Introduction
The astrocytic tumors are a class of cancer whose
treatment appears to be one of the greatest challenges in oncology.
Glioblastoma multiforme (GBM) is the most common form of astrocytic
tumor and is known to be particularly aggressive due to its speed
in invading the adjacent brain structures (1). This tumor represents the 40% of all
primary brain tumors and 78% of the malignant tumors of the central
nervous system (CNS) (2). Surgical
resection, current chemotherapy or/and radiotherapy have not yet
produced appreciable results in order to improve the survival or
the well-being of patients (3).
Therefore, a number of studies are in progress to search for new
drugs known to be more effective and with fewer side-effects,
compared to existing chemotherapeutic. Among the putative
molecules, phenolic compounds are of great interest in
epidemiologic studies. These studies have demonstrated the
protective effects of phenolic compounds against damage induced by
exogenous and endogenous mutagens (4,5).
These effects are believed to occur through the regulation of the
signaling pathways, such as nuclear factor-κB (NF-κB), activator
protein-1 (AP-1) or the mitogen-activated protein kinase (MAPK)
(6,7). By modulating these cell signaling
pathways, polyphenols activate cell death signals and induce
apoptosis in precancerous or malignant cells resulting in the
inhibition of cancer development or progression (8,9).
However, regulation of cell signaling pathways by dietary
polyphenols can also lead to cell proliferation/survival or
inflammatory responses due to the increased expression of several
genes. Many recent studies have shown that polyphenols are able to
influence gene expression at the epigenetic level inducing the
transcription of small non-coding RNAs, particularly microRNAs
(miRNAs), that are able to inhibit the expression of target genes
interfering with the translation of their RNA messengers (10,11).
MicroRNAs are single strand non-coding RNAs ~19–25 nt in length,
that are transcribed starting from intergenic and intronic
sequences. They are released into the extracellular compartment by
various proteins, lipids or exosomes, inducing a spread of
molecular signals in biological fluids (12). Many studies have confirmed the
effects of different miRNAs in various physiological processes such
as proliferation, apoptosis and cell development. Consequently, a
dysregulation in their expression, plays a fundamental role in the
onset, progression and dissemination of malignant cells (13–16).
Gallic acid (GA) is one of the emerging polyphenol
candidates for cancer treatment. It has been studied extensively
and has demonstrated its ability to suppress cell viability,
proliferation, invasion and angiogenesis in the human glioma cell
lines (17,18). GA has been identified as both a
pro-oxidant and an antioxidant agent (19). This dual role depends on the
resulting GA:Fe(III) ratio (20),
that comports the activity of GA or as scavengers of reactive
oxygen species (ROS) or as inductor of ROS by depletion of
glutathione (GHS) (21,22). Its anti-inflammatory (23), antimutagenic (24) and anticancer activities have been
reported (25,26). Other studies underline the
genotoxic effects of the antioxidants on human cells suggesting the
use of antioxidants as attractive candidates for improved
chemotherapeutic agents (27).
In the present study, we aimed to evaluate the in
vitro effect of gallic acid (3,4,5-trihydroxybenzoic acid) on
the T98G glioma cell line and to correlate the anti-proliferative
and cell death effects with the expression of the miRNAs
hsa-miR-17-3, hsa-miR-21-5p and hsa-miR-421-5p, already proven to
be involved in the regulation of cancer cell pathways.
hsa-miR-17-3p was reported to act as a tumor
suppressor both in vitro and in vivo for prostatic
cancer and it has been demonstrated that this ability is due to the
suppression of three critical primary mitochondrial antioxidant
enzymes: manganese superoxide dismutase (MnSOD), glutathione
peroxidase-2 (GPX-2) and thioredoxin reductase (TrxR2) (28,29).
hsa-miR-21-5p negatively regulates the expression of p21 protein in
the p53 network (30), and
hsa-miR-421, is a miRNA that downregulates the ataxia
telangiectasia mutated (ATM) gene expression inducing
changes at S-phase level of cell cycle checkpoint and an increasing
of sensitivity to ionizing radiation (31,32).
Moreover, it is involved in the transforming growth factor β
(TGF-β) pathway interfering with the DPC4/Smad4 gene
regulation (33).
Materials and methods
Drug preparation
Gallic acid was purchased from Sigma-Aldrich Co.,
and 100 mg was dissolved in 1 ml of dimethyl sulfoxide (DMSO)
(PubChem CID: 679). Furthermore, the solution was diluted (1:10)
with Eagle’s minimum essential medium (EMEM), subdivided into stock
aliquots that and stored at −20°C. The solution was further diluted
to appropriate concentrations using cell culture medium immediately
before use.
Cell culture
Human glioblastoma T98G cells were obtained from The
European Collection of Cell Cultures (ECACC). These cells were
maintained in EMEM containing 10% calf serum; 100 units/ml
penicillin/streptomycin; 1% sodium pyruvate and 2 mM L-glutamine
(Sigma-Aldrich, St. Louis, MO, USA). The cells were maintained in
exponential growth as monolayers in 75-cm2 plastic
tissue-culture flasks (Corning) and kept in a humidified atmosphere
with 5% CO2 at 37°C.
Mitotic activity index and clonogenic
assay
To evaluate the mitotic activity index (MAI),
confluent cells were harvested from flasks by trypsinization. Cells
(2×104) in a 2-ml complete medium were seeded in 2.5 cm
diameter dishes containing 18×18-mm glass coverslip. After 24 h of
incubation with serial dilution (100-1 μg/ml) of gallic acid,
during the process of taking out the drug, the coverslips were
reset and stained with MGG. The mitotic activity was assessed by
examining ~10 consecutive high-power fields (HPFs) with an Olympus
BX-41 microscope in a blind manner.
For the clonogenic assay, exponentially growing
cells were seeded in 25-cm2 flasks (Corning) at a
density of 100 cells/flask in a 5-ml culture medium. After 24 h the
culture medium was removed and the medium containing GA was added.
After a further 24 h, the medium was replaced with fresh culture
medium and the cells were grown for 12 days, with medium renewal
every 3 days. After discarding the culture medium the cells were
stained with crystal violet (0.5%) for 7 min, rinsed with PBS and
distilled water, then all viable colonies of >50 cells, were
counted. The results were normalized with the data obtained in
unexposed control and expressed as colony forming efficiency, which
is the ratio of the mean number of colonies in the treated
condition to the mean number of colonies in the control
condition.
