. Cells were washed with phosphate-buffered
saline (PBS) and re-suspended in 500 μl of binding buffer, then
incubated with 5 μl of Annexin V-PE and 5 μl of 7-amino-actinomycin
D (7-AAD) solution for 15 min in the dark at room temperature (both
from BD Biosciences, San Jose, CA, USA). Cell apoptosis were then
analyzed by FC500 Flow Cytometer (Beckman Coulter, Inc. Brea, CA,
USA).
Gene transfection
Infections of hMSCs were performed by lentiviruses.
As described previously, recombinant lentiviral vector
plenti6.3-PML-EGFP which encodes the full length human PML-cDNA
(NM_002675) and the enhanced green fluorescent protein (EGFP) was
synthesized by Life Technologies (Shanghai, China). The empty
vector, plenti6.3-EGFP, was used as control. Short hairpin RNAs
(shRNA) against PML in a lentiviral vector with green fluorescent
protein (GFP) and control vector were designed and synthesized by
GenePharma Inc. (Shanghai, China). Packaging vectors used in our
experiments was pLP1, pLP2, and pLP/VSVG (Invitrogen, Life
Technologies). Human embryonic kidney 293T cells were used as
packaging cells. High-titer lentivirus was produced in 293T and was
used to infect hMSCs which had reached 50–60% confluence. After
transfection of 72 h, the efficiency was estimated by evaluation of
GFP expression via fluorescence microscopy, and PML gene expression
was verified by quantitative real-time PCR and western blot
analysis. The DNA sequence of the PML shRNA was
5′-TGGAGGAGGAGTTCCAGTTTCTTTCAAGAGAAGAAACTGGAACTCCTCCTCCTTTTTTC-3′
and
5′-TCGAGAAAAAAGGAGGAGGAGTTCCAGTTTCTTCTCTTGAAAGAAACTGGAACTCCTCCTCCA-3′.
The control shRNA was
5′-TGTTCTCCGAACGTGTCACGTTTCAAGAGAACATGACACGTTCGGAGAACTTTTTTC-3′ and
5′-TCGAGAAAAAAGTTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACA-3′.
Statistical analysis
All statistical analyses were performed using SPSS
software (SPSS, Inc., Chicago, IL, USA) and all data were expressed
as means ± standard deviation (SD). Differences between groups were
evaluated using one-way analyses of variance or non-parametric
tests. A P-value <0.05 was considered statistically
significant.
Results
IFN-α induces cellular senescence of
hMSCs in a dose-dependent manner
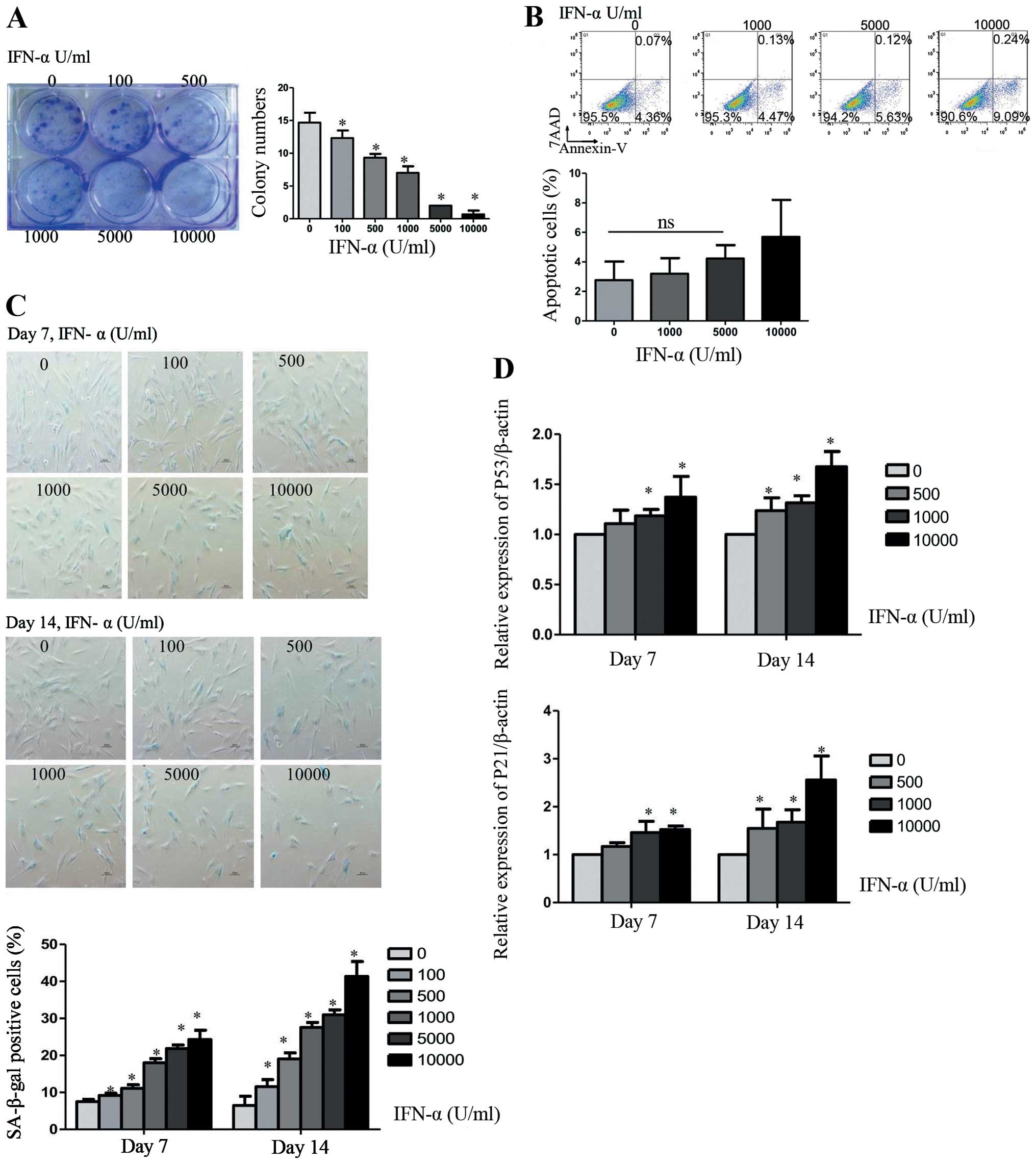
IFN-α is an important inhibitor of tumor cells, thus
we investigated the biological function of IFN-α in hMSCs. Cells
were treated with 0, 100, 500, 1,000, 5,000, or 10,000 U/ml IFN-α
every 3 days up to 14 days. Detected by colony formation assay,
IFN-α treated cell stopped growing, leading to a dramatically
decreased number of colonies in a dose-dependent manner (Fig. 1A). The number of colonies was
reduced to nearly half when IFN-α was 1,000 U/ml as compared with
no IFN-α. However, IFN-α did not induce significant cell apoptosis.
We did not find obvious difference in the percentage of apoptotic
cells when hMSCs were treated with IFN-α at 0, 1,000 and 5,000 U/ml
for 14 days (Fig. 1B). Apoptotic
cells showed a minor increase when the concentration of IFN-α
reached 10,000 U/ml. These results suggest that cell apoptosis was
not the main reason for the inhibition of IFN-α. Then we examine
whether the IFN-α induced inhibition of cell proliferation resulted
from cellular senescence, a variety of senescence-associated
detection was measured. The cellular senescence was confirmed by an
increase in SA-β-gal-positive cells in IFN-α treated as compared to
