Introduction
Cervical cancer is one of the most common
malignancies affecting women worldwide, and it is estimated that
cervical carcinoma is responsible for 274,000 deaths annually
(1). It is well established that
infection of high-risk human papillomavirus (hr-HPV) is necessary
for cervical cancer development, and hr-HPV DNA can be detected in
almost all cervical carcinomas (2). Carcinogenesis by hr-HPV relies
primarily on the expression of two virally encoded oncoproteins, E6
and E7 (3). These act
synergistically to immortalize and transform the infected cells,
partly via their ability to degrade p53 and Rb, respectively
(4). p53 is a tumor-suppressor
protein with a sequence-specific DNA-binding domain that plays an
important role in transcriptional regulation (5,6).
This protein acts via a variety of mechanisms, including cell-cycle
arrest, induction of apoptosis and cellular senescence (6). Loss of normal p53 function occurs in
a significant proportion of human tumors and primarily induces
abnormal expression of many target genes (6,7).
Noteworthy, this abnormal expression of several p53 target genes is
caused by DNA methylation (8–10).
Together with genetic factors, epigenetic factors have been
suggested as contributing mechanisms in cervical carcinogenesis
(11,12). Epigenetic modifications,
particularly DNA methylation in promoter regions, are recognized as
common molecular alterations in tumor cells and act via the
complete blockage of transcription of tumor-suppressor genes
(13,14). Previous data related to cervical
cancer showed that DAPK1, FHIT, MGMT, CDKN2A, CADM1 and
MAL were frequently methylated genes in cervical
carcinogenesis (12,15).
The cell adhesion molecule 1 (CADM1) gene
encodes a member of the immunoglobulin superfamily and is one of
the crucial tumor suppressors involved in cell adhesion. It is also
known as TSLC1, Necl-2, IgSF4A and SynCAM1 (16). The CADM1 gene is frequently
down regulated epigenetically in a variety of advanced-stage human
cancers of the lung, prostate, liver, pancreas, and breast
(16,17). Reduced CADM1 expression disrupts
cell-cell adhesion in epithelial cells and triggers tumor cell
invasion and metastasis (17).
In addition to the epidemiological studies of CADM1
in cervical cancer performed to date, the functional involvement of
CADM1 in tumor suppression has been reported by very few studies
and remains unclear (18,19). In this study, we explored the
relationship between CADM1 methylation status and its expression in
various cervical cancer cell lines. Concomitantly, we investigated
whether CADM1 expression could be restored in cervical cancer cell
lines expressing methylated CADM1 that were treated with the
demethylation reagent 5-aza-2′-deoxycytidine (5-aza-dC). In
addition, we determined the effect of CADM1 overexpression on cell
proliferation, and the role of p53 in the regulation of CADM1
expression in cervical cancer cell lines.
Materials and methods
Cell culture
The human embryonic kidney (HEK) 293T and cervical
cancer cells (C33A, HeLa, SiHa and CaSki) used in this study were
purchased from ATCC (Rockville, MD, USA). The cells were cultured
in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum at 37°C in a humidified atmosphere with 5%
CO2. The media used in this study contained 100 U/ml of
penicillin and 100 μg/ml of streptomycin (Invitrogen, Carlsbad, CA,
USA).
Kits, reagents and antibodies
5-Aza-2′-deoxycytidine (5-aza-dC) and 5-Fluorouracil
(5-FU) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
The Cell Count Kit-8 (CCK-8) was obtained from Dojindo Molecular
Technology (Tokyo, Japan). The TRIzol was purchased from
Invitrogen. The ECL western blotting kit was obtained from Amersham
(Arlington Heights, IL, USA), and Immobilon-P membranes were
obtained from Millipore Corp. (Bedford, MA, USA). Anti-p53 and
anti-β-actin antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA), the anti-CADM1 antibody was
obtained from Abnova (Walnut, CA, USA), the anti-phospho-p53
antibody was obtained from Cell Signaling Technology (Danvers, MA,
USA), and horseradish peroxidase (HRP)-conjugated anti-mouse IgG
and anti-rabbit IgG were obtained from Santa Cruz
Biotechnology.
qRT-PCR
Total RNA was extracted from cells using the TRIzol
reagent (Invitrogen) according to the manufacturer’s instructions,
and 2 μg of total RNA was transcribed using the GoScript™ Reverse
Transcription System (Promega, Madison, WI, USA) and random
primers, according to the manufacturer’s instructions. Quantitative
real-time PCR analysis was performed on a StepOnePlus™ Real-time
PCR system (Applied Biosystems, Foster City, CA, USA) with SYBR
Green. The primer sequences for CADM1 were
5′-CCACAGGTGATGGGCAGAA-3′ (forward), 5′-TCGCAACCTCTCCCTCGAT-3′
(reverse). The primer sequences for β-actin were
5′-ATGCTTCTAGGCGGACTATGA-3′ (forward), 5′-TTTCTGCGCAAGTTAGGTTTT-3′
(reverse). The expression of CADM1 relative to that of β-actin in
each sample was calculated and compared.
Preparation of cell lysates and western
blot analysis
Cell lysates were prepared by suspending various
cervical carcinoma cell lines in 1X RIPA lysis buffer (Invitrogen)
supplemented with a protease inhibitor cocktail (Roche Diagnostics,
Indianapolis, IN, USA). The quantitation of proteins was performed
using a Micro BCA kit (Pierce, Rockford, IL, USA). Equivalent
amounts of protein lysates (20 μg) were electrophoresed on 10%
Tris-glycine gel with Tris/glycine/SDS buffer. The proteins were
electrotransferred onto Immobilon-P membranes, which were incubated
overnight with primary antibodies raised against CADM1 (Abnova),
p53 (Santa Cruz Biotechnology), phospho-p53 (Cell Signaling), and
β-actin (Santa Cruz Biotechnology) at 4°C. Membranes were then
washed with Tris-buffered saline (TBS) containing 0.1% Tween-20
(TBST) and incubated with the appropriate secondary antibodies for
1 h. The detection of each protein was performed using the ECL
western blotting kit according to the manufacturer’s instructions.
