Introduction
The incidence and mortality of primary liver cancer
(PLC) are increasing year by year (1). PLC is one of the most common
malignant tumors in China, the occurrence of liver cancer is
approximately one million people all around the world each year and
most of them occur in mainland of China, accounting for 54% of all
cases worldwide (2). PLC contains
mainly three subcategories: hepatocellular carcinoma (HCC),
intrahepatic bile duct carcinoma (IHBD) and hybrid liver cancer.
HCC is the major form of PLC and is the sixth most common neoplasm
in the world (3). Over the past
several decades, a series of risk factors for HCC have been
established (4–7), including hepatitis B virus (HBV) or
hepatitis C virus (HCV) infection, aflatoxin exposure, and tobacco
smoking. However, the mechanisms of the oncogenesis of HCC have not
been completely described. Recently, several studies have strongly
suggested that nuclear factor-κB (NF-κB) may play a pivotal role in
this pathophysiological process (8–11).
NF-κB, which contains five subunits [RelA (p65),
C-Rel, NF-κB1 (P50/P105), RelB and NF-κB2 (P52/P100)], is a major
transcription regulator of the cell functions, including adhesion,
immune response, cell invasion, cell differentiation, cell
proliferation and apoptosis (12).
In the resting cells, NF-κB is a dimer and is inactivated
retentively in the cytoplasm via binding to specific inhibitor of
NF-κB: IκB family. However, NF-κB can be activated by various
stimuli (13–18). First, IκB is phosphorylated, and
NF-κB is activated and released from its IκB-bound complex. In
addition, NF-κB translocates into the nucleus from cytoplasm. The
activated NF-κB (p65) binds to κB sequences and alters the
expression of various target genes (13). The above physiologic process also
occurred in different tumor tissues or in cancer cells (14–18),
such as breast cells, and especially in liver cancer cells
(19), indicating that NF-κB is
involved in oncogenesis process of liver cancer.
Hydrogen sulfide (H2S), is an unusual
virulent gas, and has been qualified as the third gasotransmitter
following nitric oxide (NO) and carbon monoxide (CO) (20–22).
H2S can be endogenously produced mainly by
3-mercaptopyruvate sulfurtransferase (3-MST),
cystathionineb-synthase (CBS) or cystathionine-γ-lyase (CSE)
(23,24). The expression of CSE and CBS is
distinctly tissue-specific. CBS is mainly found in the central
nervous system (CNS), and CSE mostly in the cardiovascular system.
Interestingly, both CBS and CSE have been simultaneously identified
in some systems, including kidney, liver, intestine and brain
(25). In recent years, more
attention is paid to H2S for its extensive physiological
and pathophysiological properties. Accumulating studies have
demonstrated that H2S can exert cardioprotection
(26–31), angiogenesis (32–34),
antioxidant (35), pro- and
anti-inflammatory (36,37) and other wide range of physiological
functions (38–41). Furthermore, increasing evidence has
shown that H2S is involved in the pathophysiological
process of tumors (42–52). Notably, the effects of
H2S on cancer are still controversial. On the one hand,
some findings from in vivo and in vitro studies
showed that H2S is beneficial for cancer cell growth,
proliferation, migration, and invasion (42–48),
owing to its angiogenesis and vascular relaxant effects,
H2S promotes the supply of nutrients and blood to the
tumor cells and tissues (42).
Nevertheless, on the other hand it was found that H2S
exerted its potential anticancer effects on SGC-7901 gastric cancer
cells (49), oral cancer cell
lines (50), colon cancer cell
(51) and several different human
cancer cell lines (HeLa, HCT-116, Hep G2, HL-60, MCF-7, MV4-11 and
U2OS) (52). Thus, the effects of
H2S on cancer are complicated and still unclear.
Furthermore, to our knowledge, no study has been focused on the
effect of exogenous H2S on liver cancer cells and its
mechanisms. Based on recent studies (42–48),
we investigated whether exogenous H2S can contribute to
cancer progress and explored these potential effects via
amplification of NF-κB pathway in PLC/PRF/5 hepatoma cells.
Materials and methods
Materials
NaHS, a donor of H2S, was obtained from
Sigma Chemical Co. (St. Louis, MO, USA), stored at 2–4°C and
protected from sunlight. Hoechst 33258 and PDTC were also purchased
from Sigma Chemical Co. The cell counter kit-8 (CCK-8) was supplied
by Dojindo Lab (Kumamoto, Japan). Fetal bovine serum (FBS) and
RPMI-1640 medium were obtained from Gibco BRL (Grand Island, NY,
USA). Anti-MMP2 antibody, anti-COX-2 antibody, anti-p-IκB antibody,
anti-NF-κB p65 antibody and anti-p-NF-κB p65 antibody were supplied
by Cell Signaling Technology (Boston, MA, USA). Horseradish
peroxidase (HRP)-conjugated secondary antibody and BCA protein
assay kit were obtained from KangChen Bio-tech, Inc. (Shanghai,
China). Enhanced chemiluminescence (ECL) solution was purchased
from KeyGen Biotech (Nanjing, China). Enzyme-linked immunosorbent
assay (ELISA) was supplied by ExCell Bio Co. (Shanghai, China). A
sulfur-sensitive electrode was obtained from Fuji Electric (ELIT
8225, ISEIonometer, EA Instruments Ltd., UK).
Cell culture and treatments
The human hepatoma cells PLC (PLC) and the Lo2 cells
(LO2) were supplied by Sun Yat-sen University Experimental Animal
Center (Guangzhou, Guangdong, China). The PLC cells and LO2 cells
were grown in RPMI-1640 medium supplemented with 10% fetal bovine
serum under an atmosphere of 5% CO2 and at 37°C with 95%
air. The PLC cells were treatment with 500 μmol/l NaHS for 24 h or
co-treatment with 500 μmol/l NaHS and 200 μmol/l PDTC for 24 h.
Western blot analysis
After the indicated treatments, the cells were
harvested and lysed with cell lysis solution at 4°C for 30 min. The
total proteins were quantified through using the BCA protein assay
kit. Loading buffer was added to cytosolic extracts, then boiled
for 6 min, the same amounts of supernatant from each sample were
fractionated by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE), and the total proteins were transferred
into polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% fat-free milk for 60 min in fresh blocking buffer
[0.1% Tween-20 in Tris-buffered saline (TBS-T)] at room
temperature, and incubated with either anti-MMP2 antibody (1:1,000
dilution), anti-COX-2 antibody (1:1,000 dilution), anti-p-IκB
antibody (1:1,000 dilution), anti-NF-κB p65 antibody (1:1,000
dilution), and anti-p-NF-κB p65 antibody (1:1,000 dilution) in
freshly prepared TBS-T with 3% free-fat milk overnight with gentle
agitation at 4°C. Membranes were washed for 5 min with TBS-T three
times and incubated with HRP-conjugated goat anti-rabbit secondary
antibody at a concentration of 1:3,000 dilution (KangChen
Bio-tech), in TBS-T with 3% fat-free milk for 1.5 h at room
temperature. Then membranes were washed three times with TBS-T for
5 min. The immunoreactive signals were visualized via using the ECL
(enhanced chemiluminescence) detection. In order to quantify the
protein expression, the X-ray film was scanned and analyzed with
ImageJ 1.47i software. The experiment was carried out three
times.
Measurement of cell viability
The PLC cells were seeded in 96-well plates at
concentration of 1×104/ml, and incubated at 37°C, the
CCK-8 assay was employed to assess the cell viability of PLC cells.
After the indicated treatments, 10 μl CCK-8 solution at a 1/10
dilution was added to each well and then the plate was incubated
for 1.5 h in the incubator. Absorbance at 450 nm was assayed using
a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The
means of the optical density (OD) of three wells in the indicated
groups were used to calculate the percentage of cell viability
according to the formula: Cell viability (%) = (OD treatment
group/OD control group) × 100%. The experiment was carried out in
three times.
Hoechst 33258 nuclear staining for
evaluation of apoptosis
Apoptotic cell death was tested by the Hoechst 33258
staining followed by photofluorography. PLC cells were plated in
35-mm dishes at a density of 1×106 cells/well. After the
above indicated treatments, the PLC cells were fixed with 4%
paraformaldehyde in 0.1 mol/l phosphate-buffered saline (PBS, pH
7.4) for 10 min at 4°C. In addition, the slides were washed three
times with PBS. After staining followed by 5 mg/ml Hoechst 33258
for 15 min, the PLC cells were washed three times with PBS. The PLC
cells were visualized under a fluorescence microscope (Bx50-FLA;
Olympus, Tokyo, Japan). Viable PLC cells displayed a uniform blue
fluorescence throughout the nucleus and normal nuclear size.
However, apoptotic PLC cells showed condensed, distorted or
fractured nuclei. The experiment was carried out three times.
Measurement of cell culture medium
H2S levels
A sulfur-sensitive electrode (ELIT 8225,
ISEIonometer, EA Instruments Ltd.) was used to measure the
production levels of H2S in the cell culture medium.
Cell culture medium samples were obtained from all subjects and
mixed with an equal volume of antioxidant solution. The total
volume covered the electrode; usually >0.8 ml. The electrode was
activated in the deionized water for ≥2 h. The modified sulfide
electrode and a reference electrode were dipped into the above
mixture. The electrode was flushed with the deionized water after
sample determination, and the activity state was maintained through
dipping the electrode in deionized water. A standard curve was
generated using a standard S2-solution, and then the H2S
concentration was calculated according to this standard curve.
ELISA for detection of VEGF in culture
supernatant
PLC/PRF/5 hepatoma cells were cultured in 96-well
plates. After the different indicated treatments, the level of VEGF
in the culture media was tested by enzyme-linked immunosorbent
assay (ELISA) according to the manufacturer’s instruction. The
experiment was performed at least five times.
Statistical analysis
All data are presented as the mean ± SEM.
Differences between groups were analyzed by one-way analysis of
variance (ANOVA) by using SPSS 13.0 (SPSS, Chicago, IL, USA)
software, and followed by LSD post hoc comparison test.