the untreated cells (Fig. 1C). The
hMSC senescence induced by IFN-α was dose- and time-dependent.
After treated with IFN-α at 1,000 U/ml for 7 or 14 days, we found
that up to 18±1.1 or 27.56±1.33% of hMSCs became SA-β-gal-positive
as compared with 7.53±0.55 or 6.47±2.5% of untreated cells.
Real-time PCR analysis proved this process by an increase in
production of the senescence marker p53 and p21 (Fig. 1D).
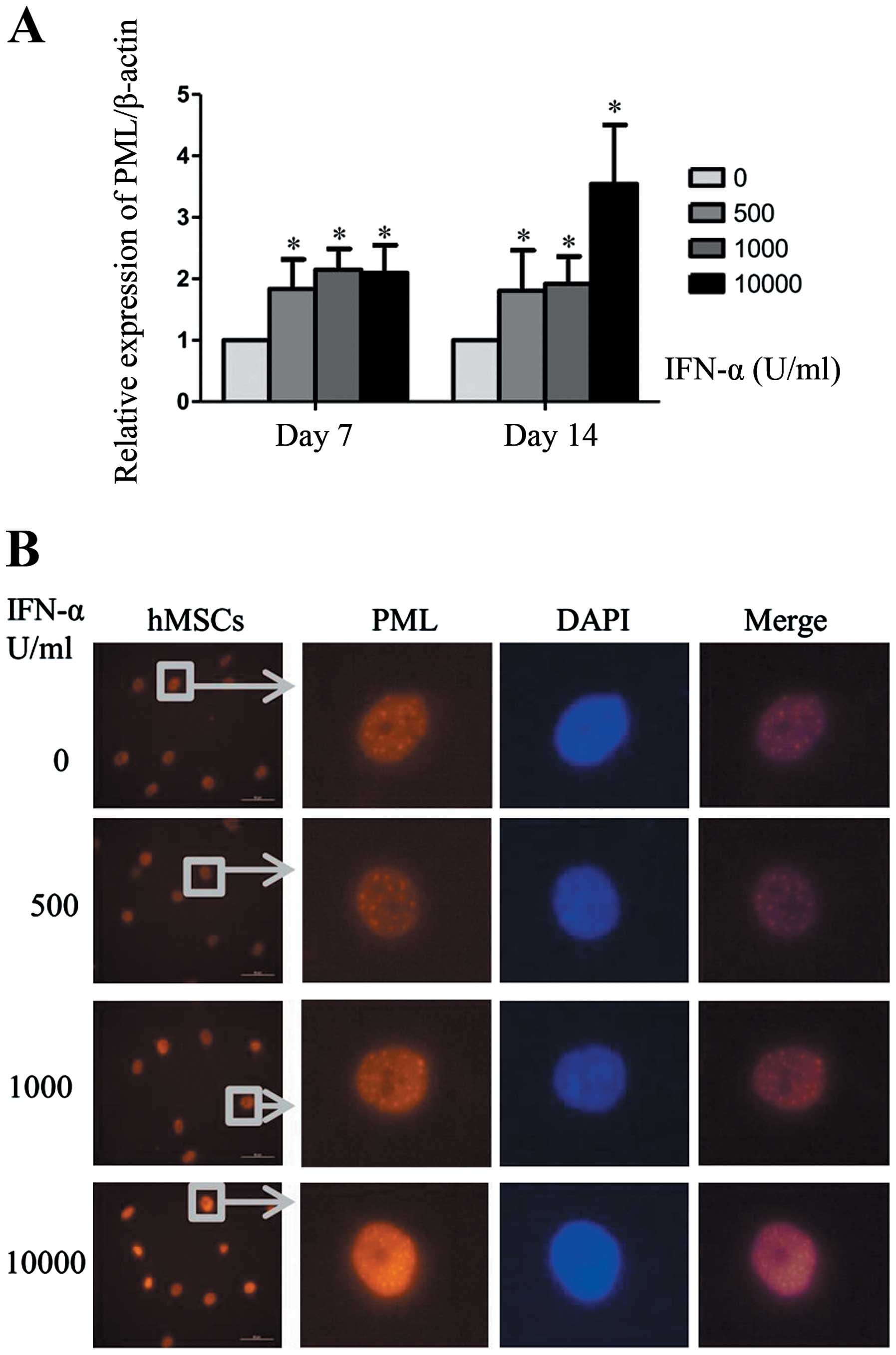
PML expression is upregulated in IFN-α
induced senescence of hMSCs
PML is one of the most interesting upregulated genes
induced by IFN-α, and PML gene has been proved to be associated
with cellular senescence in other cell types. The present study was
undertaken to evaluate the role of PML in IFN-α induced senescence
of hMSCs. Consistent with other studies, mRNA expression of PML can
be upregulated by IFN-α in hMSCs (Fig.
2A). When cells were treated with IFN-α at 1,000 U/ml for 7 or
14 days, PML gene expression in hMSCs was increased by >2-fold.
Then we analyzed PML protein level by western blot analysis.
However, we did not observed significant changes of PML protein
during the process of IFN-α treatment. The possible reason may be
that western blot analysis was not sufficiently sensitive to detect
PML proteins which were mainly distributed in the cell nucleus.
Immunofluorescence can reflect the changes of nuclear protein
expression more directly. As shown in Fig. 2B, when hMSCs were treated with
IFN-α for 7 days, both the number and size of PML-NBs were
increased markedly in a concentration-dependent manner. These
results indicate that PML protein was upregulated by IFN-α in
hMSCs.
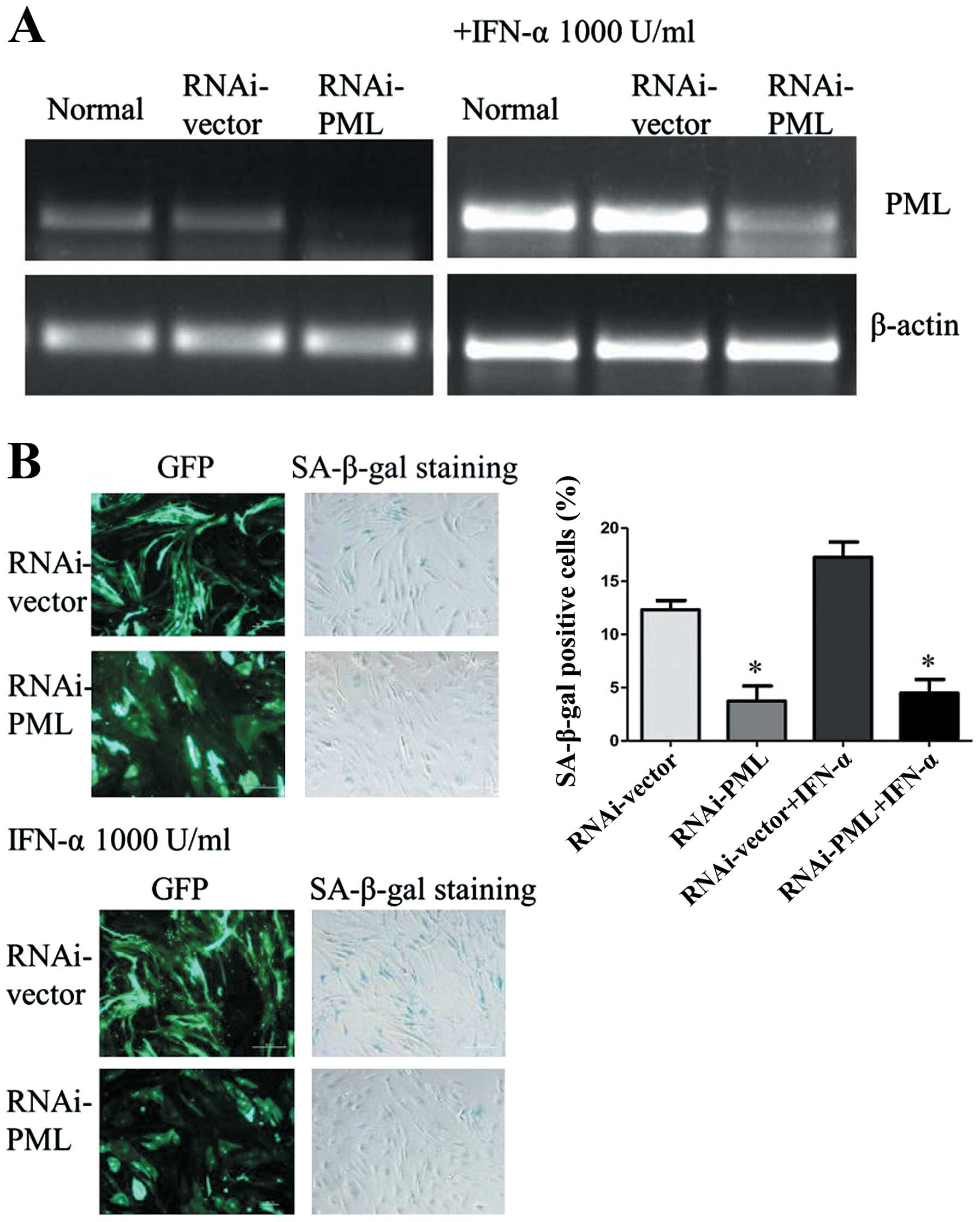