Densitometry was carried out using ImageQuant TL software
(Amersham). Arbitrary densitometric units of the protein of
interest were corrected using the densitometric units of
β-actin.
5-Aza-2′-deoxycytidine (5-aza-dC)
treatment
5-Aza-dC was dissolved in dimethylsulfoxide (DMSO)
and stored at temperatures below −20°C. Final 5-aza-dC
concentrations (1–100 μM) were prepared by adding an appropriate
amount of the stock solution directly to the culture medium. To
identify whether cells were restored after treatment with 5-aza-dC,
cells were treated with the drug for 3 days, and the media
containing 5-aza-dC was changed every 24 h. Cells were used for the
cell proliferation assay or western blot analysis.
Cell proliferation assay
Cell proliferation was determined using the Cell
Count Kit-8 (CCK-8) assay, which reflects cell viability, as
described (20). Briefly, cervical
cancer cells (C33A, HeLa, SiHa and CaSki) were cultured overnight
in 96-well plates. When cells reached a confluency of approximately
70%, they were treated with 5-aza-dC (0–100 μM) for 3 days.
Subsequently, 10 μl of the CCK-8 solution was added to each well of
the plates. After incubation for 4 h, absorbance was measured at
450 nm using a multi-well plate reader. All assays were performed
in triplicate.
Pyrosequencing
CpG island DNA methylation status was determined by
sequencing bisulfite-modified genomic DNA. Briefly, genomic DNA
samples were extracted from various cervical cancer cells using the
QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA). Genomic DNA (200
ng) was converted with the EZ DNA Methylation kit (Zymo Research,
Orange, CA, USA), according to the manufacturer’s protocol. The
primers used in pyrosequencing assays [sense,
5′-TTGTTTTGTTAATTAGGGGATTTG-3′; and antisense, 5′-(biotin)
CACACCCAATACATCTAACCTA-3′] were used for PCR amplification of the
CADM1 promoter region (nucleotides -444 to -305). The size (140 bp)
and purity of each amplicon were confirmed by agarose gel
electrophoresis. Quantitative pyrosequencing analyses were
performed using the PyroMarkQ96 ID system (Qiagen), according to
the protocol provided by the manufacturer. The results were
analyzed using PyroMark Q96 ID software 2.5 (Qiagen).
5-Fluorouracil (5-FU) treatment
Stock solutions of this compound were prepared in
dimethylsulfoxide (DMSO) and stored at temperatures below −20°C.
Further dilutions were made in DMEM before use. C33A and SiHa cells