Proliferation and MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide)
assay
To measure the cell viability, cells were seeded
onto 96-well plate at a density of 1.2×104 in 0.2 ml
medium/well. After a 24-h incubation, the culture medium was
removed and complete medium containing serial dilution (100-1
μg/ml) of gallic acid was added. Each concentration and the control
untreated cells were tested in triplicate. After 24 h of treatment,
50 μl of MTT solution was added to each well and incubated for an
additional 3 h. The medium was then discarded, and the formazan
crystals obtained were solubilized by adding 150 μl of DMSO. The
absorption of formazan in solution was measured at the wavelength
of 570 nm by an ELISA plate reader (Tecan Group Ltd., Mannedorf,
Switzerland). To measure the anti-proliferative effects of gallic
acid, 1×104 cells were seeded onto 96-well plates and
after overnight incubation were treated for 24 h with the same
concentrations of drug described above. The cells were incubated
for 24, 48 and 72 h. An MTT assay was performed at each time-point
for three independent experiments.
Cytofluorimetric analysis
To evaluate the different cell death pathway, cells
were seeded at a density of 2.5×105 in 25-cm2
flasks. After overnight incubation, the culture medium was removed
and cells were incubated with complete medium containing serial
dilutions (100-1 μg/ml) of gallic acid. After 24 h the cells were
harvested by trypsinization and centrifuged for 6 min at 1,200 rpm.
The cell pellet obtained was resuspended in 200 μl of binding
buffer 1X (HEPES/NaOH 100 mM) with 1.4 MNaCl and 25 mM
CaCl2 (pH 7.5). Finally, 7 μl of Annexin V and 3 μl
propidium iodide (PI) were added to the cell suspension, and flow
cytometric analyses were perfomed using a Partec PAS II instrument
(Partec, Munster, Germany).
Wound scratch assay
Cells were seeded at a density of 2×105
in 1-ml complete medium/well, in 24-well plates. After 24 h the
cells were treated with gallic acid as described above. At the end
of the treatment the monolayers were scratched with a 1-ml plastic
pipette tip to create a uniform wound. The wound area was then
examined after 24 h by scratch under a phase-contrast microscope at
×4 magnification. Images of three random fields were taken and the
cell migration ability was expressed by closure of gap
distance.
RNA extraction
Total RNA was extracted from cell pellets using the
RNeasy Mini kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer’s instructions. The quality of RNA was assessed by
determining the RIN (TapeStation; Agilent Technologies). A
quantitative RNA analysis was performed using fluorimetric methods
by means of the Qubit® platform (Invitrogen, Grand
Island, NY, USA) using the Quant-iT RNA assay (declared assay range
between 5–100 ng; sample starting concentration between 250 pg/μl
and 100 ng/μl): 2 μl of RNA was added to 198 μl of working solution
obtained by mixing 1 μl of Qubit™ RNA reagent to 199 μl of Qubit™
RNA buffer. The quantitation was performed following the
calibration of the instrument with the Quant-iT RNA standards (0
and 10 ng/ml).
Real-time reverse transcription-PCR
(qRT-PCR)
Quantitative real-time PCR (qRT-PCR) was performed
using cDNA obtained following the reverse transcription reaction
with the miRCURY LNA™ Universal RT microRNA PCR kit: 4 μl of total
RNA (5 ng/μl), were added to 4 μl of 5X reaction buffer, 2 μl of
enzyme mix, 1 μl of synthetic spike-in and 9 μl of nuclease-free
water and the reaction was performed using a MJ Mini thermal cycler
(Bio-Rad Laboratories, Hercules, CA, USA) for one reaction cycle at
42°C for 60 min, 95°C for 5 min and the reaction products were
immediately cooled at 4°C.
To evaluate the miRNA expression, qRT-PCR reactions
were performed using the Universal cDNA synthesis and
SYBR® Green Master Mix kits. Amplification was performed
in a 10-μl reaction mixture containing 4 μl of 1:80 diluted cDNA, 5
μl of SYBR-Green Master Mix and 1 μl of specific LNA probe.
miR-17-3p LNA probe (ACUGCAGUGAAGGC ACUUGUAG), miR-21-5p LNA probe
(UAGCUUAUCAGAC UGAUGUUGA), miR-421-5p LNA probe (AUCAACAGAC
AUUAAUUGGGCGC), provided by Exiqon, using the following reaction
conditions: a first step at 95°C for 10 min, 45 amplification
cycles of 95°C for 10 sec followed by a step at 60°C for 1 min. U6
small nuclear RNA (snU6) was used to normalize the expression data
of miRNAs and each assay was performed in triplicate using the Eco
Real-Time PCR instrument (Illumina, San Diego, CA, USA). The
results were analyzed by the comparative ct method (ΔΔct method
using the software package of the Eco Real-Time PCR system for the
calculus of the 2−ΔΔct value (34).
Statistical analysis
Data are presented as mean and standard deviation.
Statistical significance was analyzed by t-test to compare two
means using the GraphPad QuickCalcs. P-values <0.05 were
considered significant.
