Introduction
Breast cancer is the most common cancer among women
with a relatively high incidence of 20% of all malignancies and
remains one of the leading causes of cancer-related death worldwide
(1). Although chemotherapy has
improved outcomes for patients, the marginal benefits achieved with
cytotoxic agents seem to have reached a plateau (2,3).
Recently, preventive agents and targeted therapies directed at the
estrogen receptor, progesterone receptor, and human epidermal
growth factor 2 receptor have resulted in improved clinical
outcomes for many women with breast cancer (3). However, further challenges remain in
treating tumors that do not express these molecular targets or
tumor cells that become resistant for these molecular targets.
Therefore, the development of new therapeutic agents or new
combination therapy for these clinically intractable tumors is
still highly desirable.
Arsenic trioxide (ATO) is an approved treatment for
acute promyelocytic leukemia (APL). ATO induces differentiation at
lower concentrations and induces apoptosis at higher concentrations
in APL cells (4). It is now well
established that ATO induces complete remission in 80–90% of newly
diagnosed patients with APL, as well as in 60–90% of
all-trans retinoic acid refractory patients (5–7).
Furthermore, the anticancer activity of ATO was also intensively
studied in various other hematological malignancies and several
solid tumors, including breast cancer (8–13).
Although ATO is very effective in the treatment of APL, ATO has
been less successful in other malignancies at tolerable doses. The
doses of ATO required to exert detectable anticancer effects in
solid tumors are much higher than those required to inhibit
hematological malignancies (14–16).
Combination therapy is a frequently used method in clinical
practice to improve the therapeutic effect and reduce the toxicity
of anticancer drugs (17,18). Therefore, new strategies are
essential to enhance the efficacy of ATO, while reducing its dose
in order to avoid severe side-effects.
We have examined whether inducers of differentiation
in leukemia cells can control the growth of solid tumors. Cotylenin
A (CN-A), which is a fucicoccan-diterpene glycoside with a complex
sugar moiety, was originally isolated as a plant growth regulator
and has been shown to affect several physiological processes in
higher plants (19). We previously
reported that CN-A has a potent differentiation-inducing activity
in several human and murine myeloid leukemia cell lines and in
leukemia cells that were freshly isolated from patients with acute
myeloid leukemia (20–23). We previously found that treatment
with CN-A plus rapamycin, which also has a potent
differentiation-inducing activity in myeloid leukemia cells
(24), effectively inhibited the
proliferation of human breast cancer cell line MCF-7 cells
(13,25,26).
In the present study, we found that a new combination treatment
with CN-A and low doses of ATO showed marked anti-proliferative and
anti-metastatic effects in human breast cancer cells.
Materials and methods
Cell culture
Human breast cancer cell lines (MCF-7, MDA-MB-231
and T47D) and human promyelocytic leukemia cell line HL-60 cultured
in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) at 37°C
in a humidified atmosphere of 5% carbon dioxide in air.
Materials
ATO, nitroblue tetrazolium (NBT),
12-O-tetradecanoylphorbol-13-acetate (TPA) and
N-acetyl-L-cysteine (NAC) were purchased from Sigma-Aldrich (St.
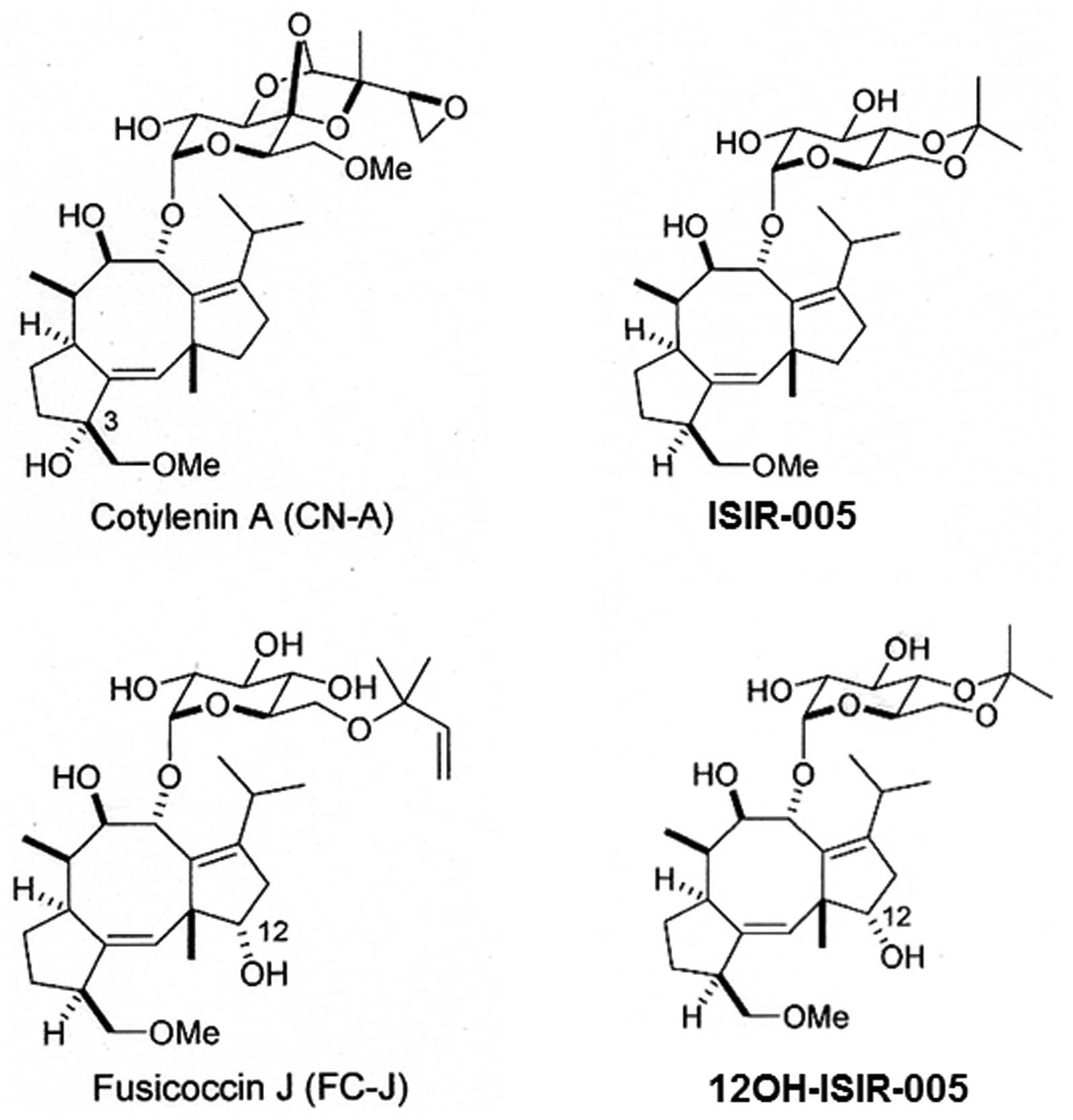
Louis, MO, USA). CN-A, ISIR-005, 12OH-ISIR-005 and Fusicoccin J
(FC-J) were prepared as previously described (19,27).
The structures of CN-A, ISIR-005, 12OH-ISIR-005 and FC-J are shown
in Fig. 1. YM155 was obtained from
Selleckchem (Houston, TX, USA). Methyl cellulose was purchased from
Wako Pure Chemical Industries (Osaka, Japan). Human apoptosis array
kit and anti-p27 antibody were obtained from R&D Systems
(Minneapolis, MN, USA). Anti-caspase-7, anti-survivin, anti-p21 and
anti-XIAP antibodies were purchased from Cell Signaling Technology
(Danvers, MA, USA). Anti-α-tubulin antibody was obtained from Santa
Cruz Biotechnology (Santa Cruz, CA, USA).
Assay of cell differentiation for
leukemia cells
NBT reduction was assayed colorimetrically as
previously described (28).
Briefly, HL-60 cells were incubated in 1 ml of serum-free medium
containing 1 mg/ml NBT and 100 ng/ml TPA at 37°C for 30 min. The
reaction was stopped by adding HCl. Formazan deposites were
solubilized in DMSO, and the absorption of the formazan solution at
560 nm was measured in a spectrophotometer.
Assay of cell growth
Cells were seeded at 1–3×104 cells/ml in
a 24-well multidish. After culture with or without test compounds
for the indicated times, viable cells were examined by a modified
MTT (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide)
assay as previously reported (25).
Assay of anchorage-independent
growth
MCF-7 cells (2×103 cells/well) and
MDA-MB-231 cells (4×103 cells/well) were plated in
RPMI-1640 supplemented with 10% fetal bovine serum and 1.0%
methylcellulose in a 24-well ultra-low attachment multidish
(Corning Inc., Corning, NY, USA). Colonies containing 10 or more
cells were counted 12 days after seeding.