were plated in six-well plates and cultured. Twenty-four hours
after plating, cells were incubated with 5-FU (50 μM) for indicated
times from 0 h to 24 h. The cells were used to detect CADM1,
phospho-p53, p53 and β-actin using western blot analysis.
Results
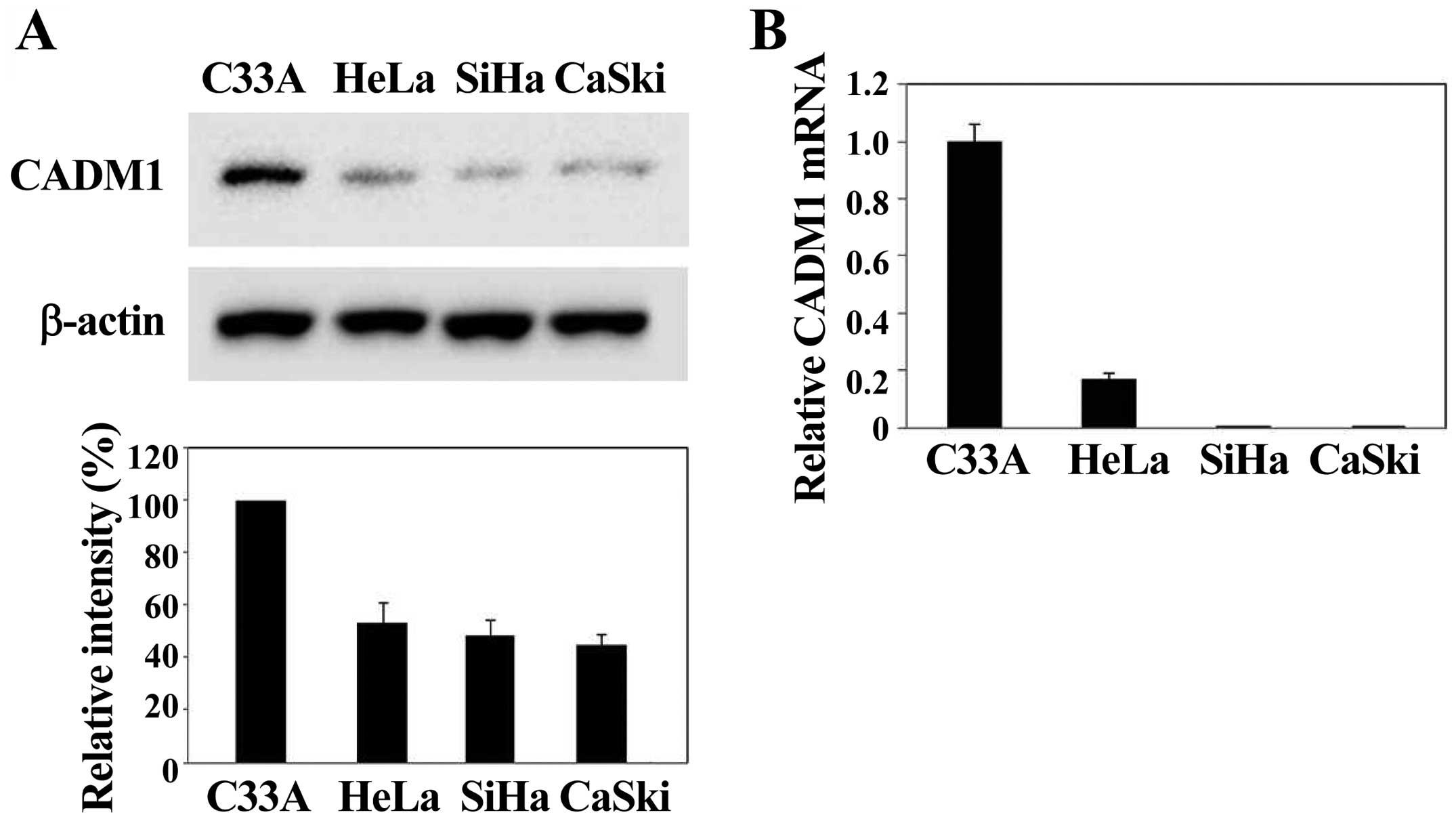
CADM1 expression is reduced in
HPV-infected cervical cancer cells
To explore whether HPV infection is related to CADM1
expression, we investigated the level of CADM1 in various cervical
cancer cells, such as C33A (HPV-negative), HeLa (HPV18-positive),
SiHa and CaSki (HPV16-positive) cells. Western blot analyses
revealed that the CADM1 protein was predominantly expressed in
HPV-negative C33A and decreased in HPV-positive HeLa, SiHa and
CaSki cells (Fig. 1A). In
addition, we measured CADM1 mRNA levels in all cell lines using
real-time quantitative RT-PCR and compared them with the average
CADM1 mRNA level. The CADM1 mRNA was mainly expressed in
HPV-negative cervical cells. In contrast, the CADM1 mRNA was
undetectable in three cervical carcinoma cell lines (i.e., HeLa,
SiHa, and CaSki) (Fig. 1B). Thus,
we observed either a complete loss of, or a marked decrease in
CADM1 mRNA expression in the HPV-infected cervical cancer cell
lines analyzed. CADM1 gene silencing was significantly
observed in HPV-induced cervical carcinoma cell lines. These data
suggest that CADM1 silencing is a critical requirement for the
development of HPV-induced cervical carcinogenesis.
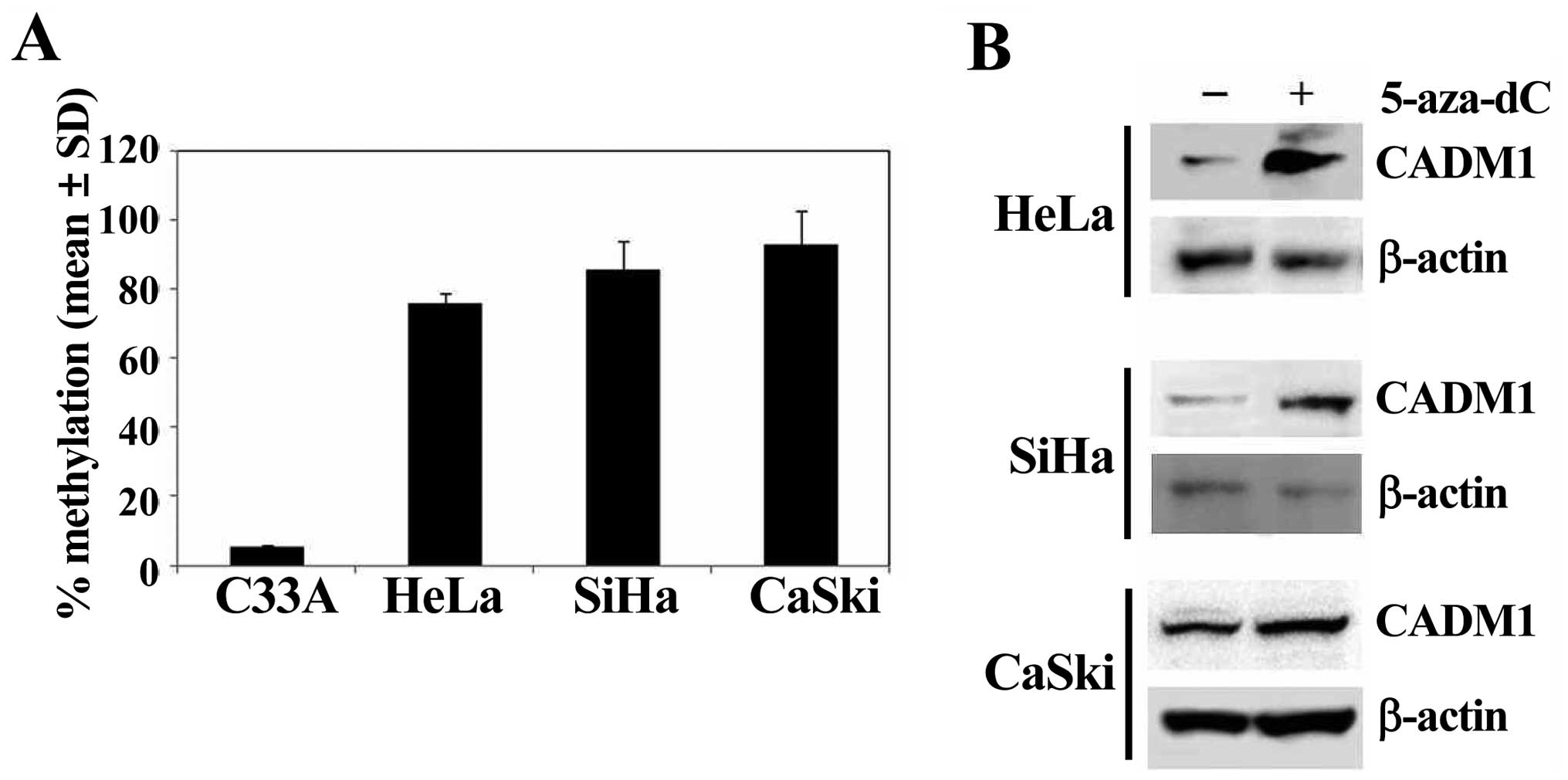
Decreased expression of CADM1 is caused
by hypermethylation of the CADM1 promoter
We examined the DNA methylation status of the
promoter region of CADM1 (nucleotides -444 to -305) to gain insight
into the contribution of gene promoter methylation toward aberrant
expression of CADM1 in HPV-positive cancer cell lines (Fig. 2A). Hypermethylation was defined as
the over 50% methylation of the specific CpG sites. The methylation
of the CADM1 promoter was highly increased in HPV-positive
HeLa (71.7%), SiHa (84.8%), and CaSki (95.2%) cells, but was very
low in HPV-negative C33A (2.6%) cells (Fig. 2A). Moreover, CADM1
hypermethylated cervical cancer cells were treated with the DNA
methylation inhibitor 5-aza-dC to determine whether methylation of
CADM1 was the reason for its silencing. Treatment with
5-aza-dC induced significant upregulation of gene expression in all
highly methylated cell lines (Fig.