Results
In the first phase of the investigation, the toxic
effects of gallic acid were studied using MTT proliferation test,
mitotic index and clonogenic assay. T98G cells were treated for 24
h with increasing concentrations of GA, ranging from 1 to 100
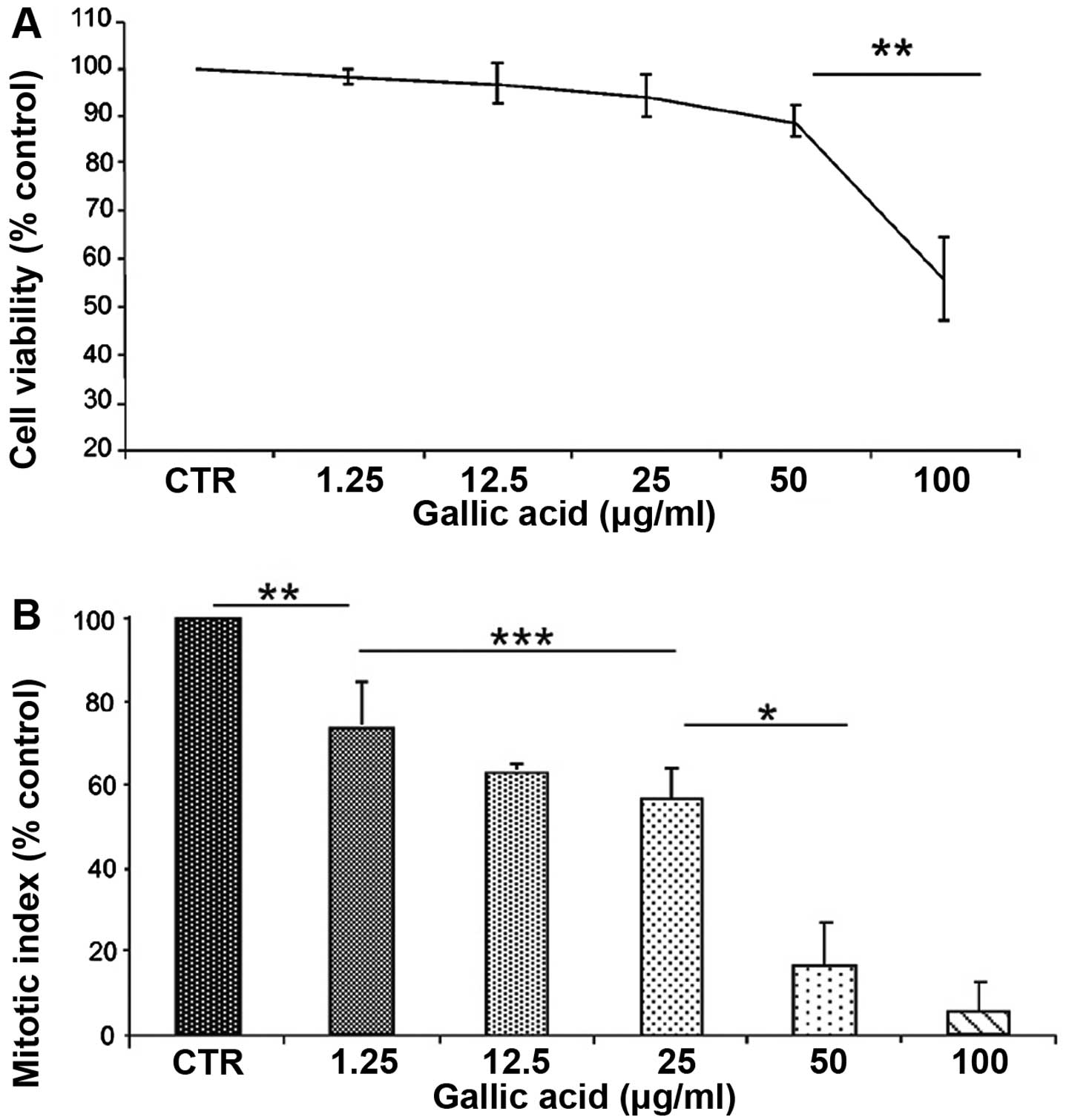
μg/ml. The toxic effect was evaluated determining the decrease in
survival percentage (Fig. 1A) and
MAI percentage (Fig. 1B) in
comparison with the untreated samples. The results, summarized in
Fig. 1, showed a toxic effect of
GA in a concentration-dependent manner that reaches the highest
value at the conditions of 50 and 100 g/ml. This highest value is
correlated to a decrease in surviving cells 12 and 45%,
respectively (P<0.01) and to a reduced mitotic index of 84 and
95% at the same concentrations (P<0.01). Considering cell
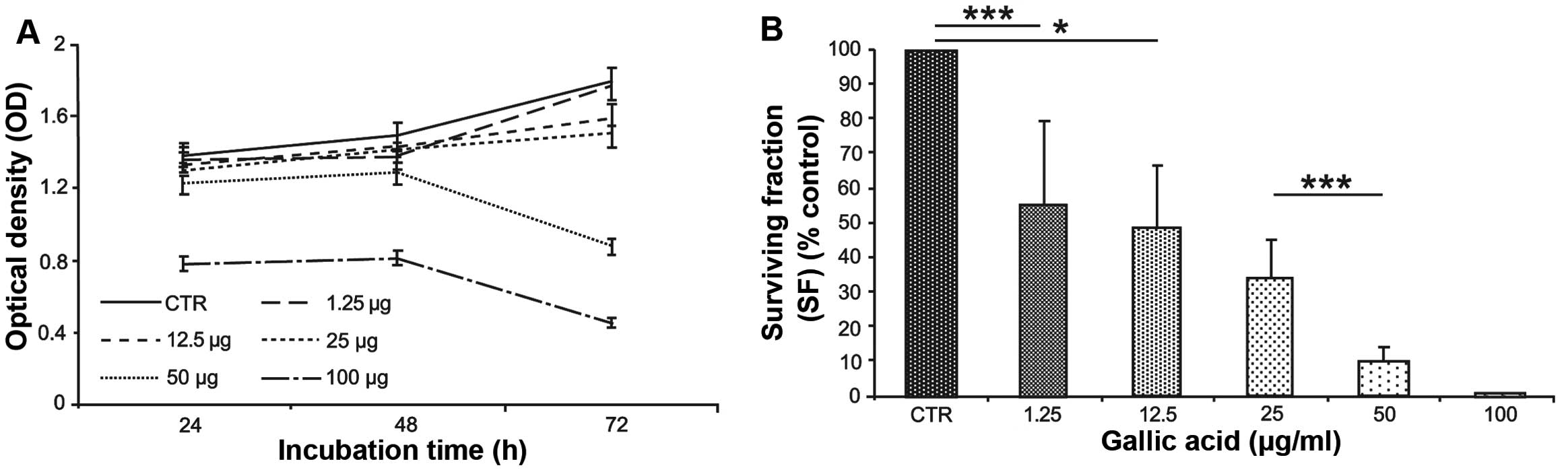
proliferation, the maximum effect is observed after 72 h of
consecutive GA treatment with a proliferation reduction of 45% at
concentration of 100 μg/ml (Fig.
2A). To evaluate the capacity to repair damage induced by GA
treatment, a colony forming assay was performed after the
treatments. It was possible to observe that the number of colonies
is progressively reduced with the increasing concentration of GA
and for cells treated with the highest concentration the clonogenic
capacity is completely compromised (Fig. 2B).
