Introduction
Breast cancer is a major health problem in women
worldwide and the main cause of cancer death among women. Many
breast cancer patients fail conventional treatment strategies of
chemotherapy, radiation and anti-estrogen therapy (1,2).
Therefore, the search for new and effective drugs to treat breast
cancer is required. Research into the molecular pathway and
biomarkers associated with the development of breast cancer is
needed to find successful therapeutic approaches.
Plant-derived drugs play an increasingly important
role in cancer therapy due to their low toxicity and high efficacy.
Natural products derived from plants, such as camptothecin,
vincristine, taxol, etoposide and paclitaxel, have received
extensive attention as potential anticancer drugs. These bioactive
phytochemicals are known to exert anticancer activity through
different mechanisms, including immune activation, suppression of
cell cycle progression and induction of apoptosis (3–11).
Naphthazarin (Naph, DHNQ, 5,8-dihydroxy-l,4-naphthoqui-none) is a
naturally occurring 1,4-naphthoquinone derivative; a lipophilic red
pigment like alkannin and shikonin. Naph derivatives are well-known
for their anti-inflammatory, antioxidant, antibacterial antifungal
and wound healing effects. They are also known for antitumor
cytotoxic effects in cancer cells (12–16).
The mechanism of antitumor cytotoxicity and the specific molecular
target of Naph in cancer cells are yet to be established, but they
probably involve the induction of differential expression of cell
cycle regulators. Cell cycle progression is governed by
cyclin-dependent kinases (CDKs) that are activated by cyclin
binding and inhibited by CDK inhibitors (17,18).
P21 is a CDK inhibitor (CKI) that plays a crucial role in arresting
cellular growth, differentiation and apoptosis. P21 gene expression
is induced by the p53 gene, thereby directly mediating p53-induced
cell cycle arrest (19–24).
UHRF1 (Ubiquitin-like containing PHD and ring finger
domains 1), also known as ICBP90 or Np95, is a multi-domain protein
associated with cellular proliferation and epigenetic regulation.
UHRF1 binds to methylated CpG dinucleotides and recruits the
transcriptional repressors DNA methyltransferase 1 (DNMT1) and
histone deacetylase 1 (HDAC1) to regulate gene expression (25–30).
In addition, UHRF1 forms a complex with DNMT1/HDAC1 and binds to
the promoter regions of tumor suppressor genes such as p16INK4A,
p14ARF and p21 in cancer cells (31–36).
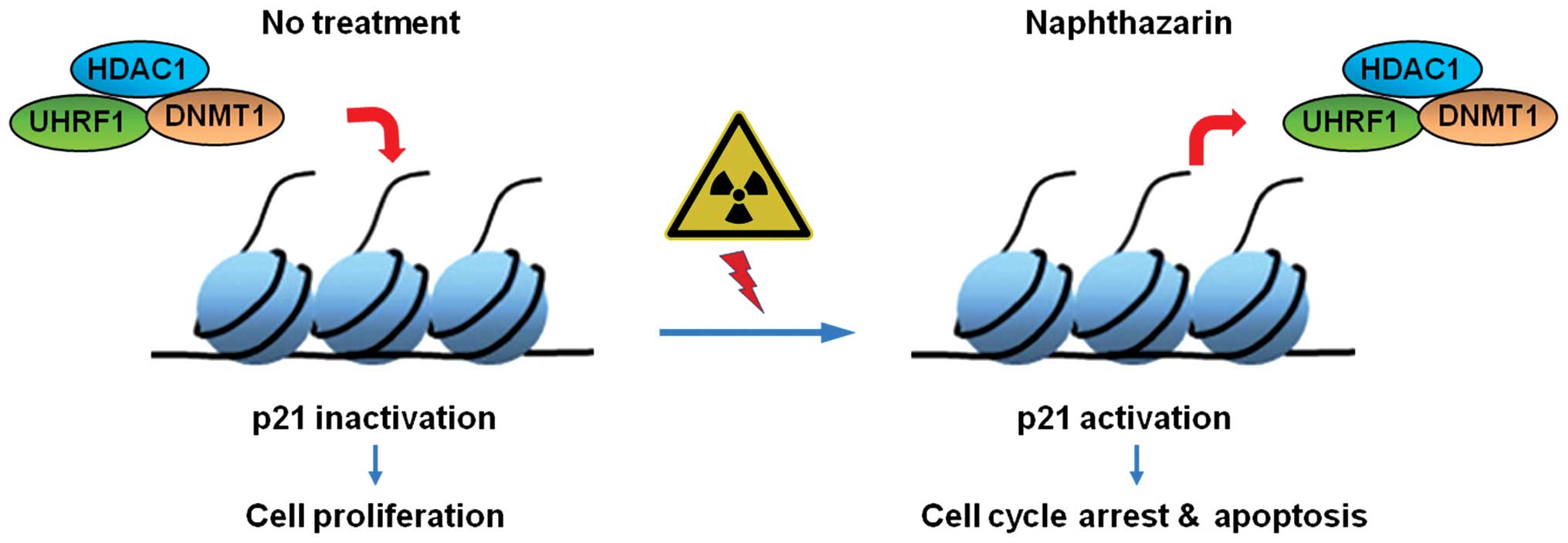
In this study, we demonstrated that treatment with Naph and
irradiation (IR) downregulates the expressions of DNMT1, UHRF1 and
HDAC1, whereas it upregulates the expression of p21 in MCF-7 cells.
Moreover, cell cycle arrest and apoptosis were significantly
increased by combinatorial treatment of Naph and IR. Collectively,
our results suggest that Naph might be an effective radiosensitizer
and adjuvant therapy for breast cancer.
Materials and methods
Cell and culture conditions
Human breast cancer cell line, MCF-7, was obtained
from the American Type Culture Collection (Rockville, MD, USA).
Cells were maintained in DMEM (Welgene, Daegu, Korea) supplemented
with 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA) and 5%
antibiotic-antimycotic (Gibco, Grand Island, NY, USA). Cells were
cultured at 37°C in a humidified atmosphere of 5%
CO2.