2B). These data indicate that hypermethylation of the
CADM1 promoter region might be an active mechanism of
silencing of CADM1 gene expression, and that HPV may play an
important role in the loss of CADM1 gene expression via DNA
methylation.
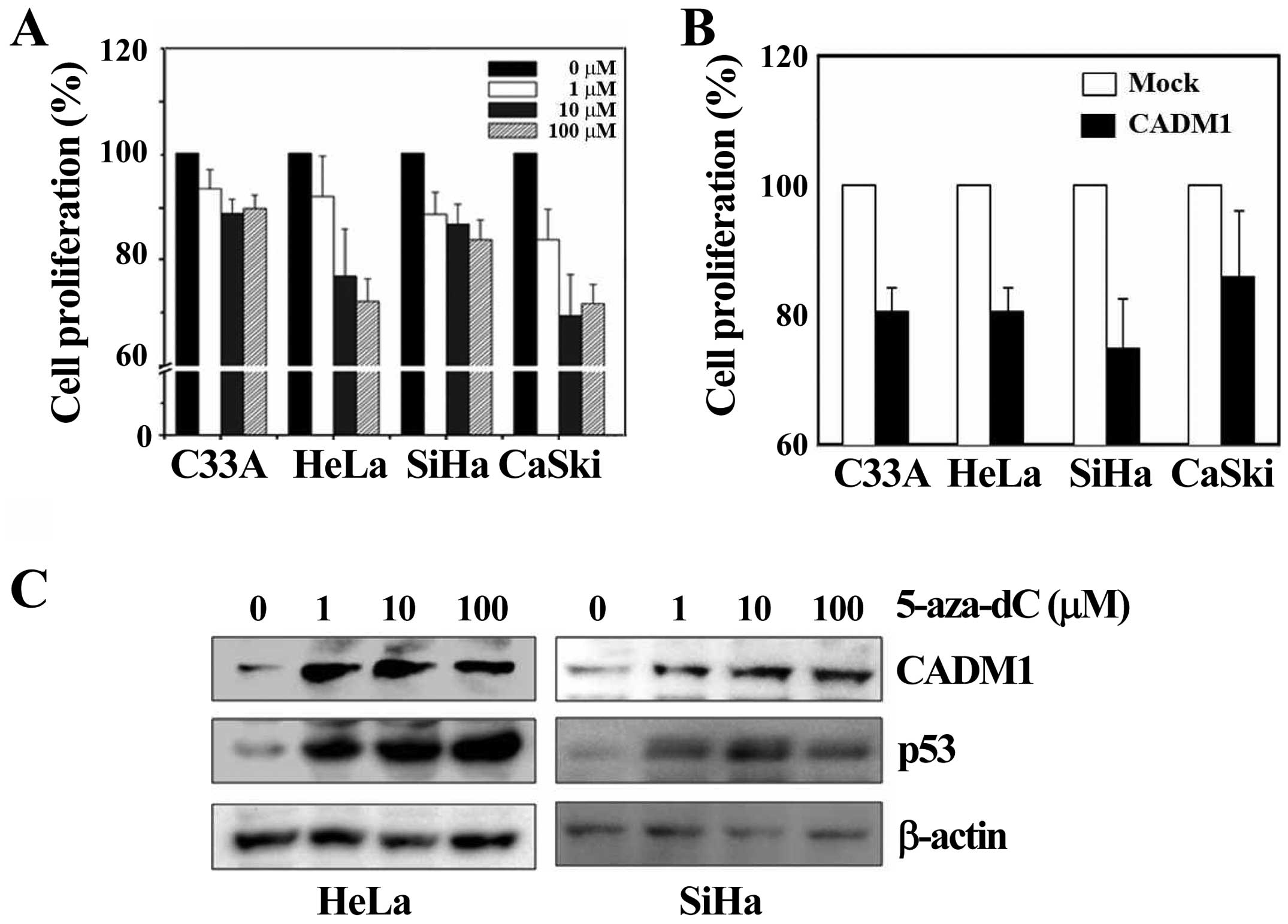
Demethylation enhances the
antiproliferative function of CADM1
To analyze the function of the CADM1 gene in
HPV-induced cervical carcinogenesis, we treated cells with the
demethylating reagent 5-aza-dC to reverse the epigenetic silencing
dose-dependently, or constructed and transfected a pCDNA3.1-CADM1
expression vector into each of the cervical cancer cell lines. We
performed the cell proliferation assay in various cell lines. In
the cervical cancer cells (C33A, HeLa, SiHa and CaSki) that were
treated with the demethylating agent 5-aza-dC for 72 h, cell
proliferation was markedly decreased in all the cell lines tested
compared with DMSO-treated samples, and the effect was
dose-dependent, although the effect observed in C33A cells (which
are negative for HPV infection) was slightly lower (Fig. 3A). In addition, overexpressed CADM1
resulted in a significant decrease in proliferation in all cell
lines compared with the control, as shown in Fig. 3B. To determine whether the effect
of 5-aza-dC on cell proliferation was induced by restoration of
CADM1, we treated HeLa and SiHa cells with 5-aza-dC for 72 h at
various concentrations, similar to the protocol used in the
proliferation assay. The level of CADM1 was significantly increased
in a dose-dependent manner in both cell lines (Fig. 3C). Therefore, these data indicate
that 5-aza-dC is effective and that demethylation on the
CADM1 promoter induces CADM1 restoration. It is known that
the demethylating agent 5-aza-dC can promote p53 expression by
inducing DNA damage (21).