Statistical significance was set at P<0.05.
Results
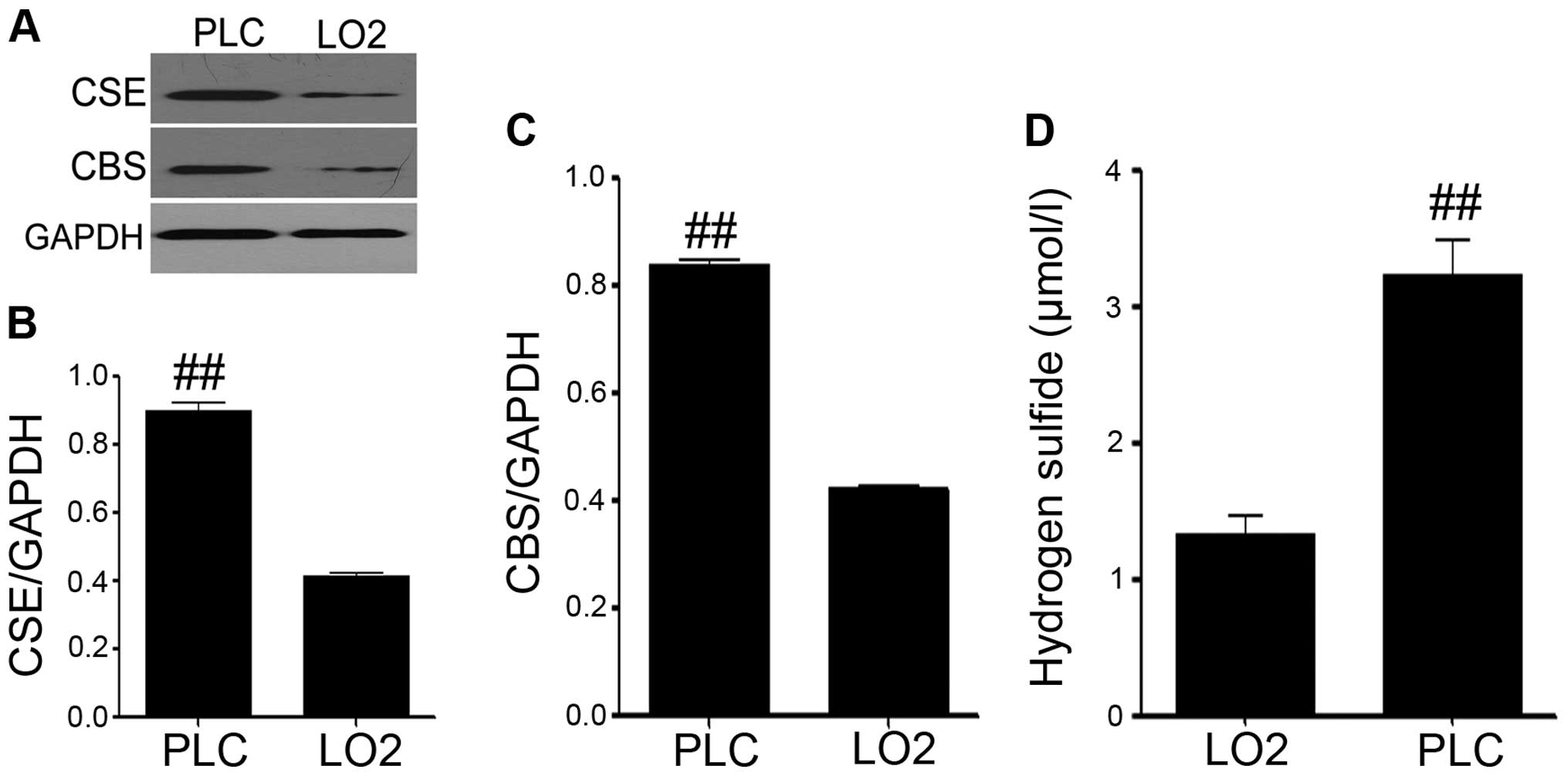
The expression levels of CBS and CSE, and
endogenous H2S production in PLC/PRF/5 hepatoma
cells
To determine the role of hydrogen sulfide
(H2S) in the oncogenesis process of liver cancer, the
expression levels of CSE and CBS were detected by western blot
assay both in PLC/PRF/5 hepatoma cells (PLC) and in the human
hepatic cell lines LO2 (LO2). As shown in Fig. 1A–C, both the expression of CSE
(Fig. 1A and B) and CBS (Fig. 1A and C) were significantly
upregulated, compared with the LO2 group. In addition, the
sulfur-sensitive electrode was used to explore the production level
of H2S in the cell culture medium. We found that the
production of hydrogen sulfide in PLC/PRF/5 hepatoma cells was
distinctly increased, compared with the LO2 group (Fig. 1D). So we hypothesized that
endogenous H2S might take part in the development of
liver cancer.
NaHS promotes cells proliferation in
PLC/PRF/5 hepatoma cells
In order to test the effect of exogenous
H2S in human liver cancer cell proliferation, the
dose-response study with varying doses (10, 50, 100, 200, 300, 400,
500, 600, 700 and 1,000 μmol/l) of NaHS (a donor of H2S)
for 24 h was performed to calculate the effective doses of NaHS. As
shown in Fig. 2A, low
concentrations of NaHS (10, 50 and 100 μmol/l) did not alter cell
viability. Whereas, the doses of NaHS from 200 to 1,000 μmol/l
markedly promoted cell proliferation, leading to an increase in
cell viability and reaching a peaking at 500 μmol/l. Therefore, 500
μmol/l NaHS was used in the subsequent time-response study with
different treatment times (12, 24, 36, 48, 60 and 72 h). As shown
in Fig. 2B, treatment of PLC cells
with 500 μmol/l NaHS for the indicated times all dramatically
promoted cell proliferation, reaching the maximal proliferative