Western blot analysis
Cells were packed after washing with cold PBS and
then lysed at a concentration of 1×107 cells/ml in lysis
buffer CelLytic™ M (Sigma-Aldrich) supplemented with a proteinase
inhibitor cocktail and phosphatase inhibitor cocktail 1/2
(Sigma-Aldrich). Equal amounts of protein were separated on 5–20%
SDS-polyacrylamide gels (Wako Pure Chemical Industries). Proteins
were electrophoresed on gels and transferred to an Immobilon-P
membrane (Millipore, Bedford, MA, USA) using the primary
antibodies. An anti-rabbit or anti-mouse IgG HRP-linked antibody
(Cell Signaling Technology) was used as a secondary antibody
(1:2,000 dilution). Bands were identified by treatment with
Immune-Star™ HRP chemiluminescence (Bio-Rad Laboratories, Hercules,
CA, USA) for 5 min at room temperature and detected using a Fuji
Lumino Image Analyser LAS-4000 system (Fuji Film Co., Ltd., Tokyo,
Japan) (28). All western blots
shown are representative of at least 3 independent experiments.
Apoptosis array
Cells were plated in 100-mm plastic dishes at a
density of 4×104 cells/ml and incubated with CN-A (3.5
μg/ml) and ATO (4 μM) at 37°C for 96 h. The cells were washed with
PBS twice and solubilized at 1×107 cells/ml in lysis
buffer (Human Apoptosis Array kit). The lysates were resuspended
and rocked gently at 2–8°C for 30 min. After centrifuging, the
supernatant was transferred into a clean tube. Then, 400 μg of
total protein was used for the Human Apoptosis Array kit according
to the manufacturer’s protocol as previously described (29).
In vitro cell invasion assay
Cell invasion activity was measured by real-time
monitoring of cell invasion using xCELLigence Real-Time Cell
Analyzer (Roche-Diagnostics Japan, Tokyo, Japan). For continuous