Consistently, we also observed that p53 levels were dramatically
increased in both cell lines after treatment with 5-aza-dC
(Fig. 3C). We further investigated
a potential mechanistic link between p53 and CADM1 expression in
cervical cancer.
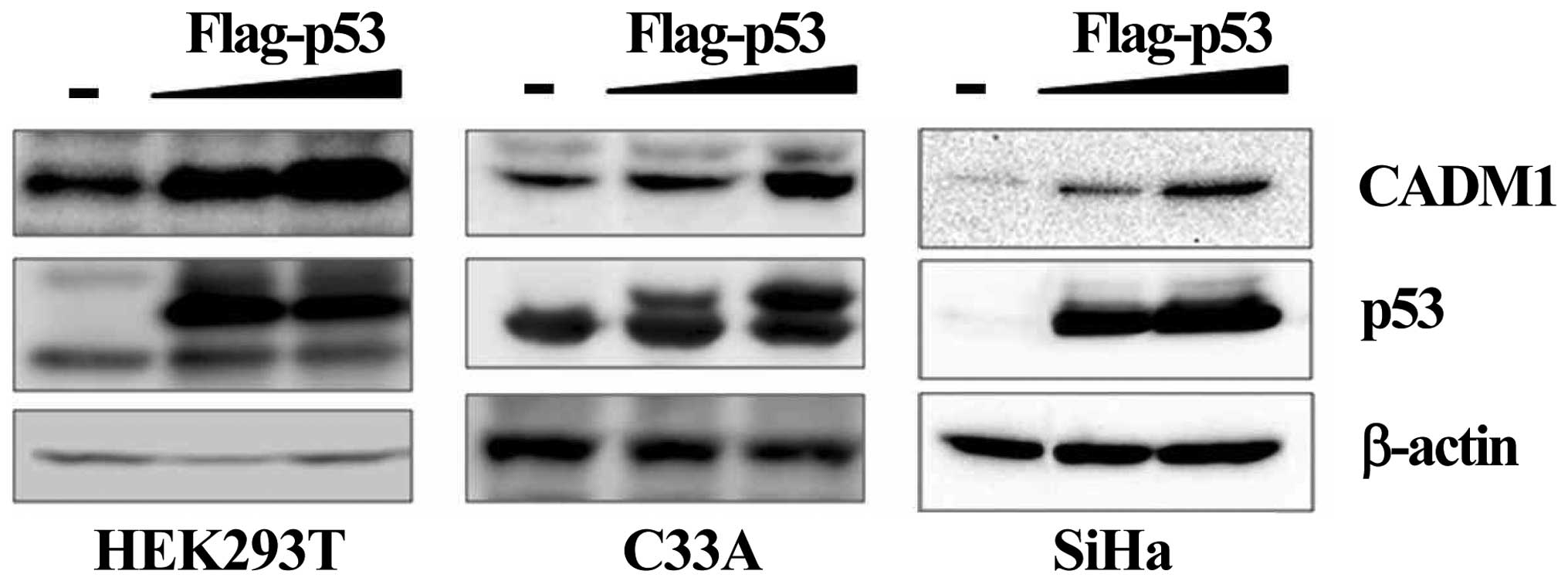
Effect of p53 on the regulation of CADM1
expression
Degradation of the p53 protein via hr-HPV E6 is a
crucial factor for cervical carcinogenesis. In our data (Fig. 3C), treatment with the demethylating
agent 5-aza-dC upregulated the p53 protein and induced the CADM1
reactivation by demethylation. To investigate a potential
mechanistic link between the upregulation of p53 and CADM1
expression in cervical cancer cells, we selected three cell lines,
including HEK293T (non-cervical cancer cell line), C33A, and SiHa
(cervical cancer cell line), for transfection with a flag-tagged
wild-type p53 expression vector (22) or an empty vector. Successful
transfection was confirmed by western blotting, which demonstrated
the upregulation of p53 in the transfected cell lines compared with
mock transfectants (Fig. 4).