Drug treatment and IR exposure
Stock solutions of 10 mM naphthazarin
(Sigma-Aldrich, St. Louis, MO, USA) were dissolved in dimethyl
sulfoxide (DMSO) and diluted in culture medium to the indicated
final concentration for cell treatment. Cells were incubated at
37°C overnight and then treated with naphthazarin (Naph). After 2
h, cells were exposed to gamma-rays from a 137Cs γ-ray source
(Eckert & Ziegler, Berlin, Germany) at a dose rate of 2.6
Gy/min. Following IR at a 10 Gy dose, the cells were incubated
under naphthazarin conditions for the indicated times.
Cell proliferation assay and cell
morphology
Cell proliferation was assessed using the MTT
colorimetric assay. MCF-7 cells (2×105 cells/well) were
seeded in 6-well plates and incubated at 37°C overnight (O/N).
After 24 h of culture, the medium was removed and replaced with
experimental medium. Cells were pretreated with Naph (respective
concentration) before 2 h and then exposed to IR at a 10 Gy dose
for 24 h. Subsequently, cells were washed twice with PBS and 5
mg/ml MTT in PBS was added to each well for 4 h. After removal of
the MTT solution, a solubilization solution (DMSO/EtOH, 1:1 ratio)
was added to each well to dissolve the formazan crystals. The
absorbance at 570 nm was measured using a Paradigm™ Detection
Platform (Beckman Coulter, Inc., Fullerton, CA, USA). For
investigation of morphological changes, Naph or/and IR treated
cells were examined under an inverted light microscope (Nikon)
after 48 h.
RNA isolation and quantitative real-time
PCR
Total RNA was isolated from the MCF-7 breast cancer
cells using TRI-Solution (Bio Science Technology, Rockaway, NJ,
USA) according to the manufacturer’s protocol. The quantity of
isolated RNA was measured using NanoDrop (Thermo Scientific,
Rockford, IL, USA) and 1 μg of RNA was reverse-transcribed using
the iScript™ cDNA synthesis kit (Bio-Rad). The following qPCR
primers were used: sense HDAC1 5′-TGGAAATCTATCG CCCTCAC -3′ and
antisense HDAC1 5′-TCTCTGCATCTGCT TGCTGT-3′; sense DNMT1
5′-GAGCTACCACGCAGAC ATCA-3′ and antisense DNMT1 5′-CGAGGAAGTAGAA
GCGGTTG-3′; sense UHRF1 5′-CTGGGGGATGATTCT CTGAA-3′ and antisense
UHRF1 5′-CTCTTCCGTCTCA TGGGGT-3′; sense p21 5′-ATGGAACTTCGACTTTGTC
ACC-3′ and antisense p21 5′-AGGCACAAGGGTACAA GACAGT-3′; sense
β-actin 5′-AGCGAGCATCCCCCAAA GTT-3′ and antisense β-actin
5′-GGGCACGAAGGCTC ATCATT-3′.
Western blot analysis
Total cell lysates were loaded onto SDS-PAGE and
transferred to PVDF (GE Healthcare Life Sciences, Piscataway, NJ,
USA). The membranes were incubated overnight at 4°C with the
primary antibodies. The following primary antibodies were used:
anti-DNMT1 (Sigma-Aldrich), anti-UHRF1 (BD Bioscience), anti-HDAC1
(Abcam), anti-p53 (Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA), anti-p21 (Abcam) and anti-β-actin (Sigma-Aldrich). On the
following day, the membranes were washed for 10 min, 3 times each,
and incubated with the secondary antibody: polyclonal anti-rabbit
antibody (Invitrogen, Carlsbad, CA, USA) or monoclonal anti-mouse
antibody (Invitrogen). The immunoreactive proteins were detected
using enhanced chemiluminescence (Thermo Scientific). Immunoblots
were quantified using the ImageMaster densitometry program.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed using the Magna ChIP kit
(Millipore) according to the manufacturer’s protocol. The following
primers were used: sense p21 5′-TGGACTGGGCACTCTTGTCC -3′ and
antisense p21 5′-CA-GAGTAACAGGCTAAGGTT-3′. Anti-DNMT1
(Sigma-Aldrich), anti-UHRF1 (BD Biosciences), anti-HDAC1 (Abcam)
and anti-p53 (Santa Cruz Biotechnology Inc.) antibodies were used
to immunoprecipitate chromatin fragments.
Cell cycle analysis
Cells were treated with 1 μM Naph or/and 10 Gy IR
for 24 h. The cells were trypsinized and resuspended in PBS. Then,
cells were centrifuged and washed in PBS. Following fixation in
cold 70% ethanol for 30 min at 4°C, the cells were stained with PI
(40 μg/ml) and RNAse A (50 μg/ml) prior to analysis. The stained
cells were subjected to cell cycle analysis using FACSAria (BD
Biosciences).
Apoptosis analysis
The Annexin V analysis was carried out using the PE
Annexin V apoptosis detection kit (BD Biosciences). After treatment
of 1 μM Naph or/and 10 Gy, cells were washed in cold PBS and then
resuspended in 1X binding buffer and incubated with PE Annexin V
(2.5 μg/ml)-conjugated primary antibody and 7-amino-actinomycin
(7-AAD, 5 μl) for 15 min on ice. Following incubation, PI (10
μg/ml) was added to the suspension, and the cells were analyzed by
FACSAria (BD Biosciences).