monitoring of cell invasion 5×104 cells were seeded in a
5% (v/v) Matrigel-coated CIM-Plate 16 with 10% serum serving as the
chemoattractant in the lower chamber according to the
manufacturer’s protocol.
Results
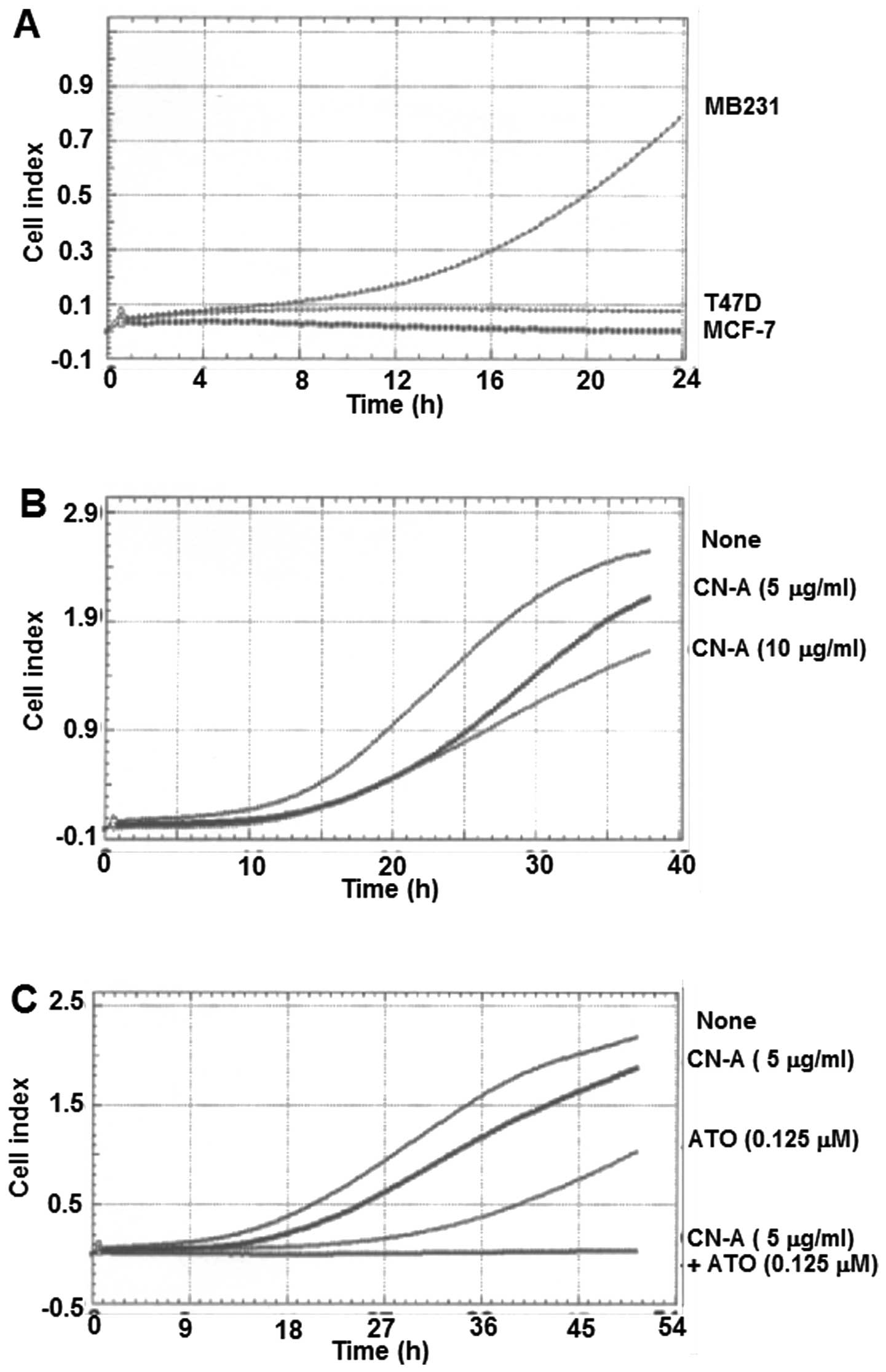
CN-A sensitizes ATO-induced growth
inhibition of human breast cancer MCF-7 and MDA-MB-231 cells
We and others have reported that CN-A and ATO (at
low doses) alone could induce the differentiation of human myeloid
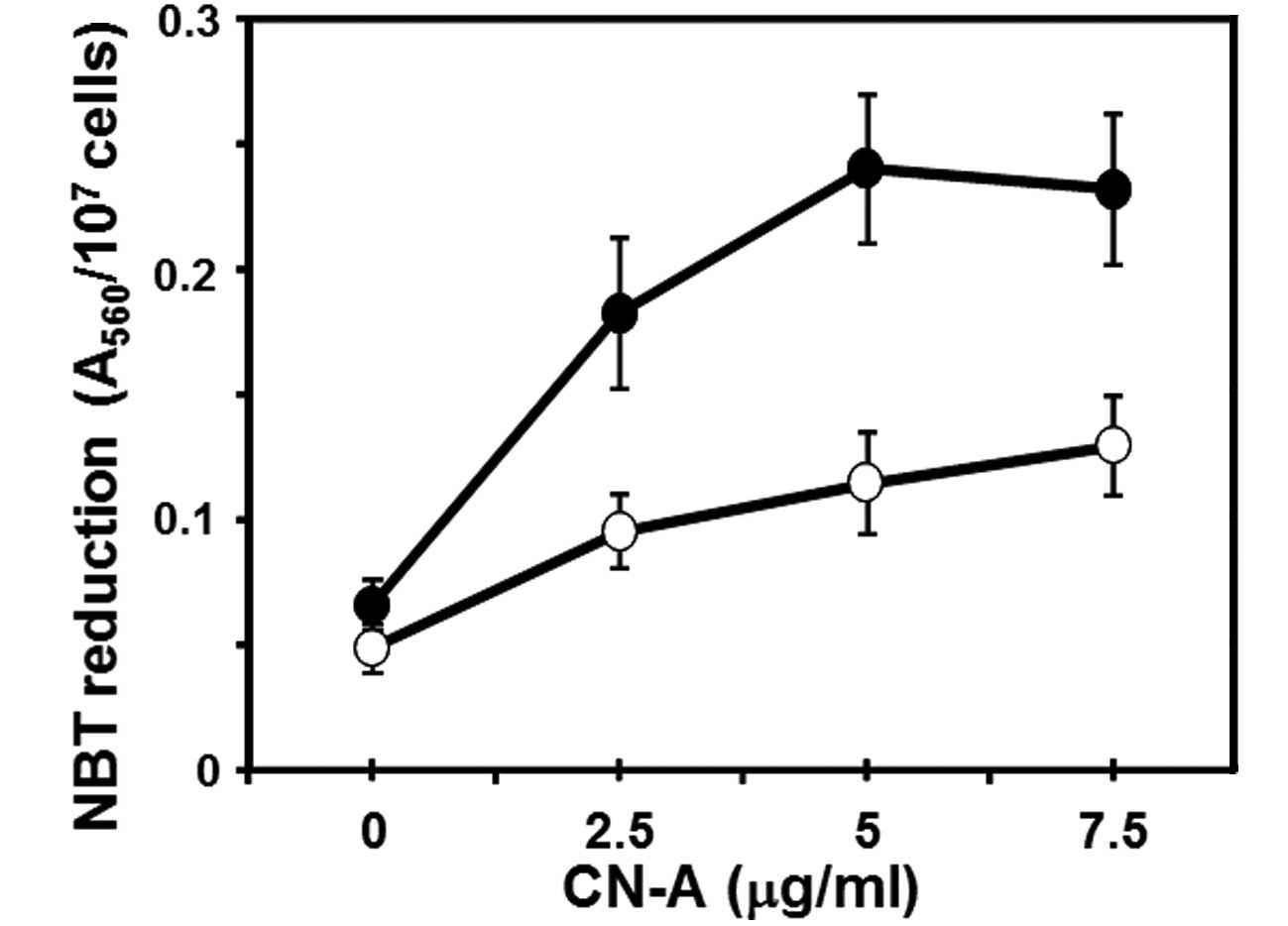
leukemia cells (4,21). Firstly, we found that CN-A and 0.25
μM ATO synergistically induced the NBT reduction (one of typical
differentiation markers of human leukemia cells) (Fig. 2). Then we examined whether these
combined treatment also could be effective in the suppression of
the proliferation of solid tumor cells including breast cancer
cells. CN-A and ATO synergistically inhibited the proliferation of
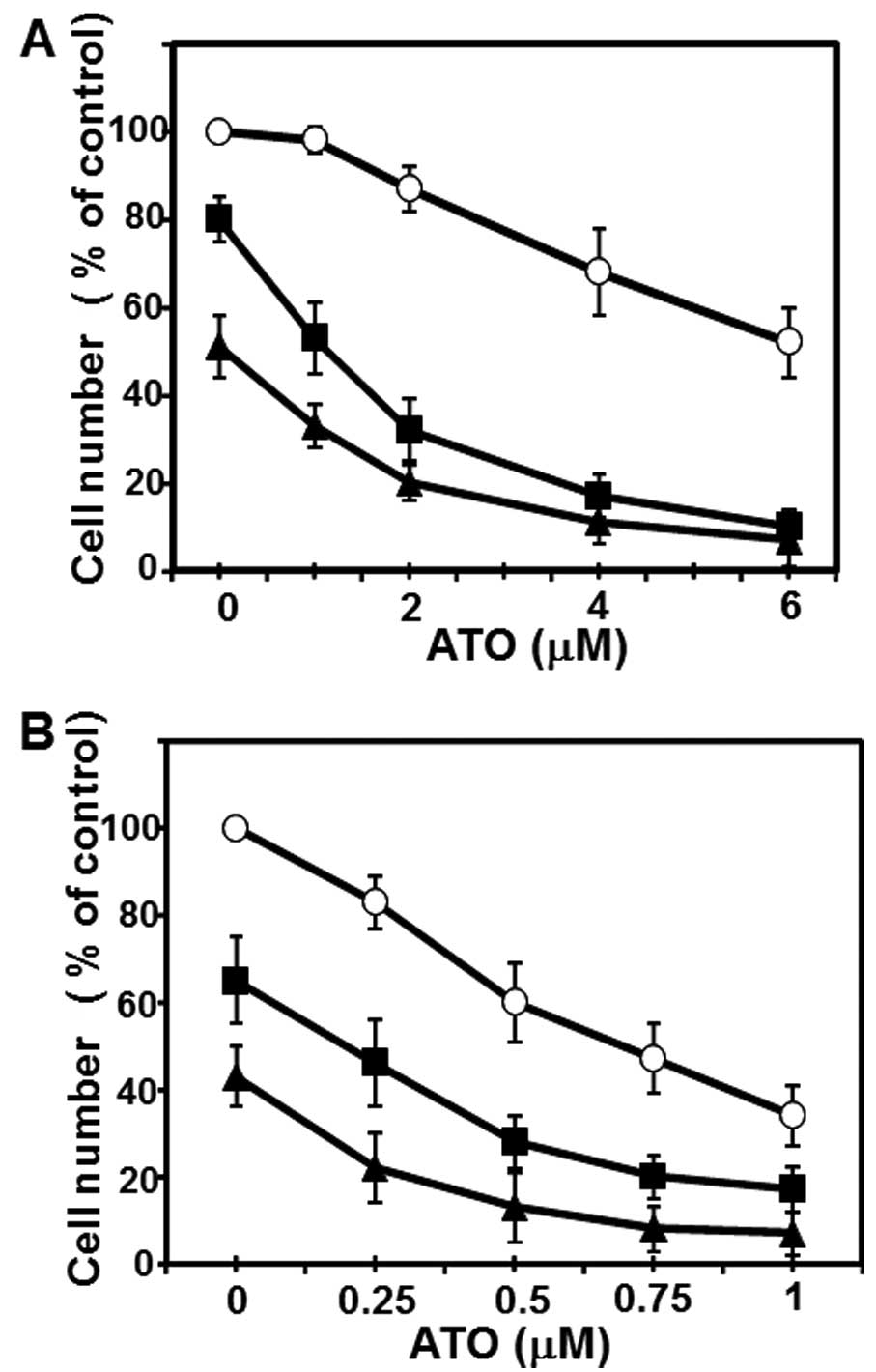
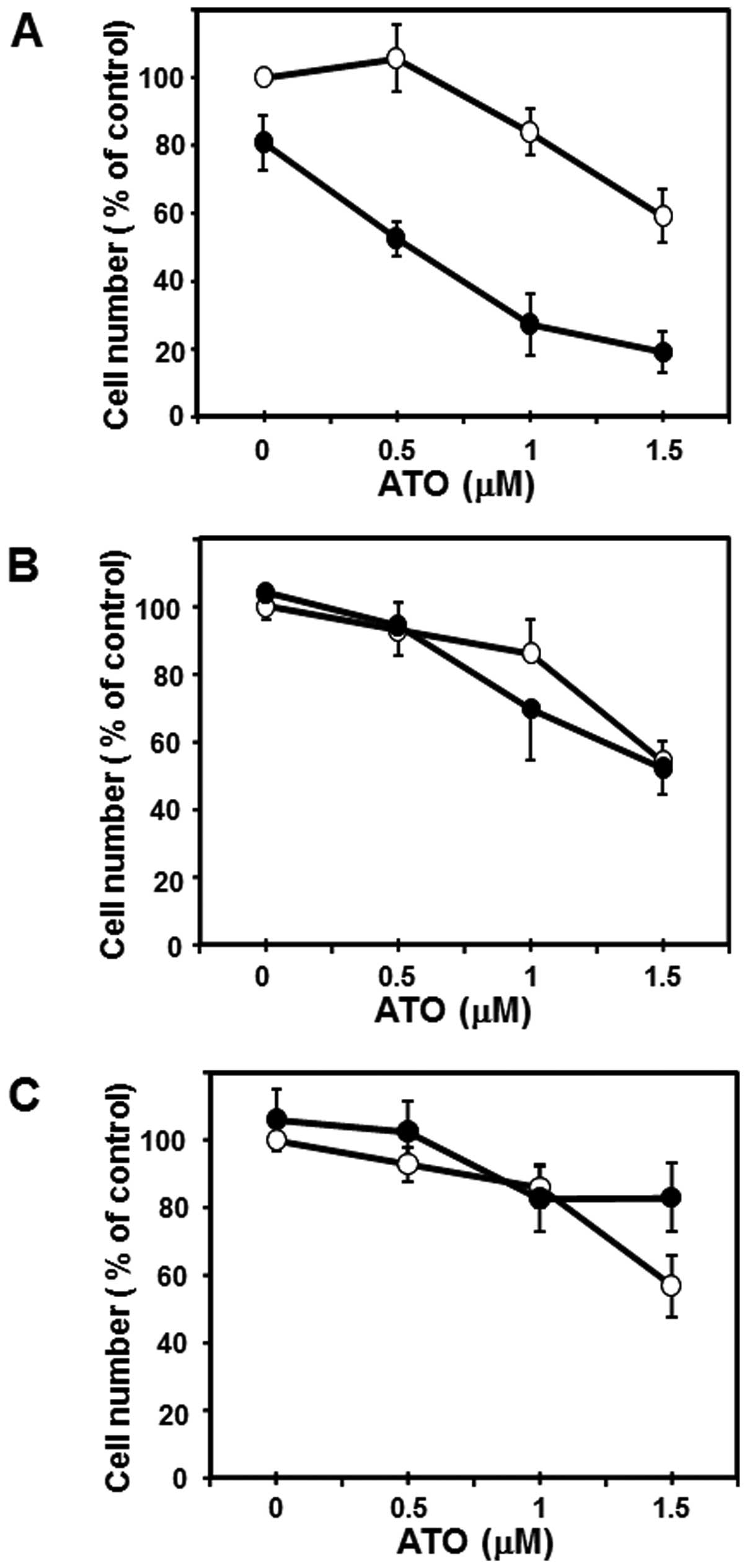
human breast cancer cell line MCF-7 cells (Fig. 3A). Although ATO alone even at a
higher concentration (6 μM) inhibited the growth of MCF-7 cells to
~50% of control and CN-A (1.25 μg/ml) alone slightly inhibited the
growth of MCF-7 cells, in the presence of CN-A (1.25 μg/ml) ATO at
1.5–4 μM, which is in or close to the range of clinically
achievable concentrations (30,31),
could inhibit the growth of MCF-7 cells to <50% of control
(Fig. 3A). Unexpectedly,
MDA-MB-231 cells were more sensitive to ATO: at 1 μM ATO alone
could inhibit the proliferation of MDA-MB-231 cells to <50% of
control (Fig. 3B). CN-A also
effectively enhanced the ATO-induced growth inhibition of
MDA-MB-231 cells (Fig. 3B).
