Introduction
About a third of breast cancer patients have tumors
that either do not express estrogen receptor (ER) or very low
levels and are therefore de novo resistant to endocrine
therapy, as also are a significant proportion of patients whose
tumors are ER+. Of the patients that do respond, the
vast majority acquire resistance during therapy (1). We have previously shown that
shRNA-mediated silencing of ER in MCF7 breast cancer cells leads to
endocrine insensitivity (2). This
is accompanied by transformation from an epithelial to a
mesenchymal-like cell (commonly referred to as epithelial to
mesenchymal transition; EMT) as evidenced both by their modified
gene expression profile (resembling that of MDA-MB-231 cells, a
line that is derived from an ER− breast tumor) and their
more fibroblast-like morphological appearance (3). During the EMT process described in
vivo, epithelial cells undergo unique changes which are
accompanied by diminished intracellular adhesion, resulting in
enhanced motility and invasion, paralleled with poor clinical
outcome (4,5). Tamoxifen resistance has been
associated with changes in a large variety of molecules that
include growth factors, their tyrosine kinase receptors and
downstream signaling mediators such as MAPK, ERK, PI3K, AKT and
SRC, cell cycle regulators and ER associated transcription factors
and co-activators. Many of these are also implicated in EMT
(1), providing a compelling
argument for the parallelism between the two processes, as we have
observed in our model system; as a result, our ER silenced cells
have acquired enhanced proliferative, motile and invasive
capabilities, with chemotactic attraction towards various growth
factors and chemokines (6).
Of the many aspects of the tumor microenvironment
that play a critical role in cancer cell invasion and metastasis,
one that is receiving increased attention is the pH within a
growing tumor mass. Current thinking is that, in order to maintain
optimum slightly alkaline cytoplasmic pH and to avoid acidosis,
cancer cells undergoing excessive fermentative glycolysis (due to
poor vascular perfusion and regional hypoxia) extrude lactate and
protons, thereby contributing to acidification of the extracellular
matrix (7–9), a phenomenon linked to increasing
tumor aggressiveness (7,8,10).
The associated ion movements are facilitated through various
transporters and enzymes that include carbonic anhydrases, vacuolar
H+-ATPases, the H+/Cl− symporter,
the monocarboxylate transporter, the Na+-dependent
Cl−/HCO3− exchangers, and the
Na+/K+ and Na+/H+
ATPases (11–13). An acidic matrix has been suggested
to promote tumor metastasis (14,15),
by facilitating cancer cell clonal evolution through induction of
chromosomal instability and gene mutations (16,17)
and extracellular matrix degradation, in part through enhanced
activity of proteases (such as cathepsins) and matrix
metalloproteinases (particularly MMP2/9) (14,18).
Several therapeutic interventions, aiming to reduce tumor acidity
through administration of alkaline buffers (200 mM sodium
bicarbonate), claim to have raised the extracellular pH (pHe)
without altering the intracellular pH (pHi) (19), to have reduced lung metastasis
(20), and suppressed the
formation of spontaneous prostate tumors (21). In addition, several groups have
found that treatment with proton pump inhibitors reduced neoplastic
development of esophageal adenocarcinoma and hepatoblastoma
(22,23). Other reports however, have
suggested that acidosis inhibits cancer cell proliferation, induces
stress response and apoptosis (24–27),
and reduces activation of Akt, whose upregulation is frequently
correlated with poor prognosis (28).
We have recently reported that brief exposure of
specifically ER silenced (but not ER expressing) breast cancer
cells, to an alkaline pH environment, can induce a marked
morphological change whereby individual cells rapidly shrink and
become spherical, with increased tendency to disaggregate from the
cluster of cells (termed contractolation). This phenomenon is
paralleled by changes in the level of expression and/or activity of
various signaling molecules. It is also co-incident with enhanced
invasive potential, with elevated MMP2/9 activity. All of these
morphological and functional changes can be inhibited by drugs
targeting two major ion pumps; Na+/K+ and the
Na+/H+ exchangers (29).
In the present study, we have further characterized
the morphological changes mentioned above, and identify the
components of the alkaline pH induced ring-like structures or blebs
on the outer membrane of ER− cells. These appear to be
related to cell movement and polarity, and their formation is
dependent upon rearrangement and integrity of the actin
cytoskeleton. We show that acidic conditions fail to induce a
similar response and do not modify their metastatic behaviour.
These data suggest that environmental pH needs to be more
extensively investigated, and the notion of a purely acidic driven
mechanism for tumor dissemination may be over-simplified, at least
in respect of endocrine resistant cells.
Materials and methods
Cell lines
MCF7 breast cancer cells were obtained from the
American Type Culture Collection (VA, USA). YS1.2 (ER+)
and pII (ER−) cell lines were established in this
laboratory by transfection of MCF7 with ER directed shRNA plasmid
as described previously (2,3). The
YS1.2 is a transfected line that failed to downregulate the ER. For
routine culture, all cell lines were maintained as monolayers in
advanced Dulbecco’s minimum essential medium (DMEM) containing
phenol red and supplemented with 5% fetal bovine serum (FBS), 600
μg/ml L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and
6 ml/500 ml 100X non-essential amino acids (all from Invitrogen,
CA, USA), and grown at 37°C in an incubator gassed with an
atmosphere of 5% CO2 and maintained at 95% humidity. For
YS1.2, the maintenance medium also contained G418 (1 mg/ml) but
this was omitted during experiments. The DMEM requires an
atmosphere of 5% CO2 to produce HCO3
buffering capacity to maintain pH at 7.4 for normal cell growth.
Unless otherwise specified, cells were always grown under these
conditions. Upon exposure to normal atmosphere, (and consequent
loss of the ‘CO2-enriched’ environment) this medium
reaches a maximum pH value of ~8.3 within a few minutes in either
flasks with a loosened cap or in tissue culture dishes/microtiter
plates with free air flow. The term ‘alkaline conditions’ in the
text refers to this pH value of 8.3. In order to maintain the
medium at acidic pH (6.5), or to culture the cells at specific
alkaline pH without raising it gradually through exposing the cells
to the normal atmosphere, CO2-independent medium
(Invitrogen) was used to stabilize the pH value independently of
the surrounding atmosphere.
Antibodies
The following antibodies were obtained from Cell
Signaling, USA: Akt (cat no. 9272), FAK (cat no. 9330), vimentin
(cat no. 3932), and actin (cat no. 4967). The following antibodies
were obtained from Abcam: integrin-α2 (cat no. ab55340), and JAM-1
(cat no. ab52647).
Microscopic analysis of morphological
changes in response to pH
For each cell line, 105 cells were seeded
into individual wells of a 12-well plate and allowed to settle at
37°C for 24 h. To induce a gradual increase in pH, culture plates
were removed from the gassed incubator (i.e., from the 5%
CO2 atmosphere needed to maintain the buffering capacity
of the DMEM) and placed in an ungassed incubator at 37°C. In
another experimental set-up, cells were cultured at specific acidic
or alkaline pH by using the CO2-independent medium
adjusted to pH 6.5 or 8.3 and placed in an ungassed incubator at
37°C. Several fields containing colonies were marked and
photographed immediately and after several time-points (as
indicated in the text) using an Olympus inverted microscope fitted
with a camera. Resultant changes in cell size and shape in each
photographed field were quantified in terms of the field area
occupied by cells, using Adobe Photoshop CS4 Measuring Tool.
Live cell imaging
The effect of pH change on pII cells was monitored
by time-lapse photography using a live cell imager (Cell Observer
HS, Zeiss, Germany). Cell monolayers grown in 5% CO2 in
a 25-cm2 tissue culture flask were placed inside the
imaging chamber maintained at 37°C and then exposed to normal
atmosphere by loosening the cap (to induce a gradual increase in
the pH to 8.3). In separate experiments, images were recorded every
5 min over a 72-h period, at either ×20, ×40 or ×60 magnification.
AxioVision software (Zeiss) was used to combine all the individual
snap shots to generate a video using Windows Movie Maker software
(Microsoft). The effect of amiloride or zoniporide (10 μM) was
tested by pre-treatment of cells with either agent for 1 h before
placing in the imager.
Electron microscopy
Coverslips (22×22 mm) were coated with 50 μg/ml of
poly-D lysine for 4 h, air-dried at room temperature and sterilized
by UV light for 15 min. After that, pII cells (1×104)
were seeded in 6-well plates containing the coated coverslip and
allowed to grow for 2 days. Cells were washed with PBS and fixed
with 3% glutaraldehyde for 3 h at room temperature. After that,
cells were washed with Millong’s phosphate buffer three times and
then treated with 1% osmium tetroxide for 2 h. Then the cells were
washed with Millong’s phosphate buffer three times. The coverslips
were carefully removed and adhered to double-sided carbon tabs on
aluminum stubs. The coverslips were then gold coated with Autofine
coater JFC-1600 (Jeol, Japan) for 40 sec to have a uniform fine
conducting layer of electrons. The coated coverslips was observed
under scanning electron microscope (SEM) Carryscope JCM-5700
(Jeol).
Apoptosis assay
pII cells were cultured in 6-well plates at various
pH (7.4, 8.3 and 6.5) using the CO2-independent medium.
In addition, another batch of cells were cultured in normal DMEM
and then transferred to an ungassed incubator at 37°C for 1 h to
induce a gradual increase in the pH to 8.3. All of the cells were
then trypsinized, pelleted by centrifugation at 1000 g for 3 min
and washed twice by re-suspension and centrifugation in ice-cold
PBS and once in Annexin V binding buffer [10 mM HEPES/NaOH (pH
7.4), 0.14 M NaCl, 2.5 mM CaCl2]. The final cell pellet
was re-suspended in 100 μl of Annexin V binding buffer at
5×106 cells/ml and processed for FACS analysis using the
PE Annexin V apoptosis detection kit I from BD Pharmingen (USA).
Cells were stained in the following manner: a) cells only (negative
control); b) with 10 μl of Annexin V-PE; c) with 20 μl of 7AAD; and
d) with 10 μl of Annexin V-PE plus 20 μl 7AAD. All incubations were
performed in the dark at room temperature (RT) for 15 min.
Actin staining
YS1.2 and pII cells grown overnight at 37°C, 5%
CO2 in 8-well glass chambered slides (Lab-Tek, USA) were
treated in the following manner: a) continued to be cultured at 37
°C, 5% CO2 (pH 7.4); b) medium changed to CO2
independent media pH 6.5 for 1 h at 37°C; or c) medium changed to
CO2 independent media pH 8.3 for 1 h at 37°C. All cells
were then fixed with 3.7% paraformaldehyde and stained with either
phalloidin (red stain) or phallotoxin (green stain) to visualize
F-actin, and examined by confocal microscopy.
In a separate experimental set-up, cells were
pre-treated with various concentrations of cytochalasin-D
(StressMarq, USA), a rho kinase inhibitor (Millipore, USA), or
myosin light chain kinase (MLCK) inhibitor peptide 18 (Millipore)
for 1 h and either fixed directly with 3.7% paraformaldehyde, or
after exposure to alkaline pH (as above) for 1 h. To visualize the
nuclei, cells were finally incubated with DAPI (blue stain).
Immunostaining
YS1.2 and pII cells were cultured and fixed as
described above, washed with 1% BSA and incubated overnight at 4°C
with primary antibody diluted in 1% BSA: anti-integrin α-2 (1:50
dilution), anti-vimentin (1:150 dilution), anti-AKT (1:500
dilution), and anti-JAM-1 (1:100 dilution). After several washes in
PBS, followed by incubation with secondary antibody (1:500 dilution
in 1% BSA) for 1 h in the dark at RT, cells were mounted and
examined by fluorescence confocal microscopy.