Results
Naph and IR inhibit proliferation of
human MCF-7 breast cancer cells
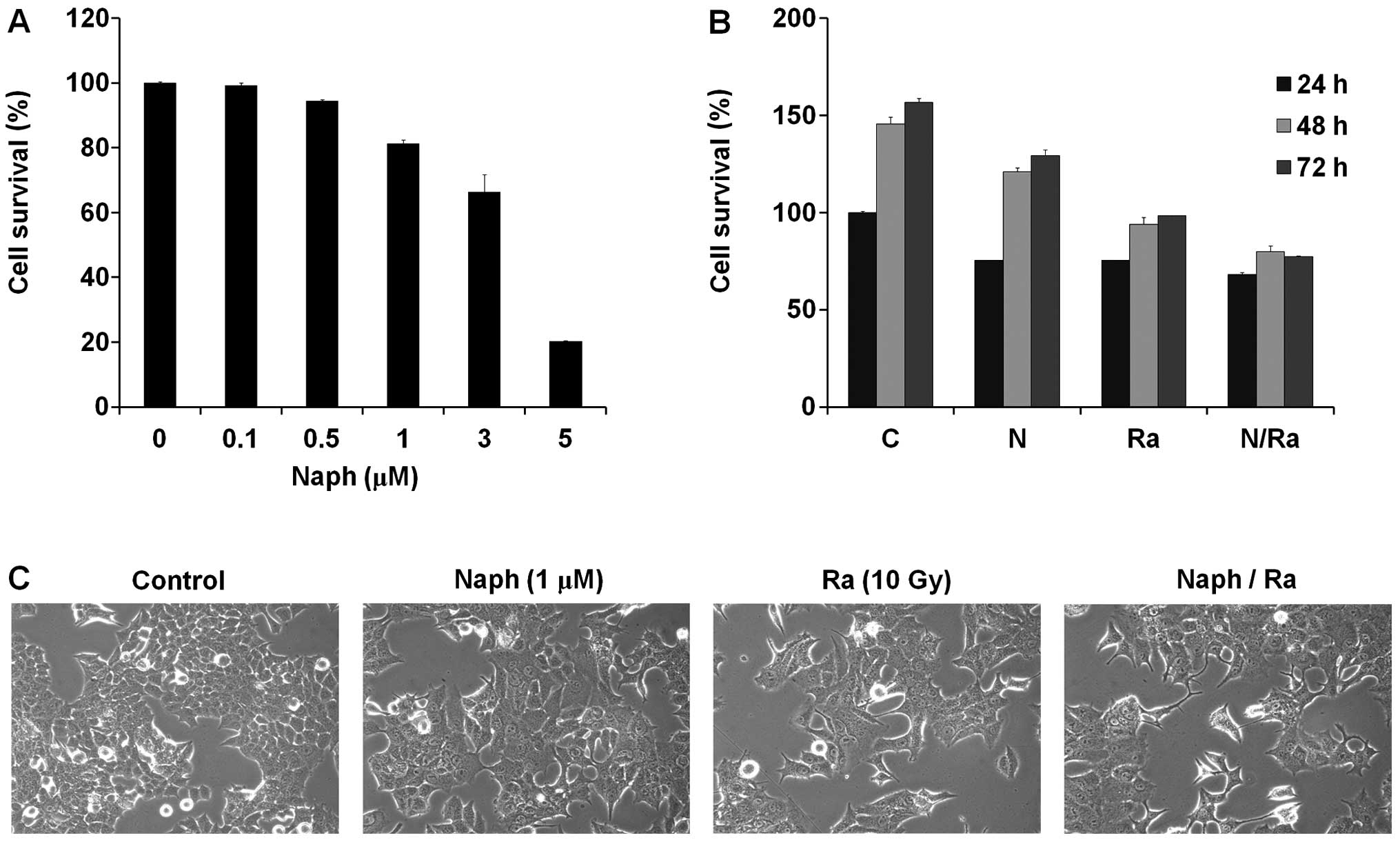
Since Naph and/or ionizing radiation (IR) modulate
cell proliferation or viability, we first examined the regulatory
effect of Naph and IR on cell growth of MCF-7 cells. We treated
MCF-7 cells with Naph for 48 h and cell viability was measured by
the MTT assay. The viability of MCF-7 cells treated with Naph was
decreased in a dose-dependent manner (Fig. 1A). We used the 1 μM of Naph for
further experiments to observe its synergistic effect with
radiation. To investigate the synergistic effect of Naph and IR,
MCF-7 cells were treated for 24, 48 and 72 h with Naph or 10 Gy of
IR as a single treatment, or in combination. The results from the
MTT assay showed that the combination of Naph and IR was more
robust than that of Naph or IR single-treatment (Fig. 1B). MCF-7 cells typically exhibit a
cobblestone-like appearance and tight cell-cell junction which is
the characteristic of the epithelial phenotype (37,38),
but when the cells were treated with Naph and IR, morphological
changes and less number of prominent cells were observed (Fig. 1C).
Naph and IR upregulate p21 expression and
downregulate the expression of DNMT1, UHRF1 and HDAC1
Several studies have shown that the
DNMT1/UHRF1/HDAC1 complex negatively regulates the expression of
p21, which inhibits cell proliferation (31–36).
Since Naph and IR decrease MCF-7 cell proliferation, we first
determined the expression levels of p21 and its corepressors,
DNMT1, UHRF1 and HDAC1 after treatment with Naph. We treated the
MCF-7 cells with Naph at a concentration of 1, 3 and 5 μM for 24 h.
The results of Real-time PCR indicated that Naph downregulated
DNMT1, UHRF1 and HDAC1 mRNA expressions in a dose-dependent manner
(Fig. 2A). In order to determine
the combinatorial effect of Naph and IR on the expression of these
genes, we treated the MCF-7 cells with Naph and IR under the
indicated conditions. The MCF-7 cells were pre-incubated with Naph
for 2 h prior to exposure to IR. The results showed that Naph
significantly enhanced IR-induced p21 mRNA induction via decrease
in DNMT1, UHRF1 and HDAC1 in MCF-7 cells (Fig. 2B). Since p21 is a downstream target
of p53 which plays a key role in the regulation of cell cycle and
apoptosis, we measured the protein levels of p53 and p21 together
with levels of repressive factors including DNMT1, UHRF1 and HDAC1.
In MCF-7 cells, the expression levels of p53 and its downstream p21
were increased after 10 Gy IR.