CN-A and ATO synergistically inhibit
anchorage-independent growth of MCF-7 and MDA-MB-231 cells
Since anchorage-independent growth is well
correlated with tumorigenic potential, we next examined whether
this combined treatment with CN-A and ATO could effectively inhibit
the anchorage-independent growth of these breast cancer cells.
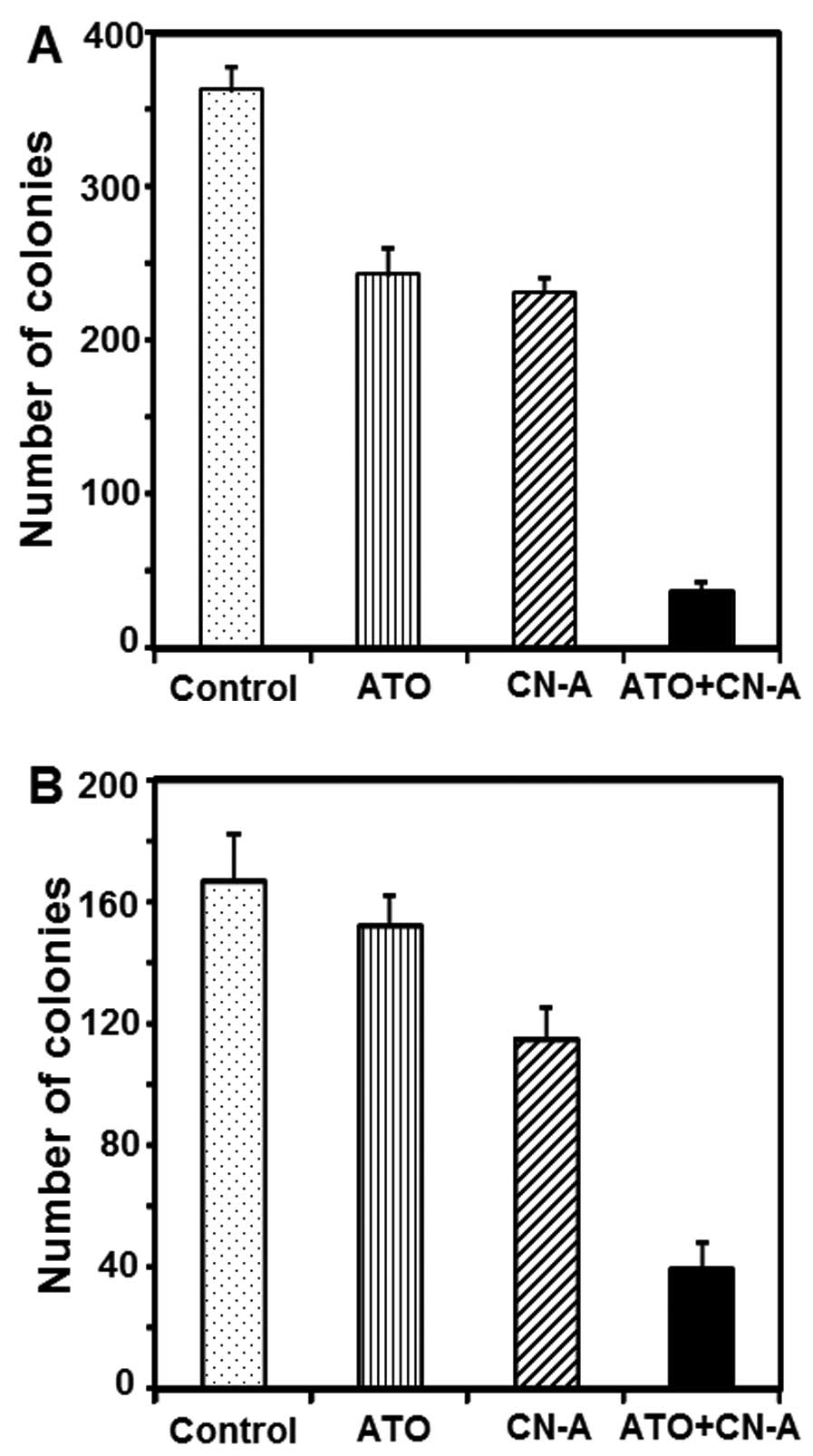
Although ATO (1 μM) or CN-A (1 μg/ml) alone inhibited colony
formation of MCF-7 cells to ~67 or 64% of controls, respectively,
combined treatment with ATO and CN-A inhibited colony formation to
10% of controls (Fig. 4A).
Although ATO (0.2 μM) alone slightly inhibited colony formation of
MDA-MB-231 cells (<10% inhibition) and CN-A (2.5 μg/ml) alone
inhibited colony formation of MDA-MB-231 cells to ~69% of controls,
combined treatment with ATO and CN-A inhibited colony formation to
23% of controls (Fig. 4B).
Effects of CN-A analogues on the growth
of MCF-7 cells in the presence of ATO
We next examined whether the active CN-A analogue
and ATO also cooperatively inhibited the growth of MCF-7 cells.
Although ISIR-005, a synthetic CN-A-derivative, at 6 μg/ml slightly
inhibited the growth of MCF-7 cells (~20% inhibition) after the
5-day treatment, combined treatment with ISIR-005 plus ATO
synergistically inhibited the growth of MCF-7 cells (Fig. 5A). On the other hand, FC-J, a
CN-A-related natural product, at 6 μg/ml scarcely inhibited the
growth of MCF-7 cells after the 5-day treatment and also could not
enhance ATO-induced growth inhibition (Fig. 5B). Furthermore, similar results
were obtained from ATO treatment plus 12OH-ISIR-005, an inactive
analogue of ISIR-005 (Fig.
5C).
CN-A and ATO synergistically increased
the expression of cleaved caspase-7 in MCF-7 cells
We next examined whether the combined treatment with
CN-A and ATO inhibited growth of MCF-7 cells through the induction
of apoptosis. Although cleaved caspase-3 is used for one of the
markers of apoptosis (32), MCF-7
cells lack expression of caspase-3 as a result of a 47-bp deletion
in exon 3 of the CASP3 gene (33).
Since there were reports that in MCF-7 cells apoptosis was induced
through the activation of caspase-7 instead of caspase-3 (34), we examined whether the combined
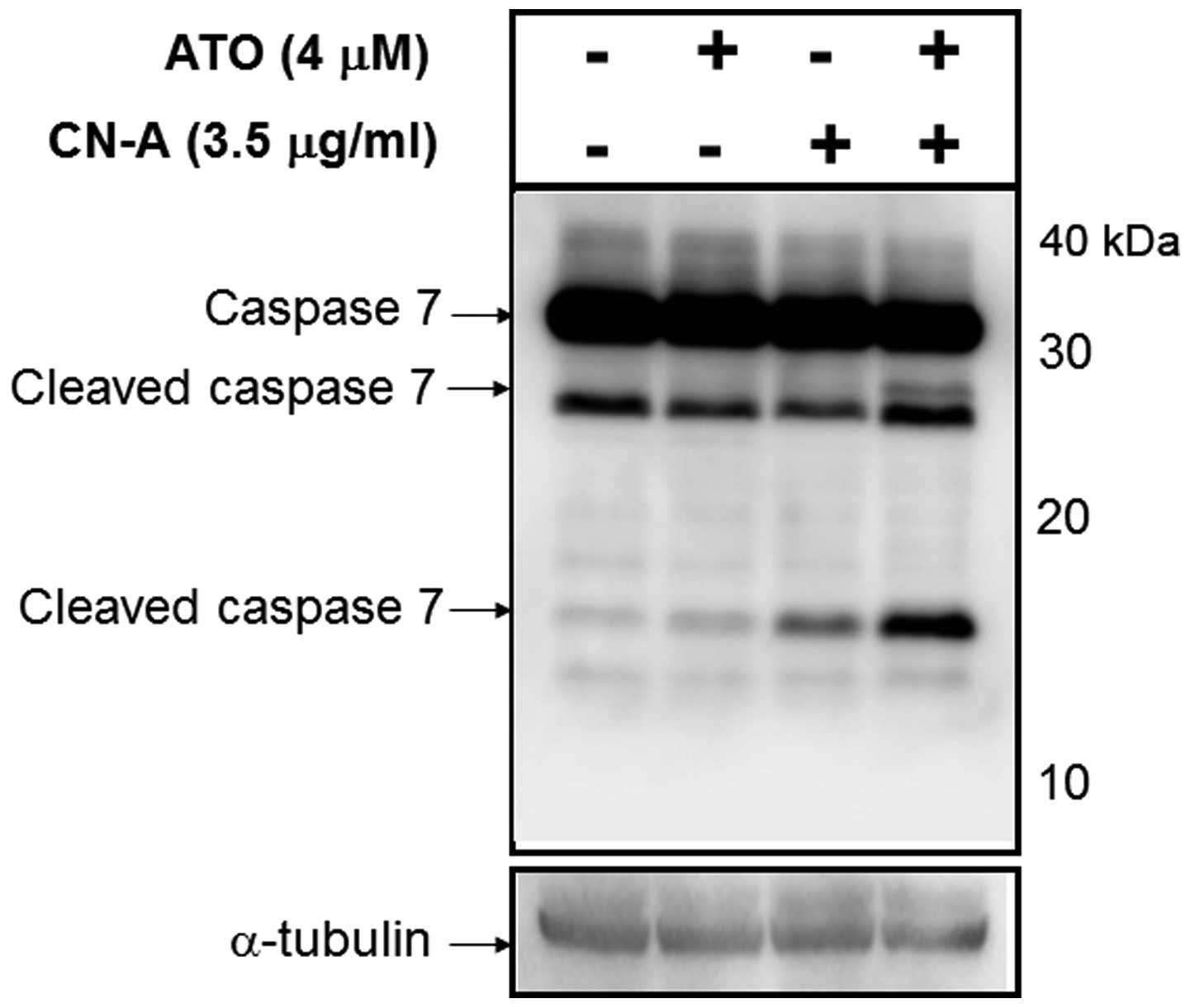
treatment with CN-A and ATO could induce cleaved caspase-7. MCF-7
cells were cultured with 4 μM ATO, 3.5 μg/ml CN-A or both ATO and
CN-A for 96 h. Although ATO alone scarcely induced cleaved
caspase-7 and CN-A alone only weakly induced cleaved caspase-7,
this combined treatment markedly induced cleaved caspase-7 in MCF-7
cells (Fig. 6). These results
suggest that the combined treatment with CN-A and ATO induced
apoptosis through the activation of caspase-7.