PML plays a key role in IFN-α induced
cellular senescence of hMSCs
In order to investigate the role of PML in IFN-α
induced cellular senescence of hMSCs, PML expression was inhibited
using an RNAi-mediated PML knockdown system. As shown in Fig. 3A, PML gene significantly
downregulated in hMSCs, as compared with normal and cells
transfected with empty plasmid. Even though hMSCs were treated with
IFN-α at 1,000 U/ml for 7 days, PML gene showed no obvious
upregulation in PML knockdown cells. Then the expression of
SA-β-gal was analyzed. Compared with normal, cells transduced with
lentivirus became easier senescent. However, there were a lower
percentage of SA-β-gal-positive cells in PML knockdown hMSCs than
the control RNAi (3.74±1.42 vs. 12.32±0.87%, P<0.05). After
treated with IFN-α at 1,000 U/ml for 7 days, hMSCs senescence can
be rescued by the knockdown of PML. The percentage of
SA-β-gal-positive cells in PML knockdown hMSCs showed a significant
decrease as compared with cells transfected with control-shRNA
(4.49 ±1.27 vs. 17.26±1.44%, P<0.05) (Fig. 3B). Therefore, these results
suggested that PML may associate with IFN-α induced cellular
senescence.
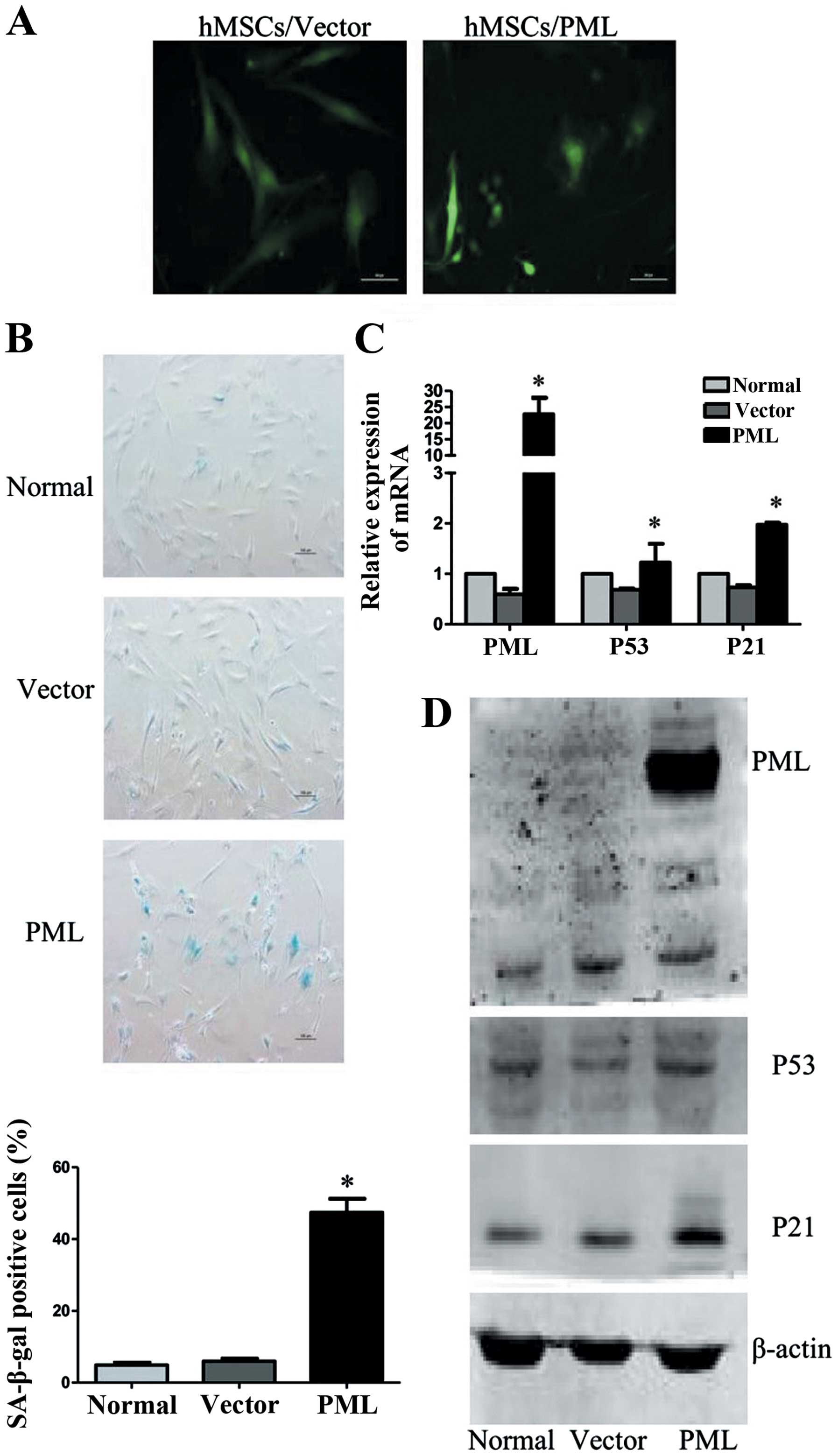
Effect of PML on cellular senescence in
hMSCs
To further characterize the effect of PML on
cellular senescence in hMSCs, PML-overexpressed hMSCs were
analyzed, while normal and empty vector transfected cells were used
as controls. As shown in Fig. 4A,
PML-overexpressing hMSCs remained viable for two weeks. However,
these cells became flat and enlarged. Detected 7 days
post-transfection, cells were strongly positive for SA-β-gal
activity (47.43±3.8%), as compared with normal and empty vector