Membrane ruffling and polarity
assays
For assessment of membrane ruffling, pII cells were
stimulated with EGF (Sigma, USA) (10 ng/ml) for 10–30 min at 37°C,
5% CO2 followed by paraformaldehyde fixation and
phalloidin staining. For polarity experiments, pII cells were
cultured on a polylysine coated glass coverslip and allowed to
adhere overnight. The coverslip was then embedded in a
10-cm2 petri dish in contact with previously added
solidified 0.5% ultra-pure agarose (Invitrogen) containing either
PBS (control) or 0.1 mg/ml EGF. DMEM was carefully pipetted into
the dish and cells incubated overnight at 37°C, 5% CO2
followed by paraformaldehyde fixation and phalloidin staining. In
another experimental setup, after the overnight incubation with
EGF, cells were incubated for 1 h in a 37°C ungassed incubator to
induce a gradual increase in pH to 8.3, then fixed and stained with
phalloidin.
Statistical analysis
Differences between means of individual groups were
assessed by the Student’s two tailed unpaired t-test: p≤0.05 was
considered statistically significant.
Results
Effect of acidic pH on cell
morphology
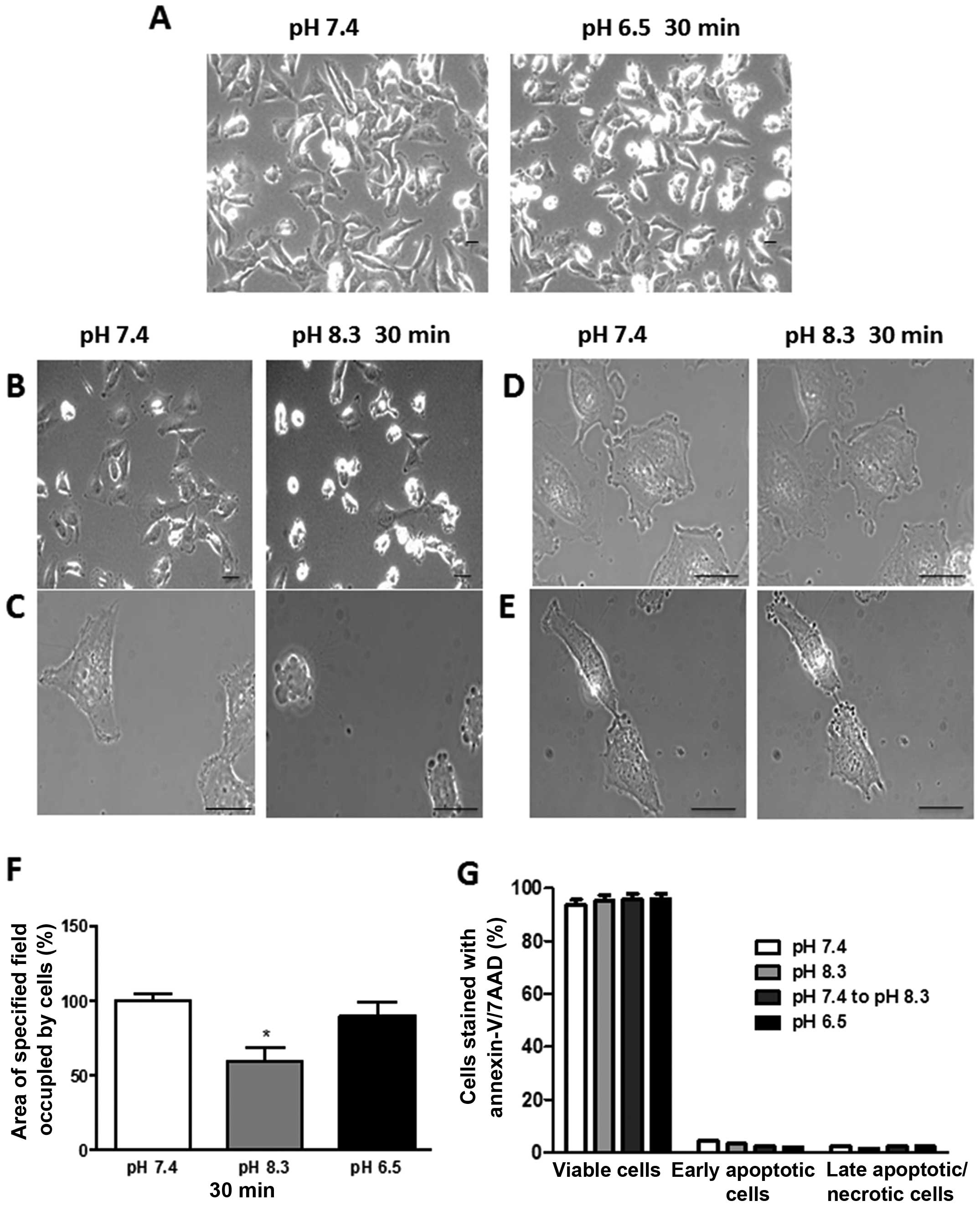
No specific morphological changes were observed by
exposure of either the ER+ YS1.2 or the ER−
pII cells to extracellular acidic pH. This is illustrated for pII
cells in Fig. 1A. The left panel
shows pII cells photographed immediately after removal from the
incubator when the culture medium was at pH 7.4, while the right
panel shows pII cells after transfer to acidic pH medium for 30 min
at 37°C.
Effect of short exposure to alkaline pH
on morphology and viability of pII cells
The left panel in Fig.
1B shows pII cells photographed immediately after removal from
the incubator when the culture medium was at pH 7.4, while the
right panel shows the same cells cultured in an ungassed incubator
at 37°C for 30 min, at which time the pH of the medium had become
noticeably alkaline as evidenced by the purple color of the phenol
red indicator and confirmed by pH meter. A dramatic morphological
change was observed, where cells separated from each other and
became spherical (contractolation). These alkaline pH-induced
morphological changes also occurred when cells were cultured in
serum-free media (data not shown). Fig. 1C shows higher magnification views;
multiple ring or vesicle-like bleb structures were observed on the
outer cell membrane, which all disappeared within 2–3 h of
returning the cells to pH 7.4. Prior exposure of cells to either
amiloride or zoniporide inhibited both contractolation and bleb
formation as shown in Fig. 1D and
E, respectively, which suggests that these two processes occur
simultaneously. Fig. 1F shows that
there was a significant reduction in the culture area occupied by
pII cells (30–40%) after exposure to alkaline but not acidic pH.
Since membrane blebbing has been observed as an early event
preceding cell death, we analysed the cells for any indication that
the short exposure to alkaline or acidic pH had a damaging effect.
Fig. 1G shows the percentage of
viable, apoptotic and necrotic pII cells after exposure to neutral,
acidic or alkaline pH for 1 h. In the experiment performed, most of
the cells (90–95%) were viable when cultured at pH 7.4 and no
significant difference was observed after exposure to either acidic
or alkaline pH, suggesting that the formation of blebs seen rapidly
after exposure to alkaline pH is not associated with cell
apoptosis.
Effect of prolonged (overnight) exposure
to alkaline pH
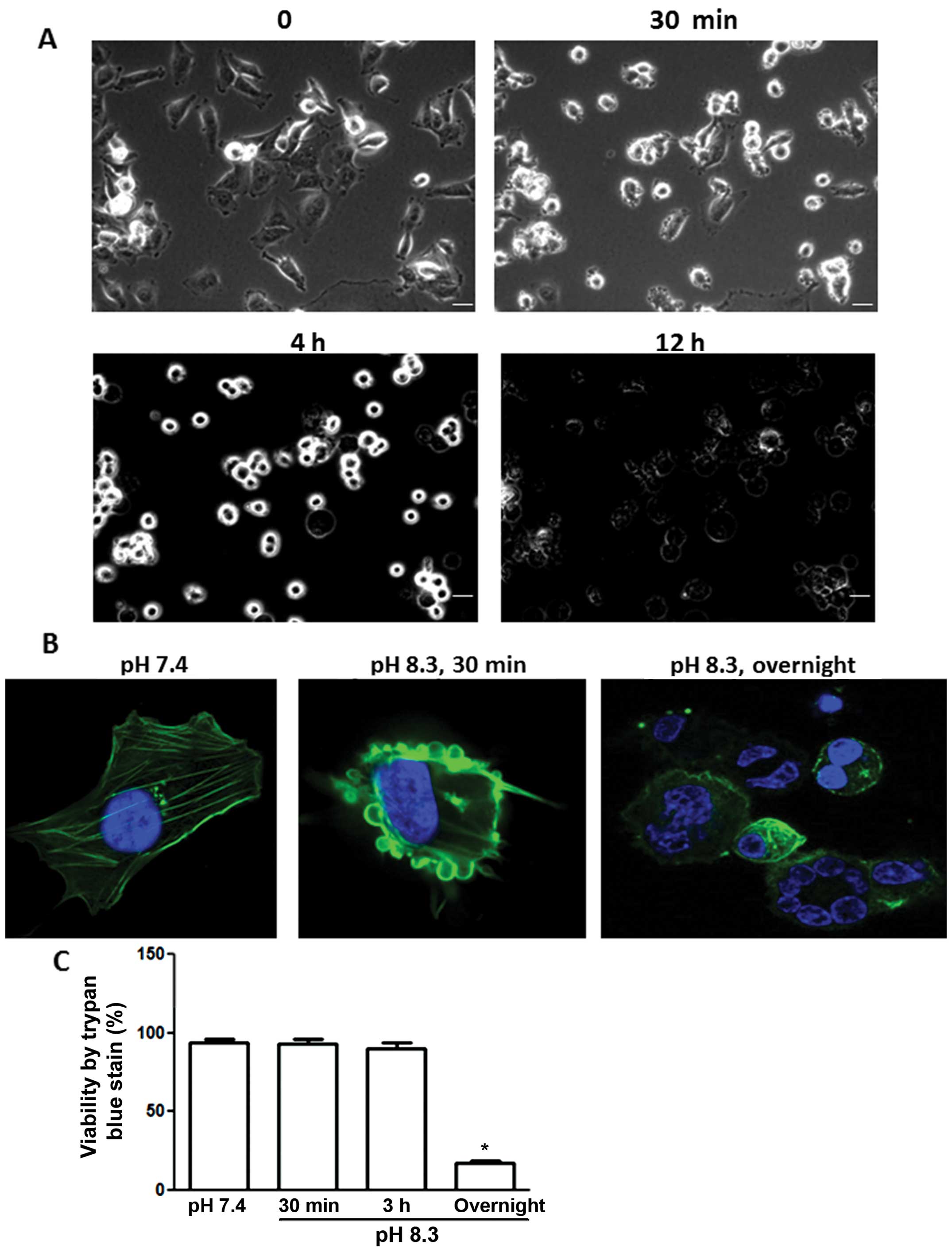
Fig. 2A shows pII
cells photographed at various times after exposure to alkaline pH.
Cells started to detach from the plate after 4 h and beyond and
they were unable to re-adhere or grow when they were subsequently
placed back at pH 7.4. Fig. 2B
shows phalloidin staining of the actin cytoskeleton for pII cells
at neutral pH (left panel), after brief exposure to alkaline pH
(contractolation, middle panel) or, after prolonged (overnight)
exposure to alkaline pH (right panel) when cells lost their
membrane integrity and demonstrated nuclear condensation,
suggesting cell death. Fig. 2C
shows the percentage of viable pII cells after various exposure
times to alkaline pH as determined by trypan blue exclusion.
Approximately 80% of cells were stained with trypan blue after an
overnight exposure, confirming the immunofluorescence data
(Fig. 2B, right panel). Neither
pII nor YS1.2 cell lines appeared to be adversely affected by
overnight incubation in acidic pH (data not shown), as judged by
their ability to continue normal growth when returned back to a pH
7.4 environment.