Characterization of the combined
treatment-induced apoptosis
In order to investigate further the mechanism by
which both CN-A and ATO induce apoptosis, various apoptosis-related
proteins were examined using apoptosis array analysis. MCF-7 cells
were cultured with 4 μM ATO, 3.5 μg/ml CN-A or both ATO and CN-A
for 96 h. Whole cell lysates were used for each apoptosis array
spotted with 35 antibodies specific to apoptosis-related proteins.
As mentioned above, the expression of caspase-3 was not detected in
this array (Fig. 7A spot number
1). The expression of death receptors [DR5 (TRAIL receptor 2) and
Fas] and inhibitors of cell cycle (p21/CIP1 and p27/Kip1) were
significantly induced (Fig. 7A and
B). On the other hand, among the inhibition of apoptosis (IAP)
family, expressions of cIAP-1 and survivin were clearly decreased
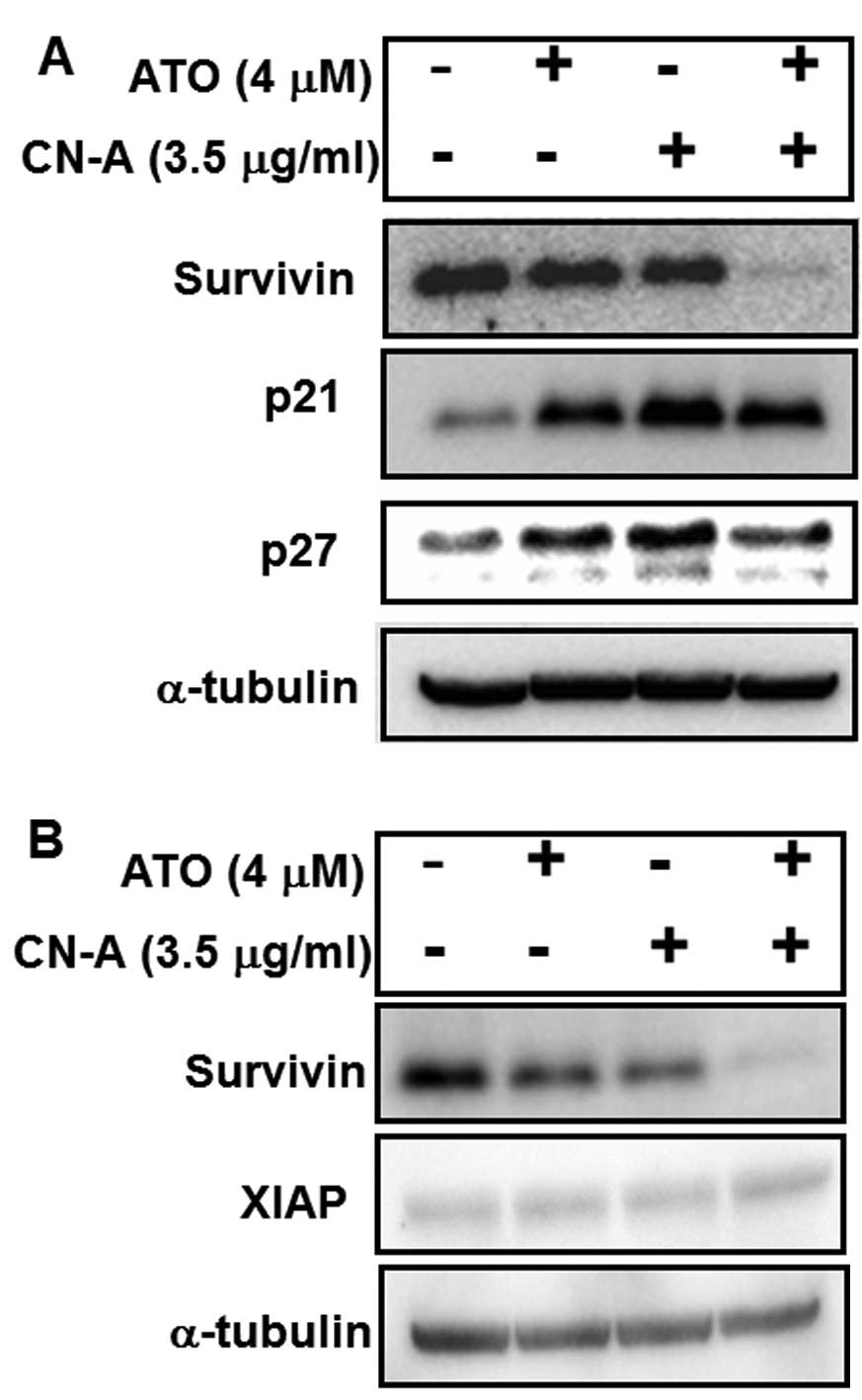
but the expression of XIAP was not significantly changed (Fig. 7A and B). We further examined
expressions of survivin, XIAP, p21/CIP1 and p27/Kip1 proteins by
western blot analysis (Fig. 8). We
found that the expression of survivin in MCF-7 cells was
dramatically decreased in the presence of both CN-A and ATO,
although the expression of survivin was only slightly decreased by
CN-A or ATO alone (Fig. 8A and B).
On the other hand, the expression of XIAP was not significantly
modulated by CN-A and/or ATO (Fig.
8B).