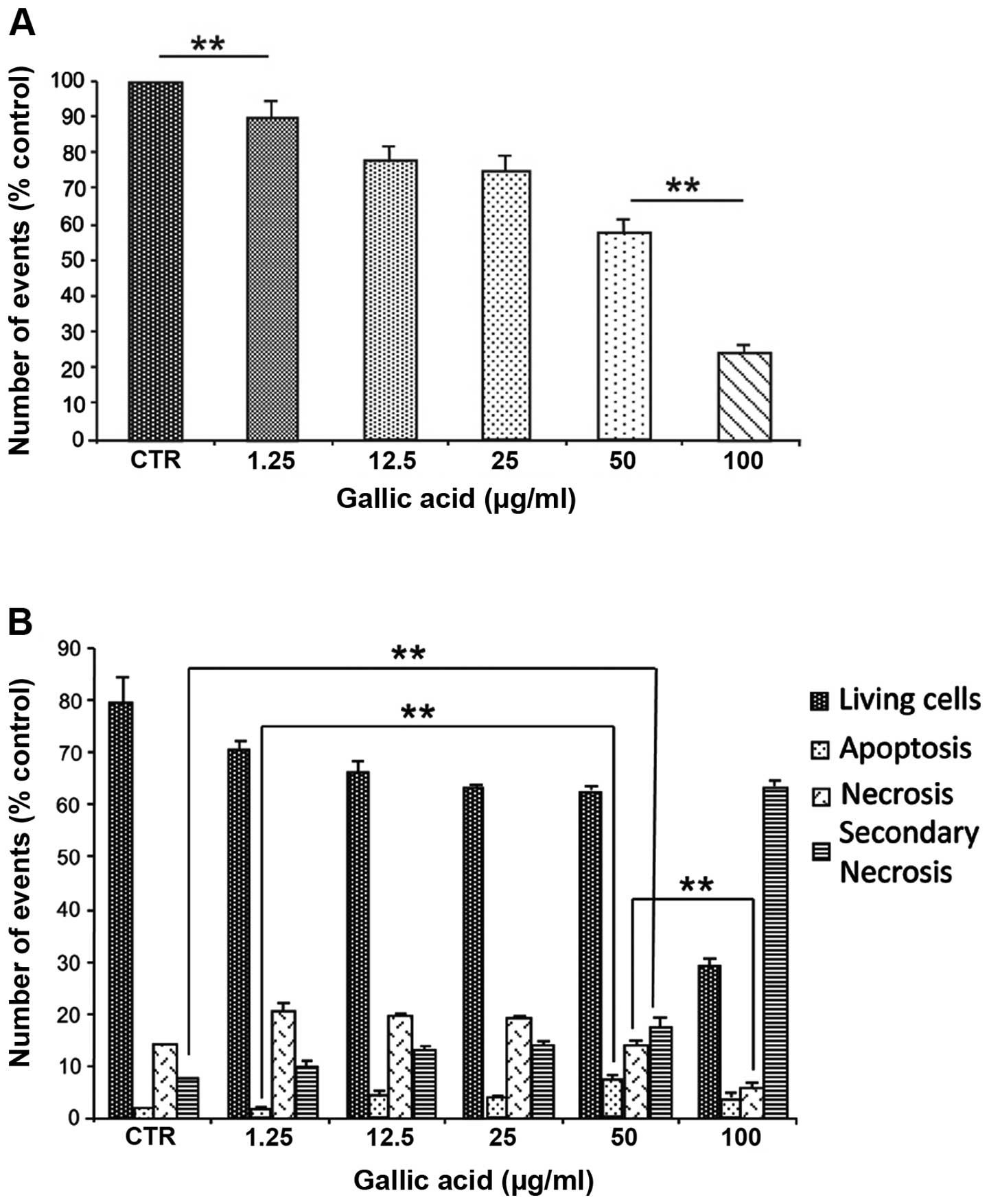
To evaluate the cell death pathway induced by GA a
cytofluorimetric analysis was performed. The results indicated a
progressive decrease in cell numbers with the increasing GA
concentration (Fig. 3A) due to
increase of necrotic (propidium iodide positive cells) and
apoptosis cell fractions (Annexin V- positive cells) (Fig. 3B). At the lowest concentrations, up
to 25 μg/ml, an increase in both necrotic and apoptotic cells were
observed. On the contrary, at higher concentrations, the damaged
cells appeared positive both to Annexin V and propidium iodide
emphasizing a massive damage induced by the GA treatment (Fig. 3B).
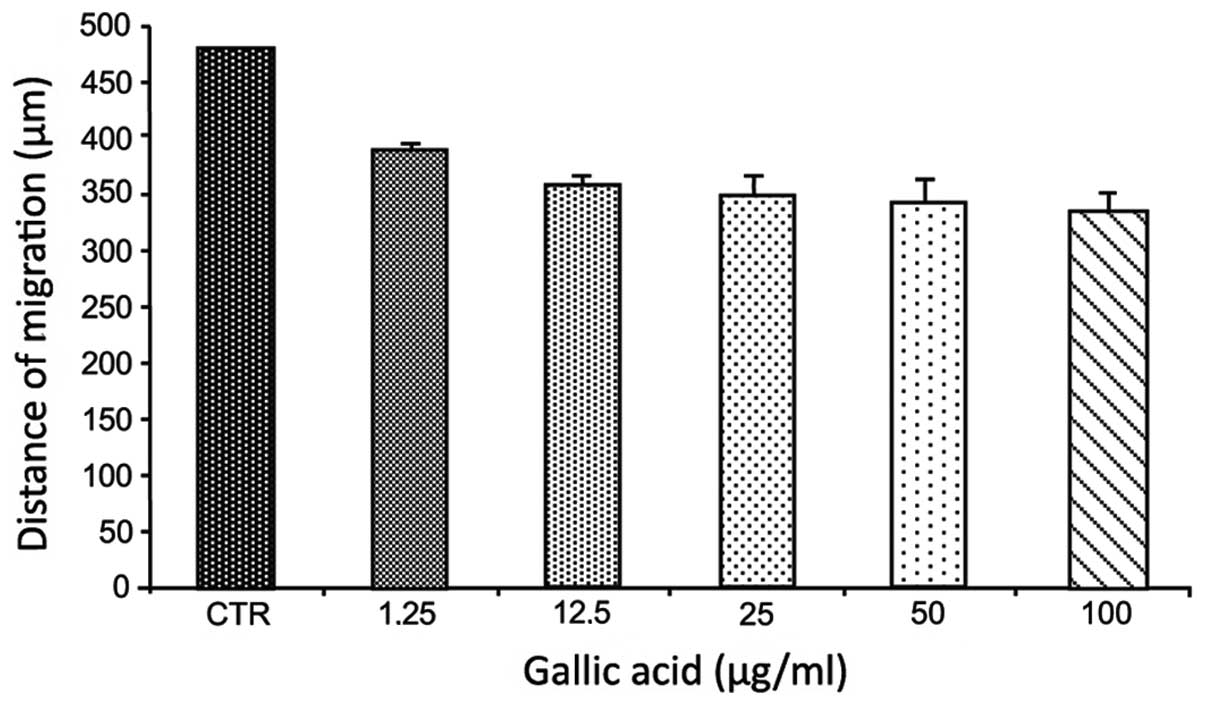
The wound scratch assay was carried out to examine
the effect of GA on migration of T98G glioma cells. As compared
with the control, the gap distance was not significantly reduced by
gallic acid even at the highest concentration of 100 μg/ml
(Fig. 4).