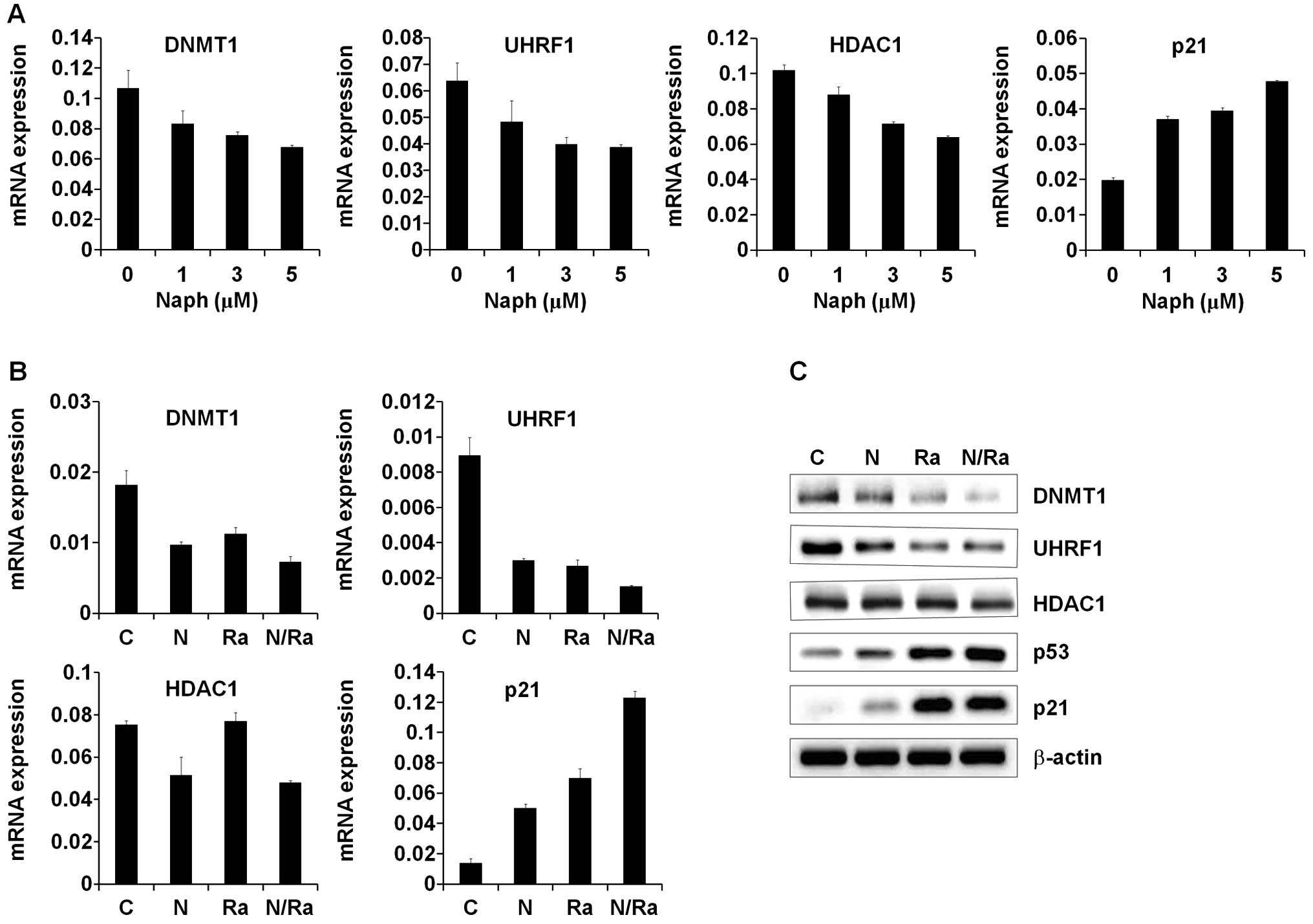 | Figure 2Expression of p21 and
DNMT1/UHRF1/HDAC1 in MCF-7 cells after treatment with Naph and/or
IR. (A) DNMT1, UHRF1, HDAC1 and p21 mRNA expression patterns in
MCF-7 cells under 1, 3 and 5 μM. Naph conditions were assessed by
quantitative RT-PCR. C, untreated condition; N1, naphthazarin (1
μM); N3, naphthazarin (3 μM); N5, naphthazarin (5 μM). (B) DNMT1,
UHRF1, HDAC1 and p21 mRNA expression patterns in MCF-7 cells
treated with 1 μM Naph, IR and/or combined conditions assessed by
quantitative RT-PCR. Results represent mRNA levels normalized to
the levels of GAPDH mRNA. Relative DNMT1, UHRF1, HDAC1 and p21 mRNA
levels are shown as mean ± standard deviation of three independent
experiments. C, untreated condition; N, naphthazarin (1 μM); R,
ionizing radiation (IR, 10 Gy); NR, Naph (1 μM) + IR (10 Gy). (C)
Western blot analyses of DNMT1, UHRF1, HDAC1, p53 and p21 protein
expression patterns in MCF-7 cells treated with Naph (1 μM) and IR
and/or combined conditions. |
Furthermore, expression levels of p53 and p21 were
enhanced in cells treated with a combination of 1 μM Naph and 10 Gy
IR. On the contrary, the expression levels of DNMT1, UHRF1 and
HDAC1 were decreased after 10 Gy of irradiation. The decreased
expression levels of DNMT1, UHRF1 and HDAC1 were further reduced in
cells treated with a combination of 1 μM Naph and 10 Gy IR
(Fig. 2C). These data suggest that
Naph is a potential radiosensitizer that increases the sensitivity
to IR-induced cell death in breast cancer.
DNMT1/UHRF1/HDAC1 complex and p53 are
reciprocally localized on the p21 promoter in MCF-7 cells under
treatment with Naph and IR
UHRF1 recruits transcriptional repressors DNMT1 and
HDAC1 through its distinct domains. Moreover, it is known that
UHRF1 recruits and cooperates with DNMT1 and HDAC1 on the promoter
of p21, thereby inhibiting the expression of p21 (31–36).
In order to investigate the occupancy of DNMT1/UHRF1/HDAC1 and p53
on the p21 promoter under various conditions such as Naph, IR and
NaphsIR treatments, we performed the chromatin immunoprecipitation
(ChIP) assay. As expected, binding of DNMT1, UHRF1 and HDAC1 to the
p21 promoter after IR and Naph treatment gradually decreased when
compared to that under untreated conditions (Fig. 3A–C). In contrast, the occupancy of
p53 on the p21 promoter dramatically increased after combinatorial
treatment of IR and Naph (Fig.
3D). Taken together, these results demonstrate that
dissociation of DNMT1/UHRF1/HDAC1 from the p21 promoter during
treatment with Naph and IR enhances the recruitment of p53 to the
p21 promoter, thereby activating the transcription of p21 in MCF-7
cells.