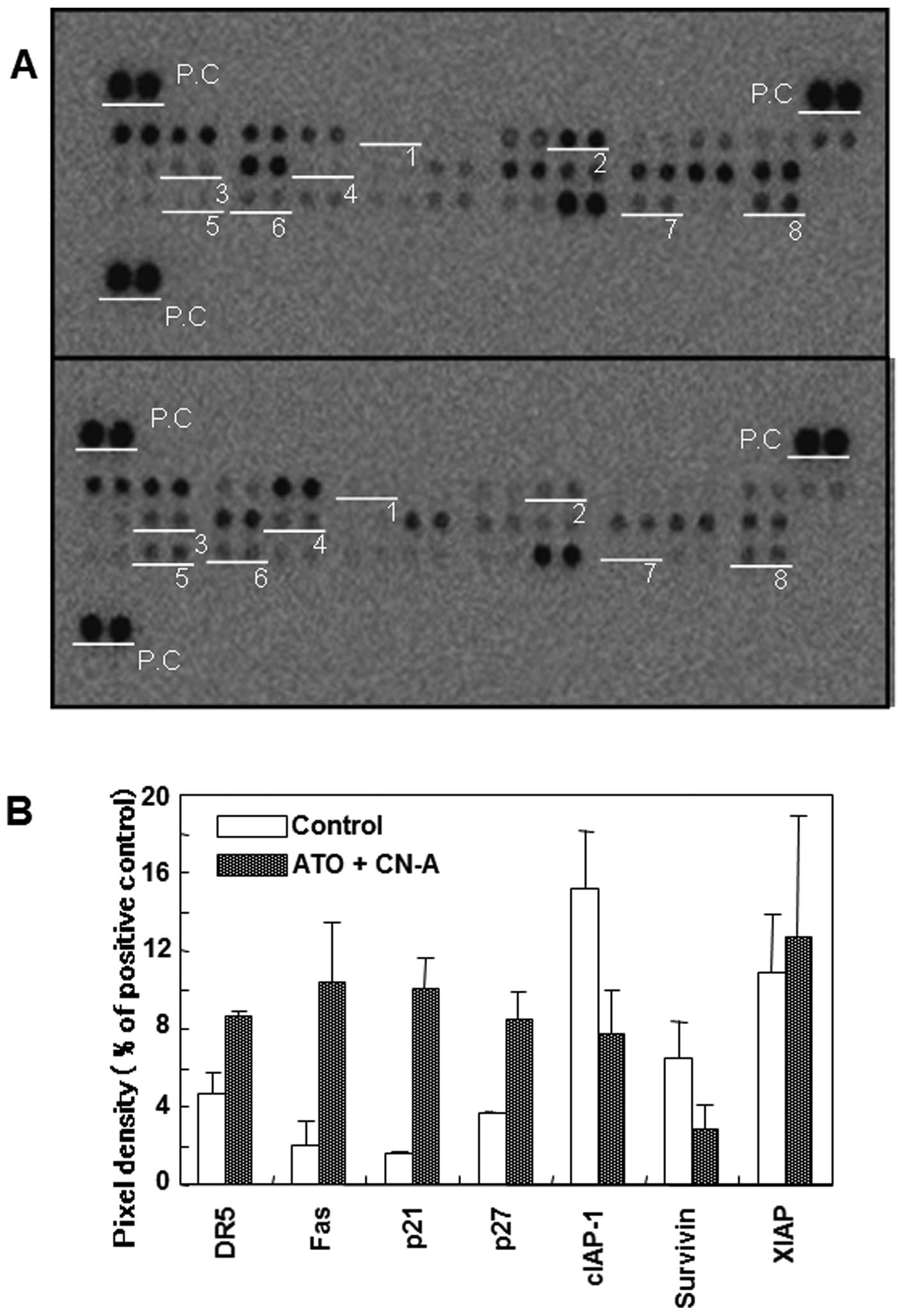 | Figure 7Effects of CN-A and ATO on the
expression of apoptosis-associated proteins in MCF-7 cells.
Apoptosis array analysis. (A) MCF-7 cells were cultured with 4 μM
ATO, 3.5 μg/ml CN-A or both ATO and CN-A for 96 h. Whole cell
lysate were used for each apoptosis array. White underline show
spots for: 1, pro-caspase-3; 2, cIAP-1; 3, TRAIL R2/DR5; 4, Fas; 5,
p21/CIP1; 6, p27/Kip1; 7, survivin; and 8, XIAP. P.C, positive
control. The results are representative of 3 independent
experiments. (B) Expression levels of these apoptosis-related
proteins were quantified using an image analyzer. The expression
levels are shown as percentages of positive control spots. Data
represent the mean ± standard deviation of three independent
apoptosis array analyses. |
CN-A or ATO alone clearly induced p21/CIP1 and
p27/Kip1 proteins. The combined treatment with CN-A and ATO did not
further increase the p21/CIP1 and p27/Kip1 proteins (Fig. 8A).
Effect of YM155 (survivin inhibitor) on
the CN-A-induced or ATO-induced growth inhibition of MCF-7
cells
As mentioned above, our results suggest that the
induction of cleaved caspase-7 and the inhibition of survivin are
important events in the corporative inhibition of growth of MCF-7
cells by both CN-A and ATO. Since survivin is a direct inhibitor of
caspasse-3 and -7 (35), we
examined the effect of the survivin inhibitor YM155 (36), on the CN-A-induced or ATO-induced
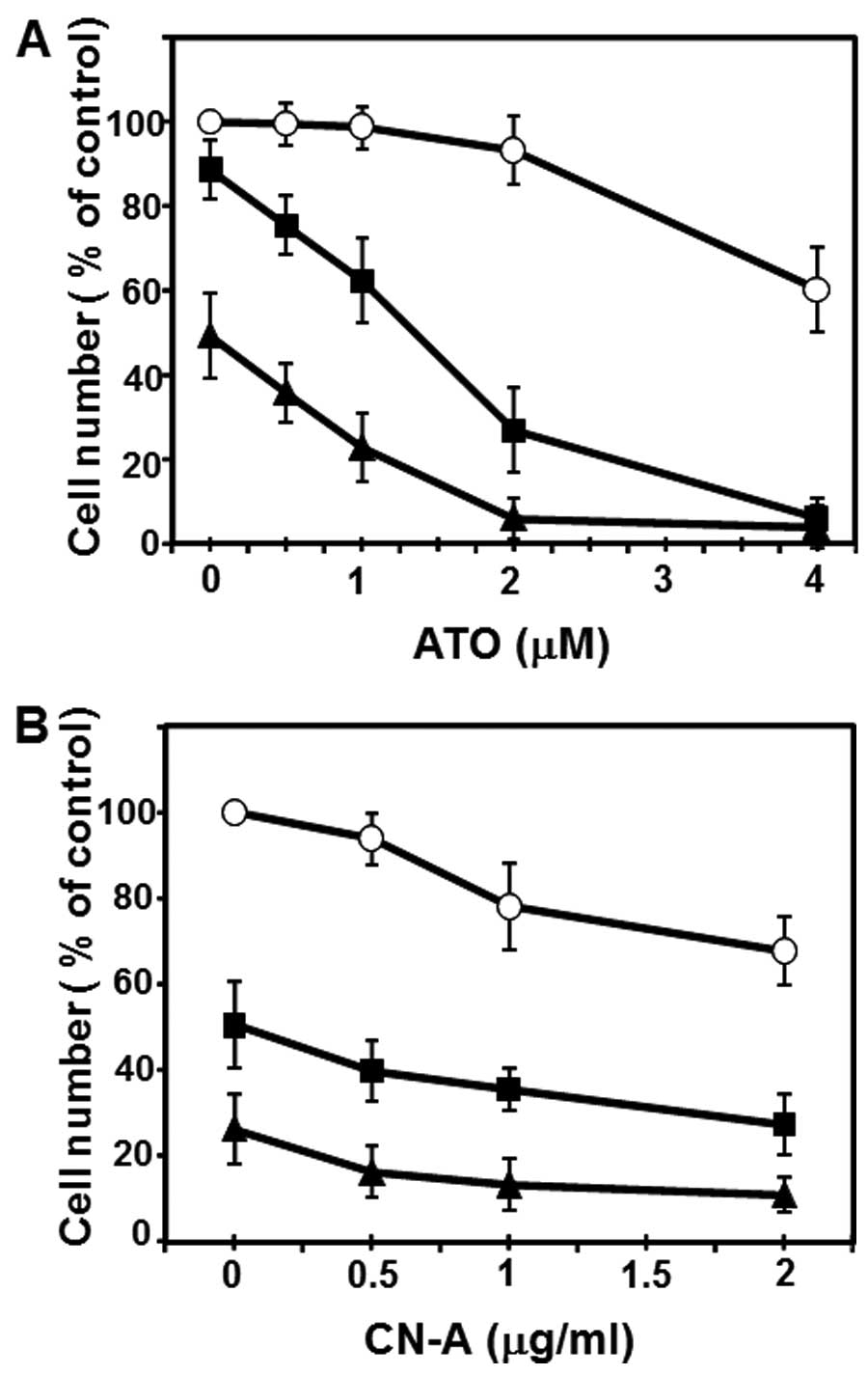
growth inhibition of MCF-7 cells. As shown in Fig. 9A, YM155 and ATO synergistically
inhibited the growth of MCF-7 cells. Whereas ATO at 2 μM or YM155
at 2.5 nM alone scarcely inhibited the growth of MCF-7 cells, the
combined treatment with ATO and YM155 inhibited growth to <30%
of control (Fig. 9A). On the other
hand, the combined treatment with CN-A and YM155 showed only
additive growth inhibition of MCF-7 cells (Fig. 9B). These results suggest that the
synergistic growth inhibition by both CN-A and ATO was, at least in
part, due to the synergistic inhibition of survivin induced by both
CN-A and ATO.