effect at 24 h. Based on the above results, PLC/PRF/5 hepatoma
cells were treated with 500 μmol/l NaHS for 24 h in all subsequent
experiments.
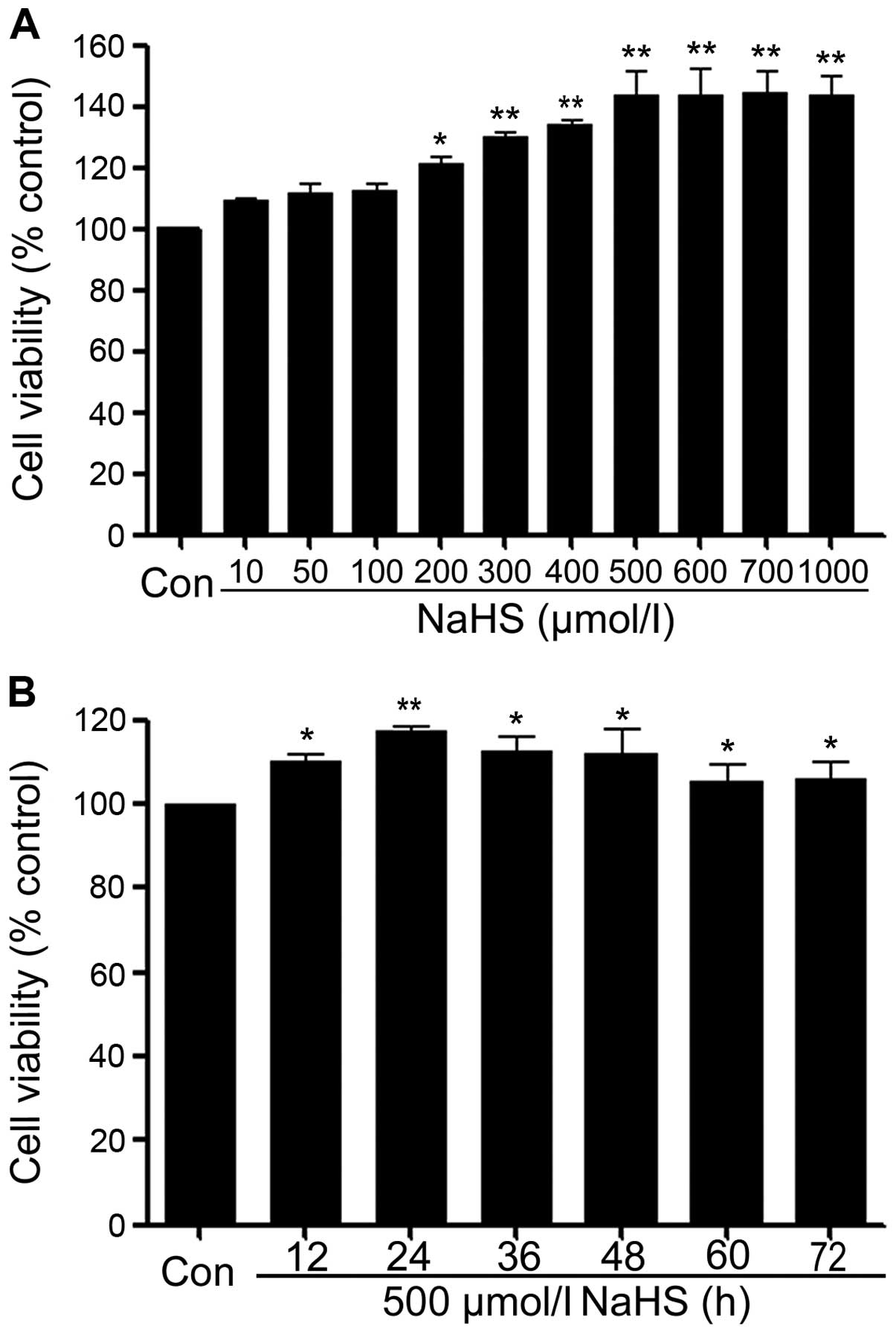 | Figure 2NaHS promotes cells proliferation in
PLC/PRF/5 hepatoma cells. Cell viability was tested by using the
cell counter kit (CCK-8). (A) PLC cells were treated with different
doses of NaHS (10, 50, 100, 200, 300, 400, 500, 600, 700 and 1,000
μmol/l) for 24 h. (B) Cells were treated with 500 μmol/l NaHS for
the indicated times (12, 24, 36, 48, 60 and 72 h). Data are the
mean ± SEM (n=3). *p<0.05, **p<0.01
compared with the control group. Con, the control group; NaHS, a
donor of H2S. |
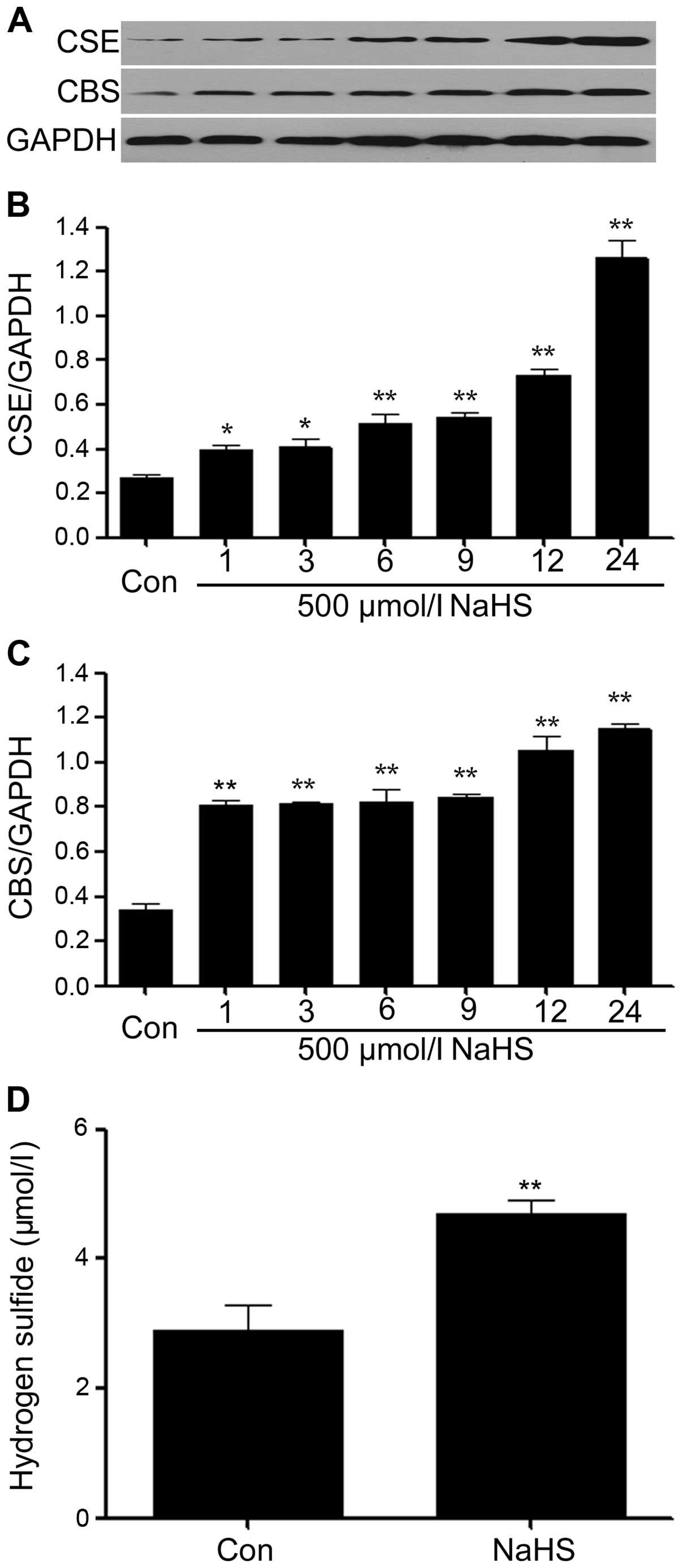
NaHS increases the expression levels of
CSE and CBS and production of H2S in PLC/PRF/5 hepatoma
cells
We observed the effects of NaHS on the expression
levels of CSE and CBS. As shown in Fig. 3A–C, exposure of PLC/PRF/5 hepatoma
cells for the indicated time (1, 3, 6, 9, 12 and 24 h) to 500
μmol/l NaHS markedly enhanced the expression levels of CSE and CBS,
reaching a peak at 24 h. In addition, at the same time, we found
that the production of H2S was significantly increased
in the cell culture medium, compared with the control group.
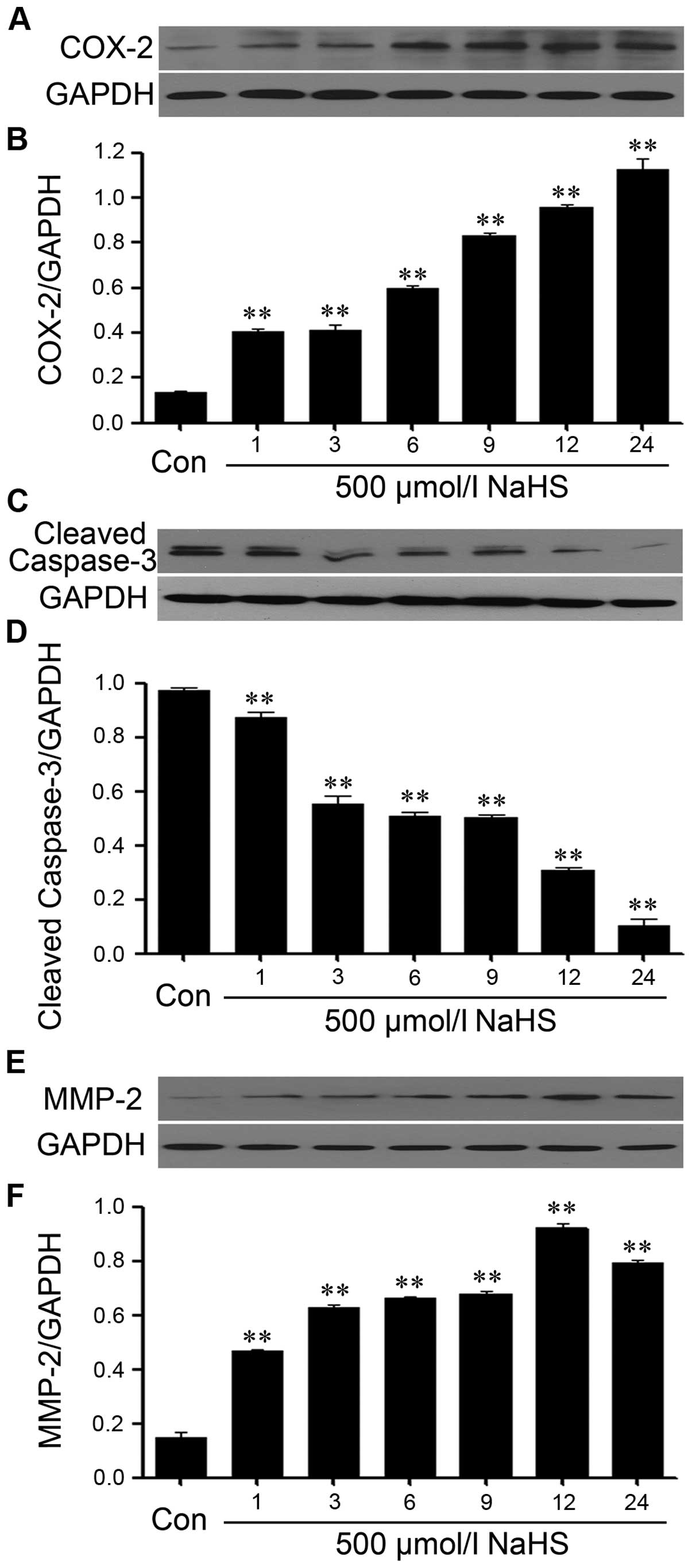
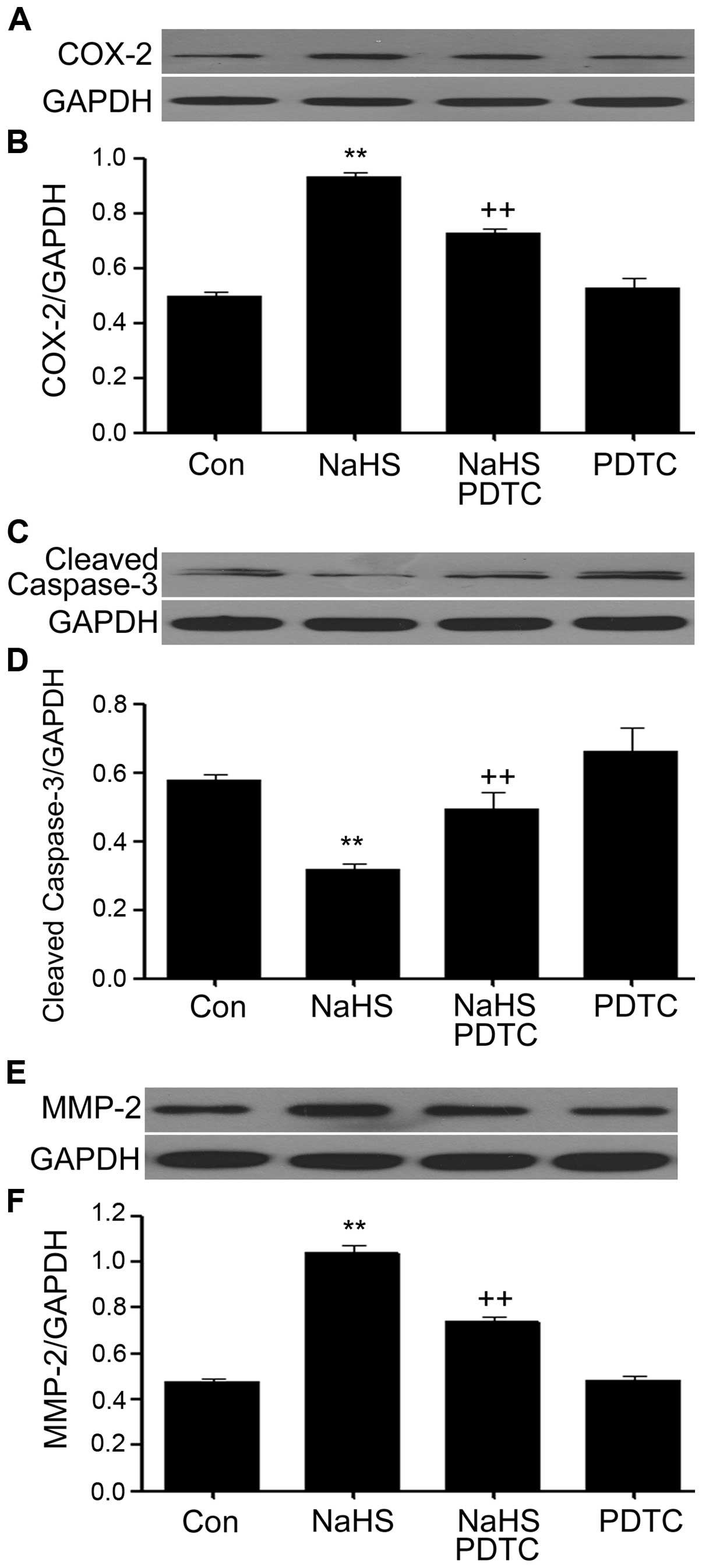
NaHS alleviates the expression level of
caspase-3 and upregulates the expression levels of COX-2 and MMP-2
in PLC/PRF/5 hepatoma cells
In order to observe the effects of NaHS on the
expression levels of caspase-3, COX-2 and MMP-2 in PLC/PRF/5
hepatoma cells, exposure of PLC/PRF/5 hepatoma cells to 500 μmol/l
NaHS for different times (1, 3, 6, 9, 12 and 24 h). As shown in
Fig. 4, NaHS significantly
enhanced the expression levels of COX-2 and MMP-2, and reaching a
peak at 12 h, whereas, the expression level of caspase-3 was
markedly decreased.