Expression of the CADM1 protein was increased by exogenous
expression of p53 in a dose-dependent manner in all cells. We
further analyzed the effect of endogenous p53 on CADM1 expression
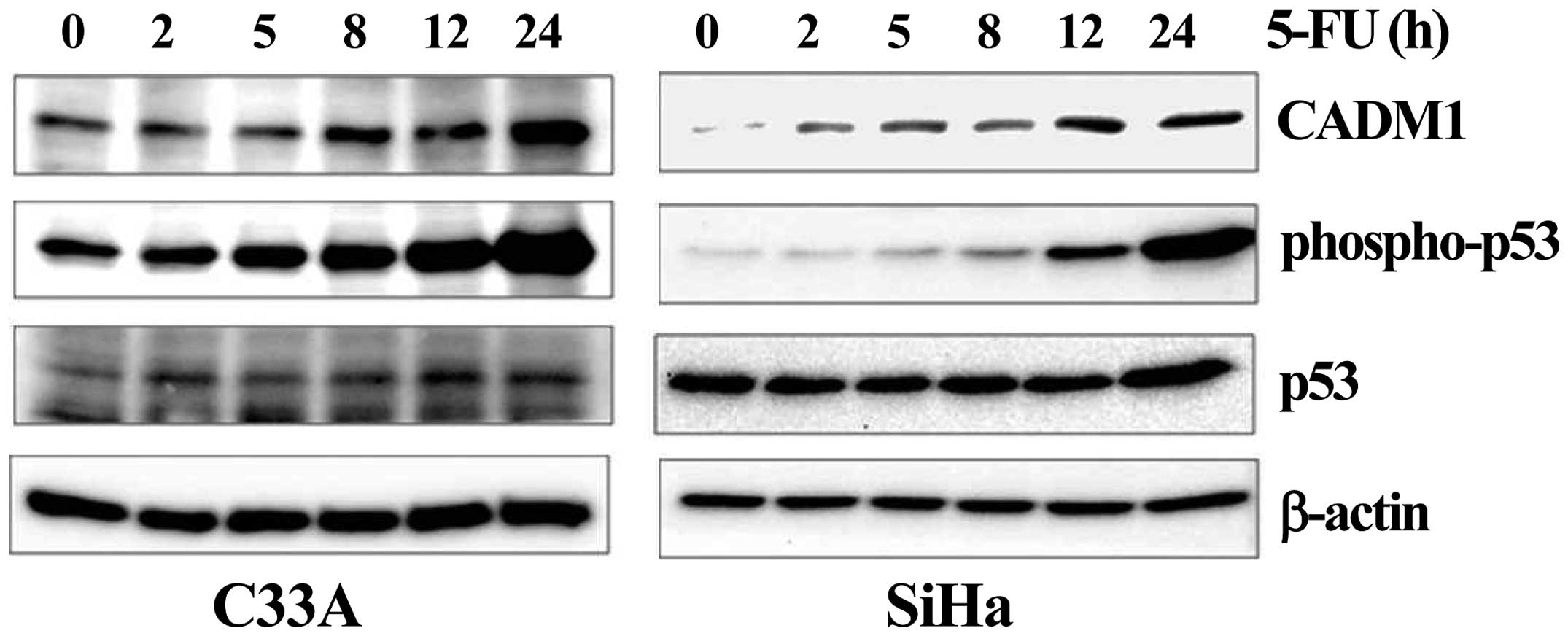
by treatment of C33A and SiHa cell lines with 5-fluorouracil
(5-FU), an inducer of endogenous p53 activation. Induction of the
expression of the phosphorylated p53 active protein was achieved in
the selected cell lines after 5-FU treatment. We observed that, in
all cell lines, increased phospho-p53 markedly enhanced the
expression of CADM1 (Fig. 5).
These results demonstrated that the expression of the CADM1
tumor-suppressor gene maybe under the control of p53 and that CADM1
may be another target of the p53 protein.
Discussion
The cell adhesion molecule 1 (CADM1) gene,
which is also known as TSLC1 or Necl-2, has been
generally investigated as a tumor-suppressor gene in various
tumors, including prostate, esophageal, nasopharyngeal, non-small
cell lung and cervical cancers (16,18,23–25).
Many studies have shown that CADM1, as a tumor-suppressor
gene, is associated with inhibition of cell proliferation, as well
as invasion and induction of apoptosis in various tumor cells,
including the non-small cell lung cancer A549 cell line (26), human esophageal carcinoma Eca109
cells (27), and a hepatocellular
carcinoma cell line (28).
CADM1 is downregulated in many cancers, frequently via
promoter hypermethylation (16,17).
However, to date, little is known about the role of the
hypermethylation of CADM1 in cervical cancer.
In our study, we analyzed the protein and mRNA
expression of CADM1 in various cervical cancer cell lines,
including C33A (HPV-negative), HeLa (HPV18 positive), and SiHa and
CaSki (HPV16-positive) cells, and found that CADM1 was
significantly downregulated in three HPV-induced cervical cell
lines. Moreover, we explored the mechanism underlying the
downregulation of CADM1 in each of the cell lines. It is now known
that CADM1 is expressed universally in human tissues and is
frequently silenced in a variety of human carcinomas, such as lung,
prostate, liver, stomach, pancreatic, and breast carcinoma
(26,29). The silencing of CADM1, which
was first described by Murakami et al (30), was explained by loss of
heterozygosity on chromosome 11q23 (30) and promoter hypermethylation
(16,17). Similarly, the expression of
CADM1 is modulated by genetic and/or epigenetic mechanisms.
In our study, we assessed the methylation status of the promoter
region of CADM1 by pyrosequencing. In three HPV-positive
cancer cell lines (SiHa, HeLa and CaSki), the average methylation
level of CADM1 was 83.9%. Treatment with 5-aza-dC increased
CADM1 expression levels in three cervical carcinoma cell lines that
lack the endogenous CADM1 protein, thus confirming that promoter
methylation is involved in the silencing of CADM1 in
cervical carcinoma. However, an HPV-negative cervical cancer cell
line, C33A, exhibited a methylation level of 2.6%. Based on our
data, the methylation levels of C33A (HPV-negative cervical cancer
cell line) were significantly lower than those of SiHa, HeLa and
CaSki cells (HPV-positive cervical cancer cell lines). Thus,
CADM1 gene silencing might be associated with HPV-mediated
malignant transformation.
A previous study showed that new tumor suppressors,
such as the secreted frizzled-related protein (SFRP) gene,
exhibited higher levels of methylation in the HPV-positive than in
the HPV-negative group in ovarian cancer, and that the increase in
the methylation pattern of the SFRP1 gene might be due to
viral infection and integration into host cells (31). Some authors have also suggested
that HPV interferes with the cellular DNA methylation machinery,
either to conceal itself or as part of its viral cycle (32). The aberrant promoter methylation of
tumor-suppressor genes may result from mistargeted host defenses
during viral integration or as a consequence of genomic instability
caused by HPV infection. To date, there is no evidence that the
silencing of specific genes is linked to the functions of the viral
genes expressed after HPV infection. Further studies are needed to
determine whether CADM1 silencing is induced by HPV
infection.