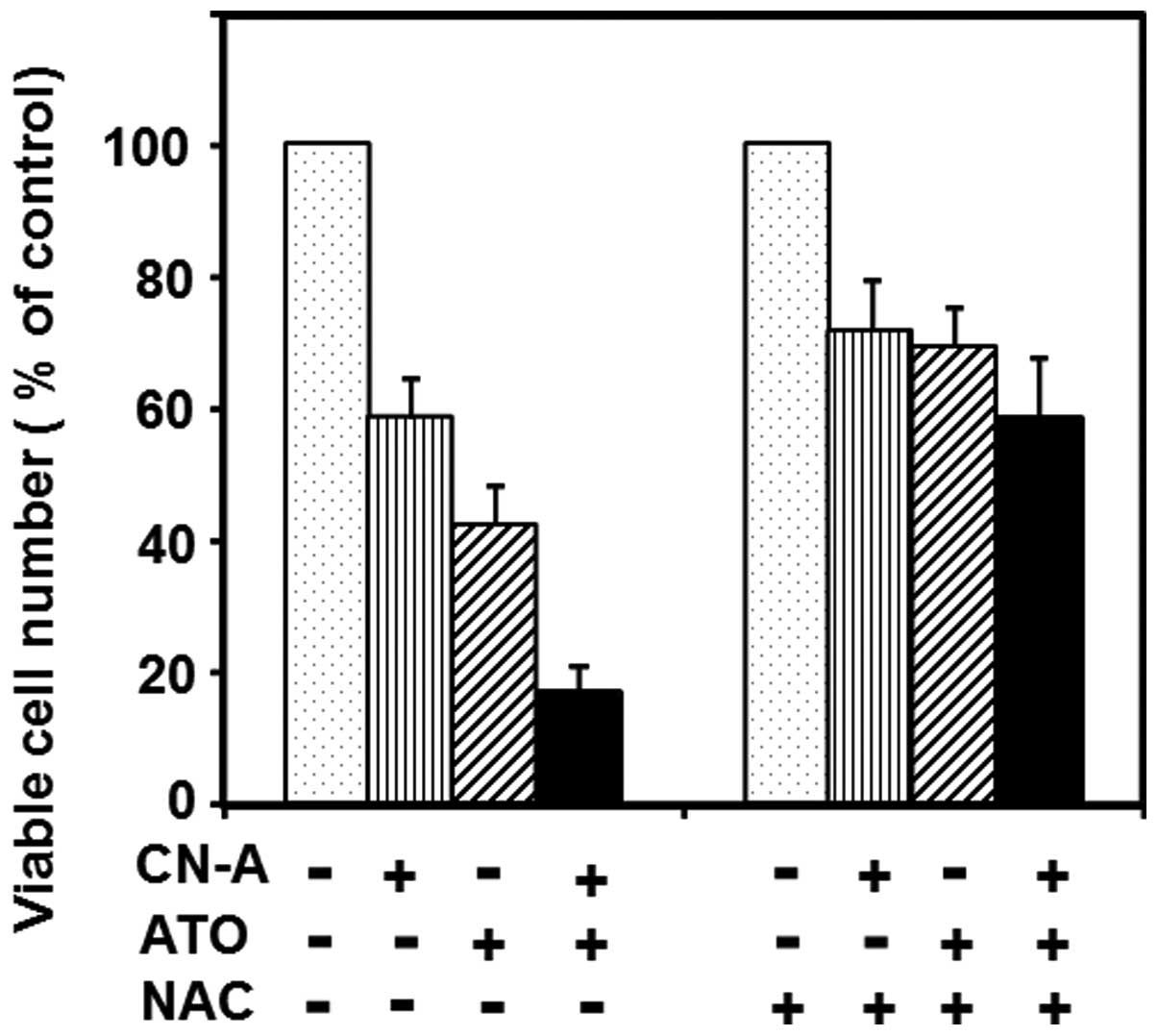
Effect of N-acetylcysteine (antioxidant
compound) on combined treatment-induced growth inhibition of MCF-7
cells
Next, we determined whether oxidative stress was
involved in the combined treatment with CN-A and ATO -induced
growth inhibition. An antioxidant compound N-acetylcysteine (NAC)
that can broadly scavenge reactive oxygen species (ROS) was used to
reduce ROS-induced cellular stress. When cell growth rate was
assessed, pretreatment with NAC significantly reduced the
combination treatment-induced cell growth inhibition (Fig. 10). Furthermore, we found that
hydrogen peroxide at low doses could enhance the CN-A-induced or
ATO-induced growth inhibition of MCF-7 cells (data not shown).
These data suggest that oxidative response plays an essential role
in the combination treatment-induced apoptosis.
CN-A and ATO synergistically inhibit cell
invasion capacity of MDA-MB-231 cells
Finally, we examined whether the combined treatment
with CN-A and ATO also could be effective in the suppression of the
invasive capacity of MDA-MB-231 cells. For the detection of
MDA-MB-231 cell invasion, we used the impedance-based xCELLigence
Real-Time Cell Analysis (RTCA) technology. Matrigel (5%, v/v) as
extracellular matrix component was added on the top of the
microporous membranes of upper chambers. Under this condition,
MDA-MB-231 cells could invade time-dependently to lower chambers,
whereas MCF-7 and T47D cells could not invade (Fig. 11A). We found that CN-A alone could
dose-dependently inhibit the invasion activity of MDA-MB-231 cells
(Fig. 11B). As previously
reported using standard Matrigel-coated Transwell assay, we
confirmed that ATO alone also inhibited the invasion activity in
this assay (Fig. 11C).
Furthermore, we found that the combined treatment with CN-A and ATO
completely suppressed the invasion activity (Fig. 11C).
Discussion
ATO is an approved treatment for APL. In addition to
APL, the antitumor activity of ATO has been reported in a variety
of solid tumor cell lines including breast, esophageal, cervical,
lung, liver, prostate and liver carcinoma (8–13).
However, it was reported that many solid tumors are less sensitive
to ATO than APL. The requirement of higher doses of ATO for the
induction of effective growth inhibition of solid tumor cells was
associated with the risk of severe adverse effects such as
leukopenia, anemia, fever and vomiting (14–16,37).
Combination therapy is a frequently used method in clinical
practice to improve the therapeutic effect and reduce the toxicity
of anticancer drugs (17,18). Therefore, novel strategies of
treatment which can potentiate the antitumor activity and alleviate
toxicity are needed for employment of ATO on patients with solid
tumors. In the present study, we showed that low doses of ATO and
CN-A, which is a potent differentiation inducer of myeloid leukemia
cells, could inhibit cooperatively the cell proliferation of human
breast cancer MCF-7 cells and MDA-MB-231 cells measured by both MTT
assay and methylcellulose colony-formation assay. These results
suggest that CN-A is an attractive enhancer for ATO-induced
anticancer activities in human breast cancer.
Cleaved caspase-3 is used for a marker of apoptosis
induction in several types of cancer cells (32). Although MCF-7 cells lack expression
of caspase-3 as a result of a 47-bp deletion in exon 3 of the CASP3
gene (33), there is a report that
MCF-7 cells induced apoptosis through the activation of caspase-7
instead of caspase-3 (34). The
combined treatment with 4 μM ATO and 3.5 μg/ml CN-A markedly
induced cleaved caspase-7 in MCF-7 cells, although ATO alone
scarcely induced cleaved caspase-7 and CN-A alone only weakly
induced cleaved caspase-7 (Fig.
6). These results suggest that the combined treatments with
CN-A and ATO induced apoptosis through the activation of caspase-7.
Accompanying with this synergistic induction of cleaved caspase-7
by the treatment with CN-A plus ATO, we also found that the
expression of survivin, which is a member of IAP family and a
direct inhibitor of caspase-3 and -7 (35), significantly decreased in MCF-7
cells treated with both CN-A and ATO (Figs. 7 and 8), although the expression of survivin
was only slightly decreased by CN-A or ATO alone (Fig. 8). Furthermore, we found that ATO
and the survivin inhibitor YM155 also synergistically inhibited the
growth of MCF-7 cells (Fig. 9).
These results suggest that the induction of cleaved caspase-7 and
inhibition of survivin are important events in the corporative
inhibition of growth of MCF-7 cells by both CN-A and ATO.
The pretreatment with antioxidant NAC significantly
reduced the combination treatment-induced cell growth inhibition
(Fig. 10). We observed that the
growth of MCF-7 cells was synergistically inhibited by the
treatment with both CN-A and low doses of hydrogen peroxide (one of
ROS) or the treatment with both ATO and hydrogen peroxide (data not
shown). These results suggest that oxidative response plays an
essential role in the combination treatment-induced apoptosis and
also suggest that ROS-inducing drugs or substances could further
enhance ATO-induced, CN-A-induced, or the combined treatment with
CN-A and ATO-induced growth inhibition of tumor cells. Indeed,
recently Nakaoka et al (38) reported that ATO and cisplatin (a
ROS inducer) showed synergistic anticancer activity in oral
squamous cell carcinoma cells. On the other hand, we found that
CN-A and cisplatin showed synergistic anticancer activity in MCF-7
cells (data not shown).
In addition to inhibiting cell proliferation of
cancer cells, suppression of cell invasion capacity of cancer cells
is very important for development of effective cancer treatment.
Therefore, we also examined the effects of CN-A, ATO or combined
treatment with CN-A and ATO on the cell invasion capacity of human
breast cancer cells by using the impedance-based xCELLigence RTCA
technology. We confirmed that invasion capacity was observed in
MDA-MB-231 cells but not in MCF-7 and T47D cells using xCELLigence
RTCA technology as previously reported (39). We found that CN-A alone could
dose-dependently inhibit the invasion capacity of MDA-MB-231 cells.