NaHS amplifies the activation of NF-κB
and p-IκBα in PLC/PRF/5 hepatoma cells
We observed the effects of NaHS on NF-κB p65
phosphorylation and IκBα phosphorylation in PLC/PRF/5 hepatoma
cells. PLC/PRF/5 hepatoma cells were exposed to 500 μmol/l NaHS for
the indicated times (3, 6, 9, 12 and 24 h), the expression levels
of p-NF-κB p65 were significantly upregulated, reaching a peak at
12 h (Fig. 5A and B), and the
t-NF-κB p65 expression was unchanged. Interestingly, the expression
levels of p-IκBα were also markedly increased from 3 h, reaching
the maximum at 24 h. Subsequently, we explored the effect of NaHS
on the nuclear translocation of NF-κB p65 subunit. NaHS treatment
significantly increased the nuclear translocation (Fig. 5E and F), with ameliorating amounts
of NF-κB p65 in the cytosol (Fig. 5G
and H). These results suggested that NaHS amplifies the
activation and nuclear translocation of NF-κB in PLC/PRF/5 hepatoma
cells.
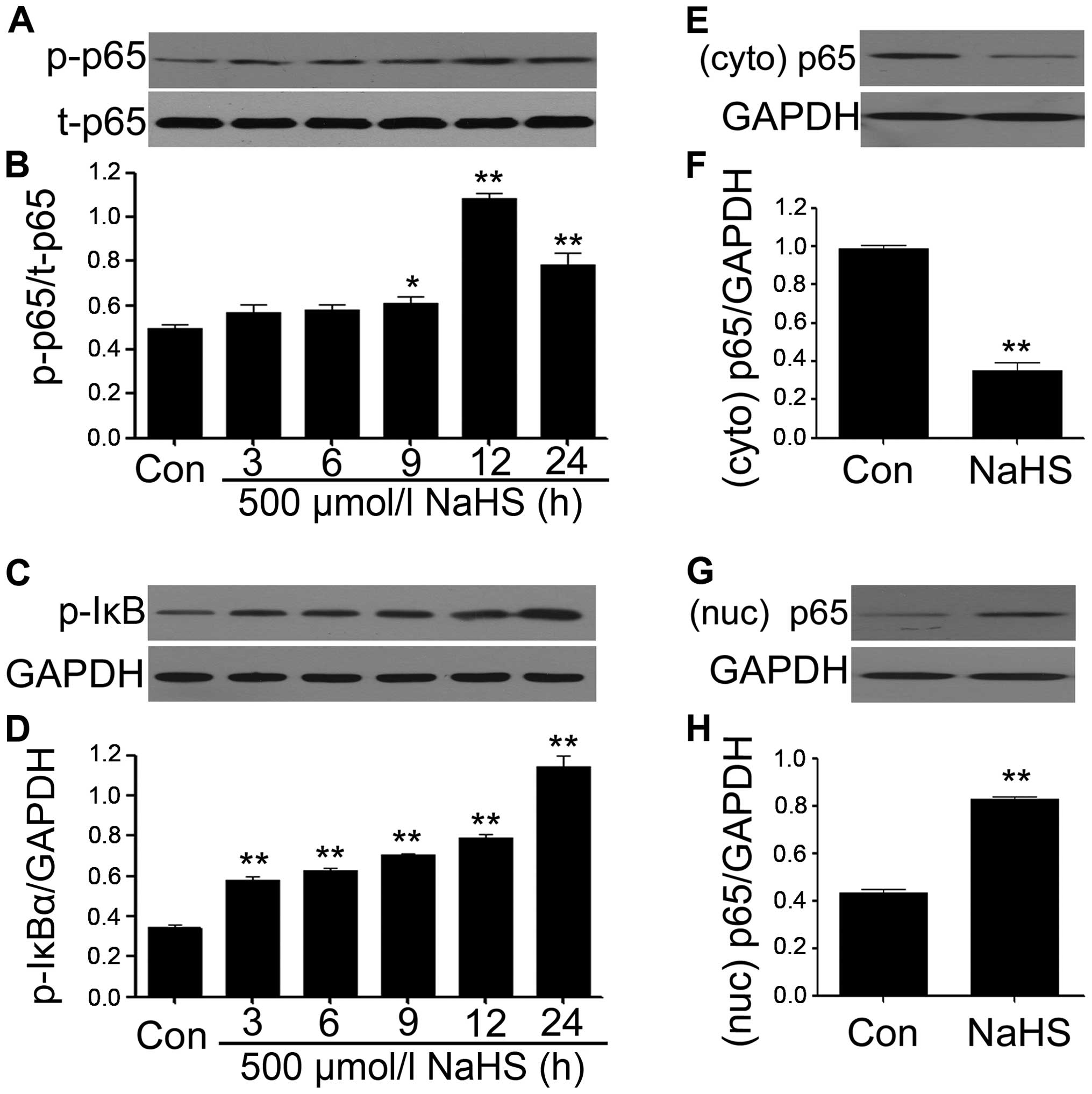 | Figure 5NaHS amplifies the activation of
NF-κB in PLC/PRF/5 hepatoma cells. (A–D) PLC/PRF/5 hepatoma cells
were exposed to 500 μmol/l NaH for the indicated times (3, 6, 9, 12
and 24 h). To test the effects of NaHS on NF-κB p65
phosphorylation, the cells were exposed to 500 μmol/l NaHS for 24
h. Cytoplasm (E and F) and nuclear (G and H) extracts were
extracted. The expression of p65 was analyzed by western blot
analysis. (B, D F and H) The data in (A, C, E and G) was quantified
by densitometric analysis with ImageJ 1.47i software. Data are
shown as the mean ± SEM (N=3). *P<0.05,
**P<0.01 versus with the control group; Con, the
control group; Nuc, nuclear, Cyto, cytoplasm. |
PDTC alleviates NaHS-induced increased
cell viability in PLC/PRF/5 hepatoma cells
As shown in Fig. 6,
exposure of PLC/PRF/5 hepatoma cells to 500 μmol/l NaHS for 24 h
obviously induced cell proliferation, leading to an increase in
cell viability. However, the increased cell viability was repressed
by co-treatment with different doses PDTC (a specific inhibitor of
NF-κB pathway) for 24 h.