transfected cells (4.9±0.7 and 5.97±0.75%). As important senescence
markers, mRNA levels of P53 and P21 were also enhanced in
PML-overexpressed hMSCs. The upregulation of P21 protein was
confirmed by western blot analysis. However, we did not observe
obvious changes of P53 protein expression. These results
collectively proved that PML alone is sufficient to promote
cellular senescence of hMSCs.
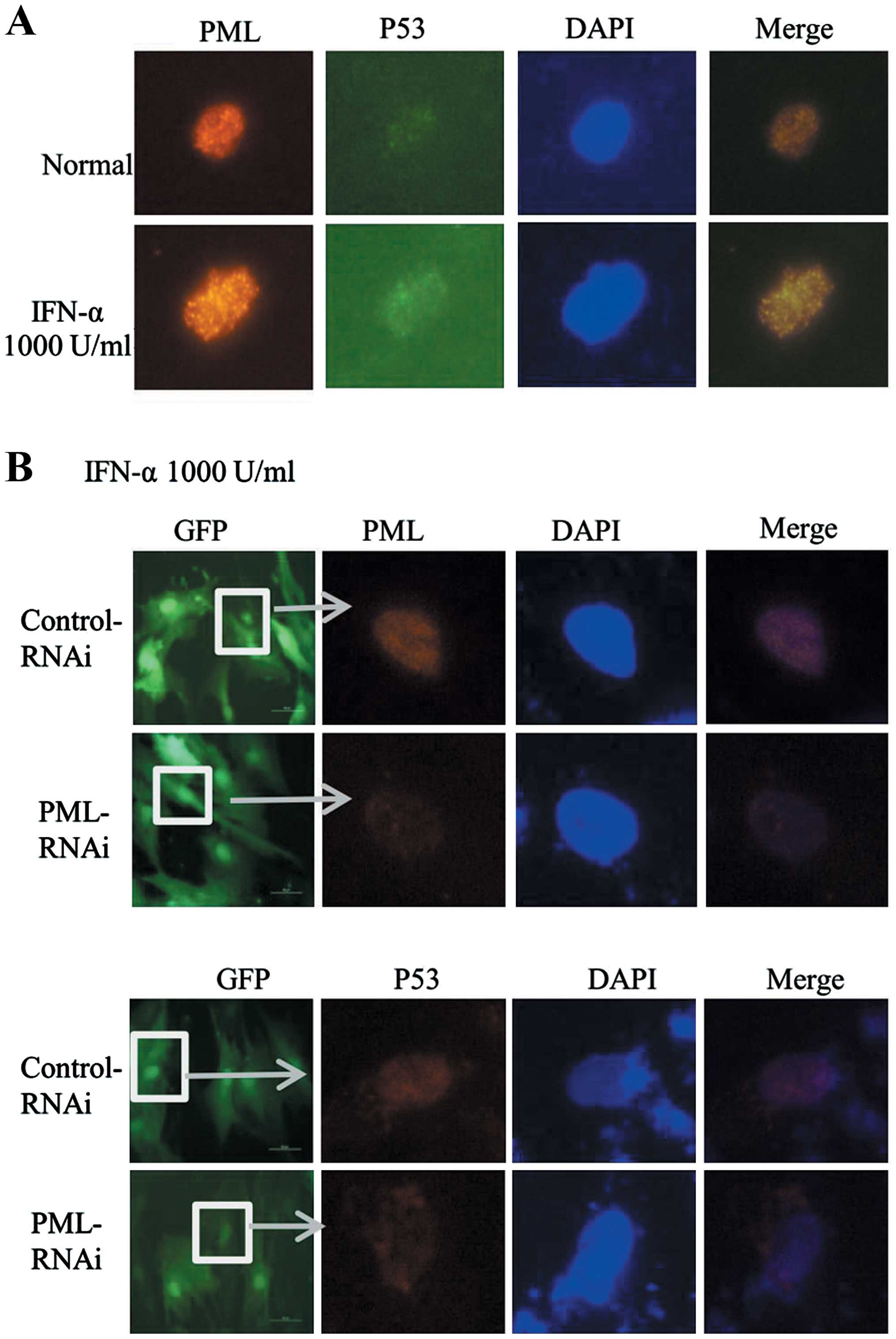
IFN-α promotes the co-localization of P53
and PML in hMSCs
P21 as an important downstream effector of P53 was
enhanced in PML-transfected hMSCs. Then, we wondered whether
upregulation of PML induced by IFN-α has a relationship with P53
pathway in hMSCs. Previous studies have proved that P53 can be
recruited to the PML-NBs, leading to enhancement of its
transcriptional activity. In the process of IFN-α induced hMSCs
senescence, the co-localization of PML and P53 was observed by
immunofluorescence assay. As shown in Fig. 5A, an increasing co-localization of
PML (red) and P53 (green) was observed in IFN-α treated cells
(1,000 U/ml, 7 days) as compared with untreated cells. To further
confirm whether or not the change of P53 location was mediated
through the upregulation of PML, we knocked down the expression of
PML in hMSCs. Then, cells were treated with IFN-α (1,000 U/ml, 7
days), however, we did not find significant location of P53 (red)
in PML knockdown cells as compared with control (Fig. 5B). Taken together, these results
delineate that upregulated expression of PML induced by IFN-α can
recruit P53 to PML-NBs, and to participate in the process of
promoting cell senescence.
Discussion
IFN-α has been used widely in treatment of many