According to a recent report using Transwell assay (40), ATO also attenuated the invasion
capacity of MDA-MB-231 cells in xCELLigence assay. Finally, we
found that the combined treatment with CN-A and ATO markedly
suppressed the invasion capacity (Fig. 11). Although the combined treatment
with CN-A and ATO in these experiments did not induce apoptosis
(data not shown), the mechanism of this marked suppression of the
invasion capacity is still not known and further studies are
needed.
In conclusion, CN-A and ATO cooperatively suppress
cell proliferation and cell invasion capacity of human breast
cancer cells. These results suggest that CN-A is an attractive
enhancer for the ATO-induced anticancer activities in human breast
cancer.
Acknowledgements
The present study was supported partly by a grant
from the Ministry of Education, Culture, Sports, Science, and
Technology of Japan.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham MA and Jemal A:
Cancer statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurvitz S, Hu Y, O’Brien N and Finn RS:
Current approaches and future directions in the treatment of
HER2-positive breast cancer. Cancer Treat Rev. 39:219–229. 2013.
View Article : Google Scholar
|
|
4
|
Gianni M, Koken MH, Chelbi-Alix MK, Benoit
G, et al: Combined arsenic and retinoic acid treatment enhances
differentiation and apoptosis in arsenic-resistant NB4 cells.
Blood. 91:4300–4310. 1998.PubMed/NCBI
|
|
5
|
Shen ZX, Chen GQ, Ni XS, et al: Use of
arsenic trioxide (As2O3) in the treatment of
acute promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360.
1997.PubMed/NCBI
|
|
6
|
Deuer D and Tallman MS: Arsenic trioxide:
new clinical experience with an old medication in hematologic
malignancies. J Clin Oncol. 23:2396–2410. 2005. View Article : Google Scholar
|
|
7
|
Platanias L: Biological responses to
arsenic compounds. J Biol Chem. 284:18583–18587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emadi A and Gore SD: Arsenic trioxide-An
old drug rediscovered. Blood Rev. 24:191–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi S: Combination therapy with
arsenic trioxide for hematological malignancies. Anticancer Agents
Med Chem. 10:504–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Su Y and Sun Z: Opposite effects
of arsenic trioxide on the Nrf2 pathway in oral squamous cell
carcinoma in vitro and in vivo. Cancer Lett. 18:93–98. 2012.
View Article : Google Scholar
|
|
11
|
Kryeziu K, Jungwirth U, Hoda MA, et al:
Synergistic anticancer activity of arsenic trioxide with erlotinib
is based on inhibition of EGFR-mediated DNA double-strand break
repair. Mol Cancer Ther. 12:1073–1084. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu W, Gong Y, Li H, et al:
Arsenic-induced growth arrest of breast cancer MCF-7 cells
involving FOXO3a and IkB kinase β expression and localization.
Cancer Biother Radiopharm. 27:504–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasukabe T, Okabe-Kado J, Haranosono Y,
Kato N and Honma Y: Inhibition of rapamycin-induced Akt
phosphorylation by cotylenin A correlated with their synergistic
growth inhibition of cancer cells. Int J Oncol. 42:767–775.
2013.
|
|
14
|
Vuky J, Yu R, Schwartz L and Motzer RJ:
Phase II trial of arsenic trioxide in patients with metastatic
renal cell carcinoma. Invest New Drugs. 20:327–330. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KB, Bedikian AY, Camacho LH,
Papadopoulos NE and McCullough C: A phase II trial of arsenic
trioxide in patients with metastatic melanoma. Cancer.
104:1687–1692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL
and Yang CH: Arsenic trioxide in patients with hepatocellular
carcinoma: a phase II trial. Invest New Drugs. 25:77–84. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abe O, Abe R, Enomoto K, et al: Effects of
chemotherapy and hormonal therapy for early breast cancer on
recurrence and 15-year survival: an overview of the randomized
trials. Lancet. 365:1687–1717. 2005. View Article : Google Scholar
|
|
18
|
Zhao XY, Yang S, Chen YR, Li PC, Dou MM
and Zhang J: Resveratrol and arsenic trioxide act synergistically
to kill tumor cells in vitro and in vivo. PloS One. 9:e989252014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sassa T, Tojyo T and Munakata K: Isolation
of a new plant growth substance with cytokinin-like activity.
Nature. 227:3791970. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asahi K, Honma Y and Hazeki K: Cotylenin
A, a plant-growth regulator, induces the differentiation in murine
and human myeloid leukemia cells. Biochem Biophys Res Commun.
238:758–763. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto-Yamaguchi Y, Yamada K, Ishii Y,
Asahi KI, Tomoyasu S and Honma Y: Induction of the monocytic
differentiation of myeloid leukemia cells by cotylenin A, a plant
growth regulator. Br J Haematol. 112:697–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada K, Honma Y, Asahi KI, Sassa T, Hino
KI and Tomoyasu S: Differentiation of human acute myeloid leukemia
cells in primary culture in response to cotylenin A, a plant growth
regulator. Br J Haematol. 114:814–821. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Honma Y: Cotylenin A: a plant growth
regulator as a differentiation-inducing agent against myeloid
leukemia. Leuk Lymphoma. 43:1169–1178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto-Yamaguchi Y, Okabe-Kado J,
Kasukabe T and Honma Y: Induction of differentiation of human
myeloid leukemia cells by immunosuppressant macrolides (rapamycin
and FK506) and calcium/calmodulin-dependent kinase inhibitors. Exp
Hematol. 29:582–588. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kasukabe T, Okabe-Kado J, Kato N, Sassa T
and Honma Y: Effects of combined treatment with rapamycin and
cotylenin A, a novel differentiation-inducing agent, on human
breast carcinoma MCF-7 cells and xenografts. Breast Cancer Res.
7:R1097–R1110. 2005. View
Article : Google Scholar
|
|
26
|
Kasukabe T, Okabe-Kado J and Honma Y:
Cotylenin A, a new differentiation inducer, and rapamycin
cooperatively inhibit growth of cancer cells through induction of
cyclin G2. Cancer Sci. 99:1693–1698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawakami K, Hattori M, Inoue T, et al: A
novel Fusicoccin derivative preferentially targets hypoxic tumor
cells and inhibits tumor growth in xenografts. Anticancer Agents
Med Chem. 12:791–800. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakamaki T, Okabe-Kado J,
Yamamoto-Yamaguchi Y, et al: Role of MmTRA1b/phospholipid
scramblase 1 gene expression in the induction of differentiation of
human myeloid leukemia cells into granulocytes. Exp Hematol.
30:421–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hagiwara Y, Kasukabe T, Kaneko Y, Niitsu N
and Okabe-Kado J: Ellagic acid a natural compound, induces
apoptosis and potentiates retinoic acid-induced differentiation of
human leukemia HL-60 cells. Int J Hematol. 92:136–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou C, Boggess J, Bae-Jump V and Gehrig
PA: Induction of apoptosis and inhibition of telomerase activity by
arsenic trioxide (As2O3) in endometrial
carcinoma cells. Gynecol Oncol. 105:218–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar P, Gao Q, Ning Y, Wang Z, Krebsbach
PH and Polverini PJ: Mol Cancer Ther. 7:2060–2069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng Y, Pan TC, Pant DK, et al: SPSB1
promotes breast cancer recurrence by potentiating c-MET signaling.
Cancer Discov. 4:790–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janicke RU, Sprenger ML, Wati MR and
Porter AG: Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Biol Chem.
273:9357–9360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aziz MY, Omar AR, Subramani T, et al:
Damnacanthal is a potent inducer of apoptosis with anticancer
activity by stimulating p53 and p21 genes in MCF-7 breast cancer
cells. Oncol Lett. 7:1479–1484. 2014.PubMed/NCBI
|
|
35
|
Shin S, Sung BJ, Cho YS, et al: An
anti-apoptotic protein human survivin is a direct inhibitor of
caspase-3 and -7. Biochemistry. 40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakahara T, Takeuchi M, Kinoyama I, et al:
YM155, a novel small-molecule survivin suppressant, induces
regression of established human hormone-refractory prostate tumor
xenografts. Cancer Res. 67:8014–8021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li W, Wang M, Wang L, Ji S, Zhang J and
Zhang C: Icariin synergizes with arsenic trioxide to suppress human
hepatocellular carcinoma. Cell Biochem Biophys. 68:427–436. 2014.
View Article : Google Scholar
|
|
38
|
Nakaoka T, Ota A, Ono T, et al: Combined
arsenic trioxidecisplatin treatment enhances apoptosis in oral
squamous cell carcinoma cells. Cell Oncol. 37:119–129. 2014.
View Article : Google Scholar
|
|
39
|
Limame R, Wouters A, Pauwels B, et al:
Comparative analysis of dynamic cell viability, migration and
invasion assessments by novel real-time technology and classic
endpoint assays. PloS One. 7:e465362012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Si L, Jiang F, Li Y, et al: Induction of
the mesenchymal to epithelial transition by demethylation-activated
microRNA-200c is involved in the anti-migration/invasion effects of
arsenic trioxide on human breast cancer cells. Mol Carcinog. Apr
14–2014. View Article : Google Scholar : (Epub ahead of
print).
|