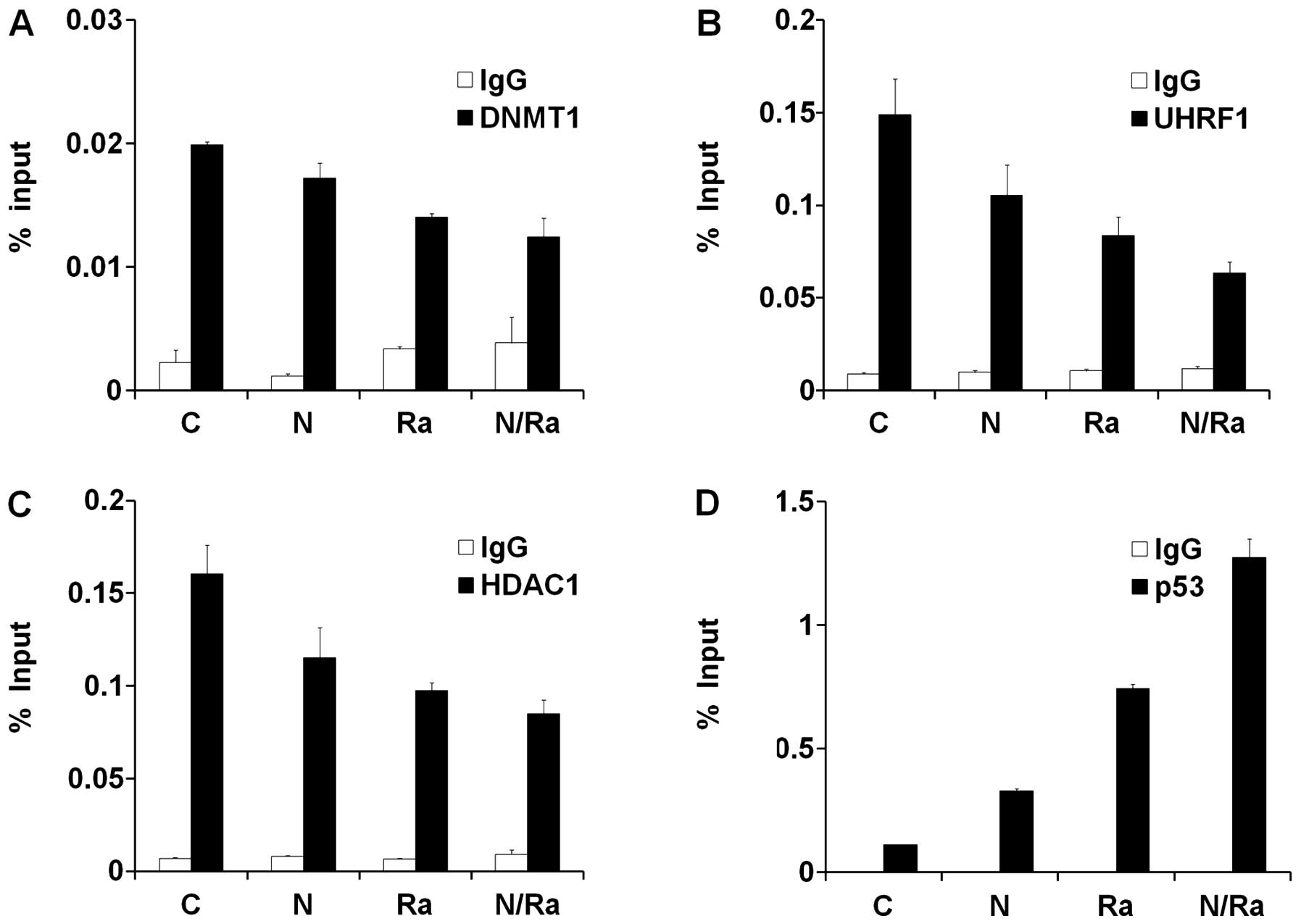 | Figure 3Localization of DNMT1/UHRF1/HDAC1
complex and p53 on the p21 promoter in MCF-7 cells after treatment
with Naph and IR. Chromatin immunoprecipitation (ChIP) assay for
DNMT1 (A), UHRF1 (B), HDAC1 (C) and p53 (D) at the p21 gene
promoter region in MCF-7 cells under 1 μM Naph, 10 Gy IR, and/or
combined conditions. Cross-linked and sheared chromatin was
immunoprecipitated with the anti-DNMT1 antibody (white bar, left
upper panel), the anti-UHRF1 antibody (white bar, right upper
panel), the anti-HDAC1 antibody (white bar, left bottom panel), the
anti-p53 antibody (white bar, right bottom panel), and the anti-IgG
antibody (black bar). The results are shown as a percentage of the
chromatin input. ChIP samples were quantified by RT-PCR. Data
represent mean ± standard deviation of triplicates. Representative
data from three independent experiments. |
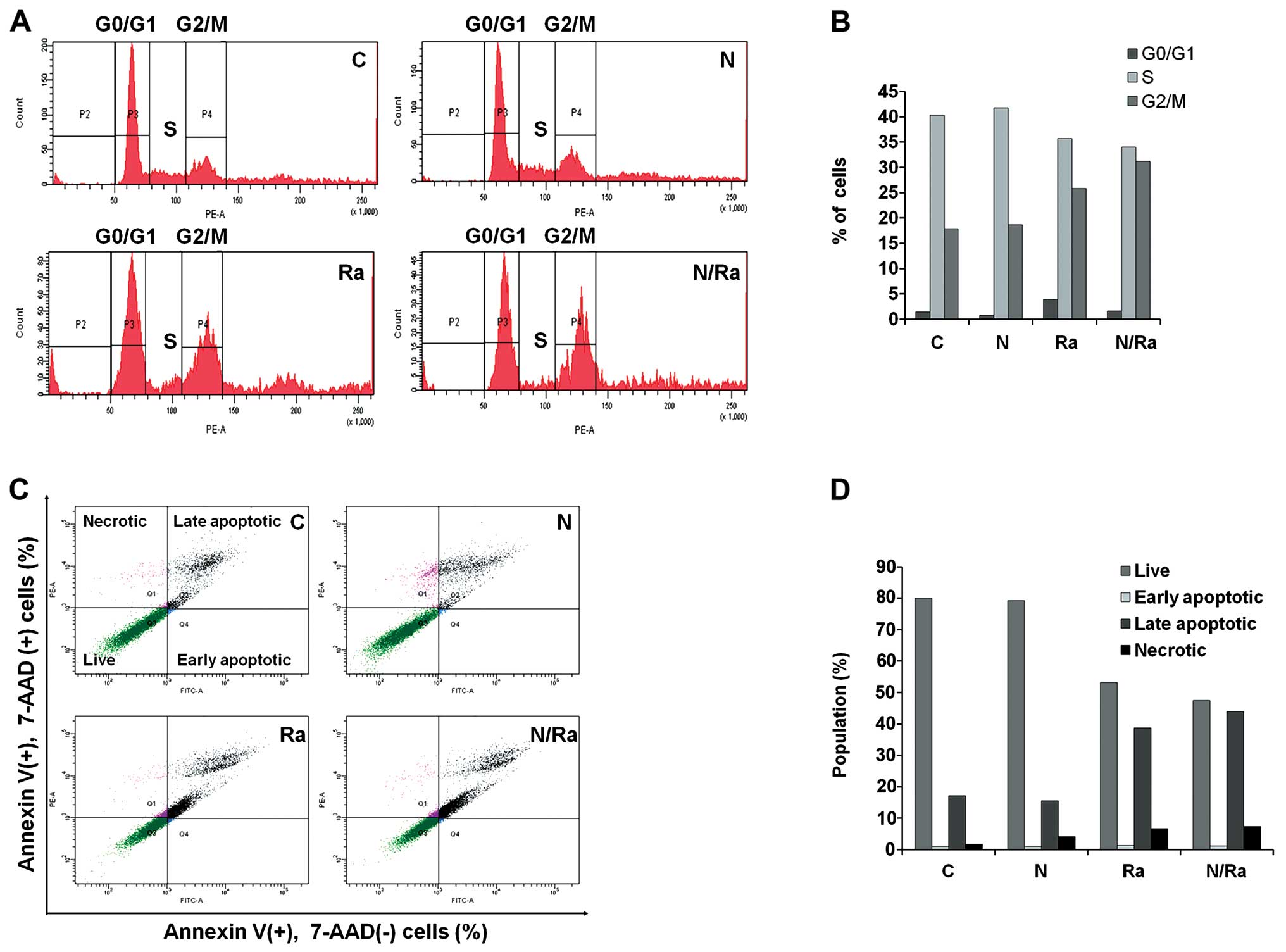
Combination of Naph and IR induces G2/M
cell cycle arrest and apoptosis in MCF-7 cells
Since we identified that mRNA and protein levels of
cell cycle related p53 and p21 are regulated by Naph and/or IR, we
next investigated whether Naph and IR induce cell cycle arrest in
the MCF-7 cells using flow cytometry (FACSAria). In controls, flow
cytometry analysis showed 1.5% of cells in G0/G1 phase, 40.3% of
cells in S phase, and 17.9% of cells in G2/M phase. In contrast, in
cells treated with 1 μM Naph, the proportions of cells in G0/G1, S,
and G2/M phases were 0.8, 41.8 and 18.7%, respectively, and the
proportions of cells exposed to 10 Gy IR in G0/G1, S, and G2/M
phases were 3.9, 35.7 and 25.8%, respectively. When cells were
treated with 1 μM Naph and IR, the proportions of cells in G0/G1,
S, and G2/M phases were 1.6, 34.0 and 31.2%, respectively. These
results suggest that combinatorial treatment of Naph and IR induces
G2/M phase arrest compared with single treatment (Fig. 4A and B).
Next, we investigated whether Naph and IR induce
MCF-7 cell apoptosis using the Annexin V/7-AAD double staining kit.
The combinatorial effect of Naph and IR was evaluated after 48 h of
treatment. Cells negative for 7-AAD and positive for PE Annexin V
were regarded as early apoptotic cells (Annexin V+,
7-AAD−); 7-AAD and Annexin V positive cells were defined
as late apoptotic cells (Annexin V+, 7-AAD+);
7-AAD positive and PE Annexin V negative cells were considered as
necrotic cells (Annexin V−, 7-AAD+). The
combined treatment of 1 μM Naph and 10 Gy IR in MCF-7 cells
significantly increased the apoptotic effect compared with single
treatment of IR or Naph. Increased number of late apoptotic cells
were observed in the combined treatment group (43.9%) compared with
the Naph alone group (15.6%) and IR alone group (38.7%). However,
the cells treated with Naph and IR showed a slightly increased