diseases. Recent developments have renewed interest in IFN-α for
CML therapy (4). However the
precise mechanism remains unclear. In the present study, we
investigated this problem starting from hMSCs. Our study showed
that IFN-α induced cellular senescence of hMSCs in a dose-dependent
manner and PML was involved in this process.
Type 1 interferons have been described as negative
regulator of the cell cycle and potent proliferation inhibitor of
several cells, such as human uterine cells, and osteoprogenitor
cells (8–10). We also found that IFN-α can
significantly inhibit the proliferation of hMSCs in a
concentration-dependent manner. In general, the inhibition of cell
proliferation is mostly associated with cell cycle arrest and cell
apoptosis. However, when hMSCs were treated with IFN-α, cell
apoptosis was not obvious until IFN-α concentration of 10,000 U/ml.
Therefore, cell apoptosis was not the main reason for the
inhibition effect of IFN-α on hMSCs. The cell cycle was evaluated
by flow cytometry, but we did not find significant cell cycle
changes in hMSCs (data not shown). The possible mechanism may be
that the major proportion of hMSCs was in G0/G1 phase under normal
culture (19) and it is difficult
to detect cell cycle arrest in hMSCs. So, as another important form
of proliferative potential inhibition in vitro, cellular
senescence was assessed.
Cellular senescence is a program activated by normal
cells in response to various types of stress, such as shortening of
telomere, DNA damage, oxidative stress and others (20,21).
Once cells have entered senescence, they cease to divide and
undergo a series of morphologic and metabolic changes (22,23).