In the second phase of the investigation we
investigated the ability of gallic acid to induce variations in the
levels of hsa-miR-17-3p, hsa-miR-21-5p and hsa-miR-421. These
miRNAs downregulate, respectively, the expression of three critical
primary mitochondrial antioxidant enzymes (MnSOD, GPX-2 and TrxR2),
the expression of the activator transcriptional factors E2F1 and
E2F2 involved in cell cycle progression, and finally the expression
of ATM gene involved in DNA repair.
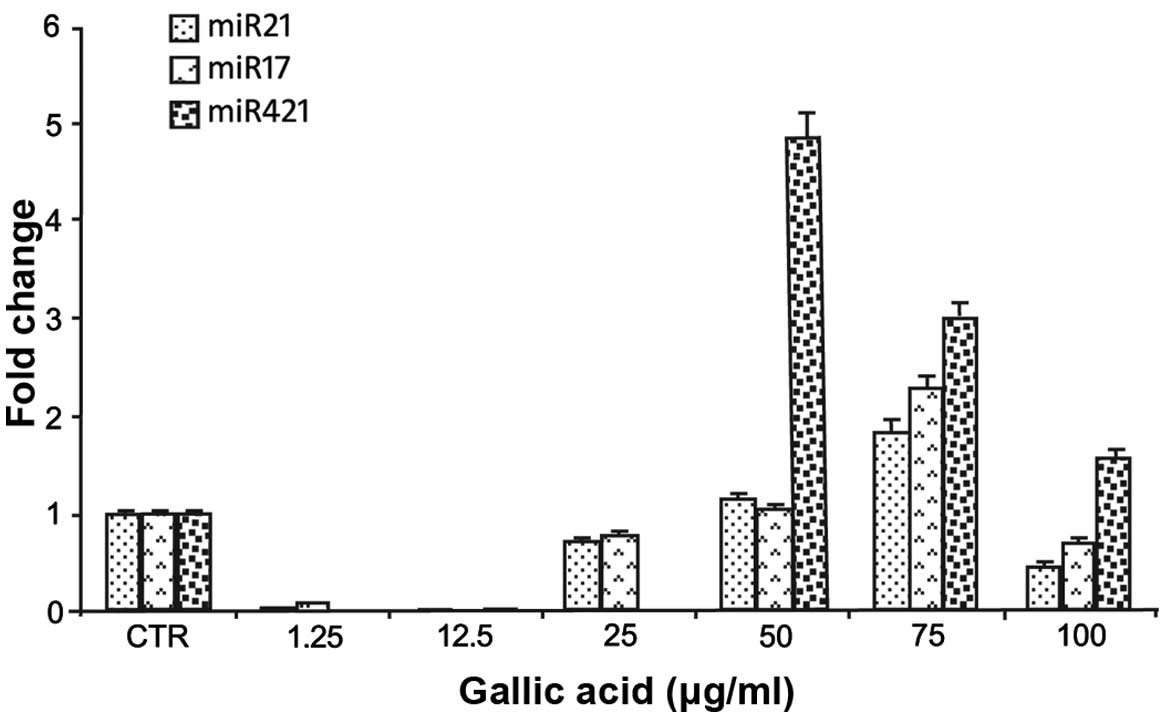
The expression of these miRNAs was determined by
qRT-PCR (see Materials and methods) and the results are reported in
Fig. 5. The miRNAs considered
showed a variation in their expression after GA treatment
displaying a common reduced expression at low GA concentrations
(1.2, 12.5 and 25 μg/ml) and an increased expression at
concentrations >25 μg/ml (Fig.
5). The expression of the three different miRNAs seems to be
modulated by different GA concentrations with a significant
increase at the concentration of 75 μg/ml and a reduction at the
highest concentration of 100 μg/ml (Fig. 5).
Discussion
Our data demonstrated the anti-proliferative effects
of GA on the T98G glioma cell line so confirming the anti-tumoral
effects reported previously on other cell models of glioma
(17). Moreover, our findings
demonstrate that GA influences the expression of some miRNAs that
control significant pathways involved in anti-oxidant activities,
in cell cycle progression and in cell death. The effects observed
are in relation with GA concentration: at low concentrations up to
25 μg/ml the expression of all the miRNA considered was reduced
compared to the untreated control cells, but at concentrations
>25 μg/ml a progressive induction of miRNA synthesis was
observed. The reduction of miR-17 expression at low GA
concentrations is indicative of an upregulation of the
mitochondrial antioxidant activities and this function can be
considered a beneficial effect exerted by GA as radical scavenger
(20). Many epidemiological
studies correlate the beneficial effects on population health to
food containing polyphenols with the antioxidant property of these
molecules (4,5). Following the GA concentration
increase, a progressive increase of the expression levels of
hsa-miR-17 occurs, which is indicative of a progressive
downregulation of antioxidant activities. These results are in
agreement with the toxic effects (i.e. reduction of mitotic index,
increased cell death, reduced cell recovery, reduced clonogenic
ability and increased apoptosis) that were significantly enhanced
at concentrations of ≥50 μg/ml GA. Our results confirm recent
studies which demonstrated that the tumor suppressor ability of
hsa-miR-17-3p is correlated with the downregulation of the three
critical primary mitochondrial antioxidant enzymes: manganese
superoxide dismutase (MnSOD), glutathione peroxidase-2 (GPX-2) and
thioredoxin reductase (TrxR2) (29).