necrotic portion (7.3%) and there was a moderate decrease in the
number of live cells (47.5%) in comparison with cells treated with
Naph or IR treatment alone (Fig. 4C
and D). These data suggest that combinatorial treatment of Naph
and IR has a potential synergistic effect on the regulation of cell
cycle arrest and apoptosis in MCF-7 cells.
Discussion
Radiotherapy is used in over 50% of patients during
the course of cancer treatment both as a curative modality and for
palliation. However, radioresistance and toxic side effects
impeding dose escalation are major obstacles to the success of
radiation therapy (39,40). Therefore, there is a critical need
for the discovery of a novel radiosensitizer that can improve the
efficacy of radiotherapy. Several studies have reported that
plant-derived natural products have cancer chemopreventive and
chemotherapeutic properties. The use of natural products as
antitumor agents or radiosensitizers for the management of human
cancers is an attractive idea because they are readily available
and exhibit little or no toxicity (3–11).
Naph is a natural component of the roots of several
members of the genus Boraginaceae and is well-known to have
anti-tumor cytotoxic effects in cancer cells (12–16).
However, the biological role and mechanism of cytotoxicity and the
specific molecular target of Naph in cancer cells are yet to be
established. In this study, we demonstrated the role and mechanism
of Naph as an effective radiosensitizer and adjuvant therapy in
breast cancer. We first evaluated the effect of Naph and IR on the
proliferation of MCF-7 breast cancer cells. The results showed that
the growth of MCF-7 cells treated with Naph was decreased in a
concentration-dependent manner (Fig.
1A).
To diminish the toxic side effect of Naph, we used a
low dose (1 μM) of Naph for further experiments to observe its
synergistic effect with radiation. Indeed, we observed that the
viability of cells treated with a low concentration (1 μM) of Naph
and IR of 10 Gy was decreased more than that of MCF-7 cells treated
with single Naph and 10 Gy of IR. We also demonstrated that the
cells treated with Naph and IR of 10 Gy showed morphologic changes
and less number of the prominent cells than untreated cells
(Fig. 1C). Therefore, these
results suggest that combinational treatment of Naph and IR more
effectively inhibits cancer cell viability and proliferation than a
single treatment.
Several studies have reported that DNMT1/UHRF1/HDAC1
complex negatively regulates the expression of p21, which inhibits
cell proliferation (31–36). To determine the mechanism of
antitumor cytotoxicity and the specific molecular target of Naph,
we next examined the expression of p21 and its regulatory factors
(DNMT1/UHRF1/HDAC1). Our results of Real-time PCR showed that Naph
downregulated DNMT1, UHRF1, and HDAC1 mRNA expressions in a
dose-dependent manner (Fig. 2A).