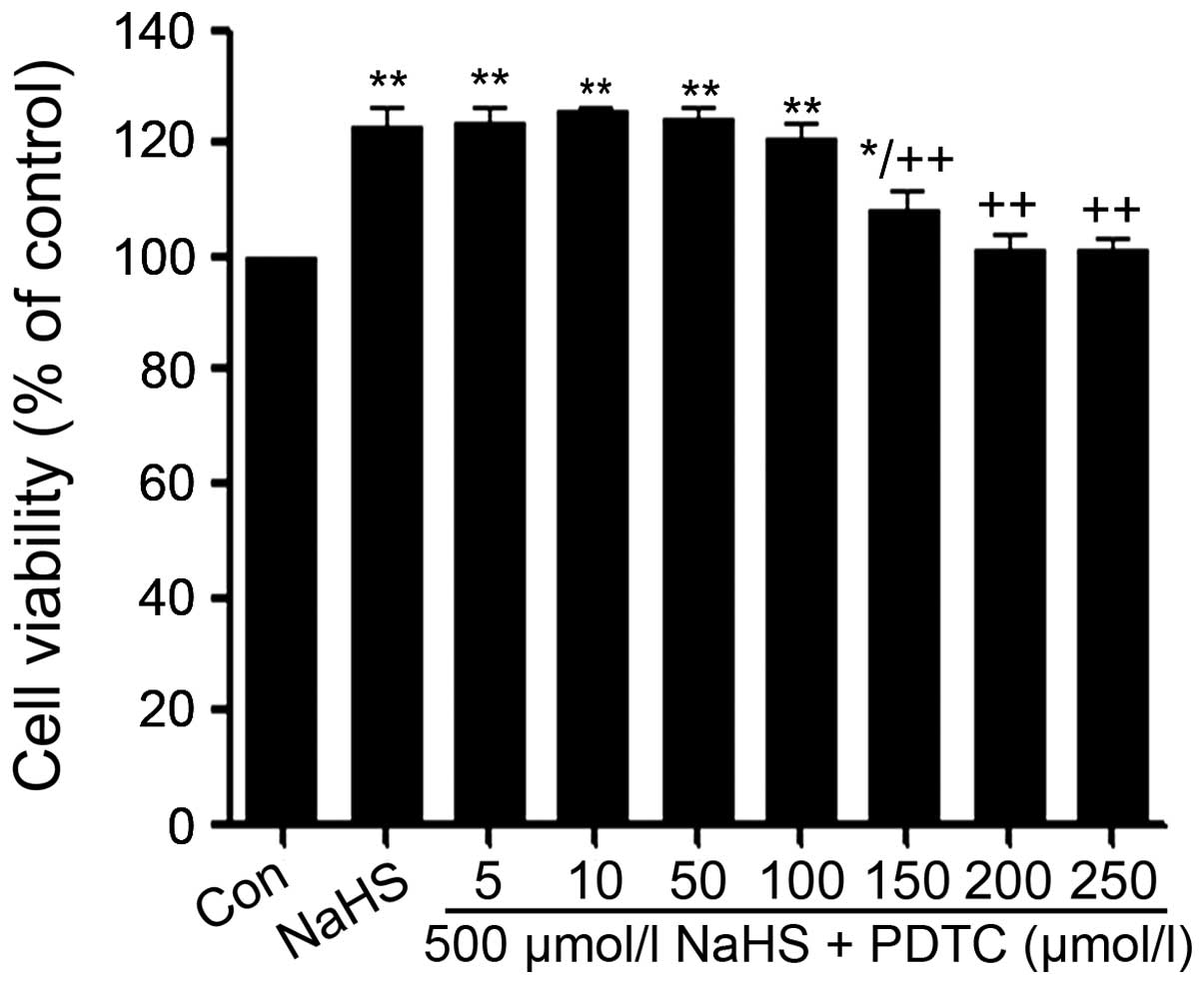 | Figure 6PDTC alleviates NaHS-induced cell
proliferation in human liver cancer cell line PLC/PRF/5 hepatoma
cells. PLC/PRF/5 hepatoma cells were co-conditioned with 500 μmol/l
NaHS and different doses of PDTC (0, 5, 10, 50, 100, 200 and 250
μmol/l) for 24 h. Data are the mean ± SEM (n=3).
*p<0.05, **p<0.01 compared with the
control group. ++p<0.01 compared with the NaHS group.
Con, the control group; NaHS, a donor of H2S; PDTC,
pyrrolidine dithiocarbamate, a specific inhibitor of NF-κB
pathway. |
As shown in Fig. 6,
at the dose of PDTC from 5 to 100 μmol/l did not change in the cell
viability. On the contrary, the dose of PDTC from 150 to 250 μmol/l
significantly suppressed the cells proliferation, leading to a
decrease in cell viability and reaching the minimum at 200 μmol/l.
According to the above results, PLC/PRF/5 hepatoma cells were
co-treated with 500 μmol/l NaHS and 200 μmol/l PDTC for 24 h in all
following experiments.
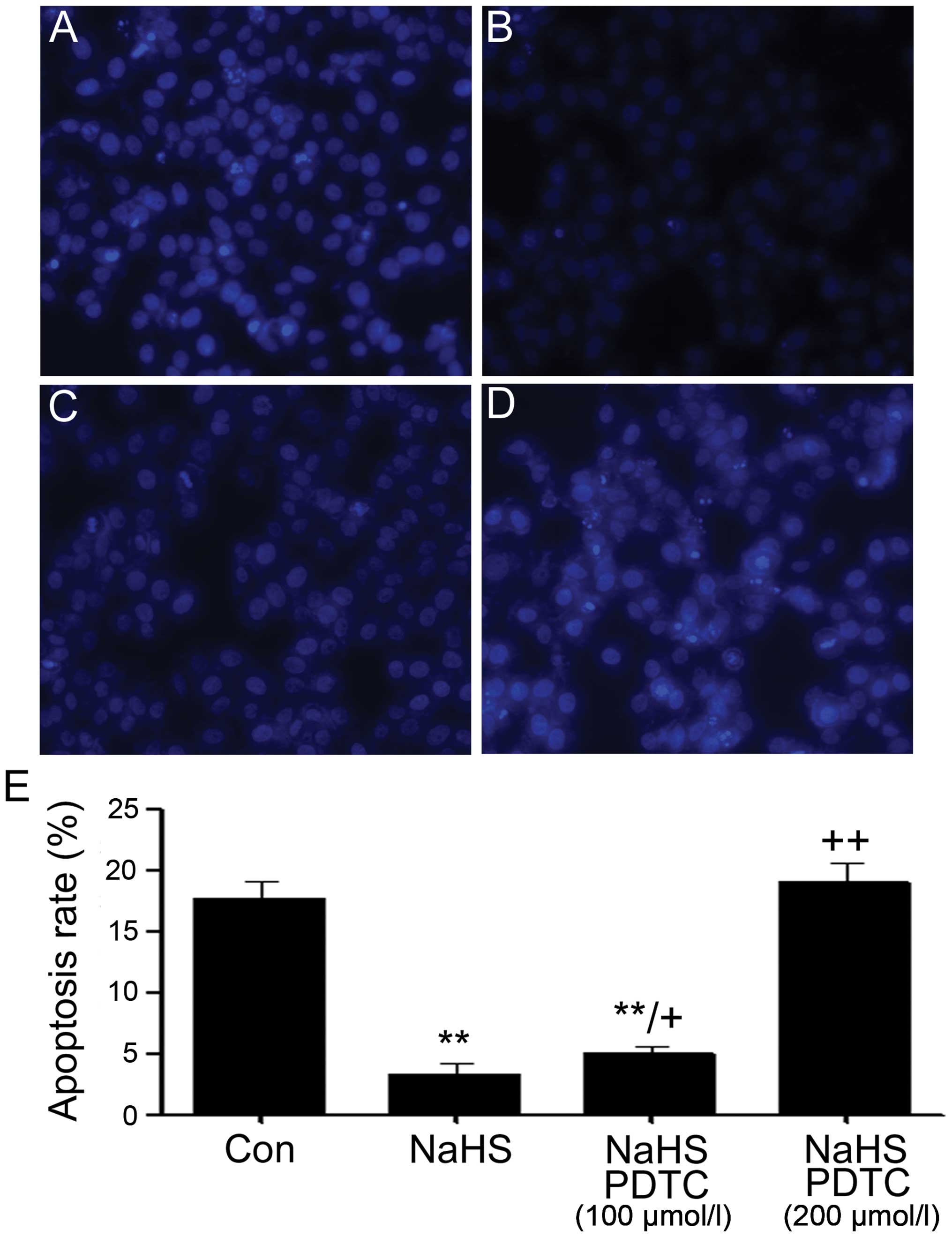
PDTC increases NaHS-induced decreased
cell apoptosis in PLC/PRF/5 hepatoma cells
We observed the effect of PDTC against NaHS-induced
decreased cell apoptosis in PLC/PRF/5 hepatoma cells. It was showed
that exposure of cells to 500 μmol/l NaHS for 24 h markedly
enhanced proliferation, as evidenced by a decrease in apoptotic
cells (Fig. 7B). In addition, the
above proliferation was partly inhibited by co-treating PLC/PRF/5
hepatoma cells with 500 μmol/l NaHS and 100 μmol/l PDTC for 24 h or
almost completely inhibited by co-treating PLC/PRF/5 hepatoma cells
with 500 μmol/l NaHS and 200 μmol/l PDTC for 24 h.
PDTC inhibits NaHS-induced increased
expression levels of MMP-2 and COX-2 and upregulates NaHS-induced
decreased caspase-3 expression in PLC/PRF/5 hepatoma cells
As shown in Fig. 8,
PLC/PRF/5 hepatoma cells were exposed to 500 μmol/l NaHS for 24 h,
the expression levels of MMP-2 and COX-2 were significantly
increased, on the contrary, the expression level of caspase-3 was
markedly decreased. Notably, co-treatment of PLC/PRF/5 hepatoma
cells with 500 μmol/l NaHS and 200 μmol/l PDTC for 24 h
considerably depressed NaHS-induced increased expression levels of
MMP-2 and COX-2, however, caspase-3 expression was obviously
downregulated. Treatment of cells with 200 μmol/l PDTC for 24 h did
not alter the basal expression levels of MMP-2, COX-2 and
caspase-3.