Two intracellular oncoproteins, E6 and E7, play
important roles in the malignant transformation of HPV-infected
cells (4). As the main player in
cervical carcinogenesis, E6 activities are mediated by E6-dependent
degradation of the tumor-suppressor protein p53. Aberrant
regulation of p53 is crucial for cervical carcinogenesis and, most
importantly, for the maintenance of the malignant phenotype
(4). p53 was activated by
5-aza-dC. 5-Aza-dC treatment is perceived as being DNA damaging and
leads to the activation of the G1 checkpoint regulator p53
(21). Our data showed that p53
expression was increased after 5-aza-dC treatment, independent of
the presence of HPV. The abnormal expression of p53 target genes is
caused by DNA methylation (8–10).
Thus, it was of great interest to investigate whether the
upregulation of p53 affects the regulation of CADM1. In cancer cell
lines with CADM1 methylation, the induction of CADM1
expression after transfection with p53 was achieved.
We further tested the role of p53 by inducing its
endogenous expression using 5-fluorouracil (5-FU). This
chemotherapeutic agent is used to treat cancer cells, as it causes
irreparable DNA damage, thus inducing these aberrant cells to
undergo cell death. It has been reported that 5-FU activates p53
expression and induces p53 target genes during damaged DNA repair
(33). Treatment of the cervical
cancer cell lines with 5-FU led to the increase of the level of
activated p53. Activation of endogenous p53 by 5-FU markedly
enhanced the expression of CADM1 in the cell lines with
unmethylated CADM1. Our data showed that exogenous
expression of p53 or increased phosphorylation of p53 regulated
CADM1 expression both in the cell line (C33A) with less
CADM1 DNA methylation and in the cell line (SiHa) with a
high level of CADM1 methylation. We presumed that CADM1
expression was regulated by p53, even though we do not know exactly
whether p53 binds to the CADM1 promoter directly or via
other mediators. In fact, 5-aza-dC and 5-FU have been considered as
a part of a combination therapy with other anticancer agents to
treat ovarian, breast, prostate, gastric, lung, pancreatic and
colon cancers via the relief of DNA hypermethylation (34) or the growth arrest of cancer cells
(35). These results suggest that
a therapeutic approach that combines DNA methyltransferase
inhibitor drugs that induce the expression or activation of p53 may
be a useful strategy for the treatment of cervical cancer. However,
the genes that are induced by combination therapy are not limited
to CADM1, and the involvement of many unknown or known genes
that were regulated by the demethylation or p53 should be
considered; further studies are necessary to analyze this issue
from different aspects in the context of cervical cancer
treatment.
In conclusion, we demonstrated that the inactivation
of CADM1 was associated with its hypermethylation in
HPV-induced cervical cancer. To our knowledge, this is first study
of the reduction of CADM1 protein levels by epigenetic silencing,
and that CADM1 exerts its tumor-suppressive effect via the
inhibition of cell proliferation in cervical cancer cell lines.
Moreover, CADM1 expression was regulated by exogenously expressed
p53 and activated p53 induced by 5-FU treatment. Based on our
results, we suggest that CADM1 methylation is one of the
common epigenetic alterations in HPV-infected cervical cancers. In
this respect, recovery of CADM1 gene expression may be a
proper target of demethylating agents and p53-regulating drugs in
cervical carcinoma. Although much remains to be clarified regarding
the aberrant methylation of CADM1, further studies should
shed light on the mechanism of cancer development, as well as
identify more effective cancer therapies.
Acknowledgements
This work was supported by an intramural grant (No.
2013-NG51003-00) from the Korea National Institute of Health,
Ministry of Health and Welfare, Republic of Korea.
References
|
1
|
WHO/ICO Information Centre on HPV and
Cervical Cancer. Human papillomavirus and related cancers in
Brazil. Summary Report. 2010, (cited May 12, 2010). Available from:
www.who.int/hpvcentrehttps://www.who.int/hpvcentre.
|
|
2
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ganguly N and Parihar SP: Human
papillomavirus E6 and E7 oncoproteins as risk factors for
tumorigenesis. J Biosci. 34:113–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moody CA and Laimins LA: Human
papillomavirus oncoproteins: Pathways to transformation. Nat Rev
Cancer. 10:550–560. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kern SE, Kinzler KW, Bruskin A, Jarosz D,
Friedman P, Prives C and Vogelstein B: Identification of p53 as a
sequence-specific DNA-binding protein. Science. 252:1708–1711.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akeno N, Miller AL, Ma X and
Wikenheiser-Brokamp KA: p53 suppresses carcinoma progression by
inhibiting mTOR pathway activation. Oncogene. 34:589–599. 2015.
View Article : Google Scholar
|
|
8
|
Karakoula K, Jacques TS, Phipps KP,
Harkness W, Thompson D, Harding BN, Darling JL and Warr TJ:
Epigenetic genome-wide analysis identifies BEX1 as a candidate
tumour suppressor gene in paediatric intracranial ependymoma.
Cancer Lett. 346:34–44. 2014. View Article : Google Scholar
|
|
9
|
Christoph F, Kempkensteffen C, Weikert S,
Krause H, Schostak M, Miller K and Schrader M: Frequent epigenetic
inactivation of p53 target genes in seminomatous and
nonseminomatous germ cell tumors. Cancer Lett. 247:137–142. 2007.
View Article : Google Scholar
|
|
10
|
Suzuki H, Itoh F, Toyota M, Kikuchi T,
Kakiuchi H and Imai K: Inactivation of the 14-3-3 sigma gene is
associated with 5′ CpG island hypermethylation in human cancers.
Cancer Res. 60:4353–4357. 2000.PubMed/NCBI
|
|
11
|
Sova P, Feng Q, Geiss G, Wood T, Strauss
R, Rudolf V, Lieber A and Kiviat N: Discovery of novel methylation
biomarkers in cervical carcinoma by global demethylation and
microarray analysis. Cancer Epidemiol Biomarkers Prev. 15:114–123.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banzai C, Nishino K, Quan J, Yoshihara K,
Sekine M, Yahata T and Tanaka K: Gynecological Cancer Registry of
Niigata: Promoter methylation of DAPK1, FHIT, MGMT, and CDKN2A
genes in cervical carcinoma. Int J Clin Oncol. 19:127–132. 2014.