We did not observe a significant effect of GA
treatment on the reduction of cell migration of glioma cells. This
effect could be explained with the ability of miR-17-3p to target
the phosphatase and tensin homolog gene (PTEN) (35). PTEN is an oncosuppressor
gene that inhibits tumor cell growth and motility by blocking the
PI3K/Akt pathway. Its decrease in some malignant cancers, causes a
Akt hyperactivation and the promotion of cell proliferation,
migration, invasion and angiogenesis. The decrease in hsa-miR-17
levels, induced by low concentrations of GA, causes the
upregulation of PTEN with a consequent decrease in cell
migration. On the contrary, the increase in hsa-miR-17 levels,
after treatment with concentrations >25 μg/ml of GA, induces the
downregulation of PTEN and could explain the non-significant
difference observed for wound scratch assay in all the conditions
tested in (Fig. 4).
miR-421 regulates the cell cycle S-phase checkpoint
and cellular radiosensitivity by suppressing ATM expression, a
serine/threonine protein kinase that regulates DNA damage-induced
at the G1-S and S phases of the cell cycle checkpoints (31). The increased expression of
hsa-miR-421 downregulates ATM so reducing the capacity of
T98G cells to repair radiation damage. This activity of GA is
particularly important to control tumor cell proliferation after
radiation treatments to avoid death escape of radiation-resistant
cells.
hsa-miR-21-5p has been recently shown to be one of
the five most abundantly expressed miRNAs in patients with
colorectal cancer (36). The
pathways with the most significant gene-enrichment for this miRNA
belong to the ‘Pathways in cancer’, ‘Colorectal cancer’, ‘Hepatitis
B’, ‘MAPK signalling pathway’, ‘Cell cycle’ and ‘Glioma’. Focusing
on this latter pathway, the target genes are E2F1,
E2F2, two important activator transcriptional factors,
involved in cell cycle progression, in particular in the G1/S
transition, that binds the proto-oncogene epithelial growth factor
receptor (EGFR) (37). The
binding of a ligand to EGFR leads to proliferation, differentiation
and inhibition of apoptosis through the activation of different
pathways, such as MAPK, phosphatidylinositide 3-kinases (PIK3),
signal transducer and activator of transcription (STAT),
cyclin-dependent kinase 6 (CDK6), PIK3R1 and PTEN.
Additionally, hsa-miR-21-5p has been found, deregulated in
pediatric cancer stem cells and in clear renal cell carcinoma (RCC)
(38,39).
Fig. 6 summarizes a
possible network describing the principal mechanisms influenced by
GA and the microRNAs tested. All the three microRNAs tested are
involved in p53 pathway, inducing variations in expression of
p21.
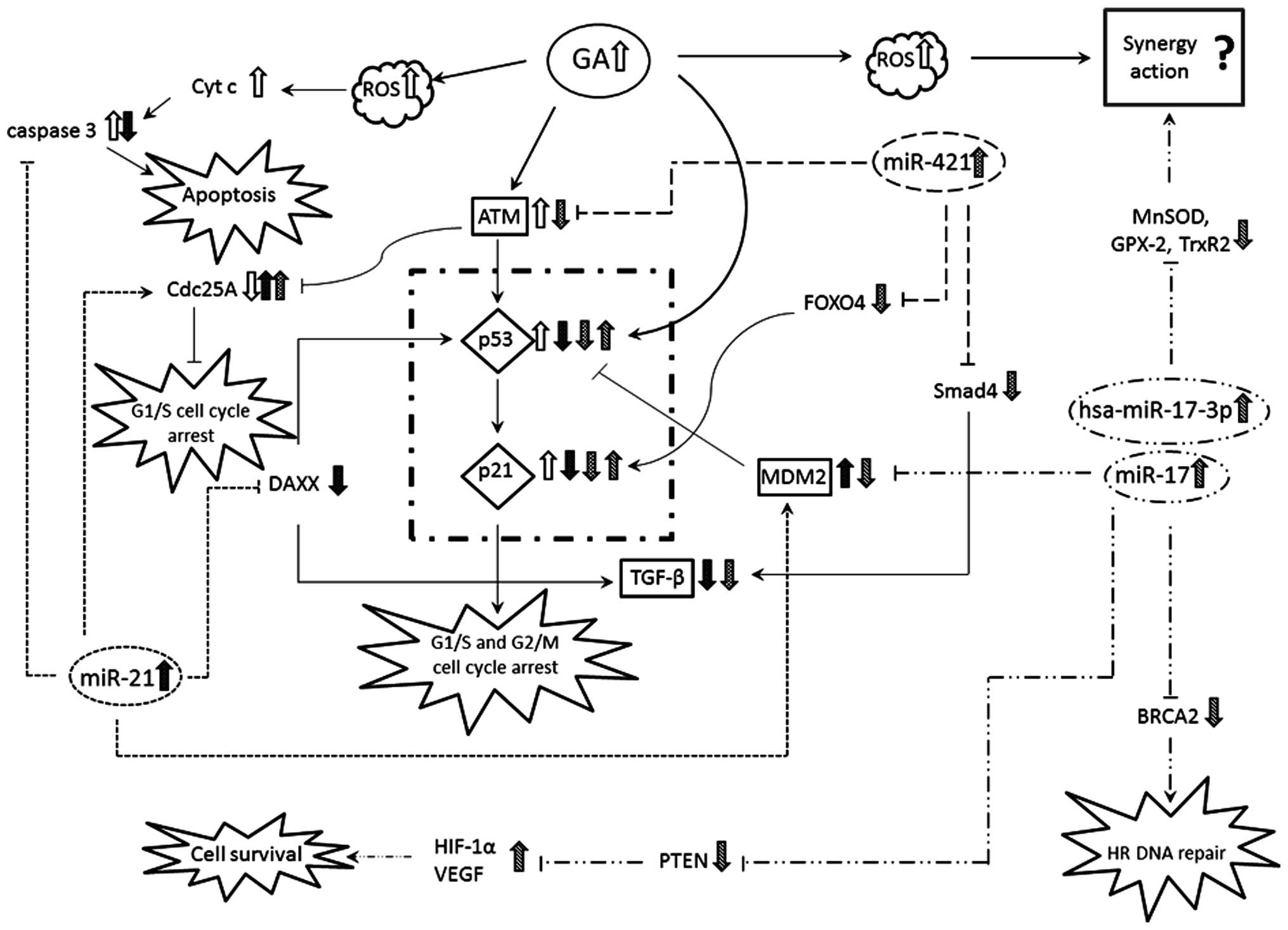 | Figure 6Summary diagram of the relationships
between miR-21, miR-17 and miR-421 and the related targets: GA,
gallic acid; ROS, reactive oxygen species; Cyt c, cytochrome
c; CdC25A, cell division cycle 25 homolog A; DAXX,
death-associated protein 6; ATM, ataxia telangiectasia mutated;
p53, genome reparative protein; G1/S phase; p21, cell cycle
regulator protein; TGF-β, transforming growth factor β; HIF-1α,
hypoxia-inducible factor 1-α; VEGF, vascular endothelial growth
factor; PTEN, phosphatase and tensin homolog; FOXO4, forkhead box
protein O4; Smad4, SMAD family member 4; MnSOD, manganese
superoxide dismutase; GPX-2, glutathione peroxidase 2; TrxR2,
thioredoxin reductase-2; BRCA2, breast cancer 2, early onset. |
In conclusion, based on the present study, GA at low
concentrations inhibits all the three miRNAs considered, indicating
that the increase in mitochondrial antioxidant capacity (decrease
of 17-3p), increases cell proliferation by stimulating the
regeneration of cells and tissues (decrease of miR-21), and
increases the ability to repair damage caused by chemicals and
radiation (decrease of miR-421). This scenario is in agreement with
the observations reported by a number of epidemiological studies
that have observed a lower incidence of cancer and aging delay in
populations with a diet rich in polyphenols (4,5).