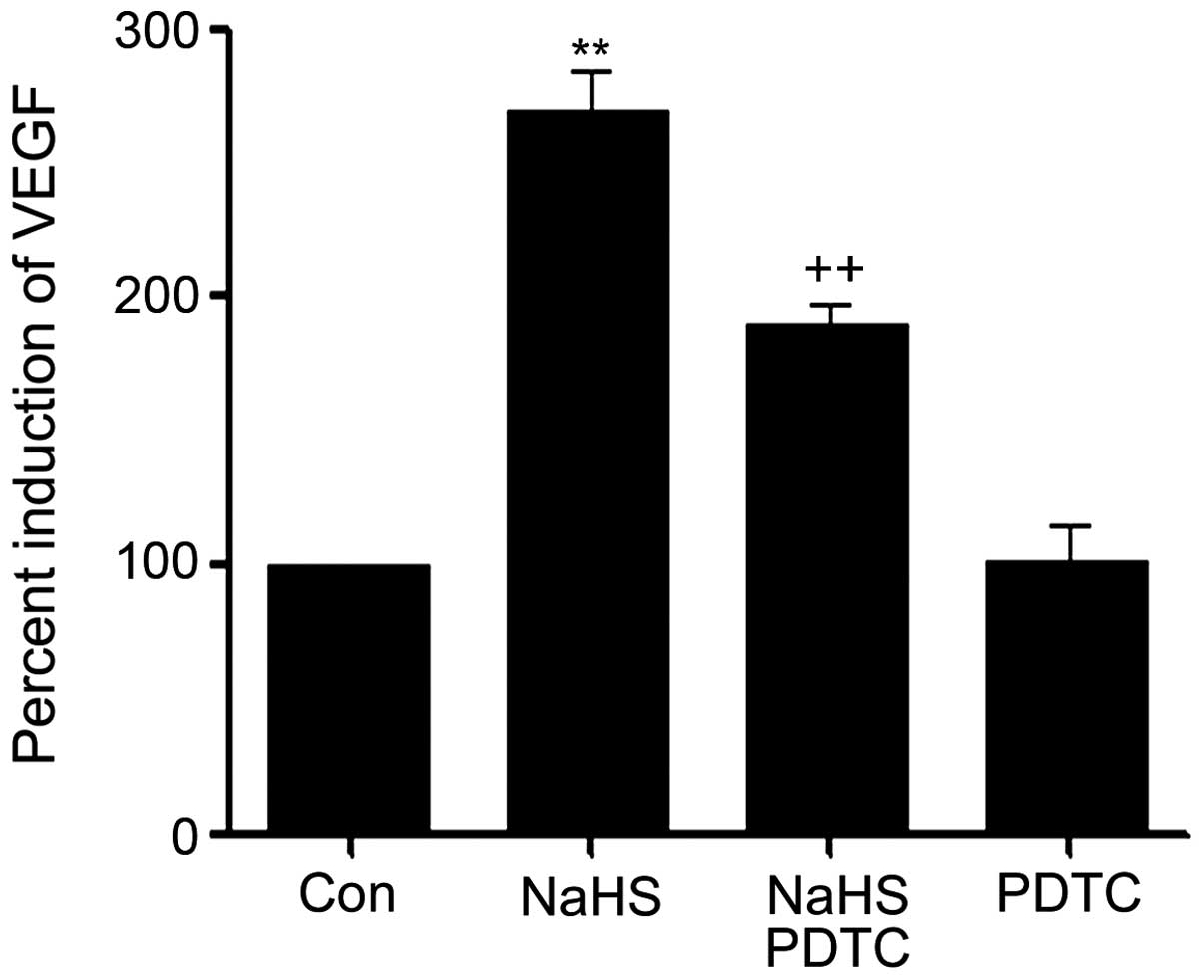
PDTC suppresses NaHS-induced upregulated
production of VEGF in PLC/PRF/5 hepatoma cells
As shown in Fig. 9,
the level of VEGF was markedly increased in NaHS-induced PLC/PRF/5
hepatoma cells, compared with the control group (P<0.01).
However, the increased level of VEGF was significantly suppressed
by co-treatment cells with PDTC and NaHS.
H2S demonstrates
pro-proliferation, anti-apoptosis, angiogenesis, invasion and
migration effects on PLC/PRF/5 hepatoma cells via amplifying the
activation of NF-κB pathway
We found that NaHS upregulated both CSE and CBS
activity resulting in an elevated rate of H2S production
level, which in turn modulates protein expressions of caspase-3,
MMP-2 and VEGF. The downregulated caspase-3 directly induced
anti-apoptosis, led to decreased apoptosis and increased cell
viability of PLC/PRF/5 hepatoma cells. MMP-2 contributes to cancer
cell invasion and migration. The increased production of VEGF
stimulates angiogenesis, promoting the supply of nutrients and
blood to the tumor. Conversely, the above properties of
H2S were significantly inhibited by the co-condition of
500 μmol/l NaHS and 200 μmol/l PDTC for 24 h.
Discussion
In this study, we demonstrated a novel finding in
tumor development of H2S on PLC/PRF/5 hepatoma cells and
also provide data to revel its potential mechanisms. Herein we
report that: i) the production of H2S was dramatically
increased in the PLC/PRF/5 hepatoma cells compared with the human
LO2 hepatocyte cells group, along with overexpression levels of CSE
and CBS, ii) NaHS upregulated the expression levels of CBS and CSE,
as well as the production of H2S, iii) NaHS caused an
increase in cell viability, iv) NaHS induced a decrease in cell
apoptosis, due to the increased expression level of caspase-3, v)
NaHS activated the NF-κB pathway and promoted NF-κB nuclear
translocation, vi) NaHS induced increased expression levels of
COX-2, MMP-2 and VEGF, vii) co-treatment of PLC/PRF/5 hepatoma
cells with NaHS and PDTC (an inhibitor of NF-κB) was able to
largely suppress the above NaHS-induced effects.
H2S, as the third gaseous transmitter
following NO and CO, modulates an array of cellular and molecular
mechanism, physiological and pathophysiological processes. It can
be endogenously catalyzed by CBS or CSE or both and contributes to
cardioprotection (26–31,53),
angiogenesis (32–34), antioxidant (35), pro- and anti-inflammatory (36,37)
and other wide range of physiological functions (38–41)
in a variety of animal or human non-tumor cells. However, the
effects of H2S on the cancer cells are comparatively
complicated and extremely controversial. Accumulating evidence has
demonstrated that H2S may possess anticancer functions
by reason of its anti-inflammatory effect (54), activation of MAPKs pathway (p38
MAPK, ERK1/2 and JNK) (49), and
pro-apoptosis performance (49).
Significantly, it has been reported that H2S can exert
totally opposite properties, in the main mechanisms related to
AKT/ERK pathways (47), and
angiogenesis (42,49). NaHS, a donor of H2S, is
actively being investigated on account of the above effects of
H2S. In the present study, our results suggested that
both CSE and CBS were expressed in PLC/PRF/5 hepatoma cells and
significantly increased compared with the human hepatic cell line
LO2, suggesting H2S might be closely linked to liver
cancer progression. Previous studies have demonstrated that
H2S can exert anti-apoptosis and pro-proliferation on
various diversified cells (26–31,53,55,56).
In this study, we treated PLC/PRF/5 hepatoma cells with NaHS and
found interesting results. Our findings demonstrated that NaHS
improved PLC/PRF/5 hepatoma cells proliferation at the
concentrations ranging from 100 to 1,000 μmol/l and the optimal
concentration of NaHS that induced maximal effect of proliferation
was 500 μmol/l, leading to increased cell viability, which
indicates that H2S might participate in the PLC cancer
growth. Treatment of PLC/PRF/5 hepatoma cells with 500 μmol/l NaHS
for 24 h markedly diminished cell apoptosis, and decreased the
expression level of caspase-3, an apoptotic factor. The above
result was consistent with previous reports (13–19).
These results demonstrate that H2S induces cell
proliferation via exerting its dual cytoprotective and
anti-apoptosis effects. A large number of experiments have shown
that H2S can contribute to VEGF production (42,57–61).
In agreement we found that H2S notably increased the
production of VEGF in PLC/PRF/5 hepatoma cells, compared with the
control group. VEGF is one of the most potent and pivotal
angiogenic factors and is crucial for the persistent proliferation
and metastasis of tumor cells (62). Therefore, we hypothesized that
H2S promotes the supply of blood and nutrients to the
tumor via angiogenesis effect. Further studies are needed to
explore our hypothesis in vivo. Fourthly, we studied the
MMP-2 and found for the first time that treatment with 500 μmol/l
NaHS significantly upregulated the expression level of MMP-2, and
reached a peak at 12 h. The upregulated expression of MMPs,
particularly the gelatinase (MMP-2 and MMP-9), is high associated
with metastasis potential in several types of carcinomas (63–66).
It indicated that H2S was involved in PLC/PRF/5 hepatoma
cell invasion and migration. In our observational study, treatment
with 500 μmol/l NaHS for 24 h obviously promoted protein COX-2
secretion in PLC/PRF/5 hepatoma cells. Importantly, accumulating
evidence has demonstrated that cyclooxygenase (COX)-2 is
overexpressed in several types of cancers cells (73–75)
including hepatocellular carcinoma (HCC) (76). COX-2 expression in cells and animal
models is closely associated with tumor cell growth and is a
crucial molecule in the development of malignant tumors, including
promotion of angiogenesis (77),
anti-apoptotic effects (78),
invasiveness of tumor cells (74),
and tumor cell proliferation (79), demonstrating that COX-2 pathway is
implicated in NaHS-induced PLC/PRF/5 hepatoma cell proliferation,
anti-apoptosis, angiogenesis and migration.
To investigate the complicated mechanism for
NaHS-induced pro-proliferative effect, anti-apoptosis, angiogenesis
and migration in PLC/PRF/5 hepatoma cells, we studied the NF-κB
pathway, which has been demonstrated previously linked to cancer
progression by mean of cell invasion, cell differentiation, cell
proliferation, and apoptosis (12). It has been reported that NF-κB can
be activated by various stimuli both in normal cells (13) and in cancer cells (13–19).
Herein, we found that NaHS not only activated NF-κB in PLC/PRF/5
hepatoma cells, leading to increased phosphorylation of NF-κB p65
and IκBα, but also increased NF-κB nuclear translocation.
Interestingly, PDTC, an inhibitor of NF-κB, blocked NaHS-induced
NF-κB activation, along with NaHS-induced pro-proliferative effect,
anti-apoptosis, angiogenesis and migration in PLC/PRF/5 hepatoma
cells, because of decreased expression levels of MMP-2, VEGF, and
COX-2, and increased caspase-3 expression. These results suggest
that NF-κB activation is necessary in NaHS-induced PLC/PRF/5
hepatoma cell progression.
NO, which is another important gaseous transmitter,
can also exert a wide variety of biological properties. It is hard
to prove that treatment of PLC/PRF/5 hepatoma cells with 500 μmol/l
NaHS for 24 h significantly reduced NO production in culture medium
(data not shown). Thefore, the interaction of H2S and NO
in PLC/PRF/5 hepatoma cells is still unclear and need to be further
investigated.