Effect of extracellular pH on the actin
cytoskeleton
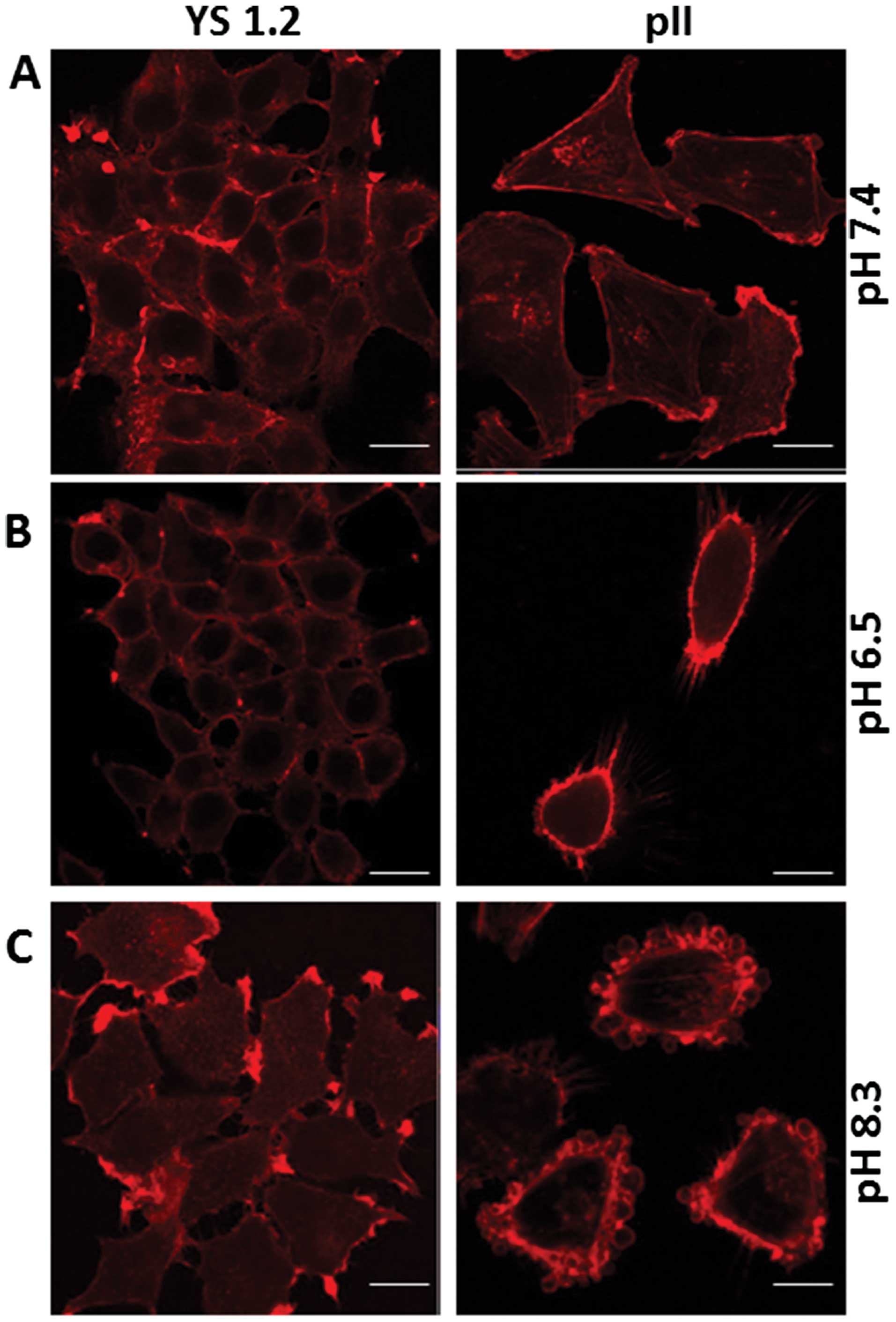
Fig. 3A shows
phalloidin staining of the actin cytoskeleton for YS1.2 and pII
cells at pH 7.4. When YS1.2 cells were cultured in either acidic or
alkaline pH for 1 h, no marked difference in staining was observed
(Fig. 3B and C, left panels). pII
cells cultured in alkaline medium showed a re-distribution of
phalloidin staining from the cytoplasm to the outer membranes with
particularly intense staining of the membranes of the newly formed
blebs (Figs. 2B, middle panel, and
3C, right panel). When pII cells were cultured in acidic pH medium,
some cells did exhibit minor increased membrane staining and some
rounding was observed in a few cells but without bleb formation
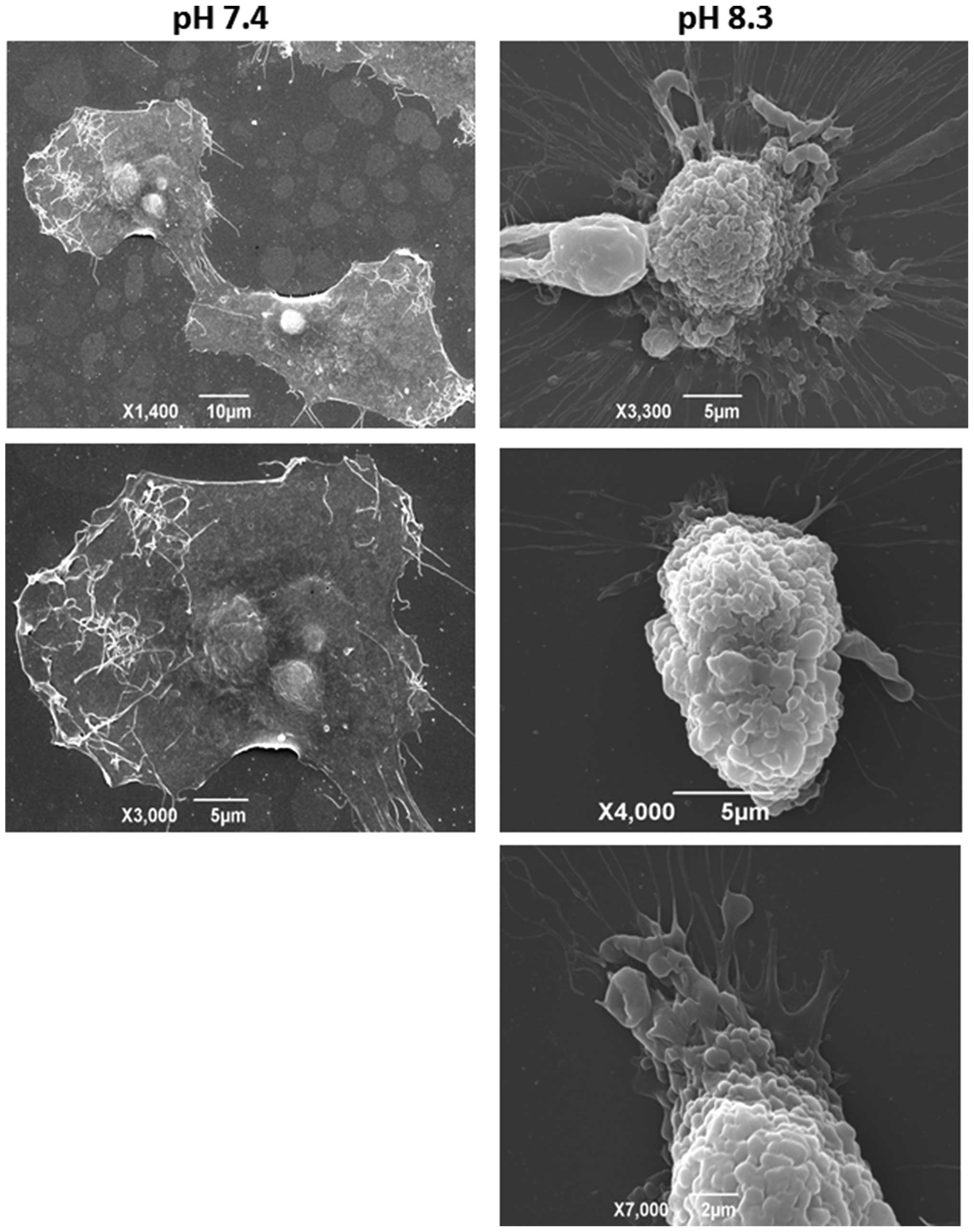
(Fig. 3B, right panel). Fig. 4 shows the ultastructural changes in
the plasma membrane of pII cells upon exposure to alkaline pH as
visualised by scanning electron microscopy. At neutral pH, there
are few lamellipodia or other protruding structures apparent on the
cell’s outer membrane (Fig. 4,
left panels). After brief exposure to alkaline pH, pII cells
exhibited a rounded shape with numerous invaginations and prominent
blebs covering the entire cell surface, confirming and further
extending the live cell imaging and immunofluorescence data
(Fig. 4, right panels). What
appeared to be hair-like extensions under the light microscope are
seen here as flowing cytoplasmic protrusions.
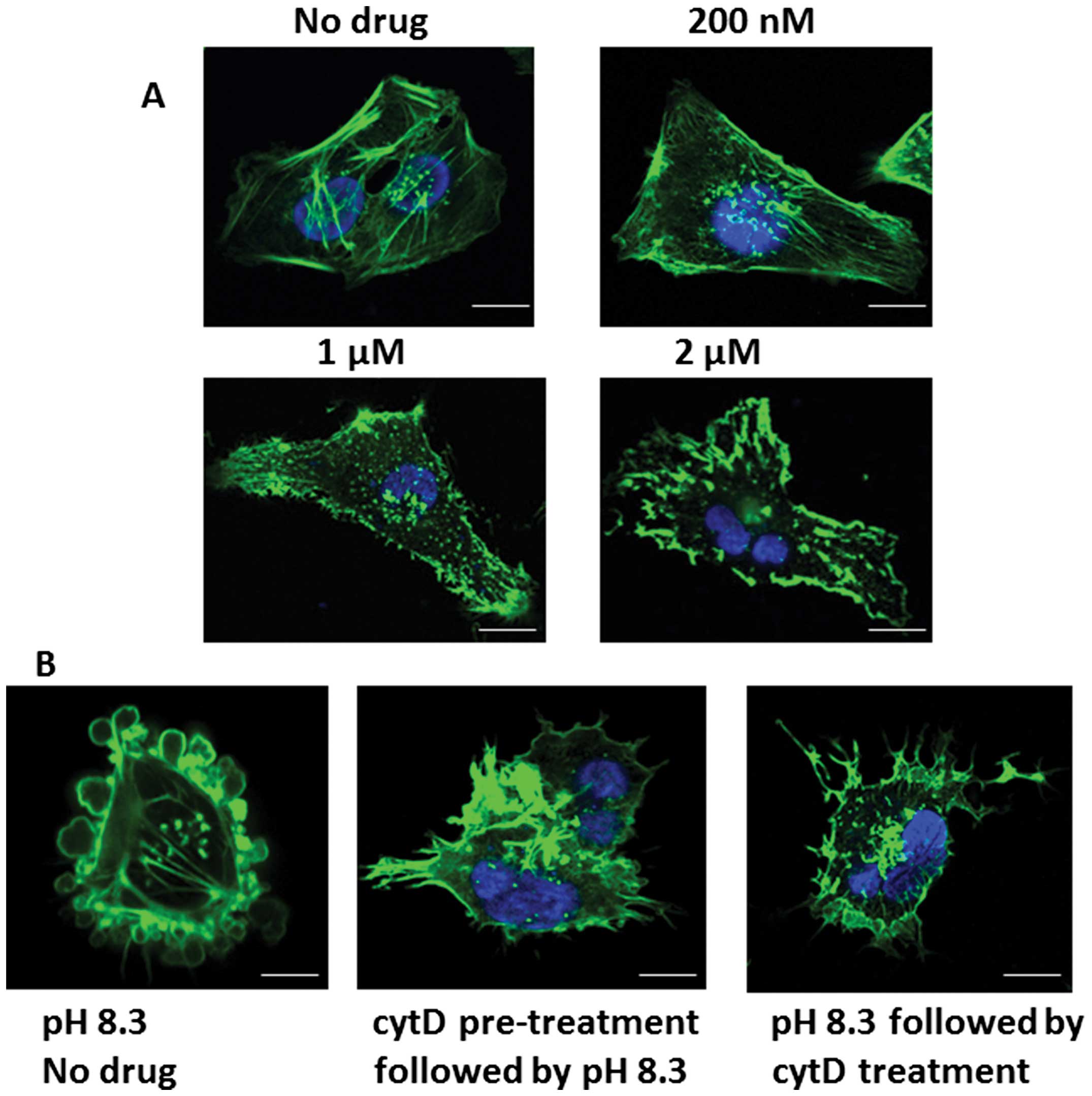
Effect of cytochalasin-D on phallotoxin
staining of pII cells
Fig. 5A shows actin
distribution in pII cells using phallotoxin staining after exposure
to various concentrations of the potent actin polymerization
inhibitor cytochalasin-D. At 1 μM and higher, the staining pattern
reflected a marked disruption of the membrane structure. We chose a
lower concentration (200 nM) of the drug, at which it exhibited
minimal effect on the actin structure at pH 7.4, to test its effect
on the alkaline induced morphological changes in pII cells. At this
concentration it completely inhibited or reversed the morphological
changes and the re-distribution of actin associated with alkaline
pH when it was added to the cells either before or after exposure
to alkaline pH (Fig. 5B).