Gallic acid at high doses causes a reduction in
mitochondrial antioxidant activity (increased miR-17 levels); slows
cell proliferation (increased miR-21 levels) and decreases the
ability to repair the damage (increased miR-421 levels).
The functionality of polyphenols has been proven in
numerous publications, but there are still unclear points regarding
the useful concentrations. GA is a molecule that is found intact in
biological fluids having a 1.2–1.5 h elimination half-life and a
better absorption capacity when compared with other polyphenols
(40). Moreover, it was observed
that <60% of GA excreted in the urine is metabolized to its
glucuroni-dated form 4-O-methylgallic acid (4OMGA) (41). Studies of the pharmacokinetics,
bioavailability and toxicity on experimental models in vivo
can provide useful information for the use of GA in the treatment
or in the prevention of cancer and neurodegenerative diseases.
Abbreviations:
|
GBM
|
glioblastoma multiforme
|
|
CNS
|
central nervous system
|
|
NF-κB
|
nuclear factor-κB
|
|
AP-1
|
activator protein-1
|
|
MAPK
|
mitogen-activated protein kinases
|
|
miRNA
|
microRNA
|
|
GA
|
gallic acid
|
|
ROS
|
reactive oxygen species
|
|
GHS
|
glutathione
|
|
MnSOD
|
manganese soperoxide dismutase
|
|
GPX-2
|
glutathione peroxidase-2
|
|
TrxR2
|
thioredoxin reductase
|
|
DMSO
|
dimethyl sulfoxide
|
|
EMEM
|
Eagle’s minimum essential medium
|
|
ECACC
|
European Collection of Cell
Cultures
|
|
MAI
|
mitotic activity index
|
|
HPFs
|
high-power fields
|
|
qRT-PCR
|
quantitative real-time PCR
|
|
PTEN
|
phosphatase and tensin homolog
gene
|
|
EGFR
|
epidermal growth factor receptor
|
|
4OMGA
|
4-O-methylgallic acid
|
|
ATM
|
ataxia telangiectasia mutated
|
|
PIK3
|
phosphatidylinositide 3-kinases
|
|
STAT
|
signal transducer and activator of
transcription
|
|
TGF-β
|
transforming growth factor β
|
|
CDK6
|
cyclin-dependent kinase 6
|
|
RCC
|
renal cell carcinoma
|
References
|
1
|
Burger PC, Heinz ER, Shibata T and
Kleihues P: Topographic anatomy and CT correlations in the
untreated glioblastoma multiforme. J Neurosurg. 68:698–704. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller CR and Perry A: Glioblastoma. Arch
Pathol Lab Med. 131:397–406. 2007.PubMed/NCBI
|
|
3
|
Kilic T, Alberta JA, Zdunek PR, Acar M,
Iannarelli P, O’Reilly T, Buchdunger E, Black PM and Stiles CD:
Intracranial inhibition of platelet-derived growth factor-mediated
glioblastoma cell growth by an orally active kinase inhibitor of
the 2-phenylami-nopyrimidine class. Cancer Res. 60:5143–5150.
2000.PubMed/NCBI
|
|
4
|
Mukhtar H and Ahmad N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71:1698S–1702S. 2000.PubMed/NCBI
|
|
5
|
Fresco P, Borges F, Diniz C and Marques
MP: New insights on the anticancer properties of dietary
polyphenols. Med Res Rev. 26:747–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong AN, Yu R, Hebbar V, Chen C, Owuor E,
Hu R, Ee R and Mandlekar S: Signal transduction events elicited by
cancer prevention compounds. Mutat Res. 480–481:231–241. 2001.
View Article : Google Scholar
|
|
8
|
Ji BC, Hsu WH, Yang JS, Hsia TC, Lu CC,
Chiang JH, Yang JL, Lin CH, Lin JJ, Suen LJ, Gibson Wood W and
Chung JG: Gallic acid induces apoptosis via caspase-3 and
mitochondrion-dependent pathways in vitro and suppresses lung
xenograft tumor growth in vivo. J Agric Food Chem. 57:7596–7604.
2009. View Article : Google Scholar
|
|
9
|
Liu Z, Li D, Yu L and Niu F: Gallic acid
as a cancer-selective agent induces apoptosis in pancreatic cancer
cells. Chemotherapy. 58:185–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Curti V, Capelli E, Boschi F, Nabavi SF,
Bongiorno AI, Habtemariam S, Nabavi SM and Daglia M: Modulation of
human miR-17-3p expression by methyl 3-O-methyl gallate as
explanation of its in vivo protective activities. Mol Nutr Food
Res. 58:1776–1784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar
|
|
14
|
Hiyoshi Y, Kamohara H, Karashima R, Sato
N, Imamura Y, Nagai Y, Yoshida N, Toyama E, Hayashi N, Watanabe M
and Baba H: MicroRNA-21 regulates the proliferation and invasion in
esophageal squamous cell carcinoma. Clin Cancer Res. 15:1915–1922.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu
Z and You Y: hsa-mir-181a and hsa-mir-181b function as tumor
suppressors in human glioma cells. Brain Res. 1236:185–193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weiss FU, Marques IJ and Woltering JM:
Retinoic acid receptor antagonists inhibit miR-10a expression and
block metastatic behavior of pancreatic cancer. Gastroenterology.