A novel finding of our present study is the
interaction between H2S and its catalyzing enzyme (CSE
and CBS) in PLC/PRF/5 hepatoma cells. On the one hand, naturally,
H2S can be catalyzed by CBS or CSE and treatment of
PLC/PRF/5 hepatoma cells with NaHS significantly increased its
production compared with the control group. Unexpectedly, treatment
of PLC/PRF/5 hepatoma cells with NaHS significantly upregulated
both CSE and CBS expression. This finding means that there may be
positive regulation mechanism between H2S and its
catalyzing enzyme (CSE and CBS) in PLC/PRF/5 hepatoma cells. The
positive regulation mechanism might play a crucial role in
NaHS-induced liver cancer cell progression. However, this mechanism
remains to be further elucidated.
In conclusion, H2S induced cells
proliferation, anti-apoptosis, angiogenesis, and migration in
PLC/PRF/5 hepatoma cells. These effects might be mediated by the
activation of NF-κB pathway, leading to overexpression levels of
MMP-2, COX-2 and VEGF, down-expression of caspase-3, increased cell
viability, and decreased number of apoptotic cells. In PLC, the
findings provide novel insight into a unified concept and identify
CBS- and CSE-derived H2S as an endogenous
tumor-promoting factor and anticancer drug target. The interaction
of H2S and NO in PLC/PRF/5 hepatoma cells is still
unclear and needs to be further investigated.
References
|
1
|
Nordenstedt H, White DL and El-Serag HB:
The changing pattern of epidemiology in hepatocellular carcinoma.
Dig Liver Dis. 42(Suppl 3): S206–S214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu MC and Yuan JM: Environmental factors
and risk for hepatocellular carcinoma. Gastroenterology. 127(Suppl
1): S72–S78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chuang SC, La Vecchia C and Boffetta P:
Liver cancer: Descriptive epidemiology and risk factors other than
HBV and HCV infection. Cancer Lett. 286:9–14. 2009. View Article : Google Scholar
|
|
6
|
Liang X, Bi S, Yang W, et al:
Epidemiological serosurvey of hepatitis B in China - declining HBV
prevalence due to hepatitis B vaccination. Vaccine. 27:6550–6557.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang H, Wang J, Xiao H, Wang D, Wei W,
Qiao Y and Boffetta P: Estimation of cancer incidence and mortality
attributable to alcohol drinking in China. BMC Public Health.
10:7302010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arsura M and Cavin LG: Nuclear
factor-kappaB and liver carcinogenesis. Cancer Lett. 229:157–169.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He G and Karin M: NF-κB and STAT3 - key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar
|
|
11
|
Berasain C, Castillo J, Perugorria MJ,
Latasa MU, Prieto J and Avila MA: Inflammation and liver cancer:
New molecular links. Ann NY Acad Sci. 1155:206–221. 2009.
View Article : Google Scholar
|
|
12
|
Baldwin AS Jr: Series introduction: The
transcription factor NF-kappaB and human disease. J Clin Invest.
107:3–6. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Werner SL, Barken D and Hoffmann A:
Stimulus specificity of gene expression programs determined by
temporal control of IKK activity. Science. 309:1857–1861. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Curran JE, Weinstein SR and Griffiths LR:
Polymorphic variants of NFKB1 and its inhibitory protein NFKBIA,
and their involvement in sporadic breast cancer. Cancer Lett.
188:103–107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang P, Wei Q, Li X, Wang K, Zeng H, Bu H
and Li H: A functional insertion/deletion polymorphism in the
promoter region of the NFKB1 gene increases susceptibility for
prostate cancer. Cancer Genet Cytogenet. 191:73–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lo SS, Chen JH, Wu CW and Lui WY:
Functional polymorphism of NFKB1 promoter may correlate to the
susceptibility of gastric cancer in aged patients. Surgery.
145:280–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu Y, Liu H, Jin M, Zhang M, Pan Y, Zhang
S, Li Q and Chen K: The joint association of REST and NFKB1
polymorphisms on the risk of colorectal cancer. Ann Hum Genet.
76:269–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin CW, Hsieh YS, Hsin CH, Su CW, Lin CH,
Wei LH, Yang SF and Chien MH: Effects of NFKB1 and NFKBIA gene
polymorphisms on susceptibility to environmental factors and the
clinicopathologic development of oral cancer. PLoS One.
7:e350782012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Xu HL, Gao S, et al: Genetic
polymorphism of NFKB1 and NFKBIA genes and liver cancer risk: A
nested case-control study in Shanghai, China. BMJ Open.
4:e0044272014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang R: Two’s company, three’s a crowd:
Can H2S be the third endogenous gaseous transmitter?
FASEB J. 16:1792–1798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guidotti TL: Hydrogen sulfide: Advances in
understanding human toxicity. Int J Toxicol. 29:569–581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kilburn KH, Thrasher JD and Gray MR:
Low-level hydrogen sulfide and central nervous system dysfunction.
Toxicol Ind Health. 26:387–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan BH, Wong PT and Bian JS: Hydrogen
sulfide: A novel signaling molecule in the central nervous system.
Neurochem Int. 56:3–10. 2010. View Article : Google Scholar
|
|
24
|
Rong W, Kimura H and Grundy D: The
neurophysiology of hydrogen sulfide. Inflamm Allergy Drug Targets.
10:109–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kamoun P: Endogenous production of
hydrogen sulfide in mammals. Amino Acids. 26:243–254. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo R, Wu K, Chen J, Mo L, Hua X, Zheng D,
Chen P, Chen G, Xu W and Feng J: Exogenous hydrogen sulfide
protects against doxorubicin-induced inflammation and cytotoxicity
by inhibiting p38MAPK/NFκB pathway in H9c2 cardiac cells. Cell
Physiol Biochem. 32:1668–1680. 2013.
|
|
27
|
Guo RM, Xu WM, Lin JC, Mo LQ, Hua XX, Chen
PX, Wu K, Zheng DD and Feng JQ: Activation of the p38 MAPK/NF-κB
pathway contributes to doxorubicin-induced inflammation and
cytotoxicity in H9c2 cardiac cells. Mol Med Rep. 8:603–608.
2013.PubMed/NCBI
|
|
28
|
El-Seweidy MM, Sadik NA and Shaker OG:
Role of sulfurous mineral water and sodium hydrosulfide as potent
inhibitors of fibrosis in the heart of diabetic rats. Arch Biochem
Biophys. 506:48–57. 2011. View Article : Google Scholar
|
|
29
|
Dong XB, Yang CT, Zheng DD, et al:
Inhibition of ROS-activated ERK1/2 pathway contributes to the
protection of H2S against chemical hypoxia-induced
injury in H9c2 cells. Mol Cell Biochem. 362:149–157. 2012.
View Article : Google Scholar
|
|
30
|
Yang Z, Yang C, Xiao L, Liao X, Lan A,
Wang X, Guo R, Chen P, Hu C and Feng J: Novel insights into the
role of HSP90 in cyto-protection of H2S against chemical
hypoxia-induced injury in H9c2 cardiac myocytes. Int J Mol Med.
28:397–403. 2011.PubMed/NCBI
|
|
31
|
Chen SL, Yang CT, Yang ZL, Guo RX, Meng
JL, Cui Y, Lan AP, Chen PX and Feng JQ: Hydrogen sulphide protects
H9c2 cells against chemical hypoxia-induced injury. Clin Exp
Pharmacol Physiol. 37:316–321. 2010. View Article : Google Scholar
|
|
32
|
Coletta C, Papapetropoulos A, Erdelyi K,
et al: Hydrogen sulfide and nitric oxide are mutually dependent in
the regulation of angiogenesis and endothelium-dependent
vasorelaxation. Proc Natl Acad Sci USA. 109:9161–9166. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T
and Zhu YC: The novel proangiogenic effect of hydrogen sulfide is
dependent on Akt phosphorylation. Cardiovasc Res. 76:29–40. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Papapetropoulos A, Pyriochou A, Altaany Z,
et al: Hydrogen sulfide is an endogenous stimulator of
angiogenesis. Proc Natl Acad Sci USA. 106:21972–21977. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kimura H: Hydrogen sulfide: From brain to
gut. Antioxid Redox Signal. 12:1111–1123. 2010. View Article : Google Scholar
|
|
36
|
Fiorucci S: Hydrogen sulfide: From
physiology to pharmacology. Inflamm Allergy Drug Targets. 10:77–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hegde A and Bhatia M: Hydrogen sulfide in
inflammation: Friend or foe? Inflamm Allergy Drug Targets.
10:118–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H and Bhatia M: Hydrogen sulfide: A
novel mediator of leukocyte activation. Immunopharmacol
Immunotoxicol. 30:631–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mok YY and Moore PK: Hydrogen sulphide is
pro-inflammatory in haemorrhagic shock. Inflamm Res. 57:512–518.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gong QH, Wang Q, Pan LL, Liu XH, Huang H
and Zhu YZ: Hydrogen sulfide attenuates lipopolysaccharide-induced
cognitive impairment: A pro-inflammatory pathway in rats. Pharmacol
Biochem Behav. 96:52–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Sio SW, Moochhala S and Bhatia M:
Role of hydrogen sulfide in severe burn injury-induced inflammation
in mice. Mol Med. 16:417–424. 2010.PubMed/NCBI
|
|
42
|
Szabo C, Coletta C, Chao C, Módis K,
Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived
hydrogen sulfide, produced by cystathionine-β-synthase, stimulates
bioenergetics, cell proliferation, and angiogenesis in colon
cancer. Proc Natl Acad Sci USA. 110:12474–12479. 2013. View Article : Google Scholar
|
|
43
|
Pupo E, Pla AF, Avanzato D, Moccia F, Cruz
JE, Tanzi F, Merlino A, Mancardi D and Munaron L: Hydrogen sulfide
promotes calcium signals and migration in tumor-derived endothelial
cells. Free Radic Biol Med. 51:1765–1773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Du SX, Xiao J, Guan F, Sun LM, Wu WS, Tang
H, Du JB, Tang CS and Jin HF: Predictive role of cerebrospinal
fluid hydrogen sulfide in central nervous system leukemia. Chin Med
J (Engl). 124:3450–3454. 2011.
|
|
45
|
Levine J, Ellis CJ, Furne JK, Springfield
J and Levitt MD: Fecal hydrogen sulfide production in ulcerative
colitis. Am J Gastroenterol. 93:83–87. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rose P, Moore PK, Ming SH, Nam OC,
Armstrong JS and Whiteman M: Hydrogen sulfide protects colon cancer
cells from chemopreventative agent beta-phenylethyl isothiocyanate
induced apoptosis. World J Gastroenterol. 11:3990–3997.