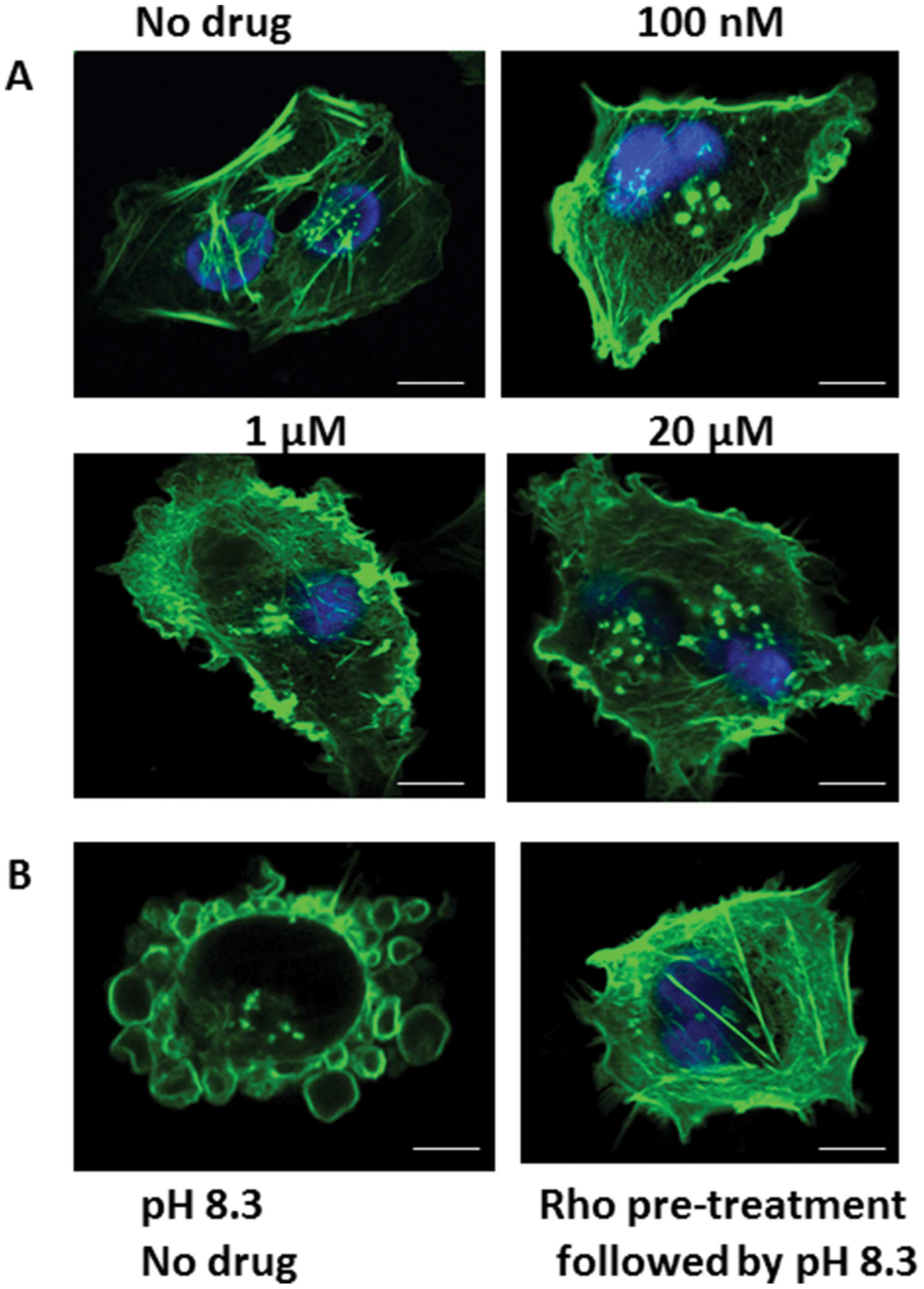
Effect of Rho kinase inhibitor on
phallotoxin staining of pII cells
Fig. 6A shows
phallotoxin staining of pII cells after treatment with various
concentrations of the Rho kinase inhibitor. At 1 μM and higher,
there was a marked disruption of the membrane and actin
cytoskeleton structure. At 100 nM the drug had minimal effect on
the actin distribution, but completely inhibited the morphological
changes associated with alkaline pH when it was added to the cells
1 h before culturing in alkaline pH (Fig. 6B).
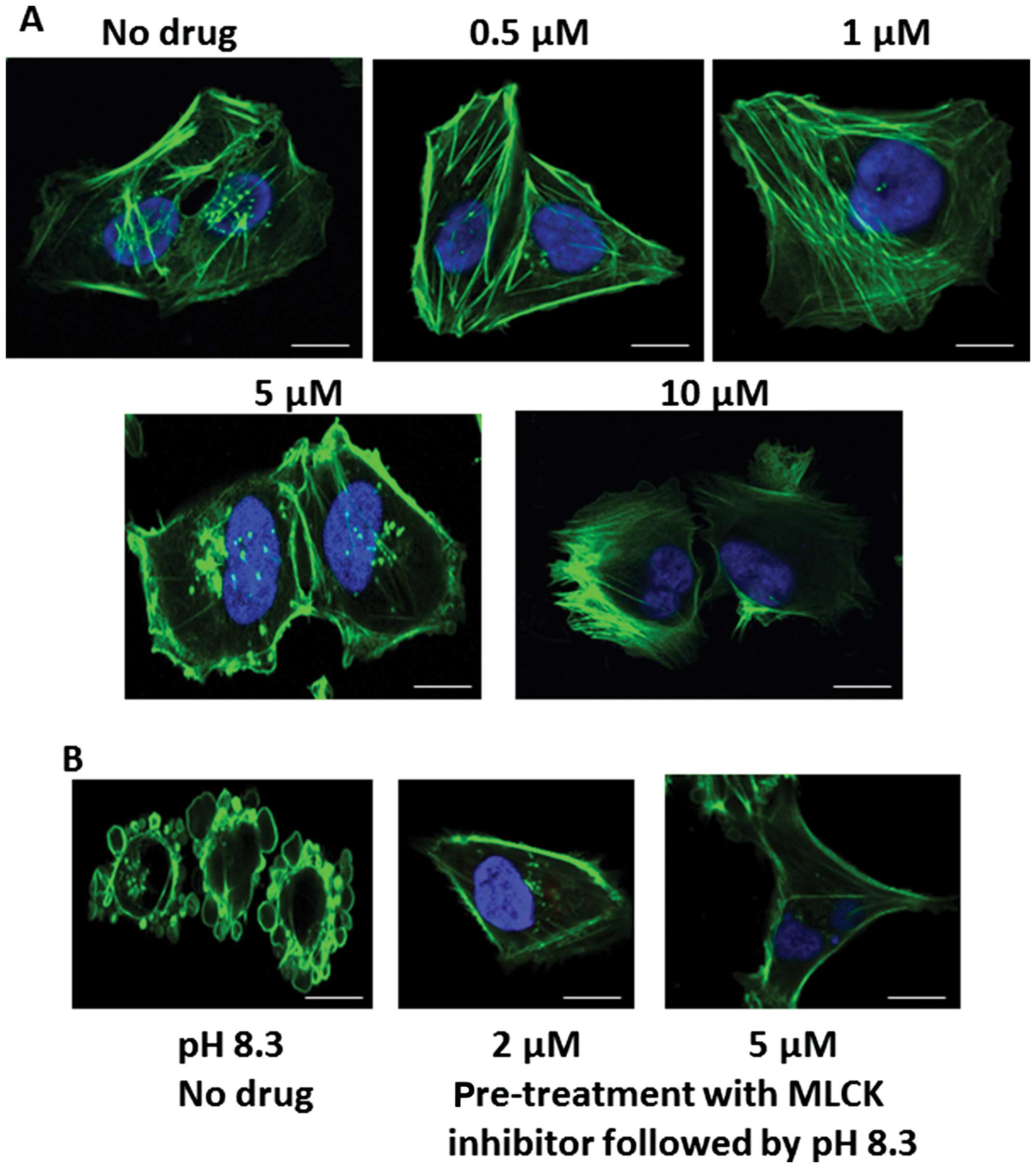
Effect of myosin light chain kinase
(MLCK) inhibitor on phallotoxin staining of pII cells
Fig. 7A shows
phallotoxin staining of pII cells after treatment with various
concentrations of the MLCK inhibitor peptide 18. At 5 μM and
higher, there was a marked disruption of the membrane and actin
cytoskeleton structure. At 2–5 μM the drug had minimal effect on
the actin distribution. At this concentration it completely
inhibited the morphological changes associated with alkaline pH
when it was added to the cells 1 h before culturing in alkaline pH
(Fig. 7B).
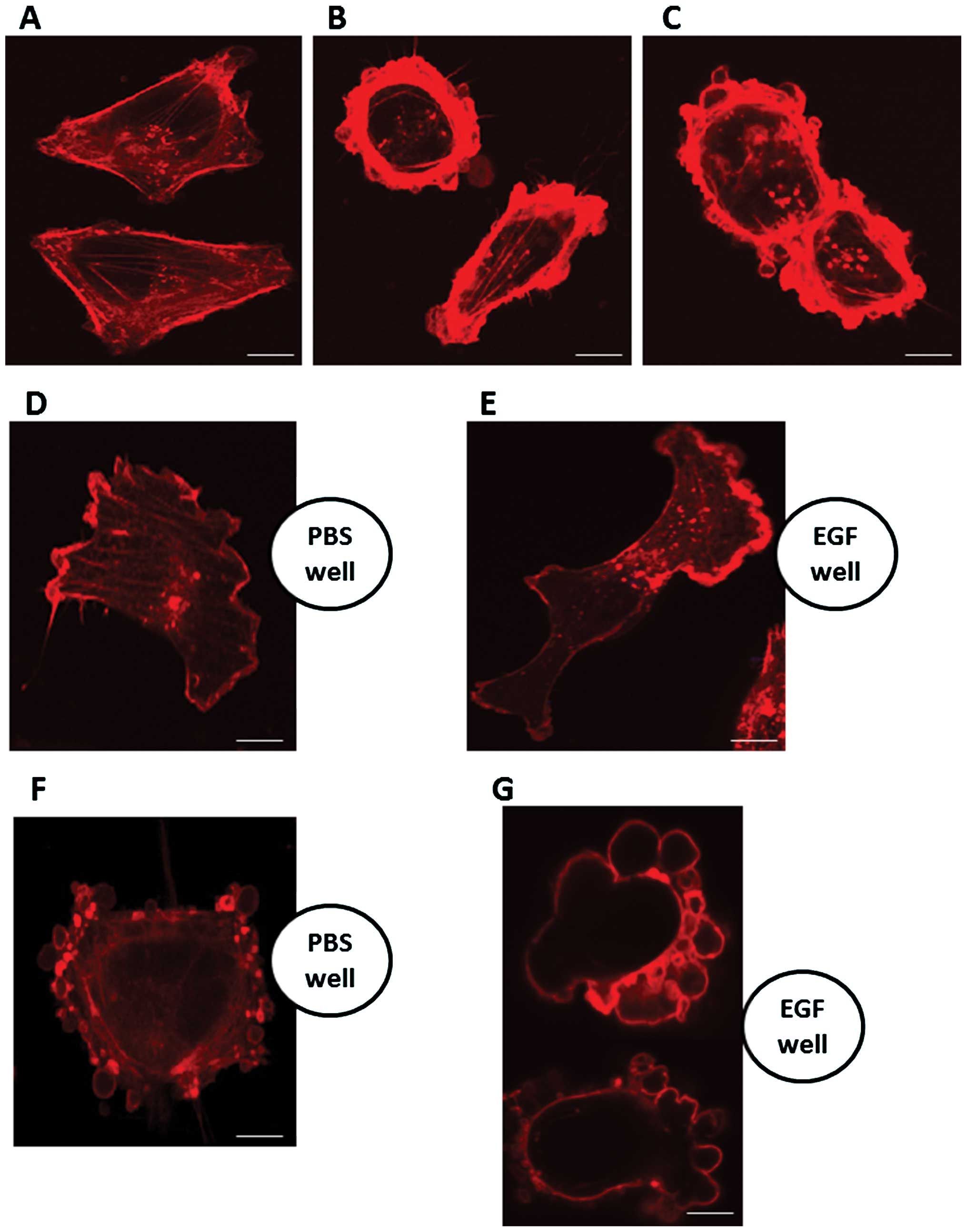
Effect of epidermal growth factor (EGF)
on phalloidin staining of pII cells
Fig. 8A shows
phalloidin staining of pII cells at neutral pH. Exposure to medium
containing EGF (10 ng/ml) for 10 and 30 min at pH 7.4 (Fig. 8B and C respectively) resulted in
more intense membrane staining, reflecting increased membrane
ruffling, but there was no indication of formation of blebs. When
cells were placed near a source of EGF (in a strip of agarose gel)
they tended to move in that direction (Fig. 8E), showing polarization, reflected
in the distribution of phalloidin staining. This was not seen with
PBS-containing gel (Fig. 8D). pII
cells exposed to alkaline pH (by transfer of the dish to normal
atmosphere) for 1 h before fixation and staining, exhibited
contractolation as expected but with formation of blebs principally
on the surface of the cell facing the EGF source (Fig. 8G), in marked contrast with the more
random distribution of the blebs when the adjacent gel contained
PBS only (Fig. 8F).