Moreover, we identified that the expression level of p21 was
dramatically upregulated by co-treatment of Naph and IR, whereas
the expression levels of DNMT1, UHRF1, and HDAC1 were downregulated
(Fig. 2B and C).
It is well documented that p21 is a downstream
target of p53, which regulates transcription, DNA repair, cell
cycle arrest, differentiation, senescence, genomic instability,
apoptosis, and survival as well as glucose metabolism, oxidative
stress, and angiogenesis (31–34).
Therefore, we measured the protein levels of p53 and p21 together
with levels of repressive factors, including DNMT1, UHRF1, and
HDAC1. Our results showed that the expression levels of p53 and its
downstream p21 were enhanced in cells co-treated with 1 μM Naph and
10 Gy IR compared to Naph or IR alone treated cells (Fig. 2C). These results support that Naph
increases the sensitivity to IR-induced cell cycle arrest or
apoptosis through the p53-p21 pathway in breast cancer. In order to
identify the regulatory mechanism of p21 expression, we performed
the ChIP analysis. Our ChIP results revealed that the repressive
factors, DNMT1/UHRF1/HDAC1 were dissociated from the p21 promoter
by treatment with Naph and IR compared with that under untreated
conditions. On the contrary, the occupancy of p53 on the p21
promoter was significantly increased after combinational treatment
of Naph and IR (Fig. 3).
Therefore, these results demonstrate that DNMT1/UHRF1/HDAC1 complex
and p53 are reciprocally localized on the p21 promoter under
treatment with Naph and IR, thereby regulating the transcription of
p21 in MCF-7 cells.
Deregulation of the cell cycle and apoptosis are
frequent occurrences in cancer development. In this study, p53 and
its target gene, p21, were dependently regulated by Naph and/or IR.
We investigated whether Naph and IR induce cell cycle arrest in the
MCF-7 cells using flow cytometry. The cells treated with 1 μM Naph
or 10 Gy IR alone showed a slight increase in the proportion of
cells in G2/M phase compared with the untreated cells. However, the
cells treated with 1 μM Naph and 10 Gy IR together showed a
significant increase in the proportion of cells in G2/M phase
compared with the cells treated with Naph and IR alone (Fig. 4A and B). These results suggest that
Naph and IR induce G2/M phase arrest. Moreover, the
radiosensitizing effect of Naph was assessed through the apoptosis
assay. Our data showed that combined treatment of 1 μM Naph and 10
Gy IR in MCF-7 cells significantly increased the apoptotic effect
compared with single treatment of IR or Naph (Fig. 4C and D).
In conclusion, our findings show that combinational
treatment of Naph and IR dramatically inhibits cell proliferation.
Furthermore, the dissociation of DNMT1/UHRF1/HDAC1 from the p21
promoter during treatment with Naph and IR enhances the recruitment
of p53 to the p21 promoter, thereby inducing cell cycle arrest and
apoptosis in MCF-7 cells. These findings lead us to believe that
Naph might be a potential radiosensitizer in breast cancer
(Fig. 5).
Acknowledgements
This study was supported by the National R&D
Program through the Dongnam Institute of Radiological & Medical
Sciences (DIRAMS) (50595–2014) and the [Basic Science Research
Program] through the National Research Foundation of Korea (NRF)
(NRF-2014 M2A2A 7043665) funded by the Ministry of Science, ICT and
Future Planning.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bozovic-Spasojevic I, Azambuja E,
McCaskill-Stevens W, Dinh P and Cardoso F: Chemoprevention for
breast cancer. Cancer Treat Rev. 38:329–339. 2012. View Article : Google Scholar
|
|
3
|
Zhang Y, Luo M, Zu Y, et al: Dryofragin, a
phloroglucinol derivative, induces apoptosis in human breast cancer
MCF-7 cells through ROS-mediated mitochondrial pathway. Chem Biol
Interact. 199:129–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krifa M, Pizzi A, Mousli M, Chekir-Ghedira
L, Leloup L and Ghedira K: Limoniastrum guyonianum aqueous gall
extract induces apoptosis in colorectal cancer cells by inhibiting
calpain activity. Tumour Biol. 35:7877–7885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ziech D, Anestopoulos I, Hanafi R, et al:
Pleiotrophic effects of natural products in ROS-induced
carcinogenesis: The role of plant-derived natural products in oral
cancer chemoprevention. Cancer Lett. 327:16–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Magee PJ, Owusu-Apenten R, McCann MJ, Gill
CI and Rowland IR: Chickpea (Cicer arietinum) and other
plant-derived protease inhibitor concentrates inhibit breast and
prostate cancer cell proliferation in vitro. Nutr Cancer.
64:741–748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang Y, DeMarco VG and Nicholl MB:
Resveratrol enhances radiation sensitivity in prostate cancer by
inhibiting cell proliferation and promoting cell senescence and
apoptosis. Cancer Sci. 103:1090–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, Fan H, Liu Y, et al: Curcumin
enhances the radio-sensitivity in nasopharyngeal carcinoma cells
involving the reversal of differentially expressed long non-coding
RNAs. Int J Oncol. 44:858–864. 2014.PubMed/NCBI
|
|
9
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srivastava V, Negi AS, Kumar JK, Gupta MM
and Khanuja SP: Plant-based anticancer molecules: A chemical and
biological profile of some important leads. Bioorg Med Chem.
13:5892–5908. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kourounakis AP, Assimopoulou AN,
Papageorgiou VP, Gavalas A and Kourounakis PN: Alkannin and
shikonin: Effect on free radical processes and on inflammation - a
preliminary pharmacochemical investigation. Arch Pharm (Weinheim).
335:262–266. 2002. View Article : Google Scholar
|
|
12
|
Acharya BR, Bhattacharyya S, Choudhury D
and Chakrabarti G: The microtubule depolymerizing agent
naphthazarin induces both apoptosis and autophagy in A549 lung
cancer cells. Apoptosis. 16:924–939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kundaković T, Stanojković T, Juranić Z and
Kovacević N: Cytotoxicity in vitro of naphthazarin derivatives from
onosma arenaria. Phytother Res. 20:602–604. 2006. View Article : Google Scholar
|
|
14
|
Johansson AC, Norberg-Spaak L and Roberg
K: Role of lysosomal cathepsins in naphthazarin- and fas-induced
apoptosis in oral squamous cell carcinoma cells. Acta Otolaryngol.