2005.PubMed/NCBI
|
|
47
|
Cai WJ, Wang MJ, Ju LH, Wang C and Zhu YC:
Hydrogen sulfide induces human colon cancer cell proliferation:
Role of Akt, ERK and p21. Cell Biol Int. 34:565–572. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cao Q, Zhang L, Yang G, Xu C and Wang R:
Butyrate-stimulated H2S production in colon cancer
cells. Antioxid Redox Signal. 12:1101–1109. 2010. View Article : Google Scholar
|
|
49
|
Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan
BK and Zhu YZ: H2S donor, S-propargyl-cysteine,
increases CSE in SGC-7901 and cancer-induced mice: Evidence for a
novel anti-cancer effect of endogenous H2S? PLoS One.
6:e205252011. View Article : Google Scholar
|
|
50
|
Murata T, Sato T, Kamoda T, Moriyama H,
Kumazawa Y and Hanada N: Differential susceptibility to hydrogen
sulfide-induced apoptosis between PHLDA1-overexpressing oral cancer
cell lines and oral keratinocytes: Role of PHLDA1 as an apoptosis
suppressor. Exp Cell Res. 320:247–257. 2014. View Article : Google Scholar
|
|
51
|
Chattopadhyay M, Kodela R, Olson KR and
Kashfi K: NOSH-aspirin (NBS-1120), a novel nitric oxide- and
hydrogen sulfide-releasing hybrid is a potent inhibitor of colon
cancer cell growth in vitro and in a xenograft mouse model. Biochem
Biophys Res Commun. 419:523–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH,
Li L, Moore PK and Deng LW: The slow-releasing hydrogen sulfide
donor, GYY4137, exhibits novel anti-cancer effects in vitro and in
vivo. PLoS One. 6:e210772011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu W, Wu W, Chen J, Guo R, Lin J, Liao X
and Feng J: Exogenous hydrogen sulfide protects H9c2 cardiac cells
against high glucose-induced injury by inhibiting the activities of
the p38 MAPK and ERK1/2 pathways. Int J Mol Med. 32:917–925.
2013.PubMed/NCBI
|
|
54
|
Kashfi K: Anti-cancer activity of new
designer hydrogen sulfide-donating hybrids. Antioxid Redox Signal.
20:831–846. 2014. View Article : Google Scholar :
|
|
55
|
Shi S, Li QS, Li H, Zhang L, Xu M, Cheng
JL, Peng CH, Xu CQ and Tian Y: Anti-apoptotic action of hydrogen
sulfide is associated with early JNK inhibition. Cell Biol Int.
33:1095–1101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rinaldi L, Gobbi G, Pambianco M, Micheloni
C, Mirandola P and Vitale M: Hydrogen sulfide prevents apoptosis of
human PMN via inhibition of p38 and caspase 3. Lab Invest.
86:391–397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Holwerda KM, Burke SD, Faas MM, et al:
Hydrogen sulfide attenuates sFlt1-induced hypertension and renal
damage by upregulating vascular endothelial growth factor. J Am Soc
Nephrol. 25:717–725. 2014. View Article : Google Scholar :
|
|
58
|
Polhemus DJ, Kondo K, Bhushan S, Bir SC,
Kevil CG, Murohara T, Lefer DJ and Calvert JW: Hydrogen sulfide
attenuates cardiac dysfunction after heart failure via induction of
angiogenesis. Circ Heart Fail. 6:1077–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Köhn C, Dubrovska G, Huang Y and Gollasch
M: Hydrogen sulfide: Potent regulator of vascular tone and
stimulator of angiogenesis. Int J Biomed Sci. 8:81–86. 2012.
|
|
60
|
Bir SC, Kolluru GK, McCarthy P, Shen X,
Pardue S, Pattillo CB and Kevil CG: Hydrogen sulfide stimulates
ischemic vascular remodeling through nitric oxide synthase and
nitrite reduction activity regulating hypoxia-inducible factor-1α
and vascular endothelial growth factor-dependent angiogenesis. J Am
Heart Assoc. 1:e0040932012. View Article : Google Scholar
|
|
61
|
Tao BB, Liu SY, Zhang CC, et al: VEGFR2
functions as an H2S-targeting receptor protein kinase
with its novel Cys1045-Cys1024 disulfide bond serving as a specific
molecular switch for hydrogen sulfide actions in vascular
endothelial cells. Antioxid Redox Signal. 19:448–464. 2013.
View Article : Google Scholar :
|
|
62
|
Leung WK, To KF, Go MY, Chan KK, Chan FK,
Ng EK, Chung SC and Sung JJ: Cyclooxygenase-2 upregulates vascular
endothelial growth factor expression and angiogenesis in human
gastric carcinoma. Int J Oncol. 23:1317–1322. 2003.PubMed/NCBI
|
|
63
|
Jones JL, Shaw JA, Pringle JH and Walker
RA: Primary breast myoepithelial cells exert an invasion-suppressor
effect on breast cancer cells via paracrine down-regulation of MMP
expression in fibroblasts and tumour cells. J Pathol. 201:562–572.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li C, Li F, Zhao K, Yao J, Cheng Y, Zhao
L, Li Z, Lu N and Guo Q: LFG-500 inhibits the invasion of cancer
cells via downregulation of PI3K/AKT/NF-κB signaling pathway. PLoS
One. 9:e913322014. View Article : Google Scholar
|
|
65
|
Puzovic V, Brcic I, Ranogajec I and
Jakic-Razumovic J: Prognostic values of ETS-1, MMP-2 and MMP-9
expression and co-expression in breast cancer patients. Neoplasma.
61:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ruan M, Zhang Z, Li S, Yan M, Liu S, Yang
W, Wang L and Zhang C: Activation of Toll-like receptor-9 promotes
cellular migration via up-regulating MMP-2 expression in oral
squamous cell carcinoma. PLoS One. 9:e927482014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li Y, Liu G, Cai D, Pan B, Lin Y, Li X, Li
S, Zhu L, Liao X and Wang H: H2S inhibition of chemical
hypoxia-induced proliferation of HPASMCs is mediated by the
upregulation of COX-2/PGI2. Int J Mol Med. 33:359–366. 2014.
|
|
68
|
Ang SF, Sio SW, Moochhala SM, MacAry PA
and Bhatia M: Hydrogen sulfide upregulates cyclooxygenase-2 and
prostaglandin E metabolite in sepsis-evoked acute lung injury via
transient receptor potential vanilloid type 1 channel activation. J
Immunol. 187:4778–4787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Terzuoli E, Meini S, Cucchi P, Catalani C,
Cialdai C, Maggi CA, Giachetti A, Ziche M and Donnini S: Antagonism
of bradykinin B2 receptor prevents inflammatory responses in human
endothelial cells by quenching the NF-κB pathway activation. PLoS
One. 9:e843582014. View Article : Google Scholar
|
|
70
|
Tsagaraki I, Phenekos C, Tsilibary E and
Tzinia A: Calcitonin-induced NF-κB activation up-regulates
fibronectin expression in MG63 osteosarcoma cells. Anticancer Res.
33:4901–4906. 2013.PubMed/NCBI
|
|
71
|
Hsu CJ, Wu MH, Chen CY, Tsai CH, Hsu HC
and Tang CH: AMP-activated protein kinase activation mediates
CCL3-induced cell migration and matrix metalloproteinase-2
expression in human chondrosarcoma. Cell Commun Signal. 11:682013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Son SW, Kim HG, Han JM, Lee JS, Choi MK,
Lee JS and Son CG: Anti-melanoma activity of Cynanchi atrati Radix
is mediated by regulation of NF-kappa B activity and pro-apoptotic
proteins. J Ethnopharmacol. 153:250–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jana D, Sarkar DK, Ganguly S, Saha S, Sa
G, Manna AK, Banerjee A and Mandal S: Role of cyclooxygenase 2
(COX-2) in prognosis of breast cancer. Indian J Surg Oncol.
5:59–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wu X, Cai M, Ji F and Lou LM: The impact
of COX-2 on invasion of osteosarcoma cell and its mechanism of
regulation. Cancer Cell Int. 14:272014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xu K, Wang L and Shu HK: COX-2
overexpression increases malignant potential of human glioma cells
through Id1. Oncotarget. 5:1241–1252. 2014.PubMed/NCBI
|
|
76
|
Dong X, Li R, Xiu P, et al: Meloxicam
executes its antitumor effects against hepatocellular carcinoma in
COX-2-dependent and -independent pathways. PLoS One. 9:e928642014.
View Article : Google Scholar
|
|
77
|
Tegeder I, Niederberger E, Israr E,
Gühring H, Brune K, Euchenhofer C, Grösch S and Geisslinger G:
Inhibition of NF-kappaB and AP-1 activation by R- and
S-flurbiprofen. FASEB J. 15:2–4. 2001.
|
|
78
|
Seo KW, Coh YR, Rebhun RB, et al:
Antitumor effects of celecoxib in COX-2 expressing and
non-expressing canine melanoma cell lines. Res Vet Sci. pii:
S0034–5288. 00051–00054. 2014.
|
|
79
|
Sui W, Zhang Y, Wang Z, Wang Z, Jia Q, Wu
L and Zhang W: Antitumor effect of a selective COX-2 inhibitor,
celecoxib, may be attributed to angiogenesis inhibition through
modulating the PTEN/PI3K/Akt/HIF-1 pathway in an H22 murine
hepatocarcinoma model. Oncol Rep. 31:2252–2260. 2014.PubMed/NCBI
|