137:2136–2145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu Y, Jiang F, Jiang H, Wu K, Zheng X, Cai
Y, Katakowski M, Chopp M and To SS: Gallic acid suppress cell
viability, proliferation, invasion and angiogenesis in human glioma
cells. Eur J Pharmacol. 641:102–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SH, Kim JK, Kim DW, Hwang HS, Eum WS,
Park J, Han KH, Oh JS and Choi SY: Antitumor activity of methyl
gallate by inhibition of focal adhesion formation and Akt
phosphorylation in glioma cells. Biochim Biophys Acta.
1830.4017–4029. 2013.
|
|
19
|
Sakagami H and Satoh K: Prooxidant action
of two antioxidants: ascorbic acid and gallic acid. Anticancer Res.
17:221–224. 1997.PubMed/NCBI
|
|
20
|
Strlic M, Radovic T, Kolar J and Pihlar B:
Anti- and prooxidative properties of gallic acid in fenton-type
systems. J Agric Food Chem. 50:6313–6317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK,
Hsieh YC, Chen CC and Yuan SS: Gallic acid, a major component of
Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer
activity in human prostate cancer cells. Cancer Lett. 286:161–171.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Savi LA, Leal PC, Vieira TO, Rosso R,
Nunes RJ, Yunes RA, Creczynski-Pasa TB, Barardi CR and Simões CM:
Evaluation of anti-herpetic and antioxidant activities, and
cytotoxic and genotoxic effects of synthetic alkyl-esters of gallic
acid. Arzneimittelforschung. 55:66–75. 2005.PubMed/NCBI
|
|
23
|
Kroes BH, van den Berg AJ, Quarles van
Ufford HC, van Dijk H and Labadie RP: Anti-inflammatory activity of
gallic acid. Planta Med. 58:499–504. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gichner T, Pospísil F, Velemínský J,
Volkeová V and Volke J: Two types of antimutagenic effects of
gallic and tannic acids towards N-nitroso-compounds-induced
mutagenicity in the Ames Salmonella assay. Folia Microbiol.
32:55–62. 1987. View Article : Google Scholar
|
|
25
|
Mirvish SS, Cardesa A, Wallcave L and
Shubik P: Induction of mouse lung adenomas by amines or ureas plus
nitrite and by N-nitroso compounds: effect of ascorbate, gallic
acid, thiocyanate and caffeine. J Natl Cancer Inst. 55:633–636.
1975.PubMed/NCBI
|
|
26
|
Inoue M, Suzuki R, Sakaguchi N, Li Z,
Takeda T, Ogihara Y, Jiang BY and Chen Y: Selective induction of
cell death in cancer cells by gallic acid. Biol Pharm Bull.
18:1526–1530. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fox JT, Sakamuru S, Huang R, Teneva N,
Simmons SO, Xia M, Tice RR, Austin CP and Myung K: High-throughput
geno-toxicity assay identifies antioxidants as inducers of DNA
damage response and cell death. Proc Natl Acad Sci USA.
109:5423–5428. 2012. View Article : Google Scholar
|
|
28
|
Zhang X, Ladd A, Dragoescu E, Budd WT,
Ware JL and Zehner ZE: MicroRNA-17-3p is a prostate tumor
suppressor in vitro and in vivo, and is decreased in high grade
prostate tumors analyzed by laser capture microdissection. Clin Exp
Metastasis. 26:965–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, Fang F, Zhang J, Josson S, St Clair
WH and St Clair DK: miR-17* suppresses tumorigenicity of prostate
cancer by inhibiting mitochondrial antioxidant enzymes. PLoS One.
5:e143562010. View Article : Google Scholar
|
|
30
|
Papagiannakopoulos T, Shapiro A and Kosik
KS: MicroRNA-21 targets a network of key tumor-suppressive pathways
in glioblastoma cells. Cancer Res. 68:8164–8172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu H, Du L, Nagabayashi G, Seeger RC and
Gatti RA: ATM is down-regulated by N-Myc-regulated microRNA-421.
Proc Natl Acad Sci USA. 107:1506–1511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mansour WY, Bogdanova NV, Kasten-Pisula U,
Rieckmann T, Köcher S, Borgmann K, Baumann M, Krause M, Petersen C,
Hu H, Gatti RA, Dikomey E, Dörk T and Dahm-Daphi J: Aberrant
overexpression of miR-421 downregulates ATM and leads to a
pronounced DSB repair defect and clinical hypersensitivity in SKX
squamous cell carcinoma. Radiother Oncol. 106:147–154. 2013.
View Article : Google Scholar
|
|
33
|
Hao J, Zhang S, Zhou Y, Liu C, Hu X and
Shao C: MicroRNA 421 suppresses DPC4/Smad4 in pancreatic cancer.
Biochem Biophys Res Commun. 406:552–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
35
|
Gao Y, Luo LH, Li S and Yang C: miR-17
inhibitor suppressed osteosarcoma tumor growth and metastasis via
increasing PTEN expression. Biochem Biophys Res Commun.
444:230–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schee K, Lorenz S and Worren MM: Deep
sequencing the MicroRNA transcriptome in colorectal cancer. PLoS
One. 8:e661652013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O’Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005. View Article : Google Scholar
|
|
38
|
Sanchez-Diaz PC, Hsiao TH, Chang JC, Yue
D, Tan MC, Chen HI, Tomlinson GE, Huang Y, Chen Y and Hung JY:
De-regulated microRNAs in pediatric cancer stem cells target
pathways involved in cell proliferation, cell cycle and
development. PLoS One. 8:e616222013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gowrishankar B, Ibragimova I, Zhou Y,
Slifker MJ, Devarajan K, Al-Saleem T, Uzzo RG and Cairns P:
MicroRNA expression signatures of stage, grade, and progression in
clear cell RCC. Cancer Biol Ther. 15:329–341. 2014. View Article : Google Scholar :
|
|
40
|
Manach C, Williamson G, Morand C, Scalbert
A and Rémésy C: Bioavailability and bioefficacy of polyphenols in
humans. I. Review of 97 bioavailability studies. Am J Clin Nutr.
81:230S–242S. 2005.PubMed/NCBI
|
|
41
|
Shahrzad S, Aoyagi K, Winter A, Koyama A
and Bitsch I: Pharmacokinetics of gallic acid and its relative
bioavailability from tea in healthy humans. J Nutr. 131:1207–1210.
2001.PubMed/NCBI
|