Effect of extracellular pH on expression
of vimentin
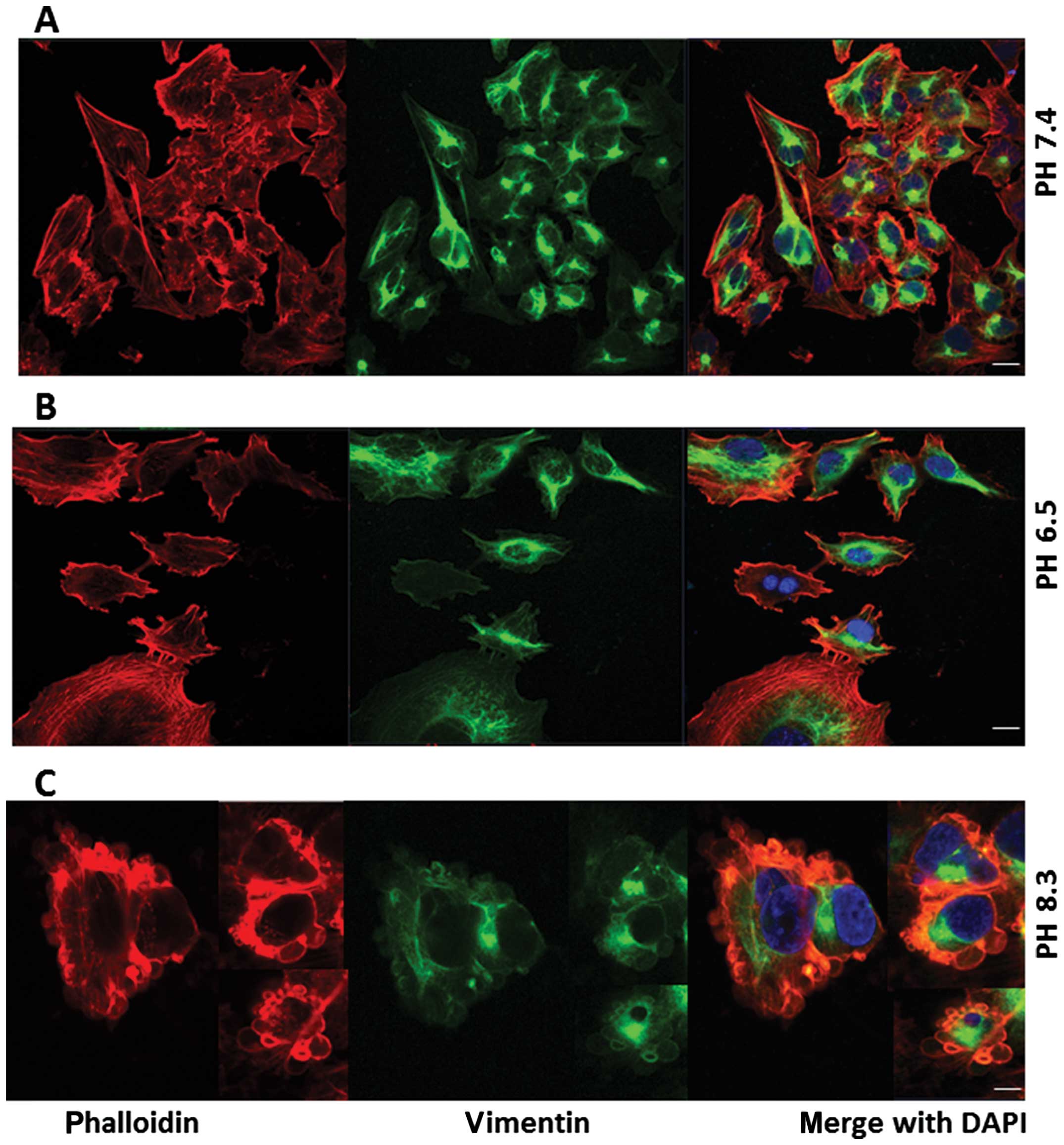
Fig. 9A shows
vimentin staining (green stain) in pII cells (which have undergone
EMT and express mesenchymal markers) at neutral pH with a polarized
peri-nuclear pattern. The distribution of this protein was
unaffected by exposure to either acidic or alkaline conditions for
1 h (Fig. 9B and C respectively)
and it did not appear in the blebs.
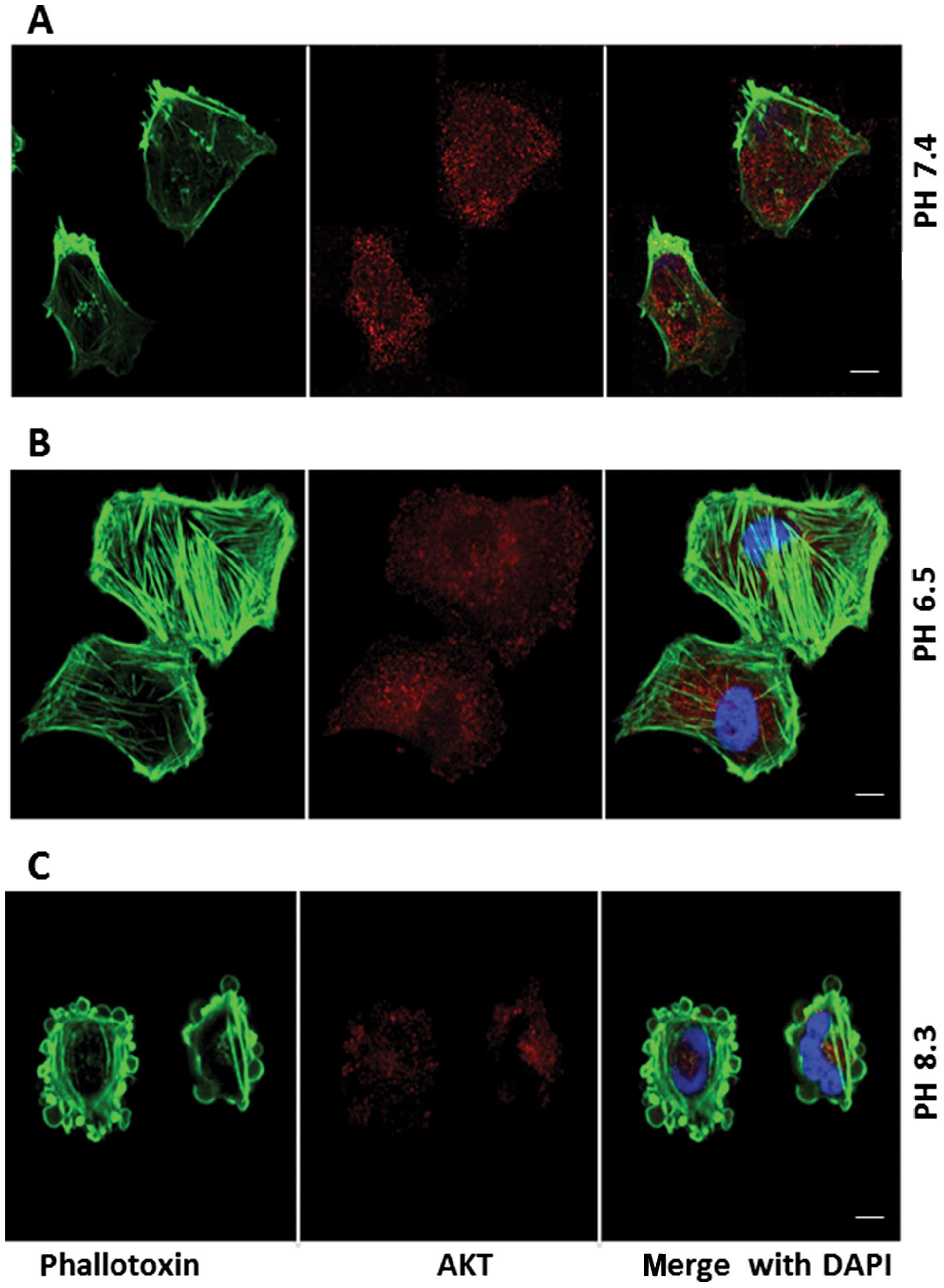
Effect of extracellular pH on expression
of AKT
Fig. 10A shows AKT
staining (red stain) in pII cells at neutral pH with a diffuse
cytoplasmic staining pattern. The distribution of this protein was
unaffected by exposure to acidic conditions for 1 h (Fig. 10B). At alkaline pH (Fig. 10C) the staining was greatly
reduced and was conspicuously absent from the blebs.