In previous research, IFN pathway involved in regulating cellular
senescence has been proved in endothelial cells and fibroblasts
(24,25). In our research, hMSC senescence
induced by IFN-α was measured by a variety of senescence-associated
detection, and it was found that IFN-α can induce cellular
senescence of hMSCs in a dose-dependent manner. In vitro,
1,000–1,500 U/ml was considered the effective concentration of
IFN-α (26). The hMSCs studied
here exhibited an increased senescence phenotype and upregulation
of P53 and P21 expression upon treatment with IFN-α at 1,000 U/ml
for 7 or 14 days. Then, we wonder which downstream gene of IFN
pathway might participate in regulating cellular senescence of
hMSCs.
PML, which is known as a tumor suppressor, can be
directly induced by IFN-α (13).
Previously, PML attracted our interest due to its role in
regulating stem cells function. We have proved that PML is stably
expressed in hMSCs and plays a vital role in maintaining the normal
proliferation capacity of hMSCs. At the same time, several studies
have proven that PML is an important regulator in cellular
senescence (27,28). Therefore, the role of PML in IFN-α
induced cellular senescence was studied.
Our results here showed that PML expression was
upregulated in IFN-α treated hMSCs, and was
concentration-dependent. To better understand the role of PML in
senescence of hMSCs, we changed the expression of PML in hMSCs.
When PML was downregulated, cellular senescence induced by IFN-α
can be inhibited. While PML was overexpressed, hMSCs showed changes
in morphology, and were strongly positive for SA-β-gal activity.
Our conclusion was that PML was required for the IFN-α induced
cellular senescence of hMSCs.
P53 is a key regulator of the senescence response
and PML can regulate P53 activity via direct interaction (29–31).
We did not find significant changes of P53 protein expression.
However, PML overexpression induced upregulation of p21, a
transcriptional target of p53. Then we considered whether the
activity, but not the expression of P53, was changed in this
process.
In normal cells, P53 protein is expressed at low
levels and has a short half-life due to rapid turnover mediated by
ubiquitination or proteolysis (32,33).
Phosphorylation and acetylation was the most important
post-translation modification form of P53 and determined the
activity and function of P53 (34). PML-NBs play an important role in
this process. P53 can be recruited to the PML-NBs, leading to
enhancement of its transcriptional activity (30,35).
In the process of IFN-α induced hMSCs senescence, we found
increased colocalization of PML and P53. Treatment of IFN-α in the
PML-knockdown cells, showed no significant changes of P53 location
as compared with control. These results indicated that upregulated
expression of PML induced by IFN-α can recruit P53 to PML-NBs, and
may thus participate in the process of promoting cell
senescence.
In conclusion, our present study showed the effect
of IFN-α on cellular senescence of hMSCs, and revealed a previously
unknown role of PML in this process. Briefly, IFN-α can induce
cellular senescence of hMSCs in a dose-dependent manner, which is
accompanied by an upregulation of PML. Downregulation of PML can
rescue cellular senescence induced by IFN-α in hMSCs. Upregulation
of PML alone can also promote cellular senescence. One of the
mechanisms may be that the co-localization of P53 and PML was
increased in IFN-α treated hMSCs. However, the interconnections of
PML and P53 in hMSCs need further investigation. Our results offer
new insights into the role of IFN-α in hMSCs and may translate into
a clinical strategy for wider use of IFN-α with fewer adverse
effects.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (81100364).
References
|
1
|
Borden EC, Sen GC, Uze G, et al:
Interferons at age 50: past, current and future impact on
biomedicine. Nat Rev Drug Discov. 6:975–990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Preudhomme C, Guilhot J, Nicolini FE, et
al: Imatinib plus peginterferon alfa-2a in chronic myeloid
leukemia. N Engl J Med. 363:2511–2521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simonsson B, Gedde-Dahl T, Markevarn B, et
al: Combination of pegylated IFN-alpha2b with imatinib increases
molecular response rates in patients with low- or intermediate-risk
chronic myeloid leukemia. Blood. 118:3228–3235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Talpaz M, Hehlmann R, Quintas-Cardama A,
Mercer J and Cortes J: Re-emergence of interferon-alpha in the
treatment of chronic myeloid leukemia. Leukemia. 27:803–812. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin LH, Tabe Y, Konoplev S, et al: CXCR4
up-regulation by imatinib induces chronic myelogenous leukemia
(CML) cell migration to bone marrow stroma and promotes survival of
quiescent CML cells. Mol Cancer Ther. 7:48–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang B, Li M, McDonald T, et al:
Microenvironmental protection of CML stem and progenitor cells from
tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin
signaling. Blood. 121:1824–1838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plimack ER, Desai JR, Issa JP, et al: A
phase I study of decitabine with pegylated interferon alpha-2b in
advanced melanoma: impact on DNA methylation and lymphocyte
populations. Invest New Drugs. 32:969–975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abukawa H, Kaban LB, Williams WB, Terada
S, Vacanti JP and Troulis MJ: Effect of interferon-alpha-2b on
porcine mesenchymal stem cells. J Oral Maxillofac Surg.