View Article : Google Scholar
|
|
13
|
Portela A and Esteller M: Epigenetic
modifications and human disease. Nat Biotechnol. 28:1057–1068.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Esteller M: Cancer epigenomics: DNA
methylomes and histone-modification maps. Nat Rev Genet. 8:286–298.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bierkens M, Hesselink AT, Meijer CJ,
Heideman DA, Wisman GB, van der Zee AG, Snijders PJ and Steenbergen
RD: CADM1 and MAL promoter methylation levels in hrHPV-positive
cervical scrapes increase proportional to degree and duration of
underlying cervical disease. Int J Cancer. 133:1293–1299. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuramochi M, Fukuhara H, Nobukuni T, Kanbe
T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura
T, et al: TSLC1 is a tumor-suppressor gene in human non-small-cell
lung cancer. Nat Genet. 27:427–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murakami Y: Involvement of a cell adhesion
molecule, TSLC1/IGSF4, in human oncogenesis. Cancer Sci.
96:543–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steenbergen RD, Kramer D, Braakhuis BJ,
Stern PL, Verheijen RH, Meijer CJ and Snijders PJ: TSLC1 gene
silencing in cervical cancer cell lines and cervical neoplasia. J
Natl Cancer Inst. 96:294–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Overmeer RM, Henken FE, Snijders PJ,
Claassen-Kramer D, Berkhof J, Helmerhorst TJ, Heideman DA, Wilting
SM, Murakami Y, Ito A, et al: Association between dense CADM1
promoter methylation and reduced protein expression in high-grade
CIN and cervical SCC. J Pathol. 215:388–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang WS, Park SO, Yoon AR, Yoo JY, Kim MK,
Yun CO and Kim CW: Suicide cancer gene therapy using pore-forming
toxin, streptolysin O. Mol Cancer Ther. 5:1610–1619. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karpf AR, Moore BC, Ririe TO and Jones DA:
Activation of the p53 DNA damage response pathway after inhibition
of DNA methyltransferase by 5-aza-2′-deoxycytidine. Mol Pharmacol.
59:751–757. 2001.PubMed/NCBI
|
|
22
|
Yoon CH, Rho SB, Kim ST, Kho S, Park J,
Jang IS, Woo S, Kim SS, Lee JH and Lee SH: Crucial role of TSC-22
in preventing the proteasomal degradation of p53 in cervical
cancer. PLoS One. 7:e420062012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukuhara H, Kuramochi M, Fukami T,
Kasahara K, Furuhata M, Nobukuni T, Maruyama T, Isogai K, Sekiya T,
Shuin T, et al: Promoter methylation of TSLC1 and tumor suppression
by its gene product in human prostate cancer. Jpn J Cancer Res.
93:605–609. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ito T, Shimada Y, Hashimoto Y, Kaganoi J,
Kan T, Watanabe G, Murakami Y and Imamura M: Involvement of TSLC1
in progression of esophageal squamous cell carcinoma. Cancer Res.
63:6320–6326. 2003.PubMed/NCBI
|
|
25
|
Lung HL, Cheung AK, Xie D, Cheng Y, Kwong
FM, Murakami Y, Guan XY, Sham JS, Chua D, Protopopov AI, et al:
TSLC1 is a tumor suppressor gene associated with metastasis in
nasopharyngeal carcinoma. Cancer Res. 66:9385–9392. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao X, Seidlitz E, Truant R, Hitt M and
Ghosh HP: Re-expression of TSLC1 in a non-small-cell lung cancer
cell line induces apoptosis and inhibits tumor growth. Oncogene.
23:5632–5642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang QL, Wang BR, Li ZY, Chen GQ and Zhou
Y: Effect of TSLC1 gene on growth and apoptosis in human esophageal
carcinoma Eca109 cells. Arch Med Sci. 8:987–992. 2012. View Article : Google Scholar
|
|
28
|
Qin L, Zhu W, Xu T, Hao Y, Zhang Z, Tian Y
and Yang D: Effect of TSLC1 gene on proliferation, invasion and
apoptosis of human hepatocellular carcinoma cell line HepG2. J
Huazhong Univ Sci Technolog Med Sci. 27:535–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shingai T, Ikeda W, Kakunaga S, Morimoto
K, Takekuni K, Itoh S, Satoh K, Takeuchi M, Imai T, Monden M, et
al: Implications of nectin-like
molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion
and transmembrane protein localization in epithelial cells. J Biol
Chem. 278:35421–35427. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murakami Y, Nobukuni T, Tamura K, Maruyama
T, Sekiya T, Arai Y, Gomyou H, Tanigami A, Ohki M, Cabin D, et al:
Localization of tumor suppressor activity important in nonsmall
cell lung carcinoma on chromosome 11q. Proc Natl Acad Sci USA.
95:8153–8158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Shabanah OA, Hafez MM, Hassan ZK,
Sayed-Ahmed MM, Abozeed WN, Alsheikh A and Al-Rejaie SS:
Methylation of SFRPs and APC genes in ovarian cancer infected with
high risk human papillomavirus. Asian Pac J Cancer Prev.
15:2719–2725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leonard SM, Wei W, Collins SI, Pereira M,
Diyaf A, Constandinou-Williams C, Young LS, Roberts S and Woodman
CB: Oncogenic human papillomavirus imposes an instructive pattern
of DNA methylation changes which parallel the natural history of
cervical HPV infection in young women. Carcinogenesis.
33:1286–1293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pritchard DM, Watson AJ, Potten CS,
Jackman AL and Hickman JA: Inhibition by uridine but not thymidine
of p53-dependent intestinal apoptosis initiated by 5-fluorouracil:
Evidence for the involvement of RNA perturbation. Proc Natl Acad
Sci USA. 94:1795–1799. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shames DS, Minna JD and Gazdar AF: DNA
methylation in health, disease, and cancer. Curr Mol Med. 7:85–102.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|