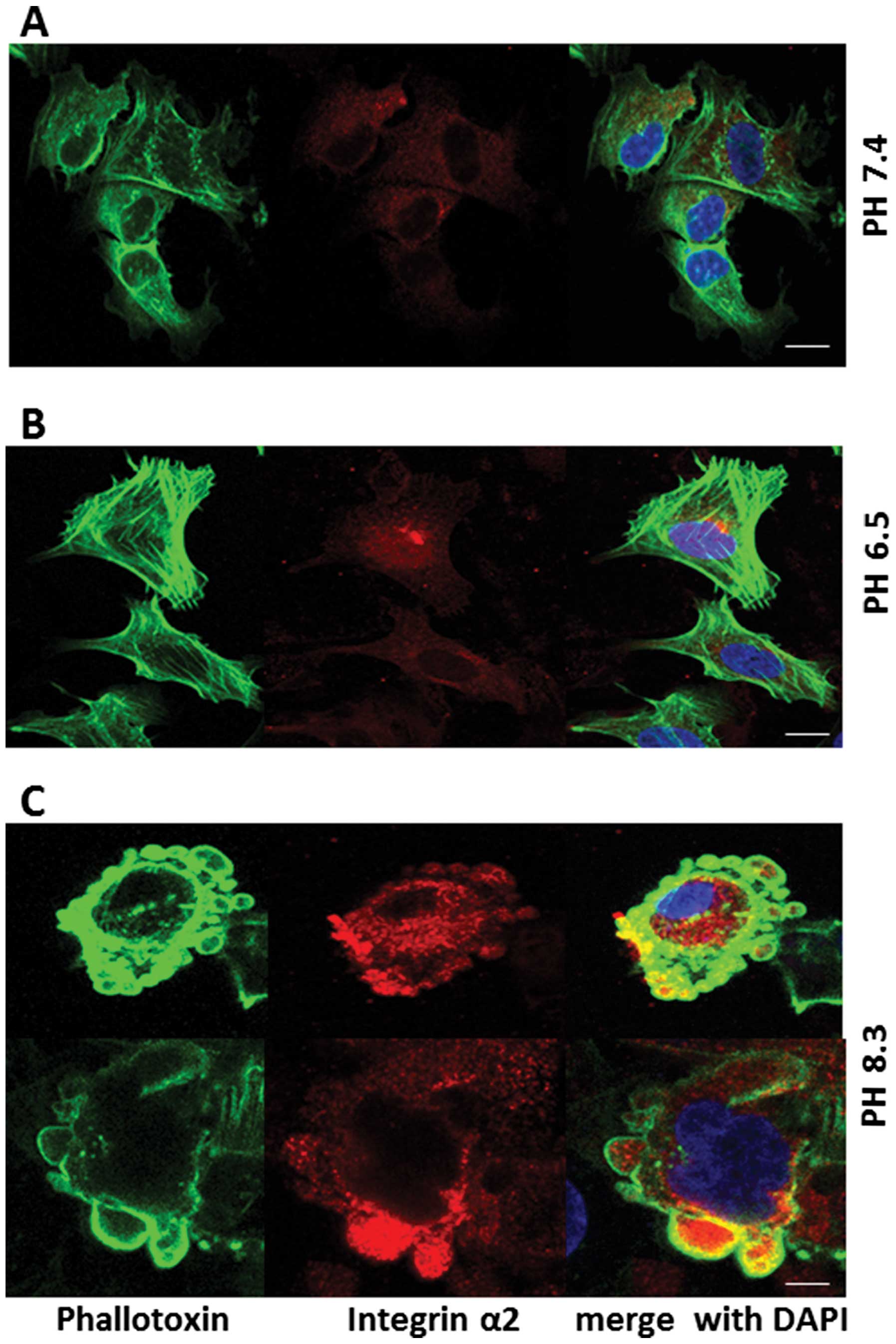
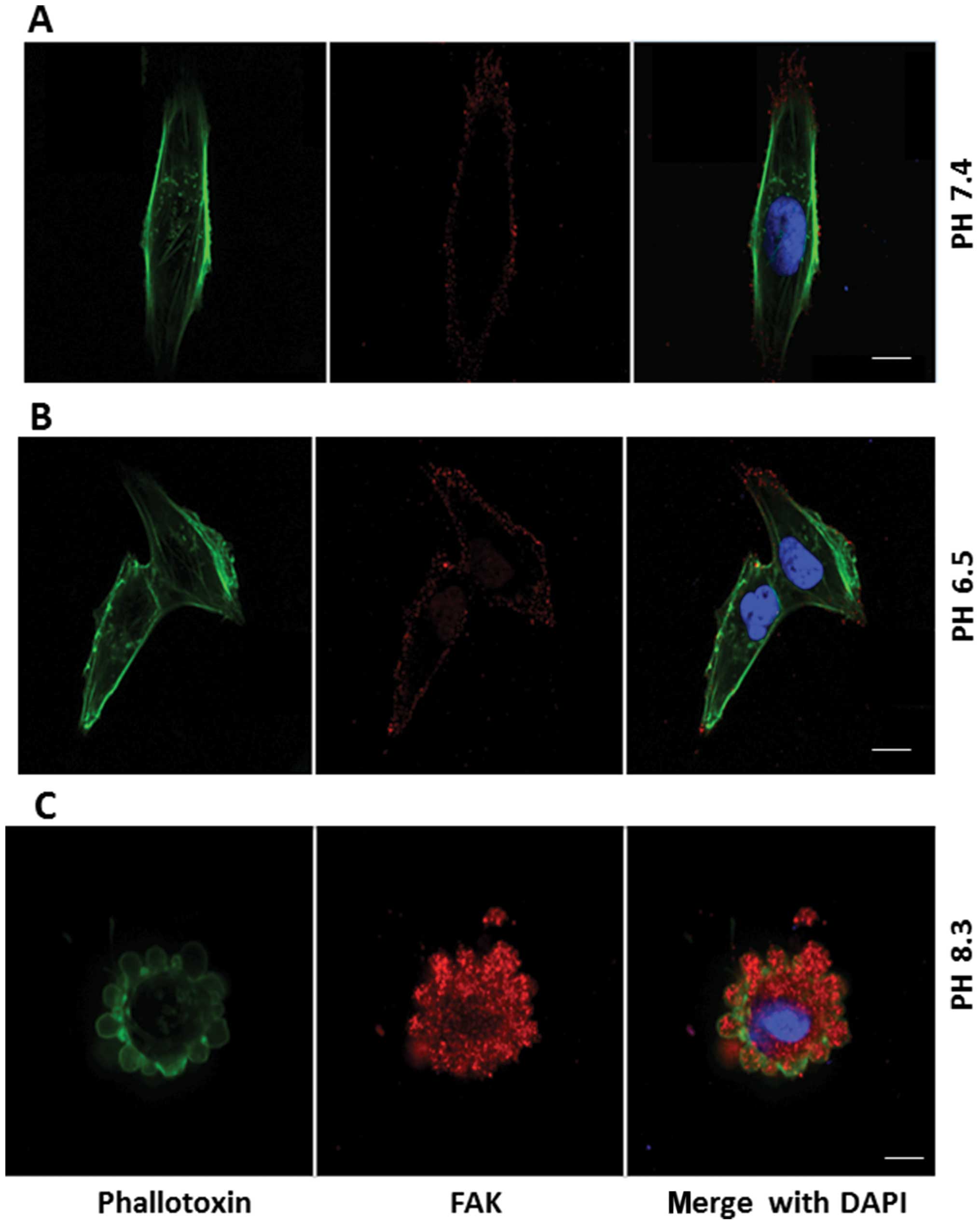
Effect of extracellular pH on expression
of adhesion molecules
Figs. 11–13 show distribution of integrin-α2,
junctional adhesion molecule-1 (JAM-1), and focal adhesion kinase
(FAK) (red stain), respectively, in pII cells, after 1-h exposure
to neutral, acidic or alkaline pH. These molecules show diffuse
cytoplasmic expression in neutral pH conditions (panels A); this
pattern was not changed upon exposure to acidic pH (panels B).
Under conditions of alkaline pH, these molecules were seen inside
the newly formed blebs, but did not appear to be part of the
membrane, which was stained with phalloidin (panels C). It was
noted that the intensity of staining for FAK was markedly increased
upon exposure to alkaline pH when compared to neutral or acidic
conditions (Fig. 13) under which
it was very poorly expressed.
Discussion
Acidic vs. alkaline pH
The majority of the data in the literature appears
to support the idea that the tumor microenvironment is acidic in
nature and aids in tumor development and progression through
various mechanisms (reviewed in ref. 13). This has prompted exploration of
alkalinization-based therapeutic measures to nullify this acidity
(30,31) and such proposals have already
entered the popular press as a new type of cancer treatment. We
have previously shown that alkaline conditions have a dramatic
effect, though only on ER− cells, and in particular on
those that have acquired functional loss of ER as opposed to those
that are de novo ER− (29).
In the present study we observed that even prolonged
(overnight) exposure to acidic conditions did not significantly
affect either the ER+ or the ER− cell lines
with respect to either the type of morphological changes (Fig. 1) or enhancement of cell motility
and invasion induced by very brief exposure to alkaline pH (data
not shown). In contrast with this observation, both mouse B16-F1-
melanoma (32) and human A-07,
D-12, or T-22 melanoma (33) are
reported to acquire enhanced invasive ability in acidic pH,
suggesting that the pH effect might also be tissue specific.
Indeed, consistent with our observations, exposure of human colonic
adenoma and carcinoma cell lines to acidic pH significantly reduced
their proliferative capacity (24). In addition, expression of the
universal inhibitor of cell cycle dependent kinases WAF1 was
increased by exposure of human glioblastoma cells to acidic pH and
resulted in cell cycle arrest in G1 (34–36).
With respect to the ability of cells to survive over longer periods
under the stressful conditions of acid/alkaline pH (24), reported enhancement in
proliferative capacity of human colonic adenoma and carcinoma
derived cell lines that had previously been exposed to alkaline pH
for 4 days (37) observed that
exposure of their murine fibrosarcoma cell line Fsa-II to alkaline
conditions caused cell death by generation of reactive oxygen
species (within 90 min), DNA fragmentation (within 24 h) and
mitochondrial damage (within 6 h). Whilst all our cell lines appear
able to survive well in acidic conditions, alkaline pH starts to
induce cell death of pII and YS2.5 [both ER silenced lines
(3)] after ~4-h exposure. With
exposures of <2 h they are able to revert to their original
morphology and continue normal growth if restored to pH 7.4 medium.
The ER+ MCF7 cells are able to survive for longer
periods, with significant cell death evident from ~8–10 h onwards
(data not shown). This might be due to the stronger cell-cell
contact in ER+ cells compared to more loose contacts in
ER− cells (due to the loss of adherent and tight
junctions proteins during the EMT process), or perhaps to a higher
threshold of resistance to alkaline-induced death in ER+
vs ER− cells. Thus it seems brief exposure to alkaline
pH confers advantages to endocrine resistant cancer cells, in terms
of increased motility and invasive capacity, while a longer period
is detrimental.
Contractolation
The alkaline induced shrinking and rounding
phenomenon of the cell that we documented previously, when examined
more closely at higher magnification, indicates thickening of the
plasma membrane (often also referred to as ruffling) and formation
of vesicular invaginations closely resembling cytoplasmic
‘blebbing’ described during early embryogenic migration (38). In a more general context, and as
distinct from the more extensively studied membrane protrusions
enabling motile functions such as lamellipodia and filopodia, blebs
are associated with motility (39), cell spreading (40), cytokinesis (41) and mitosis (42–45).
Membrane blebbing (46,47)
is also seen during the execution phase of apoptosis which
subsequently leads to DNA fragmentation, chromatin condensation and
apoptotic cell death (48–50). Treatment of PC6-3 cells with the
caspase inhibitor z-VAD-FMK for 24 h generated similar blebs
(51), and these were also
correlated with cell death. The time needed to form blebs in PC6-3
cells was significantly longer compared to what we have observed in
pII cells upon exposure to alkaline pH (24 h vs ≤10 min). In
general however, bleb dynamics are quite rapid (52).