64:1214–1220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oreffo RO, Romberg S, Virdi AS, Joyner CJ,
Berven S and Triffitt JT: Effects of interferon alpha on human
osteoprogenitor cell growth and differentiation in vitro. J Cell
Biochem. 74:372–385. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee BS, Stewart EA, Sahakian M and Nowak
RA: Interferon-alpha is a potent inhibitor of basic fibroblast
growth factor-stimulated cell proliferation in human uterine cells.
Am J Reprod Immunol. 40:19–25. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leaman DW, Chawla-Sarkar M, Jacobs B, et
al: Novel growth and death related interferon-stimulated genes
(ISGs) in melanoma: greater potency of IFN-beta compared with
IFN-alpha2. J Interferon Cytokine Res. 23:745–756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hatzfeld A, Eid P, Peiffer I, et al: A
sub-population of high proliferative potential-quiescent human
mesenchymal stem cells is under the reversible control of
interferon α/β. Leukemia. 21:714–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crowder C, Dahle O, Davis RE, Gabrielsen
OS and Rudikoff S: PML mediates IFN-alpha-induced apoptosis in
myeloma by regulating TRAIL induction. Blood. 105:1280–1287. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Everett RD and Chelbi-Alix MK: PML and PML
nuclear bodies: implications in antiviral defence. Biochimie.
89:819–830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou W and Bao S: PML-mediated signaling
and its role in cancer stem cells. Oncogene. 33:1475–1484. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salomoni P: Stemming out of a new PML era?
Cell Death Differ. 16:1083–1092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salomoni P, Dvorkina M and Michod D: Role
of the promyelocytic leukaemia protein in cell death regulation.
Cell Death Dis. 3:e2472012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J, Fu S, Zhong W and Huang H: PML
overexpression inhibits proliferation and promotes the osteogenic
differentiation of human mesenchymal stem cells. Oncol Rep.
30:2785–2794. 2013.PubMed/NCBI
|
|
19
|
Conget PA and Minguell JJ: Phenotypical
and functional properties of human bone marrow mesenchymal
progenitor cells. J Cell Physiol. 181:67–73. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sperka T, Wang J and Rudolph KL: DNA
damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol
Cell Biol. 13:579–590. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ben-Porath I and Weinberg RA: When cells
get stressed: an integrative view of cellular senescence. J Clin
Invest. 113:8–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campisi J: Aging, cellular senescence, and
cancer. Annu Rev Physiol. 75:685–705. 2013. View Article : Google Scholar
|
|
23
|
Sikora E, Arendt T, Bennett M and Narita
M: Impact of cellular senescence signature on ageing research.
Ageing Res Rev. 10:146–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Upreti M, Koonce NA, Hennings L, Chambers
TC and Griffin RJ: Pegylated IFN-alpha sensitizes melanoma cells to
chemotherapy and causes premature senescence in endothelial cells
by IRF-1 mediated signaling. Cell Death Dis. 1:e672010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Q, Tang L, Roberts PC, et al:
Interferon regulatory factors IRF5 and IRF7 inhibit growth and
induce senescence in immortal Li-Fraumeni fibroblasts. Mol Cancer
Res. 6:770–784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burchert A, Wolfl S, Schmidt M, et al:
Interferon-alpha, but not the ABL-kinase inhibitor imatinib
(STI571), induces expression of myeloblastin and a specific T-cell
response in chronic myeloid leukemia. Blood. 101:259–264. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bischof O, Kirsh O, Pearson M, Itahana K,
Pelicci PG and Dejean A: Deconstructing PML-induced premature
senescence. EMBO J. 21:3358–3369. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vernier M, Bourdeau V, Gaumont-Leclerc MF,
et al: Regulation of E2Fs and senescence by PML nuclear bodies.
Genes Dev. 25:41–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kruse JP and Gu W: Modes of p53
regulation. Cell. 137:609–622. 2009. View Article : Google Scholar
|
|
30
|
Sung KS, Lee YA, Kim ET, Lee SR, Ahn JH
and Choi CY: Role of the SUMO-interacting motif in HIPK2 targeting
to the PML nuclear bodies and regulation of p53. Exp Cell Res.
317:1060–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue Y, Li L, Zhang D, et al: Telomerase
suppression initiates PML-dependent p53 activation to inhibit
bladder cancer cell growth. Oncol Rep. 24:1551–1559.
2010.PubMed/NCBI
|
|
32
|
Midgley CA and Lane DP: p53 protein
stability in tumour cells is not determined by mutation but is
dependent on Mdm2 binding. Oncogene. 15:1179–1189. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Michael D and Oren M: The p53-Mdm2 module
and the ubiquitin system. Semin Cancer Biol. 13:49–58. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dai C and Gu W: p53 post-translational
modification: deregulated in tumorigenesis. Trends Mol Med.
16:528–536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carracedo A, Ito K and Pandolfi PP: The
nuclear bodies inside out: PML conquers the cytoplasm. Curr Opin
Cell Biol. 23:360–366. 2011. View Article : Google Scholar : PubMed/NCBI
|