126:70–81. 2006. View Article : Google Scholar
|
|
15
|
Song GY, Kim Y, You YJ, Cho H, Kim SH, Sok
DE and Ahn BZ: Naphthazarin derivatives (VI): Synthesis, inhibitory
effect on DNA topoisomerase-I and antiproliferative activity of 2-
or 6-(1-oxyiminoalkyl)-5,8-dimethoxy-1,4-naphthoquinones. Arch
Pharm (Weinheim). 333:87–92. 2000. View Article : Google Scholar
|
|
16
|
Kim JA, Lee EK, Park SJ, Kim ND, Hyun DH,
Lee CG, Lee JH, Yang KM, Heo K and Son TG: Novel anti-cancer role
of naphthazarin in human gastric cancer cells. Int J Oncol.
40:157–162. 2012.
|
|
17
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YH and Bae YS: Phospholipase D2
downregulation induces cellular senescence through a reactive
oxygen species-p53-p21Cip1/WAF1 pathway. FEBS Lett. 588:3251–3258.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saha K, Adhikary G, Kanade SR, Rorke EA
and Eckert RL: p38δ regulates p53 to control p21Cip1 expression in
human epidermal keratinocytes. J Biol Chem. 289:11443–11453. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yadav V, Sultana S, Yadav J and Saini N:
Gatifloxacin induces S and G2-phase cell cycle arrest in pancreatic
cancer cells via p21/p27/p53. PLoS One. 7:e477962012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan M, Rasul A, Yi F, Zhong L and Ma T:
Jaceosidin induces p53-dependent G2/M phase arrest in U87
glioblastoma cells. Asian Pac J Cancer Prev. 12:3235–3238.
2011.PubMed/NCBI
|
|
23
|
Powell BL, van Staveren IL, Roosken P,
Grieu F, Berns EM and Iacopetta B: Associations between common
polymorphisms in TP53 and p21WAF1/Cip1 and phenotypic features of
breast cancer. Carcinogenesis. 23:311–315. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Darcy KM, Brady WE, McBroom JW, et al:
Associations between p53 overexpression and multiple measures of
clinical outcome in high-risk, early stage or
suboptimally-resected, advanced stage epithelial ovarian cancers A
Gynecologic Oncology Group study. Gynecol Oncol. 111:487–495. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Unoki M, Nishidate T and Nakamura Y:
ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG
through its SRA domain. Oncogene. 23:7601–7610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bostick M, Kim JK, Estève PO, Clark A,
Pradhan S and Jacobsen SE: UHRF1 plays a role in maintaining DNA
methylation in mammalian cells. Science. 317:1760–1764. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Achour M, Jacq X, Rondé P, et al: The
interaction of the SRA domain of ICBP90 with a novel domain of
DNMT1 is involved in the regulation of VEGF gene expression.
Oncogene. 27:2187–2197. 2008. View Article : Google Scholar
|
|
28
|
Achour M, Fuhrmann G, Alhosin M, et al:
UHRF1 recruits the histone acetyltransferase Tip60 and controls its
expression and activity. Biochem Biophys Res Commun. 390:523–528.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alhosin M, Sharif T, Mousli M,
Etienne-Selloum N, Fuhrmann G, Schini-Kerth VB and Bronner C:
Down-regulation of UHRF1, associated with re-expression of tumor
suppressor genes, is a common feature of natural compounds
exhibiting anti-cancer properties. J Exp Clin Cancer Res.
30:412011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bronner C, Achour M, Arima Y, Chataigneau
T, Saya H and Schini-Kerth VB: The UHRF family: Oncogenes that are
drugable targets for cancer therapy in the near future. Pharmacol
Ther. 115:419–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Unoki M, Brunet J and Mousli M: Drug
discovery targeting epigenetic codes: The great potential of UHRF1,
which links DNA methylation and histone modifications, as a drug
target in cancers and toxoplasmosis. Biochem Pharmacol.
78:1279–1288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JK, Estève PO, Jacobsen SE and Pradhan
S: UHRF1 binds G9a and participates in p21 transcriptional
regulation in mammalian cells. Nucleic Acids Res. 37:493–505. 2009.
View Article : Google Scholar :
|
|
33
|
Yamaguchi T, Cubizolles F, Zhang Y,
Reichert N, Kohler H, Seiser C and Matthias P: Histone deacetylases
1 and 2 act in concert to promote the G1-to-S progression. Genes
Dev. 24:455–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saldanha SN, Kala R and Tollefsbol TO:
Molecular mechanisms for inhibition of colon cancer cells by
combined epigenetic-modulating epigallocatechin gallate and sodium
butyrate. Exp Cell Res. 324:40–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tien AL, Senbanerjee S, Kulkarni A, et al:
UHRF1 depletion causes a G2/M arrest, activation of DNA damage
response and apoptosis. Biochem J. 435:175–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vijayaraghavalu S, Dermawan JK, Cheriyath
V and Labhasetwar V: Highly synergistic effect of sequential
treatment with epigenetic and anticancer drugs to overcome drug
resistance in breast cancer cells is mediated via activation of p21
gene expression leading to G2/M cycle arrest. Mol Pharm.
10:337–352. 2013. View Article : Google Scholar :
|
|
37
|
Zhang X, Li X, Zhang N, Yang Q and Moran
MS: Low doses ionizing radiation enhances the invasiveness of
breast cancer cells by inducing epithelial-mesenchymal transition.
Biochem Biophys Res Commun. 412:188–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH,
Way TD and Chen WJ: 3,5,4′-Trimethoxystilbene, a natural
methoxylated analog of resveratrol, inhibits breast cancer cell
invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin
signaling cascades and reversal of epithelial-mesenchymal
transition. Toxicol Appl Pharmacol. 272:746–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo H, Wang L, Schulte BA, Yang A, Tang S
and Wang GY: Resveratrol enhances ionizing radiation-induced
premature senescence in lung cancer cells. Int J Oncol.
43:1999–2006. 2013.PubMed/NCBI
|
|
40
|
Hall EJ and Wuu CS: Radiation-induced
second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol
Biol Phys. 56:83–88. 2003. View Article : Google Scholar : PubMed/NCBI
|