It has been well documented that actin is the main
component of most of these membrane blebs (53–57),
with other known constituents being myosin and ezrin (52). Formation of blebs, such as in
Walker 256 carcinosarcoma cells, have also been described as areas
where cortical actin is initially depolymerized and constitutes a
boundary separating the blebs from the rest of the cell (58); this would generally be followed by
reconstitution of an actin cortex. It is considered that the major
role of the actin cytoskeleton in formation of membrane blebs is to
increase the intracellular hydrostatic pressure by cortical
contraction, which results in protrusions at sites where the
elastic resistance is reduced. Myosin also plays a critical role in
the formation of membrane blebs. The myosin II contractile activity
is stimulated through phosphorylation of myosin light chain on
serine 19 by MLCK which catalyzes the interaction between myosin
and actin needed to produce sliding forces for cell contraction and
movement (59,60). Microinjection of catalytically
active MLCK can induce bleb formation (41). In addition, the small GTPases Rho,
Rac and CDC42 [which are elevated in pII cells (3)] play an important role in cytoskeletal
rearrangement and actin stress fiber formation (61,62);
treatment with the actin de-polymerizer cytochalasin-D, or
inhibitors of MLCK and Rho kinase activity, all inhibited bleb
formation in serum-deprived z-VAD-FMK treated PC6-3 cells (51). We found that F-actin is the main
component of the membranes of the blebs formed in response to
exposure to alkaline pH. Molecules critical for adhesion and
motility, such as JAM-1, FAK and integrin-α2, were seen to flow
from their diffuse cytoplasmic locations into the newly formed
blebs, but were not part of the membrane. This shows that the blebs
are continuous with the rest of the cell; however, other molecules
such as vimentin, exemplifying the mesenchymal phenotype (in place
of the keratin found only in the ER+ cells), did not
alter their perinuclear distribution and were not seen inside the
blebs (Fig. 9). Cytochalasin-D,
MLCK and Rho kinase inhibitors all completely disrupted the plasma
membrane of all our cells. Pre-treatment of pII cells, prior to
exposure to alkaline pH, with lower concentrations, at which the
integrity of plasma membranes was maintained (reflected in the
continuity of actin staining), prevented bleb formation. Moreover,
these drugs also caused retraction of already formed blebs when
added to pII cultures that had been previously shifted to pH 8.3
(Figs. 5–7) (51)
had previously found that MLCK inhibitors were able to decrease
blebbing. Involvement of RhoA-ROCK and myosin in bleb formation has
also been previously documented (46,63),
with (64) suggesting that
bleb-associated motility could be reflective of reduced substratum
adhesion, as indeed would be true of our pII cells that lack many
critical adhesion proteins such as E-cadherin (65) previously showed that blebs can be
either stationary or move to the leading edge or backward over the
cell body. We observed that the formation of blebs, in response to
alkaline pH, is relatively uniform along the entire cell body but
they can re-distribute towards a chemoattractant source (in this
case EGF) in a polarized manner (Fig.
8D–G). It is interesting that while the chemoattractant is also
clearly able to cause cell polarization in its direction at pH 7.4,
with increased mobilization of actin into a thickened membrane, it
did not induce any blebbing.
These observations are consistent with our previous
data that suggest that the blebs may enhance the directional
invasive ability of endocrine resistant breast cancer cells
(29). It would appear that in
fact, blebs are normal protrusions of locomoting cells both in
vitro and in vivo (66–68)
and they can transform into lamellipodia and vice versa (38,69,70).
Migrating dictyostelium cells can switch from pseudopods to
blebs (71). In addition, the
mesenchymal type of tumor cells (such as pII) have characteristics
of fibroblast-like motility with elongated spindle-like shape and
obvious leading edge towards the source of the chemotactic agent
(72). On the other hand, an
amoeboid-like movement was observed in various leukocytes (73,74)
and tumor cells (75–78) and is characterized by rounded shape
of the cell during its motility. Cells with amoeboid type of
movement have enhanced contractility and invasive capacity due to
their rounded shape, which would conceivably enable them to squeeze
through the small spaces in the extracellular matrix (ECM) fibers
and exert higher force to deform the surrounding ECM (73–77).
Furthermore, supra-stimulation of pancreatic acinar cells with CCK,
induced rapid membrane actin cytoskeleton-rich blebs within 2–3
min, and the cells exhibited amoeboid shape (79). The formed blebs were rapidly
absorbed when the agonist level was reduced, indicating that
blebbing is a reversible, not necessarily a terminal event, which
is in agreement with our data. Indeed, formation and retraction of
blebs is not an uncommonly reported phenomenon. In view of these
data a possible explanation of the behaviour of pII cells is that
alkaline pH induces an amoeboid-like spherical shape (Figs. 3C and 4), enabling enhanced invasive potential,
mediated in part through elevated MMP2/9 activity (29) or possibly other secreted factors
(currently under investigation).
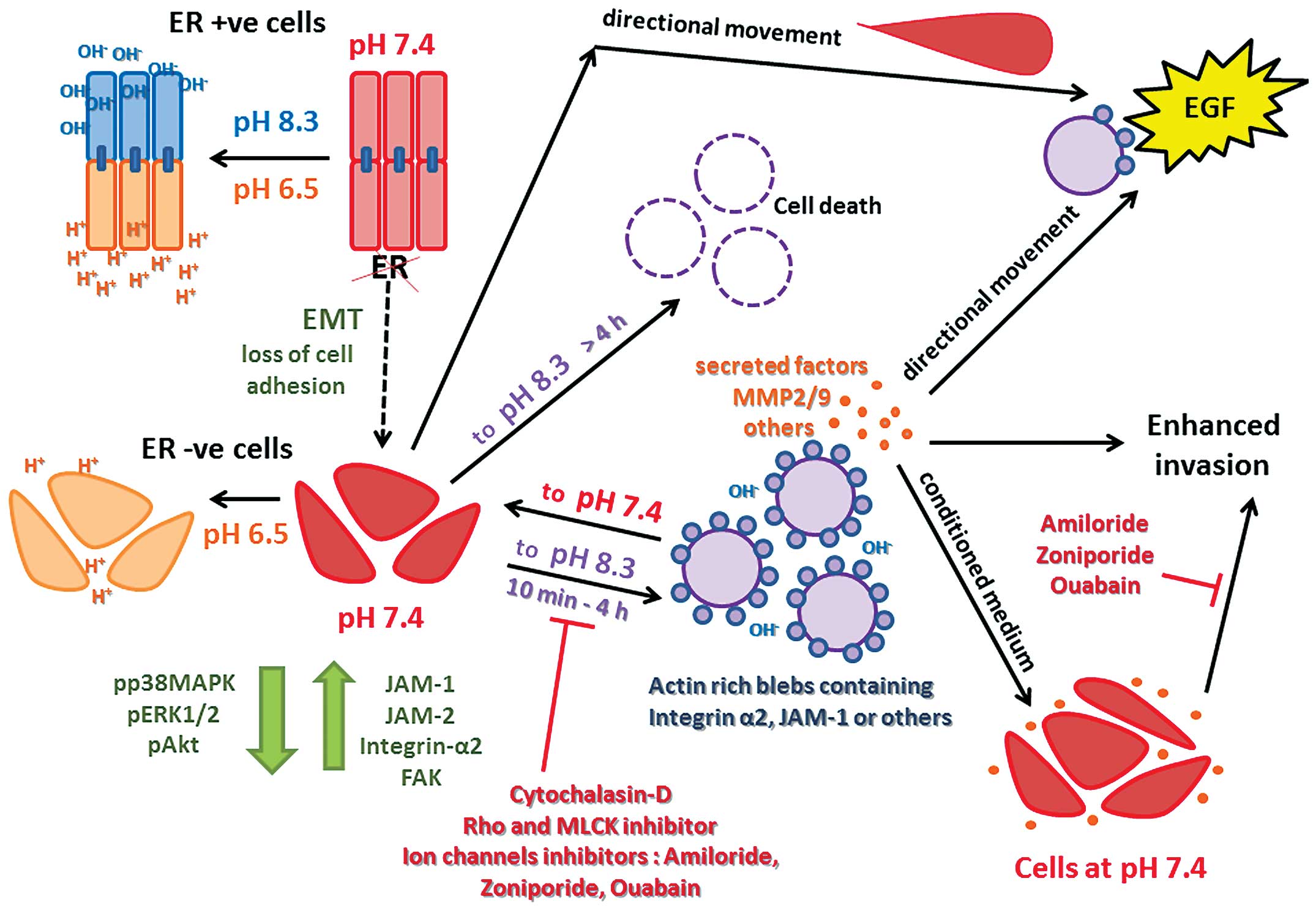
In conclusion, we have demonstrated that ER silenced
breast cancer cells, when shifted for brief periods from neutral to
alkaline, but not acidic conditions, markedly change their
morphological and functional properties. Cells tend to become
spherical (ameoboid-like) and segregate further from each other and
form dynamic and reversible blebs and other long cytoplasmic
protrusions which are made up of actin/myosin filaments on the
outer membrane, with molecules critical for cell adhesion and
motility in the cytoplasmic compartment of the formed blebs. These
are uniformly distributed but can polarize in the direction of
movement towards EGF, which by itself cannot elicit formation of
blebs though it can induce polarization and membrane thickening
through actin aggregation. Interruption of formation, as well as
disruption of pre-formed blebs, can be achieved with drugs that
interfere with several GTPase associated effector proteins. The
blebs also appear to be dependent upon ion channel activity and
confer increased invasive capacity. The differential longer term
detrimental effects of alkaline pH are being further investigated
as a possible means of selective cell killing. Fig. 14 shows a summary schematic
representation of the events described in this study.
Acknowledgements
This study was supported by Kuwait University
Research Sector grant PT02/11. Parts of this study were supported
by grant SRUL02/13 to the Research Unit for Genomics, Proteomics
and Cellomics Studies (OMICS), Kuwait University. We also thank the
Electron Microscopy Unit of the Faculty of Medicine and the
Nanoscopy Science Centre of Faculty of Science, Kuwait University
for assistance with the scanning electron microscopy.
References
|
1
|
Al Saleh S, Sharaf LH and Luqmani YA:
Signalling pathways involved in endocrine resistance in breast
cancer and associations with epithelial to mesenchymal transition
(Review). Int J Oncol. 38:1197–1217. 2011.PubMed/NCBI
|
|
2
|
Luqmani YA, Al Azmi A, Al Bader M, Abraham
G and El Zawahri M: Modification of gene expression induced by
siRNA targeting of estrogen receptor alpha in MCF7 human breast
cancer cells. Int J Oncol. 34:231–242. 2009.
|
|
3
|
Al Saleh S, Al Mulla F and Luqmani YA:
Estrogen receptor silencing induces epithelial to mesenchymal
transition in human breast cancer cells. PLoS One. 6:212011.
View Article : Google Scholar
|
|
4
|
Roxanis I: Occurrence and significance of
epithelial-mesenchymal transition in breast cancer. J Clin Pathol.
66:517–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giuliano M, Schifp R, Osborne CK and
Trivedi MV: Biological mechanisms and clinical implications of
endocrine resistance in breast cancer. Breast. 9776:70293–70294.
2011.
|
|
6
|
Khajah MA, Al Saleh S, Mathew PM and
Luqmani YA: Differential effect of growth factors on invasion and
proliferation of endocrine resistant breast cancer cells. PLoS One.
7:302012. View Article : Google Scholar
|
|
7
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: a review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
8
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hashim AI, Zhang X, Wojtkowiak JW,
Martinez GV and Gillies RJ: Imaging pH and metastasis. NMR Biomed.
24:582–591. 2011.PubMed/NCBI
|
|
10
|
Cairns R, Papandreou I and Denko N:
Overcoming physiologic barriers to cancer treatment by molecularly
targeting the tumor microenvironment. Mol Cancer Res. 4:61–70.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Izumi H, Torigoe T, Ishiguchi H, et al:
Cellular pH regulators: potentially promising molecular targets for
cancer chemotherapy. Cancer Treat Rev. 29:541–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boedtkjer E, Moreira JM, Mele M, et al:
Contribution of Na+, HCO3(−)-cotransport to
cellular pH control in human breast cancer: a role for the breast
cancer susceptibility locus NBCn1 (SLC4A7). Int J Cancer.
132:1288–1299. 2013. View Article : Google Scholar
|
|
13
|
Abaza M and Luqmani YA: The influence of
pH and hypoxia on tumor metastasis. Expert Rev Anticancer Ther.
13:1229–1242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rozhin J, Sameni M, Ziegler G and Sloane
BF: Pericellular pH affects distribution and secretion of cathepsin
B in malignant cells. Cancer Res. 54:6517–6525. 1994.PubMed/NCBI
|
|
15
|
Brisson L, Reshkin SJ, Gore J and Roger S:
pH regulators in invadosomal functioning: proton delivery for
matrix tasting. Eur J Cell Biol. 91:847–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao H, Li TK, Yang JM and Liu LF: Acidic
pH induces topoisomerase II-mediated DNA damage. Proc Natl Acad Sci
USA. 100:5205–5210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morita T, Nagaki T, Fukuda I and Okumura
K: Clastogenicity of low pH to various cultured mammalian cells.
Mutat Res. 268:297–305. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsubara T, Diresta GR, Kakunaga S, Li D
and Healey JH: Additive influence of extracellular pH, oxygen
tension, and pressure on invasiveness and survival of human
osteosarcoma cells. Front Oncol. 3:20132013. View Article : Google Scholar
|
|
19
|
Raghunand N, Altbach MI, van Sluis R, et
al: Plasmalemmal pH-gradients in drug-sensitive and drug-resistant
MCF-7 human breast carcinoma xenografts measured by 31P magnetic
resonance spectroscopy. Biochem Pharmacol. 57:309–312. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robey IF, Baggett BK, Kirkpatrick ND, et
al: Bicarbonate increases tumor pH and inhibits spontaneous
metastases. Cancer Res. 69:2260–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ibrahim-Hashim A, Cornnell HH, Abrahams D,
et al: Systemic buffers inhibit carcinogenesis in TRAMP mice. J
Urol. 188:624–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morimura T, Fujita K, Akita M, Nagashima M
and Satomi A: The proton pump inhibitor inhibits cell growth and
induces apoptosis in human hepatoblastoma. Pediatr Surg Int.
24:1087–1094. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kastelein F, Spaander MC, Steyerberg EW,
et al: Proton pump inhibitors reduce the risk of neoplastic
progression in patients with Barrett’s esophagus. Clin
Gastroenterol Hepatol. 11:382–388. 2013. View Article : Google Scholar
|
|
24
|
Williams AC, Collard TJ and Paraskeva C:
An acidic environment leads to p53 dependent induction of apoptosis
in human adenoma and carcinoma cell lines: implications for clonal
selection during colorectal carcinogenesis. Oncogene. 18:3199–3204.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Putney LK and Barber DL: Na-H
exchange-dependent increase in intracellular pH times G2/M entry
and transition. J Biol Chem. 278:44645–44649. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohtsubo T, Wang X, Takahashi A, et al:
p53-dependent induction of WAF1 by a low-pH culture condition in
human glioblastoma cells. Cancer Res. 57:3910–3913. 1997.PubMed/NCBI
|
|
27
|
Smallbone K, Maini PK and Gatenby RA:
Episodic, transient systemic acidosis delays evolution of the
malignant phenotype: Possible mechanism for cancer prevention by
increased physical activity. Biol Direct. 5:1745–6150. 2010.
View Article : Google Scholar
|
|
28
|
Chen JL, Lucas JE, Schroeder T, et al: The
genomic analysis of lactic acidosis and acidosis response in human
cancers. PLoS Genet. 4:52008. View Article : Google Scholar
|
|
29
|
Khajah MA, Almohri I, Mathew PM and
Luqmani YA: Extracellular alkaline pH leads to increased metastatic
potential of estrogen receptor silenced endocrine resistant breast
cancer cells. PLoS One. 8:20132013. View Article : Google Scholar
|
|
30
|
Calorini L, Peppicelli S and Bianchini F:
Extracellular acidity as favouring factor of tumor progression and
metastatic dissemination. Exp Oncol. 34:79–84. 2012.PubMed/NCBI
|
|
31
|
McCarty MF and Whitaker J: Manipulating
tumor acidification as a cancer treatment strategy. Altern Med Rev.
15:264–272. 2010.PubMed/NCBI
|
|
32
|
Kato Y, Nakayama Y, Umeda M and Miyazaki
K: Induction of 103-kDa gelatinase/type IV collagenase by acidic
culture conditions in mouse metastatic melanoma cell lines. J Biol
Chem. 267:11424–11430. 1992.PubMed/NCBI
|
|
33
|
Rofstad EK, Mathiesen B, Kindem K and
Galappathi K: Acidic extracellular pH promotes experimental
metastasis of human melanoma cells in athymic nude mice. Cancer
Res. 66:6699–6707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
el-Deiry WS, Tokino T, Velculescu VE, et
al: WAF1, a potential mediator of p53 tumor suppression. Cell.
75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Noda A, Ning Y, Venable SF, Pereira-Smith
OM and Smith JR: Cloning of senescent cell-derived inhibitors of
DNA synthesis using an expression screen. Exp Cell Res. 211:90–98.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Majima HJ, Oberley TD, Furukawa K, et al:
Prevention of mitochondrial injury by manganese superoxide
dismutase reveals a primary mechanism for alkaline-induced cell
death. J Biol Chem. 273:8217–8224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Trinkaus JP: Surface activity and
locomotion of Fundulus deep cells during blastula and gastrula
stages. Dev Biol. 30:69–103. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bergert M, Chandradoss SD, Desai RA and
Paluch E: Cell mechanics control rapid transitions between blebs
and lamellipodia during migration. Proc Natl Acad Sci USA.
109:14434–14439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Norman LL, Brugues J, Sengupta K, Sens P
and Aranda-Espinoza H: Cell blebbing and membrane area homeostasis
in spreading and retracting cells. Biophys J. 99:1726–1733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fishkind DJ, Cao LG and Wang YL:
Microinjection of the catalytic fragment of myosin light chain
kinase into dividing cells: effects on mitosis and cytokinesis. J
Cell Biol. 114:967–975. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boss J: Mitosis in cultures of newt
tissues. IV The cell surface in late anaphase and the movements of
ribonucleoprotein. Exp Cell Res. 8:181–187. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boss J: Mitosis in cultures of newt
tissues. III Cleavage and chromosome movements in anaphase. Exp
Cell Res. 7:443–456. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boss J: Mitosis in cultures of newt
tissues. I A critical study of the methods and material. Exp Cell
Res. 7:215–231. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moes MJ, Bijvelt JJ and Boonstra J:
Attachment of HeLa cells during early G1 phase. Histochem Cell
Biol. 136:399–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Charras G and Paluch E: Blebs lead the
way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol.
9:730–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Charras GT: A short history of blebbing. J
Microsc. 231:466–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: the significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Earnshaw WC: Nuclear changes in apoptosis.
Curr Opin Cell Biol. 7:337–343. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jacobson MD, Weil M and Raff MC:
Programmed cell death in animal development. Cell. 88:347–354.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mills JC, Stone NL, Erhardt J and Pittman
RN: Apoptotic membrane blebbing is regulated by myosin light chain
phosphorylation. J Cell Biol. 140:627–636. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bovellan M, Fritzsche M, Stevens C and
Charras G: Death-associated protein kinase (DAPK) and signal
transduction: blebbing in programmed cell death. FEBS J. 277:58–65.
2010. View Article : Google Scholar
|
|
53
|
Cotter TG, Lennon SV, Glynn JM and Green
DR: Microfilament-disrupting agents prevent the formation of
apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res.
52:997–1005. 1992.PubMed/NCBI
|
|
54
|
Cunningham CC: Actin polymerization and
intracellular solvent flow in cell surface blebbing. J Cell Biol.
129:1589–1599. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Laster SM and Mackenzie JM Jr: Bleb
formation and F-actin distribution during mitosis and tumor
necrosis factor-induced apoptosis. Microsc Res Tech. 34:272–280.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pitzer F, Dantes A, Fuchs T, Baumeister W
and Amsterdam A: Removal of proteasomes from the nucleus and their
accumulation in apoptotic blebs during programmed cell death. FEBS
Lett. 394:47–50. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vemuri GS, Zhang J, Huang R, Keen JH and
Rittenhouse SE: Thrombin stimulates wortmannin-inhibitable
phosphoinositide 3-kinase and membrane blebbing in CHRF-288 cells.
Biochem J. 314:805–810. 1996.PubMed/NCBI
|
|
58
|
Keller H and Eggli P: Protrusive activity,
cytoplasmic compartmentalization, and restriction rings in
locomoting blebbing Walker carcinosarcoma cells are related to
detachment of cortical actin from the plasma membrane. Cell Motil
Cytoskeleton. 41:181–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kohama K, Ye LH, Hayakawa K and Okagaki T:
Myosin light chain kinase: an actin-binding protein that regulates
an ATP-dependent interaction with myosin. Trends Pharmacol Sci.
17:284–287. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gallagher PJ, Herring BP and Stull JT:
Myosin light chain kinases. J Muscle Res Cell Motil. 18:1–16. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ridley AJ and Hall A: The small
GTP-binding protein rho regulates the assembly of focal adhesions
and actin stress fibers in response to growth factors. Cell.
70:389–399. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chrzanowska-Wodnicka M and Burridge K:
Rho-stimulated contractility drives the formation of stress fibers
and focal adhesions. J Cell Biol. 133:1403–1415. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jia Z, Vadnais J, Lu ML, Noel J and Nabi
IR: Rho/ROCK-dependent pseudopodial protrusion and cellular
blebbing are regulated by p38 MAPK in tumour cells exhibiting
autocrine c-Met activation. Biol Cell. 98:337–351. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fackler OT and Grosse R: Cell motility
through plasma membrane blebbing. J Cell Biol. 181:879–884. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Keller HU and Cottier H: Crawling-like
movements and polarisation in non-adherent leucocytes. Cell Biol
Int Rep. 5:3–7. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Johnson KE: Circus movements and blebbing
locomotion in dissociated embryonic cells of an amphibian, Xenopus
laevis. J Cell Sci. 22:575–583. 1976.PubMed/NCBI
|
|
67
|
Haston WS and Shields JM: Contraction
waves in lymphocyte locomotion. J Cell Sci. 68:227–241.
1984.PubMed/NCBI
|
|
68
|
Grinnell F: Migration of human neutrophils
in hydrated collagen lattices. J Cell Sci. 58:95–108.
1982.PubMed/NCBI
|
|
69
|
Keller HU, Naef A and Zimmermann A:
Effects of colchicine, vinblastine and nocodazole on polarity,
motility, chemotaxis and cAMP levels of human polymorphonuclear
leukocytes. Exp Cell Res. 153:173–185. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Keller HU and Zimmermann A: Shape changes
and chemokinesis of Walker 256 carcinosarcoma cells in response to
colchicine, vinblastine, nocodazole and taxol. Invasion Metastasis.
6:33–43. 1986.PubMed/NCBI
|
|
71
|
Zatulovskiy E, Tyson R, Bretschneider T
and Kay RR: Bleb-driven chemotaxis of Dictyostelium cells. J Cell
Biol. 204:1027–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pankova K, Rosel D, Novotny M and Brabek
J: The molecular mechanisms of transition between mesenchymal and
amoeboid invasiveness in tumor cells. Cell Mol Life Sci. 67:63–71.
2010. View Article : Google Scholar :
|
|
73
|
Mandeville JT, Lawson MA and Maxfield FR:
Dynamic imaging of neutrophil migration in three dimensions:
mechanical interactions between cells and matrix. J Leukoc Biol.
61:188–200. 1997.PubMed/NCBI
|
|
74
|
Friedl P, Borgmann S and Brocker EB:
Amoeboid leukocyte crawling through extracellular matrix: lessons
from the Dictyostelium paradigm of cell movement. J Leukoc Biol.
70:491–509. 2001.PubMed/NCBI
|
|
75
|
Sahai E and Marshall CJ: Differing modes
of tumour cell invasion have distinct requirements for Rho/ROCK
signalling and extracellular proteolysis. Nat Cell Biol. 5:711–719.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wolf K, Mazo I, Leung H, et al:
Compensation mechanism in tumor cell migration:
mesenchymal-amoeboid transition after blocking of pericellular
proteolysis. J Cell Biol. 160:267–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wyckoff JB, Pinner SE, Gschmeissner S,
Condeelis JS and Sahai E: ROCK- and myosin-dependent matrix
deformation enables protease-independent tumor-cell invasion in
vivo. Curr Biol. 16:1515–1523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rosel D, Brabek J, Tolde O, et al:
Up-regulation of Rho/ROCK signaling in sarcoma cells drives
invasion and increased generation of protrusive forces. Mol Cancer
Res. 6:1410–1420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Torgerson RR and McNiven MA: The
actin-myosin cytoskeleton mediates reversible agonist-induced
membrane blebbing. J Cell Sci. 111:2911–2922. 1998.PubMed/NCBI
|